Abstract
Women and men differ in their risk for developing stress-related conditions such as alcohol use and anxiety disorders and there are gender differences in the typical sequence in which these disorders co-occur. However, the neural systems underlying these gender-biased psychopathologies and clinical course modifiers in humans are poorly understood and may involve both central and peripheral mechanisms regulating the limbic-hypothalamic-pituitary-adrenal axis. In the present randomized, double blind, placebo-controlled, triple-dummy crossover study, we juxtaposed a centrally-acting, citalopram (2 mg/unit BMI) neuroendocrine stimulation test with a peripherally-acting, dexamethasone (Dex) (1.5 mg)/corticotropin-releasing factor (CRF) (1 μg/kg) test in euthymic women (N = 38) and men (N = 44) with (54%) and without histories of alcohol dependence to determine whether sex, alcohol dependence or both influenced the adrenocorticotropic hormone (ACTH) and cortisol responses to the pharmacological challenges and to identify the loci of these effects. We found that central serotonergic mechanisms, along with differences in pituitary and adrenal sensitivity, mediated sexually- diergic ACTH and cortisol responses in a stressor-specific manner regardless of a personal history of alcohol dependence. Specifically, women exhibited a greater response to the Dex/CRF test than they did the citalopram test while men exhibited the opposite pattern of results. Women also had more robust ACTH, cortisol and body temperature responses to Dex/CRF than men, and exhibited a shift in their adrenal glands’ sensitivity to ACTH as measured by the cortisol/log (ACTH) ratio during that session in contrast to the other test days. Our findings indicate that central serotonergic and peripheral mechanisms both play roles in mediating sexually dimorphic, stressor-specific endocrine responses in humans regardless of alcohol dependence history.
Keywords: Dex/CRF test, Alcohol use disorder, Sex differences, Serotonin (5-HT), Citalopram stimulation test, Cortisol
1. Introduction
Women and men differ in their risk for developing stress-related mental health conditions such as alcohol dependence (AD) (Keyes et al., 2008), post-traumatic stress disorder (PTSD) (Kessler et al., 1995) and major depression (Kessler et al., 1994). For example, men develop AD at roughly twice the rate of women; while for PTSD, the female to male prevalence rate is 2:1 in the opposite direction. The role of stress in the development and maintenance of AD also differs between the sexes. Thus, in contrast to AD men where alcohol problems usually antedate the onset of anxiety and depression, AD women typically suffer from stress-related anxiety or mood disorders prior to the onset of AD (Hesselbrock et al., 1985; Kessler et al., 1997), and experience increased post-abstinence depressive symptoms (Hatsukami and Pickens, 1982) or frank depressive episodes (Hasin and Grant, 2002) when they quit drinking. While the disparity between women and men is widely known, the neural mechanisms underlying these gender- biased psychopathologies and clinical course modifiers are poorly understood.
Sexual diergisms in the limbic-hypothalamic-pituitary-adrenal (LHPA) axis that regulates the neuroendocrine limb of the stress response have been proposed as important mediators of sex-specific disease risk (Bangasser and Valentino, 2014). These dimorphisms are found at all levels of the axis - from the cortical and limbic structures (e.g., prefrontal cortex, hippocampus and amygdala) that send inputs to neurons in the paraventricular nucleus (PVN) of the hypothalamus which secrete corticotropin-releasing factor (CRF) and arginine vasopressin (AVP) - to the peripheral target of these secretagogues, the pituitary, which secretes adrenocorticotropic hormone (ACTH) and stimulates the adrenal gland to release cortisol (Goel et al., 2014; Solomon and Herman, 2009). The PVN also receives stimulatory and inhibitory signals from monoaminergic afferents originating in the midbrain which themselves are influenced by sex steroids (Barth et al.,2015).
The serotonin (5-hydroxytryptamine or 5-HT) system has been implicated as playing an important role in mediating sex-biased psychopathologies such as AD (Anthenelli et al., 2001; Marcinkiewcz et al.,2016) and PTSD (Ravindran and Stein, 2009). Studies in non-human primates and humans have found sex differences or gonadal steroid effects in 5-HT synthesis (Sakai et al., 2006; Sanchez et al., 2005), presynaptic autoreceptor (e.g., 5-HT1A receptor) activity (Pecins- Thompson and Bethea, 1999), postsynaptic receptor function (Centeno et al., 2007), reuptake (Pecins-Thompson et al., 1998), and degradation (Gundlah et al., 2002). 5-HTergic neurons originating in the brainstem raphe nuclei innervate the LHPA axis centrally at the levels of the prefrontal cortex, amygdala and hippocampus, and also send branches to the PVN of the hypothalamus to form bidirectional feedback loops between the CRF and 5-HT systems (Barth et al., 2015). Thus, the 5-HT- CRF system is uniquely poised to regulate ACTH and cortisol release in a sex-sensitive manner.
Prior work from our group implicated the 5-HT-CRF system as a potential mediator of sex-specific ACTH and cortisol reactivity in longterm abstinent AD men and women and controls (Anthenelli and Maxwell, 2002; Anthenelli et al., 2001). We found that AD men and women had an exaggerated and prolonged endocrine response to the 5- HT-releaser, fenfluramine, compared with non-AD controls, and that the ACTH response to fenfluramine was increased to a larger extent in AD women compared with all other groups. However, our earlier experiments had too few women to confirm whether there was a reproducible disparity in 5-HT-induced ACTH and cortisol release, and were not designed to identify the locus of the disturbance at either a suprapituitary (i.e., 5-HT impacting the hypothalamus or limbic structures regulating the PVN) or peripheral (pituitary and/or adrenal) level. This latter distinction is important because sex differences have also been found at the levels of the pituitary corticotrophs (Gallucci et al., 1993), adrenal cortex (Figueiredo et al., 2007), and gonadal steroids influence the glucocorticoid-dependent negative feedback loop that terminates the stress response (Weiser and Handa, 2009).
In order to begin disentangling the effects of sex and AD on stress circuit function we conducted the first double-blind, placebo-controlled crossover study targeting different loci in the LHPA axis. Using a pharmacological stressor approach, we performed a citalopram stimulation test in women and men with and without histories of AD to examine whether sex, AD, or both affected the individual’s response to this selective 5-HTergic probe. We also examined glucocorticoid-dependent negative feedback, along with pituitary-adrenal sensitivity in these same women and men by administering the combined dex- amethasone/CRF stimulation test to determine sex and AD effects on those parameters. Based on our preliminary results, we hypothesized that: 1) women would have greater ACTH and cortisol responses than men to both the citalopram and dexamethasone/CRF stimulation tests; and 2) AD women would have substantially greater ACTH responses to both challenges than the other three groups.
2. Material and methods
2.1. Participants
Adult premenopausal women and men with (n = 165) and without (n = 112) remitted AD (early or sustained) were recruited from treatment facilities or the general population, respectively. One aim of the study was to determine whether longer-term abstinent AD participants differed from non-AD controls in endocrine (ACTH and cortisol), cardiovascular (blood pressure and heart rate), and subjective responses to the citalopram stimulation test, thus, AD participants had been abstinent from alcohol and all other drugs (except nicotine) for a minimum of 60 days prior to neuroendocrine testing. The study was approved by the University of Cincinnati Institutional Review Board and Cincinnati VA Research and Development Committee, and all participants provided written informed consent and were compensated for their time and effort.
Participants were excluded if they were ≥ 56 years of age, or evidenced an independent (i.e., non-substance-induced) mood, anxiety, psychotic or eating disorder within the past 12 months. Psychotropic and other systemic medications that could influence the stress response were not allowed, and all participants were in generally good health as evidenced by normal physical examinations, laboratory testing and electrocardiogram. Female participants were excluded if they were pregnant, lactating, or using any form of hormonal contraception that could alter corticosteroid-binding globulin levels and, thus, affect measurement of plasma cortisol concentrations (Kirschbaum et al., 1999).
2.2. Rationale for the double-blind, crossover study and targets of the pharmacological probes
The study was designed to assess the central effects of the citalopram stimulation test (CST) in juxtaposition to the peripheral effects of the combined dexamethasone/CRF test (Dex/CRF) while controlling for any potential placebo effects as determined by a third test administering triple-dummy placebos. Acute increases in brain 5-HT levels reliably produced by a single dose of the selective serotonin-reuptake inhibitor (SSRI), citalopram (Nadeem et al., 2004), stimulate neurons in the PVN of the hypothalamus via 5-HT1A and/or 5-HT2A/2C receptors (Hanley and Van de Kar, 2003; Heisler et al., 2007) to secrete CRF and/ or AVP (Jørgensen et al., 2003) to initiate a stress response. Whereas our prior work in AD men and women demonstrated sex-specific, exaggerated ACTH/cortisol responses to serotonergic stimulation compared with non-AD controls (Anthenelli and Maxwell, 2002; Anthenelli et al., 2001), and since these increased hormonal responses might have been of either central or peripheral origin, in the present experiment we also administered the combined Dex/CRF test. In that paradigm, administration of the synthetic glucocorticoid inhibits the pituitary release of ACTH which, in turn, suppresses cortisol secretion. The dex- amethasone suppression portion of the challenge allows one to assess differences in the glucocorticoid-dependent negative feedback loop. The CRF portion of the test is believed to measure pituitary-adrenal sensitivity as well as CRF/AVP feed forward drive of the axis in the setting of low endogenous cortisol (Heuser et al., 1994a). Thus, differential patterns of activation across the CST and Dex/CRF test days has the potential to distinguish a suprapituitary versus pituitary-adrenal locus of response.
2.3. Procedures
This was a randomized, double-blind, triple-dummy crossover study wherein participants received each of 3 separate challenge tests (i.e., placebo, CST, Dex/CRF) in counterbalanced order. Effort was made to conduct neuroendocrine tests in female participants at roughly once- monthly intervals (mean = 44.2 ± 32.3 days between sessions) and timed to correspond with the follicular phase of the menstrual cycle as determined by a calendar method (Mortola et al., 1990) and by measurements of plasma estradiol and progesterone concentrations. Testing in men occurred at shorter between-session intervals (mean = 26.0 ± 43.0 days; p < 0.01; Wilcoxon rank sum test). The neuroendocrine stimulation tests comprised visits 3–5 of an overall 6-session paradigm as illustrated in Fig. 1 and as described below.
Fig. 1.
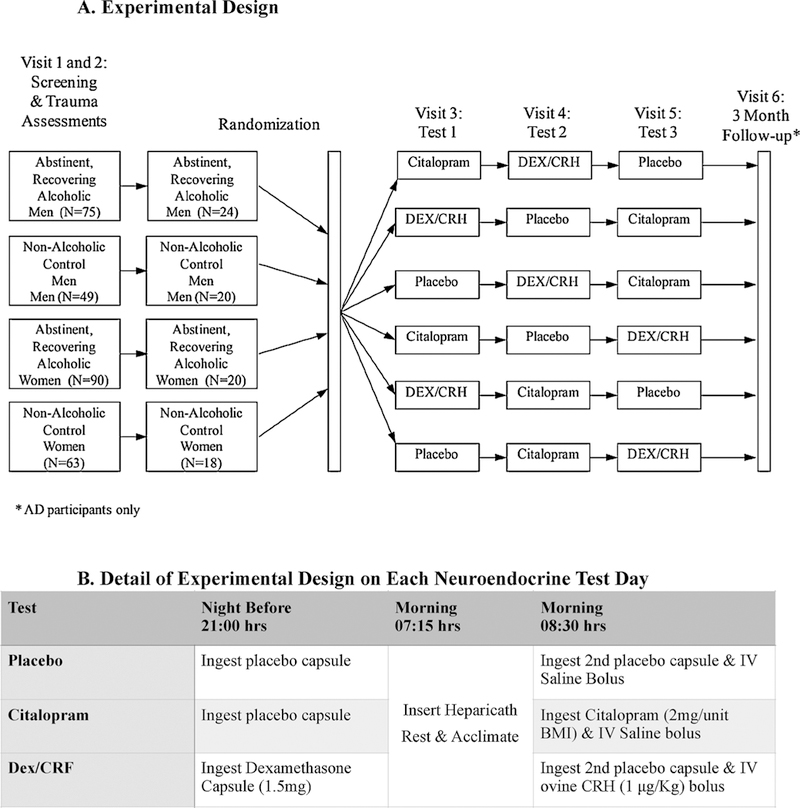
A and B Overall Study Design (1A) - Randomized, double-blind, triple dummy, crossover study conducted in adult men and women with and without histories of alcohol dependence. The 82 randomized participants received each of 3 separate challenge tests (i.e., placebo, citalopram stimulation test [Citalopram, 2 mg/unit BMI], and the combined dexamethasone [1.5 mg]/corticotropin-releasing factor [1 μ/kg] [DEX/CRF] stimulation test) in counterbalanced order as detailed in 1.B. Outcomes from the 3-month follow-up visit assessing relapse versus continued abstinence are presented in other manuscripts.
Details of Visits 1, 2 and 6 have been described previously (Heffneret al., 2011). Briefly, after a telephone screening procedure (n = 1199), 277 eligible participants were interviewed by a trained research assistant with the Semi-Structured Assessment for the Genetics of Alcoholism - Version II (SSAGA-II) (Bucholz et al., 1994) to determine in- clusionary and exclusionary diagnoses using the Diagnostic and Statistical Manual for Mental Disorders, 4th Edition, Text-Revised (DSM-IV-TR) criteria (American Psychiatric Association, 2000). Recency, frequencies and quantities of alcohol, nicotine and other drug use were codified in the relevant sections of the SSAGA-II and monitored throughout the protocol using the calendar-based TimeLine Follow-Back (TLFB) technique (Sobell and Sobell, 1992). Self-reported abstinence from drinking and drug use was verified by repeated alcohol breathalyzer testing; urine drug dipstick and confirmatory urine toxicology screening; and evaluations of state markers of heavy drinking including liver function tests (LFTs) (e.g., gamma glutamyltransferase [GGT]), mean corpuscular volume (MCV), and % carbohydrate-deficient transferrin. Most AD participants were residing in a controlled sober living environment during their participation in the protocol. This also served as another safeguard of AD participants’ abstinence during testing.
The triple-dummy design consisted of participants ingesting a capsule at 2100 h the night prior to each day of neuroendocrine testing. On two of the three nights, this capsule contained placebo, except for the one evening that preceded the CRF stimulation test when participants ingested 1.5 mg of dexamethasone as part of the combined Dex/CRF test. All capsules contained riboflavin as a tracer which was used along with ultraviolet detection, reminder notices, and phone calls as a means of monitoring adherence with taking the capsules. After reporting to the laboratory the next morning at 0715 h and following an overnight fast, a heparicath attached to a 3-way stopcock was placed in the antecubital region of the non-dominant arm. On two of the testing days, 3cc of saline was injected intravenously at approximately 0830 hrs (i.e., on the placebo and CST days), except for the Dex/CRF day, when ovine corticotropin-releasing hormone (Ferring Laboratories) (1 μg/kg) diluted in saline was injected intravenously by slow push (over 10 min). Also at 0830 hrs, participants ingested a second capsule containing either placebo (i.e., on the placebo and Dex/CRF days) or citalopram (2 mg/ unit BMI: 37 mg minimum to 60 mg maximum dosage) with 8 ounces of water.
During each laboratory session, participants reclined in a chair and were asked to stay calm throughout the session. Venous blood samples for baseline measures of cortisol, ACTH, electrolytes, LFTs and sex steroid concentrations (women only) were obtained at −45 and − 5 min pre-ingestion/-infusion of the capsule/IV solution. A Dinamap Pro 100 V2 (GE Medical Systems, Tampa, FL) and digital thermometer (Welch Allyn Sure Temp, Skaneateles Falls, NY) were used to measure blood pressure, heart rate and body temperature, respectively, at these baseline timepoints. Following administration of the study drugs, blood samples and vital sign measurements were obtained every 15 min for the first hour, and then every 30 min for the next 4 h, to monitor the endocrine and cardiovascular effects of the various challenge agents.
Subjective measures of drug and mood effects were also obtained at these same timepoints. These included the Subjective High Assessment Scale (SHAS) (Schuckit, 1984) which measures an individual’s subjective experience of a drug across 13 domains (e.g., feeling high, sleepy, etc.) using visual analog scales; and the Profile of Mood States (POMS), another self-report assessment of an individual’s emotional state that uses a Likert rating scale (McNair et al., 1971).
2.4. Stress hormone assays
Venous blood samples were collected in ice-chilled vacutainer tubes containing 0.1 ml of 15% EDTA. Plasma ACTH concentrations were measured using an immunoradiometric assay (DiaSorin; Stillwater, MN) with an assay sensitivity of ~ 1.0 pg/ml and intra- and interassay coefficients of variance < 10%. Plasma cortisol levels were measured by radioimmunoassay (Siemens Health Care Diagnostics; Los Angeles, CA). The assay has a sensitivity of ~0.3 μg/dl across a standard range from 0.5 to 50 μg/dl. The intra-and interassay coefficients of variance were < 10%
2.5. Data analyses
Demographic and clinical characteristics were analyzed using analysis of variance (ANOVA) with Tukey-adjusted post hoc tests, chi- square tests or non-parametric median tests. ANOVA models were used to analyze baseline measures, peak changes from baseline measures, and hormone area-under-the-curve (AUC) values. Mixed linear models were used to analyze the repeated measures hormonal ratio data across the 13 time points on each of the test days. Log- or square root-transformations were used on the AUC and peak change from baseline hormonal data to correct for non-normality. For clearer interpretation of the results, however, all data presented are raw data. Significance levels for all hypothesis testing were 0.05 and test were two-sided. All analyses were performed using SAS version 9.2 (Cary, N.C.).
3. Results
3.1. Demographics and clinical characteristics
Table 1 presents the demographic and clinical characteristics of the sample demonstrating the expected main effects of a diagnosis of AD on marital status, household income, educational attainment level, alcoholism severity score, drinking parameters and lifetime use of other drugs. AD men were significantly older than men and women in the other three groups, and AD women had significantly higher BMIs than participants in the other groups. AD women had been abstinent longer than AD men, and reported the highest rates of lifetime cocaine/stimulant abuse or dependence compared with the other three groups.
Table 1.
Demographic and clinical characteristics.
| Men | Women | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AD (1) | Control (2) | AD (3) | Control (4) | ||||||
| (n = 24) | (n = 20) | (n = 20) | (n = 18) | P-value | |||||
| Age | 46.2 | [5.3] | 38.2 | [8.4] | 35.1 | [6.5] | 38.9 | [7.5] | < 0.0001 (1 > 2,3,4) |
| BMI (kg/m2) | 26.4 | [3.5] | 26.0 | [3.9] | 30.5 | [5.7] | 26.0 | [6.3] | 0.01 (3 > 1,2,4) |
| Race (# Caucasian) | 17 | (71%) | 17 | (85%) | 11 | (55%) | 15 | (83%) | 0.12 |
| Marital Status (# married) | 2 | (8%) | 7 | (35%) | 3 | (15%) | 11 | (61%) | 0.0009 |
| Household income ($ thousands) | 12.9 | [12.5] | 51.5 | [43.1] | 17.0 | [21.9] | 57.3 | [43.5] | < 0.0001 (2,4 > 1,3) |
| Grade completed | 13.2 | [1.6] | 14.4 | [1.9] | 11.9 | [2.0] | 13.9 | [2.6] | 0.002 (2,4 > 3) |
| ADS total score | 17.0 | [6.5] | 3.0 | [3.3] | 20.4 | [10.4] | 2.5 | [2.2] | < 0.0001 (1,3 > 2,4) |
| Alcohol days abstinent at placebo stim. (median) | 107.0 | 4.5 | 306.5 | 4.0 | < 0.0001 | ||||
| Alcohol days abstinent at dex/CRH stim. (median) | 107.5 | 2.0 | 386.0 | 9.0 | < 0.0001 | ||||
| Alcohol days abstinent at citalopram stim. (median) | 103.0 | 2.0 | 357.0 | 9.0 | < 0.0001 | ||||
| Weeks drinking in last 6 months | 15.8 | [8.2] | 14.8 | [9.0] | 4.5 | [7.2] | 10.8 | [9.2] | 0.0001 (1,2 > 3) |
| Drinks per drinking week | 92.2 | [43.0] | 7.7 | [4.3] | 82.8 | [28.5] | 4.6 | [3.2] | < 0.0001 (1,3 > 2,4) |
| Family history of alcohol use disorder | 13 | (54%) | 2 | (11%) | 13 | (65%) | 9 | (50%) | 0.004 |
| Any illegal drug abuse/dependencea | 20 | (83%) | 6 | (30%) | 18 | (90%) | 2 | (11%) | < 0.0001 |
| Cocaine/stimulants abuse/dependencea | 11 | (46%) | 2 | (10%) | 17 | (85%) | 1 | (6%) | < 0.0001 |
| Cannabis abuse/dependencea | 16 | (67%) | 6 | (30%) | 14 | (70%) | 1 | (6%) | < 0.0001 |
| Current cigarette smokers | 18 | (75%) | 10 | (50%) | 15 | (75%) | 7 | (39%) | 0.04 |
| CRH dosage (mcg) | 91.3 | [23.8] | 87.5 | [17.2] | 86.7 | [17.0] | 73.2 | [17.7] | 0.03 (1 > 4) |
| Citalopram dosage (mg) | 52.9 | [6.2] | 51.5 | [6.3] | 56.2 | [4.6] | 51.0 | [7.9] | 0.05 |
Mean [SD] or # (%) shown. Abbreviations: AD=alcohol dependent; BMI=body mass index; ADS=Alcohol Dependence Scale; CRH=corticotropin-releasing hormone.
Lifetime diagnoses.
In order to examine which of the demographic and clinical variables should be considered for inclusion as covariates in our subsequent models of stress responsivity, Spearman correlations were examined among all variables found to significantly differ across the four groups (e.g., age, BMI, days abstinent) with the baseline and area under the curve (AUC) hormonal measures on each test day. If any demographic or clinical variable was significantly correlated with the hormonal measures, then it was included in data analyses as a covariate. Based on this analysis, three variables - age, family history of alcoholism, and any history of illicit drug abuse or dependence - were included as covariates in subsequent analyses. We also included order in the models to control for the effects that receiving the various stimulation agents in different sequences may have had on the study results.
3.2. Baseline resting AM hormonal concentrations
Fig. 2 illustrates the baseline ACTH and cortisol concentrations across the three test days. As depicted in the upper panel (Fig. 2A), women had lower resting AM ACTH levels than men on the placebo test day (main sex effect: F = 7.69; df = 1; p < 0. 007), but there were no significant sex or alcohol group effects for ACTH on either the Dex/CRF or citalopram test days. Baseline cortisol concentrations, however, did not significantly differ across the four groups on any of the test days (Fig. 2B).
Fig. 2.
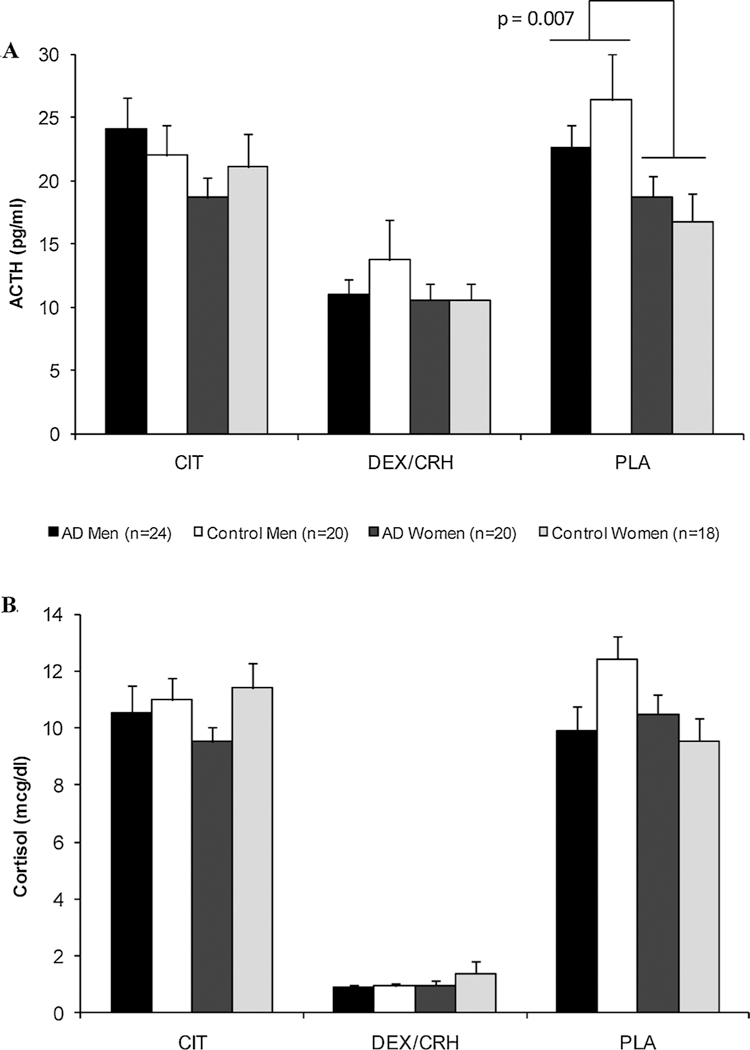
A and B Baseline, resting AM hormonal (ACTH = upper panel, 2A; cortisol = lower panel, 2B) concentrations on each of the 3 test days. Regardless of alcoholism status, women had significantly lower ACTH concentrations than men on the placebo day. Baseline cortisol levels did not differ across the groups on any test day. Abbreviations: AD = alcohol dependent; CIT = citalopram day; DEX/CRF = combined dexamethasone/corticotropin-releasing factor day; and PLA = placebo day. Mean ± SEM shown.
3.3. Placebo day differences in hormonal concentrations
Serum ACTH and cortisol levels on the placebo day are depicted in Fig. 3. After controlling for significant baseline ACTH differences (F = 520.0; df = 75; p < 0.001), results of a repeated measures ANOVA revealed a significant time effect (F = 3.4; df = 11; p < 0.001) and a sex x time interaction for ACTH (F = 2.7; df = 11; p < 0.006) indicating that women’s ACTH levels increased over time while men’s did not (see upper panel, Fig. 3A). There were no significant main effects of alcohol group (p = .11), sex (p = .25), or their interaction (p = .67).
Fig. 3.
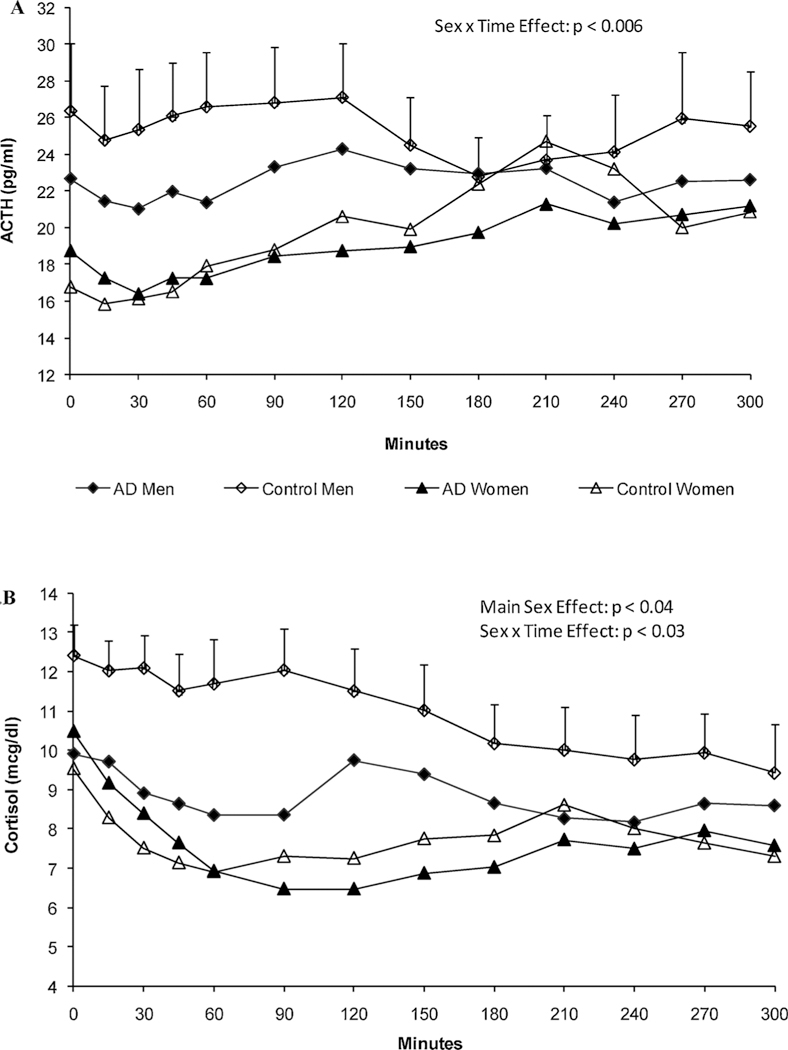
A and B Unadjusted hormonal (ACTH - upper panel, 3A; cortisol = lower panel, 3B) concentrations on the placebo test day. After adjusting for baseline effects, corticotropin levels in women increased over time compared with men (Sex x Time interaction, p < 0.006). Morning cortisol concentrations were significantly lower in women than men (main Sex effect, p < 0.04), but gradually increased over time (Sex x Time interaction, p < 0.03). Mean ± SEM shown.
After adjusting for baseline cortisol concentrations (F = 419.4; df = 75; p < 0.0001), an identical analysis for the cortisol levels on the placebo day revealed a main effect for sex (F = 4.6; df = 1; p < 0.04), time (F = 3.2; df = 11; p < 0.002), and a significant sex x time interaction (F = 2.2; df = 11; p < 0.03; see Fig. 3B). Women had lower cortisol levels than men throughout most of the morning test hours. There was no alcohol group effect (p = .64) or group x sex interaction (p = .81), but there was a significant effect of a family history of alcoholism (F = 7.0; df = 1; p < 0.01) with family history positive (FHP) individuals having lower cortisol concentrations over time compared with family history negative (FHN) participants.
Given these findings of sex differences at rest and in response to a placebo challenge, the placebo day AUC response was used as a covariate in all subsequent analyses.
3.4. Sex- and stressor-specific hormonal responses
The upper panel of Fig. 4 portrays the net integrated serum ACTH (left, Fig. 4A) and cortisol (right, Fig. 4B) responses to the three neuroendocrine stimulation tests using AUC values as the dependent variable. Regarding the ACTH response, after controlling for significant baseline ACTH (F = 102.5; df = 1,68; p < 0.001), family history of alcoholism (FHP > FHN: F = 4.1; df = 1,68; p < 0.05), and order (F = 2.5; df = 5,68; p < 0.05) effects, ANCOVA results revealed a significant sex x treatment (test day) interaction effect (F = 6.2; df = 1,68; p < 0.02) without any main sex (p = .78), AD group (p = .92) or treatment (p = .85) effects. Post hoc analyses demonstrated a trend (p < 0.1) for women to have greater ACTH release in response to the Dex/CRF test than with the citalopram test.
Fig. 4.
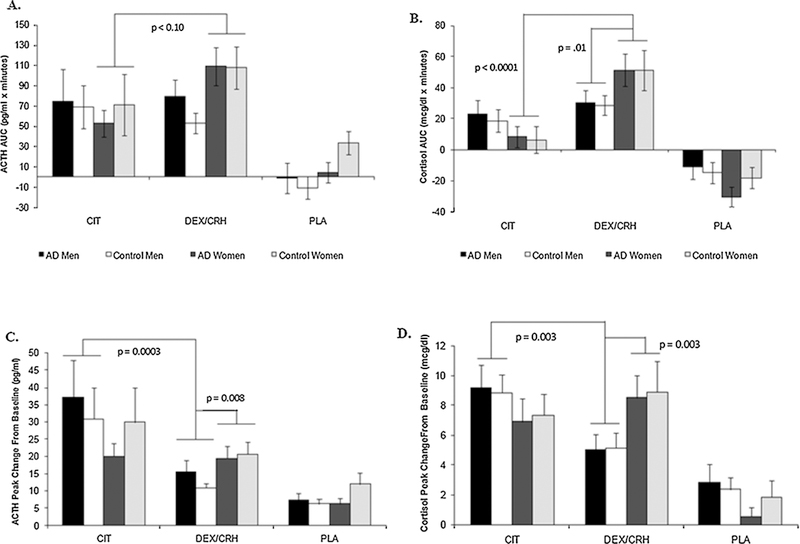
A-D ACTH (left panels, 4A and 4C) and cortisol (right panels, 4B and 4D) responses to the citalopram and DEX/CRF stimulation tests, respectively, demonstrate sex- and pharmacological stressor-specificity. There was a trend for women to have more robust ACTH responses to DEX/CRF than citalopram (upper left panel, Fig. 4A), whereas for cortisol (upper right panel, Fig. 4B), both the stressor-specific and the sexually-dimorphic response to the DEX/CRF test were clearly observed. The corollary of these findings is shown in the lower panels where peak Δ hormonal responses are displayed: men had significantly greater ACTH (lower left panel, Fig. 4C) and cortisol (lower right panel, Fig. 4D) responses following citalopram stimulation than after DEX/CRF. Women’s peak Δ responses to the DEX/ CRF test were significantly greater than men’s. Mean ± SEM shown.
Evidence for pharmacological stressor-specificity was even more apparent upon examination of the cortisol AUC measures (upper right panel, 4.B). Women had significantly greater cortisol responses to the Dex/CRF test as compared to the citalopram test (treatment x sex interaction): t = −5.09; df = 68; p < 0.0001) even after controlling for significant placebo (F = 9.0); df = 1,68; p < 0.004) and order (F = 4.20; df = 5,68; p < 0.003) effects. There was also evidence of a sexually-dimorphic response to the combined Dex/CRF test: following dexamethasone suppression, women had significantly greater cortisol responses to exogenous CRF than men (treatment x sex interaction: t = 2.49; df = 68; p < 0.02).
A similar pattern of findings emerged when the peak change from baseline hormonal measures served as the dependent variables in the ANCOVA models (lower panels, Fig. 4C and D). However, when this metric of HPA axis stimulation was used, the corollary of our previous finding was revealed with men demonstrating stressor-specific response patterns across the testing days. Regarding the ACTH response (lower left panel, Fig. 4C), after controlling for significant baseline ACTH (F = 43.1; df = 1,68; p < 0.0001), placebo day (F = 21.9; df = 1,68; p < 0.0001), and order (F = 3.1; df = 5,68; p < 0.02) effects, ANCOVA results revealed significant treatment (test day) (F = 7.3; df = 1,68; p < 0.009) and sex x treatment interaction effects (F = 6.9; df = 1,68; p < 0.015) without any AD group (p = .97) or AD group interaction effects (e.g., group x sex: p = .84). Thus, men had significantly greater ACTH responses on the citalopram test day than they did on the Dex/CRF day (treatment x sex: t = 3.8; df = 68; p = .0003). Moreover, men had significantly lower ACTH responses than women in response to the Dex/CRF test (treatment x sex: t = 2.7; df = 68; p < 0.008) after controlling for the aforementioned variables.
A nearly identical pattern of responses was observed for the peak change in cortisol responses (lower right panel, Fig. 4D), where again, after controlling for significant baseline cortisol (F = 19.2; df = 1,68; p < 0.0001), placebo day (F = 37.8; df = 1,68; p < 0.0001), age (F = 4.4; df = 1,68; p < 0.04) and order effects (F = 4.1; df = 5,68; p < 0.003), a treatment x sex interaction emerged (F = 8.9; df = 1,68; p < 0.004). Consistent with the ACTH results, men had significantly greater peak cortisol responses to citalopram than Dex/CRF (treatment x sex: t = 3.0; df = 68; p < 0.003). The sexual diergism in the response to the Dex/CRF test was again apparent with women having significantly greater peak change in cortisol responses compared with men (treatment x sex effect: t = 3.09; df = 68; p < 0.003).
3.5. Sex- and pharmacological stressor-specific differences in adrenal sensitivity
To determine whether there were sex or alcohol group effects on adrenal sensitivity, we calculated the cortisol to log (ACTH) ratios for each of the three neuroendocrine stimulation tests. This ratio has been used by others (Ulrich-Lai and Engeland, 2002) as an indirect measure of the adrenal’s response to pituitary ACTH. In the resting non-stimu- lated baseline state, cortisol to log (ACTH) ratios were similar across all four groups on each test day, albeit markedly smaller in magnitude on the Dex/CRF day when cortisol and ACTH concentrations were suppressed by the presence of the synthetic glucocorticoid (data not shown).
However, examination of these ratios over time across each of the three test sessions revealed further evidence that this indirect marker of adrenal sensitivity appears to vary by sex and fluctuate in response to different pharmacological stressors. Fig. 5 portrays the plots of the cortisol to log (ACTH) ratios on the citalopram (upper panel, Fig. 5A), Dex/CRF (middle panel, Fig. 5B), and placebo (lower panel, Fig.5C) test days, respectively. After controlling for significant baseline differences and accounting for the effects of age, illicit drug use, and family history of alcoholism, men exhibited greater degrees of adrenal sensitivity (i.e., greater cortisol to log (ACTH) ratios) than women on both the citalopram and placebo test days. That is, results of separate repeated measures ANCOVAs revealed significant main effects for sex (citalopram: F = 4.3; df = 72, p < 0.05; placebo: F = 7.2, df = 72, p < 0.01) as well as significant sex x time interactions (citalopram: F = 2.0, df = 72, p < 0.04; placebo: F = 1.9, df = 72, p = .05) on each of these test days.
Fig. 5.
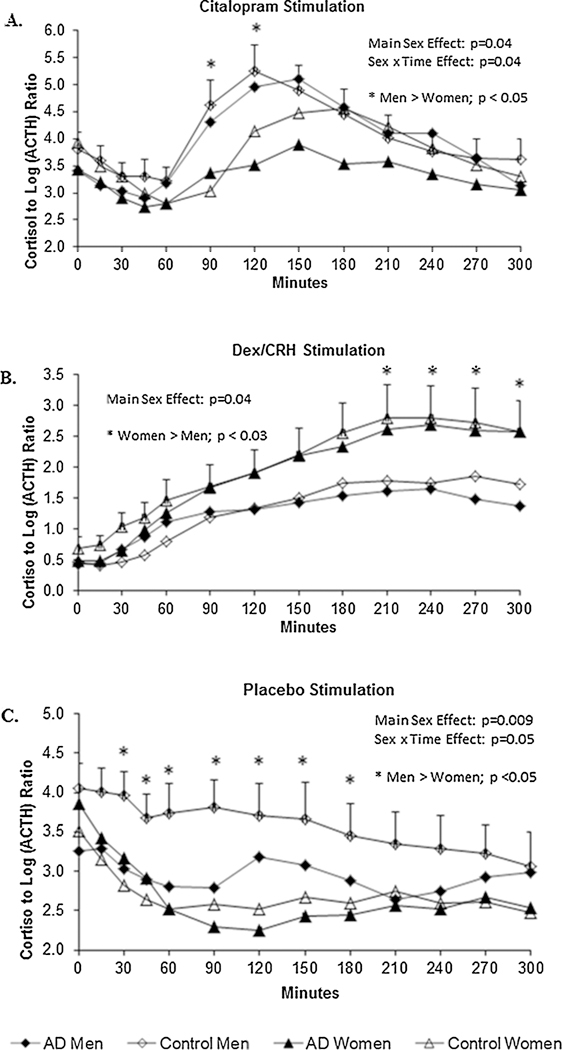
A-C Cortisol to log (ACTH) ratios - an indirect measure of adrenal sensitivity - indicated that men’s adrenal glands were more sensitive to ACTH than women’s on the Citalopram (upper panel, Fig. 5A) and Placebo (lower panel, Fig. 5C) test days. Asterisks (*) signify specific time points where significant Sex X Time interaction effects were found. An opposite pattern (i.e., women’s ratios > men’s) was observed on the DEX/CRF test day (middle panel, Fig. 5B). Mean ± SEM shown.
A different pattern of results emerged, however, when we examined these ratios on the Dex/CRF day (see Fig. 5B). After controlling for significant baseline (F = 203.3; df = 1,72; p < 0.0001) and illicit drug abuse effects (i.e., participants who had abused drugs in the past had higher ratios; p < 0.03), there was a main sex effect (F = 4.7; df = 72; p < 0.04) without any significant sex x time interaction (p = .2). Thus, under the conditions of the Dex/CRF test day, women had greater cortisol to log (ACTH) ratios than men and this sex difference did not fluctuate as a function of time.
3.6. Cardiovascular and body temperature response
Men had higher baseline systolic (p-values < 0.003) and diastolic (p-values < 0.02) blood pressure levels, respectively, compared with women on all three test days (see Supplemental Table 1). Peak changes in diastolic blood pressure were not significantly different across the four groups on any of the test days; however, the elevated systolic and diastolic blood pressures observed in men at baseline remained higher than women’s throughout each of the test sessions (data not shown). There were no significant differences in either baseline or peak change in heart rates across the groups on any of the test days.
Regarding the temperature responses, there were no significant sex or alcohol group effects on any of the test days (data not shown). However, there was a pharmacological stressor-specific elevation in peak change in body temperature: women exhibited significantly increased body temperatures compared with men on the Dex/CRF day (main sex effect: F = 11.8; df = 1; p < 0 .001) without any alcohol group (p = .9) or sex x group (p = .9) interaction effects. Body temperature changes were similar across the four groups on the citalopram and placebo test days.
3.7. Subjective drug and mood responses
Baseline total SHAS scores and peak Δ from baseline SHAS scores were examined on each test day. Since SHAS results were not normally distributed, this variable was dichotomized (i.e., no SHAS response vs. any response) and logistic regression analysis was used. There were no significant sex effects across the three test days, nor were there any significant alcohol group or group x sex interaction effects on the Dex/ CRF and placebo days (data now shown). On the citalopram day at baseline, a significant group effect (p < 0.04) was observed, and there was a trend for a group x sex interaction (p < 0.07). Peak change in SHAS scores did not significantly differ as a function of sex or alcohol group status on any of the test days (data not shown).
Supplemental Fig. 1 depicts the POMS total score at baseline across the four groups on each of the neuroendocrine challenge test days. Separate ANCOVAs controlling for age, family history of alcoholism and history of illicit drug abuse/dependence (none of which were significant) revealed a main alcohol group effect (p-values < 0.05) without any significant sex (p-values > 0.20) or group by sex (p-va- lues > 0.30) interaction effects on each of the three test days. This mild dysphoric state reported by both alcohol dependent men and women at baseline compared with controls persisted throughout each testing session without significant changes over time (data not shown).
4. Discussion
We found sex differences in ACTH and cortisol release varied in relation to the specific pharmacological stressor administered. Women mounted greater endocrine responses to the Dex/CRF test than they did the citalopram test while the opposite pattern was found in men. Women also had greater ACTH and cortisol release and elevations in body temperature than men on the Dex/CRF test day. The pharmacological stressor-specific sex differences in endocrine response appear to involve both centrally-mediated 5-HTergic pathways (Bethea et al., 2011; Goel et al., 2014) and peripheral mechanisms (Bangasser and Valentino, 2014), the latter of which fluctuated in a sex-dependent manner in the setting of subphysiological cortisol levels following dexamethasone suppression. We also found constitutive sex differences in resting morning ACTH plasma levels and systolic and diastolic blood pressure with women exhibiting lower levels than men. Contrary to our predictions, these sex differences were present regardless of whether the individual had a personal history of AD.
Our finding that women respond more robustly to the Dex/CRF test than men replicates other groups’ results (Heuser et al., 1994b; Kunugi et al., 2006) and may indicate that in the setting of markedly reduced endogenous cortisol concentrations following dexamethasone suppression there is more centrally-mediated feed forward drive (i.e., greater CRF and/or AVP secretion) of the HPA axis in women than men (Hundt et al., 2001). Alternatively, since there is evidence that women have greater ACTH release to exogenous CRF than men (Gallucci et al., 1993), it is possible that women’s pituitary corticotrophs are more sensitive to CRF than men’s. It is also conceivable that women and men differ in glucocorticoid dependent negative feedback dynamics with women having more sensitive feedback constrainment of the axis under normal physiological conditions which is mitigated following the administration of the synthetic glucocorticoid. To that end it is interesting to note that healthy adult men whose endogenous cortisol levels were suppressed following ketoconazole administration exhibited greater ACTH secretion in response to exogenous pulsatile cortisol infusions than low cortisol-clamped women - a phenomenon enhanced in both sexes by co-administration of the glucocorticoid receptor (GR) blocker, mifepristone (Roelfsema et al., 2016).
That women exhibited more reactive pituitary-adrenal responses to the Dex/CRF test than men makes our finding of female-specific blunting to the citalopram test more provocative. To our knowledge, this is the first study in humans to document sex differences in ACTH and cortisol responses to acute SSRI challenge in juxtaposition to the peripheral Dex/CRF test. That womens’ pituitary glands were more sensitive to exogenous CRF than men’s on the Dex/CRF day implies that women exhibited relatively less 5-HT-induced feed forward drive (i.e., less hypothalamic CRF and/or AVP secretion) of the HPA axis than men. That men only exhibited significant differences in pharmacological stressor-specifity when peak change from baseline measurements were analyzed further argues that men had greater feed forward drive of the HPA axis to citalopram than they did to Dex-CRF. In support of that hypothesis, positron emission tomography (PET) studies in healthy human volunteers have found that women have lower 5-HT synthesis rates than men (Sakai et al., 2006). While reduced 5-HT synthesis in women compared with men may be the most parsimonious explanation for our finding, it is important to note that sex differences in the 5-HT- CRF system are abundant and have been found at every level of 5- HTergic neurotransmission (Barth et al., 2015; Goel et al., 2014), so this interpretation must be taken cautiously until more definitive mechanistic studies can be performed.
The finding that women have lower resting morning ACTH levels than men in the absence of baseline differences in cortisol concentrations has been interpreted either that 1) women’s adrenal cortex is more sensitive to ACTH than men’s; or, as previously mentioned, 2) women have more sensitive glucocorticoid-dependent negative feedback compared with men (Roelfsema et al., 2017). The present results provide little support for the first of these two interpretations: cortisol to log (ACTH) ratios were significantly greater in men than women on both the placebo and citalopram days indicating that, if anything, the male adrenal cortex is more sensitive to ACTH than the female adrenal cortex. Regarding the second explanation, our study was not specifically designed to test this hypothesis. However, it is noteworthy that the cortisol to log (ACTH) ratio reversed following dexamethasone suppression. Thus, when morning cortisol concentrations were artificially lowered to nighttime — like nadir levels, there was a sex-specific shift in the sensitivity of the adrenal cortex to ACTH. This phase-shifted adjustment in adrenal sensitivity is reminiscent of findings in experimental animals (Ulrich-Lai et al., 2006) which have found that outputs from the circadian clock in the suprachiasmatic nucleus alter adrenal activity via both classic endocrine and autonomic mechanisms, the latter of which may be mediated via the splanchnic nerve.
A key feature of our study design was the inclusion of the doubleblind placebo condition where we also observed sex differences. Inspection of these curves (Fig. 3) and the histograms portraying the resting morning hormonal levels on the placebo day (far right panel of Fig. 2) demonstrated that women had lower ACTH levels than men - a finding frequently reported in the literature (Veldhuis et al., 2009). Cortisol levels, however, while not different at baseline, appeared to belower over the first 90 min of the placebo session in women compared with men leading to an upward sloping compensatory ACTH response in women as the glucocorticoid levels reached their nadir (see Fig. 3). The lower cortisol levels during the morning hours in these premenopausal women compared with men is consistent with results in healthy volunteers (Roelfsema et al., 2017).
The pharmacological challenge tests we performed produced little in the line of cardiovascular changes; however, there was a sex- and pharmacological stressor-specific elevation in the peak body temperature response to the Dex-CRF test with women exhibiting greater elevations than men. We speculate that these elevated body temperatures in women found only on the Dex/CRF test day reflect AVP-mediated effects on thermoregulation related to the circadian phase shift (Guzmán-Ruiz et al., 2015). We also observed that subjective drug and mood responses generally did not differ across the three test days, but alcohol dependent women and men reported modest, but significantly elevated, dysphoric mood states compared with the non-AD controls.
We found no evidence across any of the three test days that AD participants differed from non-AD controls in their ACTH or cortisol responses to the pharmacological probes. We also found no evidence of any alcohol group by sex interaction effects. We interpret these results in the context of our AD participants having been selected for their long-term abstinence, resiliency, and the absence of any current co- morbid psychopathology. It is also important to note that the 5-HT releaser, fenfluramine, and the SSRI, citalopram - while both primarily targeting the 5-HT transporter, have different mechanisms of action to enhance 5-HTergic tone. Fenfluramine promotes 5-HT transport out of neurons while also blocking reuptake (Balcioglu and Wurtman, 1998), while citalopram selectively blocks reuptake. Moreover, fenfluramine’s active metabolite, norfenfluramine, works as a 5-HT2C agonist (Balcioglu and Wurtman, 1998), and our prior work has demonstrated that the breakdown of fenfluramine to norfenfluramine is induced by tobacco smoking which is commonly associated with AD (Anthenelli and Maxwell, 2000). Thus, the discrepancy between our earlier findings using fenfluramine as the 5-HTergic probe versus these SSRI-specific findings may be related to the different effects of the 5-HTergic medications administered. That sex differences have been found in practically all aspects of 5-HTergic neurotransmission ranging from 5-HT synthesis (Sakai et al., 2006; Sanchez et al., 2005) to degradation (Gundlah et al., 2002) makes this an important area for future investigation. Regardless, when one considers how strikingly different our same-sex control and AD groups were regarding their drinking and drug use histories, the absence of alcohol group effects only further substantiates the profundity of these sex differences.
Our study had several limitations. First, we conducted targeted pharmacological neuroendocrine challenges as opposed to psychological or physiological stress tests, thus, these results may not generalize to other stressors. Second, in order to be able to compare our results with our prior work (Anthenelli and Maxwell, 2002; Anthenelli et al., 2001), and in an attempt to make the duration of the stressor more similar to the CST, we conducted the stimulation tests in the morning and used ovine CRH rather than human CRH which were both modifications to the classic Dex/CRF test. Third, we did not measure plasma dexamethasone concentrations; therefore, we cannot rule out that pharmacokinetic differences between women and men might have influenced the results. Fourth, we only studied women during the follicular phase of the menstrual cycle, thus, we are unable to determine whether the sex differences we observed might be cycle-dependent. Fifth, as mentioned previously, we selected a more resilient AD phenotype, some of whom, especially women, had achieved long-term abstinence, so these results might not generalize to more recently abstinent or dually-diagnosed AD individuals. Sixth, elevations in ACTH and cortisol levels observed following acute administration of citalopram may differ from this SSRI medication’s effects on LHPA axis function after chronic dosing. Finally, in an effort to better balance certain characteristics between our AD and control groups, we oversampled FHP and cigarette smoking individuals in our non-AD control group so these are not supernormal controls as used in some studies. Despite these caveats, the consistency of our findings; our use of a randomized, double-blind, placebo-controlled, triple dummy, crossover design; and methodological rigor in controlling for menstrual cycle phase and no concomitant use of hormonal contraceptives, lend credence to the validity of these results.
In conclusion, we have demonstrated that women and men exhibit diametrically opposite reactions to pharmacological challenges probing 5-HTergic and peripheral mediators of the endocrine stress response with women mounting greater reactions to Dex/CRF than men and to their own responses to a citalopram stimulation test. These results lend further support to the notion that the human stress response is sexually- dimorphic and stressor-specific and that these phenomena are, in part, biologically-based involving the 5-HT and pituitary-adrenal systems. The extent to which these sex differences in response to pharmacological stressors correlate with other observations that women and men respond differently to laboratory-based psychogenic stressors such as the Trier Social Stress Test (Stephens et al., 2016) or to social rejection stress paradigms (Stroud et al., 2002) remains to be determined. Unraveling these mechanisms in nonanxious, nondepressed, women and men provides a backdrop against which patients with stress-related anxiety and mood disorders can be compared.
Supplementary Material
Acknowledgements
The authors would like to thank our colleagues and collaborators at the University of Cincinnati and Cincinnati VA Medical Center for their contributions to this study. We also appreciate the generous collaboration of the Cincinnati VA Substance Dependence Program, First Step Home, Talbert House - Pathways for Women Program, Transitions Women’s Recovery Addiction Program, VA Fort Thomas Domiciliary, Center for Chemical Addictions Treatment, and The Crossroads Center, without whose support this study would not have been possible.
We would also like to thank Drs. James Herman, Stephen Woods, Thomas Geracioti, Jr., and Yve Ulrich-Lai who provided thoughtful advice in the design or interpretation of these experiments.
Sources of funding
This work was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant #sAA013307 and AA013307–05S1 and by the Department of Veterans Affairs Research Service. The writing of this manuscript was also supported by NIAAA’s Integrative Neuroscience Initiative on Alcoholism (INIA) Stress Consortium (Grant #U01 AA013641) and by National Institute on Drug Abuse (NIDA)/VA CSP Grant # 1033 and NIDA Grant #UO1 DA041731.
Footnotes
Disclosures
Dr. Anthenelli provides consulting and/or advisory services to Pfizer and US World Meds. The Pacific Treatment and Research Center has received grant support from Alkermes and Pfizer. Drs. Heffner, Daniel, McKenna, Wand and Mr. Blom have no competing interests to disclose.
Declaration of interest
Dr. Anthenelli provides consulting and/or advisory services to Pfizer and US World Meds. The Pacific Treatment and Research Center has received grant support from Alkermes and Pfizer. Drs. Heffner, Daniel, McKenna, Wand and Mr. Blom have no competing interests to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.10167j.psyneuen.2018.05.007.
References
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Text Revision, Washington, DC. [Google Scholar]
- Anthenelli RM, Maxwell RA, 2000. Cigarette smoking decreases the prolactin response to serotonergic stimulation in subgroups of alcoholics and controls. Alcohol. Clin. Exp. Res. 24, 987–995. [PubMed] [Google Scholar]
- Anthenelli RM, Maxwell RA, 2002. Independent alcohol and tobacco effects on stress axis function. Alcohol. Clin. Exp. Res. 26, 1932–1933. [DOI] [PubMed] [Google Scholar]
- Anthenelli RM, Maxwell RA, Geracioti TD, Hauger R, 2001. Stress hormone dysregulation at rest and after serotonergic stimulation among alcohol-dependent men with extended abstinence and controls. Alcohol. Clin. Exp. Res. 25, 692–703. [PubMed] [Google Scholar]
- Balcioglu A, Wurtman RJ, 1998. Effects of fenfluramine and phentermine (fen-phen) on dopamine and serotonin release in rat striatum: in vivo microdialysis study in conscious animals. Brain Res. 813, 67–72. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ, 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 35, 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J, 2015. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Lima FB, Centeno ML, Weissheimer KV, Senashova O, Reddy AP, Cameron JL, 2011. Effects of citalopram on serotonin and CRF systems in the midbrain of primates with differences in stress sensitivity. J. Chem. Neuroanat. 41, 200–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Reich T, Schmidt I, Schuckit MA, 1994. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J. Stud. Alcohol 55, 149–158. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Cameron JL, Bethea CL, 2007. Hypothalamic expression of serotonin 1A, 2A and 2C receptor and GAD67 mRNA in female cynomolgus monkeys with different sensitivity to stress. Brain Res. 1142, 1–12. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP, 2007. Estrogen potentiates adrenocortical responses to stress in female rats. Am. J. Physiol. Endocrinol. Metab. 292, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Gallucci WT, Baum A, Laue L, Rabin DS, Chrousos GP, Gold PW, Kling MA, 1993. Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health Psychol. 12, 420–425. [DOI] [PubMed] [Google Scholar]
- Goel N, Workman JL, Lee TT, Innala L, Viau V, 2014. Sex differences in the HPA axis. Compr Physiol. 4, 1121–1155. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Bethea CL, 2002. Ovarian steroid regulation of monoamine oxidase-A and — B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl.) 160, 271–282. [DOI] [PubMed] [Google Scholar]
- Guzmán-Ruiz MA, Ramirez-Corona A, Guerrero-Vargas NN, Sabath E, Ramirez- Plascencia OD, Fuentes-Romero R, León-Mercado LA, Basualdo Sigales M, Escobar C, Buijs RM, 2015. Role of the suprachiasmatic and arcuate nuclei in diurnal temperature regulation in the rat. J. Neurosci. 35, 15419–15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley NR, Van de Kar LD, 2003. Serotonin and the neuroendocrine regulation of the hypothalamic - pituitary-adrenal axis in health and disease. Vitam. Horm. 66, 189–255. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF, 2002. Major depression in 6050 former drinkers: association with past alcohol dependence. Arch. Gen. Psychiatry 59, 794–800. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Pickens RW, 1982. Posttreatment depression in an alcohol and drug abuse population. Am. J. Psychiatry 139, 1563–1566. [DOI] [PubMed] [Google Scholar]
- Heffner JL, Blom TJ, Anthenelli RM, 2011. Gender differences in trauma history and symptoms as predictors of relapse to alcohol and drug use. Am. J. Addict. 20, 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GSH, Rahilly S, Colmers WF, Elmquist JK, Tecott LH, 2007. Serotonin activates the hypothalamic-pituitary-adrenal axis via Ssrotonin 2C receptor stimulation. J. Neurosci. 27, 6956–6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock MN, Meyer RE, Keener JJ, 1985. Psychopathology in hospitalized alcoholics. Arch. Gen. Psychiatry 42, 1050–1055. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer F, 1994a. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J. Psychiatr. Res. 28, 341–356. [DOI] [PubMed] [Google Scholar]
- Heuser IJ, Gotthardt U, Schweiger U, Schmider J, Lammers CH, Dettling M, Holsboer F, 1994b. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol. Aging 15, 227–231. [DOI] [PubMed] [Google Scholar]
- Hundt W, Zimmermann U, Pottig M, Spring AK, Holsboer F, 2001. The combined dexamethasone-suppression/crh-stimulation test in alcoholics during and after acute withdrawal. Alcohol. Clin. Exp. Res. 25, 687–691. [PubMed] [Google Scholar]
- Jørgensen H, Riis M, Knigge U, Kj$r A, Warberg J, 2003. Serotonin receptors involved in vasopressin and oxytocin secretion. J. Neuroendocrinol. 15, 242–249. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H, Kendler KS, 1994. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch. Gen. Psychiatry 51, 8–19. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC, 1997. Lifetime co-occurence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the national comorbidity survey. Arch. Gen. Psychiatry 54, 313–321. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS, 2008. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend. 93, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH, 1999. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 61, 154–162. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Ida I, Owashi T, Kimura M, Inoue Y, Nakagawa S, Yabana T, Urushibara T, Kanai R, Aihara M, Yuuki N, Otsubo T, Oshima A, Kudo K, Inoue T, Kitaichi Y, Shirakawa O, Isogawa K, Nagayama H, Kamijima K, Nanko S, Kanba S, Higuchi T, Mikuni M, 2006. Assessment of the dex- amethasone/CRH test as a state-dependent marker for hypothalamic-pituitary- adrenal (HPA) axis abnormalities in major depressive episode: a multicenter study. Neuropsychopharmacology 31, 212–220. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Lowery-Gionta EG, Kash TL, 2016. Serotonin’s complex role in alcoholism: implications for rreatment and future research. Alcohol. Clin. Exp. Res. 40, 1192–1201. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppelman L, 1971. Profile of Mood States. Educational and Industrial Testing Service, San Diego. [Google Scholar]
- Mortola JF, Girton L, Beck L, Yen SS, 1990. Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: the calendar of premenstrual experiences. Obstet. Gynecol. 76, 302–307. [PubMed] [Google Scholar]
- Nadeem HS, Attenburrow MJ, Cowen PJ, 2004. Comparison of the effects of citalopram and escitalopram on 5-HT-mediated neuroendocrine responses. Neuropsychopharmacology 29, 1699–1703. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Bethea CL, 1999. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience 89, 267–277. [DOI] [PubMed] [Google Scholar]
- Pecins-Thompson M, Brown NA, Bethea CL, 1998. Regulation of serotonin re-uptake transporter mRNA expression by ovarian steroids in rhesus macaques. Brain Res. Mol. Brain Res. 53, 120–129. [DOI] [PubMed] [Google Scholar]
- Ravindran LN, Stein MB, 2009. Pharmacotherapy of PTSD: premises, principles, and priorities. Brain Res. 1293, 24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema F, Aoun P, Veldhuis JD, 2016. Pulsatile cortisol feedback on ACTH secretion is mediated by the glucocorticoid receptor and modulated by gender. J. Clin. Endocrinol. Metab. 101, 4094–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema F, van Heemst D, Iranmanesh A, Takahashi P, Yang R, Veldhuis JD, 2017. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocr. Connect. 6, 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Nishikawa M, Leyton M, Benkelfat C, Young SN, Diksic M, 2006. Cortical trapping of alpha-[(11)C]methyl-l-tryptophan, an index of serotonin synthesis, is lower in females than males. Neuroimage 33, 815–824. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL, 2005. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res. Mol. Brain Res. 135, 194–203. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, 1984. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch. Gen. Psychiatry 41, 879–884. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline follow-back — a technique for assessing selfreported alcohol consumption In: Litten R, Allen J (Eds.), Measuring Alcohol Consumption. The Human Press Inc, pp. 41–72. [Google Scholar]
- Solomon MB, Herman JP, 2009. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol. Behav. 97, 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MAC, Mahon PB, McCaul ME, Wand GS, 2016. Hypothalamic-pituitary- adrenal axis response to acute psychosocial stress: effects of biological sex and circulating sex hormones. Psychoneuroendocrinology 66, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES, 2002. Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatry 52, 318–327. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Engeland WC, 2002. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology 76, 79–92. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC, 2006. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1128–R1135. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roelfsema F, Iranmanesh A, Carroll BJ, Keenan DM, Pincus SM, 2009. Basal, pulsatile, entropic (patterned), and spiky (staccato-like) properties of ACTH secretion: impact of age, gender, and body mass index. J. Clin. Endocrinol. Metab. 94, 4045–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ, 2009. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience 159, 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


