Abstract
Background
Nitrofurantoin is widely recommended for empirical treatment of urinary tract infection (UTI), but primary care clinicians may prescribe alternative antibiotics to improve prognosis in older, sicker patients. We assessed whether prescribing alternative antibiotics was associated with reduced risk of adverse outcomes in older patients.
Methods
This retrospective cohort study included patients aged ≥65 years empirically treated for a UTI with nitrofurantoin, cefalexin, ciprofloxacin, or co-amoxiclav. We matched patients on their propensity to receive a nitrofurantoin prescription and used mixed-effects logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for reconsultation and represcription (proxy for treatment failure), hospitalization for UTI, sepsis, or acute kidney injury, and death.
Results
We identified 42 298 patients aged ≥65 years prescribed empirical nitrofurantoin, cefalexin, ciprofloxacin, or co-amoxiclav for a UTI. Compared with nitrofurantoin, patients prescribed cefalexin, ciprofloxacin, or co-amoxiclav had lower odds of reconsultation and represcription (OR for cefalexin = 0.85, 95% CI = 0.75–0.98; OR for ciprofloxacin = 0.48, 95% CI = 0.38–0.61, OR for co-amoxiclav = 0.77, 95% CI = 0.64–0.93). Patients prescribed cefalexin or ciprofloxacin had greater odds of hospitalization for sepsis (OR for cefalexin = 1.89, 95% CI = 1.03–3.47; OR for ciprofloxacin = 3.21, 95% CI = 1.59–6.50), and patients prescribed cefalexin had greater odds of death (OR = 1.44, 95% CI = 1.12–1.85).
Conclusions
Compared with nitrofurantoin, prescribing of alternative antibiotics for UTI in older people may be associated with lower rates of treatment failure but was not associated with reduced risk of UTI-related hospitalization or death.
Keywords: aged, electronic health records, primary care, urinary tract infection
Urinary tract infection (UTI) is the most common indication for antibiotic prescribing in older adults presenting to ambulatory care services [1] and those in long-term care facilities [2–4]. Approximately 60%–75% of adults presenting with suspected UTI receive empirical antibiotic therapy at the same consultation, without knowledge of microbiological susceptibilities [5–7]. Current clinical guidelines in the United States [8] and United Kingdom [9] recommend nitrofurantoin for empirical treatment of uncomplicated UTI. However, previous research found that approximately 15% of older adults empirically treated for a UTI in primary care were prescribed cefalexin, ciprofloxacin, or co-amoxiclav [10]. Cefalexin, ciprofloxacin, and co-amoxiclav are broad-spectrum antibiotics, and they are associated with increased rates of drug-related adverse events [11] and antibiotic-associated diarrhoea [12]. They are also more likely to select for drug-resistant organisms leading to subsequent antibiotic-resistant colonization or infection [8]. Qualitative research found that primary care clinicians were more likely to consider broad-spectrum antibiotics for older patients, who were frail, had comorbidities, and were judged to have more severe illness [13]. The perceived aim of broad-spectrum antibiotic prescribing was to prevent treatment failure, worsening illness, and hospitalization, events thought to be more likely if narrow-spectrum antibiotics were prescribed for that clinical scenario [13].
Meta-analysis of 3 randomized trials (n = 289) found similar clinical cure rates between patients with UTI treated with nitrofurantoin versus flouroquinolones, suggesting that flouroquinolones offer little additional benefit [14]. However, trials only included young, healthy women and were underpowered to assess risk of important but rare outcomes such as UTI-related hospitalization or death [15–17]. Previous observational studies have compared trimethoprim-sulfamethoxazole with flouroquinolones, and sulfamethiazole with pivmecillinam, but no trials or observational studies have compared nitrofurantoin with cefalexin or co-amoxiclav [18, 19].
Therefore, we used data from anonymized linked health records to compare the risk of adverse outcomes in adults aged ≥65 prescribed empirical nitrofurantoin versus cefalexin, ciprofloxacin, or co-amoxiclav for suspected UTI in primary care. Our aim was to assess whether cefalexin, ciprofloxacin, or co-amoxiclav were associated with a reduced risk of treatment failure, hospitalization for UTI, sepsis or acute kidney injury (AKI), or death. If these antibiotics were associated with risks that were similar or higher than those of nitrofurantoin, then this would support further reductions in their use, even in older, frailer, comorbid patients with more severe presenting features.
METHODS
Data Source
We used the Clinical Practice Research Datalink (CPRD), an electronic database of anonymized primary care records, covering 11.3 million patients from 674 general practices across the United Kingdom (UK) [20]. Approximately 7% of the UK population are included, and patients are broadly representative of the wider UK population in terms of age, gender, and ethnicity. The CPRD holds data on demographics, clinical encounters and diagnoses (coded using Read codes), drug prescriptions, blood tests, and referrals to specialists. Data are available once they have met a series of quality checks on completeness and reliability, and the CPRD deems them to be of the standard required for research purposes. Linked hospital and death registration data are available for patients from approximately 50% of contributing English practices. Hospital diagnoses and causes of death are recorded using version 10 of the International Classification of Disease (ICD-10).
The CPRD Independent Scientific Advisory Committee approved the study protocol (protocol number 17_250). Further ethical approval was not required because the proposed research was within the remit of the CPRD’s broad National Research Ethics Service approval. We used the Reporting of Studies Conducted using Observational Routinely-collected Health Data (RECORD) statement and checklist to guide study reporting [21].
Design and Participants
This was a retrospective cohort study using linked health record data. Patients were eligible for inclusion if, between January 1, 2010 and December 31, 2016, their data were of the quality required by CPRD, they were ≥65 years old, and eligible for data linkage. Only patients registered with practices that consented to data linkage had linked hospital and death registry data. We excluded patients if they were temporary residents or had gaps in their data coverage. Follow-up began from the latest of, study start date (January 1, 2010), patient’s 65th birthday, 6 months after they registered with the practice (to avoid including historical UTIs recorded at registration), or the date their practice met the CPRD data quality requirements. Follow-up ended on the earliest of study end date (December 31, 2016), the day the patient died or transferred out of the practice (ie, last date of CPRD data collection), or 28 days after an incident UTI event. We identified eligible patients with (1) a Read code indicating an incident primary care presentation with a suspected UTI (codes available in Supplementary Appendix 1) and (2) a same-day prescription code indicating empirical prescribing of a relevant antibiotic. We defined “incident” as a consultation occurring in a patient without a UTI-related Read code or trimethoprim or nitrofurantoin prescription in the preceding 90 days (trimethoprim and nitrofurantoin are used almost exclusively for UTI in the UK). We used the first incident episode during each patient’s follow-up period. We excluded UTI episodes with a hospital discharge in the preceding 14 days to exclude hospital-acquired infections.
Exposures
The exposure variable was the recorded empirical antibiotic prescription.
Outcomes
We estimated risk of the following adverse outcomes for patients empirically treated in primary care for an incident suspected UTI: (1) reconsultation for urinary symptoms and a same-day antibiotic represcription within 14 days after the incident UTI, as a proxy for treatment failure, ascertained through Read and prescription codes recorded in primary care records; (2) hospitalization for UTI, sepsis, or AKI within 14 days after the incident UTI ascertained from ICD-10 codes recorded in linked hospital admission data for the first episode of a hospital admission, ie, the episode most likely responsible for the admission; (3) death within 28 days after the incident UTI using linked death registration data.
We chose 14 days for the reconsultation and hospitalization outcomes to increase the likelihood that these events were related to the initial UTI. Longer time periods increase the likelihood that the outcome may have been influenced by an intervening event, eg, if a 28-day period was used, a patient could have a UTI, recover, have a cardiac event, and be hospitalized with AKI. We chose 28 days for the death outcome because the UTI could precipitate events (eg, sepsis) that take some time to evolve before death.
Statistical Analyses
We used primary care demographic and clinical codes to describe baseline characteristics for patients by prescribed antibiotic. To compare outcomes between patients prescribed nitrofurantoin versus cefalexin, ciprofloxacin, or co-amoxiclav, we matched patients on their propensity to receive a nitrofurantoin prescription. Variables included in the logistic regression models that generated the propensity score were age, Index of Multiple Deprivation score quintile [22], Charlson score [23], the presence or absence of a Read code indicating coronary heart disease, renal disease, respiratory disease, type 2 diabetes mellitus, heart failure, peripheral arterial disease, and stroke, because these variables were previously shown to be associated with antibiotic prescribing [7, 24]. We also included gender, whether the patient was housebound, had dementia, liver disease, rheumatoid arthritis, cancer, urinary incontinence or a urinary catheter, an estimated glomerular filtration rate, and polypharmacy (defined as records indicating ≥5 long-term medications per month in the year before the incident UTI), because these variables could be associated with both the clinical decision around choice of antibiotic and the UTI-related outcomes.
We used nearest neighbor matching with no replacement to match 3 patients with nitrofurantoin prescriptions to 1 patient with a cefalexin prescription. We assessed balance in measured baseline covariates between matched groups (1) by visually inspecting jitter plots and histograms of covariate distribution before and after matching and (2) by calculating standardized mean differences for covariates between groups. We regarded standardized mean differences of <0.1 as reflecting adequate balance [25, 26]. We used mixed-effects logistic regression [27] to calculated odds ratios (ORs) and 95% confidence intervals (CIs) for each outcome, accounting for clustering by general practice. We repeated the analyses by matching 3 patients with nitrofurantoin prescriptions to 1 patient with a ciprofloxacin prescription and then 3 patients with nitrofurantoin prescriptions to 1 patient with a co-amoxiclav prescription.
RESULTS
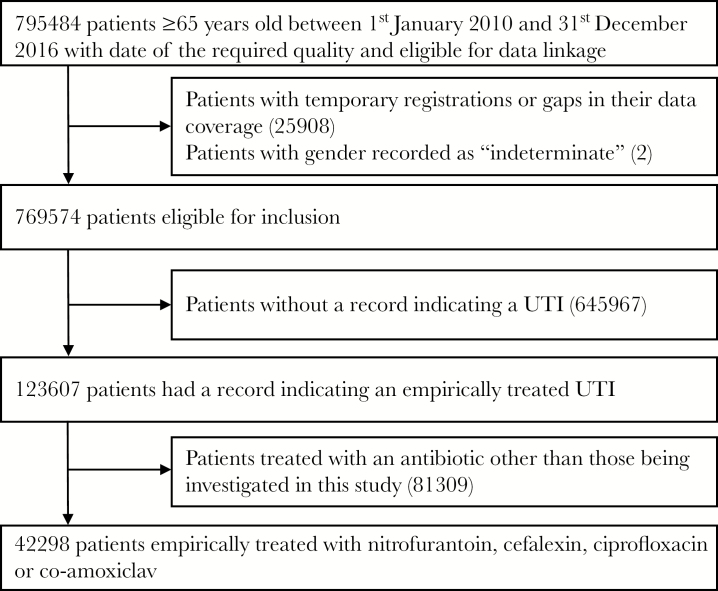
From a cohort of 795 484 patients aged 65 and over, we identified 123 607 (16%) with an incident empirically treated UTI, 42 298 of whom were prescribed nitrofurantoin, cefalexin, ciprofloxacin, or co-amoxiclav (Figure 1). In this final cohort, 11 420 (27%) patients were male, and the median age at time of incident UTI was 76 years (interquartile range, 70–83). Nitrofurantoin was the most commonly prescribed antibiotic, accounting for 60% of all prescriptions, followed by cefalexin (18%), co-amoxiclav (13%), and ciprofloxacin (9%).
Figure 1.
Flow of patients from initial identification in the database through to final cohort.
Baseline Characteristics
There were differences in baseline characteristics across the antibiotic groups. For example, 55% of the ciprofloxacin group were male compared with 23% of the nitrofurantoin group (Table 1). Compared with the nitrofurantoin group, greater proportions of the cefalexin, ciprofloxacin, and co-amoxiclav groups had comorbidities, particularly ischemic heart disease, heart failure, and renal disease. Approximately 3% of the nitrofurantoin group had a Charlson score of ≥6, compared with 5%–6% of the other groups.
Table 1.
Baseline Characteristics by Prescribed Antibiotica
| Characteristic | Cefalexin | Ciprofloxacin | Co-amoxiclav | Nitrofurantoin |
|---|---|---|---|---|
| N | 7546 (17.8) | 3868 (9.1) | 5516 (13.0) | 25 368 (60.0) |
| Men | 2150 (28.5) | 2115 (54.7) | 2229 (40.4) | 5930 (23.4) |
| Mean (SD) age | 77.5 (8.4) | 76.5 (8.3) | 77.6 (8.5) | 76.5 (8.4) |
| Mean (SD) follow-up time (years) | 4.2 (2.0) | 4.5 (2.0) | 4.2 (2.0) | 4.6 (1.9) |
| Mean (SD) prescription duration (days) | 8.2 (7.0) | 7.6 (7.0) | 8.3 (8.6) | 6.6 (3.6) |
| Index of Multiple Deprivation Decile | ||||
| 1 or 2 (least deprived) | 1708 (22.6) | 1056 (27.3) | 1354 (24.5) | 6850 (27.0) |
| 3 or 4 | 1710 (22.7) | 957 (24.7) | 1401 (25.4) | 6180 (24.4) |
| 5 or 6 | 1722 (22.8) | 850 (22.0) | 1255 (22.8) | 5214 (20.6) |
| 7 or 8 | 1254 (16.6) | 605 (15.6) | 849 (15.4) | 3970 (15.6) |
| 9 or 10 (most deprived) | 1152 (15.3) | 400 (10.3) | 657 (11.9) | 3154 (12.4) |
| Housebound | 41 (5.5) | 136 (3.5) | 265 (4.8) | 929 (3.7) |
| Respiratory disease | 1702 (22.6) | 849 (21.9) | 1198 (21.7) | 5339 (21.0) |
| Cardiac failure | 516 (6.8) | 212 (5.5) | 347 (6.3) | 1083 (4.3) |
| Dementia | 512 (6.8) | 170 (4.4) | 382 (6.9) | 1439 (5.7) |
| Peripheral vascular disease | 488 (6.5) | 252 (6.5) | 321 (5.8) | 1082 (4.3) |
| Renal disease | 2243 (29.7) | 1001 (25.9) | 1499 (27.2) | 5310 (20.9) |
| Rhuematoid arthritis | 297 (3.9) | 108 (2.8) | 188 (3.4) | 900 (3.5) |
| Cancer | 1295 (17.2) | 780 (20.2) | 949 (17.2) | 3889 (15.3) |
| Stroke | 886 (11.7) | 392 (10.1) | 673 (12.2) | 2460 (9.7) |
| Diabetes | 1474 (19.5) | 783 (20.2) | 1111 (20.1) | 4234 (16.7) |
| Liver disease | 68 (0.9) | 30 (0.8) | 36 (0.7) | 171 (0.7) |
| Ischaemic heart disease | 1602 (21.2) | 821 (21.2) | 1158 (21.0) | 4290 (16.9) |
| Urinary catheter | 372 (4.9) | 309 (8.0) | 327 (5.9) | 853 (3.4) |
| Urinary incontinence | 1199 (15.9) | 471 (12.2) | 867 (15.7) | 3972 (15.7) |
| Polypharmacy | 3299 (43.7) | 1540 (39.8) | 2376 (43.1) | 9301 (36.7) |
| eGFR | ||||
| 60–90 | 4168 (55.2) | 2344 (60.6) | 3170 (57.5) | 16 719 (65.9) |
| 45–59 | 1749 (23.2) | 811 (21.0) | 1227 (22.2) | 5237 (20.6) |
| 30–44 | 917 (12.2) | 388 (10.0) | 613 (11.1) | 1815 (7.2) |
| 15–29 | 319 (4.2) | 148 (3.8) | 208 (3.8) | 391 (1.5) |
| <15 | 47 (0.6) | 26 (0.7) | 44 (0.8) | 41 (0.2) |
| Missing | 346 (4.6) | 151 (3.9) | 254 (4.6) | 1165 (4.6) |
| Charlson Score | ||||
| 0 | 2029 (26.9) | 1073 (27.7) | 1591 (28.8) | 8845 (34.9) |
| 1 | 1515 (20.1) | 765 (19.8) | 1098 (1909) | 8845 (34.9) |
| 2 | 1406 (18.6) | 773 (20.0) | 1006 (18.2) | 4755 (18.7) |
| 3 | 1070 (14.2) | 535 (13.8) | 769 (13.9) | 3050 (12.0) |
| 4 | 658 (8.7) | 313 (8.1) | 437 (7.9) | 1567 (6.2) |
| 5 | 428 (5.7) | 197 (5.1) | 305 (5.5) | 955 (3.8) |
| ≥6 | 440 (5.8) | 212 (5.5) | 310 (5.6) | 842 (3.3) |
Abbreviations: eGFR, estimated glomerular filtration rate; SD, standard deviation.
aValues are numbers (%) unless otherwise stated.
Propensity-Score Matching
We matched 21 600 patients prescribed nitrofurantoin with 7200 patients prescribed cefalexin, 11 151 patients prescribed nitrofurantoin with 3717 patients prescribed ciprofloxacin, and 15 786 patients prescribed nitrofurantoin with 5262 patients prescribed co-amoxiclav (Table 2). Inspection of jitter plots and histograms suggested matching had improved balance of covariates across the 2 groups. Standardized mean differences were all less than 0.1.
Table 2.
Propensity-Score Matched Analyses Comparing Outcomes Between Nitrofurantoin and Other Antibiotics
| Outcomes | Cefalexin (n = 7200) Versus Nitrofurantoin (n = 21 600) | Ciprofloxacin (n = 3717) Versus Nitrofurantoin (n = 11 151) | Co-amoxiclav (n = 5262) Versus Nitrofurantoin (n = 15 786) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Reconsultation and represcription within 14 days | 0.85 (0.75–0.98) | .020 | 0.48 (0.38–0.61) | <.001 | 0.77 (0.64–0.93) | .006 |
| Hospitalized for UTI within 14 days | 0.96 (0.78–1.18) | .673 | 0.84 (0.57–1.26) | .408 | 0.94 (0.68–1.31) | .731 |
| Hospitalized for sepsis within 14 days | 1.89 (1.03–3.47) | .038 | 3.21 (1.59–6.50) | .001 | 1.91 (0.98–3.73) | .058 |
| Hospitalized for AKI within 14 days | 0.55 (0.23–1.31) | .175 | 1.53 (0.49–4.79) | .457 | 0.87 (0.40–1.90) | .727 |
| Death within 28 days | 1.44 (1.12–1.85) | .004 | 1.18 (0.83–1.68) | .353 | 1.39 (0.93–2.07) | .108 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; OR, odds ratio; UTI, urinary tract infection.
Risk of Adverse Outcomes
Compared with nitrofurantoin, patients prescribed cefalexin, ciprofloxacin, or co-amoxiclav had lower odds of reconsultation and represcription (OR for cephalexin = 0.85, 95% CI = 0.75–0.98, P = .020; OR for ciprofloxacin = 0.48, 95% CI = 0.38–0.61, P ≤ .001; OR for co-amoxiclav = 0.77, 95% CI = 0.64–0.93, P = .006) (Table 2). We found no significant difference in the odds of hospitalization for UTI between patients prescribed nitrofurantoin versus cefalexin, ciprofloxacin, or co-amoxiclav. However, compared with nitrofurantoin, patients prescribed ciprofloxacin had greater odds of hospitalization for sepsis (OR = 3.21, 95% CI = 1.59–6.50, P = .001), as did patients prescribed cefalexin (OR = 1.89, 95% CI = 1.03–3.47, P = .038). We found no significant difference in the odds of hospitalization for AKI between patients prescribed nitrofurantoin versus cefalexin, ciprofloxacin, or co-amoxiclav. Compared with nitrofurantoin, patients prescribed cefalexin had greater odds of death within 28 days of the UTI (OR = 1.44, 95% CI = 1.12–1.85, P = .004).
Sensitivity Analyses
The association between patients prescribed ciprofloxacin or cefalexin and lower odds of reconsultation and represcription could be due to the significantly increased rates of sepsis hospitalization (ciprofloxacin) and death (cefalexin) in these groups, preventing patients’ re-presenting to primary care. Therefore, we combined these 3 outcomes and found that 7.1% of patients prescribed nitrofurantoin reconsulted or were hospitalized for sepsis or died, compared with 6.3% of patients prescribed ciprofloxacin or cefalexin, with an adjusted OR for the combined outcome of 0.87 (95% CI, 0.78–0.95).
DISCUSSION
Our results show that patients prescribed cefalexin, ciprofloxacin, or co-amoxiclav had lower odds of reconsultation and represcription. Patients prescribed cefalexin or ciprofloxacin had greater odds of sepsis hospitalization, and those prescribed cefalexin had greater odds of death. Overall, compared with nitrofurantoin, we found no evidence that cefalexin, ciprofloxacin, or co-amoxiclav were associated with a reduction in the risk of UTI-related hospitalization or death.
Results in Context
The lower odds of reconsultation and represcription among patients prescribed cefalexin, ciprofloxacin, or co-amoxiclav may reflect lower odds of treatment failure. This was in contrast to previous trials that generally showed similar clinical cure rates between narrow and broad-spectrum agents [16, 28, 29]. This association remained significant when we combined the reconsultation and represcription outcome with hospitalization for sepsis or death, suggesting that, despite the higher rates of sepsis/death in the cefalexin/ciprofloxacin group, there remain a group of patients who were less likely to experience treatment failure with these agents. However, we were unable to distinguish whether patients in the nitrofurantoin group who reconsulted and received another antibiotic prescription did so because of an adverse event or intolerance, rather than for treatment failure.
We found increased odds of sepsis in patients prescribed cefalexin or ciprofloxacin. This finding may be due to residual unmeasured confounding because these patients were sicker or had more complicated infection. It may also relate to higher levels of prior fluoroquinolone exposure, previously shown to be associated with increased sepsis risk, possibly due to disruption of the gut microbiome and subsequent dysregulation of the immune response to infection [30].
Our finding of an increased risk of death in patients prescribed cefalexin is intriguing. There are several possible explanations. The antibiotic itself may increase the risk of death, particularly in this cohort, many of whom had multiple comorbidities and were prescribed multiple other drugs. This is not implausible; cefalexin use is associated with antibiotic-associated diarrhea and Clostridium difficile infection, which may result in serious and protracted illness in elderly comorbid patients [31]. It may also be due to antimicrobial resistance. For example, the 2017 English Surveillance Programme for Antimicrobial Utilisation and Resistance report showed that 10% of community-acquired Escherichia coli UTIs were resistant to cefalexin but only 2% to nitrofurantoin [32]. Finally, some of these findings could again be due to residual confounding. Patients prescribed cefalexin may have been less healthy, presented with more severe illness, and were therefore more likely to experience an adverse outcome irrespective of the prescribed antibiotic. Thus, it may be more appropriate to regard the exposure as a combination of patient and prescription factors, which is why we have related associations to the “patients prescribed cefalexin” rather than the prescription alone.
Strengths and Weaknesses of This Study
We used data from a general practice database that is broadly representative of the UK population [20]. Cohort entry was dependent on presentation and empirical treatment of UTI in primary care and thus reduced indication bias. We also reduced indication bias by propensity-score matching and achieving adequate balance of baseline characteristics across the groups.
Our study has some limitations. We attempted to capture patients presenting with UTI but had no microbiological data to support this. However, although a limitation, this is also more representative of clinical practice. Our outcomes, particularly sepsis and AKI, relied on coding and were not microbiologically or biochemically confirmed. We were unable to determine precise reasons for reconsultation and represcription and acknowledge that not all of these events may have been due to treatment failure. We were unable to determine antibiotic treatment duration and therefore could not include this potentially important variable in the propensity score model. Based on current definitions [8], some patients may have presented with “complicated” UTI, for which the recommended treatment includes some of the alternative antibiotics assessed. Therefore, we have not commented on the appropriateness (or not) of the prescribed agent. Our findings are based on prescriptions and not on dispensed or ingested drugs. Finally, despite our design, differential coding, indication bias, and residual confounding may have affected our findings.
CONCLUSIONS
Our findings highlight the challenges associated with selecting antibiotics for older patients with suspected UTI. Compared with nitrofurantoin, we found no evidence that cefalexin, ciprofloxacin, or co-amoxiclav prescribing was associated with a reduced risk of hospitalization or death, suggesting that the perceived aim expressed by clinicians in previous qualitative work was not being achieved, and thus supporting further reductions in prescribing of these agents, even in frailer, sicker patients, especially given their impact on antimicrobial resistance. Future research should explore reasons for continued use of these antibiotics for UTI in primary care and provide clinicians with information on which patients are most likely to benefit from their use.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank our lay advisors, Shanaz Dorkenoo and Eiddwen Thomas, for tongoing advice and invaluable input into this program of work.
Disclaimer. The views expressed in this publication are those of the authors and not necessarily those of the National Institute of Health Research (NIHR), NHS Wales, Health and Care Research Wales (HCRW), or the Welsh Government. The funders had no role in study design, data analysis, manuscript preparation, or decision to submit this manuscript.
Financial support. This report is independent research arising from an NIHR Doctoral Research Fellowship (awarded to H. A.) and funded by HCRW (Grant number DRF-2014-07-010).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among us ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 2. McClean P, Tunney M, Gilpin D, et al. Antimicrobial prescribing in residential homes. J Antimicrob Chemother 2012; 67:1781–90. [DOI] [PubMed] [Google Scholar]
- 3. McClean P, Hughes C, Tunney M, et al. Antimicrobial prescribing in European nursing homes. J Antimicrob Chemother 2011; 66:1609–16. [DOI] [PubMed] [Google Scholar]
- 4. McClean P, Tunney M, Gilpin D, et al. Antimicrobial prescribing in nursing homes in Northern Ireland: results of two point-prevalence surveys. Drugs Aging 2011; 28:819–29. [DOI] [PubMed] [Google Scholar]
- 5. O’Brien K, Hillier S, Simpson S, et al. An observational study of empirical antibiotics for adult women with uncomplicated UTI in general practice. J Antimicrob Chemother 2017; 59:1200–3. [DOI] [PubMed] [Google Scholar]
- 6. Butler CC, Hawking MK, Quigley A, McNulty CA. Incidence, severity, help seeking, and management of uncomplicated urinary tract infection: a population-based survey. Br J Gen Pract 2015; 65:e702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2018; 69:234–40. [DOI] [PubMed] [Google Scholar]
- 8. Gupta K, Hooton TM, Naber KG, et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 9. SIGN. Management of suspected bacterial urinary tract infection in adults Available at: https://www.sign.ac.uk/sign-88-management-of-suspected-bacterial-urinary-tract-infection-in-adults.html. Accessed 1 June 2018.
- 10. Ahmed H, Farewell D, Jones HM, et al. Incidence and antibiotic prescribing for clinically diagnosed urinary tract infection in older adults in UK primary care, 2004–2014. PLoS One 2018; 13: e0190521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shehab N, Lovegrove MC, Geller AI, et al. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA 2016; 316:2115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hood K, Nuttall J, Gillespie D, et al. Probiotics for Antibiotic-Associated Diarrhoea (PAAD): a prospective observational study of antibiotic-associated diarrhoea (including Clostridium difficile-associated diarrhoea) in care homes. Health Technol Assess 2014; 18:1–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wood F, Simpson S, Butler CC. Socially responsible antibiotic choices in primary care: a qualitative study of GPs’ decisions to prescribe broad-spectrum and fluroquinolone antibiotics. Fam Pract 2007; 24:427–34. [DOI] [PubMed] [Google Scholar]
- 14. Huttner A, Verhaegh EM, Harbarth S, et al. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015; 70:2456–64. [DOI] [PubMed] [Google Scholar]
- 15. Ludwig G, Pauthner H. Clinical experience with ofloxacin in upper and lower urinary tract infections. A comparison with co-trimoxazole and nitrofurantoin. Drugs 1987; 34(Suppl 1):95–9. [DOI] [PubMed] [Google Scholar]
- 16. Iravani A, Klimberg I, Briefer C, et al. A trial comparing low-dose, short-course ciprofloxacin and standard 7 day therapy with co-trimoxazole or nitrofurantoin in the treatment of uncomplicated urinary tract infection. J Antimicrob Chemother 1999; 43(Suppl A):67–75. [PubMed] [Google Scholar]
- 17. Ernst EJ, Ernst ME, Hoehns JD, Bergus GR. Women’s quality of life is decreased by acute cystitis and antibiotic adverse effects associated with treatment. Health Qual Life Outcomes 2005; 3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bjerrum L, Dessau RB, Hallas J. Treatment failures after antibiotic therapy of uncomplicated urinary tract infections. A prescription database study. Scand J Prim Health Care 2002; 20:97–101. [PubMed] [Google Scholar]
- 19. Lee MTG, Lee SH, Chang SS, et al. Comparative effectiveness of different oral antibiotics regimens for treatment of urinary tract infection in outpatients: an analysis of national representative claims database. Medicine (Baltimore) 2014; 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015; 44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015; 12:e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The English Index of Multiple Deprivation (IMD) 2015 – Guidance. UK Department for communities and local government. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/464430/English_Index_of_Multiple_Deprivation_2015_-_Guidance.pdf. Accessed 7 January 2018.
- 23. Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract 2010; 11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shallcross L, Beckley N, Rait G, et al. Antibiotic prescribing frequency amongst patients in primary care: a cohort study using electronic health records. J Antimicrob Chemother 2017; 72:1818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007; 26:734–53. [DOI] [PubMed] [Google Scholar]
- 26. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bates D. Package ‘lme4’. Available at: https://cran.r-project.org/web/packages/lme4/lme4.pdf. Accessed 24 January 2018.
- 28. Arredondo-García JL, Figueroa-Damián R, Rosas A, et al. Comparison of short-term treatment regimen of ciprofloxacin versus long-term treatment regimens of trimethoprim/sulfamethoxazole or norfloxacin for uncomplicated lower urinary tract infections: a randomized, multicentre, open-label, prospective study. J Antimicrob Chemother 2004; 54:840–3. [DOI] [PubMed] [Google Scholar]
- 29. Kavatha D, Giamarellou H, Alexiou Z, et al. Cefpodoxime-proxetil versus trimethoprim-sulfamethoxazole for short-term therapy of uncomplicated acute cystitis in women. Antimicrob Agents Chemother 2003; 47:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baggs J, Jernigan JA, Halpin AL, et al. Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect Dis 2018; 66:1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilcox MH, Chalmers JD, Nord CE, et al. Role of cephalosporins in the era of Clostridium difficile infection. J Antimicrob Chemother 2017; 72:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) – report, 2018. Crown. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/759975/ESPAUR_2018_report.pdf. Accessed 25 September 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.