Abstract
Age-related declines in human skeletal muscle performance may be caused, in part, by decreased responsivity of muscle fibers to calcium (Ca2+). This study examined the contractile properties of single vastus lateralis muscle fibers with various myosin heavy chain (MHC) isoforms (I, I/IIA, IIA and IIAX) across a range of Ca2+ concentrations in 11 young (24.1 ± 1.1 years) and 10 older (68.8 ± 0.8 years) men and women. The normalized pCa-force curve shifted rightward with age, leading to decreased activation threshold (pCa10) and/or Ca2+ sensitivity (pCa50) for all MHC isoforms examined. In older adults, the slope of the pCa-force curve was unchanged in MHC I-containing fibers (I, I/IIA), but was steeper in MHC II-containing fibers (IIA, IIAX), indicating greater cooperativity compared to young adults. At sub-maximal [Ca2+], specific force was reduced in MHC I-containing fibers, but was minimally decreased in MHC IIA fibers as older adults produced greater specific forces at high [Ca2+] in these fibers. Lessor pCa50 in MHC I fibers independently predicted reduced isokinetic knee extensor power across a range of contractile velocities, suggesting that the Ca2+ response of slow-twitch fibers contributes to whole muscle dysfunction. Our findings show that aging attenuates Ca2+ responsiveness across fiber types and that these cellular alterations may lead to age-related reductions in whole muscle power output.
Keywords: Myosin heavy chain isoforms, Human, Specific force, Muscle function
1. Introduction
Physical function declines with age in humans (Fielding et al., 2011). Whole muscle contractile performance is a critical determinant of physical function (Bean et al., 2002; Foldvari et al., 2000; Suzuki et al., 2001; Skelton et al., 1994), with both muscle strength and power declining precipitously with advancing age (Delmonico et al., 2009; Clark et al., 2013; Bouchard et al., 2011). Muscle size decreases with age, but atrophy alone does not explain decrements in whole muscle strength and power (Delmonico et al., 2009; Hughes et al., 2001). Whole muscle function is determined, in part, by the intrinsic contractile properties of the underlying muscle fibers (Miller et al., 2014; Bottinelli and Reggiani, 2000; Cormie et al., 2011). Thus, understanding age-related changes at the cellular level is crucial for developing targeted countermeasures to age-related muscle weakness that seek to improve physical function.
The role of calcium (Ca2 +) in regulating skeletal muscle contraction is well-known (Gordon et al., 2000; MacIntosh, 2003). However, only a small number of studies have examined the influence of age on the Ca2 + response of single skeletal muscle fibers in humans. A recent study found that older adults had a reduction of pCa50 in all myosin heavy chain (MHC) II fibers combined (e.g., MHC IIA and IIAX), but did not observe age-related differences in the Ca2 + response of MHC I fibers (Lamboley et al., 2015). Two other studies from a different research group showed no significant age-related differences in pCa10, pCa50 or slope of the pCa-force curve (Hill coefficient; h) in either MHC I or IIA fibers (Hvid et al., 2011; Hvid et al., 2013). While these studies provide valuable insights, several factors that modify age-related single fiber function need to be considered. First, experimental control for physical activity level is important, as physical inactivity and exercise can modulate Ca2 + sensitivity (Widrick et al., 1985; Mounier et al., 2009; Yamashita-Goto et al., 2001; Malisoux et al., 2006; Gejl et al., 2016; Godard et al., 2002). Previous studies did match the young and older populations using questionnaires, but self-reporting of physical activity has limitations (Sallis and Saelens, 2000). Second, previous work evaluated fibers at 120% of slack length (Lamboley et al., 2015; Hvid et al., 2011; Hvid et al., 2013), so the sarcomere length was unknown. This is important as Ca2 + sensitivity is altered with sarcomere length, due to changes in the proximity of actin to the myosin binding site (MacIntosh, 2003; Endo, 1972; Wang and Fuchs, 2000). Finally, no study has examined the relationship between the Ca2 + responsivity of single muscle fibers and in vivo contractile function to determine whether alterations in cellular function scale to the whole muscle level to contribute to age-related functional deficits.
The aim of the present study was to examine the effects of age on the Ca2 + response of chemically-skinned, single vastus lateralis muscle fibers (MHC I, I/IIA, IIA and IIAX) from young and older men and women. Notably, sarcomere length under relaxing conditions (pCa 8) was set at 2.65 μm and physical activity of the young and older groups was matched using accelerometry. Furthermore, we aimed to examine the relationship between single fiber Ca2 + sensitivity and whole muscle isokinetic power output of the knee extensors. As an exploratory analysis, we examined whether sex interacted with age to alter Ca2 + sensitivity in MHC I and IIA fibers, as we have previously observed sex specific changes in molecular, cellular and whole muscle contractile function among older adults (Miller et al., 2013; Callahan et al., 2014a).
2. Methods
2.1. Participants
Eleven young (5 men, 6 women) and 10 older (3 men, 7 women) adults participated in this study. Young and older adults had habitual physical activity levels measured over 7 days using accelerometry, as previously described (Toth et al., 2010). In order to match young and older adults for physical activity level, we recruited young adults who were minimally active (not engaged in a structured exercise program) and older adults who were moderately active (~30 min of walking on 3–5 days per week). All volunteers had no symptoms or signs of heart disease, hypertension, or diabetes (fasting blood glucose > 112 mg/dl); normal resting electrocardiogram; normal electrocardiogram response to an exercise stress test; normal thyroid function; and normal blood cell counts and blood biochemistry values. Volunteers were excluded if they currently or had participated in a weight loss or exercise training program in the past year, a history of smoking (within 1 yr), unintentional weight loss of > 2.5 kg during the last 3 mo, a body mass index > 30.0 kg/m2, a hospitalization longer than 3 days in the past 5 yr, an active neoplasm or history of one within the past 5 yr, or were taking/had taken hormone replacement therapy (combined estrogen/progestin for older women and testosterone for older men). Participants taking statins or oral corticosteroids were excluded. Young women taking oral contraceptives were included (n = 4), and all older women were postmenopausal (cessation of menses > 1 yr). Data on clinical characteristics as well as whole muscle and single fiber structure and function in MHC I and IIA fibers from young and older volunteers have been reported previously (Miller et al., 2013; Callahan et al., 2014b), as have relationships between molecular and cellular function in MHC I, I/IIA, IIA and IIAX fibers from young adults (Miller et al., 2015). The present study examines a subset of the entire cohort, and was limited to those volunteers that had pCa-force measurements performed. All participants provided written informed consent prior to enrollment in this study. The protocol was approved by the Committees on Human Research at the University of Vermont.
2.2. Experimental protocol
Eligibility for participation was determined during screening visits, which also included whole muscle strength testing, a treadmill test, and resting and exercising electrocardiograms. At least one week following the screening visit, a percutaneous needle biopsy of the vastus lateralis muscle was performed under lidocaine anesthesia. The majority of tissue was immediately placed into cold (4 °C) dissecting solution and processed for single fiber mechanical assessment. The remaining tissue was frozen in liquid nitrogen and stored at −80 °C or prepared for electron microscopy or immunohistochemical assessment, the results of which are presented elsewhere (Callahan et al., 2014b).
2.3. Whole body measurements
Body mass was measured using a digital scale (ScaleTronix, Wheaton, IL) and total and regional body composition was assessed via dual-energy X-ray absorptiometry, or DEXA (GE Lunar, Madison, WI). Leg fat-free mass was determined (Heymsfield et al., 1990) and used as a proxy for muscle mass. Maximum isokinetic knee extensor torque of the right leg was measured, after establishing range of motion and gravity correction, using a dynamometer (Humac Norm, CSMi, Stoughton, MA) at five different isokinetic testing velocities (60, 120, 180, 240 and 300°/s) in a randomized fashion. For each testing speed, participants performed 4 repetitions, and each trial was separated by a 2-min rest period. Following each trial, data were reviewed to ensure that participants attained the target isokinetic velocity in at least 3 of the 4 repetitions. The peak power output was calculated during the isovelocity phase of the contraction for each velocity where the target was achieved.
2.4. Muscle tissue processing
Biopsy tissue was immediately placed into cold (4 °C) dissecting solution (in mM: 20 N,N-bis[2-hydroxyethyl]-2-aminoethanesulfonic acid (BES), 5 ethylene glycol-bis(2-amino-ethylether)-N,N,N′,N′-tetraacetic acid (EGTA), 5 MgATP, 1 free Mg2 +, 1 dithiothreitol and 0.25 phosphate (Pi)) with an ionic strength of 175 mEq, pH 7.0, and at pCa 8.0 for isolation of single fiber bundles for mechanical measurements. Muscle bundles of approximately 50 fibers were dissected and tied to glass rods at slightly stretched lengths and placed in skinning solution (in mM: 170 potassium propionate, 10 imidazole, 5 EGTA, 2.5 MgCl2, 2.5 Na2H2ATP, 0.05 leupeptin and 0.05 antipain at pH 7.0) for 24 h at 4 °C. After skinning, fibers were placed in storage solution (identical to skinning solution, but with 1 mM sodium azide and without leupeptin and antipain) with increasing concentrations of glycerol (10% v/v glycerol for 2 h, 25% glycerol v/v for 2 h) until reaching the final storage solution (50% v/v glycerol), in which they were incubated at 4 °C for 18–20 h. Thereafter, fibers were stored at −20 °C until isolation of single fibers for mechanical measurements, which occurred within 4 weeks of the biopsy.
2.5. Experimental solutions
Constituents of all solutions used during mechanical measurements were calculated using the equations and stability constants according to Godt and Lindley (Godt and Lindley, 1982). Relaxing solution was dissecting solution with 15 mM creatine phosphate and 300 units/ml of creatine phosphokinase. Pre-activating solution was the same as relaxing solution, except at an EGTA concentration of 0.5 mM. Activating solution was the same as relaxing solution, except at pCa 4.5. All solutions were adjusted to proper ionic strength (175 mEq) using sodium methane sulfate.
2.6. Single fiber pCa-force measurements
The Ca2 + response of single muscle fibers is routinely determined by exposing a chemically- or mechanically-skinned fiber to a range of [Ca2 +] that produce minimum to maximum force and plotting the results as a pCa-force curve, where pCa = −log10[Ca2 +] and force is normalized to maximal force. The [Ca2 +] at which 10 and 50% of maximum force production occur are defined as the activation threshold (pCa10) and calcium sensitivity (pCa50), respectively. The slope of the pCa-force curve, h (Hill coefficient), represents the degree of cross-bridge cooperativity, and primarily occurs because the binding of one myosin molecule to the actin filament will increase the affinity for nearby myosins to bind actin (Gordon et al., 2000).
Muscle bundles were incubated in dissection solution containing 1% Triton X-100 (v/v) for 20 min at 4 °C. Segments (~2.5–3.0 mm) of single chemically-skinned fibers were isolated from muscle bundles and their ends fixed with glutaraldehyde, as described (Miller et al., 2010). Aluminum T-clips were placed on the fixed regions and the fiber was mounted in relaxing solution onto hooks to perform top and side diameter measurements at three positions along the length of the fiber using a filar eyepiece micrometer (Lasico, Los Angeles, CA, USA) and a right-angled, mirrored prism to calculate average cross-sectional area. The experimental apparatus specifications as well as fiber mounting and preparation procedures have been previously described (Miller et al., 2010). Briefly, the T-clipped fiber ends were attached to a piezoelectric motor (Physik Instrumente, Auburn, MA, USA) and a strain gauge (SensorNor, Horten, Norway) in relaxing solution, sarcomere length was set to 2.65 μm (IonOptix, Milton, MA, USA) and the length of the fiber’s unfixed portion was determined. Starting in relaxing solution, the fiber was slackened completely, the force gauge zeroed, the fiber pulled back to its original position, allowed to equilibrate for 1 min and relaxed isometric specific force (force per cross-sectional area) measured. The fiber was transferred to pre-activating solution for 30 s, to improve fiber stability by allowing for the rapid influx of Ca2 + into the fiber (Larsson and Moss, 1993; Moisescu, 1976), and then to activating solution at pCa 4.5, with specific force recorded at its plateau. This initial maximal Ca2 + activation was performed to verify fiber integrity (maintenance of normal sarcomere register). Afterwards, fibers were placed in relaxing solution, sarcomere length set to 2.65 μm, slackened completely, the force gauge zeroed, the fiber pulled back to its original position, allowed to equilibrate for 1 min and relaxed isometric specific force measured. The fiber was transferred to pre-activating solution for 30 s and subsequently activated in solutions containing progressively greater Ca2 + levels (pCa 8.0, 7.0, 6.5, 6.0, 5.75,5.5, 5.25, 5.0, 4.5), with specific force measured at each plateau. Normalized specific force for most individual fibers was calculated using (x - specific force at pCa 8.0)/(specific force at pCa 4.5 - specific force at pCa 8.0), where x is the tension at a given Ca2 + level. However, MHC IIAX fibers on average had their maximum specific force occur at pCa 5.0 (2% higher than values at pCa 4.5), so their normalized specific force was calculated using (x – specific force at pCa 8.0)/(specific force at pCa 5.0 - specific force at pCa 8.0). Individual recordings of normalized force were fit to the Hill equation [Ca2 +]h/([Ca2 +]50h + [Ca2 +]h, where [Ca2 +]50 = calcium concentration at half activation, pCa50 = −log [Ca2 +]50, and h = Hill coefficient. To permit comparisons with other studies, activation threshold (pCa10) was calculated by setting the normalized force to 0.1 (10% of maximal force), solving the Hill equation for [Ca2 +]10 and calculating pCa10 = −log[Ca2 +]10. Experiments were performed at 15 °C.
2.7. Gel electrophoresis
Following pCa-force measurements, single fibers were placed in 30 μl loading buffer, heated for 2 min at 65 °C and stored at −80 °C until determination of MHC isoform composition by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to identify fiber type, as described (Miller et al., 2010).
2.8. Statistical analysis
Data are expressed as means ± SE. A one-way analysis of variance (ANOVA) was used to examine the main effect of age on all outcomes, and a two-way ANOVA to determine the interaction between age and sex on the Hill fit parameters. For variables with multiple observations within the same individual (e.g., single fiber specific force measurements), a linear mixed model was used, as described (Callahan et al., 2014a). To account for clustering of observations within individuals, we included a repeated effect in the model, as described (Toth et al., 2012). Partial correlations adjusted for leg fat-free mass were conducted to examine the association between single fiber Ca2 + response and whole muscle isokinetic power. Finally, linear regression models adjusted for leg fat-free mass were created to examine the relative contributions of MHC I and IIA Ca2 + sensitivity to whole muscle power at each iso-kinetic testing velocity. All statistical analyses were conducted using SPSS for Windows version 22.0 (IBM, Armonk, NY) and SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Clinical characteristics
There were no differences between young and older adults in height, weight, body mass index or measures of body composition (Table 1, p = 0.07–0.75). Young and older adults did not differ with respect to habitual physical activity level (Table 1, p = 1.00).
Table 1.
Clinical characteristics of young and older adults.
| Young (N = 11) | Older (N = 10) | P value for age effect | |
|---|---|---|---|
| N (M/F) | 5/6 | 3/7 | _ |
| Age (y) | 24.1 ± 1.1 | 68.8 ± 0.8 | < 0.01 |
| Height (cm) | 173.2 ± 3.0 | 164.1 ± 3.8 | 0.07 |
| Weight (kg) | 67.7 ± 3.4 | 65.8 ± 4.8 | 0.75 |
| Body mass index (kg/m2) | 22.5 ± 0.9 | 24.1 ± 0.9 | 0.21 |
| Percent body fat (%) | 28.1 ± 3.0 | 33.4 ± 2.8 | 0.23 |
| Leg fat-free mass (kg) | 16.4 ± 1.1 | 13.7 ± 1.3 | 0.13 |
| Physical activity (kcal/d) | 415 ± 34 | 415 ± 50 | 1.00 |
Data are presented as mean ± SE.
Bold indicates p < 0.05
3.2. Single muscle fiber contractile properties
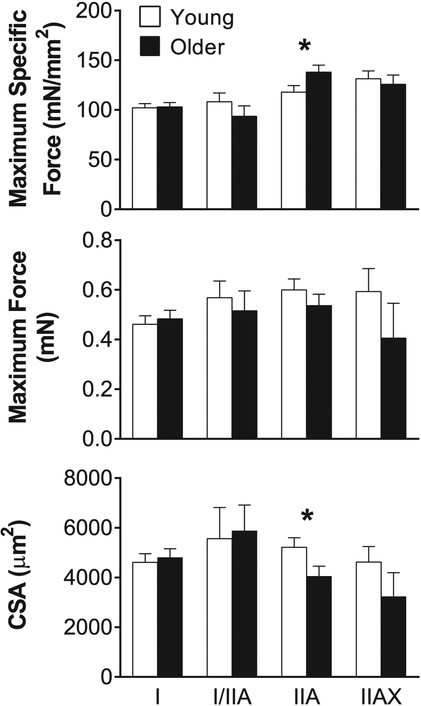
We present data from 198 single muscle fibers from 21 men and women (11 young and 10 older adults). Pure (I and IIA) and hybrid (I/IIA and IIAX) MHC fiber types were compared, as the number of MHC IIX and I/IIA/IIX fibers were insufficient to permit statistical analysis (0 IIX and 1 I/IIA/IIX fiber or 0.5% of all fibers examined). Maximum specific force (maximal Ca2 +-activated isometric force divided by CSA), maximum force and CSA were not different between young and older adults in MHC I, I/IIA or IIAX fibers (Fig. 1). However, the specific force of MHC IIA fibers was 17% greater in older fibers (young: 118 ± 7 vs. older: 138 ± 7 mN/mm2, p = 0.05), due to their maintenance of force production (0.60 ± 0.04 vs. 0.54 ± 0.05 mN, p = 0.33) despite a 22% lower CSA relative to the young (5216 ± 384 vs. 4053 ± 402 μm2, p = 0.05).
Fig. 1. Single skeletal muscle fiber maximum specific force, maximum force and cross-sectional area (CSA).
Maximum specific force (maximum force/CSA), maximum force and CSA of myosin heavy chain (MHC) I, I/IIA, IIA and IIAX single muscle fibers from young and older adults. The number of fibers analyzed in each group for each fiber type is provided by n in Table 2. Data were acquired under maximal Ca2 +-activated conditions (pCa 4.5) at 15 °C and represent means ± SE. Asterisks indicate significant difference (*p < 0.05) between young and older groups.
3.3. Single muscle fiber pCa-normalized specific force relationships
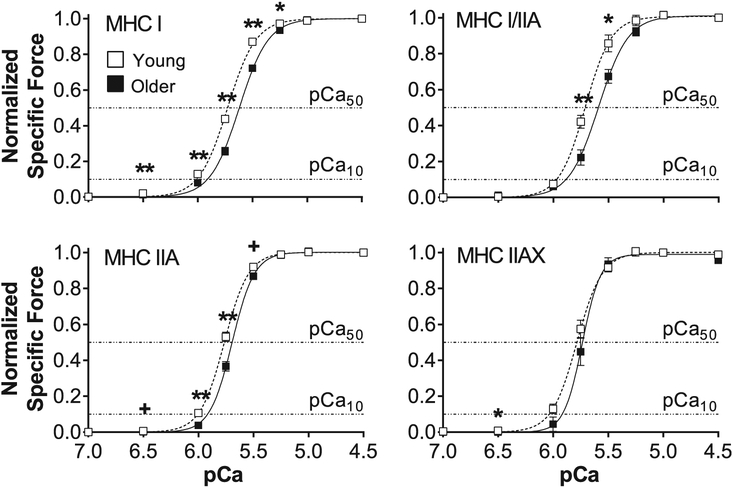
Normalized specific force was plotted versus pCa (−log10[Ca2 +]) and fit using the Hill equation for each MHC isoform in young and older adults (Fig. 2). Although specific force was measured across a range of [Ca2 +] (pCa 8.0 to 4.5), the specific force values at pCa 8.0 and 7.0 were nearly identical. Thus, all pCa-force curves are plotted showing pCa 7.0 as the lowest [Ca2 +]. In the normalized pCa-force curve, a rightward shift indicates that more Ca2 + is needed to achieve a given fraction of the maximum Ca2 +-activated force and is measured by an increase in the activation threshold (pCa10) and/or the Ca2 + sensitivity (pCa50). A rightward shift in the pCa-force curve with age was observed for all fiber types, thus causing a decline in pCa10 and/or pCa50 (Table 2). The pCa10 and pCa50 were decreased with age in the pure fiber types (MHC I and IIA), while hybrids showed either a lessor pCa50(MHC I/IIA) or pCa10 (MHC IIAX). The significant age effects on pCa50 were largest for MHC I (ΔpCa50 = −0.14) and smallest for MHC IIA (ΔpCa50 = −0.07), while the changes in pCa10 were the same for MHC I, IIA and IIAX (ΔpCa10 = −0.11). The steepness of the pCa-force curve (h) was unchanged with age in MHC I-containing fibers (I, I/IIA; Table 2). However, slope of the pCa-force curve was greater with age in MHC II-containing fibers (IIA, IIAX; Table 2).
Fig. 2. Normalized pCa-force relationships for young and older adults.
Normalized pCa-force relationships of myosin heavy chain (MHC) I, I/IIA, IIA and IIAX single muscle fibers from young and older adults. The horizontal dashed lines represent the activation threshold (pCa10) and Ca2 + sensitivity (pCa50). The number of fibers analyzed in each group for each fiber type is provided by n in Table 2. Data were acquired at 15 °C and represent means ± SE. Asterisks and plus sign indicate significant difference (*p < 0.05 or **p < 0.01) and trends (+p ≤ 0.10) between young and older groups.
Table 2.
Activation threshold (pCa10), Ca2 + sensitivity (pCa50) and pCa-force slope (Hill coefficient, h) of myosin heavy chain (MHC) I, I/IIA, IIA and IIAX single muscle fibers from young and older adults.
| n | N | pCa10 | pCa50 | h | ||||
|---|---|---|---|---|---|---|---|---|
| MHC | Y/O | Y/O | Y | O | Y | O | Y | O |
| I | 45/50 | 11/10 | 6.01 ± 0.02 | 5.90 ± 0.02 ** | 5.73 ± 0.01 a | 5.59 ± 0.02 ** a | 3.49 ± 0.13 a | 3.46 ± 0.13a |
| I/IIA | 7/5 | 3/5 | 5.96 ± 0.02 | 5.88 ± 0.03 | 5.71 ± 0.02 ab | 5.59 ± 0.02 ** a | 3.82 ± 0.10 ab | 3.43 ± 0.27a |
| IIA | 42/38 | 11/10 | 6.01 ± 0.01 | 5.90 ± 0.01 ** | 5.76 ± 0.01 bc | 5.69 ± 0.01 ** b | 4.09 ± 0.15 b | 4.65 ± 0.16 * b |
| IIAX | 7/4 | 5/2 | 6.03 ± 0.02 | 5.92 ± 0.03 * | 5.78 ± 0.02 c | 5.73 ± 0.04 b | 3.55 ± 0.12 ab | 5.47 ± 0.13 ** b |
Data are presented as mean ± SE.
Values correspond to the pCa-normalized specific force curves.
n indicates the number of fibers assessed and N indicates the number of subjects.
Y = young; O = older.
Significantly different from Young group at * = p < 0.05; ** = p < 0.01.
Where fiber type effects were observed, different letters identify pairwise differences (p < 0.05) between fiber types within an age group.
In older adults, MHC I-containing fibers had a greater Ca2 + sensitivity (higher pCa50) than MHC II-containing fibers; however, fibers that expressed the MHC II isoform had a larger h, indicating a greater degree of cooperative binding (Table 2). For the young group, pCa50 had the same pattern as the older group; lessor in MHC I- versus MHC II-containing fibers, except that the pCa50 was similar between MHC I/IIA and IIA fibers. The young group had a greater h for MHC IIA fibers compared to MHC I, similar to the older group, although hybrid slopes were similar to both MHC I and IIA fibers. There were no differences between fiber types with respect to pCa10 within the young or older groups.
3.4. Single muscle fiber pCa-specific force relationships
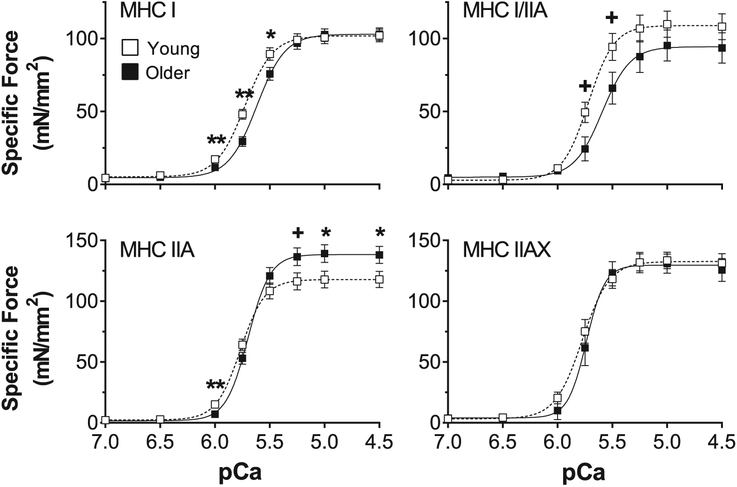
We also plotted pCa-force relationships for all fibers using specific force (Fig. 3). Similar to the results shown in Fig. 2, MHC I-containing fibers of older adults exhibited a rightward shift in the curve, indicating lower specific force production at submaximal pCa values (MHC I: pCa 6.0–5.5, MHC I/IIA: pCa 5.75–5.5) compared to young adults. However, in MHC IIA fibers, older adults generated greater specific force than young at pCa values where maximum specific force is produced (pCa 5.25–4.5) and lower specific force at pCa 6.0. MHC IIAX fibers showed no age-related differences in the pCa-specific force curve.
Fig. 3. Absolute pCa-force relationships for young and older adults.
Absolute pCa-force relationship of myosin heavy chain (MHC) I, I/IIA, IIA and IIAX single muscle fibers from young and older adults. The number of fibers analyzed in each group for each fiber type is provided by n in Table 2. Data were acquired at 15 °C and represent means ± SE. Asterisks and plus sign indicate significant difference (*p < 0.05 or **p < 0.01) and trends (+p < 0.10) between young and older groups.
3.5. Associations with whole muscle power
Absolute and adjusted isokinetic knee extension power was lower for older adults compared to young (Table 3). Relationships between absolute knee extension power and single muscle fiber Ca2 + responsiveness were examined using bivariate correlations. Absolute power at all velocities was related to pCa10 (r = 0.47–0.51, p < 0.05) and pCa50 (r = 0.45–0.49, p < 0.05), except 120°/s (r = 0.43, p = 0.06), in MHC I fibers. Isokinetic power was not related to the Hill coefficient (h) in MHC I fibers. In MHC IIA fibers, there were no significant relationships between pCa10 (r = 0.16–0.25), pCa50 (r = −0.03 − 0.00) or h (r = −0.42 to −0.32) and absolute power (all p > 0.05).
Table 3.
Isokinetic knee extension power in young and older adults.
| Isokinetic velocity (°/s) | N (Y/O) | Knee extension power (Nm) | P value for age effect | |
|---|---|---|---|---|
| Y | O | |||
| Absolute | ||||
| 60 | 11/9 | 168.8 ± 6.2 | 123.3 ± 8.2 | < 0.01 |
| 120 | 11/9 | 283.4 ± 11.1 | 205.5 ± 14.6 | < 0.01 |
| 180 | 10/9 | 353.5 ± 15.4 | 253.1 ± 19.5 | < 0.01 |
| 240 | 10/9 | 405.5 ± 16.4 | 285.5 ± 20.8 | < 0.01 |
| 300 | 10/7 | 436.7 ± 15.1 | 321.5 ± 25.8 | < 0.01 |
| Adjusted* | ||||
| 60 | 11/9 | 158.2 ± 5.6 | 120.9 ± 6.3 | < 0.01 |
| 120 | 11/9 | 262.1 ± 8.8 | 200.9 ± 9.9 | < 0.01 |
| 180 | 10/9 | 330.9 ± 13.9 | 247.3 ± 15.3 | < 0.01 |
| 240 | 10/9 | 380.4 ± 14.4 | 279.1 ± 15.9 | < 0.01 |
| 300 | 10/7 | 408.3 ± 10.2 | 319.4 ± 14.8 | < 0.01 |
Data are presented as mean ± SE.
N indicates the number of subjects.
Y = young; O = older.
Adjusted for leg fat-free mass (kg) measured from DEXA.
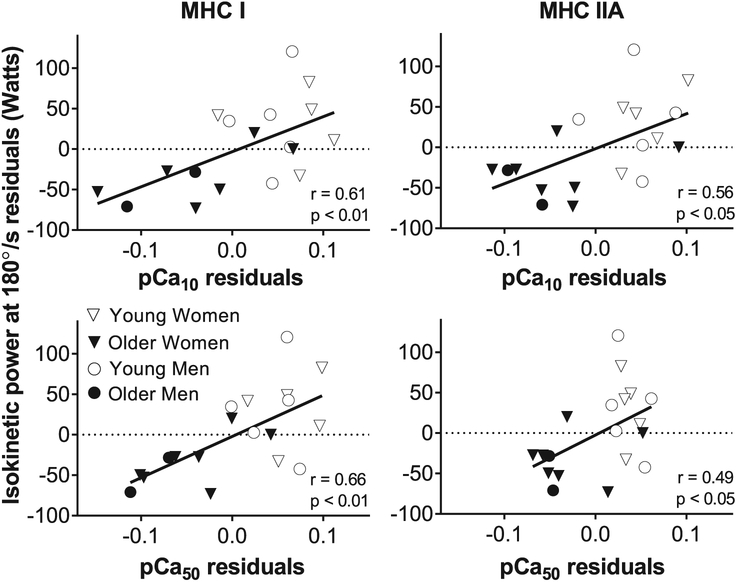
Partial correlations between single muscle fiber Ca2 + responsiveness and isokinetic muscle power were conducted by adjusting for leg fat-free mass (measured via DEXA) to account for variation among subjects in muscle size. Isokinetic power output at most contractile velocities (60 to 240°/s) was positively related (p < 0.01) to both pCa10 (MHC I: r = 0.61–0.67, MHC IIA: r = 0.50–0.56) and pCa50 (MHC I: r = 0.65–0.67, MHC IIA: r = 0.47–0.50) in MHC I and IIA fibers. Isokinetic power output at 300°/s was positively related (p < 0.05) to pCa10 (r = 0.50) and pCa50 (r = 0.56) in MHC I fibers, but no significant correlation was found for MHC IIA fibers. In contrast, and similar to bivariate correlations, isokinetic power output was not significantly correlated to h in MHC I (r = −0.25 to −0.01) or IIA (r = −0.38 to −0.33) fibers. The partial correlation between two variables is shown by plotting the residuals (observed minus predicted values) of the independent variables (i.e., isokinetic power and Ca2 + sensitivity), which are obtained by regressing each on the covariate (leg fat-free mass) (Fig. 4). We also conducted partial correlations between single fiber Ca2 + responsiveness and whole muscle power with additional adjustment for age. However, controlling for age attenuated all observed associations (p > 0.10 for MHC I and IIA fibers).
Fig. 4.
Single fiber Ca2 + sensitivity is related to iso-kinetic power output in MHC I and IIA fibers. Relationship between whole muscle isokinetic power output at 180°/s and single fiber activation threshold (pCa10) and Ca2 + sensitivity (pCa50) in myosin heavy chain (MHC) I and IIA fibers. Each variable was regressed on leg fat-free mass (measured via DEXA) and residuals (observed minus predicted) were calculated. Plotting the residuals for whole muscle isokinetic power and Ca2 + sensitivity depicts the partial correlation between these variables adjusted for leg fat-free mass. This analysis used n = 19 as two participants (one younger and one older adult) were missing data for isokinetic power output at 180°/s.
As skeletal muscle is heterogeneous and multiple fiber types contribute to whole muscle function (Bottinelli and Reggiani, 2000), we examined the relative contributions of MHC I and IIA single fiber Ca2 + sensitivity to isokinetic knee extension power using multiple linear regression models (Table 4). After adjusting for thigh fat-free mass, our analyses revealed that Ca2 + sensitivity (pCa50) of MHC I fibers was a significant independent predictor of whole muscle power output at multiple testing velocities (60–240°/s). In contrast, no relationship was evident between Ca2 + sensitivity of MHC IIA fibers and whole muscle power.
Table 4.
Linear regression analysis of Ca2 + sensitivity (pCa50) of myosin heavy chain (MHC) I and IIA single fibers for isokinetic knee extension power (Nm) in young and older adults.
| Isokinetic velocity (°/s) | pCa50 | β | SE | P value |
|---|---|---|---|---|
| 60 | MHC I | 333.91 | 122.99 | 0.02 |
| MHC IIA | −196.31 | 185.96 | 0.31 | |
| 120 | MHC I | 545.27 | 195.78 | 0.01 |
| MHC IIA | −324.10 | 296.02 | 0.29 | |
| 180 | MHC I | 790.97 | 303.21 | 0.02 |
| MHC IIA | −487.98 | 468.13 | 0.31 | |
| 240 | MHC I | 901.78 | 347.54 | 0.02 |
| MHC IIA | −540.21 | 536.56 | 0.33 | |
| 300 | MHC I | 604.84 | 428.94 | 0.18 |
| MHC IIA | −163.95 | 658.23 | 0.81 |
All models were adjusted for leg fat-free mass (kg) measured from DEXA. β represents the unstandardized regression coefficient.
Bold indicates p < 0.05
3.6. Sex differences
Because we have previously observed sex-based differences in molecular and cellular contractile function among older adults (Miller et al., 2013; Callahan et al., 2014a), we examined whether sex interacted with age to determine Ca2 + sensitivity of MHC I and IIA fibers. However, we did not observe an age × sex interaction for any of the Hill fit parameters in MHC I (p = 0.38–0.74) or MHC IIA (p = 0.11–0.92) fibers. Overall, these results indicate sex did not affect the Ca2 + response of either fiber type.
4. Discussion
This study investigated the effects of aging on the Ca2 + response of single vastus lateralis muscle fibers from young and older men and women. The major finding was that the Ca2 + response was attenuated in muscle fibers from older adults in both pure (MHC I and IIA) and hybrid (I/IIA and IIAX) fibers, showing that older adults need more Ca2 + to activate their fibers to a similar percentage of maximum force compared to young. In addition, the slope of the pCa-force curve was steeper in MHC II-containing fibers from older adults, indicating a greater degree of cooperative binding in fast-twitch fibers with age, suggesting an enhanced ability to facilitate additional myosin binding (Gordon et al., 2000). Using linear regression, we found that reduced Ca2 + sensitivity (pCa50) of MHC I fibers independently predicted lower isokinetic knee extension power across a range of velocities, indicating that age-related alterations in single fiber function may help explain the lower whole muscle performance in older adults. Importantly, young and older adults in this study were matched for physical activity level, as verified by accelerometry, improving the likelihood that our results reflect the effects of aging.
4.1. How can single fiber Ca2 + responsivity impact whole muscle function?
As whole muscle specific force or power (force or power per whole muscle size) should be directly influenced by single fiber specific force (force per fiber CSA) (Harridge et al., 1996), understanding the Ca2 + response in terms of absolute specific force is relevant for predicting alterations in whole muscle function that are independent of age-related atrophy. Older adults had reduced specific forces at sub-maximal [Ca2 +] in MHC I fibers, while MHC IIA fibers generated higher specific forces at maximal [Ca2 +] and similar specific forces at sub-maximal [Ca2 +], except at the lowest activation level (pCa 6.0) where specific force was reduced. These alterations in force production in MHC I and IIA fibers both lead to a reduced Ca2 + response, but may have different effects on whole muscle function.
How can decreased single fiber Ca2 + responsiveness lead to reduced knee extensor power output? Power is the product of contractile force and velocity, and reducing [Ca2 +] decreases both single fiber force (Lamboley et al., 2015; Hvid et al., 2011; Hvid et al., 2013; McDonald, 2000) (Figs. 2–3) and shortening velocity (Gilliver et al., 2011; Morris et al., 2003; Moss, 1986) in a sigmoidal manner. Thus, if Ca2 + sensitivity of the myofilaments or sarcoplasmic [Ca2 +] is significantly reduced, power output at the single fiber level and, in turn, the whole muscle would be reduced. Both of these phenomena may occur, as other human studies have reported an age-related decrease in the amount of Ca2 + in the sarcoplasmic reticulum (SR) (Lamboley et al., 2015) and SR Ca2 + release (Delbono et al., 1995). That single fiber Ca2 + sensitivity influences whole muscle power is suggested by studies showing pharmacologic agents that improve myofilament Ca2 + sensitivity increase diaphragm single fiber (van Hees et al., 2009) and whole muscle (Doorduin et al., 2012) function. Accordingly, the decrease in Ca2 +sensitivity we observed should lead to reduced whole muscle function through the same mechanism. While others have suggested that age-related changes in Ca2 + handling and sensitivity could have functional implications (Gordon et al., 2000; MacIntosh, 2003; Lamboley et al., 2015), to our knowledge, the present study is the first to demonstrate a relationship between single fiber Ca2 + sensitivity and in vivo contractile function. Whole muscle power is an important determinant of daily activities, such as walking speed, climbing stairs and rising from a chair (Bassey et al., 1992; Bean et al., 2003). Thus, cellular changes that reduce whole muscle power may impair physical function, in keeping with models of the disablement process (Verbrugge and Jette, 1994).
Because younger and older volunteers had similar physical activity levels by design, our results suggest that otherwise healthy older adults can maintain daily activity levels on par with younger individuals despite impaired single fiber function. Considered through the lens of interventions to improve overall activity in older adults, this result is heartening, as it suggests that impaired myofilament function does not limit the capacity of older individuals to achieve youthful daily activity levels and the associated health benefits. However, our isokinetic knee extensor data would suggest that deficits in myofilament function limit the capacity to perform tasks requiring high power output in older adults. Accordingly, activities or physical movements that require high power output, such as preventing a fall (McKinnon et al., 2017), may still be impaired. In this context, exercise and rehabilitation strategies that target these cellular and molecular deficits may help restore power output and, in turn, more effectively mitigate disability. Following this logic, knowledge of the fundamental molecular and cellular deficits contributing to impaired function in older adults across the disability spectrum would allow for more targeted, and hopefully effective, interventions to counter disability.
4.2. Correlation between age-related Ca2 + response and whole muscle function
We found associations between single fiber Ca2 + response parameters (pCa10 and pCa50) and in vivo contractile function, specifically isokinetic knee extensor power output. A substantial proportion (25–45%) of the individual variability in whole muscle power output was accounted for by pCa10 and pCa50 from MHC I fibers. Likewise, our multivariate regression analyses revealed that Ca2 + sensitivity of MHC I fibers was an independent predictor of isokinetic power output across a range of contractile velocities, indicating that participants with greater single fiber Ca2 + responsiveness had greater whole muscle power. Importantly, because all participants reached the target iso-kinetic velocity during testing at each speed (60°/s, 120°/s, etc.), the age-related deficits in muscle power were likely attributable to reduced torque production. Collectively, these findings suggest that the decline in knee extensor power output is commensurate with reductions in the Ca2 + response of the underlying MHC I fibers in vastus lateral is muscle.
Our multivariate analysis revealed MHC I fiber Ca2 + responsiveness as the best predictor of whole muscle power, which begs the question: how can function in the relatively low-power MHC I fibers dictate whole muscle power production? We have previously shown that slower MHC I fiber velocity is associated with a reduced whole muscle rate of torque development in older adults with knee osteoarthritis (Callahan et al., 2015), indicating that slower contracting fibers play an important role in dictating whole muscle function. Slower contracting myosins have a more dominant effect on contractile velocity than faster contracting myosins in pre-clinical models (Cuda et al., 1997) and human tissue (Larsson and Moss, 1993), potentially due to their longer myosin attachment times. In this context, MHC I fibers may be acting as a molecular brake for overall muscle power output. This has been shown using the in vitro motility assay (Cuda et al., 1997), where small amounts of MHC I can disproportionately reduce contractility. In an analogous manner, and considering the substantial proportion of MHC I fibers in human muscle, a reduction in power output from MHC I fibers secondary to decreased Ca2 + responsiveness could disproportionately reduce whole muscle power.
While our results suggest that single fiber Ca2 + sensitivity, primarily of MHC I fibers, partially explains decrements in isokinetic knee extension power, skeletal muscle function is influenced by a multitude of physiologic factors. In the present study, we statistically controlled for the contribution of muscle mass to isokinetic power. However, while early research suggested muscle mass was a major determinant of strength (Frontera et al., 1991), subsequent prospective studies showed that atrophy accounts for a relatively small proportion of the decrements in muscle strength (Delmonico et al., 2009; Hughes et al., 2001). These results agree with studies showing that factors other than muscle mass contribute to muscle function in older adults (Beliaeff et al., 2008; Landers et al., 2001; Jubrias et al., 1997). Thus, changes in other physiologic regulators of whole muscle function contribute to reductions with age, including single fiber contractile properties (Callahan et al., 2015), neuromuscular activation (Clark et al., 2013; Kent-Braun and Ng, 1999; Moore et al., 2014), excitation-contraction coupling (Delbono et al., 1995), slowing of myosin-actin cross-bridge kinetics (Miller et al., 2013), and fibrosis (Cadore et al., 2012), which are potentially driven by sex hormone levels (Schaap et al., 2005), systemic inflammation (Visser et al., 2002; Cesari et al., 2004), skeletal muscle lipid infiltration (Goodpaster et al., 2001) and obesity (Choi et al., 2016).
4.3. Comparison to previous Ca2 + response studies in young and older adults
The impact of aging on the Ca2 + response of human skeletal muscle fibers has received little attention, with studies generally showing no age-related changes in pCa10, pCa50 or h in MHC I (Lamboley et al., 2015; Hvid et al., 2011; Hvid et al., 2013) and IIA (Hvid et al., 2011; Hvid et al., 2013) fibers, except for one study that found a decrease in pCa50 for MHC II fibers (Lamboley et al., 2015). In this latter study, however, MHC IIA and IIAX isoforms, the two most predominant MHC II fiber types, were examined together. In contrast, our results indicate MHC I and IIA fibers show age-related Ca2 + response changes, as pCa10 and pCa50 were reduced in older adults. Variability in results may be due to methodological differences between studies or our matching for weight-bearing activity using accelerometers instead of questionnaires (Widrick et al., 1985; Mounier et al., 2009; Yamashita-Goto et al., 2001;Malisoux et al., 2006; Gejl et al., 2016; Godard et al., 2002). Regarding the latter, as inactivity decreases Ca2 + sensitivity (e.g., shifts the pCa-force curve to the right), studying young and older adults with similar activity levels increases confidence that our results are due to aging, and not age-related disuse.
There are potential differences among existing studies in the number and age of participants, as well as the number of fibers for each MHC isoform examined. The number of participants in our study (N = 21) was similar to others (N = 15–22) (Lamboley et al., 2015; Hvid et al., 2011; Hvid et al., 2013), as were the mean ages (young = 24 y and older = 69 y in current study vs. young = 22–24 y and older = 67–70 y) (Lamboley et al., 2015; Hvid et al., 2011; Hvid et al., 2013). With regard to number of fibers per fiber type, we examined 45–50 MHC I fibers for each age group, which was similar to other studies (n = 40–62), except for young adults (n = 26) in one study (Lamboley et al., 2015). The number of MHC IIA fibers examined for young and older adults was lowest in the two studies that showed no alterations in Ca2 + response with age; 16 and 11 for young and older adults, respectively, in one study (Hvid et al., 2013) and 40 and 15 in the other (Hvid et al., 2011). In contrast, our study in MHC IIA fibers (n = 42 young; n = 38 older) and another (Lamboley et al., 2015) in MHC II fibers (n = 21 young; n = 25 older) observed decreases in pCa50 of MHC IIA fibers with age.
Another important experimental consideration is the sarcomere length of the fibers examined. In our study, sarcomere length was set at 2.65 μm, while others stretched to 120% of slack length (Lamboley et al., 2015; Hvid et al., 2011; Hvid et al., 2013). A sarcomere length of 2.65 μm should occur at or near knee angles where whole muscle iso-kinetic peak power occurs (40–70°), based on sarcomere lengths measured in vivo in resting vastus lateralis muscle of young adults (Chen et al., 2016). In addition, a sarcomere length of 2.65 μm places our measurements in the middle of the force plateau on the force-sarcomere length curve for MHC I fibers from humans, meaning that our fibers should produce maximal force (Gollapudi and Lin, 2009) and be removed from the ascending or descending portion of this curve. Using a set stretch distance based on slack length, as prior studies have done, may, in contrast, increase variability in sarcomere length and, in turn, variability in single fiber force and Ca2 + sensitivity (MacIntosh, 2003). Notably, a recent study determined slack length in MHC I fibers to be ~2.22 μm (Gollapudi and Lin, 2009), such that stretching a fiber to 120% of its slack length would result in a sarcomere length of ~2.66 μm. However, the relationship between slack length and sarco-mere length may change with myofilament stiffness, which is altered with fiber type (Miller et al., 2015) and age (Miller et al., 2013), leading to variability in sarcomere length and possibly functional parameters.
In addition, our single fiber measurements were performed at a lower temperature than the other studies (15 vs. 22–23 °C). Animal studies using skinned skeletal and cardiac fibers have shown that Ca2 + sensitivity is temperature-dependent, with increased sensitivity at higher temperatures (Debold et al., 2006; Harrison and Bers, 1989; Maughan et al., 1995; Sweitzer and Moss, 1990). However, temperature affects Ca2 + sensitivity of slow- and fast-twitch mammalian skeletal muscle fibers differentially, such that slow-twitch fibers are insensitive to temperature, while fast-twitch fibers become less sensitive to Ca2 + as temperature increases from 5 to 25 °C (Stephenson and Williams, 1981). In this context, our observation of an age-related reduction in Ca2 + sensitivity of MHC IIA fibers may be influenced by the temperature at which mechanical experiments were performed.
Finally, there were also differences between studies in the approach used to remove the sarcolemma of single fibers. In the present study, we used chemically-skinned single fibers, which is the method used in studies by Hvid et al. (Hvid et al., 2011; Hvid et al., 2013), but different than Lamboley et al. (Lamboley et al., 2015), who evaluated mechanically-skinned fibers. However, this is unlikely to account for discrepant results between studies as previous research shows that the pCa-force relationship does not vary between chemically- and mechanically-skinned fibers (Endo, 1972).
4.4. Age-related Ca2 + response of hybrid fibers
Hybrid skeletal muscle fibers (MHC I/IIA and IIAX), which are expressed more frequently in older adults (D’Antona et al., 2003; Klitgaard et al., 1990; Andersen et al., 1999), similarly showed an attenuated Ca2 + response, decreasing pCa50 in MHC I/IIA and pCa10 in MHC IIAX, but a greater cooperativity in the fast-contracting MHC IIAX. Although we recognize that only a small number of hybrid fibers were examined in each age group (n = 4–7), these patterns are consistent with our observations in the pure MHC I and MHC IIA fibers. MHC isoform is the primary determinant of single fiber contractile performance (Harridge et al., 1996) and the relative expression of each MHC isoform in these hybrid fiber types may account for the similarity of these observations to pure MHC I and IIA fibers. Additonally, MHC expression also affects the isoforms of regulatory proteins on the actin filament (e.g., troponin) (O’Connell et al., 2004), and this could impact Ca2 + sensitivity (Geiger et al., 1999). Due to the limited number of MHC I/IIA and IIAX fibers examined in the present analysis, future human studies are needed to confirm the effect of age on hybrid muscle fibers.
5. Conclusion
In conclusion, our results indicate an attenuated Ca2 + response from muscle fibers of older adults, and suggest that these changes may contribute to reduced whole muscle power output. As the loss of muscle power is a determinant of age-related physical limitations, exercise and/or pharmacologic interventions that increase Ca2 + sensitivity in muscle fibers of older adults may improve whole muscle performance and reduce the risk of future physical disability.
Acknowledgements
We thank all the volunteers who dedicated their valuable time to these studies.
Funding
This work was supported by the National Institutes of Health (AG-031303 to M.S.M. and AG-033547 to M.J.T.).
Abbreviations:
- ANOVA
analysis of variance
- Ca2+
calcium
- CSA
cross-sectional area
- DEXA
dual-energy x-ray absorptiometry
- EGTA
ethylene glycol-bis(2-amino-ethylether)-N,N,N′,N′-tetraacetic acid
- h
Hill coefficient
- MHC
myosin heavy chain
- n
number of fibers
- N
number of subjects
- pCa
−log10[Ca2+]
- pCa10
[Ca2+] at which 10% of maximal force is produced (activation threshold)
- pCa50
[Ca2+] at which 50% of maximal force is produced (Ca2+ sensitivity)
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
- Andersen JL, Terzis G, Kryger A, 1999. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve 22, 449–454. [DOI] [PubMed] [Google Scholar]
- Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA, 1992. Leg extensor power and functional performance in very old men and women. Clin. Sci. (Lond.) 82, 321–327. [DOI] [PubMed] [Google Scholar]
- Bean JF, Kiely DK, Herman S, et al. , 2002. The relationship between leg power and physical performance in mobility-limited older people. J. Am. Geriatr. Soc 50, 461–467. [DOI] [PubMed] [Google Scholar]
- Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L, 2003. A comparison of leg power and leg strength within the InCHIANTI study: which in-fluences mobility more? J. Gerontol. A Biol. Sci. Med. Sci 58, 728–733. [DOI] [PubMed] [Google Scholar]
- Beliaeff S, Bouchard DR, Hautier C, Brochu M, Dionne IJ, 2008. Association between muscle mass and isometric muscle strength in well-functioning older men and women. J. Aging Phys. Act 16, 484–493. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Reggiani C, 2000. Human skeletal muscle fibres: molecular and functional diversity. Prog. Biophys. Mol. Biol 73, 195–262. [DOI] [PubMed] [Google Scholar]
- Bouchard DR, Heroux M, Janssen I, 2011. Association between muscle mass, leg strength, and fat mass with physical function in older adults: influence of age and sex. J.Aging Health 23, 313–328. [DOI] [PubMed] [Google Scholar]
- Cadore EL, Izquierdo M, Conceicao M, et al. , 2012. Echo intensity is associated with skeletal muscle power and cardiovascular performance in elderly men. Exp. Gerontol 47, 473–478. [DOI] [PubMed] [Google Scholar]
- Callahan DM, Miller MS, Sweeny AP, et al. , 2014a. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J. Physiol 592, 4555–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan DM, Bedrin NG, Subramanian M, et al. , 2014b. Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex specific: relationship to single-fiber function. J. Appl. Physiol 116 (1985), 1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan DM, Tourville TW, Slauterbeck JR, et al. , 2015. Reduced rate of knee extensor torque development in older adults with knee osteoarthritis is associated with intrinsic muscle contractile deficits. Exp. Gerontol 72, 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, et al. , 2004. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci 59, 242–248. [DOI] [PubMed] [Google Scholar]
- Chen X, Sanchez GN, Schnitzer MJ, Delp SL, 2016. Changes in sarcomere lengths of the human vastus lateralis muscle with knee flexion measured using in vivo microendoscopy. J. Biomech 49, 2989–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Files DC, Zhang T, et al. , 2016. Intramyocellular lipid and impaired Myofiber contraction in normal weight and obese older adults. J. Gerontol. A Biol. Sci. Med. Sci 71, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Pojednic RM, Reid KF, et al. , 2013. Longitudinal decline of neuromuscular activation and power in healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci 68, 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormie P, McGuigan MR, Newton RU, 2011. Developing maximal neuromuscular power: part 1–biological basis of maximal power production. Sports Med 41, 17–38. [DOI] [PubMed] [Google Scholar]
- Cuda G, Pate E, Cooke R, Sellers JR, 1997. In vitro actin filament sliding velocities produced by mixtures of different types of myosin. Biophys. J 72, 1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antona G, Pellegrino MA, Adami R, et al. , 2003. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J. Physiol 552, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debold EP, Romatowski J, Fitts RH, 2006. The depressive effect of Pi on the force-pCa relationship in skinned single muscle fibers is temperature dependent. Am. J. Physiol. Cell Physiol 290, C1041–1050. [DOI] [PubMed] [Google Scholar]
- Delbono O, O’Rourke KS, Ettinger WH, 1995. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J. Membr. Biol 148, 211–222. [DOI] [PubMed] [Google Scholar]
- Delmonico MJ, Harris TB, Visser M, et al. , 2009. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr 90, 1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, Sinderby CA, Beck J, et al. , 2012. The calcium sensitizer levosimendan improves human diaphragm function. Am. J. Respir. Crit. Care Med 185, 90–95. [DOI] [PubMed] [Google Scholar]
- Endo M, 1972. Stretch-induced increase in activation of skinned muscle fibres by calcium. Nat. New Biol 237, 211–213. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, et al. , 2011. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc 12, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldvari M, Clark M, Laviolette LC, et al. , 2000. Association of muscle power with functional status in community-dwelling elderly women. J. Gerontol. A Biol. Sci. Med. Sci 55, M192–199. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ, 1991. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J. Appl. Physiol 71 (1985), 644–650. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Sieck GC, 1999. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J. Appl. Physiol 87 (1985), 1894–1900. [DOI] [PubMed] [Google Scholar]
- Gejl KD, Hvid LG, Willis SJ, et al. , 2016. Repeated high-intensity exercise modulates Ca(2+) sensitivity of human skeletal muscle fibers. Scand. J. Med. Sci. Sports 26, 488–497. [DOI] [PubMed] [Google Scholar]
- Gilliver SF, Degens H, Rittweger J, Jones DA, 2011. Effects of submaximal activation on the determinants of power of chemically skinned rat soleus fibres. Exp. Physiol 96, 171–178. [DOI] [PubMed] [Google Scholar]
- Godard MP, Gallagher PM, Raue U, Trappe SW, 2002. Alterations in single muscle fiber calcium sensitivity with resistance training in older women. Pflugers Arch. 444, 419–425. [DOI] [PubMed] [Google Scholar]
- Godt RE, Lindley BD, 1982. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J. Gen. Physiol 80, 279–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollapudi SK, Lin DC, 2009. Experimental determination of sarcomere force-length relationship in type-I human skeletal muscle fibers. J. Biomech 42, 2011–2016. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, et al. , 2001. Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J. Appl. Physiol 90 (1985), 2157–2165. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M, 2000. Regulation of contraction in striated muscle. Physiol. Rev 80, 853–924. [DOI] [PubMed] [Google Scholar]
- Harridge SD, Bottinelli R, Canepari M, et al. , 1996. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch. 432, 913–920. [DOI] [PubMed] [Google Scholar]
- Harrison SM, Bers DM, 1989. Influence of temperature on the calcium sensitivity of the myofilaments of skinned ventricular muscle from the rabbit. J. Gen. Physiol 93, 411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Smith R, Aulet M, et al. , 1990. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am. J. Clin. Nutr 52, 214–218. [DOI] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Wood M, et al. , 2001. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J. Gerontol. A Biol. Sci. Med. Sci 56, B209–217. [DOI] [PubMed] [Google Scholar]
- Hvid LG, Ortenblad N, Aagaard P, Kjaer M, Suetta C, 2011. Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J. Physiol 589, 4745–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvid LG, Suetta C, Aagaard P, Kjaer M, Frandsen U, Ortenblad N, 2013. Four days of muscle disuse impairs single fiber contractile function in young and old healthy men. Exp. Gerontol 48, 154–161. [DOI] [PubMed] [Google Scholar]
- Jubrias SA, Odderson IR, Esselman PC, Conley KE, 1997. Decline in isokinetic force with age: muscle cross-sectional area and specific force. Pflugers Arch. 434, 246–253. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, 1999. Specific strength and voluntary muscle activation in young and elderly women and men. J. Appl. Physiol 87 (1985), 22–29. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B, 1990. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle.Acta Physiol. Scand 140, 55–62. [DOI] [PubMed] [Google Scholar]
- Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM, Lamb GD, 2015. Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J. Physiol 593, 2499–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL, 2001. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J. Gerontol. A Biol. Sci. Med. Sci 56, B443–448. [DOI] [PubMed] [Google Scholar]
- Larsson L, Moss RL, 1993. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J. Physiol 472, 595–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh BR, 2003. Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol. Sci 18, 222–225. [DOI] [PubMed] [Google Scholar]
- Malisoux L, Francaux M, Nielens H, Renard P, Lebacq J, Theisen D, 2006. Calcium sensitivity of human single muscle fibers following plyometric training. Med. Sci. Sports Exerc 38, 1901–1908. [DOI] [PubMed] [Google Scholar]
- Maughan DW, Molloy JE, Brotto MA, Godt RE, 1995. Approximating the isometric force-calcium relation of intact frog muscle using skinned fibers. Biophys. J 69, 1484–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald KS, 2000. Ca2 + dependence of loaded shortening in rat skinned cardiac myocytes and skeletal muscle fibres. J. Physiol 525, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon NB, Connelly DM, Rice CL, Hunter SW, Doherty TJ, 2017. Neuromuscular contributions to the age-related reduction in muscle power: mechanisms and potential role of high velocity power training. Ageing Res. Rev 35, 147–154. [DOI] [PubMed] [Google Scholar]
- Miller MS, VanBuren P, LeWinter MM, et al. , 2010. Chronic heart failure decreases cross-bridge kinetics in single skeletal muscle fibres from humans. J. Physiol 588, 4039–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Bedrin NG, Callahan DM, et al. , 2013. Age-related slowing of myosin actin cross-bridge kinetics is sex specific and predicts decrements in whole skeletal muscle performance in humans. J. Appl. Physiol 115 (1985), 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Callahan DM, Toth MJ, 2014. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front. Physiol 5, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Bedrin NG, Ades PA, Palmer BM, Toth MJ, 2015. Molecular determinants of force production in human skeletal muscle fibers: effects of myosin isoform expression and cross-sectional area. Am. J. Physiol. Cell Physiol 308, C473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu DG, 1976. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature 262, 610–613. [DOI] [PubMed] [Google Scholar]
- Moore AZ, Caturegli G, Metter EJ, et al. , 2014. Difference in muscle quality over the adult life span and biological correlates in the Baltimore longitudinal study of aging. J. Am. Geriatr. Soc 62, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA, Tobacman LS, Homsher E, 2003. Thin filament activation and unloaded shortening velocity of rabbit skinned muscle fibres. J. Physiol 550, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RL, 1986. Effects on shortening velocity of rabbit skeletal muscle due to variations in the level of thin-filament activation. J. Physiol 377, 487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier Y, Tiffreau V, Montel V, Bastide B, Stevens L, 2009. Phenotypical transitions and Ca2 + activation properties in human muscle fibers: effects of a 60-day bed rest and countermeasures. J. Appl. Physiol 106 (1985), 1086–1099. [DOI] [PubMed] [Google Scholar]
- O’Connell B, Nguyen LT, Stephenson GM, 2004. A single-fibre study of the relationship between MHC and TnC isoform composition in rat skeletal muscle. Biochem. J 378, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis JF, Saelens BE, 2000. Assessment of physical activity by self-report: status, limitations, and future directions. Res. Q. Exerc. Sport 71, 1–14. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Smit JH, et al. , 2005. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin. Endocrinol 63, 152–160. [DOI] [PubMed] [Google Scholar]
- Skelton DA, Greig CA, Davies JM, Young A, 1994. Strength, power and related functional ability of healthy people aged 65–89 years. Age Ageing 23, 371–377. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA, 1981. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J. Physiol 317, 281–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Bean JF, Fielding RA, 2001. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J. Am. Geriatr. Soc 49, 1161–1167. [DOI] [PubMed] [Google Scholar]
- Sweitzer NK, Moss RL, 1990. The effect of altered temperature on Ca2(+)-sensitive force in permeabilized myocardium and skeletal muscle. Evidence for force dependence of thin filament activation. J. Gen. Physiol 96, 1221–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth MJ, Shaw AO, Miller MS, et al. , 2010. Reduced knee extensor function in heart failure is not explained by inactivity. Int. J. Cardiol 143, 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth MJ, Miller MS, VanBuren P, et al. , 2012. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J. Physiol 590, 1243–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees HW, Dekhuijzen PN, Heunks LM, 2009. Levosimendan enhances force generation of diaphragm muscle from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med 179, 41–47. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM, 1994. The disablement process. Soc. Sci. Med 38, 1–14. [DOI] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, et al. , 2002. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the health ABC study. J. Gerontol. A Biol. Sci. Med. Sci 57, M326–332. [DOI] [PubMed] [Google Scholar]
- Wang YP, Fuchs F, 2000. Length-dependent effects of osmotic compression on skinned rabbit psoas muscle fibers. J. Muscle Res. Cell Motil 21, 313–319. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Norenberg KM, Romatowski JG, et al. , 1985. Force-velocity-power and force-pCa relationships of human soleus fibers after 17 days of bed rest. J. Appl. Phycol 1998 (85), 1949–1956. [DOI] [PubMed] [Google Scholar]
- Yamashita-Goto K, Okuyama R, Honda M, et al. , 2001. Maximal and submaximal forces of slow fibers in human soleus after bed rest. J. Appl. Physiol 91 (1985), 417–424. [DOI] [PubMed] [Google Scholar]