Abstract
Introduction
The clinical utility of the multi-biomarker disease activity (MBDA) test for rheumatoid arthritis (RA) management has been understudied in routine care in the U.S.
Methods
Using 2011–2015 Medicare data, we linked each RA patient to their MBDA test result. Initiation of a biologic or janus kinase inhibitor[JAKi] in the 6 months following MBDA testing was described. Multivariable adjustment evaluated the likelihood of adding or switching biologic/JAKi, controlling for potential confounders. For patients with high MBDA scores who added a new RA therapy and were subsequently re-tested, lack of improvement in the MBDA score was evaluated as a predictor of future RA medication failure, defined by the necessity to change RA medications again.
Results
Among 60,596 RA patients with MBDA testing, the proportion adding or switching biologics/JAKi among those not already on a biologic/JAKi was (9.0%, low MBDA; 11.8%, moderate MBDA, and 19.7%, high MBDA, p<0.0001). Similarly, among those already on biologics/JAKi, the proportions were 5.2%,8.3%, and 13.5% (p < 0.0001). After multivariable adjustment, referent to those with low disease MBDA scores, the likelihood of switching was 1.51-fold greater (95% CI 1.35–1.69) for patients with moderate MBDA scores, and 2.62 (2.26 – 3.05) for patients with high MBDA scores. Among those with high MBDA scores who subsequently added a biologic/JAKi and were re-tested, lack of improvement in the MBDA score category was associated with likelihood of future RA treatment failure (OR=1.61, 95% CI 1.27–2.03).
Discussion
The MBDA score was associated both with biologic and JAKi medication addition/switching and subsequent treatment outcomes.
Keywords: rheumatoid arthritis, biomarker, medication persistence, medication switching, biologics
Introduction
Management of patients with rheumatoid arthritis (RA) with respect to changing treatments is routinely informed by clinical assessment through history-taking and physical examination. Composite disease activity indices, such as the Clinical Disease Activity Index (CDAI) or the Disease Activity Score in 28 joints (DAS28), are quantitative measurements that include tender and swollen joint counts and global assessments of disease activity from patients and/or clinicians [1]. Other composite disease activity indices, such as Routine Assessment of Patient Index Data (RAPID 3), Patient Activity Scale (PAS), or PAS-II include only patient reported data [1]. While recommendations to use composite measures to quantify RA disease activity is advocated by the American College of Rheumatology (ACR) [2] and are commonplace in RA clinical trials, these measures nevertheless have their limitations, due to the inter-observer variability in performing joint counts [3] and subjectivity inherent to patient- and physician- global assessments [4]. In addition, disease activity for some RA patients may be difficult to assess due to concomitant comorbidities (e.g. fibromyalgia, obesity) [5, 6] or because of the substantial deformity resultant from longstanding RA.
Laboratory based assessment including C reactive protein (CRP) or erythrocyte sedimentation rate (ESR) may complement clinical assessments, but likewise has limitations. For example, patients with clinically active disease based on clinical examination may have CRP and ESR values within the normal reference range [7]. Thus, other lab-based or new biomarkers (e.g., results of joint ultrasound) hold promise for informing RA management. However, the strategies by which these approaches should inform disease management in RA patients are evolving. One such RA biomarker is the multi-biomarker disease activity (MBDA) test, a 12 analyte test commercially available in the U.S. since 2012. The MBDA test, a prospectively validated measure of RA disease activity [8, 9], has been shown to predict radiographic outcomes [10, 11]. Unlike CRP or ESR, the MBDA test provides cutpoints that classify disease activity in RA as low (<30), moderate (30–44), or high (>44). However, the clinical utility of MBDA testing for patients with RA has not been studied in routine practice in the U.S. Thus, the goals of our study were to: 1) describe the uptake of the MBDA testing by U.S. rheumatologists and the pattern of its use in RA patients enrolled in Medicare; 2) evaluate the likelihood of RA treatment switching conditional on the MBDA score; 3) examine improvement in the MBDA score as a predictor of future treatment response among patients who underwent repeat MBDA testing.
Methods
Cohort selection
RA patients in the U.S. Medicare program were identified using longitudinal 2011–2015 claims data obtained from the Center for Medicare and Medicaid Services (CMS). Patients with rheumatologist-diagnosed RA (ICD-9: 714.0, 14.2, and 714.81) were linked to their MBDA test results (billed to Medicare as HCPCS codes 84999, 84179, and 84190) using methods previously described [12]. Briefly, the MBDA test results available from the centralized laboratory were linked to individual patients enrolled in Medicare based on patient’s: 1) full birth date and sex; 2) National Provider Identifier (NPI) number of the ordering healthcare provider; and 3) MBDA test date. Although a minimum of a single RA diagnosis from a rheumatologist was required, the MBDA is approved for use only in RA patients; thus, linkage to the lab test was expected to confer greater specificity to the identification of RA. The study was governed by a data use agreement from the Center for Medicare and Medicaid Services (CMS), and the local institutional review board approved the analysis. Patient-level consent for data linkage was not required given that that the data was already available and had been collected for purposes other than the present research study.
Uptake and Patterns of Use of the MBDA Test
The cumulative number of RA patients ever tested, and the cumulative number of rheumatologists (identified using Medicare specialty codes) who ordered the test were plotted over time to describe the uptake of the MBDA test in the Medicare RA population. An analysis was performed using only CMS data from 2015 to describe the proportion of all RA patients in rheumatologist’s practice who were underwent MBDA testing at least once that year. Rheumatologists were classified into 5 groups: those who did not order the MBDA test (group 0), and those who ordered the test, by quartiles of usage compared to their peers (groups 1, lowest usage through 4, highest usage). This analysis was restricted to clinicians with at least 10 RA patients in their practice. The proportion of RA patients who added or switched biologics or Janus Kinase inhibitors (JAKi) then was quantified within each rheumatologist’s practice that year to test the hypothesis that physicians with the highest usage of MBDA testing in that year were more likely to change RA treatments for their patients. This process was repeated each calendar year (2011–2014); MBDA utilization was thus updated for each physician annually. For physicians who used the MBDA and with at least 10 RA patients in at least 2 consecutive years, we then identified the rheumatologists who increased their use of the MBDA ‘substantially’, which we defined as meeting two criteria: 1) a doubling in the proportion of RA patient’s tested in their practice compared to the prior year, and 2) who tested at least 15% of their RA patients tested in that calendar year. We then estimated the increase in biologic/JAKi add/switch behavior in each physician’s practice in the year before vs. after the year of MBDA increased use, compared to physicians who did not meet these criteria in that year.
Association between RA Treatment Switching and MBDA Score
Initiation of RA biologics or tofacitinib was examined in relation to the MBDA score. New RA treatments were identified using a window of 4 and 183 days from the time of each MBDA testing. We assessed treatment switching in this wide time window because MBDA results become available approximately 3 days after testing but may not be used to motivate a treatment change decision until the next visit with the rheumatologist, and to allow for the necessary time to obtain prior authorization approval or conduct other procedures (e.g. tuberculosis testing) that may be required to initiate a new RA medication. Results were stratified by whether RA patients were currently on a biologic or or other targeted therapy (e.g. Janus Kinase inhibitor) or not in the 6 months prior to MBDA testing.
Change in MBDA as a Predictor of Future Treatment Response to an RA Medication
MBDA testing can be used to monitor response to a new RA treatment. Patients who have higher MBDA levels (e.g. >44) have the most inflammation and are at the greatest risk of radiographic progression [13]. Thus, these individuals may have poorer RA-related outcomes. Because improvements in clinical disease activity index (CDAI) or the DAS28 were not available, the primary outcome of our study was ‘RA treatment failure’, defined as the necessity to switch to a new RA biologic or JAKi. This approach has been used previously in RA studies as part of a claims-based effectiveness algorithm that was validated against change in the DAS28 over time [14].
We conducted an additional analysis among patients whose initial MBDA score was >44, added a biologic or JAKi between 4 and 183 days later, and then were re-tested with the MBDA approximately 1–6 months later. We hypothesized that patients who had improvement in their MBDA score category (initially high, then improved to moderate/low) or who had the greatest numerical improvement (e.g. exceeding the minimally clinically important difference (MCID) for the MBDA of a 8-unit change, Chatzidionysiou K et. al. EULAR 2017, abstractr #100; Chernoff et. al, EULAR 2017, abstract #87) after this treatment change would have the lowest risk for RA treatment failure.
Statistical Analysis
Descriptive statistics were used to evaluate the number of MBDA tests ordered, comparing patients by MBDA score category at the time of their inital testing. Multivariable-adjusted, alternating logistic regression [15] was used to examine the likelihood of RA treatment switch, conditional on the MBDA score and controlling for potential confounders including demographics (age, sex, race), disability as the reason for Medicare entitlement, use of a wheelchair or other assistive devices, low income status reflected by state buy-in for Medicare premiums, comorbidities (fibromyalgia, chronic pulmonary disease, obesity, diabetes),Charlson comorbidity index, and measures of healthcare utilization (number of outpatient visits, recent hospitalizations). Multivariable-adjusted, alternating logistic regression also controlled for the clustering of RA patients within individual rheumatologists’ practices, because that RA treatment switching is motivated not ony by patient factors, but also by physician-related behaviors and practice styles [16]. All analyses were conducted in SAS 9.4.
Results
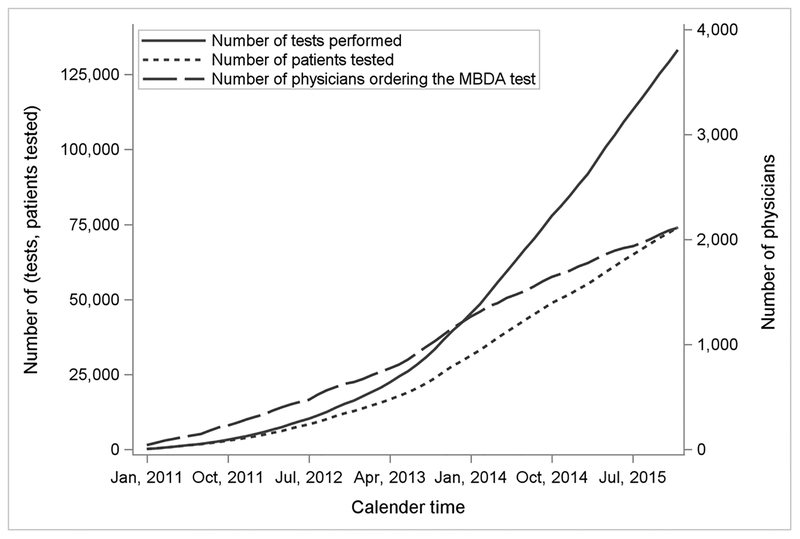
Between 2011 and 2015 more than 75,000 RA patients with fee-for-service Medicare had at least one MBDA test, and more than 125,000 MBDA tests were performed (Figure 1). As shown in Table 1, patients categorized as having low disease activity by MBDA score were somewhat younger, more likely to be male and disabled, had a higher prevalence of fibromyalgia, had a lower prevalence of other comorbidities, were more likely to be on a TNF inhibitor, and less likely to be on systemic glucocorticoids.
Figure 1.
Utilization of MBDA Testing and Uptake by U.S. Rheumatologists among Medicare Enrollees with RA
Table 1:
Descriptive Characteristics of RA Patients Tested with the MBDA, Stratified by Level of Disease Activity (N=60,596)
| MBDA Score Category | |||
|---|---|---|---|
| Low (<30) | Moderate (30–44) | High (>44) | |
| Demographics Age, mean+/-SD, years Female, % Low income, % Disability (as reason for Medicare entitlement), % |
66.4 +− 11.3 74.0 30.9 40.4 |
69.4 +− 10.2 79.1 26.5 35.5 |
69.7 +− 10.5 80.5 27.8 38.8 |
| Comorbidities, % Fibromyalgia Diabetes Chronic Pulmonary Disease Obesity (as a diagnosis) Charlson comorbidity index 0 1 2 3+ |
18.4 15.6 14.2 3.6 7.6 51.1 22.6 18.7 |
17.8 19.6 17.8 5.2 6.1 44.5 23.6 25.8 |
16.5 24.8 23.8 6.8 4.7 37.7 24.6 33.0 |
| Medication TNFi biologic nonTNFi biologic or JAKi Methotrexate HCQ, LEF, SSZ Oral glucocorticoids |
37.4 13.2 49.1 36.5 31.3 |
30.1 16.7 51.0 36.4 37.9 |
23.4 21.5 48.2 36.0 50.7 |
| Health Services Utilization | |||
| Number of ambulatory visits Any hospitalization, % |
8.0 (5.5) 6.2 |
8.5 (5.7) 8.5 |
9.4 (6.3) 14.1 |
Data shown as mean +− standard deviation or % All differences are significant at p < 0.0001 except for the comparison of HCQ/LEF/SSZ which was not significant
TNFi = tumor necrosis factor inhibitor, JAKi = janus kinase inhibitor; HCQ = hydroxychloroquine; SSZ = sulfasalazine; LEF = leflunomidie
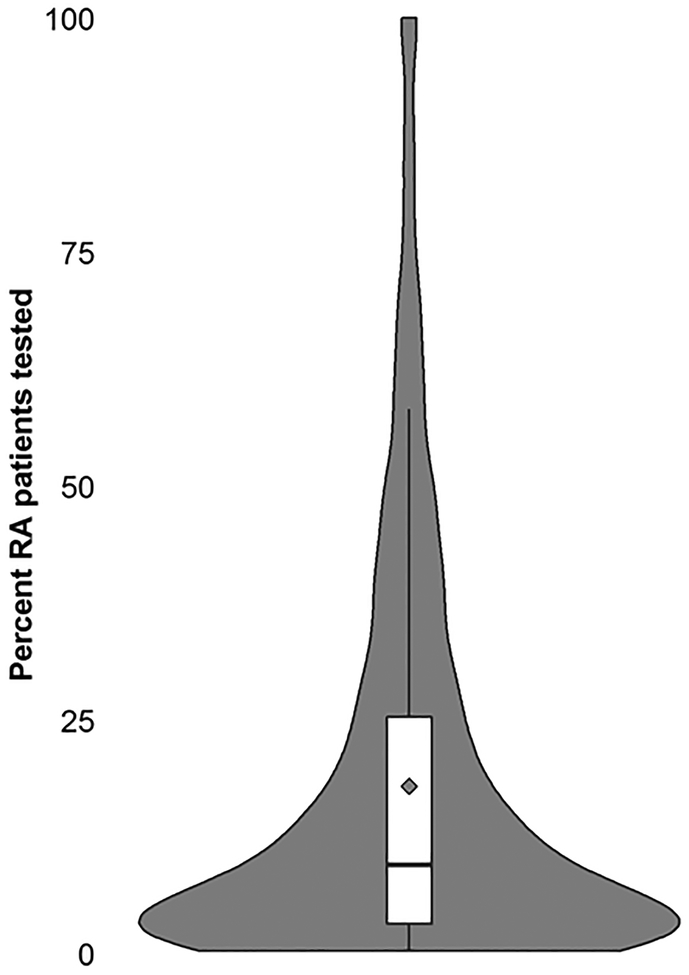
Using data specifically for 2015, we observed wide variability in patterns of MBDA testing among U.S. rheumatologists. A total of 1,426 rheumatologists (25% out of the total of 5,639 rheumatologists identified in the Medicare data who cared for at least 1 RA patient) ordered the MBDA test for at least one RA patient. There was a weak correlation (r=0.14, p < 0.0001) between the number of RA patients seen by the physician and the proportion who underwent MBDA testing. Among clinicians who tested at least 1 RA patient with the MBDA and who had at least 10 RA patients in 2015, the proportion of patients tested at least once is shown in the violin plot (Figure 2). At the 50th percentile, 11% of the rheumatologists’ RA patients were tested, and at the 75th percentile, 30% of RA patients were tested.
Figure 2.
Distribution of the Proportion of RA Patients Tested at Least Once with the MBDA within each Rheumatologist’s Practice and who had at least 10 RA patients in 2015 (n=1,426)
The solid black horizontal line refers to the proportion of patients tested in the median physician’s practice, and the diamond represents the mean proportion tested.
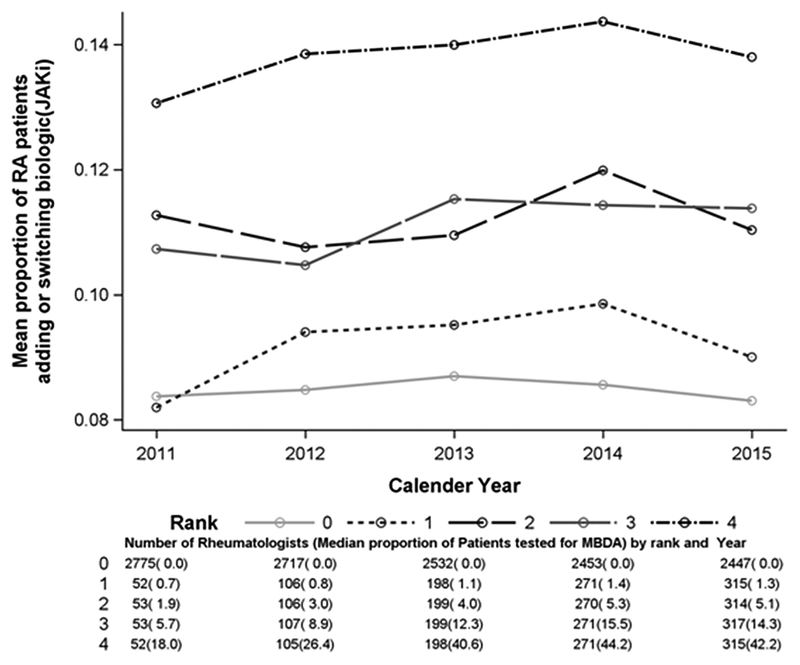
Restricting the sample of rheumatologists to those with at least 10 RA patients yielded approximately 3,708 rheumatologists (Appendix Figure). Grouping them each calendar year based on their frequency of MBDA test utilization in that year (rank 0, non-user, to rank 4, highest user), we plotted the proportion of RA patients who added or switched biologics or JAKi. In the practices of rheumatologists who did not use the MBDA at all (rank 0, n=2,447, 66% of all rheumatologists in 2015) or minimally (rank 1), approximately 9% of RA patients added or switched to a biologic or JAKi each year, compared to approximately 14% for patients of rheumatologists in the highest category of use. There was not a significant interaction between category of MBDA use and the likelihood of treatment switching (p=0.55). However, comparing rheumatologists’ rate of treatment switching in the calendar year before vs. after they ‘substantially increased’ their use of the MBDA test, there was a small (approximately 1%) but significant (p = 0.02) increase in the likelihood of adding or switching biologics or JAKi therapy in the year following a rheumatologists’ greater use of the MBDA test compared to the prior year, versus physicians who did not meet this definition, where there was no change.
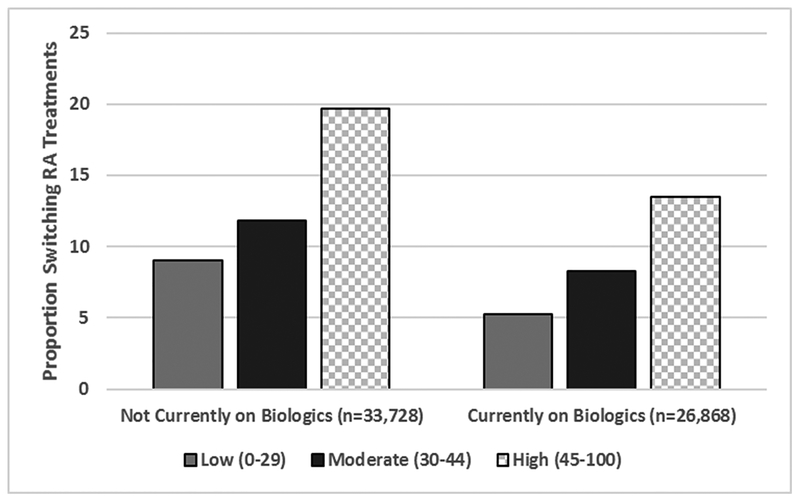
The pattern of RA treatment switch after MBDA testing is shown in Figure 3. A total of 60,596 RA patients were analyzed, of which 7,970 (13.2%) added (n=5,196) or switched (n=2,774) to a biologics or JAKi. Of these, 61% were subsequently re-tested (ever) with the MBDA. There was a strong association between the MBDA score and the likelihood of initiating a biologic or JAKi. Among patients not receiving biologics/JAKi at baseline (Figure 3, left hand panel), the likelihood to initiate a biologic or JAKi was highest (19.7%) for those with high MBDA scores (approximately half of those tested) compared to the likelihood of initiating a biologic/JAKi among patients with a moderate MBDA score (11.8%) or a low MBDA score (9.0%). Trends were similar and significant among those already on one of these these medications at the time of MBDA testing (Figure 3, right-hand panel). For patients with high MBDA scores, the median (IQR) time to switch to a new RA therapy was 36 (22, 70) days and was 60 (28, 110) days to add a therapy. Results were minimally different in the time to add or switch for patients with low or moderate MBDA scores.
Figure 3.
Patterns of Adding or Switching Biologics or JAKi*, According to MBDA Score (N=60,596)
JAKi : Janus kinase inhibitor; MBDA: Multi-biomarker disease activity.
* addition or switching between day 4 and 183 after the MBDA test
In the 60,596 analyzed, and after adjusting for potential confounders, the odds of adding or switching to a biologic or JAKi were highest among those with high MBDA scores compared to patients with low MBDA scores (Odds Ratio = 2.62, 95% CI 2.26 – 3.05) (Table 2). Patients with MBDA scores in the moderate range (30–44) were also more likely to add or switch biologics/JAKi (Odds Ratio = 1.51, 95% 1.35 – 1.69). Older patients, men, and African Americans were less likely to add or switch biologics/JAKi. Higher comorbidity scores and recent hospitalization were associated with an increased likelihood to change RA medications. Current treatment with a TNFi or non-TNFi biologic was associated with a lower likelihood of treatment change, whereas use of MTX and oral glucocorticoids was associated with a greater likelihood of treatment change.
Table 2:
Factors Associated with RA Biologic or JAKi Treatment Switch (n=60,596 patients analyzed)
| Adjusted* Odds Ratio (95% CI) | |
|---|---|
| MBDA Score Low (referent) Moderate (30–44) High (>44) |
1.0 1.51 (1.35 – 1.69) 2.62 (2.26 – 3.05) |
| Age (5 year increments) | 0.91 (0.89 – 0.92) |
| Male sex (female referent) | 0.85 (0.79 – 0.91) |
| Low income** | 1.16 (1.07 – 1.25) |
| Race (Caucasian referent) Black Other |
0.76 (0.69 – 0.84) 0.93 (0.86 – 1.01) |
| Fibromyalgia | 1.13 (1.05 – 1.21) |
| Charlson comorbidity index 0 1 2 3+ |
1.0 (referent) 1.24 (1.10–1.40) 1.25 (1.10–1.42) 1.38 (1.12–1.47) |
| Recent hospitalization | 1.18 (1.10–1.28) |
| RA medications TNFi biologic Non-TNFi biologic MTX HCQ/SSZ/LEF Glucocorticoid |
0.60 (0.52 – 0.68) 0.57 (0.47 – 0.68) 1.09 (1.02 – 1.16) 1.01 (0.95 – 1.08) 1.40 (1.33–1.48) |
JAKi = janus kinase inhibitor
also adjusted for diabetes, chronic pulmonary disease, obesity, and being disabled, none of which were significant, and also for physician clustering (OR = 1.19, 95% CI 1.06 – 1.34)
reflected by state buy-in for Medicare premiums
A total of 1,517 RA patients with high MBDA scores at baseline subsequently added a new RA medication and were included in the analysis examining lack of improvement in MBDA score upon re-testing as a predictor for failure of the recently initiated RA treatment. Over a median (IQR) follow-up time of 14.0 (8.0, 22.0) months, 28.4% of patients whose MBDA scores remained high (>44) upon re-testing subsequently changed RA treatments, compared to 20.3% of patients whose MBDA scores had decreased to moderate or low disease activity (p=0.0007). After adjusting for confounding factors, the likelihood of subsequent RA treatment failure for patients who remained with a high MBDA score was 1.61-fold greater (95% CI 1.27–2.03) compared to patients whose MBDA score category improved to low or moderate (≤ 44). Analyzing the change in MBDA as a continuous variable, and compared to patients with the most improvement in their MBDA score (>16 unit improvement, referent group), those with some improvement (>8 and <=16 unit improvement) were 1.50 (95% 1.03–2.19) more likely to change treatments, while those with minimal or no improvement (<8 unit improvement) were 2.47 (1.79–3.40) more likely to add or switch to a new RA biologic or JAki (Table 3). Older age was associated with patients less likely to add/switch to a new biologic, and already being on a biologic was associated with a greater likelihood.
Table 3:
Factors associated with Subsequent RA Treatment Failure* for Patients RA Who Added or Switched Treatments, Conditional on MBDA Score Improvement (n=1,517)
| Adjusted** Hazard Ratio (95% CI) | |
|---|---|
| MBDA score change at time of re-assessment Most Improvement (>16 units) Some Improvement (>8–16 units) Minimal to No Improvement <8 units) |
1.0 (referent) 1.50 (1.03 – 2.19) 2.47(1.79 – 3.40) |
| Age (5 year increments) | 0.88 (0.83–0.93) |
| Female sex (male referent) | 1.27 (0.94 – 1.70) |
| Low income*** | 0.95 (0.74, 1.24) |
| Race (Caucasian referent) Black Other |
0.93 (0.63–1.38) 1.26 (0.98–1.63) |
| Fibromyalgia | 1.33 (1.05, 1.70) |
| RA medications TNFi biologic Non-TNFi biologic MTX HCQ/SSZ/LEF Glucocorticoid |
1.49 (1.08 – 2.04) 1.50 (0.87 – 2.56) 0.87 (0.70 – 1.09) 1.03 (0.83 – 1.28) 1.22 (0.99–1.51) |
CI: Confidence interval; HCQ: Hydroxychloroquine; LEF: leflunomide; MBDA: Multi-biomarker disease activity; MTX: Methotrexate; RA: Rheumatoid arthritis; SSZ : Sulfasalazine; TNFi: Tumor necrosis factor inhibitor.
proxied by subsequently adding or switching to a new biologic or Janus kinase inhibitor
also adjusted for diabetes, chronic pulmonary disease, obesity, low income, disabled, inpatient hospitalization, number of ambulatory visits, and Charlson co-morbidity index, none of which were significant
reflected by state buy-in for Medicare premiums
Discussion
We found that in Medicare enrollees with RA, the MBDA test utilization increased rapidly from the time of its commercial introduction through 2015. We observed that treatment switching to biologics or JAKi was more likely for patients who had high MBDA scores, as expected. For patients who were tested, found to have high MBDA scores, initiated a biologic or JAKi, and then were re-tested for monitoring purposes, lack of improvement in MBDA score or MBDA score category was a significant predictor of subsequent RA treatment failure. RA treatment failure was proxied by the need to switch to a new targeted RA therapy, an outcome that has been used previously in RA studies and validated against longitudinal change in RA disease activity [14].
Our results are consistent with prior analyses evaluating the MBDA score as a catalyst for changing RA treatments [17]. In one analysis, 18% of patients made a change in their biologic treatment after receiving information about the MBDA score. The MBDA test has been found to be cost-effective in a comprehensive RA management program in which clinical decisions were informed by MBDA testing [18]. In our analysis, we found that RA patients with higher MBDA scores were more likely to add (or switch) biologics or JAKi compared to patients who were tested and had lower MBDA scores. While we cannot provide certainty that the main reason that clinicians switched therapies was predicated on the MBDA test result, we note that the median time to add or switch to a new RA treatment was 1–2 months after testing, lending plausibility to the MBDA test being influential in this decision. Moreover, we were able to compare the overall likelihood of treatment switching within the practices of physicians not ordering the test, or who ordered it infrequently. We found (i.e. Appendix Figure) that rheumatologists who used the test most frequently had a small but significant increase over time in the likelihood of switching their patients’ RA medications. While this ecologic association does not imply causality, it nevertheless lends evidence to physicians being more confident in switching therapies based on the results and access to MBDA testing.
Strengths of our study include a large scale evaluation of uptake, practice variability, and switching medication associated with use of the MBDA testing. In evaluating RA treatment switching, we also were able to control for factors associated with RA treatment failure (e.g. comorbidities). Despite these strengths, our findings need to be interpreted in light of several limitations. Because of the observational study design, clinicians were not randomized with respect to the access to the MBDA test, and those clinicians who ordered the test may have different practice styles than those who did not order the test. We lacked information regarding why the test was ordered or associated clinical disease activity measures (e.g. DAS28, CDAI), making it difficult to assess the incremental value of the information provided by testing above and beyond clinical measurement, or to know whether the treatment changes were ‘appropriate’ (based on long term outcome data). Additionally, although we evaluated MBDA testing with respect to adding or switching to a new RA medication, it is likely that the test was ordered for reasons that were not studied (e.g. to confirm patient’s RA was quiescent with minimal subclinical inflammation). The diversity of clinical scenarios that may motivate testing may in part explain the overall relatively low likelihood of treatment switching following MBDA testing and also likely affected the distribution of MBDA disease activity categories (low/moderate/high) observed in this cohort. We also would note that the MID of the MBDA has been made available to the rheumatology community only recently, and so clinicians were likely unaware of what change in the MBDA score might be considered actionable. Additionally, treatment switching was confined to examination of biologic and JAki addition or switching. We did not study dose changes of medications the patients were already taking, because of imprecision in the data source to quantify such dose changes. For both these reasons, our estimates regarding the likelihood of medication changes made with respect to MBDA testing likely represent a conservative under-estimate.
Clinical inertia continues to exist within RA care, and multiple studies have repeatedly shown failure to advance treatment for patients with active disease [19–22]. Even for patients who might seem to be in low disease activity based on clinical assessment, a high MBDA score has been shown to predict increased risk for radiographic progression [10,13]. As a necessary first step to evaluating RA patients and facilitating a treat-to-target management style, quantitative clinical disease activity assessment is key, yet remains underutilized in the U.S. [23]. The MBDA test can be used to complement clinical assessment and can categorize patients as being in low, moderate, or high disease activity; something that ESR, CRP, or advanced joint imaging does not allow. Ongoing clinical trials (NCT02832297) and forthcoming data (e.g. MBDA to refine the patient-specific predicted risk of future radiographic damage) will be useful to further define the optimal role for the MBDA test in clinical practice to optimize longer-term outcomes. At present, physician judgment remains key in interpreting the test result for an individual patient and acting upon it accordingly to provide personalized care.
Acknowledgements:
Dr. Curtis receives support from the NIH (P60 AR064172) and Patient Centered Outcome Research Institute (PCORI). This study had additional support by Myriad Genetics. Dr. Danila receives support from NIH (P60 AR064172). The manuscript content and decision to submit for publication was solely the purview of the authors and not conditional on external approval.
Appendix Figure
Annualized rate of biologic or JAKi addition/switching, by MBDA test score utilization (n=3,953 eligible rheumatologists)
JAKi : Janus Kinase inhibitor; MBDA: Multi-biomarker disease activity; RA: Rheumatoid arthritis.
Rank 0 refers to physicians who did not order the MBDA test for any RA patient with fee-for-service Medicare in that year. Ranks 1–4 correspond to the first (lowest) to fourth (highest) quartiles of MBDA test utilization in the practices of clinicians who ordered the test at least once inthat year. Analysis was restricted to rheumatologists who cared for at least 10 RA patients in each pairwise year comparison.
The p-value for test for interaction between year and rank is 0.55.
Footnotes
Disclosures:
JC: research grants and consulting from Amgen, Abbvie, BMS, Corrona, Janssen, Myriad, Pfizer, Roche, UCB
MD: research grants from Pfizer
Others: none
References
- 1.Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, Saag KG, O’Dell JR, Kazi S: Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012, 64(5):640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh JA, Saag KG, Bridges SL Jr., Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH et al. : 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis & rheumatology 2016, 68(1):1–26. [DOI] [PubMed] [Google Scholar]
- 3.Scott IC, Scott DL: Joint counts in inflammatory arthritis. Clin Exp Rheumatol 2014, 32(5 Suppl 85):S-7–12. [PubMed] [Google Scholar]
- 4.Curtis JR, Churchill M, Kivitz A, Samad A, Gauer L, Gervitz L, Koetse W, Melin J, Yazici Y: A Randomized Trial Comparing Disease Activity Measures for the Assessment and Prediction of Response in Rheumatoid Arthritis Patients Initiating Certolizumab Pegol. Arthritis & rheumatology 2015, 67(12):3104–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YC, Hackett J, Frits M, Iannaccone CK, Shadick NA, Weinblatt ME, Segurado OG, Sasso EH: Multibiomarker disease activity score and C-reactive protein in a cross-sectional observational study of patients with rheumatoid arthritis with and without concomitant fibromyalgia. Rheumatology (Oxford) 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis JR, Herrem C, Ndlovu N, O’Brien C, Yazici Y: A somatization comorbidity phenotype impacts response to therapy in rheumatoid arthritis: post-hoc results from the certolizumab pegol phase 4 PREDICT trial. Arthritis Res Ther 2017, 19(1):215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kay J, Morgacheva O, Messing SP, Kremer JM, Greenberg JD, Reed GW, Gravallese EM, Furst DE: Clinical disease activity and acute phase reactant levels are discordant among patients with active rheumatoid arthritis: acute phase reactant levels contribute separately to predicting outcome at one year. Arthritis Res Ther 2014, 16(1):R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis JR, van der Helm-van Mil AH, Knevel R, Huizinga TW, Haney DJ, Shen Y, Ramanujan S, Cavet G, Centola M, Hesterberg LK et al. : Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res 2012, 64(12):1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centola M, Cavet G, Shen Y, Ramanujan S, Knowlton N, Swan KA, Turner M, Sutton C, Smith DR, Haney DJ et al. : Development of a multi-biomarker disease activity test for rheumatoid arthritis. PloS one 2013, 8(4):e60635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hambardzumyan K, Bolce R, Saevarsdottir S, Cruickshank SE, Sasso EH, Chernoff D, Forslind K, Petersson IF, Geborek P, van Vollenhoven RF: Pretreatment multi-biomarker disease activity score and radiographic progression in early RA: results from the SWEFOT trial. Annals of the Rheumatic Diseases 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirata S, Li W, Kubo S, Fukuyo S, Mizuno Y, Hanami K, Sawamukai N, Yamaoka K, Saito K, Defranoux NA et al. : Association of the multi-biomarker disease activity score with joint destruction in patients with rheumatoid arthritis receiving tumor necrosis factor-alpha inhibitor treatment in clinical practice. Mod Rheumatol 2016, 26(6):850–856. [DOI] [PubMed] [Google Scholar]
- 12.Curtis JR, Chen L, Bharat A, Delzell E, Greenberg JD, Harrold L, Kremer J, Setoguchi S, Solomon DH, Xie F et al. : Linkage of a de-identified United States rheumatoid arthritis registry with administrative data to facilitate comparative effectiveness research. Arthritis Care Res (Hoboken) 2014, 66(12):1790–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Sasso EH, van der Helm-van Mil AH, Huizinga TW: Relationship of multi-biomarker disease activity score and other risk factors with radiographic progression in an observational study of patients with rheumatoid arthritis. Rheumatology (Oxford) 2016, 55(2):357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis JR, Baddley JW, Yang S, Patkar N, Chen L, Delzell E, Mikuls TR, Saag KG, Singh J, Safford M et al. : Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther 2011, 13(5):R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey V, Zeger S, Diggle P: Modelling multivariate binary data with alternating logistic regressions. Biometrika 1993, 80(3):517–526. [Google Scholar]
- 16.Curtis JR, Chen L, Harrold LR, Narongroeknawin P, Reed G, Solomon DH: Physician preference motivates the use of anti-tumor necrosis factor therapy independent of clinical disease activity. Arthritis Care Res (Hoboken) 2010, 62(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Sasso EH, Emerling D, Cavet G, Ford K: Impact of a multi-biomarker disease activity test on rheumatoid arthritis treatment decisions and therapy use. Current medical research and opinion 2013, 29(1):85–92. [DOI] [PubMed] [Google Scholar]
- 18.Michaud K, Strand V, Shadick NA, Degtiar I, Ford K, Michalopoulos SN, Hornberger J: Outcomes and costs of incorporating a multibiomarker disease activity test in the management of patients with rheumatoid arthritis. Rheumatology (Oxford) 2015, 54(9):1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrold L, Reed, George W, Harrington J. Timothy: A Cluster-Randomized Trial of a Behavioral Intervention to Incorporate a Treat-to-Target Approach in the Clinical Care of Rheumatoid Arthritis Patients in the United States. In: ACR/ARHP Annual Meeting San Francisco, CA; 2015. [Google Scholar]
- 20.Harrold LR, Harrington JT, Curtis JR, Furst DE, Bentley MJ, Shan Y, Reed G, Kremer J, Greenberg JD: Prescribing practices in a US cohort of rheumatoid arthritis patients before and after publication of the American College of Rheumatology treatment recommendations. Arthritis Rheum 2012, 64(3):630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramiro S, Landewé, Robert BM, van der Heijde Désirée, et al. : Is Treat-to-Target Really Working? a Longitudinal Analysis in Biodam. In: ACR/ARHP Annual Meeting San Francisco, CA; 2015. [Google Scholar]
- 22.Yu Z, Lu B, Agosti J, Bitton A, Corrigan C, Fraenkel L, Harrold LR, Losina E, Katz JN, Solomon DH: Implementation of Treat to Target for Rheumatoid Arthritis in the US: Analysis of Baseline Data from the TRACTION Trial. Arthritis Care Res (Hoboken) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis JR, Chen L, Danila MI, Saag KG, Parham KL, Cush JJ. Routine Use of Quantitative Disease Activity Measurements among US Rheumatologists: Implications for Treat-to-target Management Strategies in Rheumatoid Arthritis. J Rheumatol 2018;45:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]