Abstract
In the present study, comparative efficacy of natural as well as synthetic tenderizers on the quality characteristics of restructured spent hen meat slices (RSHS) was studied. Four different batches of RSHS viz. Control (without any tenderizer), T1 (1.25% calcium chloride replacing salt in formulation), T2 and T3 (1.5% each of pineapple rind and fig powder, replacing binder in the formulation) were developed in pre-standardized formulation. Vacuum tumbling was performed for 2.5 h and cooked product (RSHS) was assayed for quality attributes. Samples were packaged in aerobic conditions, stored for 21 days under refrigeration (4±1°C) and were evaluated for pH, oxidative and microbial quality parameters at regular interval of 7 days. Water holding capacity of T2 was recorded the highest and significantly higher (p<0.05) than all other samples. The textural attributes of T2 were comparable to T1 but significantly higher (p<0.05) than C and T3. The colour attributes (L*, a*, and b* value) of T2 and T3 were improved due to use of natural tenderizers. During sensory evaluation, tenderness scores for T2 samples were recorded the highest. Throughout storage period, thiobarbituric acid reactive substances (TBARS), free fatty acids (FFA) and peroxide value (PV) followed an increasing trend for control as well as treated products; however, T2 showed a significantly (p<0.05) lower value than control and other treated samples. It can be concluded that good quality RSHS with better storage stability could be prepared by utilizing 1.5% pineapple rind powder as natural tenderizer.
Keywords: spent hen meat, restructure, tenderizers, quality evaluation
Introduction
Restructure meat products are known as intermediate value products as these products fetches intermediate value in between muscle steak and traditional burger (Sheard, 2002). This process is very useful in efficiently utilization of low value carcass cuts, trimmings, meat from spent or culled animals by application of tumbling, massaging and blade tenderization. These products are more prone for lipid oxidation and discolouration. Further utilization of low value meat cuts and trimmings compromises tenderness and flavour of these products. Rapid expansion of poultry industry leads to availability of huge stock of spent or culled birds every year after the end of their productive life span. However, spent hen meat does not fetch higher price and rated poor quality meat in market due to less juiciness and higher toughness (Kumar et al., 2012). The proper economical disposal of this meat is very critical for making poultry industry, especially layer farming, remunerative and attractive.
For efficient utilization of tough meat of spent hen, various tenderizers are used to reduce its toughness and improve texture, tenderness and juiciness. These tenderizers, based on their source, can be grouped into two categories viz. synthetic (such as calcium chloride) and natural (such papain, bromelin and ficin). Calcium chloride improves tenderness by activating calpains, a proteolytic enzymes present in sarcoplasm. In addition, this improves the calcium content of meat (10 mg/100 g) and fortification of meat with calcium is highly desired for meeting RDA (recommended dietary allowance) through meat products. Calcium supplementation can be either in organic form as calcium lactate or inorganic form as calcium chloride. However, organic form contains more bioavailable calcium, bland taste and neutral aroma, but inorganic form contains higher calcium content, highly soluble in water and relatively in-expensive (Irshad et al., 2016).
With ever increasing awareness and increasing demand for natural and minimally processed meat products, focus has now shifted to develop foods without or with minimal synthetic compounds. There are huge amount of waste generated every year from fruit processing industry as by-products and a significant proportion of this is not properly utilized. It poses serious concerns for solid waste disposal and environmental hazard. These byproducts are rich in many functional ingredients such as protein, minerals, dietary fibre, carotenoids and polyphenols (Lamsal and Faubion, 2009). Their proper and efficient utilization have potential to open new arena for development of functional products.
Pineapple (Ananas comosus or Bromelia ananas) is a tropical plant of Bromeliaceae family containing coalesced berries. As per Hepton and Hodgson (2003), 35%–40% of waste generated from utilization of pineapple in the form of peel, stem and centrifugal solids. These wastes are rich source of bromelin and bioactive compounds. The raw fruit is a rich source of manganese, vitamin C and sulfur compounds. Bromelin, a mixture of proteinase enzymes, is commercially prepared from stem, fruit of pineapple, widely used in medicines due to its fibrinolytic, antithrombic, anti-inflammatory and anti-edematous properties. It also has tenderizing effect and used in meat tenderization. Pineapple peel powder is rich in polyphenols, carotenoids, dietary fibre, minerals, protein and was known to increase the growth of probiotic cultures by exerting pre-biotioc effect comparable to inulin (Diaz-Vela et al., 2013; Sah et al., 2015). Their incorporation in product development had significant effect on various functional attributes such as improved oxidative stability, rheological, textural, physico-chemical and microstructural parameters (Lucey, 2002; Sah et al., 2016).
Fig (Ficus carica) is regarded as indispensable fruits on tropics due to its higher nutritive value (Slavin, 2006). It is an integral part of Mediterarrian diet which is boasted as one of the healthiest diet associated with longetivity (Trichopoulou et al., 2006). The fruit is mostly consumed fresh with or without peeling as well as in dried form as drying extended its storage life (Verbic et al., 2008). The raw fruits get ripen early due to proteolytic action of ficin enzyme dissolving pectin. This enzyme is widely used for meat tenderization. Figs are rich in calcium, dietary fibre, minerals, phenolic compounds, vitamins and amino acids along with organic acid and sugar (Joseph and Raj, 2011; Solomon et al., 2006). Verberic et al. (2008) reported rutin as highest phenolic compounds present in fig followed by catechin, chlorogenic acid, epicatechin, gallic acid and syringic acid. Most of the phytochemicals exerting antioxidant effects (such as polyphenols, anthocyanin and flavonoids) are concentrated in skin, hence fruit with darker skin exerts higher antioxidant activity. The total sugar content of figs (10%–22% in dried figs) varies with the cultivars, drying methods and seasons (Slatnar et al., 2011) and 92% of its carbohydrates as simple sugars.
Incorporation of these powders in place of pure enzymes would have additional benefits of improving dietary fibre, mineral contents and antioxidant properties besides alleviating problem of solid waste disposal of these fruits industry. Changes in colour and development of rancidity due to lipid oxidation are major problem in restructured meat products (Akamittatha et al., 1990). This ensures better oxidative stability of incorporated meat products and extended storage life leading to improved economics. Thus present study was envisaged to evaluate efficacy of these natural tenderizers in comparison to synthetic tenderizer and its effect on quality attributes of restructured spent hen meat slices (RSHS).
Materials and Methods
Source of materials
White leghorn chickens over 72 weeks of age were procured from Directorate of Livestock Farm, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana and scientifically slaughtered in the Departmental Poultry Processing Plant by following standard protocols. These dressed chickens were chilled for 24 h at refrigeration temperature. Carcasses were deboned manually and deboned meat was stored in low density polyethylene bags at –18°C until use. Before use, the frozen meat was thawed at refrigeration temperature and cut manually into approximately 1 cm cube.
Refined salt, refined wheat flour, low density polyethylene films (200 gauges) bags, onion, ginger and garlic were procured from local market of Ludhiana, India. All spice ingredients (Verma et al., 2015) were procured from local market, cleaned, washed, dried and ground into fine powder.
Preparation of pineapple peel powder and fig powder
Pineapple peel was collected from local fruit market and juice shops, quickly brought to the department, rinsed with potable water and crushed. Non-marketable edible figs were collected from local fruit market. These fruits were cleaned and crushed. The crushed material was dried in vacuum oven (NSW, New Delhi, India) at 60°C for 24 h and ground to fine powder. The powder was packaged in polyethylene terephthalate (PET) jars and stored at ambient temperature for use.
Preparation of restructure spent hen meat slices
Based on several preliminary trails, a total number of 4 tenderizing mixtures were prepared in water containing 0.3% sodium tetra polyphosphate and 120 ppm sodium nitrite and following ingredients viz.
Mixture 1 for Control - 1.7% sodium chloride
Mixture II for T1 - 1.25% calcium chloride, 0.5% sodium chloride
Mixture III for T2 - 1.50 % pineapple peel powder
Mixture IV for T3 - 1.5% fig powder
These spent hen cubes were put for marinating for 5 h under refrigeration by mixing the respective tendering mixture along with all other ingredients as per Table 1. The mixture was put in vacuum tumbler (Promarks Vac. Co. Ltd, Taiwan) and vacuum tumbling was done for 2.5 h until the mixture became tacky exudates. The meat batter was removed from vacuum tumbler, and analyzed for pH, water holding capacity, collagen content and protein solubility. The batter was filled in aluminum moulds (7.5 cm×7.5 cm×6.0 cm) and was cooked in autoclave for 15 min at 15 psi and tempered to room temperature. The meat blocks were put for chilling at 4±1°C for overnight and cut into slices of 7 mm thickness with the help of mechanical slicer (Sirman, Auto m, 300 VV, Italy). These slices were packaged in aerobic packaging in low density polyethylene films (100 gauge) (Ramon Packaging Machine: VP-580 A Model , type 19/S/CL, Germany) and analyzed for various physico-chemical, proximate, oxidative stability and sensory parameters at a regular interval of 7 days for 21 days.
Table 1. Formulation for preparation of restructure spent hen meat slices.
| Ingredients (% w/w) | Control | Treatments | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Spent hen meat | 77.5 | 77.5 | 77.5 | 77.5 |
| Water | 10 | 10 | 10 | 10 |
| Condiments | 5 | 5 | 5 | 5 |
| Spices | 2 | 2 | 2 | 2 |
| Salt | 1.7 | 0.5 | 1.7 | 1.7 |
| Sodium tetrapolyphosphate | 0.3 | 0.3 | 0.3 | 0.3 |
| Calcium chloride | - | 1.25 | - | - |
| Sodiun nitrite | 120 | 120 | 120 | 120 |
| Pineapple peel powder | - | - | 1.5 | - |
| Fig powder | - | - | - | 1.5 |
Physico-chemical parameters
Water holding capacity (WHC) of RSHS was estimated as per method prescribed by Bosco et al. (2001) with suitable modifications. 1 g sample was put into tissue paper and centrifuged (Thermo Scientific SOR VALL ST 16R) for 4 min at 72.8×g. Centrifuged meat sample was weighed after removing tissue paper and dried at 70°C for 12 h under hot air oven.
The pH of RSHS was observed by using digital pH meter (SAB 5000, LABINDIA, Mumbai, India). The moisture percent (by oven drying), protein percent (by Kjeldahl distillation), fat percent (by Soxhlet method) and ash percent (by muffle furnace) of developed products were assessed as per method described by Association of Official Analytical Chemists (AOAC, 2000).
Cooking yield was calculated by noting the change in product weight before and after cooking–
Hydroxyproline (HP) content is used as indicator to collagen (Collagen=7.25×HP). HP of raw batter was determined by using method described by Neuman and Logan (1950) with slight modifications. 2 g of sample was hydrolyzed with 40 mL 6N HCl at 105°C for 18 h and filtered. Suitable aliquot (25 mL) with pH 7 was taken in a test tube and 1 mL each of 0.0N copper sulphate, 2.5N sodium hydroxide and 6% hydrogen peroxide were added. This was kept at room temperature for 5 min followed by putting at water bath at 80°C for 5 min. The sample was chilled in ice and 4 mL 3N sulphuric acid and 2 mL 5% DMED (4-dimenthyleaminobenzaldehyde) in n-propanol were added. The absorbance was measured at 540 nm by using Synergy Hi-hybrid reader. The HP content was expressed in % mg/g of tissue by referring to a standard graph.
Texture profile analysis
Texture profile analysis (TPA) of RSHS was conducted by the procedure described by Bourne (1978) using a texture analyzer (TMS-PRO, Food Technology Corporation, Sterling, VA, USA). The samples (1.5 cm×1.5 cm×1.5 cm) were placed on a platform in a fixture and compressed twice to 80% of their original height by a compression probe (P75) at a cross head speed of 10 mm/s through a two cycle sequence. Texture profile parameters were determined and interpreted as hardness, stringiness, cohesiveness, gumminess and resilience.
Instrumental colour profile analysis
Instrumental colour profile of RSHS were measured by recording L*, a*, and b* values by using Chroma Meter (Konica Minolta-CR-300). The instrument was calibrated by using white calibration kit supplied with equipment. The hue and chroma values were calculated by using following formula (Froehlich et al., 1983).
Determination of lipid oxidation parameters
Various lipid oxidation parameters such as thiobarbituric acid reacting substances (TBRAS) (Witte et al., 1970), free fatty acid (FFA) and peroxide value (PV) (Koniecko, 1979) were determined to assess the oxidative stability of RSHS.
Microbiological quality
Microbiological quality in terms of standard plate count (SPC), coliforms count and psychrophilic count of RSHS were determined as per method prescribed by APHA (1984). Triplicate plates were prepared and microbial counts were expressed as colony forming units per gram (Log CFU/g). All the process of sample preparation and serial dilution were performed under aseptic conditions, near flame in pre-sterilized horizontal laminar flow apparatus (Model Rescholar, M. No. RH-58-C3, Ambala Cantt, India).
Sensory evaluation
A panel of twelve experienced members comprising faculty and post-graduate students of the department assessed the developed restructured spent hen meat slices for appearance and colour, texture, flavour, juiciness and overall acceptability on 8-point descriptive scale (Keeton 1983), where 8=extremely liked and 1=extremely unliked. Samples were warmed (40°C–45°C) using a microwave oven for 90 sec before serving to panelist in sensory laboratory of the department. To check reliability of procedure, control sample was introduced in evaluations two times, randomized among other samples. Potable water was provided for rinsing mouth in between samples during sensory evaluation.
Statistical analysis
Data obtained during various experiments were analyzed on SPSS-16.0 software packages, IBM Corporation, USA (Snedecor and Cochran, 1989). Duplicate samples were drawn for each parameter and whole set of experiment was replicated six times (n=6). Means in between periods of storage were compared by two-way analysis of variance (ANOVA). The statistical significance was estimated at 5% level (p<0.05) and evaluated with Duncan’s Multiple Range Test (DMRT).
Results and Discussion
Physico-chemical parameters
Incorporation of natural and synthetic tenderizers had significant effect on physico-chemical quality of RSHS (Table 2). Inclusion of natural tenderizers resulted in decreasing pH of RSHS as compared to control in both raw batter and cooked product. The pH of raw control and T1 (product with 1.25% calcium chloride) was found comparative and significantly (p<0.05) higher than raw T2 and raw T3. Among raw products, the lowest pH was recorded for T2. In cooked products, pH of control was noted significantly (p<0.05) higher than all other treated products. The pH value of treated products varied significantly with T2 samples showing the lowest value with following order T1>T3>T2. The pH of cooked products was recorded higher than corresponding value of their raw products. This increased pH on cooking compared to raw product could be due to denaturation of meat proteins at higher temperature and formation of imidazolium compounds from amino acids having imidazole side chain as well as with reaction between ammonia and aldehydes. Kumar et al. (2018a; Kumar et al., 2018b) also reported similar increase in pH upon cooking in pork patties incorporated with sapota powder and watermelon rind extracts. The lower pH of T2 and T3 samples could be due to low pH of pineapple peel powder (4.12) and fig powder (5.63).
Table 2. Effect of synthetic and natural tenderizers on physicochemical, proximate, sensory and textural attributes of restructured spent hen meat slices (RSHS) (Mean±SE).
| Parameters | C | T1 | T2 | T3 |
|---|---|---|---|---|
| Raw batter | ||||
| pH | 6.36±0.02c | 6.31±0.01c | 6.18±0.01a | 6.22±0.01b |
| Water holding capacity (%) | 40.41±0.12a | 45.45±0.15b | 48.21±0.12d | 46.21±0.18c |
| Hydroxyproline (% HP/100 g tissue) | 0.13±0.02 | 0.15±0.03 | 0.16±0.02 | 0.16±0.03 |
| Collagen solubility (%) | 35.10±0.22a | 55.30±0.56b | 73.70±0.64d | 64.12±0.75c |
| Total soluble protein (g protein extracted/100 g total muscle protein) | 60.36±0.18a | 68.31±0.49b | 72.52±0.27c | 73.10±0.41c |
| Cooked product | ||||
| Cooking yield (%) | 86.57±0.42b | 83.88±0.31a | 89.59±0.50d | 87.56±0.36c |
| Cooking loss (%) | 13.42±0.56c | 16.11±0.56d | 10.39±0.56a | 12.41±0.56b |
| pH | 6.56±0.02c | 6.35±0.01b | 6.21±0.02a | 6.24±0.01a |
| Moisture (%) | 70.09±0.75b | 69.90±0.68a | 72.02±0.60b | 71.56±0.72b |
| Protein (%) | 21.43±0.56 | 20.42±0.62 | 21.71±0.38 | 21.05±0.47 |
| Fat (%) | 2.75±0.16 | 2.67±0.22 | 2.53±0.12 | 2.46±0.14 |
| Ash (%) | 2.30±0.04a | 2.53±0.06c | 2.42±0.05bc | 2.39±0.03ab |
| Sensory attribute | ||||
| Appearance and colour | 7.04±0.03a | 7.01±0.03a | 7.18±0.06b | 7.20±0.08b |
| Flavour | 7.06±0.05ab | 7.04±0.05ab | 7.18±0.07b | 6.96±0.06a |
| Juiciness | 7.05±0.12b | 7.03±0.07b | 7.22±0.06c | 6.71±0.07a |
| Tenderness | 7.04±0.13ab | 7.05±0.07ab | 7.25±0.06b | 6.82±0.07a |
| Overall acceptability | 7.02±0.05b | 7.06±0.09b | 7.23±0.04c | 6.85±0.02a |
| Texture profile | ||||
| Hardness | 10.96±0.19bc | 11.04±0.25c | 9.45±0.51b | 6.48±0.15a |
| Stringiness | 19.59±0.18c | 20.29±0.51c | 0.55±0.03b | 0.47±0.08a |
| Cohesiveness | 0.223±0.015 | 0.317±0.015 | 0.140±0.005 | 0.252±0.016 |
| Gumminess | 1.57±0.10 | 3.04±0.46 | 0.92±0.04 | 1.34±0.01 |
| Resilience | 0.81±0.02a | 2.73±0.21b | 0.63±0.10a | 3.37±0.11c |
Means with different superscripts differ significantly (p<0.05) in a row; n=6 for each treatment.
C, control; T1, RSHS with 1.25% calcium chloride; T2, RSHS with 1.5% pineapple peel powder; T3, RSHS with 1.5% fig powder; RSHS, restructured spent hen meat slices.
WHC is an important attributes affecting cooking yield, moisture losses during transportation and storage, organoleptic attributes of cooked meat, fading of colour, low water retention and other quality attributes (Apple and Yancey, 2013). Increased extraction and solubility of muscle proteins leads to increased binding sites for water molecules resulting in improved WHC. The increased extraction of proteins and solubility due to effect of proteolytic enzymes present in pineapple powder and fig powder could have resulted in increased binding sites for water molecules leading to higher WHC of the batter. The WHC of raw batter increased significantly (p<0.05) with the incorporation of natural and synthetic tenderizers. The WHC of T2 sample was recorded highest followed by T3 and T1 and control. van Laack (1999) reported higher WHC and lower drip loss of fresh meat with higher sarcoplasmic protein in meat. In addition to this, pH (either increase or decrease than isoelectric point of muscle proteins, pI 5.0–5.3) had direct effect on WHC by creating larger hydrophilic sites in polypeptide chain to attach water molecules (Naveena et al., 2011) due to electrostatic force.
HP content of control as well treated samples was recorded comparable. The collagen content of control was recorded lower than treated products. The synthetic tenderizers were known to initiate action of calpains responsible for controlled proteolysis. The higher value for T2 and T3 could be due to bromelain and ficin present in pineapple and fig powder, respectively in addition to calcium and minerals. A comparable collagen content in buffalo meat (Naveena et al., 2011) and in emu meat chunks (Verma et al., 2018b) in presence of different tenderizers had been also reported. Collagen solubility of T2 samples was recorded highest and significantly (p<0.05) higher than T3, which exhibited significantly (p<0.05) higher value than T1. The collagen solubility of control products recorded significantly lower value than T1. The significantly higher collagen solubility of products with natural tenderizers (T2 and T3) as compared to product with synthetic tenderizers (T1) could be due to presence of enzymes in the incorporated pineapple and fig powder in addition to salt. The higher collagen solubility of treated products could be due to increased enzymatic action on collagen in addition to actomyosin proteins, resulting high tender meat. Bromelain has been reported to exert higher enzymatic proteolysis and ability to digest collagen. Belcher et al. (2016) also noted significantly higher collagen disintegration activity of bromelain and recommended its use in removal of debris after burns. Comparative lower value of T3 (product with fig powder containing ficin) in comparison to T2 (product with pineapple powder containing bromelain) could be due to comparatively lower efficiency of digesting collagen by ficin as compared to bromelain. Ficin has ability to digest collagen in presence to heat or at higher salt concentration. The effect of these enzymes on collagen in native state was very poor and efficiency of these enzymes had been reported to improve in presence of heat, lower pH and high salt concentration. Under favourable conditions, these enzymes had ability to digest elastin to some extent. El-Gharbawi and Whitaker (1963) and Hinrichs and Whitaker (1962) also reported efficiency of bromelain, ficin and salt in tenderization of beef by digesting denatured collagen by bromelain, ficin and elastin to some extent by salt and ficin at low pH, high salt concentration and higher temperature. In the present study, the pH variations among treatments were comparable and thus the role of pH was not expected to exert any visible effect.
The findings of total soluble protein (g protein extracted per 100 g of total muscle protein) recorded significantly (p<0.05) higher value for product incorporated with natural tenderizers in comparison to product with synthetic tenderizer and control. The total soluble protein value of T2 and T3 were comparable. This could be due to enzymatic action of enzymes present in natural tenderizers on muscle proteins and collagen. The synthetic tenderizer used in the present study was known to activate proteolytic activity of calpain enzymes altering integrity of actomyosin and higher amount of extracted protein. Verma et al. (2018b) attributed the higher protein solubility to the increased permeability of myofibrils and disintegration thereof. Ramezani et al. (2003) reported increased WHC of meat tenderized by ficin and noted increased solubility of meat proteins based on electrophoresis method.
The cooking yield of T2 was recorded the highest and significantly higher than T3. This could be due to high content of dietary fibres in pineapple peel and fig powder, making these products capable to retain more water during cooking. Huang et al. (2011) reported high amount of insoluble fibre (41–48 g/100 g) in pineapple peel comprising cellulose, hemicelluloses and pectic compounds. The peel also contained high amount of lignin (60–66 g/ 100 g). These fibre rich fractions had been attributed to have higher water (8–12 mL/g) and oil binding (6–9 mL/g), swelling capability (11–18 mL/g) properties. These fractions improved WHC of raw batter and cooking yield of the product. The cooking yield of T1 was recorded as lowest value. The lower cooking yield of T1 in comparison to control could be due to higher denaturation effect of calcium chloride on meat proteins, thus having less capacity to retain juice during cooking. The moisture content of T2 and T3 were significantly (p<0.05) higher than control and T1. Protein and fat content of all samples were comparable, whereas ash content of control and T1 samples was comparable but significantly lower than ash content of T2 samples. This could be due to high mineral content of pineapple peel powder than control and other treatments.
Sensory attributes
Effects of various natural and synthetic preservatives on the sensory attributes of RSHS are presented in Table 2. The incorporation of pineapple peel powder resulted in improved sensory attributes and these were comparable to control. The incorporation of fig powder resulted in decreased sensory attributes and were significantly (p<0.05) lower than T2. This lower value of T3 could be due to poor binding and texture score due to presence of higher amount of sugar. The appearance and colour scores of T2 and T3 were significantly (p<0.05) higher than T1 and control. This could be due to presence of carotenoid compounds in these powder as well as sugar leading to non-enzymatic browning reaction (Maillard reaction). The flavour score of T3 was recorded significantly (p<0.05) lower than all other samples. T2 had the highest flavour scores which in-turn had comparable value to control and T1. Sensory attributes of product with synthetic tenderizers was found comparable to control and with T2 except overall acceptability. The overall acceptability of T2 was significantly (p<0.05) higher than control and T1, which in-turn showed rated significantly (p<0.05) higher T3. The significantly lower overall acceptability of T3, RSHS with fig powder could be due to modification flavour, binding and tenderness of these samples. Sensory panelist noted poor binding, excessive tenderness and marked flavour in T3 samples during sensory evaluation process.
Instrumental colour and texture profile analysis
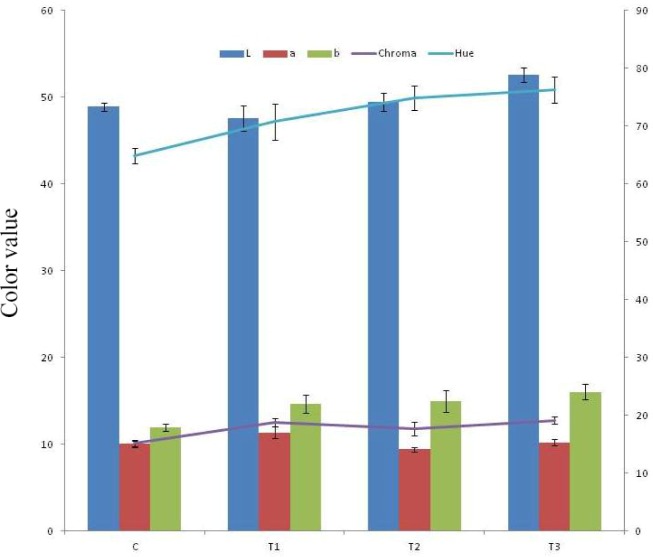
Colour and texture profile plays very important role in determining consumer acceptance and marketability of product. Addition of tenderizers affected the colour attributes of RSHS (Fig. 1). Lightness (L*) value of T3 was recorded significantly higher than control and other treatments. These values were in correlation with appearance score under sensory analysis. The redness (a*) value of T2 was significantly (p<0.05) lower than T1 and control and T3. The a* value of T1 was significantly higher than control and other treatments. This could be due to presence of calcium chloride and lower flavonoids and pigments as the case of T2 and T3 samples. Thus red colour of meat was prominent in these products. Kumar et al. (2018a) also reported decreasing redness (a*) value in pork patties upon incorporation of sapota powder and attributed it to non-enzymatic browning (Maillard reaction). Verma et al (2015) also noted the similar findings in pork patties. The higher yellowness value in T2 and T3 might be due to presence of flavonoids and carotenoids in pineapple peel powder and fig powder. Verberic et al. (2008) also noted high amount of phenolic compounds in fig fruit. Li et al. (2014) reported gallic acid, catechin, epicatechin and ferulic acids as major polyphenolic compounds in pineapple peel extracts. Fig also have perceivable amount of sugar, organic acids, minerals, vitamins and dietary fibres (Slavin, 2006; Solomon et al., 2006).
Fig. 1. Instrumental colour profile of RSHS upon incorporation of synthetic and natural tenderizers.
C, control; T1, RSHS with 1.25% calcium chloride; T2, RSHS with 1.5% pineapple peel powder; T3, RSHS with 1.5% fig powder; RSHS, restructured spent hen meat slices.
The texture profile analysis of RSHS revealed that product with synthetic tenderizer (calcium chloride) and control had higher textural attributes than product with natural tenderizers (T2 and T3) (Table 2). Out of all samples studies, T3 exhibited the lowest textural attributes. This could be due to action of ficin and presence of higher sugar in T3, leading to poor binding properties. T2 samples showed significantly (p<0.05) higher hardness than T3. This could be due to better binding ability of T3 due to presence of high amount of dietary fibres.
Storage studies
pH
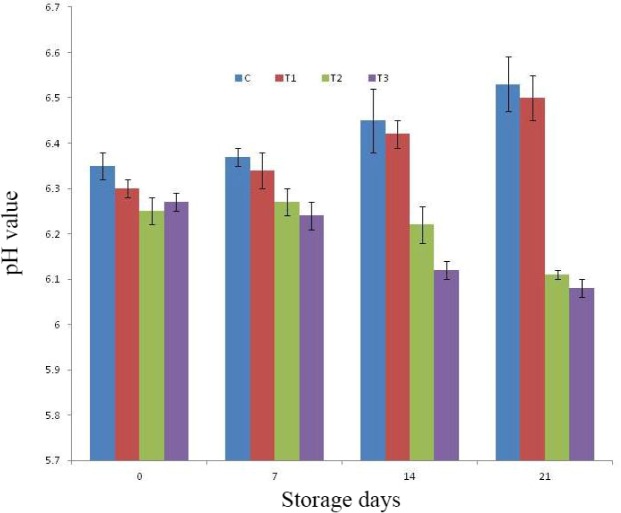
Upon refrigeration storage of RSHS, there had been significant change in pH of control as well as treated products (Fig. 2). The pH value of control and T1 had been noticed increasing trends, whereas pH value for T2 and T3 was noted decreasing trends with the advancement of storage period. Among treatments, pH value was noted comparable for all samples upto 7th day of storage afterward, on 14th day of storage study, pH value of T3 was significantly (p<0.05) lower than control and T1, whereas pH value of T2 was comparable to all other samples. The mean pH value of T2 and T3 was noted significantly (p<0.05) lower than control and T1 on 21st day of study. This could be due to presence of sugars and carbohydrates in fig powder and pineapple peel powder, which was converted to lactic acid upon storage during fermentation by microbes. The increase in pH during storage for control and T1 could be due to increase in microbial load during storage causing protein denaturation and accumulation of its metabolites. Similar findings of increasing pH were also noticed Kumar et al. (2018a) during storage of pork patties incorporated with sapota powder.
Fig. 2. pH value of RSHS during storage under refrigeration temperature (4±1°C).
C, control; T1, RSHS with 1.25% calcium chloride; T2, RSHS with 1.5% pineapple peel powder; T3, RSHS with 1.5% fig powder. RSHS, restructured spent hen meat slices.
Lipid oxidation parameters
Thiobarbituric acid reactive substances (TBARS) value
Lipid oxidation is very important criteria to determine the shelf life of meat products. This process begins due to reactions in between molecular oxygen and unsaturated phospholipid fractions of membranes and unsaturated fatty eaters, leading to generation of peroxides, hydroxides and carbonyl compounds. In presence of trace elements, there is production of several non radical compounds such as acids, aldehydes, alcohol and ketones resulting in poor sensory attributes and loss of nutritive quality (Benzie, 1996; Valenzuela and Nieto, 1996). The intermediary compounds formed during lipid oxidation process (such as free radicals, hydroperoxides, reactive oxygen species, etc.) have very short half-lives which make their detection and quantification very difficult. Thus, alternatively, oxidative stress is measured by assessing compounds which are more stable such as MDA (malondialdehyde). Thiobarbituric acid reactive substances (TBARS) (thiobarbituric acid reacting compounds) formed due to reaction in between TBA (thiobarbituric acid) and MDA is widely used to assess the degree of lipid oxidation (Liu et al., 1997). The increase in TBARS value during storage indicates production of secondary lipid oxidation products. Release of pro-oxidant compounds during storage such as ferric ions further enhances the rate of lipid oxidation (Verma et al., 2018a).
TBARS value of control as well as treatments increased significantly with the passage of storage days (Fig. 3). Among treatments, upto 14th day of storage, all samples under study were noticed with comparable TBARS value. The TBARS value of control and T1 were remained comparable throughout storage period. The rate of increase in TBARS value was higher in control and T1 samples, whereas it was recorded lowest for T2. This could be due to presence of natural antioxidant compounds such as polyphenols, flavonoids and anthocyanins in pineapple peel and fig powder, exerting inhibition effect on lipid oxidation and formation of its metabolites. Verberic et al. (2008) also noted high amount of phenolic compounds in figs such as rutin (28.7), catechin (4.03 mg), chlorogenic acid (1.71 mg) and epicatechin (0.97) on mg/ 100 g fresh weight basis. Li et al. (2014) reported gallic acid, catechin, epicatechin and ferulic acids having corresponding value (mg/100 g dry extract) 31.76, 58.51, 50.0, and 19.50 respectively, as major polyphenolic compounds in pineapple peel extracts. These compounds are known to exert antioxidant properties. Further, there has been increase in microbial population upon storage and several published studies had established positive correlation between microbial load and TBARS (Kumar et al., 2018a; Kumar et al., 2018b; Raja et al., 2014). Harzallah et al. (2016) attributed the antioxidant activity of different fruit powders in food system to presence of total polyphenols, flavonoids, ortho-diphenols, tannins and anthocyanin.
Fig. 3. Thiobarbituric acid reacting substances (TBARS) value of RSHS during storage under refrigeration temperature (4±1°C).
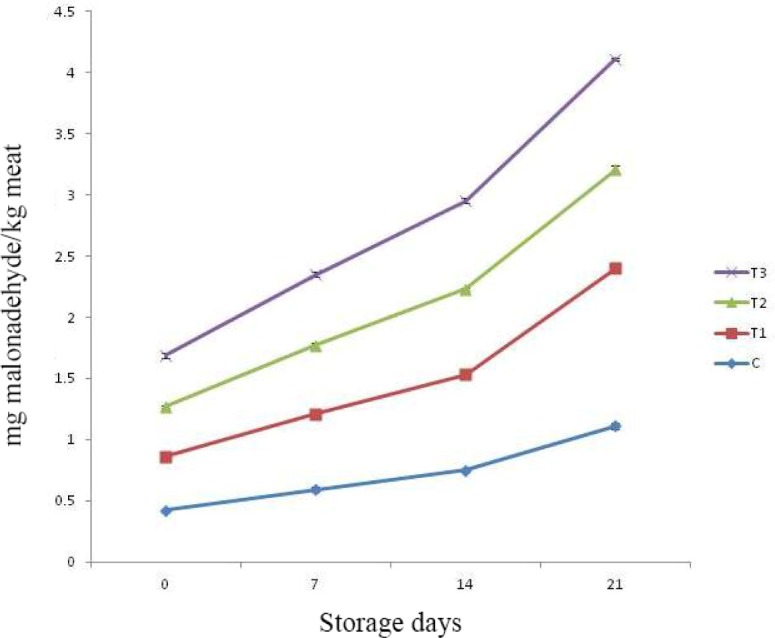
C, control; T1, RSHS with 1.25% calcium chloride; T2, RSHS with 1.5% pineapple peel powder; T3, RSHS with 1.5% fig powder; RSHS, restructured spent hen meat slices.
Free fatty acids (FFA) and peroxide value (PV)
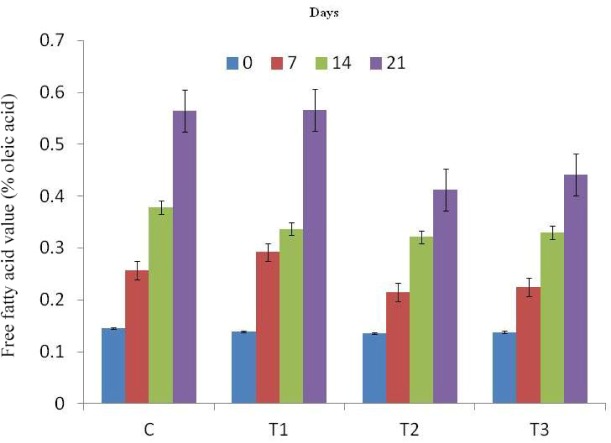
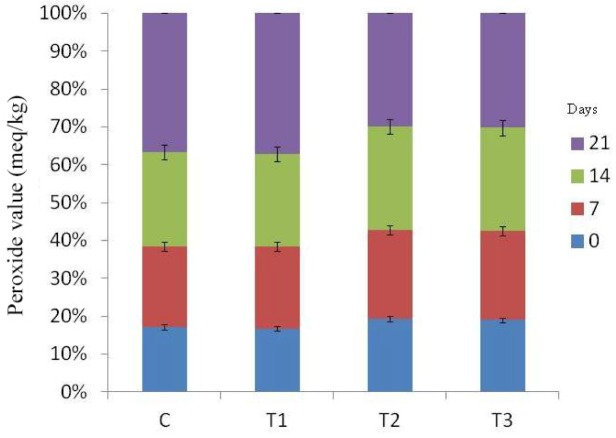
Free fatty acid (Fig. 4) and peroxide values (Fig. 5) of RSHS recorded increasing trends with elapse of storage days. FFA value of control and T1 sample was recorded highest than other samples throughout the storage period. FFA value of T2 and T3 was recorded significantly lower than T1 and comparable to control on 7th day of storage. Among treatments, PV value of all samples was comparable except on 21st day of storage. On last day of storage, PV value of T2 was the lowest value followed by T3, T1 and control. This lower value of FFA and PV in T2 and T3 samples could be due to antioxidant as well as antimicrobial effect of flavonoids and polyphenols present in natural tenderizers used in the study in the form of pineapple peel powder and fig powder.
Fig. 4. Free fatty acid (FFA) value of RSHS during storage under refrigeration temperature (4±1°C).
C, control; T1, RSHS with 1.25% calcium chloride; T2, RSHS with 1.5% pineapple peel powder; T3, RSHS with 1.5% fig powder; RSHS, restructured spent hen meat slices.
Fig. 5. Peroxide value (PV) value of RSHS during storage under refrigeration temperature (4±1°C).
C, control; T1, RSHS with 1.25% calcium chloride; T2, RSHS with 1.5% pineapple peel powder; T3, RSHS with 1.5% fig powder; RSHS, restructured spent hen meat slices.
Microbial quality
The mean SPC increased in all samples with the advancement of storage period (Fig. 6). The SPC of control and T3 increased significantly (p<0.05) with the advancement of storage period, whereas SPC of T1 and T2 were having comparable value on 7 and 14 days, but significantly (p<0.05) higher than 0 day and lower than 21st day of storage. Throughout the storage period, the highest value of SPC was recorded for control which was comparable to T1 except on 14th day of storage. Comparatively lower value of T2 and T3 samples could be due to antibacterial activity of phenolic compounds present in skin/rind of fig and pineapple. Solomon et al. (2006) and Veberic et al. (2008) also reported dried figs as rich source of polyphenols, with most of these compounds located in skin and higher levels in figs with darker skin. Puupponen-Pimia et al. (2001) also reported antimicrobial activity of phenolic compounds. Further, lower pH of these samples also contributed to the lower microbial count in these samples during storage as low pH was known to increase cell injury and prolong lag phase as noted by Kumar et al. (2018b) in pork patties incorporated with watermelon rind extract.
Fig. 6. Standard plate count (SPC) of RSHS during storage under refrigeration temperature (4±1°C).
C, control; T1, RSHS with 1.25% calcium chloride; T2, RSHS with 1.5% pineapple peel powder; T3, RSHS with 1.5% fig powder; RSHS, restructured spent hen meat slices.
In the present study, coliforms were detected during storage studies on 14th day in all samples and increased significantly (p<0.05) with elapse of storage period. The lower value of coliforms in RSHS with natural tenderizers indicated antimicrobial effect of these powders. Psychrophiles were noted on 7th day of storage in all samples including control and treatments, thereafter, a significant increase in psychrophiles was recorded in all samples. The initial absence of psychrophiles during storage could be due to lower metabolic rate of these microbes leading to extending lag phase. This detection of psychrophiles and coliforms after initial absence could be due to the reason as bacteria generally need some lag phase before starting active multiplication in log phase. On the last day of storage, the lowest microbial count was recorded in T3 and the highest in control.
Sensory quality
The main criterion for spoilage of RSHS during present study was flavour emanating at the time of opening of packets for sensory analysis. Any abnormal development in colour and sliminess were also noted. The sensory panelists have noted marked deterioration in flavour of control and T1 samples on 21st day of storage; hence, these two samples were not evaluated for sensory evaluation on last day of storage.
Sensory attributes of RSHS showed decreasing trends with the increasing storage days (Table 3). The appearance and colour score decreased with the advancement of storage period and on 14th day, these attributes were significantly (p<0.05) lower than 0th and 7th day of storage for C, T1 and T3. Among all samples studied, T2 and T3 were recorded with higher appearance and colour score. This might be due to presence of sugar in dried powders leading to non-enzymatic browning reaction and carotenoid compounds. In T2 samples, the presence of polyphenolic compounds resulted in modification of colour of RSHS, thus reducing appearance and colour value. Control and T1 samples did not have such compounds which have modifying effect on natural colour of RSHS. These results are in accordance with our instrumental colour profile of RSHS.
Table 3. Effect of synthetic and natural tenderizers on coliforms, psychrophilic count and sensory quality of RSHS stored under aerobic packaging at refrigeration temperature (4±1°C) (Mean±SE).
| Treatment | 0 | 7 | 14 | 21 |
|---|---|---|---|---|
| Microbial quality | ||||
| Coliform count (Log10CFU/g) | ||||
| C | ND | ND | 1.76±0.15aB | 1.97±0.16bB |
| T1 | ND | ND | 1.79±0.24aB | 1.99±0.18bB |
| T2 | ND | ND | 1.49±0.10aA | 1.73±0.07bA |
| T3 | ND | ND | 1.46±0.15aA | 1.82±0.16bAB |
| Psychrophilic count (Log10 CFU/g) | ||||
| C | ND | 1.86±0.164aB | 2.24±0.16bB | 2.70±0.13cB |
| T1 | ND | 1.91±0.177aB | 2.21±0.09abB | 2.60±0.31bB |
| T2 | ND | 1.48±0.150aA | 2.08±0.20abA | 2.37±0.20bA |
| T3 | ND | 1.64±0.059aAB | 2.16±0.20bAB | 2.47±0.17cAB |
| Sensory quality | ||||
| Appearance and colour | ||||
| C | 7.11±0.03b | 7.06±0.04b | 6.35±0.09a | NP |
| T1 | 7.06±0.08b | 7.03±0.08b | 6.52±0.11a | NP |
| T2 | 7.16±0.16c | 7.02±0.08c | 6.42±0.10b | 6.02±0.13a |
| T3 | 7.18±0.06c | 6.98±0.06bc | 6.52±0.13b | 6.14±0.06a |
| Flavour | ||||
| C | 7.07±0.12bAB | 6.88±0.12bAB | 6.11±0.11aA | NP |
| T1 | 7.03±0.07bAB | 6.93±0.07bAB | 6.21±0.14aAB | NP |
| T2 | 7.22±0.06cB | 7.17±0.07cB | 6.55±0.11bC | 6.17±0.13aB |
| T3 | 6.81±0.07cA | 6.73±0.07cA | 6.31±0.10bB | 5.80±0.19aA |
| Juiciness | ||||
| C | 7.07±0.12bAB | 6.88±0.12bAB | 6.19±0.11aA | NP |
| T1 | 7.03±0.07bAB | 6.93±0.07bAB | 6.21±0.14aA | NP |
| T2 | 7.22±0.06cB | 7.17±0.07cB | 6.52±0.13bB | 6.11±0.13a |
| T3 | 6.81±0.07cA | 6.73±0.07cA | 6.31±0.12bAB | 5.97±0.19a |
| Tenderness | ||||
| C | 7.01±0.12bAB | 6.91±0.11b | 6.34±0.14aA | NP |
| T1 | 7.05±0.07bAB | 6.89±0.07b | 6.36±0.15aA | NP |
| T2 | 7.25±0.06cB | 7.11±0.15c | 6.53±0.11bB | 6.10±0.05a |
| T3 | 6.82±0.07cA | 6.80±0.07c | 6.30±0.09bA | 5.85±0.16a |
| Overall acceptability | ||||
| C | 7.05±0.05bAB | 6.90±0.06bAB | 6.39±0.11a | NP |
| T1 | 7.20±0.09bAB | 7.11±0.09bB | 6.28±0.11a | NP |
| T2 | 7.25±0.04cB | 7.10±0.15cB | 6.42±0.17b | 6.11±0.05aB |
| T3 | 6.85±0.02cA | 6.78±0.02cA | 6.29±0.06b | 5.85±0.10aA |
Means with different superscripts (a, b, c and A, B, C) differ significantly (p<0.05) in a row and column respectively; n=6 for each treatment (C, control; T1, RSHS with 1.25% calcium chloride; T2, RSHS with 1.5% pineapple peel powder; T3, RSHS with 1.5% fig powder; ND, not detected; NP, not performed; RSHS, restructured spent hen meat slices.
Flavour scores of T2 were significantly (p<0.05) higher than T3 which in-turn was comparable to control and T1. A gradual decrease in flavor score could also be attributed to the expected loss of volatile flavour compounds from spices and condiments on storage of meat products (Bhat et al., 2015). The marked deterioration of flavour scores of control and T1 could be due to higher microbial growth as well as pronounced lipid oxidation thus emanating foul odour from these samples. These results were in accordance with our findings on microbial quality and lipid oxidation parameters. The presence of phenolic compounds in T2 and T3 due to pineapple peel powder and fig powder, could have exerted antioxidant effects and thus retarded the process of lipid oxidation and development of rancid flavour. Within groups, juiciness scores of T2 was observed significantly (p<0.05) higher than control and other treatments on 0th day and 7th day, whereas control, T1 and T3 showed comparable values on these two days. On 14th day of study, all samples have comparable value. The juiciness and tenderness of T3 was recorded significantly lower than all other samples. This could be due to poor binding of these samples. These findings are in accordance with texture profile analysis. The deterioration in juiciness and tenderness scores could be attributed to protein denaturation leading to change in disulphide bonds due to increasing microbial load and moisture loss. Singh et al. (2015) also reported similar trends of decreasing juiciness and tenderness scores of chevon cutlets stored under aerobic packaging condition.
Overall acceptability of RSHS also decreased upon advancement of storage period and it followed the same pattern as observed for flavour scores and a significant decrease (p<0.05) in overall acceptance of all samples were observed from 7th day onward. The overall acceptability of T3 and T2 were found close to good to moderately acceptable category (5.89–6.11) even on last day of storage study (21st day). Thus natural tenderizers have increased the storage life of RSHS due to presence of antioxidant and antimicrobial compounds in them, in addition to making this meat more tender and acceptable.
Conclusion
Thus, it can be concluded that good quality RSHS could be prepared by incorporating of pineapple peel powder and fig powder as natural tenderizers. The use of these compounds have been shown to have additional antioxidant and antimicrobial properties, thus extends the keeping quality of RSHS upon refrigerated storage.
Acknowledgements
We gratefully acknowledge financial assistance received from University Grant Commission (UGC), New Delhi, Government of India under project entitled “Development of Extended Storage Life Functional Meat Products by Incorporating Bioactive Phyto-extracts”.
Conflicts of Interest
There is no conflict of interest among authors.
Author Contributions
Conceptualization: Kumar P, Mehta N. Data curation: Kantale RA, Kumar P. Formal analysis: Kumar P. Methodology: Kumar P, Mehta N. Software: Kaur A, Chatli MK. Validation: Malav OP, Wagh RV. Investigation: Kantale RA, Kumar P. Writing - original draft: Kumar P. Writing - review & editing: Kantale RA, Kumar P, Mehta N, Chatli MK, Malav OP, Kaur A, Wagh RV.
Ethics Approval
This article does not require IRB/IACUC approval because there are no human and animal participants.
References
- Akamittath JG, Brekke CJ, Schanus EG. Lipid oxidation and color stability in restructured meat systems during frozen storage. J Food Sci. 1990;55:1513–1517. doi: 10.1111/j.1365-2621.1990.tb03557.x. [DOI] [Google Scholar]
- AOAC. Official methods of analysis. 17thed. Association of Official Analytical Chemists; Washington DC, USA: 2000. [Google Scholar]
- APHA. Compendium of methods for microbiological examination of foods. 2nd ed. American Public Health Association; Washington DC, USA: 1984. [Google Scholar]
- Apple JK, Yancey JWS. Water-holding capacity of meat. In: Kerth CR, editor. In The science of meat quality. John Wiley and Sons Inc.; Hoboken, NJ, USA: 2013. pp. 119–146. [DOI] [Google Scholar]
- Belcher MD, Kaddour-Djebbar I, Bollag WB, Davis LS. The proteolytic effect of bromelain on bullous pemphigoid antigen-2. J Am Acad Dermatol. 2016;75:838–840. doi: 10.1016/j.jaad.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Benzie IFF. Lipid peroxidation: A review of causes, consequences, measurement, and dietary influences. Int J Food Sci Nutr. 1996;47:233–261. doi: 10.3109/09637489609012586. [DOI] [PubMed] [Google Scholar]
- Bhat ZF, Kumar S, Kumar P. Effect of Aloe vera on the lipid stability and storage quality of chicken nuggets. Nutr Food Sci. 2015;45:54–67. doi: 10.1108/NFS-04-2014-0034. [DOI] [Google Scholar]
- Bosco AD, Castellini C, Bernardini M. Nutritional quality of rabbit meat as affected by cooking procedure and dietary vitamin E. J Food Sci. 2001;66:1047–1051. doi: 10.1111/j.1365-2621.2001.tb08233.x. [DOI] [Google Scholar]
- Bourne MC. Texture profile analysis. Food Technol. 1978;32:62–66. [Google Scholar]
- DiazVela J, Totosaus A, Cruz-Guerrero AE, de Lourdes Perez-Chabela M. In vitro evaluation of the fermentation of added-value agroindustrial by-products: Cactus pear (Opuntia ficus-indica L.) peel and pineapple (Ananas comosus) peel as functional ingredients. Int J Food Sci Technol. 2013;48:1460–1467. doi: 10.1111/ijfs.12113. [DOI] [Google Scholar]
- Froehlich DA, Gullett EA, Usborne WR. Effect of nitrite and salt on the color, flavor and overall acceptability of ham. J Food Sci. 1983;48:152–157. doi: 10.1111/j.1365-2621.1983.tb14811.x. [DOI] [Google Scholar]
- El‐Gharbawi M, Whitaker JR. Factors affecting enzymatic solubilization of beef proteins. J Food Sci. 1963;28:168–172. doi: 10.1111/j.1365-2621.1963.tb00177.x. [DOI] [Google Scholar]
- Harzallah A, Bhouri AM, Amri Z, Soltana H, Hammami M. Phytochemical content and antioxidant activity of different fruit parts juices of three figs (Ficus carica L.) varieties grown in Tunisia. Ind Crops Prod. 2016;83:255–267. doi: 10.1016/j.indcrop.2015.12.043. [DOI] [Google Scholar]
- Hepton A, Hodgson AS. Processing. In: Bartholomew DP, Paull RE, Rohrbach KG, editors. In The pineapple: Botany, production and uses. 1st ed. CABI Publishing; Wallingford, UK: 2003. pp. 291–320. [DOI] [Google Scholar]
- Hinrichs JR, Whitaker JR. Enzymatic degradation of collagen. J Food Sci. 1962;27:250–254. doi: 10.1111/j.1365-2621.1962.tb00089.x. [DOI] [Google Scholar]
- Huang YL, Chow CJ, Fang YJ. Preparation and physicochemical properties of fiber-rich fraction from pineapple peels as a potential ingredient. J Food Drug Anal. 2011;19:318–323. [Google Scholar]
- Irshad A, Sharma BD, Ahmed SR, Talukder S, Malav OP, Kumar A. Effect of incorporation of calcium lactate on physico-chemical, textural, and sensory properties of restructured buffalo meat loaves. Vet World. 2016;9:151–159. doi: 10.14202/vetworld.2016.151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B, Raj SJ. Pharmacognostic and phytochemical properties of Ficus carica Linn -An overview. Int J Pharmtech Res. 2011;3:8–12. [Google Scholar]
- Keeton JT. Effects of fat and NaCl/phosphate levels on the chemical and sensory properties of pork patties. J Food Sci. 1983;48:878–881. doi: 10.1111/j.1365-2621.1983.tb14921.x. [DOI] [Google Scholar]
- Koniecko ES. Handbook for meat chemists. Avery, Wayne; NJ, USA: 1979. pp. 53–55. [Google Scholar]
- Kumar P, Chatli MK, Mehta N, Malav OP, Verma AK, Kumar D, Rathour M. Antioxidant and antimicrobial efficacy of sapota powder in pork patties stored under different packaging conditions. Korean J Food Sci Anim Resour. 2018a;38:593–605. doi: 10.5851/kosfa.2018.38.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Kumar S, Bhat ZF. Effect of sex on the quality characteristics of nuggets prepared from spent Vanaraja chicken meat. Indian J Poult Sci. 2012;47:218–221. [Google Scholar]
- Kumar P, Mehta N, Malav OP, Chatli MK, Rathour M, Verma AK. Antioxidant and antimicrobial efficacy of watermelon rind extract in aerobically packaged pork patties stored under refrigeration temperature (4±1°C) J Food Process Preserv. 2018b;42:e13757. doi: 10.1111/jfpp.13757. [DOI] [Google Scholar]
- Lamsal BP, Faubion JM. The beneficial use of cereal and cereal components in probiotic foods. Food Rev Int. 2009;25:103–114. doi: 10.1080/87559120802682573. [DOI] [Google Scholar]
- Li T, Shen P, Liu W, Liu C, Liang R, Yan N, Chen J. Major polyphenolics in pineapple peels and their antioxidant interactions. Int J Food Prop. 2014;17:1805–1817. doi: 10.1080/10942912.2012.732168. [DOI] [Google Scholar]
- Liu J, Yeo HC, Doniger SJ, Ames BN. Assay of aldehydes from lipid peroxidation: Gas chromatography mass spectrometry compared with thiobarbituric acid. Anal Biochem. 1997;245:161–166. doi: 10.1006/abio.1996.9990. [DOI] [PubMed] [Google Scholar]
- Lucey JA. Formation and physical properties of milk protein gels. J Dairy Sci. 2002;85:281–294. doi: 10.3168/jds.S0022-0302(02)74078-2. [DOI] [PubMed] [Google Scholar]
- Naveena BM, Sen AR, Muthukumar M, Babji Y, Kondaiah N. Effects of salt and ammonium hydroxide on the quality of ground buffalo meat. Meat Sci. 2011;87:315–320. doi: 10.1016/j.meatsci.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Puupponen Pimia R, Nohynek L, Meier C, Kahkonen M, Heinonen M, Hopia A, Oksman Caldentey KM. Antimicrobial properties of phenolic compounds from berries. J Appl Microbiol. 2001;90:494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- Raja WH, Kumar S, Bhat ZF, Kumar P. Effect of ambient storage on the quality characteristics of aerobically packaged fish curls incorporated with different flours. SpringerPlus. 2014;3:106. doi: 10.1186/2193-1801-3-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani R, Aminlari M, Fallahi H. Effect of chemically modified soy proteins and ficin‐tenderized meat on the quality attributes of sausage. J Food Sci. 2003;68:85–88. doi: 10.1111/j.1365-2621.2003.tb14119.x. [DOI] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Effect of refrigerated storage on probiotic viability and the production and stability of antimutagenic and antioxidant peptides in yogurt supplemented with pineapple peel. J Dairy Sci. 2015;98:5905–5916. doi: 10.3168/jds.2015-9450. [DOI] [PubMed] [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT-Food Sci Technol. 2016;65:978–986. doi: 10.1016/j.lwt.2015.09.027. [DOI] [Google Scholar]
- Sheard PR. Processing and quality control of restructured meat. In: Kerry J, Kerry J, Ledward D, editors. In Meat processing: improving quality. CRC Press; Cambridge, England: 2002. pp. 332–358. [DOI] [Google Scholar]
- Singh PK, Kumar S, Bhat ZF, Kumar P, Kumar A. Effect of processed oats and clove oil on the characteristics and storage quality of aerobically packaged chevon cutlets. Indian J Small Rumin. 2015;21:76–84. doi: 10.5958/0973-9718.2015.00009.4. [DOI] [Google Scholar]
- Slatnar A, Klancar U, Stampar F, Veberic R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J Agri Food Chem. 2011;59:11696–11702. doi: 10.1021/jf202707y. [DOI] [PubMed] [Google Scholar]
- Slavin J L. Figs: Past, present, and future. Nutri Today. 2006;41:180–184. doi: 10.1097/00017285-200607000-00009. [DOI] [Google Scholar]
- Snecdecor GW, Cochran WG. Statistical methods. 8th ed. Iowa State University; Ames, IA, USA: 1989. [Google Scholar]
- Solomon A, Golubowicz S, Yablowicz Z, Grossman S, Bergman M, Gottlieb HE, Altman A, Kerem Z, Flaishman MA. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.) J Agric Food Chem. 2006;54:7717–7723. doi: 10.1021/jf060497h. [DOI] [PubMed] [Google Scholar]
- Trichopoulou A, Vasilopoulou E, Georga K, Soukara S, Dilis V. Traditional foods: Why and how to sustain them. Trends Food Sci Technol. 2006;17:498–504. doi: 10.1016/js.2006.03.005. [DOI] [Google Scholar]
- Valenzuela A, Nieto S. Synthetic and natural antioxidants: Food quality protectors. Grasay Aceites. 1996;47:186–196. doi: 10.3989/gya.1996.v47.i3.859. [DOI] [Google Scholar]
- Van Laack RLJM. The role of proteins in water-holding capacity of meat. In: Xiong YL, Ho CT, Shahidi F, editors. In Quality attributes of muscle foods. Kluwer Academic / Plenum Publishers; New York, NY, USA: 1999. pp. 309–318. (ed.) [DOI] [Google Scholar]
- Veberic R, Colaric M, Stampar F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 2008;106:153–157. doi: 10.1016/j.foodchem.2007.05.061. [DOI] [Google Scholar]
- Verma AK, Chatli MK, Kumar D, Kumar P, Mehta N. Efficacy of sweet potato powder and added water as fat replacer on the quality attributes of low-fat pork patties. Asian-Australas J Anim Sci. 2015;28:252–259. doi: 10.5713/ajas.14.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma AK, Chatli MK, Kumar P, Mehta N. Effects of inclusion of porcine blood hydrolysate on physico-chemical quality, oxidative and microbial stability of pork batter stored at (4±1°C) J Food Sci Technol. 2018a;55:4758–4769. doi: 10.1007/s13197-018-3409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma SK, Biswas S, Patra G. Tenderizing effect of Cucumis trigonus Roxb and Carica papaya on emu meat chunks. J Anim Res. 2018b;8:195–203. [Google Scholar]
- Witte VC, Krause GF, Bailey ME. A new extraction method for determining 2-thiobarbituric acid value of pork and beef during storage. J Food Sci. 1970;35:582–585. doi: 10.1111/j.1365-2621.1970.tb04815.x. [DOI] [Google Scholar]