SUMMARY
Background & aims:
Cancer patients frequently experience weight loss, with negative consequences for functionality and prognosis. The extent to which muscle atrophy contributes to weight loss, however, is not clear, as few studies have directly measured muscle fiber morphology in cancer patients.
Methods:
Whole body and regional tissue composition were measured, along with the cross-sectional area (CSA) and fiber type of mechanically-isolated, single muscle fibers, in 19 cancer patients (8 with a history of weight loss, 11 weight-stable) and 15 non-diseased controls.
Results:
Whole body fat mass was reduced in cancer patients with a history of weight loss, but no differences in whole body or leg fat-free mass were apparent. In contrast, reductions (~20%) in single muscle fiber CSA were found in both slow-twitch, myosin heavy chain (MHC) I and fast-twitch, MHC IIA fibers in both weight-stable patients and those with a history of weight loss. Fiber type distribution showed a shift towards a fast-twitch phenotype compared to controls, which may preserve muscle function in cancer patients despite atrophy, as positive relationships were found between the fractions of hybrid MHC IIAX and I/IIA fibers and 6-min walk performance.
Conclusions:
Our results suggest that, although not apparent from whole body or regional measurements, cancer is associated with reduced skeletal muscle fiber size independent of weight loss history and a shift towards fast-twitch fibers, phenotypes that resemble adaptations to muscle disuse.
Keywords: Atrophy, Single muscle fiber, Fatigue
1. Introduction
Cancer patients often experience alterations in body mass and composition during the disease and its treatment [1,2]. Estimates suggest that ~30–80% of patients experience weight loss, with variability associated with cancer site and severity [2]. Although muscle atrophy is commonly assumed to contribute to reductions in body mass, most of our knowledge of how cancer impacts skeletal muscle is derived from animal models, some of which have questionable relevance to cancer in humans [3]. Few studies have assessed skeletal muscle size in cancer patients at the tissue level [4–7] and these studies have yielded variable results, ranging from pronounced muscle atrophy to no atrophy. Similarly few studies have directly measured skeletal muscle fiber size to rigorously evaluate the extent of atrophy [8–11]. These studies generally support the notion that cancer is accompanied by muscle atrophy, but differ as to whether weight loss is a prerequisite for its occurrence. Animal models show shifts in fiber type distribution with cancer [12], but evidence to corroborate this adaptation in humans is restricted to a single study [10]. Thus, our current understanding of the effects of cancer and its treatment on skeletal muscle morphology and fiber type distribution in humans is limited.
The goal of the present study was to examine the effect of cancer and its treatment on skeletal muscle fiber size to discern the extent of atrophy and its relationship to prior weight loss. To accomplish this objective, we measured skeletal muscle fiber cross-sectional area at multiple points along the length of mechanically-isolated, single muscle fiber segments from cancer patients and controls. These fiber segments were evaluated by gel electrophoresis to identity the type of myosin heavy chain (MHC) protein(s) expressed, allowing for an unambiguous delineation of fiber type, including the ability to discern hybrid fiber types (ie, fibers expressing more than one MHC isoform). Thereafter, these data were used to evaluate whether features of muscle fiber size and MHC isoform distribution (ie, fiber type) predict functional capacity.
2. Materials and methods
2.1. Subjects
Nineteen patients (7 men, 12 women) were recruited from our multi-disciplinary oncology clinics, with clinical characteristics outlined in Table 1. These patients had diagnoses of lung (n = 10), breast (n = 6), gastrointestinal (n = 1 gastric, n = 1 pancreatic) and head-neck (n = 1 larynx) cancer, with 1 stage I,1 stage II,12 stage III and 5 stage IV patients, with 8 being characterized by prior weight loss, defined as a self-reported, unintentional weight loss >5% of body weight in the 6 months prior to evaluation and 11 patients being weight stable (ie, <5% weight loss in prior 6 months). Four patients were tested following diagnosis, prior to receiving any treatment, all of whom reported weight loss >5% of body weight (3 men, 1 woman). The rest of the patients were receiving cancer treatment at the time of testing (n = 15; 4 men/11 women; 11 weight-stable, 4 weight-losing). In these latter patients, testing was completed prior to chemotherapy cycle 4, which excludes patients who might be on longer-term maintenance chemotherapy. The average time from the start of chemotherapy to testing of 73 ± 6 d. All breast cancer patients had undergone surgical resection of their tumor prior to measurements. Three patients received radiation therapy (2 lung, 1 larynx; 2 men/1 woman) and 14 (11 non-cachectic, 3 cachectic) received dexamethasone for 1–4 days after each chemotherapy administration to diminish cancer-induced nausea. Chemotherapeutic agents are provided in Table 1. Ten patients had a history of smoking and six were current smokers (1.13 ± 0.7 packs/d). The gastric and laryngeal cancer patient did not have obstructive dysphagia, as determined by the Massey Swallowing Screen [13]. None of the patients received nutritional support or any type of anabolic therapy designed to improve muscle size or function. Data from a sub-group of this cohort (n = 10) were published previously in a manuscript examining the effects of cancer on skeletal muscle contractility [14].
Table 1.
Clinical characteristics of cancer patients.
| Cancer patients |
|||
|---|---|---|---|
| All | Weight loss | Weight-stable | |
| N | 19 | 8 | 11 |
| Cancer site (n) | |||
| Lung | 10 | 4 | 6 |
| Breast | 6 | 3 | 3 |
| Gastric | 1 | 0 | 1 |
| Pancreatic | 1 | 1 | 0 |
| Head/Neck | 1 | 0 | 1 |
| Stage (I/II/III/IV) | 1/1/12/5 | 1/1/3/3 | 0/0/9/2 |
| Histology (n) | |||
| Adenocarcinoma | 14 | 6 | 8 |
| Squamous-cell carcinoma | 4 | 1 | 3 |
| Adenosquamous carcinoma | 1 | 1 | 0 |
| Radiotherapy (n) | 3 | 0 | 3 |
| Chemotherapy (n) | 15 | 4 | 11 |
| Platinum-based | 7 | 0 | 7 |
| Taxanes | 7 | 3 | 4 |
| Pemetrexed | 3 | 0 | 3 |
| Cyclophosphamide | 5 | 3 | 2 |
| Doxorubicin | 4 | 2 | 2 |
| Herceptin | 1 | 0 | 1 |
| Gemcitabine | 1 | 1 | 0 |
| ECF | 1 | 0 | 1 |
| Etoposide | 1 | 0 | 1 |
| Dexamethasone (n) | 14 | 0 | 14 |
| Weight loss (kg) | 6.9 ± 2.2 | 11.4 ± 2.0 | 0.4 ± 0.2 |
| History of smoking (n) | 10 | 4 | 6 |
| Current smokers (n) | 6 | 3 | 3 |
Data are mean ± SE. Weight loss, indicates patients who self-report >5% loss of body mass in the 6 mos prior to evaluation; whereas, weight-stable patients self-report <5% loss of body mass over this period. Platinum-based therapies include carboplatin (n = 3) and cisplatin (n = 4). ECF, epirubicin/cisplatin/5-fluorouracil.
Controls (6 men, 9 women) were healthy based on medical screening and routine clinical/laboratory tests and had no prior history of cancer, chronic lung or cardiovascular disease, neurological or orthopedic conditions or other mobility-limiting ailments. They were moderately active, but reported not participating in any structured exercise training or weight loss programs at the time of testing and all of the controls were non-smokers. Three volunteers were on stable regimens of statins (ie, HMG CoA reductase inhibitors), but reported no muscle symptoms (eg, muscle soreness) and had normal plasma creatine kinase levels. We recently showed that stable statin therapy does not affect skeletal muscle fiber size, fiber type distribution or mitochondrial or myofilament structure/function in patients without signs or symptoms of myalgia [15]. Written informed consent was obtained from all volunteers prior to their participation, and all protocols were approved by the Committees on Human Research at the University of Vermont.
2.2. Knee extensor muscle function
Knee extensor isometric torque production (55°; peak of knee joint angle-torque relationship [16]) was measured, as described [16]. Knee extensor function measurements were not performed on the 4 cancer patients tested shortly following diagnosis.
2.3. Six-minute (6-min) walk test
The 6-min walk test was conducted in cancer patients as a measure of whole body functional capacity [17], with power production calculated as: 6-min walk power (W) = body mass (kg) * 9.8 (m/s2) * total distance walked (m)/360 s, where 9.8 represents the acceleration of gravity. 6-min walk tests were not performed on the 4 cancer patients tested shortly following diagnosis.
2.4. Total and regional body composition
Total and regional fat mass, fat-free mass and bone mass was assessed by dual energy x-ray absorptiometry, with leg fat-free tissue mass measured as described [14].
2.5. Muscle tissue processing
Protocols for muscle biopsy of the vastus lateralis have been described [18]. All solutions used for processing tissue have been previously described in detail [19]. For single fiber morphological assessments, muscle tissue was placed immediately into cold (4 °C) dissecting solution and processed as described [20], with long-term storage at −20 °C with 50% glycerol (v/v) for cryopreservation. The remainder of tissue was proportioned for mechanical and biochemical measures, as described [14].
2.6. Single muscle fiber cross-sectional area (CSA)
Single fiber CSA was assessed on segments of mechanically-isolated, chemically-skinned muscle fibers (~3 mm long), as described previously [20]. Our choice of fibers is not biased for fiber size, although we do not include fibers with diameters <40 because they can be damaged during measurements (eg, movement through air–water interface). This would bias estimates of fiber CSA towards not observing atrophy in cancer patients, as cancer patients would be expected to have more fibers with small diameters. Briefly, we measured top and side diameter at 250 μm intervals along the fiber to estimate average CSA, assuming an elliptical cross-section. This method has the advantage of over immunohistochemical approaches of allowing unambiguous delineation of pure (MHC I, IIA, IIX) and hybrid (MHC I/IIA, IIA/IIX, I/IIA/IIAX) fibers and provides an integrated estimate of CSA along the fiber length to account for any regional heterogeneity [21]. Following measurements, single fibers were placed in gel loading buffer (2% SDS, 62.5 mM Tris, 10% glycerol, 0.001% bromophenol blue, 5% β-mercaptoethanol, pH 6.8), heated (2 min at 65 °C) and stored at −80 °C until MHC assessment.
2.7. Myosin heavy chain (MHC) isoform expression
MHC isoform expression was assessed in single muscle fiber segments by gel electrophoresis, as described previously [14,20]. Briefly, a standardized amount of the single fiber volume within the linear range of detection [20] was loaded and run via SDS-PAGE (stacking gel: 4% acrylamide-bis/5% glycerol (w/v) and the resolving gel 7% acrylamide-bis/30% glycerol) at 70 V for 3.5 h, followed by 200 V for 20 h, all at 9 °C. Thereafter, the gel was silver stained (BioRad, Hercules, CA). This permits separation of the three isoforms of MHC into three distinct bands for identification of both pure (ie, MHC I, IIA, IIX) and hybrid (MHC IIA/IIX, I/IIA, I/IIA/IIX) fibers (Fig. 1).
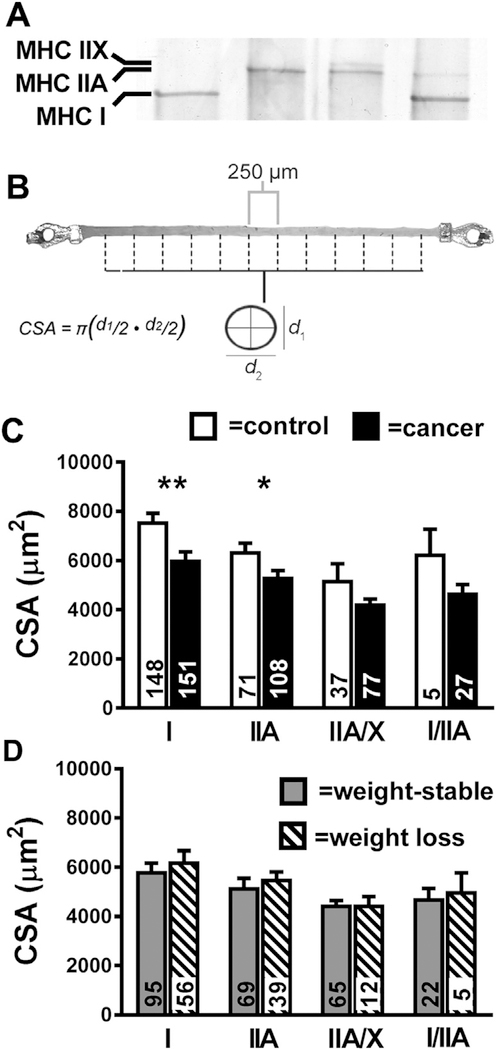
Fig. 1.
Single fiber cross-sectional area (CSA) data. Panel A shows a representative gel electrophoresis separation of MHC isoforms for single muscle fibers (Lane 1: MHC I; Lane 2: MHC IIA; Lane 3: MHC IIA/X; Lane 4: MHC I/IIA) and Panel B a description of CSA measurements. For the latter, top (d1) and side (d2) view diameter measurements were taken every 250 μm along the length of segments of chemically-skinned muscle fiber segments. Group average data are shown for MHC I, IIA, MHC IIA/X and MHC I/IIA fiber types in descending order relative to their abundance in controls (open bars) and cancer patients (closed bars) (Panel C), as well as within the cancer patients in non-cachectic (gray bars) and cachectic (hatched bars) groups (Panel D). The number of fibers evaluated is provided at the base of each bar. Data are mean ± SE. *, P < 0.05; **, P < 0.01.
2.8. Single muscle fiber type distribution
Fiber type data from all single muscle fibers evaluated for patients and controls for both morphology (ie, CSA as described above) and contractile function (described in our prior publications; [14,19,22]) were pooled (n = 46 ± 0.3 fibers/volunteer) and their fractional abundance was calculated. Muscle fibers were separated into 6 groups depending on the type of MHC(s) expressed, as determined by gel electrophoresis: three pure isoforms (I, IIA and IIX) and three hybrid isoform combinations (I/IIA, IIA/IIX and I/IIA/IIX). Based on this classification system, data from each patient was used to calculate the relative expression of each fiber type relative to the total number of fibers examined (ie, % #) and CSA (ie, % CSA). For the latter, the sum of CSAs for all fibers examined in each individual was calculated then divided by the sum of the CSAs for each fiber type to obtain the fractional expression for each fiber type.
2.9. Statistics
Differences between cancer patients and controls were determined using unpaired t-tests. Where data were not normally distributed and data transformations were unable to restore normality (eg, MHC IIA/X fiber type distribution), non-parametric statistics (Mann–Whitney U test) were used. For those variables in which multiple observations were performed within the same individual (eg, single fiber CSA), a linear mixed model (SAS Version 9.3; SAS Institute, Cary, NC) was used, with group assignment (ie, control vs. cancer) being a fixed effect and a random effect (fiber number) included to account for the clustering of observations within individuals, as described [19]. Inclusion of this effect is necessary because the general linear model assumes that each measurement is independent, which is not the case for fibers evaluated within the same volunteer (ie, fibers are related). In other words, each fiber cannot be considered a single observation. Relationships between variables within the cancer patient cohort were determined by Pearson correlation coefficients, with the normality of dependent and independent variables confirmed by Shapiro–Wilk test (SPSS version 19). In the case of data with multiple observations per participant (eg, single fiber CSA), data were averaged to provide a single value for each individual for correlation analyses. For variables that were not normally distributed (eg, MHC I/IIA fiber type distribution), data transformations were applied (eg, log10) and normality was restored. All data are reported as mean ± SEM.
3. Results
3.1. Physical characteristics
Age and physical characteristics of cancer patients and controls are shown in Table 2. Cancer patients were younger than controls (P < 0.01), but no differences in height, body mass or total or regional body composition were found. When cancer patients were divided into weight loss and weight-stable groups, the lack of differences generally persisted, except for group effects for fat mass in both absolute (kg) and relative (%) terms (P < 0.05). In both cases, post hoc comparisons showed that patients with a history of weight loss had lower fat mass than weight-stable patients (P < 0.05 for both), but not controls.
Table 2.
Physical characteristics in controls and cancer patients.
| Controls | Cancer patients |
|||
|---|---|---|---|---|
| All | Weight loss | Weight-stable | ||
| n (male/female) | 15 (9/6) | 19 (7/12) | 8 (3/5) | 11 (4/7) |
| Age (yr) | 66.1 ± 1.2 | 57.2 ± 2.4** | 55.7 ± 4.6 | 58.3 ± 2.7* |
| Body mass (kg) | 68.0 ± 3.5 | 70.4 ± 3.9 | 62.5 ± 5.8 | 76.2 ± 4.7 |
| Height (cm) | 166 ± 2 | 167 ± 2 | 167.4 ± 4.0 | 166.8 ± 3.0 |
| Fat mass (kg) | 20.6 ± 2.3 | 22.6 ± 2.8 | 15.4 ± 3.5 | 27.8 ± 3.5* |
| Fat-free tissue mass (kg) | 45.0 ± 2.4 | 45.9 ± 2.1 | 44.8 ± 3.8 | 46.7 ± 2.5 |
| Fat mass (%) | 30.9 ± 2.4 | 31.2 ± 2.6 | 24.3 ± 3.6 | 36.2 3.0* |
| Leg free tissue mass (kg) | 14.2 ± 0.8 | 14.7 ± 0.7 | 14.3 ± 1.3 | 15.0 ± 0.7 |
Data are mean ± SE. Group effect
P < 0.05
P < 0.01.
Symbols next to values in the All Cancer patient column denote group effects between controls and all cancer patients from t-tests, whereas symbols next to values in the Weight-stable Cancer patient column denote differences from ANOVA comparing control, weight loss and weight-stable groups.
3.2. Single muscle fiber CSA
Average CSA data (as shown in upper panel; average fiber length = 3091 ± 18 μm) are shown in Fig. 1. Considering that measurements were conducted every 250 μm, data are representative of ~7400 assessments of CSA across our cohort. These data show that cancer patients are characterized by substantial muscle fiber atrophy at the cellular level in MHC I (−21%; P < 0.01) and IIA (−17%; P < 0.05) fibers, whereas differences in MHC IIA/X and I/IIA fibers both showed trends (P = 0.10) towards being significant and were of similar or greater magnitude (−19 and −27%, respectively). These differences were not driven by prior weight loss, as there were no differences in CSA in MHC I, IIA, IIAX or I/IIA fibers between weight-losing and -stable patients (Panel D). We also examined the effect of cancer type by examining the two most prevalent types in our cohort: lung and breast. We found lower MHC I (−19%; P < 0.05) and IIA CSA (−19%; P < 0.05) in lung compared to breast cancer patients and a strong trend towards lower MHC IIA/X CSA (15%; P = 0.06). We did not evaluate MHC I/IIA fiber CSA because of the small number of fibers.
Muscle fibers swell with chemical skinning [23]. In a recent study [24], we found that human single muscle fibers undergo an ~20% increase in diameter following chemical skinning (121.6 ± 1.3%), which is in close agreement with previous work in lower order mammals [25]. More importantly, we found an inverse relationship between fiber size and the degree of swelling, suggesting that smaller fibers experienced greater swelling upon skinning, in keeping with recent results in the setting of genetically-induced fiber hypertrophy [26]. As myofilaments are the main osmolytes in skinned fibers, and our prior work [14] shows that the most abundant myofilament proteins, myosin and actin, do not differ in weight-stable patients or those with weight loss, it is unlikely differences in swelling would occur between patients and controls. Thus, if the skinning process skewed our measures, we would expect that any bias would underestimate the extent of atrophy in fibers from cancer patients.
3.3. MHC isoform expression in tissue homogenates and single fiber MHC fiber type distribution
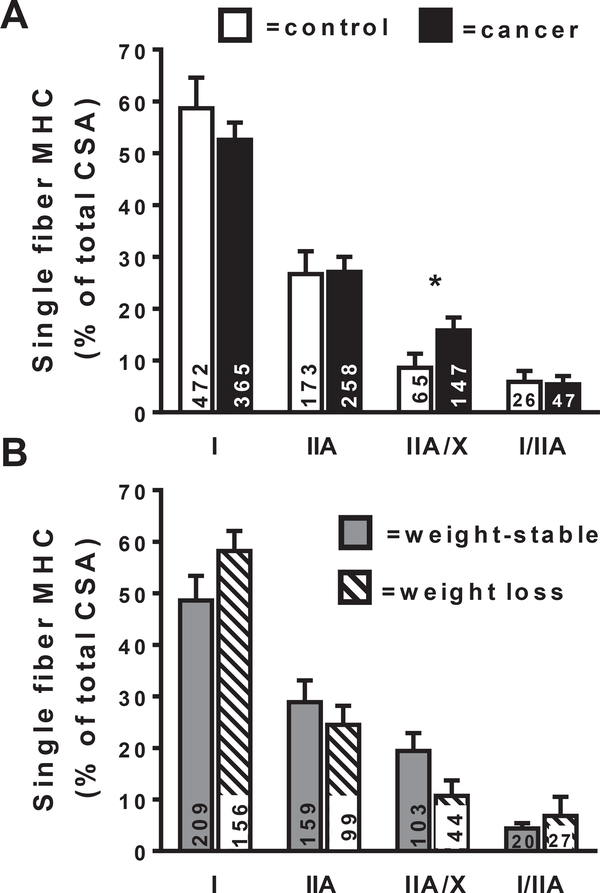
We have previously observed no group differences in the relative expression of MHC I, IIA or IIX via gel electrophoresis [14]. However, analysis of tissue homogenates does not account for variation in MHC isoforms expression within individual muscle fibers. Thus, to further interrogate fiber type distribution, we determined the admixture of fiber types in the vastus lateralis muscle tissue for all of the single muscle fibers evaluated for these patients from both mechanical and morphological analyses (Fig. 2). The prevalence of muscle fibers studied as a fraction of the total number studied (n = 1578) in descending order of abundance were MHC I (53%), IIA (27%), IIA/X (13%), I/IIA (4.6%), I/IIA/IIX (0.9%) and IIX (0.7%) in the entire cohort. Because MHC IIX (n = 11) and MHC I/IIA/IIX (n = 14) fibers each constituted less than 1% of all of the fibers examined, we did not perform statistical analyses on these populations. When we compared fiber type distributions expressed as a fractional percentage of fiber CSA, there were significant group differences. Cancer patients showed an increase in MHC IIA/X hybrid muscle fibers (P < 0.05) compared to controls, whereas differences in MHC I, IIA and I/IIA fibers were not significant. Similar results were observed when data were expressed as a fraction of the number of fibers evaluated, although there was a strong trend for differences in MHC IIA/X fibers (P = 0.06; data not shown in Figure). Sub-group analysis showed no differences between weight loss and weight-stable patients in the distribution of any fiber types (Panel B).
Fig. 2.
Myosin isoform distribution in single muscle fiber segments (MHC I, MHC IIA, MHC IIA/X and MHC I/IIA) in controls (open bars) and OA patients (closed bars) in descending order relative to their abundance (Panel A). Cancer data is also presented for non-cachectic (gray bars) and cachectic (hatched bars) groups (Panel B). Data for single fibers are expressed as a fraction of total fiber CSA, with the CSA for all of the fibers within each fiber type being summed and expressed relative to the summed total CSA from all fibers evaluated within each individual. The number of fibers evaluated is provided at the base of each bar. Note data from MHC IIX (n = 11 total fibers) and I/IIA/X (n = 14 total fibers) fibers were not analyzed because of their low numbers. Data are mean ± SE. *, P < 0.05.
3.4. Relationship between single fiber morphology/fiber type distribution and functional parameters
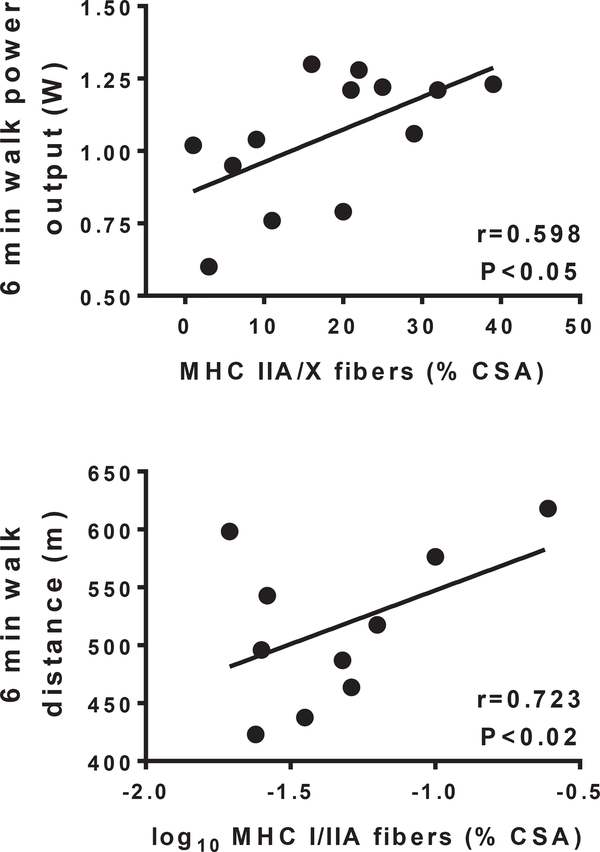
We found a strong relationship between 6-min walk power output and knee extensor peak isometric torque (r = 0.715; P < 0.01), as we have previously reported in a sub-set of the current cohort [14], with a slightly lower correlation with 6-min walk distance (r = 0.546; P < 0.05). Fiber CSA for MHC I, IIA, IIA/X or I/IIA fibers, which constitute >98% of fibers examined, did not correlate with either 6-min walk parameters or isometric knee extensor torque. In contrast, there were relationships between fiber type distribution and 6-min walk performance, but not isometric strength. The fraction of MHC IIA/X fibers were positively related to power output during the 6-min walk test (r = 0.598; P < 0.05; Fig. 3; n = 13 because no MHC IIA/X fiber were found in 2 patients). Additionally, the fraction of MHC I/IIA fibers was positively related to the distance walked (r = 0.723; P < 0.02; Fig. 3; n = 10 because no MHC I/IIA fibers were found in 5 patients).
Fig. 3.
Relationships of percentage of hybrid MHC IIA/X and I/IIA fibers to indices of 6-min walk performance in cancer patients. Note that there is n = 13 for the top panel and n = 10 for the bottom panel. The reduced n in these analyses reflects the fact that MHC IIA/X and I/IIA fibers were not observed in all cancer patients. Data for MHC I/IIA fibers in cancer patients was log10 transformed to achieve normality.
4. Discussion
4.1. Body composition
Although concurrent loss of fat and fat-free (mostly muscle) tissue is an axiom of cancer-associated weight loss [2], there is some disagreement among studies that have employed imaging techniques. Several studies have reported a more pronounced loss of fat mass [27,28], in line with our results, with the most compelling data coming from a recent longitudinal study [5]. However, a preferential loss of fat mass may be explained by the fact that radiologic- and magnetic resonance-based imaging assessments of whole body and regional tissue composition can underestimate fat-free tissue loss because of fluid retention and/ or increasing tumor mass [7], a caveat that also affects our imaging data. Indeed, studies that have attempted to account for these confounders have generally shown equivalent reductions in fat and fat-free tissue [4,7]. However, a recent longitudinal study showed reductions in fat mass, but negligible loss of arm or leg fat-free tissue mass in cancer patients over time [5], measures that should be minimally influenced by tumor mass (both arms and legs) or fluid retention (arms). Thus, there are disagreements among studies regarding adaptations in body composition with cancer. Our data may help to resolve some of these conflicting results.
4.2. Single muscle fiber morphology
Our single muscle fiber CSA data support the general notion that atrophy is present in cancer patients (Fig. 1), but these results are in contrast to no differences in whole body and leg fat-free tissue mass (Table 1). These seemingly contradictory findings may be explained by the inability of imaging techniques, such as DEXA, to accurately quantify skeletal muscle size or changes in muscle size over time [29,30] and could account for disparate results among studies detailed above. This bias may be less apparent with imaging techniques that assess muscle cross-sectional area, such as MRI and CT [29]. Indeed, one recent study showed similar reductions in muscle size in cancer patients by MRI and single fiber CSA [11]. However, caution is urged, as imaging techniques can still underestimate muscle loss in the event of fluid infiltration into the muscle interstitium [31].
To our knowledge, only four studies have evaluated single muscle fiber CSA in human patients. In two studies, atrophy was evident in weight-losing patients, defined by a loss of >10% in 6 months [8,11] compared to non-diseased controls. More recent studies have further refined these results to show that fiber atrophy is only apparent in later stage (III and IV), cachectic patients with low muscularity, with the latter being defined by CT-derived abdominal muscle size [10]. All three of these studies evaluated upper GI or pancreatic cancers. Another recent study showed, similar to our results, that atrophy is apparent in both weight-stable patients and those with a history of weight loss [9]. This study was conducted in lung cancer patients, which make up the majority of our cohort, but differ in that their patients were all treatment naïve, whereas our weight-stable patients were all tested during treatment, suggesting that muscle fiber atrophy in weight-stable patients is not specific to treatment status or its associated medications. However, we acknowledge that some cancer treatments are associated with loss of muscle/fat-free tissue mass [28,32], and dexamethasone, an adjunct to many chemotherapy regimens and which a majority of patients in the current study were taking, can cause muscle wasting [33]. Thus, on balance, these studies support the notion that both cancer alone, and the combination of cancer and its treatments, can be associated with muscle fiber atrophy, but disagree as to whether prior weight loss is a prerequisite for atrophy.
What might explain this variation among studies? One factor is the site of the muscle biopsy. We studied tissue from the vastus lateralis, as did Weber et al. [8,11] and Op den Kamp [9], whereas Johns et al. [10] obtained tissue from the rectus abdominus. One could argue that leg muscle tissue may be more susceptible to atrophy from disuse that often accompanies cancer, and in advanced stage patients specifically given their profound inactivity [34], whereas abdominal muscle tissue would be less affected. This might explain why we and others [9] observed atrophy in weight-stable patients, where biopsies were taken from the leg, whereas Johns et al. did not [10]. However, studies of experimental disuse have shown that musculature in the abdominal region undergoes atrophy to a similar degree as leg muscles [35], probably because of reduced activity of trunk musculature to maintain posture during weight-bearing movement. Therefore, tissue from both sites should be influenced by decreased weight-bearing activity to a similar degree, making it less likely that the site of the muscle biopsy would explain variation in muscle atrophy among studies.
4.3. Role for muscle disuse
Building on the above discussion, muscle disuse deserves consideration as a determinant of the skeletal muscle phenotypes in cancer patients, as disuse is known to affect both muscle size and fiber type expression [36]. For instance, the same research group that found atrophy only in advanced staged patients [10] also showed that these patients had markedly reduced physical activity levels (−45%), whereas earlier stage patients had activity levels comparable to controls [34]. Moreover, cancer therapy has been shown to be associated with reduced activity level [34,37,38], which may partially explain atrophy during treatment [28,32], in particular in weight-stable patients in the current study. A role for muscle disuse in the cancer muscle phenotype may partially explain discordant results among studies. That is, as atrophy can be a function of both disuse and weight loss, variability among patients in the presence and/or extent of either precipitant may account for the discordance between atrophy and prior weight loss in some cohorts, such as [9] and the current study.
Further evidence to substantiate an influence of disuse on the skeletal muscle phenotype in cancer patients is provided in our finding of a switch towards a more fast-twitch phenotype [36,39]. This manifested as an increased relative expression of IIA/X hybrid fibers, similar to what occurs with muscle disuse [40]. Recent studies have provided some evidence for a shift from MHC I to MHC II fibers with cancer in humans [10], but the antibody used to identify MHC II fibers would not distinguish between pure MHC IIA and hybrid MHC IIA/X fibers, the latter of which are more prevalent in cancer patients (Fig. 2). Thus, our work extends these results by providing a more definitive analysis of fiber type-specific atrophy in response to cancer and its treatment and further suggests a role for muscle disuse in the cancer muscle phenotype.
4.4. Relationship of muscle adaptations to physical function
From a functional standpoint, a switch to a more fast-twitch fiber admixture in the muscle may maintain whole muscle power output (force × velocity) in the face of declining force production secondary to atrophy (Fig. 1). Our recent studies in older adults have confirmed this reciprocal relationship between adaptations in force and velocity at the single fiber level with disuse in older adult humans [19] and our current data extend this paradigm to whole muscle/body functional measures in cancer patients. That is, a greater fractional expression of hybrid fibers reflecting a switch to a faster contractile velocity (ie, MHC I/IIA fibers reflects MHC I→IIA and MHC IIA/X fibers a switch from MHC IIA→IIX fibers; [40]) is associated with better walking performance. Indeed, both MHC I/IIA and IIA/X have greater power output than their more abundant pure fiber type (ie, MHC I and IIA, respectively) [41]. That power output would associate with 6 min walk outcomes is not unexpected, as muscle power output is a well-known determinant of walking performance [17]. However, any beneficial effects of these adaptations in MHC expression (ie, fiber type shifts) are not sufficient to overcome the aggregate negative effects of cancer on muscle performance [14].
4.5. Conclusion
In conclusion, our data suggest that cancer and its treatment are associated with muscle atrophy at the single fiber (ie, cellular) level across fiber types that is evident in both weight-stable patients and patients with a history of prior weight loss, despite the absence of differences in whole body and regional fat-free tissue mass, high-lighting the limitations in some radiologic imaging techniques to define muscle morphological adaptations in cancer patients. Moreover, we found a shift in isoform distribution towards a more fast-twitch fiber phenotype, which may serve to maintain functionality in cancer patients, as positive relationships were found between the fractions of MHC IIA/X and MHC I/IIA fibers and their 6-min walk performance. Because both of the aforementioned adaptations parallel those which are found in muscle disuse in adult humans [36], we posit that reduced weight-bearing physical activity with cancer and its treatment [34] contributes, in part, to skeletal muscle adaptations. In this context, muscle disuse may compound nutritional deficiencies to promote cancer cachexia and functional disability, both of which affect near- and long-term prognosis [42–44].
Acknowledgments
We are greatly indebted to the volunteers who dedicated their valuable time to these studies. Authors have contributed to the conception and design of the study (MJT, MEC, KD), acquisition of data (MJT, DMC, MSM, TWT, SBH, MEC, KD), analysis of data (MJT, DMC, MSM, TWT, SBH) and interpretation (MJT, KD) and drafting and/or revising the manuscript for content (MJT, DMC, MSM, TWT, SBH, MEC, KD). All authors have approved the final version of the manuscript. This study was funded by a grant from the University of Vermont College of Medicine. MSM was funded by a Mentored Research Scientist Development Award (AG-031303) and DMC by an Institutional National Research Service Award (HL-007647). The sponsors had no role in the design or conduct of the research or production of the manuscript describing the research studies.
Footnotes
Conflict of interest
Michael J. Toth, Damien M. Callahan, Mark S. Miller, Timothy W. Tourville, Sarah B. Hackett, Marion E. Couch and Kim Dittus report no conflict of interests.
References
- [1].Fearon KCH, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling and metabolic pathways. Cell Metab 2012;16:1–14. [DOI] [PubMed] [Google Scholar]
- [2].Tisdale M Cachexia in cancer patients. Nat Rev Cancer 2002;2:862–71. [DOI] [PubMed] [Google Scholar]
- [3].Johns N, Stephens NA, Fearon KCH. Muscle wasting in cancer. Int J Biochem Cell Biol 2013;45:2215–29. [DOI] [PubMed] [Google Scholar]
- [4].MacFie J, Burkinshaw L. Body composition in malignant disease. Metabolism 1987;36:290–4. [DOI] [PubMed] [Google Scholar]
- [5].Fouladiun M, Körner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care e correlations with food intake, metabolism, exercise capacity, and hormones. Cancer 2005;103: 2189–98. [DOI] [PubMed] [Google Scholar]
- [6].Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- [7].Heymsfield SB, McManus CB. Tissue components of weight loss in cancer patients: a new method of study and preliminary observations. Cancer 1985;55:238–49. [DOI] [PubMed] [Google Scholar]
- [8].Weber M-A, Kinscherf R, Krakowski-Roosen H, Aulmann M, Renk H, Künkele A, et al. Myoglobin plasma level related to muscle mass and fiber composition – a clinical marker of muscle wasting? J Mol Med 2007;85: 887–96. [DOI] [PubMed] [Google Scholar]
- [9].Op den Kamp CM, Langen RC, Snepvangers FJ, de Theije CC, Schellekens JM, Laugs F, et al. Nuclear transcription factor k B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr 2013;98:738–48. [DOI] [PubMed] [Google Scholar]
- [10].Johns N, Hatakeyama S, Stephens NA, Degen M, Degen S, Frieauff W, et al. Clinical classification of cancer cachexia: phenotypic correlates in human skeletal muscle. PLoS One 2014;9:e83618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Weber M-A, Krakowski-Roosen H, Schröder L, Kinscherf R, Krix M, Kopp-Schneider A, et al. Morphology, metabolism, microcirculation, and strength of skeletal muscles in cancer-related cachexia. Acta Oncol 2009;48:116–24. [DOI] [PubMed] [Google Scholar]
- [12].Diffee GM, Kalfas K, Al-Majid S, McCarthy DO. Altered expression of skeletal muscle myosin isoforms in cancer cachexia. Am J Physiol 2002;283: C1376–82. [DOI] [PubMed] [Google Scholar]
- [13].Massey R, Jedlicka D. The Massey bedside swallowing sceen. J Neurosci Nurs 2002;34:7–60. [DOI] [PubMed] [Google Scholar]
- [14].Toth MJ, Miller MS, Callahan DM, Sweeny AP, Nunez I, Grunberg SM, et al. Molecular mechanisms underlying skeletal muscle weakness in human cancer: reduced myosin-actin cross-bridge formation and kinetics. J Appl Physiol 2013;114:858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rengo JL, Callahan DM, Savage PD, Ades PA, Toth MJ. Skeletal muscle ultrastructure and function in statin-tolerant individuals. Muscle Nerve 2015. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Toth MJ, Shaw AO, Miller MS, VanBuren P, LeWinter MM, Maughan D, et al. Reduced knee extensor function in heart failure is not explained by inactivity. Int J Cardiol 2010;143:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bean JF, Kiely DK, Leveille SG, Herman S, Huynh C, Fielding R, et al. The 6-minute walk test in mobility-limited elders: what is being measured? J Gerontol 2002;57A:M751–6. [DOI] [PubMed] [Google Scholar]
- [18].Toth MJ, Miller MS, Ward KA, Ades PA. Skeletal muscle mitochondrial density, gene expression, and enzyme activities in human heart failure: minimal effects of the disease and resistance training. J Appl Physiol 2012;112:1864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Callahan DM, Miller MS, Sweeny AP, Tourville TW, Slauterbeck JR, Savage PD, et al. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol 2014;592:4555–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miller MS, VanBuren P, LeWinter MM, Lecker SH, Selby DE, Palmer BM, et al. Mechanisms underlying skeletal muscle weakness in human heart failure: alterations in single fiber myosin protein content and function. Circ Heart Fail 2009;2:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bua EA, McKiernan SH, Wanagat J, McKenzie D, Aiken JM. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J Appl Physiol 2002;92:2617–24. [DOI] [PubMed] [Google Scholar]
- [22].Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, et al. Resistance training alters skeletal muscle structure and function in human heart failure: effects at the tissue, cellular and molecular levels. J Physiol 2012;590:1243–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Godt RE, Maughan DW. Swelling of skinned muscle fibers of the frog: experimental observations. Biophys J 1977;19:103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Callahan DM, Tourville TW, Miller MS, Hackett SB, Sharma H, Cruickshank NC, et al. Chronic disuse and skeletal muscle structure in older adults: sex differences and relationships to contractile function. Am J Physiol Cell Physiol 2015;308:C932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Godt RE, Maughan DW. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflug Arch Eur J Physiol 1981;391:332–7. [DOI] [PubMed] [Google Scholar]
- [26].Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, et al. Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J 2009;23:3896–905. [DOI] [PubMed] [Google Scholar]
- [27].Agustsson T, Wikrantz P, Rydén M, Brismar T, Isaksson B. Adipose tissue volume is decreased in recently diagnosed cancer patients with cachexia. Nutrition 2012;28:851–5. [DOI] [PubMed] [Google Scholar]
- [28].Jackson W, Alexander N, Schipper M, Fig L, Feng F, Jolly S. Characterization of changes in total body composition for patients with head and neck cancer undergoing chemoradiotherapy using dual-energy x-ray absorptiometry. Head Neck 2014;36:1356–62. [DOI] [PubMed] [Google Scholar]
- [29].Delmonico MJ, Kostek MC, Johns J, Hurley BF, Conway JM. Can dual energy X-ray absorptiometry provide a valid assessment of changes in thigh muscle mass with strength training in older adults. Eur J Clin Nutr 2007;62:1372–8. [DOI] [PubMed] [Google Scholar]
- [30].Madden-Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact 2013;13:320–8. [PubMed] [Google Scholar]
- [31].Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 1993;148:379–85. [DOI] [PubMed] [Google Scholar]
- [32].Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol 2010;28:1054–60. [DOI] [PubMed] [Google Scholar]
- [33].Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, et al. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 2007;6:376–85. [DOI] [PubMed] [Google Scholar]
- [34].Ferriolli E, Skipworth RJE, Hendry P, Scott A, Stensteth J, Dahele M, et al. Physical activity monitoring: a responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? J Pain Symptom Manage 2012;43:1025–35. [DOI] [PubMed] [Google Scholar]
- [35].LeBlanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, et al. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J Appl Physiol 2000;89:2158–64. [DOI] [PubMed] [Google Scholar]
- [36].Narici MV, Boer MD. Disuse of the musculo-skeletal system in space and on earth. Eur J Appl Physiol 2011:111. [DOI] [PubMed] [Google Scholar]
- [37].Courneya KS, Friedenreich CM. Relationship between exercise during treatment and current quality of life among survivors of breast cancer. J Psych Oncol 1998;15:35–57. [Google Scholar]
- [38].Pinto BM, Trunzo JJ, Reiss P, Shiu S-Y. Exercise participation after diagnosis of breast cancer: trends and effects on mood and quality of life. Psycho-Oncology 2002;11:389–400. [DOI] [PubMed] [Google Scholar]
- [39].Baldwin KM, Haddad F, Pandorf CE, Roy RR, Edgerton RV. Alterations in muscle mass and contractile phenotype in response to unloading models: role of transcriptional/pretranslational mechanisms. Front Physiol 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Andersen JL, Gruschy-Knudsen T, Sandri C, Larsson L, Schiaffino S. Bed rest increases the amount of mismatched fibers in human skeletal muscle. J Appl Physiol 1999;86:455–60. [DOI] [PubMed] [Google Scholar]
- [41].Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 1996;495:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Veasey-Rodrigues H, Baracos VE, Wheler JJ, Parons HA, Hong DS, Naing A, et al. Body composition and survival in the early clinical trials setting. Eur J Cancer 2013;49:3068–75. [DOI] [PubMed] [Google Scholar]
- [43].Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Am J Med 1980;69:491–7. [DOI] [PubMed] [Google Scholar]
- [44].Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer 2012;76: 248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]