Supplemental digital content is available in the text.
Abstract
Background
Living donor liver transplantation (LDLT) is increasingly used to bridge the gap between the current supply and demand imbalance for deceased donor organs to provide lifesaving liver transplantation.
Methods
Outcomes of 135 children who underwent LDLT were compared with 158 recipients of deceased donor liver transplantation (DDLT) at the largest pediatric liver transplant program in Canada.
Results
Recipients of LDLT were significantly younger than deceased donor recipients (P ≤ 0.001), less likely to require dialysis pretransplant (P < 0.002) and had shorter wait time duration when the primary indication was cholestatic liver disease (P = 0.003). The LDLT donors were either related genetically or emotionally (79%), or unrelated (21%) to the pediatric recipients. One-, 5-, and 10-year patient survival rates were significantly higher in LDLT (97%, 94%, and 94%) compared with DDLT (92%, 87%, and 80%; log-rank P = 0.02) recipients, as were graft survival rates (96%, 93%, and 93% for LDLT versus 89%, 81.4%, and 70%, respectively, for DDLT; log-rank P = 0.001). Medical and surgical complications were not statistically different between groups. Graft failure was higher in recipients of DDLT (odds ratio, 2.60; 95% confidence interval, 1.02, 6.58) than in the LDLT group after adjustment for clinical characteristics and propensity score.
Conclusions
Living donor liver transplantation provides superior outcomes for children and is an excellent and effective strategy to increase the chances of receiving a liver transplant.
Liver transplantation (LT) is an established lifesaving treatment for children with a spectrum of etiologies including biliary atresia (BA), pediatric acute liver failure (PALF), metabolic liver conditions, tumors, autoimmune liver diseases, and other cholestatic diseases.1-3 As overall outcomes have improved, expanding indications for pediatric LT include metabolic liver conditions,4 underscoring the need for programs to address the shortage of donor organs as an impedance to access for patients requiring LT. Given an aging population and the rising incidence of obesity, it is sobering to acknowledge that the rate of high quality deceased donors (DD) organs is not anticipated to be on the increase.5 Waiting times for pediatric LT are growing worldwide.6 In Asia, live donation was developed to alleviate the lack of access to deceased organs.7 Although deceased donor liver transplantation (DDLT) remains the standard of care in North America, the supply imbalance remains problematic. Living donor liver transplantation (LDLT) is an important option for centers to meet the needs of patients requiring LT.8
The practical and theoretical advantages of LDLT include preemptive and earlier timing of hepatic replacement surgery before serious clinical decompensation, facilitation of high-quality grafts via thorough live donor (LD) evaluations, and minimization of preservation injury to the graft with decreased cold ischemia times (CITs).9-13 Potential immunological benefits for recipients of organs from genetically related donors have also been reported.14 Challenges faced by LDLT programs include reducing donor morbidity without compromise to recipient outcomes, as well as ensuring the live donation process remains voluntary, altruistic, and noncoercive.15
Published pediatric experience with LDLT in North America is limited to small patient cohorts,16-19 and do not address the many clinical variables that may impact the outcomes of LDLT compared to DDLT. The aims of this study were to determine the short- and long-term outcomes of pediatric LDLT performed in the largest pediatric LT program in Canada, to compare these outcomes with a contemporaneous cohort of pediatric recipients of DDLT, and to identify variables that predict patient and graft outcomes.
PATIENTS AND METHODS
Study Design
This was a retrospective cohort study of all consecutive patients undergoing isolated LT between 2000 and 2015 at The Hospital for Sick Children (SickKids) in Toronto, Canada. This study was approved by the SickKids Research Ethics Board. The first pediatric recipient of LDLT occurred in October 1996; however, due to program restructuring, the LDLT program was not operational until 2000 and a formal collaboration was developed with the Living Donor Office and the adult LT program at the Toronto General Hospital, University Health Network (UHN).8
All pediatric LT recipient care and follow-up take place exclusively at SickKids, as previously described.20 LT surgeons with appointments at both SickKids and UHN perform the donor and recipient surgeries. Briefly, at the time of candidate listing, all parents are provided with LD information, including contact information for the UHN Living Donor Office. Prospective donors are fully informed of their rights to terminate donor evaluation at any stage in the process. Evaluations, investigations, consultations, operations, perioperative care, and subsequent follow-up of all live liver donors are centralized at and provided by, the UHN adult LT program. All recipients were required to meet our criteria for DDLT, be formally listed and maintained on the DD waitlist until the day of LDLT. Since 2006, the UHN Living Donor office has also evaluated anonymous donors for both directed and nondirected donation,21 preferentially recommending pediatric patients when available due to the lower reported risk with left or left lateral lobe compared with right lobe resection surgery, and prioritization to those with the highest medical need (pediatric end-stage liver disease [PELD] or Model for End-Stage Liver Disease [MELD] score).8,22 An anonymous donor was defined as one who had no biological connection and whose identity was unknown to the recipient when starting the assessment and until the LT surgical date.22 When evaluating anonymous donor candidates, particular attention is paid to motivation, decision-making, prior altruism, consideration of other forms of community service, and social support. Donors are reminded that Canadian law prohibits profiting in any material way from the donation. All recipients and donors were Canadian citizens.
Study Population
Inclusion criteria included all recipients younger than 18 years at the time of first isolated LT between May 1, 2000, and December 31, 2015. Recipients of multiorgan transplants and liver retransplant recipients were excluded.
Data Collection
Information from institutional electronic patient record included demographics, clinical data (primary diagnosis, time on the waitlist, comorbid events, prior organ availability, PELD or MELD scores at the time of transplant), and targeted laboratory values. For subjects receiving LDLT, the relationship between the donor (mother, father, other genetic relatives, emotionally related [friends or neighbors], or anonymous) and recipients was recorded.
Perioperative data collected included allograft type, type of biliary anastomosis, CIT, warm ischemia time (WIT), and key immediate post-LT complications (for definitions Table S1, SDC, http://links.lww.com/TXD/A178),23 the number of days intubated, duration of stay in the pediatric intensive care unit (PICU), time to first discharge from the hospital, acute cellular rejection (ACR), chronic rejection (CR), site and histopathology of posttransplant lymphoproliferative disease (PTLD), immunosuppression details and concomitant medications were also collected. Kidney function at 1 year after LT was assessed by calculated glomerular filtration rate using the modified Schwartz formula (based on serum creatinine) and recorded utilization of antihypertensive agents.24,25
Clinical Protocols
The techniques of LD hepatectomy performed at our institution have been described previously.26-28 All study patients followed institutional protocols for immunosuppression, which were unchanged during the study duration.29,30 Briefly, standard induction immunosuppression was comprised of dual therapy including tacrolimus and corticosteroids, with steroid taper over 3 months to discontinuation. Patients with clinically noted oliguria, hepatorenal syndrome, or dialysis requirement before LT received a kidney-sparing protocol comprised of corticosteroids, either antithymocyte globulin or 2 doses of basiliximab (Simulect; Novartis, Basel, Switzerland), until normalization of creatinine or improving urine output at which time tacrolimus would be started. Mycophenolate mofetil as adjunctive therapy was also added once these patients with pre-LT renal dysfunction were eating well. For recipients with a primary diagnosis of hepatoblastoma, tacrolimus was replaced by sirolimus starting at postoperative day 30.29,30 Diagnosis of ACR required a liver biopsy for histopathological confirmation, with treatment initiated with a biopsy interpretation of Rejection Activity Index of 4 or greater of 9.31 Chronic rejection was diagnosed as per the updated Banff criteria.32
Statistical Analyses
Descriptive statistics were calculated for demographic and clinical variables. Continuous data were reported as medians and interquartile ranges (IQR). Categorical variables were reported as count and proportions. Comparisons of data between LDLT and DDLT groups were performed using χ2, t tests, and Fisher exact or rank-sum tests as appropriate. For calculation of ACR-free survival, data were censored at the first episode of biopsy-proven rejection. Patient and graft survival rates were evaluated using the Kaplan-Meier methods with censoring at the time of death or retransplantation/death and compared using the log-rank test. Multivariable Cox proportional hazard regression models were built with important clinical variables that reached a significance of P < 0.2 by univariable analysis.
We also constructed models adjusting for the propensity score using covariate analysis. The propensity score for each subject was calculated based on variables that differed between LDLT and DDLT recipients at baseline and included age, diagnosis, weight, and height at LT, dialysis requirement, wait time and serum gamma-glutamyltransferase level. Separate multiple regression models were subsequently developed with graft or patient outcome serving as the dependent variable, and allograft (DDLT vs LDLT) type as the main predictor variable while adjusting for propensity score and other variables which were significant on univariate analysis. This allowed us to estimate the graft or patient outcome associated with the allograft type of interest while reducing confounding effects by adjusting for the propensity score, the probability of receiving either DDLT or LDLT. Statistical analyses were conducted using SAS, and a P value <0.05 was considered significant.
RESULTS
Study Population
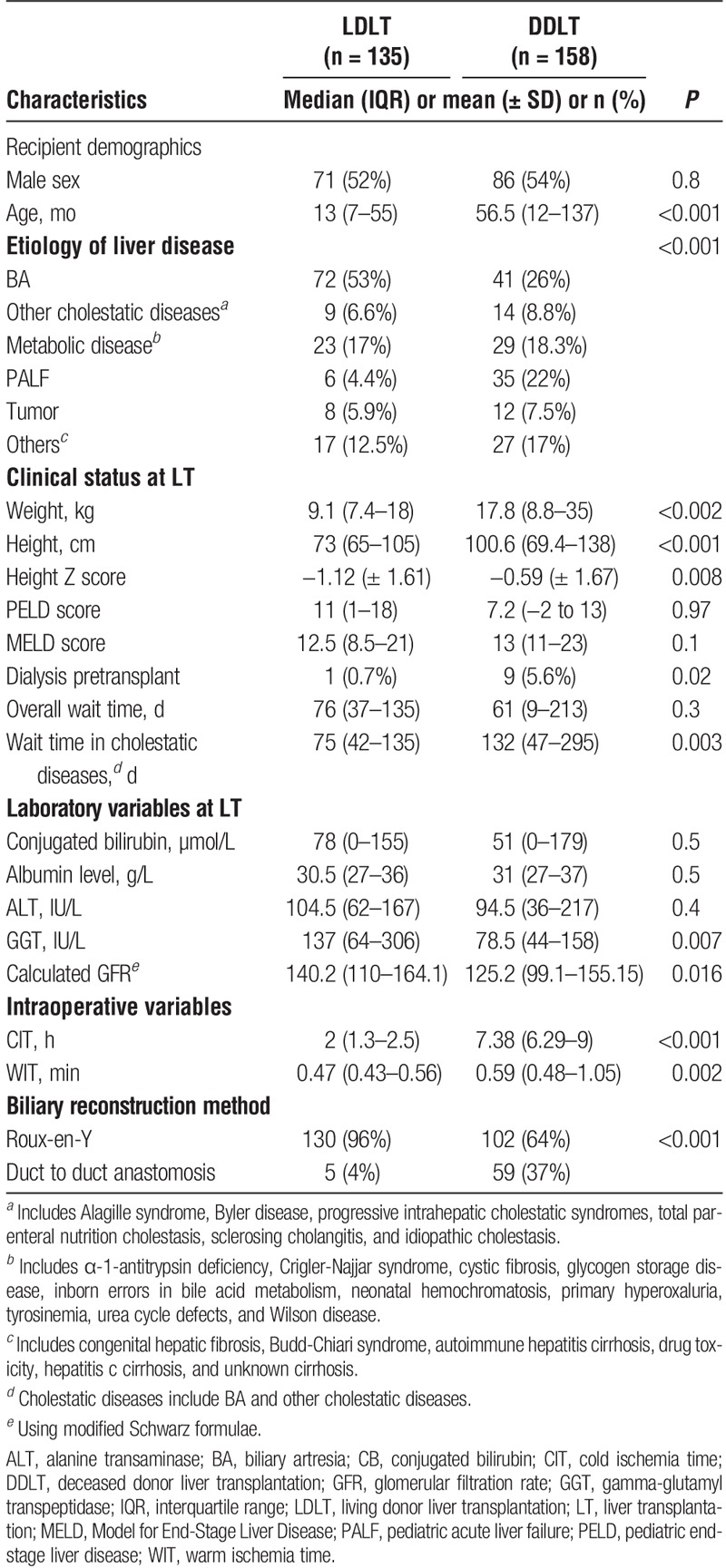
Among 293 consecutive pediatric LT recipients, 46% received LDLT (n = 135) and 54% underwent DDLT (n = 158) (Table 1). LDLT recipients were significantly younger than DDLT recipients (P ≤ 0.001). Recipients of LDLT were less likely (n = 1, 0.7%) to require dialysis pre-LT than DDLT (n = 9, 5.6% (P < 0.002). The most common indication for pediatric LT was BA (39%), occurring twice as frequently in recipients of LDLT (53%) than DDLT (26%, P = 0.001). Children presenting with PALF underwent LT with a graft from a LD less often (6/41, 10%) than from a DD (35/41, 90%). Wait times were not statistically different between DDLT (median, 61 d; IQR, 9, 213 d) and LDLT (median, 76 d; IQR, 37, 135 d; P = 0.3) recipients. However, among patients with a primary etiology of chronic cholestatic liver disease, median wait time duration was statistically shorter among those in receipt of LDLT (75 days) compared to DDLT (132 d, P = 0.003). The majority of LD were genetically related to the recipient; however, approximately 16% of children in our LDLT cohort received an allograft from an anonymous donor. Calculated PELD and MELD scores at the time of LT were not statistically significant between LDLT and DDLT groups. The median duration of follow-up was 2.2 years for LDLT and 3.5 years for DDLT recipients.
TABLE 1.
Clinical characteristics of 293 pediatric LT recipients by allograft
Intraoperative and Perioperative Courses
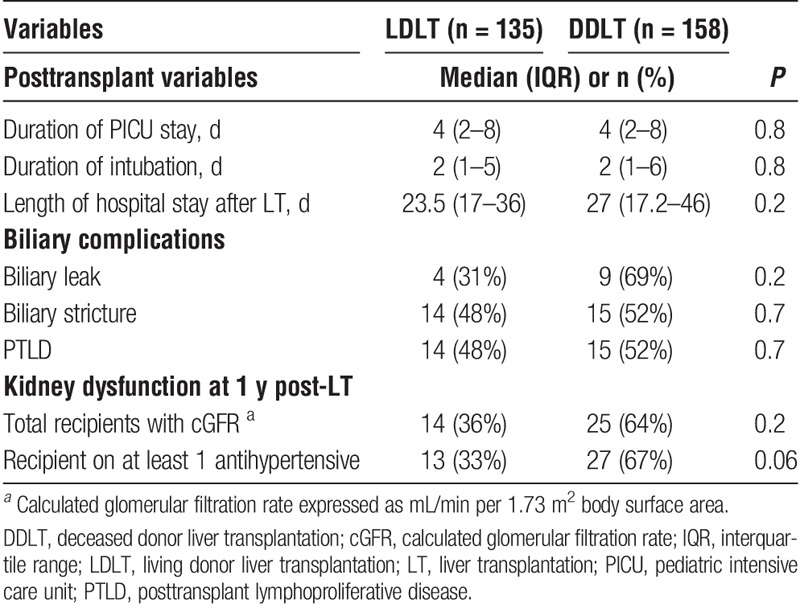
Grafts from live donors provided left lateral (n = 112, 82.8%), left (n = 5, 3.7%) and right (n = 18, 13.4%) lobes to our pediatric recipients. Genetically related donors were most frequently parents (n = 63, 61%) with allografts from mothers (n = 52/83, 63%) most frequently used. DD organs provided whole (n = 76, 48%), reduced (n = 49, 31%) and split (n = 33, 21%) allografts. CIT was significantly lower in LDLT (median, 2 h; IQR, 1.3, 2.5 h) than in DDLT (median, 7.38 h; IQR, 6.29, 9 h; P < 0.001) recipients. WIT was also significantly shorter in recipients of LDLT (47 min; IQR, 43, 56 min) than DDLT (59 min; IQR, 48, 65 min; P = 0.002). Time to extubation after LT, duration of PICU stay, as well as time to first discharge from the hospital, were not statistically different between recipients of LDLT and DDLT. Key posttransplant characteristics and complications noted during the intraoperative and perioperative course are provided in Table 2.
TABLE 2.
Posttransplantation course and complications
Surgical Complications
There were a total of 42 biliary complications identified among all LT recipients (Table 2). No difference in the prevalence of biliary or vascular complications was noted between recipients of DDLT versus LDLT. There were a total 38 vascular complications, inclusive of hepatic artery thrombosis (HAT) (LDLT n = 5; DDLT n = 5, P = 0.8), portal vein complications (LDLT, n = 12; DDLT, n = 6, P = 0.07), and hepatic vein complications (LDLT, n = 5; DDLT, n = 5, P = 0.8).
Immunosuppressive Use at 1 Year Posttransplantation
By 1 year after LT, the majority of patients have prescribed either tacrolimus (84%) or sirolimus (16%). All hepatoblastoma patients (6%) transitioned from tacrolimus over to sirolimus after postoperative day 30 as per our program practice.30 Mycophenolate mofetil was used as adjuvant therapy in 10%. Nine percent of recipients were still on steroids, with the indication being the treatment of recently diagnosed ACR. There was no statistically significant difference in immunosuppressive agent utilization at 1 year after LDLT and DDLT.
Acute Cellular Rejection and Chronic Rejection
The 1-, 3- and 5-year ACR-free survival rates were 64.4%, 61.1%, and 61.1%, respectively, in the LDLT group compared with 55%, 44.4%, and 43.4% in the DDLT group (hazard ratio [HR], 0.73; 95% confidence interval [CI], 0.51-1.04; P = 0.08) (Figure 1A). Histologically confirmed CR was diagnosed in two DDLT recipients and recorded as the indication for retransplantation at 5.4 years and 13.9 years after initial LT. There were no histologically confirmed CR episodes reported in the LDLT group.
FIGURE 1.
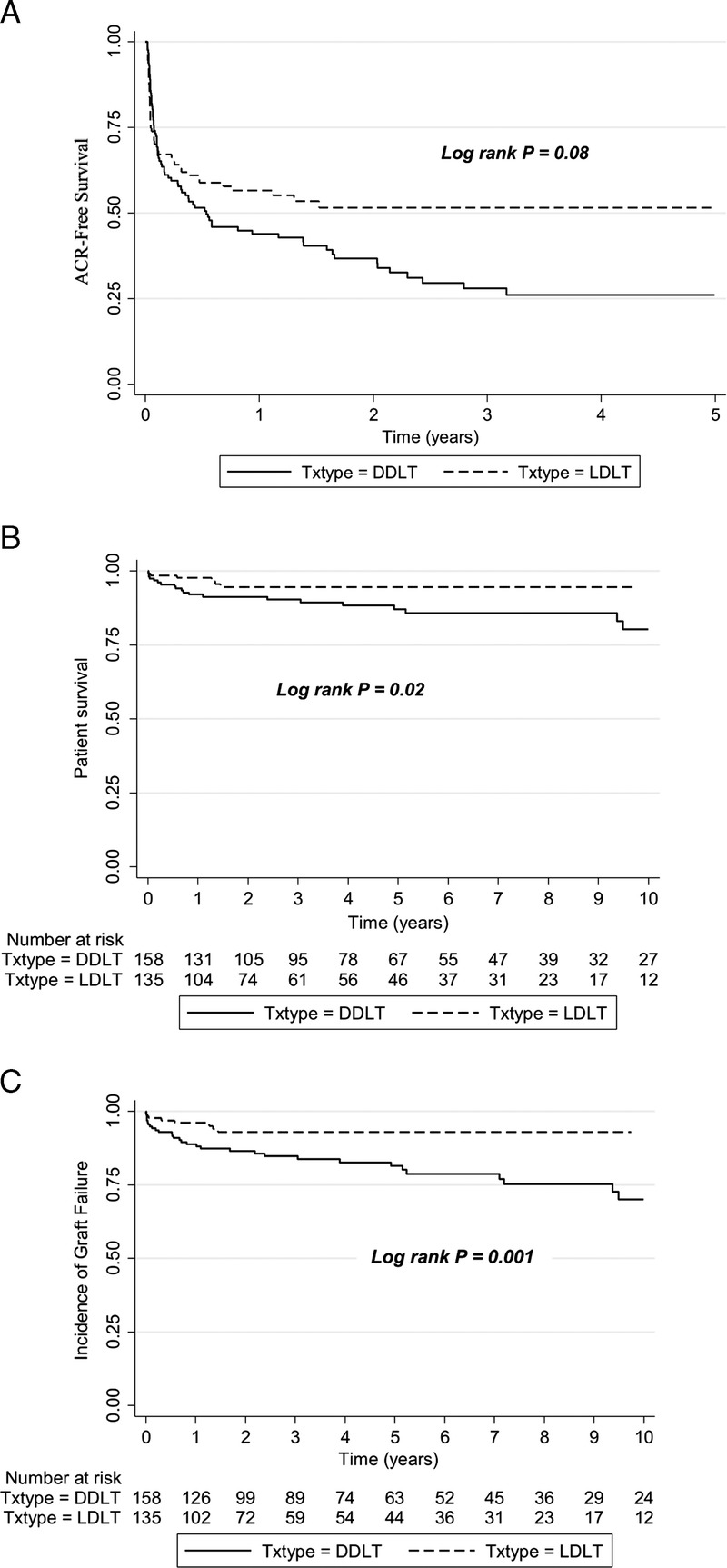
A, ACR-free survival in recipients of LDLT and DDLT at The Hospital for Sick Children, 2000 to 2015. B, Patient survival after pediatric LDLT and DDLT at The Hospital for Sick Children, 2000 to 2015. C, Graft survival after pediatric LDLT and DDLT at The Hospital for Sick Children, 2000 to 2015. ACR, acute cellular rejection; DDLT, deceased donor liver transplantation; LDLT, living donor transplantation.
Posttransplant Lymphoproliferative Disease
PTLD was diagnosed in 15 DDLT and 14 LDLT recipients. Tonsils and adenoids were the commonest PTLD sites in DDLT (40%) and LDLT (43%) recipients, representing our program's practice of preemptive otolaryngology consultation with any clinical note of snoring or clinically detected tonsillar enlargement.33 PTLD was also diagnosed in the gastrointestinal tract (n = 5, 33% DDLT and 57% LDLT) and others (27% DDLT). The prevalence of PTLD was not statistically different between DDLT versus LDLT (P = 0.7) groups.
Kidney Dysfunction
Evaluation of renal function at 1 year post-LT revealed stage 2 chronic kidney disease in 39 (13%) patients, with no statistically significant difference between those receiving LD (n = 14, 10.3%) or DD (n = 25, 16%; P = 0.2) allografts. At least one antihypertensive medication was being taken by 40 (13.6%) patients at 1 year post-LT follow-up, with no statistical difference by LDLT (n = 13) and DDLT status (n = 27; P = 0.06).
Patient and Graft Survival
Overall 1-, 5-, and 10-year patient survivals for the entire cohort was 95%, 90%, and 86%, respectively. A total of 6 (4.4%) deaths were noted among LDLT recipients, with sepsis being the most common (n = 3) cause. Among DDLT recipients, there were 21 (13.2%) deaths, with sepsis (n = 5, 24%) being again the most common cause, followed by hepatoblastoma recurrence in 3 (14%).
Overall 1-, 5-, and 10-year graft survivals for the whole cohort was 92%, 86%, and 79%, respectively. A total of 42 graft failure events occurred, in 8 (6%) LDLT and 34 (22%) DDLT subjects, leading to retransplantation in 17 (40%) and death in 24 (57%). Median time to graft failure was 9 (IQR, 0.8, 47) months. Retransplantation occurred in a total of 17 (LDLT n = 2, DDLT n = 15) subjects. Among LD recipients, graft failure was due to primary nonfunction and HAT occurring at a median 93.5 days posttransplantation. In the DDLT group, indications for retransplantation included HAT (n = 4), CR (n = 2), disease recurrence (n = 2), ischemic cholangiopathy (n = 2), and one each for primary nonfunction, de novo autoimmune hepatitis, Budd-Chiari, recurrence of hemophagocytic lymphohistiocytosis, and intestinal failure-associated liver disease.
Risk Factors for Patient and Graft Survivals
One-, 5-, and 10-year patient survival rates after LDLT (97%, 94%, and 94%) were significantly higher than after DDLT (92%, 87%, and 80%) (P = 0.02) (Figure 1B). Univariate Cox proportional hazard regression models identified allograft type (DD), higher PELD score at the time of LT, and etiology of primary liver disease (diagnosis other than BA) as risk factors for poor patient survival. In multiple regression analysis, with propensity score covariate adjustment, higher PELD score at the time of LT remained the only significant risk factor for decreased patient survival (P < 0.0001) (Table 3).
TABLE 3.
Risk factors for graft and patient survival after pediatric LT multivariate analyses
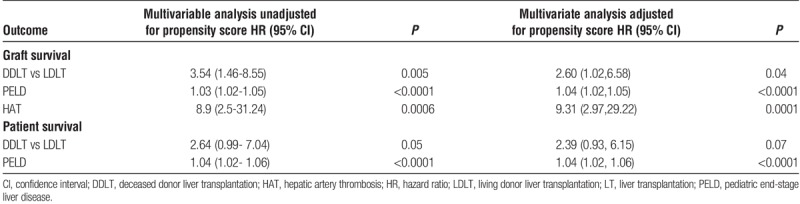
Graft survival at 1, 5, and, 10 years for LDLT recipients was 96.1%, 93%, and 93%, respectively, which was significantly higher than 88.8%, 81%, and 70% in recipients of a DD allograft (P = 0.001) (Figure 1C). We also analyzed the impact of DD allograft (whole, reduced, and split) type and LD allograft. In comparison to DD whole graft recipients, children who underwent LDLT had better graft survival (HR, 0.31; 95% CI, 0.15-0.76). There was no difference in graft survival between DD whole graft and technical variant (neither reduced [HR, 1.1; 95% CI, 0.52-2.4] nor split [HR, 1.1; 95% CI, 0.45-2.7]) allografts. Univariate analyses revealed allograft type (DD), higher PELD score, etiology of primary liver disease (diagnosis other than BA), and HAT to be closely associated with decreased graft survival. Allograft type, PELD scores, and HAT were determined to be independent factors associated with graft survival in multiple regression analysis after propensity score covariate adjustment (Table 3). The propensity score was generated using variables which were significantly different at baseline between DDLT and LDLT, including indication for pediatric LT.
Outcomes of Anonymous LDLT
A total of 22 (16%) children underwent LT with an organ from a live anonymous donor (LAD), with a median follow-up of 14 months (IQR, 3, 51 mo). Biliary atresia was the most common (n = 10, 42%) indication. Median recipient age at the time of anonymous LDLT was 14 months (IQR, 9, 55 mo). Patient and graft survival rates at 1 and 5 years after anonymous LDLT were 95% and 95%.
Effect of Donor-Recipient Relationship on Outcomes After LDLT
Amongst recipients of a LD graft from parent (n = 83), non-parent/genetically related (n = 24), and non parent/genetically unrelated emotionally related and anonymous, (n = 28) donors, no statistical differences in patient survival (P = 0.4) or graft survival (P = 0.4) were found between these three subgroups. There was also no statistically significant difference in the likelihood of ACR developing in recipients of an LDLT organ compared with any of the 3 graft types from a DD (P = 0.5).
DISCUSSION
This is the largest single-center experience of LDLT in children reported by a single North American LT program. Currently, LDLT constitutes approximately half of the pediatric LT performed each year at our center. We report LDLT outcomes with 10-year patient survival rate of greater than 94%, higher than previously reported survival rates after adjustment for confounding factors.34 There were no differences in surgical or medical complications after LDLT in comparison with a contemporaneous cohort of consecutive DDLT performed in the same period. In comparison to DD whole graft recipients, children who underwent LDLT had better graft survival. This experience suggests that access to LT with superior outcomes are achievable if more centers in the Western world in regions with prolonged wait times uniformly and vigorously embraced the option of LDLT.
Excellent outcomes after LDLT in children have previously been reported from the Japanese and Eurotransplant registry data, and single-center experiences from large pediatric programs in Europe and Asia.34-37 Graft survival at 3 years post pediatric LDLT have been reported as 90.7% and patient survival at 3 years have been reported at 91.4%.19 Data from Japanese Liver Transplant Society, the largest pediatric LDLT cohort in the world, demonstrate 5- and 20-year patient survival rates of 85.4% and 79.6%, respectively.34 We extend these findings by demonstrating significantly higher 1-, 5-, and 10-year patient survival rates in pediatric recipients of LDLT compared to DDLT in children with similar characteristics. This contrasts to findings of no difference in overall 1- and 3-year patient survival rates reported in recipients of pediatric LDLT and DDLT performed in Turkey and Wisconsin.19
The similarly excellent 1- and 5-year LDLT graft survival rates of 92% and 89%, respectively, compared with the lower 80% and 77%, respectively, among a Belgium cohort undergoing DDLT were also attributed to lower ischemic time in LDLT recipients.38 In the Scientific Registry of Transplant Recipients cohort, graft outcomes for children younger than 1 year who underwent LDLT were higher than those who underwent DDLT.39 We also found no differences in perioperative vascular and biliary or medical complications between patients receiving LDLT or DDLT. This is consistent with our center's report of an overall biliary complication rate of 16.7% with no differences between those receiving LDLT versus DDLT40 but contrasts with the published literature reporting a higher risk of biliary complications associated with LDLT in adults.41 The reason for this difference might be due to the refinement of surgical techniques, different indications for LT in children, superior vascularization of the biliary plate in left lateral segment grafts, and the predominant use of Roux-en-Y reconstruction in pediatric LDLT.
Controversy exists in the literature regarding whether ACR rates are lower in children who undergo LDLT. Studies exist showing both ACR rates to be lower,19,42 higher,38 or no difference between recipients of LDLT and DDLT.43,44 Among our patients, we did not identify any differences in rejection episodes between recipients of LDLT and DDLT. In addition, we did not find a difference in ACR-free survival rates among children who receive LD grafts from parents, from genetically related versus nongenetically related, nor anonymous donors. This perhaps suggests that HLA matching may not have an added benefit. However, limited power may be contributory due to the small sizes of our 2 subgroups. It has been postulated that the favorable effect of improved HLA matching in LDLT could be nullified by unknown effects including possible lower secretion of donor-soluble HLA antigens by the LDLT graft with a consequent lower tolerogenic effect.45 More work on the role of donor-soluble HLA and exploring the donor-recipient relationship effect on rejection is needed to address these questions in the future.
Retransplant rates among LDLT recipients were lower than among those receiving DDLT. Our higher retransplant rates amongst DDLT recipients may be the result of multiple factors including higher quality grafts from the meticulous LD work-up, as well as to lower CIT feasible in the journey to LDLT. Similar to results shown in other studies, retransplantation rates were higher in our recipients of pediatric DDLT,12 likely a result of multiple factors including increased postischemic injury from longer ischemic times, graft edema and parenchymal resistance occurring after revascularization.46,47 In our cohort, WIT and CIT were longer in DDLT as compared with LDLT. The additional WIT is most likely related to an additional venous anastomosis that is required in many DDLT. While LDLT involves a hepatic vein anastomosis and portal vein anastomosis prior to reperfusion, DDLT with whole grafts or full left lobe grafts include the IVC and require suprahepatic IVC, infrahepatic IVC, and portal vein anastomoses. The additional anastomosis adds approximately 10-15 minutes, accounting for the longer WIT in DDLT recipients.
Although DDLT remains the standard of care for LT in most jurisdictions across the world including Canada, the demand for feasible DD organs far exceeds the available supply. A recent analysis from the United States highlighted that there was an 8% wait-time mortality among children with infants being disproportionately affected.39 Yet, in the United States, despite growing waiting lists and significant waitlist mortality, only 11% of children receive a live donor graft.48,49 When considering replication of our experience at other centers in North America and Europe, we acknowledge many healthcare system advantages in Toronto. First, we candidly advise all recipient families that this is the best option to avoid the risks of death or disqualification on the waiting list, to regain better health more quickly, and to enlarge the donor poor for all children waiting for a LT. Second, our pediatric and adult LT programs have high case volumes and are tightly integrated which facilitates technology transfer, coordination of care between sites, and a strong record of donor safety.50 Third, Canada has a generous social and legal system that provides publically funded healthcare, protects employment for donors when they take time off work and reimburses the direct costs of donation. Fourth, we are prepared to accept anonymous donors who have contributed 16% of our donor population.21,22,51,52
The strengths of our study include the analysis of a contemporaneous well-characterized cohort of pediatric LDLT recipients receiving protocolized immunosuppression and clinical care that was largely unchanged over the entire study period in North America. Our excellent outcomes are reflective of the collaborative partnership with a highly experienced high-volume adult transplant program committed to addressing the unique surgical and ethical challenges in children, rigorous and comprehensive documentation of complications and long-term follow-up to minimize donor-risk and enhance donor safety, and a commitment to advance the field to ensure the continued provision of high-quality donor grafts with outcomes that are superior to our reported outcomes with DDLT. Although we used propensity score covariate analysis with a variety of variables to attempt to reduce the bias of choice of DDLT versus LDLT based on characteristics of the recipients, residual confounding may remain. We also acknowledge several limitations with our study. These include the relatively short median study follow-up when considering the expected long-life expectancy of pediatric LT recipients. Although rare, graft versus host disease has been reported as a complication occurring after LDLT.53-56 There have been no cases clinically concerning for graft versus host disease in our study cohort. However, we could not evaluate for HLA matching, because we did not have donor serum or recipient serum available for HLA typing or donor-specific antibodies estimation for LDLT.
In conclusion, LDLT is an effective lifesaving therapeutic option for children with end-stage liver disease and other selected pediatric liver conditions. Our data suggest there is an opportunity to use LDLT more widely by pediatric transplant programs to reduce or eliminate waitlist deaths, improve time to transplant, and ultimately improve longterm outcomes for children in need of LT.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge Shiyi Chen, PhD for statistical analysis support on this article.
Supporting Information: Additional Supporting Information may be found in the online version of this article.
Footnotes
Published online 27 February, 2019.
The authors declare no funding or conflicts of interest.
M.K. participated in research design, performance of the research, data analysis, writing of the article. R.S.P. participated in research design, performance of the research, data analysis, writing of the article. J.S. participated in research design, performance of the research, and writing of the article. M.DA. participated in research design, performance of the research, and writing of the article. K.V.R. participated in research design, performance of the research, and writing of the article. A.G. participated in research design, data analysis, writing of the article. M.C. participated in research design and writing of the article. A.F. participated in research design and writing of the article. S.L. participated in research design and writing of the article. B.M.K. participated in research design and writing of the article. N.J. participated in research design and writing of the article. Y.A. participated in research design, data analysis, writing of the article. D.G. participated in research design, performance of the research, data analysis, writing of the article. V.L.N. participated in research design, performance of the research, data analysis, writing of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Squires RH, Ng V, Romero R, et al. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2014;59:112–131. [DOI] [PubMed] [Google Scholar]
- 2.Ng VL, Alonso EM, Bucuvalas JC, et al. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. J Pediatr. 2012;160:820–826. e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng VL, Fecteau A, Shepherd R, et al. Outcomes of 5-year survivors of pediatric liver transplantation: report on 461 children from a North American Multicenter Registry. Pediatrics. 2008;122:e1128–e1135. [DOI] [PubMed] [Google Scholar]
- 4.Mazariegos G, Shneider B, Burton B, et al. Liver transplantation for pediatric metabolic disease. Mol Genet Metab. 2014;111:418–427. [DOI] [PubMed] [Google Scholar]
- 5.Saidi RF, Hejazii Kenari SK. Challenges of organ shortage for transplantation: solutions and opportunities. Int J Organ Transplant Med. 2014;5:87–96. [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu EK, Shaffer ML, Gao L, et al. Analysis of liver offers to pediatric candidates on the transplant wait list. Gastroenterology. 2017;153:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CL. Living donor liver transplantation: the Asian experience. Transplantation. 2014;97:53. [DOI] [PubMed] [Google Scholar]
- 8.Levy GA, Selzner N, Grant DR. Fostering liver living donor liver transplantation. Curr Opin Organ Transplant. 2016;21:224–230. [DOI] [PubMed] [Google Scholar]
- 9.Emre S. Living-donor liver transplantation in children. Pediatr Transplant. 2002;6:43–46. [DOI] [PubMed] [Google Scholar]
- 10.Olthoff KM, Smith AR, Abecassis M, et al. Defining long-term outcomes with living donor liver transplantation in North America. Ann Surg. 2015;262:465–475; discussion 473–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmer DG, Yersiz H, Ghobrial RM, et al. Early graft function after pediatric liver transplantation: comparison between in situ split liver grafts and living-related liver grafts. Transplantation. 2001;72:1795–1802. [DOI] [PubMed] [Google Scholar]
- 12.Austin MT, Feurer ID, Chari RS, et al. Survival after pediatric liver transplantation: why does living donation offer an advantage? Arch Surg. 2005;140:465–470; discussion 470–461. [DOI] [PubMed] [Google Scholar]
- 13.Chan KL, Fan ST, Lo CM, et al. Pediatric liver transplantation in Hong Kong—a domain with scarce deceased donors. J Pediatr Surg. 2009;44:2316–2321. [DOI] [PubMed] [Google Scholar]
- 14.Liu LU, Bodian CA, Gondolesi GE, et al. Marked differences in acute cellular rejection rates between living-donor and deceased-donor liver transplant recipients. Transplantation. 2005;80:1072–1080. [DOI] [PubMed] [Google Scholar]
- 15.Steel J, ed. Living Donor Advocacy: An Evolving Role Within Transplantation. New York, NY: Springer-Verlag; 2014. [Google Scholar]
- 16.Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg. 1991;214:428–437; discussion 437–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karnsakul W, Intihar P, Konewko R, et al. Living donor liver transplantation in children: a single North American center experience over two decades. Pediatr Transplant. 2012;16:486–495. [DOI] [PubMed] [Google Scholar]
- 18.Venick RS, Farmer DG, Soto JR, et al. One thousand pediatric liver transplants during thirty years: lessons learned. J Am Coll Surg. 2018;226:355–366. [DOI] [PubMed] [Google Scholar]
- 19.Yankol Y, Fernandez LA, Kanmaz T, et al. Results of pediatric living donor compared to deceased donor liver transplantation in the PELD/MELD era: experience from two centers on two different continents. Pediatr Transplant. 2016;20:72–82. [DOI] [PubMed] [Google Scholar]
- 20.Borenstein S, Diamond IR, Grant DR, et al. Outcome of pediatric live-donor liver transplantation—the Toronto experience. J Pediatr Surg. 2003;38:668–671. [DOI] [PubMed] [Google Scholar]
- 21.Wright L, Ross K, Abbey S, et al. Living anonymous liver donation: case report and ethical justification. Am J Transplant. 2007;7:1032–1035. [DOI] [PubMed] [Google Scholar]
- 22.Reichman TW, Fox A, Adcock L, et al. Anonymous living liver donation: donor profiles and outcomes. Am J Transplant. 2010;10:2099–2104. [DOI] [PubMed] [Google Scholar]
- 23.Hong JC, Yersiz H, Kositamongkol P, et al. Liver transplantation using organ donation after cardiac death: a clinical predictive index for graft failure-free survival. Arch Surg. 2011;146:1017–1023. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz GJ, Haycock GB, Edelmann CM, Jr, et al. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 26.Cattral MS, Molinari M, Vollmer CM, Jr, et al. Living-donor right hepatectomy with or without inclusion of middle hepatic vein: comparison of morbidity and outcome in 56 patients. Am J Transplant 2004;4:751–757. [DOI] [PubMed] [Google Scholar]
- 27.Shah SA, Grant DR, Greig PD, et al. Analysis and outcomes of right lobe hepatectomy in 101 consecutive living donors. Am J Transplant. 2005;5:2764–2769. [DOI] [PubMed] [Google Scholar]
- 28.Reichman TW, Sandroussi C, Azouz SM, et al. Living donor hepatectomy: the importance of the residual liver volume. Liver Transpl. 2011;17:1404–1411. [DOI] [PubMed] [Google Scholar]
- 29.Mouzaki M, Yap J, Avinashi V, et al. Basiliximab with delayed introduction of calcineurin inhibitors as a renal-sparing protocol following liver transplantation in children with renal impairment. Pediatr Transplant. 2013;17:751–756. [DOI] [PubMed] [Google Scholar]
- 30.Jimenez-Rivera C, Avitzur Y, Fecteau AH, et al. Sirolimus for pediatric liver transplant recipients with post-transplant lymphoproliferative disease and hepatoblastoma. Pediatr Transplant. 2004;8:243–248. [DOI] [PubMed] [Google Scholar]
- 31.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. [DOI] [PubMed] [Google Scholar]
- 32.Demetris A, Adams D, Bellamy C, et al. Update of the international Banff schema for liver allograft rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An international panel. Hepatology. 2000;31:792–799. [DOI] [PubMed] [Google Scholar]
- 33.L'Huillier AG, Dipchand A, Ng V, et al. Post-transplant lymphoproliferative disorder in pediatric patients: clinical sites of occurrence and related survival rates. Open Forum Infect Dis. 2016;3(Suppl 1):2342–2342. [Google Scholar]
- 34.Kasahara M, Umeshita K, Inomata Y, et al. Long-term outcomes of pediatric living donor liver transplantation in Japan: an analysis of more than 2200 cases listed in the registry of the Japanese Liver Transplantation Society. Am J Transplant. 2013;13:1830–1839. [DOI] [PubMed] [Google Scholar]
- 35.Dutkowski P, De Rougemont O, Mullhaupt B, et al. Current and future trends in liver transplantation in Europe. Gastroenterology. 2010;138:802–809. e1-4. [DOI] [PubMed] [Google Scholar]
- 36.Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European liver transplant registry (ELTR). J Hepatol. 2012;57:675–688. [DOI] [PubMed] [Google Scholar]
- 37.Gurevich M, Guy-Viterbo V, Janssen M, et al. Living donor liver transplantation in children: surgical and immunological results in 250 recipients at Universite Catholique de Louvain. Ann Surg. 2015;262:1141–1149. [DOI] [PubMed] [Google Scholar]
- 38.Bourdeaux C, Darwish A, Jamart J, et al. Living-related versus deceased donor pediatric liver transplantation: a multivariate analysis of technical and immunological complications in 235 recipients. Am J Transplant. 2007;7:440–447. [DOI] [PubMed] [Google Scholar]
- 39.Sweet SC, Wong HH, Webber SA, et al. Pediatric transplantation in the United States, 1995–2004. Am J Transplant. 2006;6(5 pt 2):1132–1152. [DOI] [PubMed] [Google Scholar]
- 40.Laurence JM, Sapisochin G, DeAngelis M, et al. Biliary complications in pediatric liver transplantation: incidence and management over a decade. Liver Transpl. 2015;21:1082–1090. [DOI] [PubMed] [Google Scholar]
- 41.Freise CE, Gillespie BW, Koffron AJ, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL retrospective cohort study. Am J Transplant. 2008;8:2569–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alonso EM, Piper JB, Echols G, et al. Allograft rejection in pediatric recipients of living related liver transplants. Hepatology. 1996;23:40–43. [DOI] [PubMed] [Google Scholar]
- 43.Reding R, de Goyet Jde V, Delbeke I, et al. Pediatric liver transplantation with cadaveric or living related donors: comparative results in 90 elective recipients of primary grafts. J Pediatr. 1999;134:280–286. [DOI] [PubMed] [Google Scholar]
- 44.Katz SM, Ozaki CF, Monsour HP, Jr, et al. Pediatric living-related and cadaveric liver transplantation: a single center experience. Transplant Proc 1994;26:145–146. [PubMed] [Google Scholar]
- 45.Davies HS, Pollard SG, Calne RY. Soluble HLA antigens in the circulation of liver graft recipients. Transplantation. 1989;47:524–527. [DOI] [PubMed] [Google Scholar]
- 46.Mor E, Schwartz ME, Sheiner PA, et al. Prolonged preservation in University of Wisconsin solution associated with hepatic artery thrombosis after orthotopic liver transplantation. Transplantation. 1993;56:1399–1402. [DOI] [PubMed] [Google Scholar]
- 47.Reding R, Wallemacq P, Moulin D, et al. Early hepatocyte, endothelial, and bile duct cell injury after pediatric liver transplantation from cadaveric or living-related donors. Transplantation. 1998;65:681–685. [DOI] [PubMed] [Google Scholar]
- 48.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant. 2018;18(Suppl 1):172–253. [DOI] [PubMed] [Google Scholar]
- 49.Zamora-Valdes D, Leal-Leyte P, Kim PT, et al. Fighting mortality in the waiting list: liver transplantation in North America, Europe, and Asia. Ann Hepatol. 2017;16:480–486. [DOI] [PubMed] [Google Scholar]
- 50.Gorgen A, Goldaracena N, Zhang W, et al. Surgical complications after right hepatectomy for live liver donation: largest single-center western world experience. Semin Liver Dis. 2018;38:133–144. [DOI] [PubMed] [Google Scholar]
- 51.Jean-Bernard O. Good Samaritan liver donor in pediatric transplantation. Pediatr Transplant. 2009;13:155–159. [DOI] [PubMed] [Google Scholar]
- 52.Jendrisak MD, Hong B, Shenoy S, et al. Altruistic living donors: evaluation for nondirected kidney or liver donation. Am J Transplant. 2006;6:115–120. [DOI] [PubMed] [Google Scholar]
- 53.Smith DM, Agura E, Netto G, et al. Liver transplant-associated graft-versus-host disease. Transplantation. 2003;75:118–126. [DOI] [PubMed] [Google Scholar]
- 54.Taylor AL, Gibbs P, Bradley JA. Acute graft versus host disease following liver transplantation: the enemy within. Am J Transplant. 2004;4:466–474. [DOI] [PubMed] [Google Scholar]
- 55.Uchiyama H, Kayashima H, Matono R, et al. Relevance of HLA compatibility in living donor liver transplantation: the double-edged sword associated with the patient outcome. Clin Transpl. 2012;26:E522–E529. [DOI] [PubMed] [Google Scholar]
- 56.Soejima Y, Shimada M, Suehiro T, et al. Graft-versus-host disease following living donor liver transplantation. Liver Transpl. 2004;10:460–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


