Abstract
Purpose
To determine whether serial cone-beam computed tomography (CBCT) images taken during head and neck radiotherapy (HNR) can improve chronic xerostomia prediction
Methods and Materials
In a retrospective analysis, parotid glands (PGs) were delineated on daily kV CBCT images using deformable image registration (DIR) for 119 HNR patients (60 or 70 Gy in 2 Gy/fraction over 6 or 7 weeks). DIR accuracy for a subset of deformed contours was quantified using the Dice similarity coefficient (DSC) and mean distance-to-agreement (MDA) in comparison to manually drawn contours. Average weekly changes in CBCT-measured mean HU intensity and volume were calculated for each PG relative to week 1. Dose-volume-histogram (DVH) statistics were extracted from each plan, and interactions between dose, volume, and intensity were investigated. Univariable analysis and penalized logistic regression were used to analyze association with observer-rated xerostomia at one year following HNR. Models including CBCT delta imaging features were compared to clinical/DVH-only models using area under the receiver-operating characteristic curve (AUC) for Grade 1 or higher (Grade≥1) and for Grade 2 or higher (Grade≥2) xerostomia prediction.
Results
All patients experienced end-treatment PG volume reduction with mean (range) ipsilateral and contralateral PG shrinkage of 19.6% (0.9-58.4%) and 17.7% (4.4-56.3%), respectively. Mid-treatment volume change was highly correlated with mean PG dose (r=−0.318, p<1e-6). Incidence of Grade≥1 and Grade≥2 xerostomia was 65% and 16%, respectively. For Grade≥1 xerostomia prediction, the delta-imaging model had AUC = 0.719 (95% CI: 0.603-0.830), compared to 0.709 (95% CI: 0.603-0.815) for the dose/clinical model. For Grade≥2 xerostomia prediction, the dose/clinical model had AUC = 0.692 (95% CI: 0.615-0.770), and the addition of contralateral PG changes modestly improved predictive performance with AUC = 0.776 (0.643-0.912).
Conclusions
The rate of CBCT-measured PG image feature changes improves prediction for chronic xerostomia prediction over dose alone. Analysis of CBCT images acquired for treatment positioning may provide an inexpensive monitoring system to support toxicity-reducing adaptive radiotherapy.
Keywords: CBCT, dose response, adaptive radiotherapy, xerostomia prediction
1. Introduction
Xerostomia is a frequent complication following head and neck cancer (HNC) radiotherapy, affecting between 60-90% of patients (1, 2), and can significantly impact long-term quality of life (3, 4). Substantial technical advancements in radiation delivery, such as intensity modulated radiotherapy (IMRT), have lowered the risk of xerostomia by limiting dose to the salivary glands without compromising treatment efficacy (5–7). Despite such advancements, persistent elevated rates of radiation-induced toxicities, often exacerbated by the use of concurrent systemic therapies, have motivated radiotherapy dose de-intensification regimens in specific HNC patient cohorts (8, 9). As treatment regimens become more personalized, it becomes more important to form accurate risk assessments. Additionally, with the establishment of novel streamlined radiotherapy planning and delivery modalities (10–12), the ability to adapt treatments based on physiological changes during the course of treatment is now practical (13, 14). However, existing normal-tissue complication probability (NTCP) models are based on planned biological dose-volume characteristics, which do not adequately address inter-patient or intra-treatment variation.
Advanced imaging techniques, such as diffusion-weighted magnetic resonance (MR) imaging, have demonstrated the ability to non-invasively characterize parotid glands (PGs) before and after radiotherapy, and correlation with physiological function as been shown (15). MR sialography has also been used to directly visualize radiation injury for quantitative xerostomia severity prediction (16). While these studies demonstrate that physiological changes to PGs can be visualized and are important for xerostomia prediction, the clinical utility of such imaging is limited. Specifically, the ideal imaging frequency during the course of radiotherapy has not been established and it is unlikely to be feasible in the general clinical setting, where access to MR scanners remains scarce.
Conversely, conventional imaging techniques such as computed tomography (CT) and ultrasound have also been shown to provide a physiological assessment of PG changes during radiotherapy (17–21). Using serial CT imaging, Feng et al. (22) and Barker et al. (23) independently showed that Hounsfield unit (HU) changes occur in gross tumor volumes (GTVs) and PGs during the course of radiotherapy. Using weekly diagnostic CT, Belli et al. (24) showed that the rates of PG density and volume change (reduction in both) were correlated with acute onset xerostomia. Further, they showed that these changes occurred early into treatment, which presents an opportunity for adaptive radiotherapy. van Dijk et al. (25) showed that PG surface area changes imaged using diagnostic CT before and 6 weeks following radiotherapy were predictive of chronic xerostomia. Fiorino et al. (26) demonstrated a significant decrease in parotid gland density (mean 5.6 HU loss) for a majority of patients. It was hypothesized that these changes are a result of acinar cell loss with a replacement of fatty tissue components, leading to average density reduction (24, 26). Still, the addition of extra imaging sessions is not ideal, as it adds cost and time to patient treatments, and the optimal imaging frequency is unknown (i.e., whether the rate of PG change is essential for accurate prediction). Since daily on-board kV cone-beam CT (CBCT) is routinely used for HNC-IMRT patient alignment (27–29), we sought to investigate whether serial CBCT imaging could provide an accurate measure of dose response and improve chronic xerostomia prediction over dose-only models. The primary aims of this study are to 1) assess whether previously shown radiation-induced volume and HU changes can be reproduced using CBCT imaging and 2) whether these features can add value to existing clinical factors for long-term xerostomia prediction. A CBCT-based assessment of dynamic radiation-induced changes is readily implementable and offers a straightforward scheme for adaptive radiotherapy in a wide range of clinical settings, with minimal extra cost. If shown feasible, the use of CBCT for frequent assessment of radiation-induced changes may be possible in a variety of body locations.
2. Materials and Methods
2. A. Patients
This study was approved by an institutional review board (IRB# HUM00119720). Of 128 patients considered, the analyzed cohort consisted of 119 patients treated with HNC volumetric modulated arc therapy (VMAT) with daily CBCT imaging between July 2013 and July 2016. Exclusion criteria included patients who received prior radiotherapy to the head or neck (N=3), the non-availability of CBCT imaging over the full treatment course (N=2), or patients for whom at least 50% of either PG was outside the CBCT field-of-view (N=4). For xerostomia outcome modeling, inclusion criteria further required a record of a follow-up visit within one month of the one-year anniversary of radiotherapy completion, during which salivary function was prospectively graded by the treating physician according to RTOG criteria. The resulting cohort of 105 patients was analyzed.
2. B. Imaging and treatment protocol
Planning CT (pCT) images were acquired using a CT simulator (Big Bore Brilliance 16-slice, Phillips Healthcare Co., Andover, MA). Normal tissue structures contoured for treatment planning, including the right and left PGs, were retrospectively analyzed. All contours and plans were reviewed by a radiation oncologist with over 25 years’ experience treating HNCs with radiotherapy (AE). The right and left PG dose-volume histograms (DVHs) were extracted from each patient’s 6MV VMAT plan totaling 60 Gy or 70 Gy in 2 Gy fractions over 6 or 7 weeks. Inverse planning objective functions included a mean dose of <22 Gy for each PG. Plan preparation was performed using a commercial treatment planning system (Eclipse, Varian Medical Systems, Palo Alto, CA), and patients were treated on one of three institutional linear accelerators (C-Series, Varian Medical Systems, Palo Alto, CA) with on-board kV imaging systems (OBI). Daily CBCT images were taken using the Standard Dose Head protocol (100 kV and 20 mA tube current in full-fan mode). All CBCT images had in-plane voxel resolution of 0.65 × 0.65 mm and 1 mm or 2.5 mm slice thickness. Patients were aligned using on-board co-registration of the planning CT and kV CBCT images. All CBCT images had been transferred to our ARIA oncology information system (Varian Medical Systems, Palo Alto, CA), where they were subsequently processed and analyzed for the purposes of this work.
2. C. CBCT Quality Assurance and Stability
Institutional QA procedures for ensuring Hounsfield unit (HU) constancy, uniformity, and noise were based on AAPM TG-142 (30) and AAPM TG-179 (31) guidelines and implemented over the time of the cohort’s treatments. Specifically, CT imaging quality assurance phantoms (CatPhan, The Phantom Laboratory, Salem, NY) were regularly scanned to ensure that HU measurements of known materials were consistent within manufacturer recommendations. In addition, daily CBCT alignment accuracy was verified by a qualified medical physicist each day a machine was used for patient treatments requiring CBCT imaging. Since the primary intent of these QA procedures is verification of positional accuracy, versus quantitative CT number measurements, the daily CBCT HU measurement reproducibility was further studied. For this purpose, daily CBCT images of an acrylic cube phantom taken over the course of a year, totaling 630 image volumes (212 from OBI 1, 239 from OBI 2, and 179 from OBI 3), were analyzed. Images with acquisition parameters deviating from the institutional QA technique (120 kVp, 80 mA, 1 mm slice thickness) or with inappropriate fan filter were excluded. Mean CT number and standard deviation within a 1 × 1 × 1 cm3 VOI automatically selected inside the 5 × 5 × 5 cm3 phantom were calculated from these image volumes. The standard error and corresponding 95% confidence interval were calculated for global and OBI-specific image metrics to assess the baseline noise of serial CBCT measurements. This noise level estimates CBCT HU stability and the minimum change required to detect a true change in physical density.
2. D. Patient demographics, follow-up and clinical outcomes
Patient characteristics, including age, sex, smoking history, primary tumor site, stage, systemic therapy, relevant surgical history, and neck treatment laterality are tabulated in Table 1. Baseline salivary function was assessed by a physician at the first treatment visit, and no patient had reported baseline xerostomia symptoms. Patients were seen for regular follow-up visits every 3 months after radiotherapy completion. Follow-up documentation taken between 11 and 13 months after radiotherapy was analyzed for information regarding xerostomia, and physician-graded xerostomia was scored using RTOG criteria (Grades 1 to 3). The number of patients with Grade 1, 2, and 3 RTOG xerostomia persistent at one-year was 51 (49%), 15 (14%), and 2 (2%), respectively, for this cohort, which agreed well with historical experience in our institution since the adoption of IMRT for HN cancers.
Table 1:
Patient Characteristics (N = 108). SD = standard deviation. Patients were staged according to the 7th Edition of the American Joint Committee on Cancer Staging Manual.
| Age at diagnosis, years | |
| Mean (SD) | 58.6 (8.8) |
| Median (range) | 57 (39-84) |
| Female sex | 19 (17.6%) |
| Smoking history | |
| Never | 45 (41.7%) |
| Former* | 32 (29.6%) |
| Current | 31 (28.7%) |
| Mean pack years (SD) | 20.6 (26.9) |
| Median pack years (range) | 10 (0-150) |
| Primary tumor site | |
| Oropharynx | 65 (60.2%) |
| Oral cavity | 16 (14.8%) |
| Larynx | 16 (14.8%) |
| Hypopharynx | 4 (3.7%) |
| Nasopharynx | 3 (2.8%) |
| Unknown primary | 3 (2.8%) |
| Synchronous oropharynx and larynx | 1 (0.9%) |
| T classification | |
| x | 3 (2.8%) |
| is | 1 (0.9%) |
| 1 | 23 (21.3%) |
| 2 | 31 (28.7%) |
| 3 | 14 (13.0%) |
| 4 | 36 (33.3%) |
| N classification | |
| 0 | 14 (13.0%) |
| 1 | 12 (11.1%) |
| 2 | 59 (54.6%) |
| 3 | 23 (21.3%) |
| Group stage | |
| 0 | 1 (0.9%) |
| I | 1 (0.9%) |
| II | 5 (4.6%) |
| III | 12 (11.1%) |
| IV | 89 (82.4%) |
| Concurrent systemic therapy** | |
| Yes | 90 (83.3%) |
| Head and neck surgery | |
| Prior to RT or within 1 year of RT | 34 (31.5%) |
| Prior to RT with removal of major salivary gland | 11 (10.2%) |
| Within 1 year of RT with removal of major salivary gland | 0 |
| Bilateral neck treatment | |
| Yes | 98 (90.7%) |
Former smoking status defined as having quit smoking at least 1 year prior to cancer diagnosis.
Concurrent systemic therapy refers to cytotoxic chemotherapy and cetuximab.
2. E. Image processing and analysis
2. E.I. Contour propagation
Deformable alignment (SmartAdapt, Varian Medical Systems, Palo Alto, CA) was used to register the pCT to CBCT images. For initial registration, a rigid downhill simplex optimization was used for maximizing mutual information between the two image sets. A “demons”-like deformable image registration (DIR) algorithm was subsequently applied (32). Deformed structure sets, including the right and left PG contours, were transferred to CBCT images and visually inspected for accuracy. For the majority of patients (N=80) CBCTs from every fraction were used; and for the remaining patients at least 2 fractions were included from each treatment week, totaling 3558 deformed contour propagations.
To evaluate the accuracy of the DIR algorithm used in this study, propagated parotid contours for a representative subset of the patient cohort (N=10) were compared to physician drawn contours. Parotid glands were manually delineated by a physician on CBCT images from three treatment fractions (3rd, 15th, and 30th) for each patient. The structures were propagated using the deformable vector fields, then transferred to a commercial tool (Velocity, v3.2, Varian Medical Systems, Palo Alto CA) for calculation of Dice similarity coefficient (DSC), mean distance-to-agreement (MDA), and standard deviation of the MDA (MDA SD) between the DIR-propagated contours and manually delineated contours, in accordance with AAPM TG-132 (33) recommendations.
2. E. II. Patient CBCT image harmonization
Small but measureable daily and OBI-dependent variation in phantom images was initially observed, motivating development of a CBCT image harmonization strategy. For this purpose, the mean HU within a 4 cc VOI placed in posterior neck muscle (medial nape region) within each patient image was used to linearly re-scale CBCT voxel values (using nominal HU values for air and muscle of −1000 and 40, respectively). The medial nape region was used since its physical density is assumed to remain unchanged over a treatment course due to relatively low dose in typical HN VMAT treatments. The linear transform normalizes each patient image using a stable anatomic landmark and, thus, the image HU better approximates relative physical density. Although a bilinear transform is commonly applied (34, 35), our analysis is limited to parotid tissue with fatty components and mean HU values typically near or slightly less than zero, where CT-to-density conversion is nearly linear.
2. E.III. Intensity and DVH metric extraction
Three dimensional image intensity profiles in their native voxel resolution from each CBCT PG and the pCT were extracted for all patients using a batch-processed Eclipse API plugin written in-house, and PG volume was calculated. After harmonization, voxels outside −200 to 0 HU were excluded and the mean HU of remaining voxels within each PG was calculated. This intensity range helps mitigate the effects of contour propagation variation and reduces non-PG tissues, calcifications, and artifacts included in the contour. DVH statistics were extracted for each patient from the initial treatment plan if the patient had a single plan over the full treatment course or from the as-treated plan sum if there were any treatment modifications, such as a re-plan or coned-down treatment boost. Five DVH metrics were used in subsequent analysis for the ipsilateral and contralateral gland: mean physical dose and equivalent dose in 2 Gy fractions (EQD2) with α/β = 2.5 Gy, and the volumes receiving at least 15, 30, and 45 Gy (V15, V30 and V45), as these metrics have been shown to correlate with salivary production (36, 37). All DVH metrics were used for investigation of CBCT-measured dose-response and to build a baseline late-xerostomia prediction model.
2.E.IV. Dynamic modeling
To reduce dimensionality for modeling purposes, PG measurements were discretized by averaging over each treatment week. For volume the percentage change in cc and for mean intensity the absolute HU change was calculated relative to baseline (week 1 mean). In this way, treatment-related changes were determined over time on an average weekly basis, while still incorporating information from each available fraction. An example of dynamic feature profiling is shown in Supplementary material (Item 1) for a representative patient’s volume change over the treatment course.
2. F. Statistical analysis
2. F. I. Dose-response characterization
To test the hypothesis that CBCT-measured PG features are influenced by dose, Spearman rank inter-correlation was calculated for all DVH and imaging features. Linear regression was applied to estimate the effect magnitude (slope) of dose on a subset of statistically significant imaging features in pair-wise rank correlation (p<0.05).
2. F. II. Xerostomia prediction
Association between one-year xerostomia of any grade and each of the clinical factors was assessed by Fisher’s exact test or two-sample t-test, as appropriate, and any clinical variables with statistically significant association (p<0.05) were saved for further analysis. Univariable association between average weekly HU change and outcome was assessed by two-sample t-test, and test p-values less than 0.05 were considered statistically significant. Because inter-correlation between dose, volume, and mean intensity change was expected, penalized logistic regression using the least absolute shrinkage and selection operator (LASSO) was used on the combination of all measured features for xerostomia prediction. To avoid overfitting, the minimum deviance of 10-fold cross validation was used for lambda selection. Receiver-operating characteristic (ROC) analysis was performed, and the area under the curve (AUC) and 95% confidence intervals from 2000 bootstrap replicates were calculated. Internal validation using bootstrapping was performed to correct for optimism of the apparent model according to TRIPOD guidelines (38). The corrected AUC (AUCcorrected) was calculated as the difference between the apparent AUC and the average optimism of the 100 bootstrap models. These methods were repeated for DVH and clinical input variables only (DVH/clinical model), and AUCs were compared. These statistical methods were performed separately for Grade 1 or higher (Grade≥1) and Grade 2 or higher (Grade≥2) one-year xerostomia prediction.
3. Results
3.A. CBCT HU measurement QA and stability
Mean (95% CI, standard deviation) acrylic phantom intensity measurement for all OBI systems combined was 120.0 HU (119.2-120.7 HU, 7.8 HU), indicating rather stable CBCT intensity in standard measurement conditions. Individual metrics for the 3 OBIs were 125.2 HU (124.4-126.0 HU, 5.1 HU), 116.3 HU (115.1-117.5 HU, 6.6 HU), and 115.1 HU (113.5-116.6 HU, 7.8 HU), indicating slight variations in the absolute output due to the particular OBI. Assuming normal distribution, these results indicate a baseline 95% CI of 2 to 3 HU for 15 to 30 serial CBCT images on a random assortment of OBIs. To reduce this potentially confounding effect, we applied a CBCT harmonization strategy using intra-patient normalization and weekly HU averaging described in sections 2. E. II-IV. After harmonization the standard deviation of the mean global PG intensity distributions and OBI-dependent differences decreased, as shown in Supplementary material (Item 2).
3.B. Contour propagation accuracy
The DIR algorithm demonstrated good performance with population average registration metrics of 0.83 (DSC), 1.7 mm (MDA), and 1.8 mm (MDA SD). Comparison of registration results using a Welch T-test between ipsilateral and contralateral PGs demonstrated slight but significant differences for MDA (0.3 mm, p = 0.013) and MDA SD (0.7 mm, p < 0.01), but not for DSC (0.04, p = 0.14). There were no fraction-dependent trends in DIR metrics. Box plots describing all registration metrics are shown in Supplementary material (Item 3).
3.C. Dose-response modeling
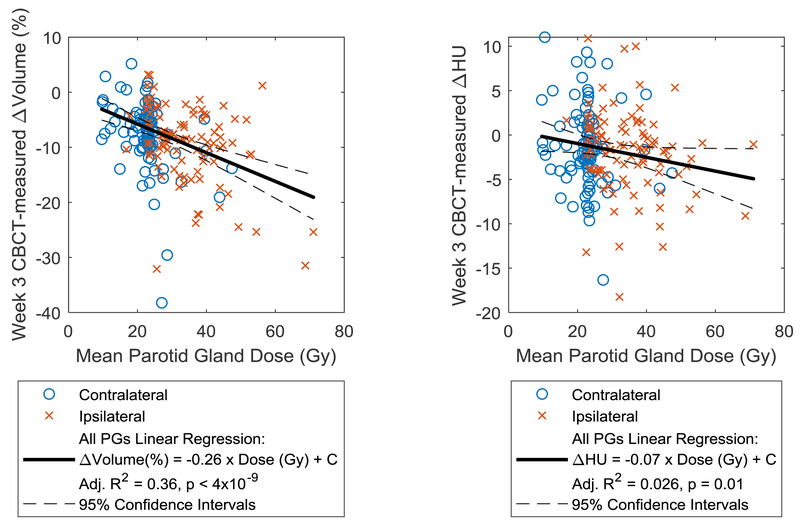
All patients experienced PG volume loss by the end of radiotherapy, with median (min-max) ipsilateral and contralateral shrinkage of 19.6 % (0.9 – 58.4%) and 17.7 % (4.4 – 56.3%), respectively. Loss of mid-treatment CT density was evident in most patients, with mean (95% CI) ipsilateral and contralateral loss of 3.3 HU (1.8 – 4.8 HU) and 4.6 HU (2.8 – 6.5 HU), respectively. A heat map indicating statistically significant Spearman rank correlations (Rs) for all features is shown in Supplementary material (Item 4). All ipsilateral DVH metrics were correlated with week 3 and 4 ipsilateral volume change, with the strongest correlation found between ipsilateral mean dose and week 3 volume change (Rs = −0.35, p < 6e-4). Most of the contralateral dose metrics were correlated with contralateral volume changes at any week, with contralateral V45 most strongly correlated with week 3 volume change (Rs = −0.44, p < 2e-6). Ipsilateral DVH metrics were weakly correlated with ipsilateral intensity change, with strongest correlation between mean dose and week 2 intensity change (Rs = −0.29, p = 0.02). Contralateral dose was generally not correlated with intensity changes. Using linear regression with mean PG dose, the magnitude of week 3 ipsilateral and contralateral volume loss was 0.31 %/Gy (95%CI: 0.19-0.43 %/Gy) and 0.31 %/Gy (95%CI: 0.15-0.46 %/Gy), respectively. For all parotid glands combined, the magnitude was 0.26 %/Gy (95%CI: 0.18-0.35 %/Gy). For ipsilateral intensity loss, the dose effect magnitude was 0.09 HU/Gy (95%CI: 0.02-0.18 HU/Gy). Regression plots and fit statistics are shown in Figure 1.
Figure 1:
Week 3 CBCT-measured change in volume (left) and intensity (right) as a function of dose and associated linear regression fits, indicating a moderate correlation of dose with volume change and weak correlation with intensity changes
3.D. Xerostomia outcome prediction
3.D.I. Imaging findings
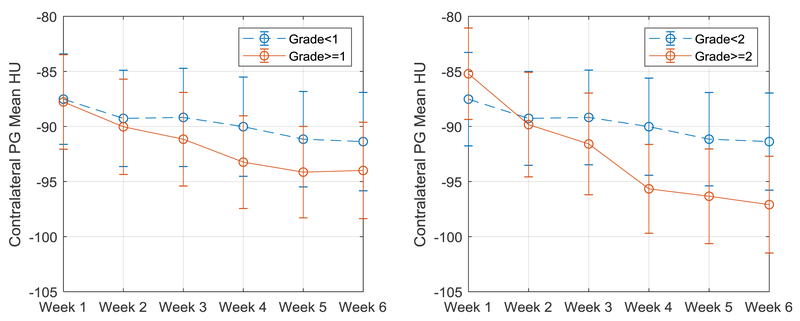
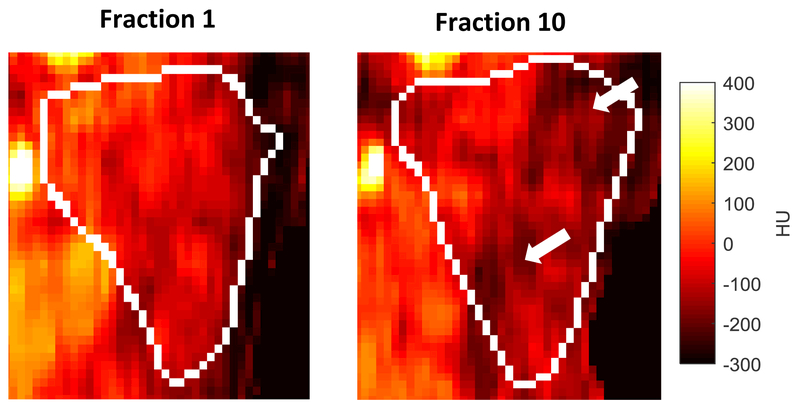
Figure 2 shows the average weekly contralateral HU measurement for all patients, stratified by whether or not they experienced Grade≥1 or Grade≥2 toxicity. Similar plots for ipsilateral HU and bilateral volume are shown in Supplementary material (Item 5). Example CBCT PG images taken from fractions 1 and 10 (end of week 2) are shown in Figure 3 for a representative patient with chronic (1-year) xerostomia, showing regions with marked density loss.
Figure 2:
Mean contralateral intensity (HU) over time across all patients stratified by (left) Grade≥1 (+N=68, −N=37) and (right) Grade≥2 (+N=88, −N=17) one-year xerostomia. Error bars represent 1 standard deviation of the mean.
Figure 3:
Example coronal CBCT ipsilateral parotid gland images for a representative patient with 1-year chronic xerostomia on the same color window (−300 to 400 HU). Note loss of high density regions between fraction 1 (left) and fraction 10 (right) which would be in accordance with mean intensity loss
3.D.II. Univariable analysis
The incidence of chronic Grade≥1 and Grade≥2 xerostomia at one year was 65% and 16%, respectively. The single clinical variable that reached statistical significance was the primary site of oropharyngeal cancer (OPC) with p<0.001 and p<0.002 in Fisher’s exact test for Grade≥1 and Grade≥2, respectively. For Grade≥1, all ipsilateral DVH features were significant, but none of the DVH metrics were associated with Grade≥2. Volume change was also not independently associated with either clinical endpoint. Week 4, 5, and 6 contralateral mean intensity loss were all associated with Grade≥2 (p= 0.006, 0.022, and 0.030, respectively). Week 3 ipsilateral and week 4 contralateral intensity loss trended toward but did not reach statistical significance for Grade≥1 (p= 0.086 and 0.101, respectively). Detailed univariable results are shown for mean intensity changes in Table 2 and for all tested variables in Supplementary material (Item 6).
Table 2:
Weekly averaged ipsilateral and contralateral parotid gland mean change in CBCT-measured HU (relative to week 1) and t-test p-value for grade≥1 and grade≥2 xerostomia with significance level p<0.05
| Ipsilateral Mean ΔHU | Contralateral Mean ΔHU | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | Grade<1 (N=37) |
Grade≥1 (N=68) |
p | Grade<2 (N=88) |
Grade≥2 (N=17) |
p | Grade<1 (N=37) |
Grade≥1 (N=68) |
p | Grade<2 (N=88) |
Grade≥2 (N=17) |
p |
| 2 | −1.40 | −2.13 | 0.469 | −2.02 | −1.20 | 0.525 | −1.49 | −1.31 | 0.858 | −1.22 | −2.17 | 0.437 |
| 3 | −1.90 | −4.20 | 0.086 | −3.59 | −2.35 | 0.474 | −1.23 | −2.90 | 0.239 | −1.96 | −4.02 | 0.257 |
| 4 | −2.32 | −3.73 | 0.410 | −3.54 | −1.77 | 0.416 | −1.88 | −4.58 | 0.101 | −2.70 | −8.43 | 0.006 |
| 5 | −2.88 | −3.57 | 0.666 | −3.24 | −3.73 | 0.812 | −3.33 | −5.38 | 0.297 | −3.70 | −9.39 | 0.022 |
| 6 | −3.18 | −2.92 | 0.875 | −3.11 | −2.54 | 0.785 | −4.70 | −5.82 | 0.552 | −4.57 | −9.72 | 0.030 |
| 7 | −4.53 | −3.81 | 0.706 | −3.99 | −4.40 | 0.860 | −4.11 | −6.23 | 0.340 | −4.59 | −9.64 | 0.064 |
3.D.III. Penalized logistic regression models
Using LASSO regularized regression with only DVH and clinical inputs, OPC and V30 were retained for the Grade≥1 model with an AUC of 0.709 (0.603-0.815) (AUCcorrected = 0.643). When imaging features were included, OPC, week 5 ipsilateral volume, and week 3 intensity change were selected, with an AUC of 0.719 (0.603-0.830) (AUCcorrected = 0.649). For Grade≥2 xerostomia, the optimal LASSO model contained only OPC with AUC of 0.692 (0.615-0.770) (AUCcorrected = 0.607). Adding imaging variables to the LASSO search produced an optimal model containing OPC, week 4 contralateral intensity change and week 6 ipsilateral volume change with an AUC of 0.776 (0.643-0.912) (AUCcorrected = 0.688). Plots of the ROC curve and corresponding 95% CI are shown in Supplementary material (Item 7), and model details are shown in Table 3, for both Grade≥1 and Grade≥2 xerostomia prediction.
Table 3:
Model characteristics for 1 yr. xerostomia prediction
| LASSO Model | Origin | WK | Feature | Normalized Coefficient |
AUC (95% CI) | AUCcorrected |
|---|---|---|---|---|---|---|
| 1 yr. Grade ≥ 1 xerostomia | ||||||
| DVH/Clinical | I | 0 | V30 | 0.137 | 0.709 (0.603-0.815) | 0.643 |
| n/a | 0 | OPC | 0.378 | |||
| I | 3 | Δ Mean HU | −0.412 | |||
| DVH/Clinical + Imaging | I | 5 | Δ Volume | 0.368 | 0.719 (0.603-0.830) | 0.649 |
| n/a | 0 | OPC | 0.357 | |||
| 1 yr. Grade ≥ 2 xerostomia | ||||||
| DVH/Clinical | n/a | 0 | OPC | 0.782 | 0.692 (0.615-0.770) | 0.607 |
| C | 4 | Δ Mean HU | −0.297 | |||
| DVH/Clinical + Imaging | I | 6 | Δ Volume | 0.148 | 0.776 (0.643-0.912) | 0.688 |
| n/a | 0 | OPC | 0.455 | |||
OPC = Oropharyngeal primary tumor. AUCcorrected = AUC corrected for optimism using internal bootstrap validation
Imaging for head and neck radiotherapy (HNR) normal tissue treatment response typically requires advanced techniques and/or extra sessions, limiting wide adoption and clinical utility. Parotid glands were delineated on daily CBCT images during the course of HNR for 105 patients who had xerostomia grade scored at one year following treatment. CBCT-measured dynamic intensity changes were associated with chronic xerostomia, with improved prediction over dose alone, which may have profound implications for adaptive radiotherapy.
4. Discussion
To our knowledge, this is the first report on the quantitative use of CBCT image analysis for modeling normal tissue changes during the course of radiotherapy for the purpose of toxicity prediction in HNC. Other investigators have shown that imaging-based changes measured using in-room CT (18, 23), or within additional MR (15) or CT imaging sessions (20, 24, 39), are descriptive of PG changes and may quantify xerostomia risk. However, the method presented here can be readily applied in a number of centers already employing on-board kV CBCT. Additionally, our findings could be prospectively incorporated into personalized radiotherapy trials where intensity changes in the parotid may be used to further limit dose to the affected glands in oropharynx response-adapted trials.
Our characterization of CBCT voxel intensity showed that under standard measurement conditions, the CBCT stability is on the order of a few HUs. Although patient imaging invariably increases image noise, the use of serial images (weekly averaging) and anatomical normalization can mitigate the effect of slight variations in absolute HU measurements. Further, since the relative location of the parotid glands and the normalization regions are highly similar among patients, the effects of geometric CBCT intensity variation due inter-patient scatter differences (40, 41) are likely minimal.
Our population average DIR metrics of 0.83 (DSC) and 1.9 (MDA) are consistent with previous investigations of deformable PG contour propagation to CBCTs (42–44). These metrics are have been shown to be representative of inter-observer variability of CT-based PG contouring (45). Further, these fall within with the suggested tolerances of AAPM TG-132 [Table III in ref. (33)] of 0.8 (DSC) and 3 mm (MDA), which demonstrates confidence in using this DIR method for automated PG delineation on CBCT imaging. The use of deformable registration for PG contour propagation has shown to be adequate in previous work (42, 46, 47), and we believe this study provides preliminary validation for its use in a novel application of serial CBCT imaging for xerostomia prediction.
Nearly all patients experienced PG volume and density loss during the course of radiotherapy, but the response dynamics and characteristics varied among patients. In agreement with other investigations, a correlation between dose and volume change was found (18, 19). Further, the magnitude of volume and HU changes over the course of radiotherapy were in agreement with previous CT imaging studies (22, 26), which indicates coherence between imaging modalities for measuring in-vivo density changes. In the context of previous during-treatment imaging studies, it was somewhat surprising that no volumetric features were directly associated with chronic xerostomia though they were strongly associated with dose. In their study on the use of weekly diagnostic CT imaging to predict xerostomia, Belli et al. (24), reported that early rates of PG volume and density change are important predictors of xerostomia. However, the investigators used acute xerostomia as the endpoint. As such, it is possible that volume changes are more temporary and recoverable over time than severe changes in internal gland anatomy, possibly quantified by the rate of density change. Further investigation is warranted to substantiate this claim.
Although most patients experienced loss of density for both PGs over the treatment course, Grade 2 or higher xerostomia was marked by a higher rate of intensity loss of the contralateral PG specifically. However, contralateral intensity changes had little association with dose, which suggests that patient-specific PG radiation sensitivity may play a role in late-xerostomia. This finding would be in accordance with other studies which indicate that preservation of a single PG reduces the risk of xerostomia (48, 49). In a result that is well-suited for adaptive radiotherapy, the Grade≥2-associated features are derived early during radiotherapy.
The Grade≥1 model with imaging had only marginal performance improvement over the clinical/DVH-only model. This could have been due to the relatively low number of patients with no xerostomia in addition to the presence of two variables (OPC and ipsilateral dose) directly associated with this endpoint. Further investigation with a larger, more controlled cohort is warranted. However, the clinical relevance of Grade 1 toxicity prediction should also be considered. In accordance with Gabryś et al (50), no association between PG dose and late Grade≥2 xerostomia was observed, limiting the discriminative performance of the clinical/DVH-only model. The modest improvement in Grade≥2 prediction by including mean intensity loss is thought to reflect a measured increase in fatty (i.e., non-functional) parotid tissue, which would be in accordance with the observations of previous investigators (21, 24, 26, 39). Since ipsilateral parotid dose was independently predictive of low-grade xerostomia, it is expected that dose adaptation (i.e., further PG dose reduction) based on CBCT-derived imaging characteristics would reduce the risk for chronic xerostomia on a patient-specific level. A prospective study which includes serial multi-modality imaging (e.g., fan-beam CT, multi-parametric MRI, or 18FDG-PET (51)) in conjunction with CBCT is warranted to test these hypotheses and help inform the roles of CBCT and deformable registration in quantitative imaging. As on-board imaging becomes more refined and commonplace, a plethora of high-quality image data is likely to be available at a large number of radiotherapy centers. Such data facilitates rapid external validation and center-specific xerostomia model calibration.
The current methodology is not without limitations. First, underlying CBCT image quality is reduced relative to diagnostic CT imaging. CBCT image quality improvement is an active area of research, and substantial advancements have recently been made (52–55). We believe that the frequent imaging, harmonization, and use of relative HU changes associated with our methodology helped alleviate underlying image quality issues. Yet, even after harmonization, slight in differences in absolute HU measurements across OBIs were apparent, which suggests that future work is warranted to characterize these differences and to prospectively harmonize machine parameters (e.g., absolute HU calibration). Second, it is well-known that non-PG tissues, such as the submandibular glands and oral cavity, contribute to salivary function and play a role in radiation-induced xerostomia (56, 57). The higher association between OPC and xerostomia, relative to other primary tumor sites, is consistent with the fact that the OPC patients in our cohort were more likely to receive prescription doses to the retropharyngeal nodes, likely increasing nearby salivary gland doses. Further, a recent study by Miah et al. (58) suggests that dose to specific PG sub-volumes (lobes) may play a significant role in xerostomia prediction. In both cases, we believe that this work presents a framework for CBCT-based normal tissue characterization which can be adapted for other body sites or for clinically relevant sub-volumes. An extension of our study might include daily dose re-calculation using the daily image information as density input, which may improve DVH-only prediction of late xerostomia. Third, since a secondary goal of this work was to demonstrate feasibility for the use of CBCT-measured changes in an adaptive radiotherapy platform, clinical workflows and clinically released software were used whenever possible. As such, the DIR process was laborious and time-consuming, as each image pair required manual selection and initial coarse alignment. These issues have been addressed by the AAPM TG-132 (33), and we expect that better integration into clinical systems will arise as DIR becomes more widely used. Such advancements may offer efficient and accurate daily dose calculation, by incorporating daily changes in patient positioning and anatomy, which may improve DVH-based NTCP models (59). Further, apart from visual inspection and subset analysis, high-throughput quality control of propagated contours was limited. The use of deformable registration algorithms for CBCT is an active area of research, and significant variation in accuracy may be achieved, depending on the specific algorithms and regions-of-interest considered (42). Finally, physician reported xerostomia was used in our analysis, which we have previously shown to underestimate patient reported xerostomia quality of life (QOL) (60). It is possible that our results may have been more significant if patient-reported QOL was available for all patients in our cohort.
5. Conclusions
A methodology for the use of on-board CBCT to quantify treatment-related PG changes during the course of HNC radiotherapy has been presented. Early treatment CBCT-measured PG density changes were shown to be associated with long-term xerostomia and improved predictions over PG dose alone. Moreover, the CBCT analysis may be performed with minimal extra cost, which is attractive for a clinically feasibly adaptive radiotherapy platform.
Supplementary Material
6. Acknowledgements
The authors would like to thank Dr. Carlos Anderson and Dr. Martha Matuszak for the batch Eclipse API script used for DVH extraction. This work was supported by NIH grant: R01-CA184153-04.
Acknowledgements: This work was supported by NIH grant: R01-CA184153-04. The authors would also like to thank Dr. Carlos Anderson and Dr. Martha Matuszak of the Michigan Medicine Department of Radiation Oncology for the batch Eclipse API script used for DVH extraction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- 1.Jiang N, Zhao Y, Jansson H, et al. Experiences of xerostomia after radiotherapy in patients with head and neck cancer: a qualitative study. J. Clin. Nurs 2017. [DOI] [PubMed] [Google Scholar]
- 2.Dirix P, Nuyts S, Vander Poorten V, et al. The influence of xerostomia after radiotherapy on quality of life. Support. Care Cancer 2008;16:171–179. [DOI] [PubMed] [Google Scholar]
- 3.Boscolo-Rizzo P, Stellin M, Fuson R, et al. Long-term quality of life after treatment for locally advanced oropharyngeal carcinoma: surgery and postoperative radiotherapy versus concurrent chemoradiation. Oral Oncol. 2009;45:953–957. [DOI] [PubMed] [Google Scholar]
- 4.Verdonck-de Leeuw IM, Buffart LM, Heymans MW, et al. The course of health-related quality of life in head and neck cancer patients treated with chemoradiation: a prospective cohort study. Radiother. Oncol 2014;110:422–428. [DOI] [PubMed] [Google Scholar]
- 5.Chao KC, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int. J. Radiat. Oncol. Biol. Phys 2001;49:907–916. [DOI] [PubMed] [Google Scholar]
- 6.Kam MK, Leung S-F, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J. Clin. Oncol 2007;25:4873–4879. [DOI] [PubMed] [Google Scholar]
- 7.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly JR, Husain ZA, Burtness B. Treatment de-intensification strategies for head and neck cancer. Eur. J. Cancer 2016;68:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nevens D, Duprez F, Daisne JF, et al. Reduction of the dose of radiotherapy to the elective neck in head and neck squamous cell carcinoma; a randomized clinical trial. Effect on late toxicity and tumor control. Radiother. Oncol 2017;122:171–177. [DOI] [PubMed] [Google Scholar]
- 10.Chang AT, Hung AW, Cheung FW, et al. Comparison of planning quality and efficiency between conventional and knowledge-based algorithms in nasopharyngeal cancer patients using intensity modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys 2016;95:981–990. [DOI] [PubMed] [Google Scholar]
- 11.Hussein M, South CP, Barry MA, et al. Clinical validation and benchmarking of knowledge-based IMRT and VMAT treatment planning in pelvic anatomy. Radiother. Oncol 2016;120:473–479. [DOI] [PubMed] [Google Scholar]
- 12.Thomas E, Phillips H, Popple R, et al. Development of a Knowledge Based Model (RapidPlan) for Brain Metastasis Stereotactic Radiosurgery and Validation with Automated Non-coplanar Treatment Planning (HyperArc). Int. J. Radiat. Oncol. Biol. Phys 2017;99:E727–E728. [Google Scholar]
- 13.Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys 2012;83:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castelli J, Simon A, Louvel G, et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat. Oncol 2015;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dirix P, De Keyzer F, Vandecaveye V, et al. Diffusion-weighted magnetic resonance imaging to evaluate major salivary gland function before and after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys 2008;71:1365–1371. [DOI] [PubMed] [Google Scholar]
- 16.Wada A, Uchida N, Yokokawa M, et al. Radiation-induced xerostomia: objective evaluation of salivary gland injury using MR sialography. Am. J. Neuroradiol 2009;30:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryan RN, Miller RH, Ferreyro RI, et al. Computed tomography of the major salivary glands. Am. J. Roentgenol 1982;139:547–554. [DOI] [PubMed] [Google Scholar]
- 18.Lee C, Langen KM, Lu W, et al. Assessment of parotid gland dose changes during head and neck cancer radiotherapy using daily megavoltage computed tomography and deformable image registration. Int. J. Radiat. Oncol. Biol. Phys 2008;71:1563–1571. [DOI] [PubMed] [Google Scholar]
- 19.Sanguineti G, Ricchetti F, Thomas O, et al. Pattern and predictors of volumetric change of parotid glands during intensity modulated radiotherapy. Br. J. Radiol 2013;86:20130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scalco E, Fiorino C, Cattaneo GM, et al. Texture analysis for the assessment of structural changes in parotid glands induced by radiotherapy. Radiother. Oncol 2013;109:384–387. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Tridandapani S, Beitler JJ, et al. Ultrasound GLCM texture analysis of radiation-induced parotid-gland injury in head-and-neck cancer radiotherapy: an in vivo study of late toxicity. Med. Phys 2012;39:5732–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng M, Yang C, Chen X, et al. Computed Tomography Number Changes Observed During Computed Tomography–Guided Radiation Therapy for Head and Neck Cancer. Int. J. Radiat. Oncol 2015;91:1041–1047. [DOI] [PubMed] [Google Scholar]
- 23.Barker JL, Garden AS, Ang KK, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int. J. Radiat. Oncol. Biol. Phys 2004;59:960–970. [DOI] [PubMed] [Google Scholar]
- 24.Belli ML, Scalco E, Sanguineti G, et al. Early changes of parotid density and volume predict modifications at the end of therapy and intensity of acute xerostomia. Strahlenther. Onkol 2014;190:1001–1007. [DOI] [PubMed] [Google Scholar]
- 25.van Dijk LV, Brouwer CL, van der Laan HP, et al. Geometric Image Biomarker Changes of the Parotid Gland Are Associated With Late Xerostomia. Int. J. Radiat. Oncol. Biol. Phys 2017;99:1101–1110. [DOI] [PubMed] [Google Scholar]
- 26.Fiorino C, Rizzo G, Scalco E, et al. Density variation of parotid glands during IMRT for head-neck cancer: Correlation with treatment and anatomical parameters. Radiother. Oncol 2012;104:224–229. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Zhu XR, Zhang L, et al. Comparison of 2D radiographic images and 3D cone beam computed tomography for positioning head-and-neck radiotherapy patients. Int. J. Radiat. Oncol. Biol. Phys 2008;71:916–925. [DOI] [PubMed] [Google Scholar]
- 28.Den RB, Doemer A, Kubicek G, et al. Daily image guidance with cone-beam computed tomography for head-and-neck cancer intensity-modulated radiotherapy: a prospective study. Int. J. Radiat. Oncol. Biol. Phys 2010;76:1353–1359. [DOI] [PubMed] [Google Scholar]
- 29.Graff P, Kirby N, Weinberg V, et al. The residual setup errors of different IGRT alignment procedures for head and neck IMRT and the resulting dosimetric impact. Int. J. Radiat. Oncol. Biol. Phys 2013;86:170–176. [DOI] [PubMed] [Google Scholar]
- 30.Klein EE, Hanley J, Bayouth J, et al. Task Group 142 report: quality assurance of medical accelerators. Med. Phys 2009;36:4197–4212. [DOI] [PubMed] [Google Scholar]
- 31.Bissonnette J-P, Balter PA, Dong L, et al. Quality assurance for image-guided radiation therapy utilizing CT-based technologies: A report of the AAPM TG-179. Med. Phys 2012;39:1946–1963. [DOI] [PubMed] [Google Scholar]
- 32.Thirion J-P. Image matching as a diffusion process: an analogy with Maxwell’s demons. Med. Image Anal 1998;2:243–260. [DOI] [PubMed] [Google Scholar]
- 33.Brock KK, Mutic S, McNutt TR, et al. Use of Image Registration and Fusion Algorithms and Techniques in Radiotherapy: Report of the AAPM Radiation Therapy Committee Task Group No. 132 Med. Phys 2017. [DOI] [PubMed] [Google Scholar]
- 34.Saw CB, Loper A, Komanduri K, et al. Determination of CT-to-density conversion relationship for image-based treatment planning systems. Med. Dosim 2005;30:145–148. [DOI] [PubMed] [Google Scholar]
- 35.Yadav P, Ramasubramanian V, Paliwal B. Feasibility study on effect and stability of adaptive radiotherapy on kilovoltage cone beam CT. Radiol. Oncol 2011;45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int. J. Radiat. Oncol. Biol. Phys 2010;76:S58–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco AI, Chao KC, El Naqa I, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int. J. Radiat. Oncol. Biol. Phys 2005;62:1055–1069. [DOI] [PubMed] [Google Scholar]
- 38.Moons KGM, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med 2015;162:W1. [DOI] [PubMed] [Google Scholar]
- 39.van Dijk LV, Brouwer CL, van der Schaaf A, et al. CT image biomarkers to improve patient-specific prediction of radiation-induced xerostomia and sticky saliva. Radiother. Oncol 2017;122:185–191. [DOI] [PubMed] [Google Scholar]
- 40.Thing RS, Bernchou U, Mainegra-Hing E, et al. Patient-specific scatter correction in clinical cone beam computed tomography imaging made possible by the combination of Monte Carlo simulations and a ray tracing algorithm. Acta Oncol. Stockh. Swed 2013;52:1477–1483. [DOI] [PubMed] [Google Scholar]
- 41.Zhu L, Xie Y, Wang J, et al. Scatter correction for cone-beam CT in radiation therapy. Med. Phys 2009;36:2258–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Zhang Y, Shi Y, et al. Comprehensive evaluation of ten deformable image registration algorithms for contour propagation between CT and cone-beam CT images in adaptive head & neck radiotherapy. PLOS ONE. 2017;12:e0175906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hvid CA, Elstrøm UV, Jensen K, et al. Accuracy of software-assisted contour propagation from planning CT to cone beam CT in head and neck radiotherapy. Acta Oncol. 2016;55:1324–1330. [DOI] [PubMed] [Google Scholar]
- 44.Zhang T, Chi Y, Meldolesi E, et al. Automatic Delineation of On-Line Head-And-Neck Computed Tomography Images: Toward On-Line Adaptive Radiotherapy. Int. J. Radiat. Oncol 2007;68:522–530. [DOI] [PubMed] [Google Scholar]
- 45.Faggiano E, Fiorino C, Scalco E, et al. An automatic contour propagation method to follow parotid gland deformation during head-and-neck cancer tomotherapy. Phys. Med. Biol 2011;56:775. [DOI] [PubMed] [Google Scholar]
- 46.Castadot P, Lee JA, Parraga A, et al. Comparison of 12 deformable registration strategies in adaptive radiation therapy for the treatment of head and neck tumors. Radiother. Oncol 2008;89:1–12. [DOI] [PubMed] [Google Scholar]
- 47.Castadot P, Geets X, Lee JA, et al. Assessment by a deformable registration method of the volumetric and positional changes of target volumes and organs at risk in pharyngo-laryngeal tumors treated with concomitant chemo-radiation. Radiother. Oncol 2010;95:209–217. [DOI] [PubMed] [Google Scholar]
- 48.Eisbruch A, Ship JA, Martel MK, et al. Parotid gland sparing in patients undergoing bilateral head and neck irradiation: techniques and early results. Int. J. Radiat. Oncol. Biol. Phys 1996;36:469–480. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Eisbruch A. IMRT for head and neck cancer: reducing xerostomia and dysphagia. J. Radiat. Res. (Tokyo) 2016;57:i69–i75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabryś HS, Buettner F, Sterzing F, et al. Parotid gland mean dose as a xerostomia predictor in low-dose domains. Acta Oncol. 2017;56:1197–1203. [DOI] [PubMed] [Google Scholar]
- 51.van Dijk LV, Noordzij W, Brouwer CL, et al. 18F-FDG PET image biomarkers improve prediction of late radiation-induced xerostomia. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol 2018;126:89–95. [DOI] [PubMed] [Google Scholar]
- 52.Kawahara D, Ozawa S, Nakashima T, et al. Absorbed dose and image quality of Varian TrueBeam CBCT compared with OBI CBCT. Phys. Med 2016;32:1628–1633. [DOI] [PubMed] [Google Scholar]
- 53.Park JC, Song B, Kim JS, et al. Fast compressed sensing-based CBCT reconstruction using Barzilai-Borwein formulation for application to on-line IGRT. Med. Phys 2012;39:1207–1217. [DOI] [PubMed] [Google Scholar]
- 54.Ren L, Yin F-F, Chetty IJ, et al. Feasibility study of a synchronized-moving-grid (SMOG) system to improve image quality in cone-beam computed tomography (CBCT). Med. Phys 2012;39:5099–5110. [DOI] [PubMed] [Google Scholar]
- 55.Jin J-Y, Ren L, Liu Q, et al. Combining scatter reduction and correction to improve image quality in cone-beam computed tomography (CBCT). Med. Phys 2010;37:5634–5644. [DOI] [PubMed] [Google Scholar]
- 56.Hawkins PG, Lee JY, Mao Y, et al. Sparing all salivary glands with IMRT for head and neck cancer: Longitudinal study of patient-reported xerostomia and head-and-neck quality of life. Radiother. Oncol 2017. [DOI] [PubMed] [Google Scholar]
- 57.Redman RS. Histologic Changes in the Salivary Glands Following Radiation Therapy In: Salivary Gland Development and Regeneration Springer; 2017:75–91. [Google Scholar]
- 58.Miah AB, Gulliford SL, Morden J, et al. Recovery of Salivary Function: Contralateral Parotid-sparing Intensity-modulated Radiotherapy versus Bilateral Superficial Lobe Parotid-sparing Intensity-modulated Radiotherapy. Clin. Oncol 2016;28:e69–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shelley LEA, Scaife JE, Romanchikova M, et al. Delivered dose can be a better predictor of rectal toxicity than planned dose in prostate radiotherapy. Radiother. Oncol 2017;123:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meirovitz A, Murdoch-Kinch CA, Schipper M, et al. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys 2006;66:445–453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.