Abstract
There are many differences in fluoroscopy-guided lumbar puncture (FG-LP) technique among radiologists. Even within the same institution, there are a variety of preferences among proceduralists with individual perspectives based on the literature, training, and/or experience. Our aim is to provide familiarity with various techniques involved in FG-LP and provide insight on how to improve patient outcomes. The pertinent anatomy and physiology, indications, contraindications, patient management, complications of the procedure, and procedural techniques for performing an FG-LP are reviewed in detail. Potentially controversial topics regarding FG-LP are also addressed.
There are many differences in fluoroscopy-guided lumbar puncture (FG-LP) technique among radiologists (1). Even within the same institution, there are a variety of individual preferences among physicians with different perspectives based on a combination of literature familiarity, training, and personal experience. Our aim is to provide familiarity with various techniques involved in FG-LP, improve efficiency, and improve patient outcomes. We will also address possible controversial issues regarding FG-LPs using an evidence-based approach.
Relevant anatomy and physiology
Over the years, we have observed an increase in the number of requests for FG-LP, attributed to several contributing factors. For instance, FG-LPs are less likely to result in a traumatic tap when compared to non-image-guided lumbar punctures (LP) (2). A traumatic tap may affect laboratory results, potentially leading to elevated cell counts and cerebrospinal fluid (CSF) protein levels. Second, an increasing number of patients with degenerative spondylosis and/or obesity, could lead to an increase in failed bedside attempts. Additionally, fear of malpractice litigation might urge some practitioners to shift the responsibility of such procedures to interventionalists. Moreover, with the increased number of complex spine surgeries, surgeons may request more computed tomography (CT) myelography examinations instead of magnetic resonance imaging (MRI) due to magnetic susceptibility artifacts from the surgical hardware and to assess surgical complications such as CSF leak. Lastly, an increase in intrathecal medication regimens and a preference for imaging confirmation might sway providers to favor FG-LP. Therefore, familiarity with CSF physiology and the pertinent anatomy is essential for the interventionalist to increase the success rate of this simple diagnostic and therapeutic intervention, to avoid potential complications, and to best manage complications when they occur.
Most of the CSF is produced by the choroid plexus, while small amounts are secreted by the ependymal surfaces of the ventricles and by the arachnoid membranes (3). CSF passes through the ventricular system and exits from the fourth ventricle through the foramina of Luschka and Magendie, entering the contiguous subarachnoid space surrounding the brain and spinal cord. CSF is absorbed through a combination of lymphatic absorption and by arachnoid villi within the venous sinuses, ultimately returning to the systemic circulation (4).
In an average adult, there is 150 cc of CSF within the subarachnoid spaces of the brain (75 cc) and spine (75 cc) (3, 4). The production rate of CSF is about 500 cc/day, which amounts to 3–4 times the total volume (3) with a production rate of about 0.35 cc/min or 20 cc/h. That means if 10 cc of CSF is collected during an LP in a healthy normally hydrated patient, that amount should be replaced in about half an hour.
The normal range of CSF opening pressure (CSF-OP) is between 6 and 20 cm H2O. A pressure of <6 cm H2O is indicative of low intracranial pressure, and a pressure of >25 cm H2O indicates idiopathic intracranial hypertension, also known as pseudotumor cerebri (3, 5, 6).
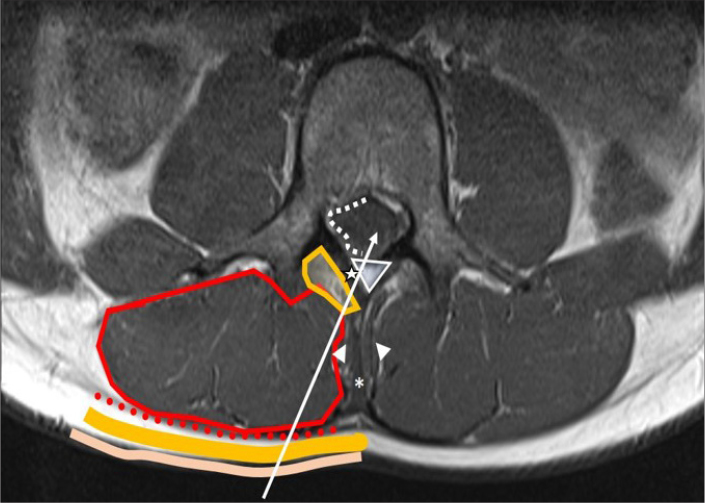
The needle path during an LP is illustrated in Fig. 1. Within the spinal canal, the dura-mater is the thick outer membrane of the thecal sac. The web-like arachnoid membrane is avascular and lies directly beneath, but is not attached to, the dura-mater. Within the subarachnoid space, the spinal cord tapers to the conus medullaris, ending usually between T12 and L2. FG-LP should be performed below the conus, using prior imaging as a guide, when available. The nerve roots extending below the conus grossly resemble a horse’s tail, cauda equina in Latin (Fig. 2). The needle might touch these nerve roots during an FG-LP, potentially causing an electrical sensation in the corresponding dermatome.
Figure 1.
The journey of a needle during LP. From an interlaminar approach, starting from the skin (beige), the needle passes through the subcutaneous fat (yellow), posterior layer of thoracolumbar fascia (red dots), multifidus muscles/erector spinae muscles (encircled in red) adipose tissue around the muscles (encircled in yellow), ligamentum flavum (star), epidural space (triangle), dura mater/arachnoid mater (white dots) and finally the subarachnoid space inside the thecal sac. From an interspinal approach, additionally the needle passes through the thicker supraspinous (*) and interspinous ligaments (arrowheads).
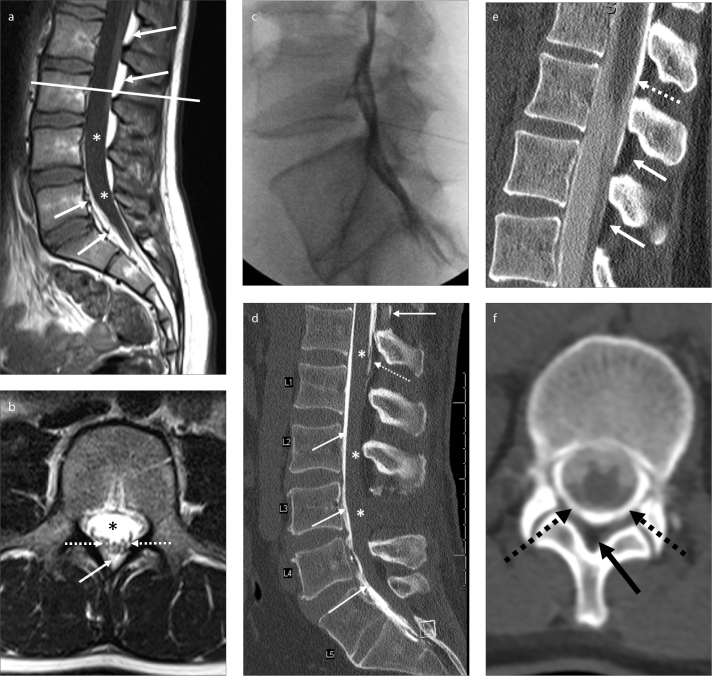
Figure 2. a–f.
Normal MRI appearance of lumbar spine on T1-weighted image (a) shows CSF filled thecal sac (asterisk) surrounded by T1 hyperintense fatty epidural space (white arrows). On the corresponding axial T2-weighted image at L3 (b), the nerve roots of the cauda equina (white dashed arrows) are visible within the CSF filled thecal sac (asterisk). On axial images, the posterior epidural space is in triangular shape (white arrow). An example of inadvertent mixed epidural/subdural contrast injection during a myelography attempt (c). Although there is contrast dispersal on the lateral fluoroscopic image, CT (d) demonstrates opacification of the anterior epidural space at all levels and minimal posterior epidural enhancement at T12 (white arrow). Note there is also tapering subdural contrast at T12 and L1 (dashed arrow). No contrast is present in the subarachnoid space (asterisk). Another example of an attempted myelogram (e, f), showing inadvertent mixed subarachnoid-subdural contrast injection. Note the hypodense nonenhancing epidural fat (e, white arrows; f, black arrow). There is contrast within the subarachnoid space, but there is more hyperdense subdural contrast injection surrounding the thecal sac (e, dotted white arrow; f, dotted black arrows).
The space between the outer margins of the vertebral canal and the thecal sac is the epidural space which contains fat and blood vessels. The subdural space is a potential space between the dura and arachnoid-mater which might enlarge if CSF or blood leaks into this space or might be iatrogenically filled with contrast during a myelogram. The subarachnoid space is the CSF-filled anatomic space deep into the arachnoid-mater (Fig. 2). Familiarity with the appearance of the lumbar vertebrae on anteroposterior (AP) and oblique fluoroscopic views is of utmost importance while planning the needle trajectory (Fig. 3).
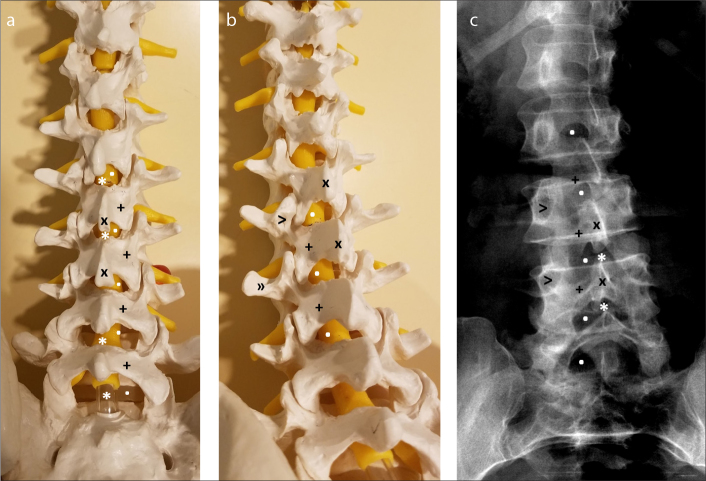
Figure 3. a–c.
Pictures of a spine model in AP (a) and 10° left/10° caudal oblique plane (b). Note how the interlaminar space is widened with this oblique view. AP spine radiograph (c) of a patient. This scoliotic patient is a great example of how the appearance of the interlaminar space changes with obliquity. Also, note how narrow the interspinal space is compared to the interlaminar space. Thus, aiming toward the interlaminar space makes the procedure much easier. Interlaminar space (dot), interspinal space (asterisk), lamina (+), spinous process (x), pedicle (eye of the Scotty dog, >), transverse process (nose of the Scotty dog, »)
Preprocedure patient assessment
The indications, contraindications, and relative contraindications for the procedure are summarized in Table 1 (7–9). Prior to the procedure, the radiologist must answer these following questions: What is the indication? Are there any contraindications? Why do we need fluoroscopy? Has someone else already tried and failed? What is the coagulation status? Is there prior brain and/or spine imaging? Is the patient medically stable? Can the patient give consent? If not, is there someone else eligible to give consent? Is there any possibility of pregnancy? Do we need sedation?
Table 1.
Indications, absolute contraindications, and relative contraindications for a lumbar puncture under fluoroscopic guidance
| Indications | |
|---|---|
| CSF for laboratory analysis | Bacterial meningitis, viral meningitis, tuberculous meningitis, fungal meningitis |
| Chemical meningitis | |
| Leptomeningeal carcinomatosis | |
| Multiple sclerosis | |
| Sarcoidosis | |
| Guillian-Barre disease | |
| Paraneoplastic syndromes | |
| Leukencephalopathies | |
| Subarachnoid hemorrhage | |
| Mitochondrial disorders | |
|
| |
| Opening CSF pressure | Idiopathic intracranial hypertension |
| Intracranial hypotension | |
|
| |
| Intrathecal drug administration | |
|
| |
| Myelography or cisternography | To demonstrate the CSF leakage point |
| Nondiagnostic MRI due to surgical hardware | |
| Surgical planning | |
| Inability to obtain MRI due to claustrophobia, patient size, presence of MRI incompatible hardware such as pacemaker, metallic implant | |
|
| |
| Absolute contraindications | |
|
| |
| Uncorrected coagulopathy | |
|
| |
| Use of anticoagulation | |
|
| |
| At risk for downward herniation | Intracranial mass, obstructive hydrocephalus, cerebral edema |
|
| |
| Relative contraindications | |
|
| |
| Lack of informed consent | Document medical necessity if needed |
|
| |
| Medically unstable or uncooperative patient | Do with sedation if needed |
| Infection in the puncture site such as overlying cellulitis or abscess | Consider cervical puncture |
| Consider transforaminal puncture | |
|
| |
| Fluoroscopy related issues | Pregnancy |
| Patient weight exceeds table limit | |
|
| |
| Myelography related risks | Seizure risk |
| Contrast allergy | |
CSF, cerebrospinal fluid; MRI, magnetic resonance imaging.
For a diagnostic FG-LP, the radiologist should be aware of which laboratory tests will be performed on the obtained CSF. Usually, 10 cc of CSF is adequate for routine laboratory evaluation. However, specific tests may require additional tubes. For instance, at least 5 cc of CSF in one tube is required for M. tuberculosis culture or flow cytometry studies at our institution. Of course, laboratory protocols may differ; therefore, one should be familiar with their institutional standards.
Absolute contraindications
Coagulopathy, anticoagulant agents and antiplatelet agents
Several literature sources cite an INR >1.5 or platelet counts <50 000/μL as an absolute contraindication for LP (7, 10, 11). According to the ACR-ASNR guidelines for myelography and cisternography, “historical or laboratory evidence of bleeding disorder or coagulopathy” is cited as a relative contraindication (9). In 2012, The Society of Interventional Radiology prepared a consensus guideline in which LP was classified as category 2, which includes procedures with moderate bleeding risk, and an INR <1.5 and platelet counts >50 000/μL are recommended (12). In that consensus, one study from 1982 was referenced in which significant spinal subarachnoid hematomas had developed in two of 13 patients with platelet counts <20 000/μL after LP and possibly led to patient death (13). However, these LPs were performed without imaging guidance. In a large retrospective study, 4309 LPs were performed on 959 children with acute lymphocytic leukemia, wherein 378 procedures were performed on patients with platelet counts <25 000/μL without significant bleeding complications. However, a higher incidence of traumatic LP associated with worsening thrombocytopenia was reported (14). Another study of 75 LPs performed on patients with platelet counts <50 000/μL did not identify any significant bleeding complications although reported a statistically significant increase in the occurrence of traumatic procedures in patients with the lowest platelet counts (15). Based on these sources, it would be wise to proceed with an INR of ≤1.5 and platelet count of ≥50 000/μL if there is no urgency. However, when an FG-LP is considered emergently essential in a patient with an abnormal INR or platelet count, FG-LP might be cautiously performed while carefully considering the risks and benefits.
Patients who are referred for FG-LP often have multiple comorbidities. It is not uncommon to encounter a patient taking either an anticoagulation agent, an antiplatelet agent, or both. There are several excellent reviews which can serve as a guide to the interventionalist in following the best approach even in the setting of more recent anticoagulation medications or complex combinations of these drugs (12, 16–19). In Table 2, we provide a summary of their findings, hoping to provide a simple approach for patients on anticoagulation and antiplatelet agents.
Table 2.
Guidelines for management of patients with coagulopathy and/or on anticoagulation/antiplatelet agents based on references (12, 16–19)
| Laboratory values | |
|---|---|
| INR | Correct to ≤1.5 |
|
| |
| Platelet | Correct to ≥50 000/μL |
|
| |
| aPTT | Recommended to check in patients receiving intravenous unfractionated heparin. Although there is no consensus, correct values larger than 1.5 times of normal |
|
| |
| Hematocrit | No recommendation |
|
| |
| Anticoagulant agents | |
|
| |
| Warfarin (Coumadin) | Withhold therapy for 3–5 d before the procedure and check INR. Resume therapy within 12 h after the procedure |
|
| |
| Unfractionated heparin | Subcutaneous: No consensus. If administered <10 000 U, wait for 4 h after the last dose and do the procedure. If ≥10 000 U, check aPTT. Return to therapy 1 h after the procedure |
| Intravenous: Wait for at least 4 h, check aPTT. Resume therapy 1 h after the procedure | |
|
| |
| Low molecular weight heparin | |
| • Enoxaparin (Lovenox) | Withhold last dose or wait for 12 h after the last dose. Resume therapy 24 h after the procedure |
|
| |
| Factor Xa inhibitors | |
| • Fondaparinux (Arixtra) | For Fondaparinux, withhold 48 h before the procedure. Resume therapy after 48 h. For Rivaroxaban, withhold 24–72h before the procedure. Resume therapy after 24 h |
| • Rivaroxaban (Xarelto) | |
|
| |
| Direct thrombin inhibitors (Argotraban, Desirudin) | Wait for 4 h after the last dose to do the procedure. Resume therapy after 1 h |
|
| |
| Antiplatelet agents | |
|
| |
| Aspirin | If low dose (81 mg “baby” aspirin) daily, no contraindication. If high dose (e.g., 325 mg/day), withhold for 5 d |
|
| |
| NSAIDs | No contraindication |
|
| |
| Thienopyridins | |
| • Clopidogrel (Plavix) | Withhold 5 d. Resume therapy the day after the procedure |
| • Ticlopidine (Ticlid) | Withhold 5 d. Resume therapy the day after the procedure |
|
| |
| Glycoprotein IIb/IIIa Inhibitors | |
| • Abciximab (Reopro) | Withhold 24 h. Resume therapy after 8 h |
| • Tirofiban (Aggrastat) | Withhold 4 h. No consensus about resuming therapy |
INR, international normalized ratio; aPTT, activated partial thromboplastin time; d, day; h, hour; NSAIDs, nonsteroid antiinflammatory drugs.
Even in patients with normal coagulation parameters and no history of anticoagulation medication usage, rare hemorrhagic complications might still occur. Although, we have not identified a published case of an atypical hemorrhage following FG-LP, intracranial subdural hematoma, spinal epidural hematoma, and spinal subdural hematoma have been reported after non-image-guided LP (20, 21).
Risk of cerebral/cerebellar herniations
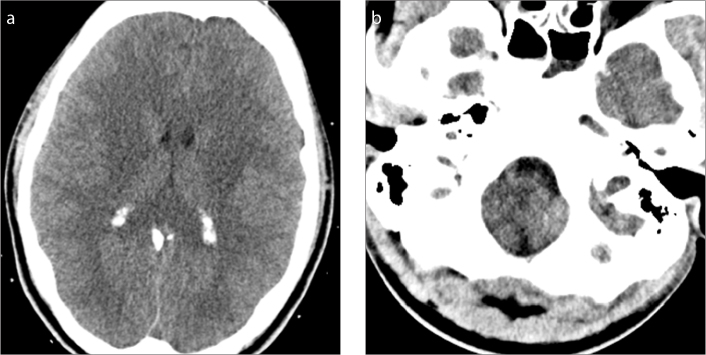
LP might facilitate herniation in conditions with diffuse brain edema, mass effect, or increased intracranial pressure. The free flow of CSF caudally may create a negative pressure gradient from below, thus adding to the compression effects from above. If the patient is comatose, has papilledema, or if there is any other reason to be suspicious of an intracranial space occupying mass, a CT or MRI of the brain could be performed to exclude any potential for tonsillar herniation prior to the FG-LP (22). In a cohort of 235 patients who had CT before LP, only 4 (2%) had a risk for downward herniation, and 2 (1%) eventually died due to increased brain edema and herniation in a week without undergoing LP (23). An example of a case in which LP is contraindicated given a possibility of herniation is shown in Fig. 4.
Figure 4. a, b.
A 22-year-old male with encephalopathy. ER providers requested an LP. However, head CT demonstrated slit-like ventricles with diffuse swelling of the entire brain (a) and cerebellar herniation (b). The LP request was declined by the radiologist after evaluation of the CT. At follow-up, the patient was found to have acute liver failure with hepatic encephalopathy.
Relative contraindications
Seizure and allergy risk in myelography
Seizures were reported in 2 out of 1350 myelography procedures among non-epileptic patients using 10.2 grams of iopamidol for an incidence of 0.15% (24). Another study reported only one seizure out of 1883 myelography patients after a cervical myelogram with FG-LP using iohexol for an incidence of 0.05% (25). In one case report, a patient seized within minutes after myelography performed with 18 cc of 240 μg/mL iohexol and died despite appropriate emergent response (26). Certain medications such as antipsychotics, antidepressants, and muscle relaxants lower the seizure threshold, and therefore it is recommended to discontinue these medications at least 24–72 hours before the procedure (9, 11, 27). However, in some emergent cases, when the benefits outweigh the risk, one might consider proceeding without holding these medications preprocedurally after discussion with the referring provider and the patient.
To our knowledge, there is only one published case report of laryngeal edema developing after the administration of iohexol into the subarachnoid space for a myelogram (28). In a retrospective study of 1005 patients who underwent myelography using iopamidol, 50 had a history of possible allergy against iodine and iodine products, and none developed any adverse effects (29).
Infection at the puncture site
Rarely, an infection involving the skin and/or subcutaneous tissue overlying the puncture site might be encountered. One might also encounter an abscess or epidural infection within the lumbar spine which is only visible on imaging. In such cases, the puncture site and needle trajectory should be carefully planned to avoid contaminating the CSF and spreading the infection to the central nervous system. If there is no safe course via a lumbar approach, an image-guided transforaminal LP or a cervical puncture should be considered (30, 31).
Medically unstable or uncooperative patient
When dealing with an unstable/uncooperative patient, the procedure might be performed under sedation using midazolam and/or fentanyl with appropriate oxygen support and continuous monitoring (32). Rarely, general anesthesia may be necessary.
Complications/risks
Aside from the complications discussed above, the risk of infection, headache, CSF leak, radiation exposure, and some more unusual complications merit discussion.
Infection
Fortunately, infection is a very rare complication. A study from 1982 reported 8 cases of streptococcal meningitis developing within 24 hours after myelography (33). In a large study, consisting of 2141 patients who underwent FG-LP, none of them developed a documented infection attributed to the procedure in the 48–72 hours after the FG-LP (25). Most of the reported iatrogenic LP-related infections occurred in infants who had non-image-guided LP (34, 35). In one case report, arachnoiditis developed 10 days after a diagnostic LP (36). In general, FG-LP is a very safe procedure when good sterile technique is performed with appropriate skin preparation, sterile equipment usage, gloves, and proper hand washing.
Some hospitals and regulatory bodies require facemasks for standardization of infection control. While some authors also favor wearing masks for infection control due to a theoretical reduction in contamination (37, 38). A small survey demonstrated that only 37.5% of the physicians wear facemasks during an LP (38).
Headache and CSF leak
The most common complication of LP is post-lumbar puncture headache (PLPH). It typically occurs/worsens in the upright position and improves/resolves with lying down (39). It usually starts within 24–48 hours after the LP and spontaneously resolves within days (39, 40). The exact pathophysiology of PLPH is unclear. Presumably, it is related to lack of closure of the dural puncture site, leading to a persistent CSF leak and a resultant decrease in intracranial CSF volume and pressure (40). Younger age and female gender have been identified as significant risk factors for PLPH (25, 39). The volume of the CSF removed has not been identified as a risk factor for PLPH (39, 40). On the contrary, in patients with idiopathic intracranial hypertension, large volumes of CSF (20–40 cc) can be removed to alleviate symptoms (41).
The incidence of PLPH varies between 5.5% and 32% in non-image-guided LP with different techniques and instrumentation (39, 42). A recent study reported PLPH in 2.2% of the 2141 FG-LPs; and only 0.8% (18 of the 48 with PLPH) underwent an epidural blood patch (EBP) within 48–72 hours (25). At our institution, over a 3-year time period, we found a rate of 1.8% of patients, requiring EBP following FG-LP (43).
Of the patients with PLPH, 72% have spontaneous symptom resolution within 7 days without any treatment (44). Nonsteroidal antiinflammatory drugs might be useful for pain relief until the PLPH resolves. In cases with longstanding PLPH, EBP can be applied for the treatment of CSF leakage (45, 46).
A recent Cochrane meta-analysis evaluating the role of bedrest and fluid intake after LP to prevent PLPH concluded that “there was no evidence suggesting that routine bedrest after dural puncture is beneficial for the prevention of PLPH onset. The role of fluid supplementation in the prevention of PLPH remains unclear” (47). Another meta-analysis also concluded that “there was no evidence that longer bedrest after cervical or lumbar puncture was better than immediate mobilization or short bedrest in reducing the incidence of headache” (48). Oral and intravenous caffeine has also been suggested for PLPH treatment; however, the supporting evidence is limited (49).
Fortunately, there are some technical considerations that can be implemented to reduce the incidence of PLPH incidence. These will be discussed in more detail further below.
Technique
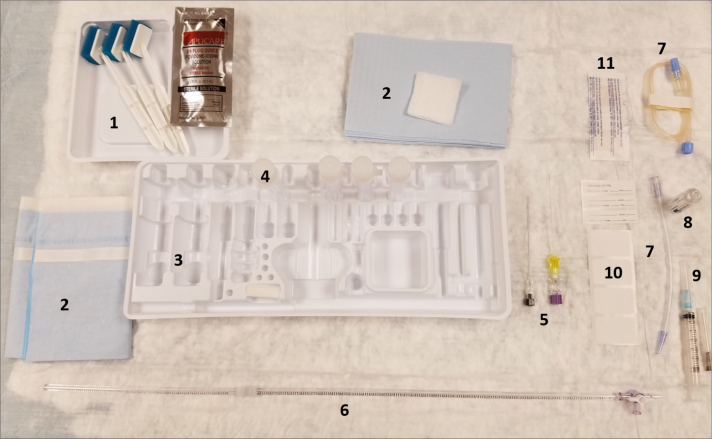
Here, the steps involved in performing an FG-LP are reviewed. Technical considerations that can be implemented to reduce the incidence of PLPH are also discussed. A typical LP tray is shown in Fig. 5.
Figure 5.
Materials in a standard LP set. 1) Sterile cleaning solution, 2) Sterile drape and gauze, 3) Plastic tray, 4) Tubes, 5) Needles, 6) Manometer, 7) Extension tubes, 8) Local anesthetic, 9) Syringes, 10) Labels, 11) Band-aid.
Patient position: prone vs. lateral decubitus
According to a large survey, 88% of the neuroradiologists prefer performing FG-LP with the patient in the prone position, while the remaining 12% preferred making the initial puncture with the patient in the lateral decubitus position (1). Although every radiologist has their own personal preference, some patients may not be comfortable in either the prone or lateral decubitus position. Furthermore, other issues such as the presence of surgical incisions, drainage catheters, respiratory equipment, or injuries might necessitate a particular position. Therefore, radiologists should be familiar with performing the procedure in either position.
The prone position is often considered to be an easier orientation which has the advantages of better anatomical visualization and relatively stable patient position less prone to motion. Ensuring the appropriate needle trajectory is also simplified without the effects of gravity weighing down the needle hub. Moreover, the C-arm is less obstructive, permitting the interventionalist to stand closer to the needle and the patient. Especially in obese patients, this position spreads the abdominal fat and bowel loops, decreasing patient thickness, allowing better visualization of the osseous spinal anatomy. The disadvantages are generally narrower interlaminar spaces and slower CSF flow. In fact, in patients with low CSF-OP, it may not be possible to achieve spontaneous CSF return.
In the lateral decubitus position, the patient can be more readily positioned with the lumbar spine in flexion. This technique promotes widening of the interlaminar spaces, and faster initial CSF flow. Some proceduralists also favor the lateral decubitus position when measuring CSF-OP. However, the effect of patient position on CSF-OP is limited, as discussed below.
Planning the needle trajectory and puncture
The most crucial part of the procedure is determining the initial needle puncture site and the needle trajectory. A carefully selected skin entry point can ensure an efficient use of time and minimize radiation exposure. If available, review of prior imaging can enable accurate numbering of lumbar-type vertebrae, identification of transitional segments when present, estimation of patency of the spinal canal, awareness of prior surgical change, and avoidance of potential fluid collections. Usually, the L2–3, L3–4 and L4–5 levels are preferred for puncture (1, 50). A higher position of the conus may permit performing the puncture at L1–L2. The likelihood of traumatic puncture at L4–5 is nearly double compared to L2–3 and L3–4, which could be due to increased degeneration in the lower spine (50). The L5-S1 level is often avoided due to its deeper anatomic location, preponderance for degenerative change, and typically narrower thecal sac.
A double-blinded randomized controlled study did not find any difference between the interlaminar and interspinal approaches with regard to the incidence of PLPH (51). Interspinal approach is a common method in the absence of imaging guidance, given the ability to palpate the spinous processes (8, 51). However, the needle will traverse additional layers comprised of the supraspinous and interspinous ligaments, which might be calcified especially in elderly patients. Furthermore, hypertrophic spinal processes might severely narrow the interspinal space.
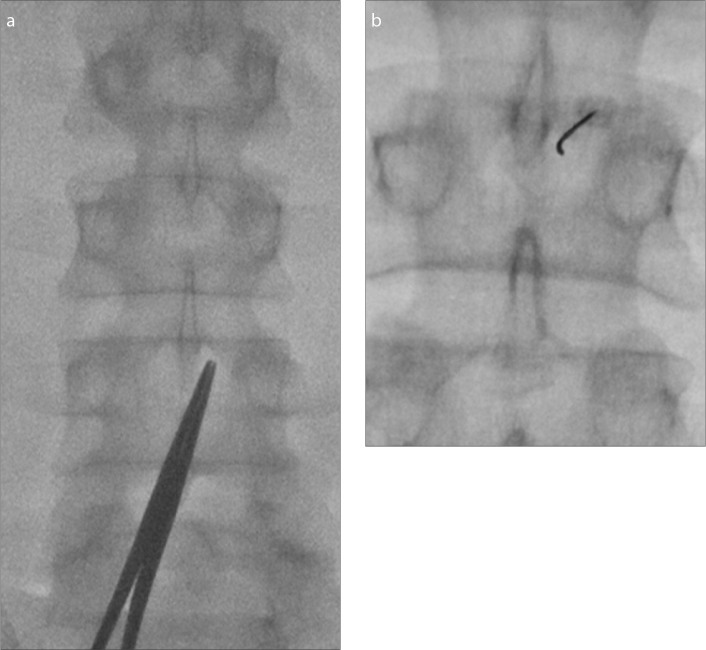
While the interlaminar spaces are usually readily visualized in younger patients (Fig. 6), degenerative osteophytes, kyphosis, and scoliosis might obscure the interlaminar space in some cases. Rotating the C-arm image intensifier 5–10 degrees laterally and 5–10 degrees caudally usually results in good visualization of the interlaminar space; however, every patient’s unique anatomy and position should be accounted for when determining the best approach. A metallic clamp can be used to mark the skin entrance point under fluoroscopic guidance (Figs. 7 and 8). This region should then be sterilized and draped appropriately.
Figure 6. a, b.
Planning needle trajectory on a young patient with an AP view (a). The right interlaminar space at L3–4 is widely patent and marked with a metallic clamp. After advancing the needle, a fluoroscopic image (b) shows the distal needle tip is slightly curved. The interventionalist also felt stiffness, attributable to the ligamentum flavum. The needle is now very close to thecal sac. It is a good time to rotate the bevel to a horizontal orientation.
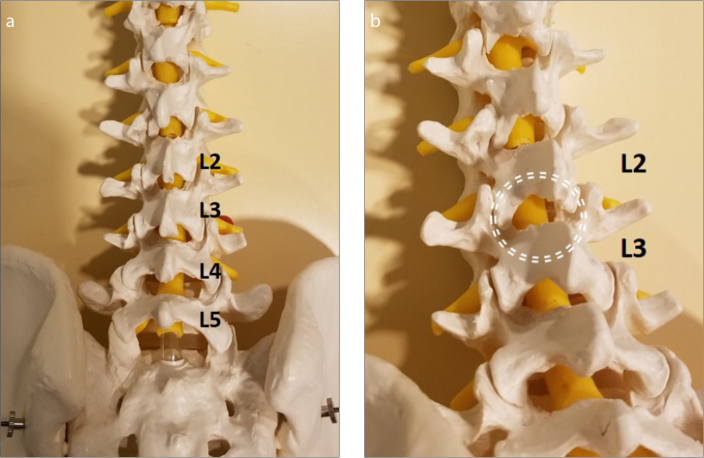
Figure 7. a, b.
Planning a needle trajectory on a spine model. Anterior posterior view of L2–3 (a). The left interlaminar space is relatively obscured by the laminae and spinous process. Panel (b) shows the picture obtained after 10° left oblique and 10° caudal angulation to widen the target for an ideal interlaminar approach.
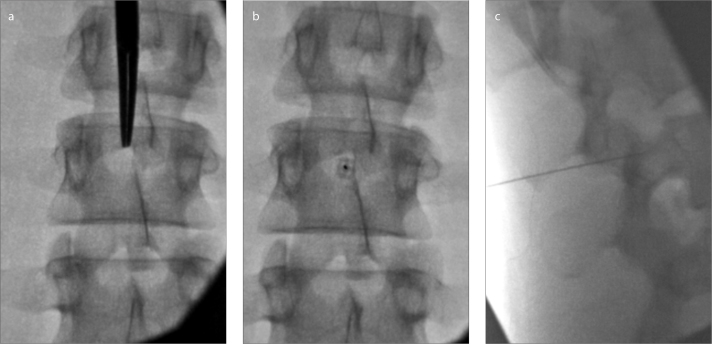
Figure 8. a–c.
Planning a needle trajectory on a young patient. Panel (a) shows 10° left oblique and 10° caudal angulation to widen the interlaminar space at L2–3. Bull’s eye needle view (b) with an ideal needle trajectory for LP. With lateral imaging (c), the needle is not yet in the thecal sac. Removal of the stylet will not yield CSF. The needle should be advanced to the center of the spinal canal.
The next step is injecting the local anesthetic along the projected needle trajectory, making certain to produce an adequate skin wheal. Amide agents are typically used, such as lidocaine or bupivacaine. Usually, 5 cc of local anesthetic is sufficient. Although these medications are generally safe, there is a <1% risk of an allergic reaction after subcutaneous lidocaine injection (52). In the setting of a known allergy to lidocaine or bupivacaine, an ester agent such as tetracaine, chloroprocaine, or benzocaine might be used (53). If these anesthetics are not available, diphenhydramine, an antihistamine, might be used as an alternative to obtain short-term local anesthesia (54). In such instances, a 1% solution of diphenhydramine (10 mg/cc) can be prepared by mixing 10 cc of 5% diphenhydramine with 40 cc of saline to achieve the optimum effect (55).
At our institution, Quincke needles are used for FG-LPs. These needles have a bevel. The notch at the needle hub points to the open side of the needle bevel. There are a few ways to guide a Quincke needle in a desired direction, as demonstrated in Fig. 9.
Figure 9. a–d.
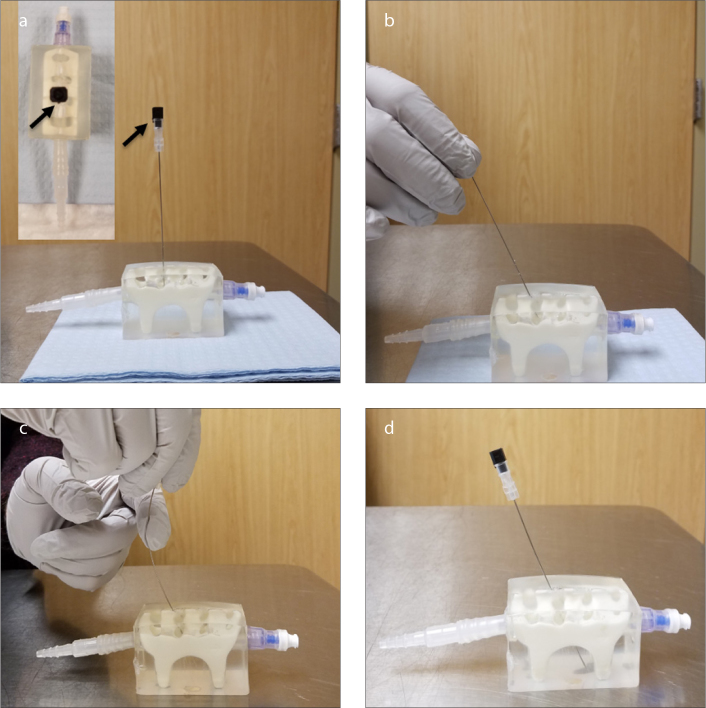
How to direct a Quincke needle. Panel (a) shows the notch (arrows) in the needle hub pointing toward the bevel, the open side of the needle tip. The very tip of the needle is thus opposite to the notch. The needle tends to advance opposite to the needle notch. Panel (b) shows the leverage method, using the skin as a fulcrum. When the needle hub is moved in one direction, the needle tip tends to go to the opposite side. Panel (c) shows the finger fulcrum method. In this technique, the hub is pulled toward the intended direction of the needle tip. Then, the proximal portion of the needle is pulled to the opposite side with the other hand, curving the needle. In panel (d), the needle tip is easily directed from the skin entry point to its target.
The needle advances easily through the skin, subcutaneous fat and the muscles. When entering the thicker stiffer ligamentous structures, the interventionalist usually feels resistance, potentially accompanied by a slight bend or curve of the needle tip under fluoroscopy. That is a generally a sign that the needle is very close to the thecal sac and one may elect to switch to a lateral projection to evaluate the needle depth.
When approaching the thecal sac, it is recommended that the needle be rotated such that the bevel is parallel to the dural fibers (pointed toward the patient’s right or left), to decrease the likelihood of PLPH, as showed in a randomized double blinded study with PLPH occurring in 3.8% of patients with the needle entering the thecal sac parallel to the course of the spine, compared to 22.6% of patients when the needle entering the thecal sac perpendicular to the spine (56). This finding is corroborated in an earlier prospective study (57). Although it was previously hypothesized that the dural fibers are oriented parallel to the course of the spine, cadaveric and histopathologic studies have shown that the dura-mater consists of collagen/elastic fibers arranged in several layers that do not demonstrate a specific orientation; they can be found in longitudinal, transverse or mixed orientations (58). No difference was found in the size of the puncture hole between the two puncture methods in an in vitro study using 22G Quincke needle (59). Thus, it remains unclear why the bevel orientation has such an effect on the incidence of PLPH.
Removing the needle
Many recommend removing the needle only after reinserting the stylet to reduce the PLPH incidence. In fact, a randomized prospective study reported PLPH in 5% of the patients who underwent LP with reinsertion of the stylet compared with 16.3% who underwent LP without reinsertion of the stylet (60). Notably, the patients in this study had their LP performed with an atraumatic 21G Sprotte needle. As such, the American Academy of Neurology recommended reinsertion of the stylet before needle removal when a non-cutting type needle is used (39). However, there is no similar study using the Quincke needle in the setting of FG-LP. A recent randomized study demonstrated that reinserting the stylet does not affect the rate of PLPH in patients who had spinal anesthesia using a 25G Quincke needle; comparing two groups consisting of 315 patients in each, the rate of PLPH was found 10.5% and 11.1% respectively (61). As such, more randomized studies are needed to evaluate the effect of reinserting the stylet using Quincke needle. Some report that reinserting the stylet prevents the occurrence of a suction effect when removing the needle which might otherwise result in trauma or entrapment of nerve fibers, although there is no sufficient evidence to support these claims (7, 62). At our institution, we err on the side of caution and reinsert the stylet before removing the needle.
Needle size
Usually, a 3.5-inch-long needle is sufficient for an FG-LP, although in patients with increased body mass index (BMI), a 5 or even 7-inch-long needle might be required. In small children and infants, needle lengths of 1–2 inches are preferred. Measuring the distance between the skin and thecal sac in prior cross-sectional studies can be very useful for selecting the needle length.
If a Quincke needle is used, the larger the needle diameter, the higher the risk of PLPH is (39, 63). However, using the smallest needle is not always practical. The CSF flow might be significantly slower with a 25G needle compared to 22G, and it may substantially increase procedure time. While using a 25G or a smaller needle in a patient with low CSF-OP, obtaining 3–5 cc of CSF might take 30 minutes or more. Notably, smaller needles are more readily bent and deformed during FGLP. In fact, needle fracture within the interspinous ligament has been reported with a 27G Quincke needle (64). Carson et al. (63) evaluated different types of needles and their ability to measure the exact CSF-OP and their flow rates for an optimum LP in an in vitro model. Accordingly, needles smaller than 22G failed to reflect 90% of the actual CSF pressure; therefore they suggested the use of 22G or larger needles to measure CSF-OP quickly and accurately. The fastest and the most accurate CSF-OP reading was obtained using 20G Sprotte and Quincke needles, followed by 22G Sprotte, Whitacre and Quincke needles. According to their results, it takes about 2 minutes to obtain an accurate reading of CSF-OP using a 22G Quincke needle. Ultimately, the radiologist should decide which needle size to use depending on the patient’s condition. If a large amount of CSF is needed, or there is a condition that dictates quick termination of the procedure such as patient instability, a 20G needle might be preferred. On the contrary, if there is an increased risk of CSF leakage, one might consider using a 22G or 25G needle.
Needle types
Apart from the classical Quincke needle with a cutting tip, there are two “atraumatic” needles with a blunt tip, Sprotte and Whitacre, which are primarily used by anesthesiologists and neurologists. The Sprotte needle has been demonstrated to have a smaller dural puncture area compared to the Quincke needle (65). Due to their “non-cutting” pen point geometry, these needles do not pierce the skin; therefore a separate introducer needle is needed to puncture the skin (Fig. 10) (8).
Figure 10. a–c.
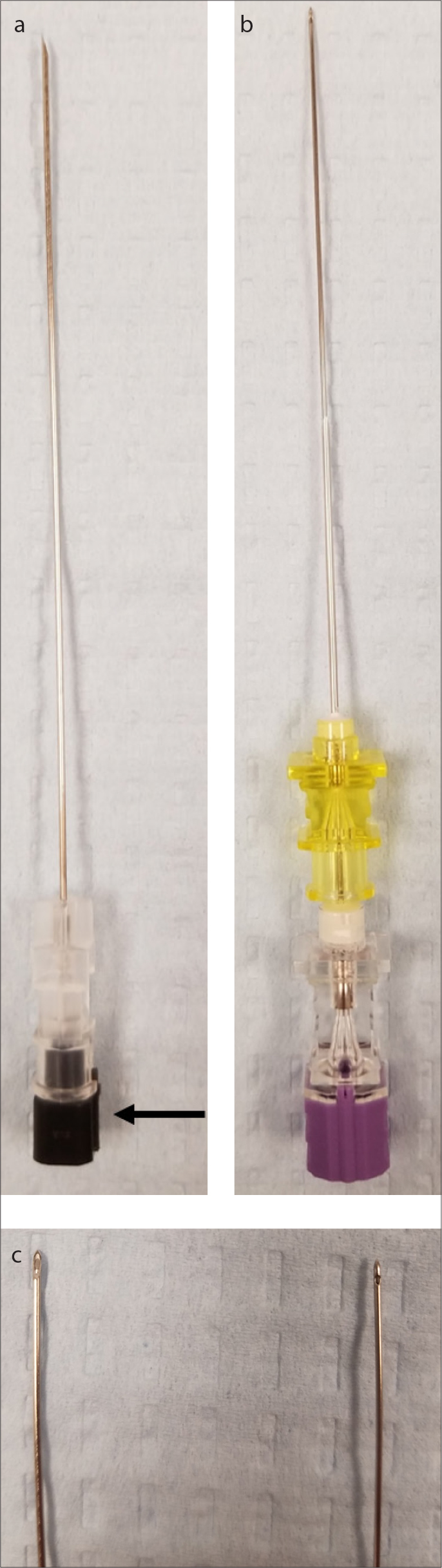
Panel (a) shows a 22G Quincke needle on the left. The notch (black arrow) points to the direction of the bevel, meaning that the needle tends to move opposite the direction of the notch. Panel (b) shows a 22G Sprotte needle with short yellow introducer and purple needle. In panel (c), note the cutting tip of a Quincke needle (left) compared to a Sprotte needle (right) which has side holes located at the side rather than the tip and blunt tip.
Some neurology and anesthesiology literature recommends the usage of atraumatic needles based on studies reporting a lower PLPH incidence compared with the Quincke needle (8, 42, 66). This recommendation is also supported by a more recent meta-analysis (67). However, we approach this recommendation with caution. 22G Sprotte and Quincke needles were compared in a prospective randomized trial consisting of 115 LP patients (65). PLPH was reported with incidences of 24.4% and 12.2% in the Quincke and Sprotte groups, respectively, and the PLPH incidence requiring an EBP was not evaluated. Also, the incidence of severe PLPH was similar in both groups (8.1% vs. 7.3%). In another smaller prospective study, 22G Whitacre and Quincke needles were compared in the setting of diagnostic LP (66), with PLPH more commonly associated with the Quincke needle, although no patients underwent eventual EBP.
In another study comparing 22G Quincke and Sprotte needles, incidences of 22.4% and 8.5% PLPH were reported with median PLPH duration of 4 days and 1 days in the Quincke and Sprotte groups, respectively (68). However, it is notable that the initial LP attempt in nearly 40% of the patients within the Sprotte group was unsuccessful, necessitating completion of the LP using a Quincke needle. The relatively high failure rate with the Sprotte needle in this study should be carefully considered when selecting the needle type. There is no data regarding the eventual need for EBP in either group.
Other studies have focused on image-guided lumbar punctures. PLPH incidences were compared in a prospective FG-LP study in patients who had myelography performed with 22G Whitacre or Quincke needles (69). The rate of PLPH with the Quincke needle was 15.6% and with the Whitacre needle was 9.6% (P > 0.05). Notably, over 15% of patients in the Whitacre group had an unsuccessful initial attempt, and the myelography was therefore completed using a Quincke needle. Two patients from each group required an EBP. In a retrospective study of patients who underwent FG-LP with a 22G Whitacre, 22G Quincke, or 20G Quincke needle, the EBP rates were 4%, 15%, and 30%, respectively (70). Although these results demonstrate a statistically significant decrease in the need for EBP following FG-LP with a Whitacre needle, EBP was performed in almost 10% of all patients after FG-LP. This rate is interestingly high compared with the study by Rodriguez et al. (25), where PLPH was observed in 2.2% of patients undergoing FG-LP using either a 22 or 25G Quincke needle; and only 0.8% of all underwent an EBP.
In summary, there appears to be a lower likelihood of PLPH with atraumatic needle usage. However, the initial procedural success rate with these atraumatic needles is reportedly inferior relative to the Quincke needle and may result in a need to switch needle types. Of course, operator experience and familiarity with these needle types play an important role in needle selection. Furthermore, the many other technical considerations discussed herein play a key role in ensuring a successful FG-LP beyond simply the needle type.
Measuring CSF-OP
Plastic tubing can be connected to the needle after observation of CSF in the hub. Then, a stopcock and manometer are attached to the other end of the tubing. The tubing is maintained in a horizontal position. During measurement, the patient is encouraged to relax and breathe normally. One should wait until the meniscus stops rising within the manometer and only respiratory fluctuation is apparent. If the patient is in the prone, the manometer is kept at the level of the needle hub and the needle length is added in centimeters to the manometer measurement. In the lateral decubitus, the manometer is kept at the same level as the needle, and the measurement on the manometer is directly recorded.
According to a survey, of the radiologists performing the FG-LP with the patient in the prone position, 72% prefer to measure the CSF-OP in prone, while 28% prefer to rotate the patient to the lateral decubitus to measure the pressure. Interestingly, 21% of those who measure the pressure in the prone, do not add the needle length to the manometer measurement (1), which might lead to a significant underestimation of the CSF-OP.
Schwartz et al. (6) investigated the effect of patient position on CSF-OP measurement. They reported a statistically significant difference of mean CSF-OP values between prone and lateral decubitus positions. They also stated that the prone position resulted in an overestimation of the pressure, concluding that measurements should be performed in the lateral decubitus position. However, the mean difference between the two groups was only 2.7 cm H2O. In another study by Abel et al. (71), a mean difference of 1.2 cm H2O was reported between two similar groups, and it was neither statistically nor clinically significant. The demographics between these two studies are notably different. In the study of Schwartz et al. (6), there were more patients with normal CSF-OP. In the study by Abel et al. (71), there were more female patients and patients with abnormal opening pressure, mostly due to a high rate of idiopathic intracranial hypertension. Even though there were more patients with a higher opening pressure in the study of Abel et al. (71), the patient position did not affect the results. Based on their results, the patient position generally does not have a significant effect on the CSF-OP measurements which can impact the clinical course.
Radiation dose
Fluoroscopy time is a variable, largely affected by operator experience. Additional contributors to a variable fluoroscopy time are the patient’s BMI and ability to remain still throughout the procedure. Even in patients with an average BMI of 29 kg/m2 who underwent FG-LP, a mean effective radiation dose of 2.9 mSv (0.54–8.19 mSv) was reported (72), which is roughly the equivalent of two spine radiographs, one intravenous pyelogram, or one year of natural background radiation (73).
Nerve injury
Many patients ask about the likelihood of periprocedural nerve injury. Although theoretically possible, the authors are not aware of any direct nerve damage that has occurred in conjunction with an FG-LP, either in the literature or at our institution where we perform approximately 1000 FG-LPs/year.
Some practical points
A few maneuvers may hasten free CSF flow: putting the patient in the reverse Trendelenburg position; Valsalva maneuver or coughing; or rotating the patient from the prone to lateral decubitus position. However, good CSF flow may not be achieved in some instances despite implementation of these maneuvers. The needle tip may indent the anterior dura-mater or an adjacent nerve root might obstruct the flow into the needle. In those instances, rotating the needle 90–180 degrees might improve CSF flow. If that does not help, consider reinserting the stylet and gently withdrawing or advancing the needle by 1–3 mm.
With atraumatic needles, the needle holes are at the lateral margins of the needle rather than at the tip. Even if the needle tip is located centrally within the spinal canal, it may simply be displacing the dura anteriorly and require further advancement to pierce the dura to enter the subarachnoid space. If the location of the needle tip is uncertain, a small amount of contrast might be injected to better determine the location of the needle tip, although this may impact the results of CSF cultures as some forms of iodinated contrast are bacteriostatic or bactericidal.
Aspiration of CSF with a syringe is not routinely recommended unless the flow is particularly slow and other maneuvers to hasten flow have been unsuccessful. Based on experience, even gentle aspiration occasionally results in pain, presumably a result of the negative pressure pulling a nerve up against the needle. Also, while aspirating, it is not uncommon to lose CSF flow due to presumed plugging of the needle tip by a nerve or arachnoid membrane. The effect of syringe size during aspiration has also been evaluated. The strength of vacuum generated with aspiration is solely determined by the volume displaced by the plunger for all syringe sizes and not directly related to the cross-sectional diameter of the plunger (74). Larger syringes can thus generate a greater maximum vacuum than smaller syringes due to a larger potential for volume displacement. That means whether you use 3 cc, 5 cc or 10 cc syringes, if you pull the plunger back by 2 cc, the vacuum effect would be the same in all syringes. However, we should note that pulling the plunger of a smaller syringe is easier. Lastly, even if you do everything right, there will be rare occasions that you may not get a very good CSF flow. At those times, it is best to restart the FG-LP at a different location, usually at a different spinal level.
Postprocedural assessment
The puncture site is cleaned, and a sterile bandage is applied. At our institution, the patients are typically monitored with bedrest for 1 hour. Discharge instructions include avoiding excessive physical activity, preferably resting as much as possible for the remainder of the day, ensuring adequate hydration, and avoiding submersion of the puncture site in water (e.g., bathtubs, pools). Nonsteroidal antiinflammatory drugs can be considered to treat headache. Patients are informed of the signs and symptoms of CSF-leakage, infection, and hemorrhage, such as positional headache, gradually increasing back pain, new onset of neurologic symptoms, or fever, and instructed to call for further evaluation if needed.
Conclusion
With an appropriate degree of experience and familiarity with the evidence described above, it is possible to develop a standard FG-LP technique while employing techniques intended to decrease the likelihood of associated complications and optimize patient outcome.
Main points.
There are many differences in fluoroscopy-guided lumbar puncture (FG-LP) technique among radiologists.
<6 cm H2O is indicative of low intracranial pressure; >25 cm H2O indicates idiopathic intracranial hypertension.
In general, FG-LP is a very safe procedure when good sterile technique is performed.
The most common complication of FG-LP is post-lumbar puncture headache (PLPH).
Younger age, female gender, needle size, needle orientation, and stylet reinsertion are important factors impacting PLPH.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
Part of this review was published as an “educational electronic poster” during annual RSNA 2017 meeting in Chicago, IL, USA.
References
- 1.Abel AS, Brace JR, McKinney AM, Harrison AR, Lee MS. Practice patterns and opening pressure measurements using fluoroscopicallyguided lumbar puncture. AJNR Am J Neuroradiol. 2012;33:823–825. doi: 10.3174/ajnr.A2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eskey CJ, Ogilvy CS. Fluoroscopy-guided lumbar puncture: decreased frequency of traumatic tap and implications for the assessment ofct-negative acute subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2001;22:571–576. [PMC free article] [PubMed] [Google Scholar]
- 3.Hall JE. Cerebral blood flow, cerebrospinal fluid, and brain metabolism. In: Hall JE, editor. Guyton and hall textbook of medical physiology. 13th ed. Philadelphia: Elsevier; 2016. pp. 787–794. [Google Scholar]
- 4.Corbett JJ, Haines DE, Ard MD, Lancon JA. The ventricles, choroid plexus, and cerebrospinalfluid. In: Haines DE, editor. Fundamental neuroscience for basic and clinical applications. 4th ed. Philadelphia: Elsevier; 2013. pp. 82–94. [DOI] [Google Scholar]
- 5.Friedman DI, Jacobson DM. Diagnostic criteriafor idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495. doi: 10.1212/01.WNL.0000029570.69134.1B. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz KM, Luetmer PH, Hunt CH, et al. Position-related variability of CSF opening pressure measurements. AJNR Am J Neuroradiol. 2013;34:904–907. doi: 10.3174/ajnr.A3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauley KA. Fluoroscopically guided lumbar puncture. AJR Am J Roentgenol. 2015;205:W442–450. doi: 10.2214/AJR.14.14028. [DOI] [PubMed] [Google Scholar]
- 8.Doherty CM, Forbes RB. Diagnostic lumbarpuncture. Ulster Med J. 2014;83:93–102. [PMC free article] [PubMed] [Google Scholar]
- 9.ACR–ASNR practice guideline for the performance of myelography and cisternography 2008. [Accessed August 13, 2018]. Available at: https://www.asnr.org/wp-content/uploads/2016/12/Myelography.pdf.
- 10.Johnson KS, Sexton DJ. Lumbar puncture:technique, indications, contraindications, andcomplications in adults. [Accessed August 13, 2018]. Available at: http://www.uptodate.com/contents/lumbar-puncture-technique-indications-contraindications-and-complications-in-adults#H6.
- 11.Pomerantz SR. Myelography: Modern technique and indications. Handb Clin Neurol. 2016;135:193–208. doi: 10.1016/B978-0-444-53485-9.00010-6. [DOI] [PubMed] [Google Scholar]
- 12.Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management ofcoagulation status and hemostasis risk in percutaneous image-guided interventions. J VascInterv Radiol. 2012;23:727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Breuer AC, Tyler HR, Marzewski DJ, Rosenthal DS. Radicular vessels are the most probable source of needle-induced blood in lumbar puncture: Signif-icance for the thrombocytopenic cancer patient. Cancer. 1982;49:2168–2172. doi: 10.1002/1097-0142(19820515)49:10<2168::AID-CNCR2820491031>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 14.Howard SC, Gajjar A, Ribeiro RC, et al. Safety of lumbar puncture for children with acute lymphoblastic leukemia and thrombocytopenia. JAMA. 2000;284:2222–2224. doi: 10.1001/jama.284.17.2222. [DOI] [PubMed] [Google Scholar]
- 15.Vavricka SR, Walter RB, Irani S, Halter J, Schanz U. Safety of lumbar puncture for adults withacute leukemia and restrictive prophylactic platelet transfusion. Ann Hematol. 2003;82:570–573. doi: 10.1007/s00277-003-0707-0. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe TA, Raiff D, Ho LM, Kim CY. Management of anticoagulant and antiplatelet medications in adults undergoing percutaneous interventions. AJR Am J Roentgenol. 2015;205:421–428. doi: 10.2214/AJR.14.13342. [DOI] [PubMed] [Google Scholar]
- 17.Layton KF, Kallmes DF, Horlocker TT. Recommendations for anticoagulated patients undergoing image-guided spinal procedures. AJNRAm J Neuroradiol. 2006;27:468–470. [PMC free article] [PubMed] [Google Scholar]
- 18.Patel IJ, Davidson JC, Nikolic B, et al. Addendumof newer anticoagulants to the SIR consensusguideline. J Vasc Interv Radiol. 2013;24:641–645. doi: 10.1016/j.jvir.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Narouze S, Benzon HT, Provenzano DA, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications: Guidelines from the american society of regional anesthesia and pain medicine, theeuropean society of regional anaesthesia andpain therapy, the american academy of painmedicine, the international neuromodulationsociety, the north american neuromodulationsociety, and the world institute of pain. RegAnesth Pain Med. 2015;40:182–212. doi: 10.1097/AAP.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 20.Samdani A, Garonzik IM, Zahos P. Subdural hematoma after diagnostic lumbar puncture. Am J Emerg Med. 2004;22:316–317. doi: 10.1016/j.ajem.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair AJ, Carroll C, Davies B. Cauda equinasyndrome following a lumbar puncture. J ClinNeurosci. 2009;16:714–716. doi: 10.1016/j.jocn.2008.07.079. [DOI] [PubMed] [Google Scholar]
- 22.van Crevel H, Hijdra A, de Gans J. Lumbar puncture and the risk of herniation: When should we first perform CT? J Neurol. 2002;249:129–137. doi: 10.1007/PL00007855. [DOI] [PubMed] [Google Scholar]
- 23.Hasbun R, Abrahams J, Jekel J, Quagliarello VJ. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345:1727–1733. doi: 10.1056/NEJMoa010399. [DOI] [PubMed] [Google Scholar]
- 24.Klein KM, Shiratori K, Knake S, et al. Status epilepticus and seizures induced by iopamidol myelography. Seizure. 2004;13:196–199. doi: 10.1016/S1059-1311(03)00077-3. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez D, Branstetter BFt, Agarwal V, et al. Journal club: Incidence of complications following fluoroscopically guided lumbar punctures and myelograms. AJR Am J Roentgenol. 2016;206:20–25. doi: 10.2214/AJR.15.14664. [DOI] [PubMed] [Google Scholar]
- 26.Alimohammadi H, Abdalvand A, Safari S, Mazinanian A. Status epilepticus after myelography with iohexol (omnipaque) Am J Emerg Med. 2012;30:2092–2093. doi: 10.1016/j.ajem.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Fedutes BA, Ansani NT. Seizure potential of concomitant medications and radiographic contrast media agents. Ann Pharmacother. 2003;37:1506–1510. doi: 10.1345/aph.1C464. [DOI] [PubMed] [Google Scholar]
- 28.Agildere AM, Haliloglu M, Cila A, Ozmen M. Laryngeal edema, following the injection of iohexol into the subarachnoid space. Neuroradiology. 1991;33:290. doi: 10.1007/BF00588241. [DOI] [PubMed] [Google Scholar]
- 29.Ebersold MJ, Houser OW, Quast LM. Iopamidol myelography: Morbidity in patients with previous intolerance to iodine derivatives. J Neurosurg. 1991;74:60–63. doi: 10.3171/jns.1991.74.1.0060. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs WNS, MR, Kim PE, Go JL, Law M. C1–2 puncture: A safe, efficacious, and potentially underused technique. Neurographics. 2017;7:1–8. doi: 10.3174/ng.1170183. [DOI] [Google Scholar]
- 31.Nascene DR, Ozutemiz C, Estby H, McKinney AM, Rykken JB. Transforaminal lumbar puncture: An alternative technique in patients with challenging access. AJNR Am J Neuroradiol. 2018 doi: 10.3174/ajnr.A5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim TH. Safety and effectiveness of moderate sedation for radiologic non-vascular intervention. Korean J Radiol. 2006;7:125–130. doi: 10.3348/kjr.2006.7.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlesinger JJ, Salit IE, McCormack G. Streptococcal meningitis after myelography. Arch Neurol. 1982;39:576–577. doi: 10.1001/archneur.1982.00510210046010. [DOI] [PubMed] [Google Scholar]
- 34.Smaoui H, Hariga D, Hajji N, et al. Iatrogenic meningitis after diagnosis lumbar puncture: 3 cases reports in the paediatric children’s hospital of Tunis. Bull Soc Pathol Exot. 2011;104:10–13. doi: 10.1007/s13149-010-0084-6. [DOI] [PubMed] [Google Scholar]
- 35.da Silva PS, de Souza Loduca RD. Intramedullary spinal cord abscess as complication of lumbar puncture: A case-based update. Childs Nerv Syst. 2013;29:1061–1068. doi: 10.1007/s00381-013-2093-9. [DOI] [PubMed] [Google Scholar]
- 36.Gurbuz MS, Erdogan B, Yuksel MO, Somay H. Postlumbar puncture arachnoiditis mimicking epidural abscess. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-200169. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baer ET. Iatrogenic meningitis: The case for face masks. Clin Infect Dis. 2000;31:519–521. doi: 10.1086/313991. [DOI] [PubMed] [Google Scholar]
- 38.Malhotra R, Kelly S. Wearing facemasks when performing lumbar punctures: A snapshot of current practice amongst trainee doctors. Local Reg Anesth. 2010;3:133–135. doi: 10.2147/LRA.S15828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans RW, Armon C, Frohman EM, Goodin DS. Assessment: Prevention of post-lumbar puncture headaches: Report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2000;55:909–914. doi: 10.1212/WNL.55.7.909. [DOI] [PubMed] [Google Scholar]
- 40.Ahmed SV, Jayawarna C, Jude E. Post lumbar puncture headache: diagnosis and management. Postgrad Med J. 2006;82:713–716. doi: 10.1136/pgmj.2006.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thurtell MJ, Wall M. Idiopathic intracranial hypertension (pseudotumor cerebri): recognition, treatment, and ongoing management. Curr Treat Options Neurol. 2013;15:1–12. doi: 10.1007/s11940-012-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armon C, Evans RW. Therapeutics, technology assessment subcommittee of the american academy of neurology. Addendum to assessment: prevention of post-lumbar puncture headaches: R\report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2005;65:510–512. doi: 10.1212/01.wnl.0000173034.96211.1b. [DOI] [PubMed] [Google Scholar]
- 43.Özütemiz C, Köksel YK, Huang H, Rubin N, Rykken JB. The efficacy of fluoroscopy-guided epidural blood patch in the treatment of spontaneous and iatrogenic cerebrospinal fluid leakage. Eur Radiol. 2018 doi: 10.1007/s00330-018-5828-x. doi: 10.1007/s00330-018-5828-x. [DOI] [PubMed] [Google Scholar]
- 44.Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–729. doi: 10.1093/bja/aeg231. [DOI] [PubMed] [Google Scholar]
- 45.Mihlon F, Kranz PG, Gafton AR, Gray L. Computed tomography-guided epidural patching of postoperative cerebrospinal fluid leaks. J Neurosurg Spine. 2014;21:805–810. doi: 10.3171/2014.7.SPINE13965. [DOI] [PubMed] [Google Scholar]
- 46.Hayek SM, Fattouh M, Dews T, Kapural L, Malak O, Mekhail N. Successful treatment of spontaneous cerebrospinal fluid leak headache with fluoroscopically guided epidural blood patch: a report of four cases. Pain Med. 2003;4:373–378. doi: 10.1111/j.1526-4637.2003.03037.x. [DOI] [PubMed] [Google Scholar]
- 47.Arevalo-Rodriguez I, Ciapponi A, Roque i Figuls M, Munoz L, Bonfill Cosp X. Posture and fluids for preventing post-dural puncture headache. Cochrane Database Syst Rev. 2016;3:CD009199. doi: 10.1002/14651858.CD009199.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thoennissen J, Herkner H, Lang W, Domanovits H, Laggner AN, Mullner M. Does bed rest after cervical or lumbar puncture prevent headache? a systematic review and meta-analysis. CMAJ. 2001;165:1311–1316. [PMC free article] [PubMed] [Google Scholar]
- 49.Lin W, Geiderman J. Myth: Fluids, bed rest, and caffeine are effective in preventing and treating patients with post-lumbar puncture headache. West J Med. 2002;176:69–70. doi: 10.1136/ewjm.176.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu SD, Chen MY, Johnson AJ. Factors associated with traumatic fluoroscopy-guided lumbar punctures: A retrospective review. AJNR Am J Neuroradiol. 2009;30:512–515. doi: 10.3174/ajnr.A1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosaffa F, Karimi K, Madadi F, Khoshnevis SH, Daftari Besheli L, Eajazi A. Post-dural puncture headache: A comparison between median and paramedian approaches in orthopedic patients. Anesth Pain Med. 2011;1:66–69. doi: 10.5812/aapm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batinac T, Sotosek Tokmadzic V, Peharda V, Brajac I. Adverse reactions and alleged allergy to local anesthetics: Analysis of 331 patients. J Dermatol. 2013;40:522–527. doi: 10.1111/1346-8138.12168. [DOI] [PubMed] [Google Scholar]
- 53.Schatz M. Allergic reactions to local anesthetics. [Accessed August 13, 2018]. Available at: http://www.uptodate.com/contents/allergic-reactions-to-local-anesthetics.
- 54.Dire DJ, Hogan DE. Double-blinded comparison of diphenhydramine versus lidocaine as a local anesthetic. Ann Emerg Med. 1993;22:1419–1422. doi: 10.1016/S0196-0644(05)81989-4. [DOI] [PubMed] [Google Scholar]
- 55.Pavlidakey PG, Brodell EE, Helms SE. Diphenhydramine as an alternative local anesthetic agent. J Clin Aesthet Dermatol. 2009;2:37–40. [PMC free article] [PubMed] [Google Scholar]
- 56.Flaatten H, Thorsen T, Askeland B, et al. Puncture technique and postural postdural puncture headache. a randomised, double-blind study comparing transverse and parallel puncture. Acta Anaesthesiol Scand. 1998;42:1209–1214. doi: 10.1111/j.1399-6576.1998.tb05279.x. [DOI] [PubMed] [Google Scholar]
- 57.Lybecker H, Moller JT, May O, Nielsen HK. Incidence and prediction of postdural puncture headache. a prospective study of 1021 spinal anesthesias. Anesth Analg. 1990;70:389–394. doi: 10.1213/00000539-199004000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Dittmann M, Schafer HG, Ulrich J, Bond-Taylor W. Anatomical re-evaluation of lumbar dura mater with regard to postspinal headache. effect of dural puncture. Anaesthesia. 1988;43:635–637. doi: 10.1111/j.1365-2044.1988.tb04145.x. [DOI] [PubMed] [Google Scholar]
- 59.Reina MA, Lopez A, Badorrey V, De Andres JA, Martin S. Dura-arachnoid lesions produced by 22 gauge quincke spinal needles during a lumbar puncture. J Neurol Neurosurg Psychiatry. 2004;75:893–897. doi: 10.1136/jnnp.2003.017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strupp M, Brandt T, Muller A. Incidence of post-lumbar puncture syndrome reduced by reinserting the stylet: a randomized prospective study of 600 patients. J Neurol. 1998;245:589–592. doi: 10.1007/s004150050250. [DOI] [PubMed] [Google Scholar]
- 61.Sinikoglu NS, Yeter H, Gumus F, Belli E, Alagol A, Turan N. Reinsertion of the stylet does not affect incidence of post dural puncture headaches (pdph) after spinal anesthesia. Braz J Anesthesiol. 2013;63:188–192. doi: 10.1016/S0034-7094(13)70213-7. [DOI] [PubMed] [Google Scholar]
- 62.Trupp M. Stylet injury syndrome. JAMA. 1977;237:2524. doi: 10.1001/jama.237.23.2524. [DOI] [PubMed] [Google Scholar]
- 63.Carson D, Serpell M. Choosing the best needle for diagnostic lumbar puncture. Neurology. 1996;47:33–37. doi: 10.1212/WNL.47.1.33. [DOI] [PubMed] [Google Scholar]
- 64.Cruvinel MG, Andrade AV. [needle fracture during spinal puncture: Case report]. Rev Bras Anestesiol. 2004;54:794–798. doi: 10.1590/S0034-70942004000600007. [DOI] [PubMed] [Google Scholar]
- 65.Strupp M, Schueler O, Straube A, Von Stuckrad-Barre S, Brandt T. “Atraumatic” sprotte needle reduces the incidence of post-lumbar puncture headaches. Neurology. 2001;57:2310–2312. doi: 10.1212/WNL.57.12.2310. [DOI] [PubMed] [Google Scholar]
- 66.Lavi R, Yarnitsky D, Rowe JM, Weissman A, Segal D, Avivi I. Standard vs atraumatic whitacre needle for diagnostic lumbar puncture: a randomized trial. Neurology. 2006;67:1492–1494. doi: 10.1212/01.wnl.0000240054.40274.8a. [DOI] [PubMed] [Google Scholar]
- 67.Nath S, Koziarz A, Badhiwala JH, et al. Atraumatic versus conventional lumbar puncture needles: a systematic review and meta-analysis. Lancet. 2018;391:1197–1204. doi: 10.1016/S0140-6736(17)32451-0. [DOI] [PubMed] [Google Scholar]
- 68.Castrillo A, Tabernero C, Garcia-Olmos LM, et al. Postdural puncture headache: impact of needle type, a randomized trial. Spine J. 2015;15:1571–1576. doi: 10.1016/j.spinee.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 69.Peterman SB. Postmyelography headache rates with whitacre versus quincke 22-gauge spinal needles. Radiology. 1996;200:771–778. doi: 10.1148/radiology.200.3.8756930. [DOI] [PubMed] [Google Scholar]
- 70.Hatfield MK, Handrich SJ, Willis JA, Beres RA, Zaleski GX. Blood patch rates after lumbar puncture with whitacre versus quincke 22- and 20-gauge spinal needles. AJR Am J Roentgenol. 2008;190:1686–1689. doi: 10.2214/AJR.07.3351. [DOI] [PubMed] [Google Scholar]
- 71.Abel AS, Brace JR, McKinney AM, et al. Effect of patient positioning on cerebrospinal fluid opening pressure. J Neuroophthalmol. 2014;34:218–222. doi: 10.1097/WNO.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 72.Brook AD, Burns J, Dauer E, Schoendfeld AH, Miller TS. Comparison of CT and fluoroscopic guidance for lumbar puncture in an obese population with prior failed unguided attempt. J Neurointerv Surg. 2014;6:324–328. doi: 10.1136/neurintsurg-2013-010745. [DOI] [PubMed] [Google Scholar]
- 73.Radiation dose in X-RAY and CT exams. [Accessed August 13, 2018]. Available at: http://www.radiologyinfo.org/en/info.cfm?pg=safety-xray.
- 74.Haseler LJ, Sibbitt RR, Sibbitt WL, Jr, Michael AA, Gasparovic CM, Bankhurst AD. Syringe and needle size, syringe type, vacuum generation, and needle control in aspiration procedures. Cardiovasc Intervent Radiol. 2011;34:590–600. doi: 10.1007/s00270-010-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]