Abstract
Melanoma represents a significant challenge in cancer treatment due to the high drug resistance of melanomas and the patient mortality rate. This study presents data indicating that nanomolar concentrations of the hormonally active form of vitamin D, 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], its non-calcemic analogues 20S-hydroxyvitamin D3 and 21-hydroxypregnacalciferol, as well as the low-calcemic synthetic analog calcipotriol, modulate the efficacy of the anticancer drugs cisplatin and dacarbazine. It was observed that vitamin D analogs sensitized melanoma A375 cells to hydrogen peroxide used as an inducer of oxidative stress. On the other hand, only 1α,25(OH)2D3 resulted in a minor, but significant effect on the proliferation of melanoma cells treated simultaneously with dacarbazine, but not cisplatin. Notably, cisplatin (300 µM) exhibited a higher overall antiproliferative activity than dacarbazine. Cisplatin treatment of melanoma cells resulted in an induction of apoptosis as demonstrated by flow cytometry (accumulation of cells at the subG1 phase of the cell cycle), whereas dacarbazine caused G1/G0 cell cycle arrest, with the effects being improved by pre-treatment with vitamin D analogs. Treatment with cisplatin resulted in an initial increase in the level of reactive oxygen species (ROS). Dacarbazine caused transient stimulation of ROS levels and the mitochondrial membrane potential (Δψm) (after 1 or 3 h of treatment, respectively), but the effect was not detectable following prolonged (24 h) incubation with the drug. Vitamin D exhibited modulatory effects on the cells treated with dacarbazine, decreasing the half maximal inhibitory concentration (IC50) for the drug, stimulating G1/G0 arrest and causing a marked decrease in Δψm. Finally, cisplatin, dacarbazine and 1α,25(OH)2D3 displayed modulatory effects on the expression of ROS and vitamin D-associated genes in the melanoma A375 cells. In conclusion, nanomolar concentrations of 1,25(OH)2D3 only had minor effects on the proliferation of melanoma cells treated with dacarbazine, decreasing the relative IC50 value. However, co-treatment with vitamin D analogs resulted in the modulation of cell cycle and ROS responses, and affected gene expression, suggesting possible crosstalk between the signaling pathways of vitamin D and the anticancer drugs used in this study.
Keywords: vitamin D, vitamin D analogs, hydroxyvitamin D, melanoma, reactive oxygen species, oxidative stress, cisplatin, dacarbazine, chemotherapy
Introduction
1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], or vitamin D, is a lipid-soluble secosteroid produced by skin subjected to ultraviolet (UV)B radiation (1-3). Apart from its widely known beneficial role in the regulation of calcium homeostasis, vitamin D exerts pleiotropic effects, including regulation of the cell cycle, proliferation, differentiation and apoptosis (4-6). The active forms of vitamin D are important in the protection against DNA damage (7-9) and UVB-induced carcinogenesis in the skin (10-15). An inverse correlation between the concentration of vitamin D in serum and total cancer incidence and mortality has recently been described (16), implying, that vitamin D deficiency is a serious cancer risk factor (13,17). An inverse correlation has also been demonstrated between the expression of the vitamin D receptor (VDR) and 25-hydroxyvitamin D3 1-α-hydroxylase (CYP27B1) with melanoma progression and disease outcome (15,18,19). Therefore, active forms of vitamin D are now considered for therapeutic use in cancer prevention and treatment, supported by numerous epidemiological and preclinical studies (20-30). It should be emphasized that active forms of vitamin D used in combined therapy enhance the effectiveness of a number of anticancer drugs, including cisplatin (31,32), doxorubicin (33) and proton therapy (34). A previous study indicated that vitamin D analogs enhance the antiproliferative activity of cisplatin on keratinocytes (35). Furthermore, vitamin D and its analogs are currently being tested in clinical trials on various types of cancer, including melanoma (36,37).
Melanoma, while accounting for only 4% of skin cancers, is linked to 80% of mortalities due to skin tumors, and therefore represents a significant public health problem (30,38-42). This tumor is aggressive, but potentially curable by surgical excision if it is diagnosed at the early stages of development, including melanoma in situ or at the radial growth phase. However, with progression of the disease to the vertical growth phase, melanoma cells become resistant to the majority of forms of treatment, and acquire the ability to metastasize (38,39,43). Furthermore, the incidence of melanoma has been rising in the Caucasian population worldwide over recent decades (38,39). In 2017, melanoma was expected to be the fifth most common cancer in males and sixth most common in females in the USA (44). In recent years, major progress has been made with respect to our understanding of the molecular nature of melanoma and the interaction of melanoma cells with the immune system. Unfortunately, despite the marked expansion of advanced treatment options, primary or acquired resistance develops in patients, emphasizing the requirement for additional effort to develop effective melanoma therapy (42,45).
The aim of the present study was to investigate the modulation of the anticancer properties of selected anti-melanoma chemotherapy agents by vitamin D and its non- or low-calcemic analogs 20S-hydroxyvitamin D3 [20(OH)D3], 21-hydroxypregnacalciferol [21(OH)pD] and calcipotriol (46-50), since the use of the hormonally active form of vitamin D, 1α,25(OH)2D3, at high doses is limited due to the risk of toxic effects, including hypercalcemia (51,52). Notably, 20(OH)D3 is a natural product synthesized in the human body and detectable in human serum (53-55). It was hypothesized that vitamin D analogs would sensitize melanoma cells to classic chemotherapeutic drugs, based on a recent study documenting the association between vitamin D and oxidative stress in keratinocytes with a high proliferative potential, and the effect of vitamin D analogs on the sensitivity of these cells to cisplatin (35). Even though it is known that cisplatin induces DNA damage (56), it should be noted that the mechanism of action of cisplatin partially relies on the generation of reactive oxygen species (ROS) (57). Therefore the effects of dacarbazine, still used in melanoma therapy and also known to produce ROS in cells (58), and cisplatin, used in combination with vitamin D or its low calcemic analogs, were tested on the human malignant melanoma A375 cell line.
Materials and methods
Chemicals
1,25(OH)2D3, hydrogen peroxide (30%), cisplatin and dacarbazine were Sigma-Aldrich products (Merck KGaA, Darmstadt, Germany). 21(OH)pD was synthesized according to Zmijewski et al (50) by ProChimia Surfaces Sp. z o. o. (Sopot, Poland). 20(OH)D3 was synthesized and purified as described previously (59). Calcipotriol was a gift from the Pharmaceutical Research Institute (Warsaw, Poland).
Cell culture
Human melanoma A375 cells (CRL-1619) were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (both Sigma-Aldrich; Merck KGaA) and 1% penicillin/streptomycin in an incubator with 5% CO2 at 37°C. DMEM medium supplemented with 2% charcoal-stripped FBS was used for all experimental procedures where the effects of vitamin D derivatives were examined.
Proliferation assay
The sulphorhodamine B (SRB) assay was performed as previously described (35). Briefly, the human melanoma A375 cells were seeded in 96-well plates (7,000 cells per well), cultured overnight and then treated with serial dilutions of the compounds (vitamin D, 10−12-10−6 M; hydrogen peroxide, 0.004-0.250 mM; cisplatin, 0.19-300 µM; and dacarbazine, 0.15-10 µM) being tested for an additional 24 or 48 h. Following cell fixation with 10% trichloroacetic acid for 1 h at 4°C, the plates were washed 5 times with distilled water and air-dried. Staining solution comprising of 0.4% SRB (Sigma-Aldrich; Merck KGaA) in acetic acid was added to each well for 15 min, followed by washing with 1% acetic acid. The SRB dye was solubilized using a solution of 10 mM buffered Tris Base (pH 10.5) and the absorbance was measured at 570 nm using an Epoch™ microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). The relative IC50 value was calculated as the concentration at which half the maximum inhibition was observed, i.e., the mid-point between no inhibition and the maximum observed decrease in proliferation (60,61).
Cell cycle analysis
The cell cycle was analyzed by quantification of DNA content using flow cytometry. Trypsinized cells and cells from culture medium were fixed in 70% ethanol for 24-48 h at 4°C, treated with ribonuclease in order to remove any contaminating RNA, and the DNA was stained with propidium iodide (PI; Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. The fluorescence of the PI-stained cells was measured by flow cytometry (excitation, 536 nm; emission, 617 nm; FACSCalibur™; Becton, Dickinson and Company, Franklin, Lakes, NJ, USA). The results were analyzed using the CellQuest™ Pro Software version 6.0 (Becton, Dickinson and Company) and expressed as a percentage of cells with DNA content corresponding to apoptotic/necrotic cells (subG1 fraction) or cells in G1, S and G2/M phases of the cycle.
Measurement of changes in the mitochondrial membrane potential (Δψm)
The detection of changes in the inner electrochemical Δψm in living cells was performed as described previously (35), using the cationic, lipophilic JC-1 dye (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Carbonyl cyanide 3-chlorophenylhydrazone (CCCP; Sigma-Aldrich; Merck KGaA), a mitochondrial potential disrupter, was used as a control. The melanoma A375 cells were pre-treated with secosteroids at a concentration of 100 nM and then exposed to 2.4 and 12 µM cisplatin or 2.0 and 10 µM dacarbazine for an additional 3 h, or to 75 nM hydrogen peroxide for 1-3 h. Following the treatment with the selected compounds, the cells were harvested and suspended in 1 ml PBS at room temperature. CCCP solution in dimethylsulfoxide (DMSO) was added to the positive control tube only (2 µM final concentration) and the cells incubated at 37°C for 5 min. JC-1 solution (2 µM in DMSO) was added to all tubes and the cells were incubated at 37°C for 15 min, then centrifuged at 1,000 × g for 10 min at room temperature, and resuspended in 500 µl PBS. The samples were kept on ice until they were analyzed on the FACSCalibur flow cytometer using the CellQuest Pro analysis software.
Detection of intracellular ROS production
The intracellular production of ROS was measured using H2DCFDA (Thermo Fisher Scientific, Inc.). Cells were incubated with 100 nM 1,25(OH)2D3 for 24 h followed by exposure to 24 µM cisplatin or 6 µM dacarbazine for 1 or 24 h. H2DCFDA was added to a final concentration of 10 µM 30 min before the end of the incubation. The cells were washed and suspended in cold PBS. The samples were kept on ice until they were analyzed using the FACSCalibur flow cytometer using CellQuest Pro analysis software.
Measurement of mRNA levels
The relative mRNA levels of particular genes were determined by a reverse transcription-quantitative polymerase chain reaction (qPCR) assay. Total RNA was isolated using the Total RNA Mini kit (A&A Biotechnology, Gdynia, Poland), according to the manufacturer’s instructions. The concentration and quality of RNA samples were determined using the Epoch spectrophotometer. A total of 1 μg RNA was used for reverse transcription using the RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) by incubating at 42°C for 1 h. The qPCR reaction comprised 1 µl cDNA and 150 nM of each primer (Table I), and was performed using the SensiFAST™ SYBR No-ROX kit (Bioline Reagents Limited, London, UK) in a total volume of 20 µl on the StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: Initial denaturation at 95°C for 2 min, followed by 40 cycles of 95°C for 5 sec, 55-63°C for 10 sec, 72°C for 15 sec and 79°C for 10 sec. The melting curve analysis of the PCR products was performed following the qPCR reaction and consisted of 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec. The reactions were run in duplicate and the resulting data were averaged prior to analysis with the StepOnePlus version 2.2.2. software (Thermo Fisher Scientific, Inc.). The RPL37 gene was used as a control to normalize the values by the 2−ΔΔCq quantification method (62).
Table I.
Primer sequences.
| Gene | Forward primer (3′-5′) | Reverse primer (3′-5′) |
|---|---|---|
| RPL37A | TTCTGATGGCGGACTTTACC | CACTTGCTCTTTCTGTGGCA |
| SOD1 | CCACACCTTCACTGGTCCAT | CTAGCGAGTTATGGCGACG |
| SOD2 | TAGGGCTGAGGTTTGTCCAG | CACCGAGGAGAAGTACCAGG |
| CAT | ACGGGGCCCTACTGTAATAA | AGATGCAGCACTGGAAGGAG |
| VDR | CCAGTTCGTGTGAATGATGG | GTCGTCCATGGTGAAGGA |
| PDIA3 | CTCCGACGTGCTAGAACTCA | CAGGTGTTAGTGTTGGCAGT |
| CYP2R1 | AGAGACCCAGAAGTGTTCCAT | GTCTTTCAGCACAGATGAGGTA |
| CYP3A4 | AAGGCACCACCCACCTATGATACT | TACTTTGGGTCACGGTGAAGAGCA |
| CYP27B1 | TGTTTGCATTTGCTCAGA | CCGGGAGAGCTCATACAG |
| CYP24A1 | GCAGCCTAGTGCAGATTT | ATTCACCCAGAACTGTTG |
| CYP11A1 | TGGGTCGCCTATCACCAGTAT | CCACCCGGTCTTTCTTCCA |
RPL37A, ribosomal protein L37a; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2; CAT, catalase; VDR, vitamin D receptor; PDIA3, protein disulfide isomerase A3; CYP2R1, vitamin D 25-hydroxylase; CYP3A4, cytochrome P450 3A4; CYP27B1, 25-hydroxyvitamin D3 1-α-hydroxylase; CYP24A1, vitamin D 24-hydroxylase; CYP11A1 cholesterol side-chain cleavage enzyme.
Statistical analysis
Statistical analysis was performed using Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA) or GraphPad Prism version 6.03 (GraphPad Software, Inc., La Jolla, CA, USA). The data were subjected to Student’s t-test for the comparison of two groups, one-way analysis of variance followed by Dunnett’s or Tukey’s multiple comparison post hoc tests. The data are expressed as the mean ± standard deviation (n=3-6). Differences were considered statistically significant when P<0.05.
Results
Vitamin D analogs modulate the cytotoxic effects of hydrogen peroxide in human malignant melanoma A375 cells
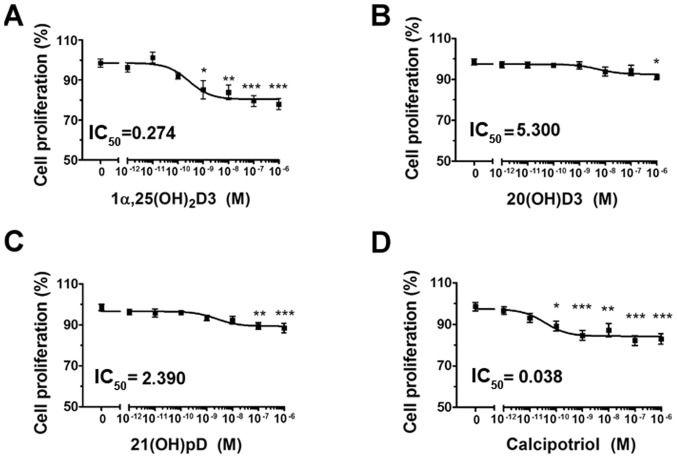
In agreement with previous studies (35,49,50,63,64) vitamin D analogs 1,25(OH)2D3, 20(OH)D3, 21(OH)pD and calcipotriol, effectively inhibited the proliferation of human melanoma A375 cells, as demonstrated by the SRB assay (Fig. 1A–D). A decrease of ≤20% in cell proliferation was observed, significant at the highest tested concentration of vitamin D analogs (10−6 M). The relative IC50 values ranged from 5.3 nM for 20(OH)D3 to ~0.274 nM for 1,25(OH)2D3 and 0.038 nM for calcipotriol (Fig. 1A–D).
Figure 1.
The effect of vitamin D derivatives on the proliferation of human melanoma A375 cells treated with H2O2. The cells were treated with serial dilutions (10−12-10−6 M) of (A) 1,25(OH)2D3, (B) 20(OH)D3, (C) 21(OH)pD or (D) calcipotriol. *P<0.05, **P<0.005 and ***P<0.0005 versus control using one-way analysis of variance. The cells were treated with serial dilutions of H2O2 (0.0039-0.25 mM) alone or in combination with (E) 10 nM 1,25(OH)2D3, (F) 20(OH)D3, (G) 21(OH)pD or (H) calcipotriol for 24 h. The results presented are representative of three experiments (n=6). *P<0.05, **P<0.01 and ***P<0.001 between the two treatments at each H2O2 concentration, using one-way analysis of variance followed by Tukey’s multiple comparison test. The same control data is plotted in each graph. In order to investigate the effect of secosteroid pre-treatment on mitochondrial transmembrane potential, human melanoma A375 cells were treated with (I) 100 nM 1,25(OH)2D3 or calcipotriol for 24 h, and subsequently exposed to 7.5 µM H2O2 for 1 or 3 h, then stained with JC-1 and analyzed by flow cytometry. The data are presented as mean ± standard deviation of 3 independent experiments. ***P<0.001 versus untreated control or between the two groups indicated by the bracket using one-way analysis of variance followed by Tukey’s multiple comparison test. The positive control was exposed to CCCP for 5 min prior to staining with JC-1. IC50, half maximal inhibitory concentration; 1α,25(OH)2D3, 1α, 25-dihydroxyvitamin D3; 20(OH)D3, 20S-hydroxyvitamin D3; 21(OH)pD, 21-hydroxypregnacalciferol; H2O2, hydrogen peroxide; CCCP, carbonyl cyanide 3-chlorophenylhydrazone.
The effects of vitamin D derivatives on the sensitivity of A375 cells to ROS were also tested. Hydrogen peroxide, an oxidative stress-generating compound, inhibited the proliferation of the cells with a relative IC50 of 17 µM (Fig. 1E–H). Simultaneous treatment with hydrogen peroxide and 1α,25(OH)2D3, 20(OH)D3 or calcipotriol at a concentration of 10 nM (Fig. 1E, F and H) for 24 h resulted in a further decrease in the proliferation of the melanoma cells. The effect was more pronounced for 1α,25(OH)2D3 (Fig. 1E) and calcipotriol (Fig. 1H), however we did not observe any significant decrease between the calculated IC50 values (Table II). It has been suggested that altered mitochondrial activity may be a signature of certain melanoma cells (65). In the present study, changes in Δψm were monitored using the JC-1 dual-emission potential-sensitive probe, by flow cytometry. The results revealed that the pre-incubation of the A375 cells with calcipotriol, but not 1α,25(OH)2D3, for 24 h modulated the effect of hydrogen peroxide on the Δψm (Fig. 1I). Notably, the pre-treatment with calcipotriol resulted in a protective effect on Δψm in melanoma cells treated with hydrogen peroxide for 1 h (Fig. 1I). Prolonged exposure to hydrogen peroxide (3 h) in combination with pre-treatment of melanoma cells with either 1,25(OH)2D3 or calcipotriol triggered a decrease in Δψm (Fig. 1I), although the observed differences were not significant.
Table II.
Summary of the relative IC50 values for inhibition of proliferation of human melanoma A375 cells by H2O2 (0.004-0.250 mM), cisplatin (0.19-300 µM) or dacarbazine (0.15-10 µM) in the presence or absence of the tested secosteroids.
| Incubation time, h | Tested compound | Relative IC 50
|
||||
|---|---|---|---|---|---|---|
| Monotreatment | +10 nM 1α,25(OH)2D3 | +10 nM 20(OH)D3 | +10 nM 21(OH)pD | +10 nM calcipotriol | ||
| 24 | H2O2 | 0.017±0.07 | 0.011±0.001 | 0.013±0.0006 | 0.017±0.002 | 0.012±0.0006 |
| 24 | Cisplatin | 4.81±2.2 | 11.61±0.98a | 14.08±3.29b | 9.37±1.64 | 15.23±6.15b |
| 48 | Cisplatin | 2.57±0.19 | 1.97±0.22 | 3.47±1.04 | 3.71±1.90 | 2.13±1.01 |
| 48 | Dacarbazine | 1.07±0.31 | 0.45±0.35a | 1.17±0.40 | 1.04±0.36 | 0.85±0.39 |
Data are presented as the mean ± standard deviation of three independent experiments (n=6 each). The data were subjected to analysis of variance followed by Tukey’s multiple comparison test.
P<0.05 and
P<0.001 vs. monotreatment. IC50, half maximal inhibitory concentration; H2O2, hydrogen peroxide; 1α,25(OH)2D3, 1α,25-dihydroxyvitamin D3; 20(OH)D3, 20S-hydroxyvitamin D3; 21(OH)pD, 21-hydroxypregnacalciferol.
Vitamin D analogs modulate the cytotoxic effects of cisplatin and dacarbazine on human malignant melanoma A375 cells
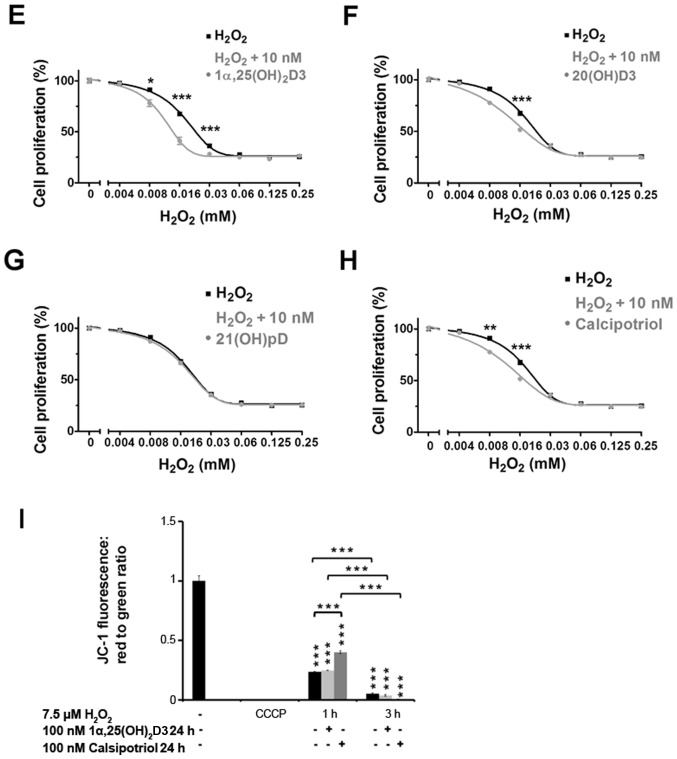
It is well established that oxidative stress and the resulting cell damage is one of the mechanisms of cell death induced by anticancer drugs. Thus, based on the aforementioned results with hydrogen peroxide (Fig. 1E–I), the effect of the treatment of A375 human melanoma cells with 1α,25(OH)2D3, 20(OH) D3, 21(OH)pD or calcipotriol, on the ability of cisplatin or dacarbazine to inhibit proliferation, was investigated. These two drugs are widely used in melanoma treatment and their activity, at least partially, relies on ROS generation (35,57,58,66). The anti-melanoma effects of cisplatin (Fig. 2A–D) or dacarbazine (Fig. 3A–D) alone or with 10 nM 1α,25(OH)2D3, 20(OH)D3, 21(OH)pD or calcipotriol were investigated in A375 cells using the SRB assay. Simultaneous treatment with vitamin D analogs and cisplatin for 24 h resulted in an unexpected increase in the cisplatin relative IC50, suggesting protective effects of the secosteroids (Table II). However, during prolonged incubation with cisplatin (48 h), the addition of 1,25(OH)2D3, but not 20(OH)D3, 21(OH)pD or calcipotriol, resulted in a decreasing trend in the relative IC50 in comparison to cisplatin alone (Fig. 2; Table II), however the observed differences were not significant.
Figure 2.
The effect of vitamin D derivatives on the proliferation of human melanoma A375 cells that were treated with cisplatin. Melanoma A375 cells were treated with serial dilutions of cisplatin (0.019-300 µM) in combination with 10 nM (A) 1α,25(OH)2D3, (B) 20(OH)D3, (C) 21(OH)pD or (D) calcipotriol for 48 h. The results are representative of three experiments (n=6). The same control data is plotted in each graph. *P<0.05 between the two treatments at each cisplatin concentration, using one-way analysis of variance followed by Tukey’s multiple comparison test. 1α,25(OH)2D3, 1α, 25-dihydroxyvitamin D3; 20(OH)D3, 20S-hydroxyvitamin D3; 21(OH)pD, 21-hydroxypregnacalciferol.
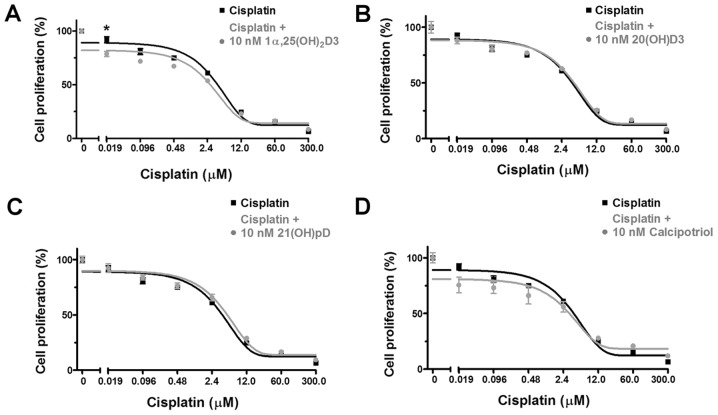
Figure 3.
The effect of vitamin D derivatives on the proliferation of human melanoma A375 cells that were treated with dacarbazine. melanoma A375 cells were treated with serial dilutions of dacarbazine (0.15-10 µM) in combination with 10 nM (A) 1α,25(OH)2D3, (B) 20(OH)D3, (C) 21(OH)pD or (D) calcipotriol for 48 h. The results are representative of three experiments (n=6). The same control data is included in all graphs. *P<0.05 between the two treatments at each dacarbazine concentration, using one-way analysis of variance followed by Tukey’s multiple comparison test. 1α,25(OH)2D3, 1α, 25-dihydroxyvitamin D3; 20(OH)D3, 20S-hydroxyvitamin D3; 21(OH)pD, 21-hydroxypregnacalciferol.
Dacarbazine inhibited the proliferation of human melanoma A375 cells during a 48 h incubation with a relative IC50 of 1.07 µM (Table II). The results from the combined treatment with the vitamin D analogs revealed that 1α,25(OH)2D3, but not 20(OH)D3, 21(OH)pD or calcipotriol, decreased the relative IC50 observed with dacarbazine alone by 2.3-fold (Table II).
Pre-treatment of human malignant melanoma A375 cells with vitamin D derivatives alters the distribution of the cells in the cell cycle phases following treatment with dacarbazine, but not cisplatin
To investigate the mechanism of proliferation inhibition of melanoma A375 cell by the combination of vitamin D analogs and the tested drugs, changes in the distribution of the cells in the cell cycle phases were investigated by flow cytometry. The cells were pre-treated with the vitamin D analogs for 24 h and then incubated with cisplatin or dacarbazine for an additional 24 or 48 h. The initial experiments revealed no significant effects of pre-treatment of melanoma cells for 24 h with 10 nM secosteroids in combination with additional incubation with cisplatin for 24 h on the cell cycle distribution (data not shown). Since the results of the aforementioned SRB tests (Fig. 1A–D) demonstrated a plateau in the inhibition of cell proliferation at 10 and 100 nM concentrations, and taking into consideration that vitamin D is widely used at higher concentrations (100-1,000 nM) in in vitro studies (67-69), the concentration of vitamin D analogs was raised to 100 nM for the present assay. Additionally, the time of incubation with cisplatin or dacarbazine was increased to 48 h, similar to the conditions used during proliferation tests, and their concentrations were increased to 24 and 6 µM, respectively, to maximize the observed effect.
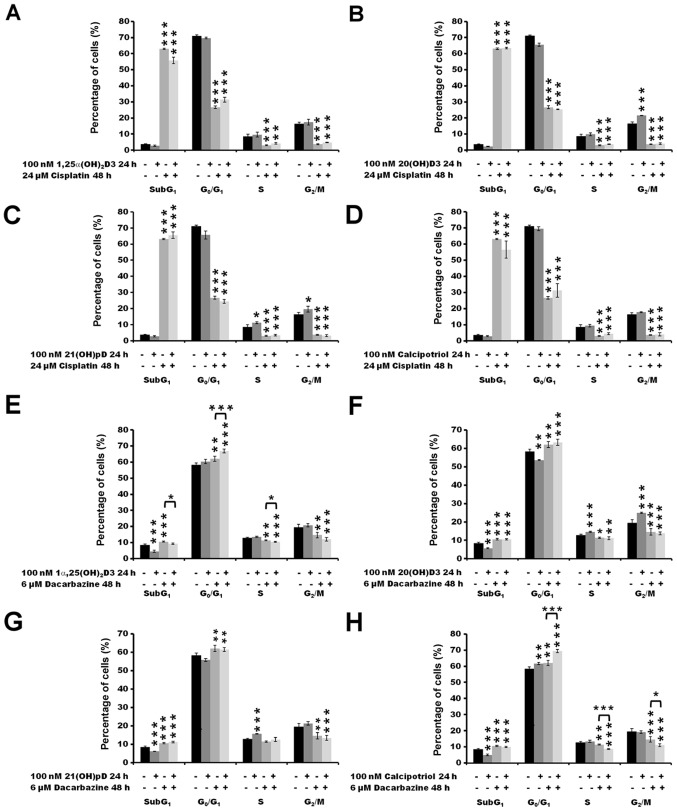
The treatment of melanoma A375 cells with 24 µM cisplatin alone for 48 h resulted in an increase in the number of SubG1 cells (P<0.001), indicating induction of apoptosis with a concomitant decrease in the number of cells in the G0/G1 (P<0.001), S (P<0.001) and G2/M (P<0.001) phases (Fig. 4A–D). No impact of the vitamin D pre-treatment was observed on the distribution of cisplatin-treated melanoma cells in the cell cycle. The effect of pre-treatment of melanoma A375 cells with vitamin D analogs prior to incubation with dacarbazine was also tested (Fig. 4E–H). The treatment with 6 µM dacarbazine alone for 48 h resulted in an increase in the fraction of cells in G0/G1 compared with untreated cells (P<0.01), with a minor effect on the SubG1 fraction (P<0.001) in comparison with cells treated with cisplatin alone (<10 vs. >60% of all cells analyzed at SubG1 following treatment with dacarbazine or cisplatin, respectively; P<0.001 cisplatin versus untreated cells; P<0.001 dacarbazine versus untreated cells; Fig. 4A and E). In addition, 24 h pre-treatment with 100 nM 1α,25(OH)2D3 or calcipotriol prior to dacarbazine treatment resulted in an increase in the percentage of cells in the G0/G1 phase compared with that observed with dacarbazine alone (P<0.001; Fig. 4E and H). The effect was accompanied by a decrease in the percentage of cells in the G2/M phase for 1α,25(OH)2D3 (P<0.05; Fig. 4E), and in S and G2/M phases for calcipotriol (P<0.001 and P<0.05, respectively; Fig. 4H).
Figure 4.
The effect of secosteroids and cisplatin or dacarbazine on the distribution of human melanoma A375 cells through the cell cycle. Cells that were treated with 24 µM cisplatin for 48 h had been pre-treated with (A) 100 nM 1α,25(OH)2D3, (B) 20(OH)D3, (C) 21(OH)pD or (D) calcipotriol for 24 h. Similarly, cells that were treated with 6 µM dacarbazine 48 h had been pre-treated with (E) 100 nM 1α,25(OH)2D3, (F) 20(OH)D3, (G) 21(OH)pD or (H) calcipotriol for 24 h. The cells were harvested, stained with propidium iodide and analyzed by flow cytometry. The data are presented as the mean ± standard deviation (n=3). *P<0.05, **P<0.01 and ***P<0.001, calculated using one-way analysis of variance followed by Tukey’s multiple comparison test versus untreated control or between the two groups indicated by the bracket. SubG1, apoptotic/necrotic cells; G1, growth; S, DNA synthesis; G2/M, preparation for mitosis/mitosis; 1α,25(OH)2D3, 1α, 25-dihydroxyvitamin D3; 20(OH)D3, 20S-hydroxyvitamin D3; 21(OH)pD, 21-hydroxypregnacalciferol.
Pre-treatment with vitamin D derivatives changes the Δψm in human melanoma A375 cells and alters the cisplatin- or dacarbazine-induced production of ROS
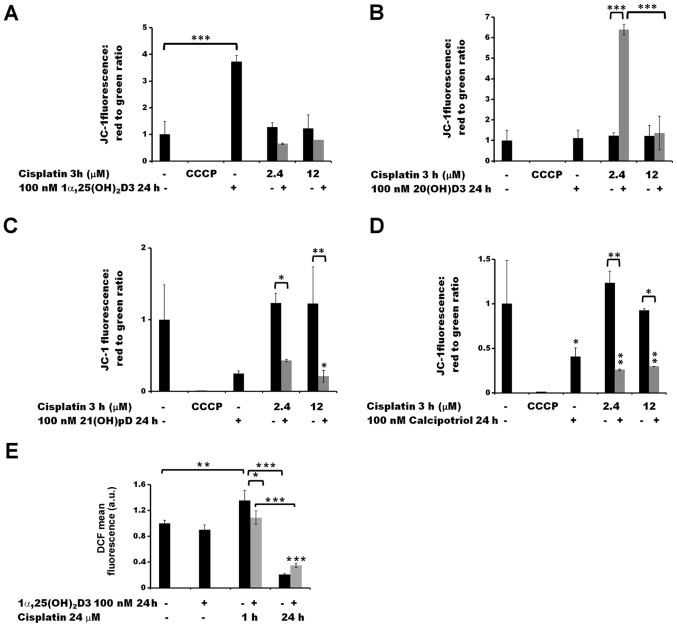
The effects of the anti-cancer drugs on the Δψm of the melanoma A375 cells were analyzed by measuring JC-1 fluorescence by flow cytometry (Figs. 5 and 6). Treatment with cisplatin alone for 3 h did not influence the Δψm, at either of the two concentrations tested (2.4 and 12 µM; Fig. 5A–D). A 24 h pre-treatment with 21(OH) pD (Fig. 5C) or calcipotriol (Fig. 5D) resulted in a decrease in Δψm following treatment with cisplatin, compared to the cisplatin effect observed without pre-treatment. Notably, pre-treatment of the melanoma cells with 20(OH)D3 resulted in an increase in Δψm (Fig. 5B) following exposure to 2.4 µM cisplatin (P<0.001). However, this effect was not observed at higher concentration of the drug, or without the drug treatment.
Figure 5.
The effect of pre-treatment of human melanoma A375 cells with vitamin D derivatives on the cisplatin-induced changes in the mitochondrial membrane potential and ROS levels. A375 cells were treated with (A) 100 nM 1α,25(OH)2D3, (B) 20(OH)D3, (C) 21(OH)pD or (D) calcipotriol for 24 h, and subsequently exposed to 2.4 or 12 µM cisplatin for 3 h. The cells were stained with JC-1 and analyzed by flow cytometry. The positive control was exposed to CCCP for 5 min prior to staining with JC-1. (E) The effect of 1α,25(OH)2D3 on ROS levels. The cells were treated with 100 nM 1,25(OH)2D3 for 24 h and subsequently exposed to 24 µM cisplatin for 1 or 24 h. The cells were stained with H2DCFDA and analyzed by flow cytometry. The data are presented as the mean ± standard deviation (n=3). *P<0.05; **P<0.01; and ***P<0.001, calculated using one way analysis of variance followed by Tukey’s multiple comparison test between the two groups indicated by the bracket or compared with the untreated control. 1α,25(OH)2D3, 1α, 25-dihydroxyvitamin D3; 20(OH)D3, 20S-hydroxyvitamin D3; 21(OH)pD, 21-hydroxypregnacalciferol; CCCP, carbonyl cyanide 3-chlorophenylhydrazone; ROS, reactive oxygen species.
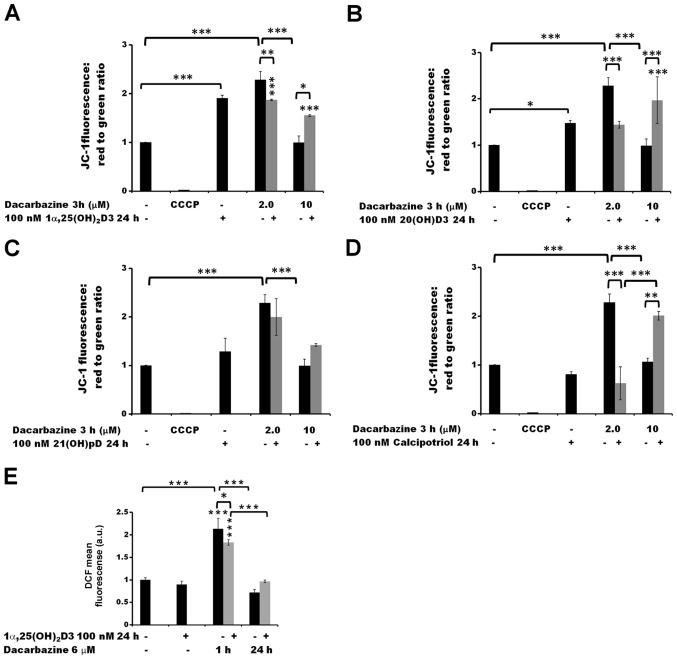
Figure 6.
The effect of pre-treatment of human melanoma A375 cells with vitamin D derivatives on the dacarbazine-induced changes in the mitochondrial membrane potential and ROS levels. A375 cells were treated with (A) 100 nM 1α,25(OH)2D3, (B) 20(OH)D3, (C) 21(OH)pD or (D) calcipotriol for 24 h, and subsequently exposed to 2.0 or 10 µM dacarbazine for 3 h The cells were stained with JC-1 and analyzed by flow cytometry. The positive control was exposed to CCCP for 5 min prior to staining with JC-1. (E) The effect of 1α,25(OH)2D3 on ROS levels. The cells were treated with 100 nM 1,25(OH)2D3 for 24 h and subsequently exposed to 6 µM dacarbazine for 1 or 24 h. The cells were stained with H2DCFDA and analyzed by flow cytometry. The data are presented as the mean ± standard deviation (n=3). *P<0.05, **P<0.01 and ***P<0.001, calculated using one way analysis of variance followed by Tukey’s multiple comparison test between the two groups indicated by the bracket or compared with the untreated control. 1α,25(OH)2D3, 1α,25-dihydroxyvitamin D3; 20(OH)D3, 20S-hydroxyvitamin D3; 21(OH)pD, 21-hydroxypregnacalciferol; CCCP, carbonyl cyanide 3-chlorophenylhydrazone; ROS, reactive oxygen species.
A 3 h treatment with 2.0 µM dacarbazine alone led to an increase in the Δψm of melanoma A375 cells (P<0.001) but this was not the case at the higher concentration (10 µM) (Fig. 6A–D). The 24 h pre-treatment of the cells with 1α,25(OH)2D3 (Fig. 6A), 20(OH)D3 (Fig. 6B) or calcipotriol (Fig. 6D) resulted in a decrease in Δψm following exposure to 2.0 µM dacarbazine (P<0.01 for 1α,25(OH)2D3 and P<0.001 for 20(OH)D3 and calcipotriol versus dacarbazine alone). In the case of 21(OH)pD (Fig. 6C), the effect was not statistically significant. In contrast, at the higher concentration of dacarbazine (10 µM), the pre-treatment of the cells with 1,25(OH)2D3, 20(OH)D3 or calcipotriol resulted in an increase in Δψm (P<0.05, P<0.001 and P<0.01, respectively, versus 10 µM dacarbazine alone). No significant difference was observed in the case of pre-treatment with 21(OH)pD.
A pre-treatment of malignant melanoma A375 cells with 100 nM 1,25(OH)2D3 for 24 h did not influence the production of ROS in comparison with untreated cells, as determined by the H2DCFDA assay (Figs. 5E and 6E). However, this pre-treatment affected the ROS production following treatment with either cisplatin (Fig. 5E) or dacarbazine (Fig. 6E). The observed effect was time-dependent. Exposure of the cells to cisplatin or dacarbazine alone for 1 h, without vitamin D pre-treatment, led to a significant increase in the ROS levels (P<0.01 for cisplatin and P<0.001 for dacarbazine; Figs. 5E and 6E, respectively). However, 24 h pre-treatment of melanoma cells with 1α,25(OH)2D3 decreased the effect that the 1 h cisplatin or dacarbazine treatment had on the ROS levels (P<0.05 versus no pre-treatment; Fig. 5E). In contrast, prolonged exposure (24 h) to cisplatin or dacarbazine alone tended towards a decrease in the ROS levels in the melanoma cells, whereas the 1α,25(OH)2D3 pre-treatment alleviated the effect of the 24 h cisplatin or dacarbazine treatment on the ROS levels, although the observed differences were not significant.
Modulation of the expression of selected genes by cisplatin or dacarbazine in the presence or absence of 1α,25(OH)2D3
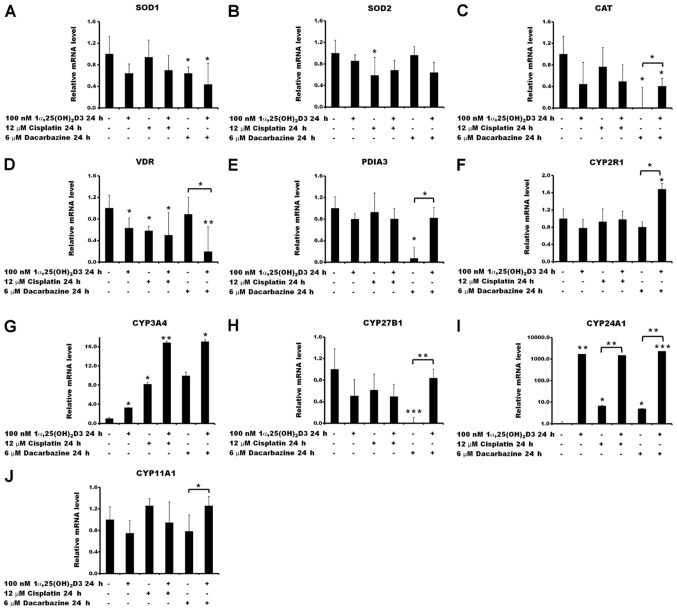
In order to verify the aforementioned changes in ROS generation and the Δψm, the impact of 1α,25(OH)2D3 pre-treatment on the expression of the selected ROS-associated genes was tested in melanoma A375 cells treated with cisplatin or dacarbazine (Fig. 7). No significant effect was observed in the expression of superoxide dismutases 1 and 2 (SOD1 and SOD2) or catalase (CAT) by 1α,25(OH)2D3 under the experimental conditions used (Fig. 7A–C). Treatment of the cells with the anticancer drugs had a limited effect on the mRNA levels of the selected ROS-associated genes. A decrease in SOD2 gene expression was observed under the influence of cisplatin alone (P<0.05 vs. no treatment control; Fig. 7B), as well as in SOD1 and CAT gene expression following treatment with dacarbazine alone (both P<0.05 vs. no treatment control; Fig. 7A and C, respectively). Pre-treatment of the cells with 1α,25(OH)2D3 prior to incubation with dacarbazine resulted in an increase of CAT mRNA compared with cells treated solely with dacarbazine (P<0.05; Fig. 7C).
Figure 7.
Relative mRNA quantification of reactive oxygen species- and vitamin D-associated genes. Effects of cisplatin or dacarbazine treatment on the mRNA levels of (A) SOD1, (B) SOD2, (C) CAT, (D) VDR, (E) PDIA3, (F) CYP2R1, (G) CYP3A4, (H) CYP27B1, (I) CYP24A1 and (J) CYP11A1 gene expression in human melanoma A375 cells pre-treated with 1,25(OH)2D3. The cells were incubated with 100 nM 1,25(OH)2D3 for 24 h, followed by exposure to 12 µM cisplatin or 6 µM dacarbazine for an additional 24 h. The mRNA levels were measured by reverse transcription-quantitative polymerase chain reactions. The data are presented as the mean ± standard deviation of 3 independent experiments carried out in duplicate. *P<0.05, **P<0.01 and ***P<0.001, calculated using Student’s t-test vs. untreated control or between the two groups indicated by the bracket. 1α,25(OH)2D3, 1α, 25-dihydroxyvitamin D3; SOD, superoxide dismutase; CAT, catalase; VDR, vitamin D receptor; PDIA3, protein disulfide-isomerase A3; CYP2R1, vitamin D 25-hydroxylase; CYP3A4, cytochrome P450 3A4; CYP27B1, 25-hydroxyvitamin D3 1-α-hydroxylase; CYP24A1, vitamin D 24-hydroxylase; CYP11A1, cholesterol side-chain cleavage enzyme.
Subsequently, the effect of cisplatin or dacarbazine on the expression of vitamin D-associated genes, including ones encoding vitamin D receptors VDR and protein disulfide-isomerase A3 (PDIA3), and vitamin D metabolizing hydroxylases that belong to the cytochrome P450 (CYP) family, CYP2R1, CYP3A4, CYP27B1, CYP24A1 and CYP11A1, was investigated in melanoma A375 cells, as well as the consequences of pre-treatment with 1α,25(OH)2D3. The results revealed that 1α,25(OH)2D3 and cisplatin, used alone, decreased VDR mRNA levels in the A375 cells (P<0.05; Fig. 7D). The effect of dacarbazine was statistically significant only in the case of the 1α,25(OH)2D3 pre-treatment (P<0.05; Fig. 7D). In contrast, 1α,25(OH)2D3 and cisplatin had no effect on PDIA3 mRNA levels (Fig. 7E), whereas dacarbazine alone led to a significant decrease (P<0.05). Notably, the effect of dacarbazine alone was reversed by the 1α,25(OH)2D3 pre-treatment (P<0.05).
Although the transcription of CYP2R1 was not affected by 1α,25(OH)2D3, cisplatin or dacarbazine alone, pre-treatment of the A375 cells with 1α,25(OH)2D3 with subsequent exposure to dacarbazine resulted in an increase in its mRNA (P<0.05; Fig. 7F) compared with that in the cells treated with dacarbazine alone. Stimulation of CYP3A4 expression was observed with all combinations of the drugs tested. The effect was further exacerbated by a 24 h 1α,25(OH)2D3 pre-treatment (Fig. 7G). Treatment with 1α,25(OH)2D3 or cisplatin alone or in combination had no statistically significant effect on the CYP27B1 mRNA levels (Fig. 7H). However, treatment with dacarbazine alone resulted in a decrease (P<0.001) and this effect was reversed by prior administration of 1α,25(OH)2D3 (P<0.01). As expected, pre-treatment of the cells with 1α,25(OH)2D3 resulted in a strong stimulation of CYP24A1, which encodes the vitamin D deactivation enzyme, 24-hydroxylase (Fig. 7I). An increase in CYP24A1 mRNA levels was also observed for cisplatin or dacarbazine alone, although to a lesser extent [7- and 5-fold increase, respectively, versus a 1,700-fold increase for 1α,25(OH)2D3]. Furthermore, cisplatin or dacarbazine had no effect on the level of CYP24A1 mRNA following pre- treatment with 1α,25(OH)2D3, compared with cells treated solely with 1α,25(OH)2D3 (Fig. 7I). Treatment of the A375 cells with 1α,25(OH)2D3, cisplatin or dacarbazine alone did not affect the transcription levels of the CYP11A1 gene (Fig. 7J). Finally, the pre-treatment with 1α,25(OH)2D3 followed by treatment with dacarbazine resulted in a small, but significant, increase in the CYP11A1 mRNA level (P<0.05 vs. dacarbazine alone).
Discussion
It is well established that UV radiation is a major skin carcinogen that serves an important role in melanomagenesis (14,70,71). However, UVB is also indispensable for the production of vitamin D in the skin (1-3). Considering the antiproliferative and differentiation-promoting function of vitamin D and its analogs, it seemed advantageous to explore their efficacy as anticancer drugs and their potential for positive interactions with other antimelanoma drugs or therapeutic approaches (34). The effects of the active forms of vitamin D require VDR activation, which results in the modulation of the expression in ~3,000 target genes in humans (72), including those involved in DNA repair and the oxidative stress response (73). Vitamin D deficiency is considered to contribute to carcinogenesis, and notably, to poor prognosis due to multidrug resistance (74,75). Recently published data suggest an inverse correlation between the vitamin D serum level and the relative risk of melanoma and non-melanoma skin cancer, as well as melanoma thickness at diagnosis (30,75,76). Wyatt et al (77) also suggested that vitamin D deficiency at the time of melanoma diagnosis is not only associated with a higher Breslow thickness but also with a poorer prognosis. Ogbah et al (78) reported that even in patients living in the sunny Mediterranean area, 1α,25(OH)2D3 levels were sub-optimal at the time of melanoma diagnosis. Patients with metastatic melanoma, who were initially vitamin D deficient, had significantly poorer outcomes in comparison to individuals who, being initially deficient, exhibited a >20 ng/ml increase in their 25-hydroxyvitamin D3 [25(OH) D3] concentration during the therapy period (75). Vitamin D deficient patients with stage IV metastatic melanoma also had significantly poorer prognosis (75). Therefore, the administration of vitamin D is potentially beneficial in cancer therapy.
A previous study has revealed that melanoma A375 cells are ≥10 times more sensitive to hydrogen peroxide than human immortalized HaCaT keratinocytes. The interaction between hydrogen peroxide, as a model oxidative stress inducer, and vitamin D analogs were investigated (35). First, as reported for HaCaT keratinocytes (35), the incubation of melanoma A375 cells with vitamin D analogs resulted in higher sensitivity of the cells to hydrogen peroxide treatment (Fig. 1). It should be emphasized that HaCaT keratinocytes represent a cellular model of epithelial cells, whereas melanocytes are derived from neural crest cells (79) and therefore represent a different cellular model. Hence, the present study focused on human malignant melanoma cells. Hydrogen peroxide treatment was used to investigate the association between ROS levels and vitamin D analogs, and subsequently the interaction between vitamin D analogs and anticancer drugs was explored.
Secondly, similar effects to those discussed above for hydrogen peroxide were observed for dacarbazine, but not cisplatin, following treatment with 1α,25(OH)2D3, since sensitization of 1α,25(OH)2D3-treated melanoma cells to this drugs was observed. Notably, the highest concentration of cisplatin (300 µM) resulted in a decrease in cell proliferation as measured using the SRB assay, by >90%, whereas treatment with 10 μM dacarbazine decreased proliferation by 50%. Incubation of the melanoma A375 cells with 1α,25(OH)2D3, 20(OH)D3, 21(OH)pD or calcipotriol for 24 h resulted in up to a 20% decrease in cell proliferation at the highest concentrations tested. This inhibitory effect of the vitamin D analogs, with the exception of 20(OH)D3, is consistent with previous studies (80,81), however certain differences were noted in the relative IC50 values [i.e., 1α,25(OH)2D3 relative IC50, 0.274 vs. 6.4 nM reported by Wasiewicz et al (81)]. The variation among the relative IC50 values could be explained by variable experimental conditions, including a shorter incubation time with vitamin D analogs (24 vs. 48 h), as well as a lower FBS concentration in the medium. It is already known that vitamin D inhibits cell proliferation and promotes their differentiation (80,82,83). Therefore, the inhibition of melanoma cell proliferation by vitamin D should not be considered as a direct cytotoxic effect, but rather reveals its antiproliferative potential.
Thirdly, it appears that these two drugs inhibit melanoma cell proliferation via distinct mechanisms (58,66). It should be noted that even though cisplatin and dacarbazine function primarily based on the induction of DNA damage (56,84), it is apparent that these drugs also lead to the generation of ROS inside treated cells (57,58). The current study design was based on a 24 h pre-treatment with vitamin D analogs at a low concentration (100 nM). This corresponds to the optimal level of 25(OH)D3 in the serum (75-125 nM) (28), since, according to Timerman et al (75), vitamin D deficiency is associated with a poorer prognosis in metastatic melanoma. As demonstrated by the cell cycle analyses, induction of apoptosis (increase in SubG1 cell fraction) was observed for the cells treated with cisplatin, consistent with other studies (85,86). On the other hand, the inhibition of melanoma cell proliferation by dacarbazine probably results from cell cycle arrest, as observed from an increase in the number of cells in the G0/G1 fraction (P<0.01) and decreases in the S and G2/M phases (both P<0.01). As expected based on previous studies (15,17,80,82,83), pre-treatment with active forms of vitamin D resulted in an increase in the number of cells in G0/G1, with this effect being observed in cells treated with dacarbazine, but not cisplatin. Notably, the two anticancer drugs exhibited similar effects on oxidative stress. Treatment of the melanoma cells with the two drugs resulted in an initial significant increase in oxidative stress (at 1 h), whereas prolonged incubation (24 h) resulted in a downward trend of 2′,7′-dichlorofluorescein fluorescence in cells treated with cisplatin or dacarbazine compared with the untreated control. The pre-incubation with vitamin D analogs, however, resulted in a drug-specific effect on Δψm. In the case of cisplatin, a significant decrease in Δψm was only observed in cells pre-treated with vitamin D derivatives, 21(OH)pD and calcipotriol (P<0.05 and P<0.01, respectively). It has been reported that cisplatin-resistant lung cancer cells exhibit increased Δψm in comparison with cisplatin-sensitive counterparts (87). Therefore, a decrease in Δψm in cisplatin-treated cells elicited by vitamin D analogs possibly reflects their drug-sensitization potential. The effect of the incubation of melanoma cells with the secosteoids, with the exception of 21(OH)pD, prior to treatment with dacarbazine was dose-dependent, with a decrease in Δψm in cells treated with a low concentration of the drug (2 μM), and an increase at the high concentration (10 μM).
The analyses of the expression levels of selected genes involved in the response to ROS or the modulation of vitamin D activity, revealed potential regulatory properties of dacarbazine. This drug resulted in significant inhibition of the expression of CAT, the alternative vitamin D binding protein encoded by PDIA3, and CYP27B1, with these effects being reversed by pre-treatment with 1α,25(OH)2D3. All the tested analogs efficiently induced the expression of CYP3A4, with the effect of cisplatin or dacarbazine treatment being enhanced by secosteroid pre-treatment. This observation indicates the induction of an anti-xenobiotic response in the melanoma A375 cells. Similar results for cisplatin and other anticancer compounds were reported in hepatocyte-derived HepG2 cells, in which chemotherapeutic agents activated cellular tumor antigen p53 protein to induce the expression of the main enzymes involved in the systemic clearance of these drugs (88). On the other hand, dacarbazine, a prodrug, requires activation by the cytochrome P450 (CYP450) enzyme family, to which the product of CYP3A4 belongs (89). Therefore, the observed induction of CYP3A4 expression may also suggest more efficient oxidation of dacarbazine to its active metabolite in melanoma cells pre-treated with vitamin D. Cells overexpressing another CYP450 family member, CYP450 2E1 (CYP2E1), were revealed to be more sensitive to cisplatin treatment with respect to cell viability and ROS production, compared with cells lacking CYP2E1 expression (90).
CYP24A1 is pivotal for vitamin D homeostasis, since it regulates the serum and tissue levels of 25(OH)D3 and 1α,25(OH)2D3, being the major vitamin D inactivating enzyme (91). A strong induction of CYP24A1 expression was observed with 100 nM 1α,25(OH)2D3 (Fig. 7I). This observation is consistent with previous reports for melanoma A375 cells (81) and HaCaT keratinocytes (35). Notably, an increase in CYP24A1 expression was also observed in cells treated with 12 µM cisplatin or 6 μM dacarbazine alone (Fig. 7I). A similar induction of CYP24A1 expression by cisplatin has been observed in HepG2 cells in a p53-dependent manner (88). Furthermore, dacarbazine is a well known powerful alkylating agent that activates p53 (92). Therefore, the induction of CYP24A1 expression in A375 melanoma cells by this chemotherapeutic agent may involve a p53-dependent mechanism. However, this hypothesis requires further investigation.
Similarities and differences were noted in the phenotypic effects between 1α,25(OH)2D3 and calcipotriol versus non-calcemic 20(OH)D3 and 21(OH)pD. These can be explained by the different receptors targeted by each of these molecules. Although the VDR is the primary target for 1α,25(OH)2D3 and calcipotriol, 20(OH)D3 acts only as a biased agonist on the VDR and can act as a reverse agonist on retinoic acid orphan receptors (46,93-96), whereas its downstream metabolite, 20,23(OH)2D3, acts as an agonist on the aryl hydrocarbon receptor (97). In the case of 21(OH)pD, its nuclear receptor remains to be identified, since it has low or no affinity for the VDR (98). Defining the precise mechanism of action for each secosteroid is a future goal.
In vitro studies require further validation by in vivo animal studies prior to the use of vitamin D in combination with cisplatin or dacarbazine in melanoma treatment. However, pre-clinical models of human melanoma, including cell line-transplantable mouse models, genetically engineered mouse models or immunodeficient mice with patient-derived xenografts (PDOX), do not reflect the true nature of the primary tumor, being controversial in their ability to translate the effectiveness of immunotherapeutic strategies in clinical trials (99,100). Nevertheless, animal models, including PDOX, are the next logical step to discovering novel targets for more efficient combinatorial therapy and approaches to overcome emerging resistance of melanoma cells to any form of treatment (101-103).
Despite not observing pronounced enhancement of anti-melanoma activity by the tested chemotherapeutics under the described experimental conditions, the results of the present study have demonstrated that vitamin D analogs modulate the response of melanoma cells to dacarbazine. In conclusion, low- and non-calcemic vitamin D analogs may serve as beneficial adjuvant agents in chemotherapy, particularly in patients suffering from vitamin D deficiency.
Acknowledgments
Not applicable.
Abbreviations
- 1α,25(OH)2D3
1α,25-dihydroxyvitamin D3
- 20(OH) D3
20S-hydroxyvitamin D3
- 21(OH)pD
21-hydroxypregnacalciferol
- 25(OH)D3
25-hydroxyvitamin D3
- CAT
catalase
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- PDIA3
protein disulfide-isomerase A3
- PDOX
patient-derived orthotropic xenograft
- ROS
reactive oxygen species
- SOD1
superoxide dismutase 1
- SOD2
superoxide dismutase 2
- SRB
sulphorhodamine B
- UV
ultraviolet
- VDR
vitamin D receptor
- Δψm
mitochondrial membrane potential
Funding
This study was supported by grant no. MN 01-0250/08/280 from the Medical University of Gdansk (Gdansk, Poland) to AP, and in part by NIH grants (nos. 1R01AR073004-01A1 and 1RO1AR071189-01A1) and a VA merit grant (no. 1I01BX004293-01A1) to ATS.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
MAZ and AP conceived and designed and supervised the study; AP, JW and AR performed the experiments; RCT provided vitamin D analogs; MAZ, AP, JW, RCT and ATS analyzed the data; AP, MAZ, RCT and ATS wrote the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Piotrowska A, Wierzbicka J, Żmijewski MA. Vitamin D in the skin physiology and pathology. Acta Biochim Pol. 2016;63:17–29. doi: 10.18388/abp.2015_1104. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D: Evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 3.Bikle DD. Vitamin D and the skin: Physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev. 2008;66(Suppl 2):S116–S124. doi: 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- 5.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 6.Kubis AM, Piwowar A. The new insight on the regulatory role of the vitamin D3 in metabolic pathways characteristic for cancerogenesis and neurodegenerative diseases. Ageing Res Rev. 2015;24(Pt B):126–137. doi: 10.1016/j.arr.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Slominski AT, Janjetovic Z, Kim TK, Wasilewski P, Rosas S, Hanna S, Sayre RM, Dowdy JC, Li W, Tuckey RC. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J Steroid Biochem Mol Biol. 2015;148:52–63. doi: 10.1016/j.jsbmb.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon-Thomson C, Gupta R, Tongkao-on W, Ryan A, Halliday GM, Mason RS. 1α,25 dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem Photobiol Sci. 2012;11:1837–1847. doi: 10.1039/c2pp25202c. [DOI] [PubMed] [Google Scholar]
- 9.Fedirko V, Bostick RM, Long Q, Flanders WD, McCullough ML, Sidelnikov E, Daniel CR, Rutherford RE, Shaukat A. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: A randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19:280–291. doi: 10.1158/1055-9965.EPI-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang YJ, Teichert AE, Fong F, Oda Y, Bikle DD. 1α,25(OH)2-dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the β-catenin pathway. J Steroid Biochem Mol Biol. 2013;136:229–232. doi: 10.1016/j.jsbmb.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, Posner GH, Ishizuka S, Halliday GM, Reeve VE, et al. Skin cancer prevention: A possible role of 1,25dihydroxyvitamin D3 and its analogs. J Steroid Biochem Mol Biol. 2005;97:137–143. doi: 10.1016/j.jsbmb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Wong G, Gupta R, Dixon KM, Deo SS, Choong SM, Halliday GM, Bishop JE, Ishizuka S, Norman AW, Posner GH, et al. 1,25-Dihydroxyvitamin D and three low-calcemic analogs decrease UV-induced DNA damage via the rapid response pathway. J Steroid Biochem Mol Biol. 2004;89–90:567–570. doi: 10.1016/j.jsbmb.2004.03.072. [DOI] [PubMed] [Google Scholar]
- 13.Bikle DD, Jiang Y, Nguyen T, Oda Y, Tu CL. Disruption of Vitamin D and Calcium Signaling in Keratinocytes Predisposes to Skin Cancer. Front Physiol. 2016;7:296. doi: 10.3389/fphys.2016.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick MF. Sunlight, ultraviolet radiation, vitamin D and skin cancer: How much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16. [PubMed] [Google Scholar]
- 15.Slominski AT, Brożyna AA, Skobowiat C, Zmijewski MA, Kim TK, Janjetovic Z, Oak AS, Jozwicki W, Jetten AM, Mason RS, et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J Steroid Biochem Mol Biol. 2018;177:159–170. doi: 10.1016/j.jsbmb.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin L, Ordóñez-Mena JM, Chen T, Schöttker B, Arndt V, Brenner H. Circulating 25-hydroxyvitamin D serum concentration and total cancer incidence and mortality: A systematic review and meta-analysis. Prev Med. 2013;57:753–764. doi: 10.1016/j.ypmed.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Reichrath J, Zouboulis CC, Vogt T, Holick MF. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: An historical view and outlook. Rev Endocr Metab Disord. 2016;17:405–417. doi: 10.1007/s11154-016-9353-4. [DOI] [PubMed] [Google Scholar]
- 18.Brożyna AA, Jóźwicki W, Janjetovic Z, Slominski AT. Expression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1) decreases during melanoma progression. Hum Pathol. 2013;44:374–387. doi: 10.1016/j.humpath.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brożyna AA, Jóźwicki W, Slominski AT. Decreased VDR expression in cutaneous melanomas as marker of tumor progression: New data and analyses. Anticancer Res. 2014;34:2735–2743. [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Trump DL, Johnson CS. Vitamin D in combination cancer treatment. J Cancer. 2010;1:101–107. doi: 10.7150/jca.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan AV, Swami S, Feldman D. Equivalent anticancer activities of dietary vitamin D and calcitriol in an animal model of breast cancer: Importance of mammary CYP27B1 for treatment and prevention. J Steroid Biochem Mol Biol. 2013;136:289–295. doi: 10.1016/j.jsbmb.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris HA. Vitamin D activities for health outcomes. Ann Lab Med. 2014;34:181–186. doi: 10.3343/alm.2014.34.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell D. The relationship between vitamin D and cancer. Clin J Oncol Nurs. 2011;15:557–560. doi: 10.1188/11.CJON.557-560. [DOI] [PubMed] [Google Scholar]
- 24.Welsh J, Wietzke JA, Zinser GM, Byrne B, Smith K, Narvaez CJ. Vitamin D-3 receptor as a target for breast cancer prevention. J Nutr. 2003;133(Suppl):2425S–2433S. doi: 10.1093/jn/133.7.2425S. [DOI] [PubMed] [Google Scholar]
- 25.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: Molecular mechanisms. Nat Rev Cancer. 2003;3:601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 26.Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: A reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011;94:1144–1149. doi: 10.3945/ajcn.111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant WB. 25-hydroxyvitamin D and breast cancer, colorectal cancer, and colorectal adenomas: Case-control versus nested case-control studies. Anticancer Res. 2015;35:1153–1160. [PubMed] [Google Scholar]
- 28.Płudowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna-Sokół D, Czech-Kowalska J, Dębski R, Decsi T, Dobrzańska A, Franek E, et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe - recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64:319–327. doi: 10.5603/EP.2013.0012. [DOI] [PubMed] [Google Scholar]
- 29.Skobowiat C, Oak AS, Kim TK, Yang CH, Pfeffer LM, Tuckey RC, Slominski AT. Noncalcemic 20-hydroxyvitamin D3 inhibits human melanoma growth in in vitro and in vivo models. Oncotarget. 2017;8:9823–9834. doi: 10.18632/oncotarget.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slominski AT, Brożyna AA, Zmijewski MA, Jóźwicki W, Jetten AM, Mason RS, Tuckey RC, Elmets CA. Vitamin D signaling and melanoma: Role of vitamin D and its receptors in melanoma progression and management. Lab Invest. 2017;97:706–724. doi: 10.1038/labinvest.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Yu WD, Trump DL, Johnson CS. 1,25D3 enhances antitumor activity of gemcitabine and cisplatin in human bladder cancer models. Cancer. 2010;116:3294–3303. doi: 10.1002/cncr.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rassnick KM, Muindi JR, Johnson CS, Balkman CE, Ramnath N, Yu WD, Engler KL, Page RL, Trump DL. In vitro and in vivo evaluation of combined calcitriol and cisplatin in dogs with spontaneously occurring tumors. Cancer Chemother Pharmacol. 2008;62:881–891. doi: 10.1007/s00280-008-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wietrzyk J, Nevozhay D, Filip B, Milczarek M, Kutner A. The antitumor effect of lowered doses of cytostatics combined with new analogs of vitamin D in mice. Anticancer Res. 2007;27(5A):3387–3398. [PubMed] [Google Scholar]
- 34.Podgorska E, Drzal A, Matuszak Z, Swakon J, Slominski A, Elas M, Urbanska K. Calcitriol and Calcidiol Can Sensitize Melanoma Cells to Low(−)LET Proton Beam Irradiation. Int J Mol Sci. 2018;19:19. doi: 10.3390/ijms19082236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piotrowska A, Wierzbicka J, Ślebioda T, Woźniak M, Tuckey RC, Slominski AT, Żmijewski MA. Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes. Steroids. 2016;110:49–61. doi: 10.1016/j.steroids.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saw RP, Armstrong BK, Mason RS, Morton RL, Shannon KF, Spillane AJ, Stretch JR, Thompson JF. Adjuvant therapy with high dose vitamin D following primary treatment of melanoma at high risk of recurrence: A placebo controlled randomised phase II trial (ANZMTG 02.09 Mel-D) BMC Cancer. 2014;14:780. doi: 10.1186/1471-2407-14-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pettijohn E, Martone B, Rademaker A, Kuzel T. A phase I study of high-dose calcitriol in combination with temozolomide for patients with metastatic melanoma. J Pers Med. 2014;4:448–458. doi: 10.3390/jpm4040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batus M, Waheed S, Ruby C, Petersen L, Bines SD, Kaufman HL. Optimal management of metastatic melanoma: Current strategies and future directions. Am J Clin Dermatol. 2013;14:179–194. doi: 10.1007/s40257-013-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah DJ, Dronca RS. Latest advances in chemotherapeutic, targeted, and immune approaches in the treatment of metastatic melanoma. Mayo Clin Proc. 2014;89:504–519. doi: 10.1016/j.mayocp.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold M, de Vries E, Whiteman DC, Jemal A, Bray F, Parkin DM, Soerjomataram I. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int J Cancer. 2018;143:1305–1314. doi: 10.1002/ijc.31527. [DOI] [PubMed] [Google Scholar]
- 41.Mattia G, Puglisi R, Ascione B, Malorni W, Carè A, Matarrese P. Cell death-based treatments of melanoma:conventional treatments and new therapeutic strategies. Cell Death Dis. 2018;9:112. doi: 10.1038/s41419-017-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slominski AT, Carlson JA. Melanoma resistance: A bright future for academicians and a challenge for patient advocates. Mayo Clin Proc. 2014;89:429–433. doi: 10.1016/j.mayocp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo A, Ficili B, Candido S, Pezzino FM, Guarneri C, Biondi A, Travali S, McCubrey JA, Spandidos DA, Libra M. Emerging targeted therapies for melanoma treatment (Review) Int J Oncol. 2014;45:516–524. doi: 10.3892/ijo.2014.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 45.Johnson DB, Pollack MH, Sosman JA. Emerging targeted therapies for melanoma. Expert Opin Emerg Drugs. 2016;21:195–207. doi: 10.1080/14728214.2016.1184644. [DOI] [PubMed] [Google Scholar]
- 46.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014;144(Pt A):28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, Sweatman T, Li W, Zjawiony J, Miller D, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, Postlethwaite AE, Miller D, Li W. 20-hydroxyvitamin D3 inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–746. [PMC free article] [PubMed] [Google Scholar]
- 49.Wasiewicz T, Szyszka P, Cichorek M, Janjetovic Z, Tuckey RC, Slominski AT, Zmijewski MA. Antitumor effects of vitamin d analogs on hamster and mouse melanoma cell lines in relation to melanin pigmentation. Int J Mol Sci. 2015;16:6645–6667. doi: 10.3390/ijms16046645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zmijewski MA, Li W, Chen J, Kim TK, Zjawiony JK, Sweatman TW, Miller DD, Slominski AT. Synthesis and photochemical transformation of 3β,21-dihydroxypregna-5,7-die n-20-one to novel secosteroids that show anti-melanoma activity. Steroids. 2011;76:193–203. doi: 10.1016/j.steroids.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koul PA, Ahmad SH, Ahmad F, Jan RA, Shah SU, Khan UH. Vitamin d toxicity in adults: A case series from an area with endemic hypovitaminosis d. Oman Med J. 2011;26:201–204. doi: 10.5001/omj.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Podgorska E, Sniegocka M, Mycinska M, Trybus W, Trybus E, Kopacz-Bednarska A, Wiechec O, Krzykawska-Serda M, Elas M, Krol T, et al. Acute hepatologic and nephrologic effects of calcitriol in Syrian golden hamster (Mesocricetus auratus) Acta Biochim Pol. 2018;65:351–358. doi: 10.18388/abp.2018_2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, Benson HA, Korik E, Janjetovic Z, Chen J, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–3915. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK, Tuckey RC. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015;5:14875. doi: 10.1038/srep14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, Tuckey RC. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol. 2015;151:25–37. doi: 10.1016/j.jsbmb.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, Doetsch PW. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One. 2013;8:e81162. doi: 10.1371/journal.pone.0081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pourahmad J, Amirmostofian M, Kobarfard F, Shahraki J. Biological reactive intermediates that mediate dacarbazine cytotoxicity. Cancer Chemother Pharmacol. 2009;65:89–96. doi: 10.1007/s00280-009-1007-8. [DOI] [PubMed] [Google Scholar]
- 59.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aykul S, Martinez-Hackert E. Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal Biochem. 2016;508:97–103. doi: 10.1016/j.ab.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalliokoski T, Kramer C, Vulpetti A, Gedeck P. Comparability of mixed IC50 data - a statistical analysis. PLoS One. 2013;8:e61007. doi: 10.1371/journal.pone.0061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 63.Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, Nguyen MN, Tuckey RC. Novel vitamin D hydroxy-derivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 2012;32:3733–3742. [PMC free article] [PubMed] [Google Scholar]
- 64.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, Pfeffer SR, Pfeffer LM, Slominski AT. High basal NF-κB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–1884. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Corazao-Rozas P, Guerreschi P, Jendoubi M, André F, Jonneaux A, Scalbert C, Garçon G, Malet-Martino M, Balayssac S, Rocchi S, et al. Mitochondrial oxidative stress is the Achille’s heel of melanoma cells resistant to Braf-mutant inhibitor. Oncotarget. 2013;4:1986–1998. doi: 10.18632/oncotarget.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le TYL, Ogawa M, Kizana E, Gunton JE, Chong JJH. Vitamin D Improves Cardiac Function After Myocardial Infarction Through Modulation of Resident Cardiac Progenitor Cells. Heart Lung Circ. 2018;27:967–975. doi: 10.1016/j.hlc.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Cai L, Luo L, Tang Z, Meng X. Combined antitumor effects of 1,25 dihydroxy vitamin D3 and Notch inhibitor in liver cancer. Oncol Rep. 2018;40:1515–1524. doi: 10.3892/or.2018.6549. [DOI] [PubMed] [Google Scholar]
- 69.Corachan A, Ferrero H, Aguilar A, Garcia N, Monleon J, Faus A, Cervelló I, Pellicer A. Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway. Fertil Steril. 2019;111:397–407. doi: 10.1016/j.fertnstert.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How UV Light Touches the Brain and Endocrine System Through Skin, and Why. Endocrinology. 2018;159:1992–2007. doi: 10.1210/en.2017-03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2 vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 73.Moukayed M, Grant WB. Molecular link between vitamin D and cancer prevention. Nutrients. 2013;5:3993–4021. doi: 10.3390/nu5103993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Timerman D, McEnery-Stonelake M, Joyce CJ, Nambudiri VE, Hodi FS, Claus EB, Ibrahim N, Lin JY. Vitamin D deficiency is associated with a worse prognosis in metastatic melanoma. Oncotarget. 2017;8:6873–6882. doi: 10.18632/oncotarget.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caini S, Boniol M, Tosti G, Magi S, Medri M, Stanganelli I, Palli D, Assedi M, Marmol VD, Gandini S. Vitamin D and melanoma and non-melanoma skin cancer risk and prognosis: A comprehensive review and meta-analysis. Eur J Cancer. 2014;50:2649–2658. doi: 10.1016/j.ejca.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 77.Wyatt C, Lucas RM, Hurst C, Kimlin MG. Vitamin D deficiency at melanoma diagnosis is associated with higher Breslow thickness. PLoS One. 2015;10:e0126394. doi: 10.1371/journal.pone.0126394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogbah Z, Visa L, Badenas C, Ríos J, Puig-Butille JA, Bonifaci N, Guino E, Augé JM, Kolm I, Carrera C, et al. Serum 25-hydroxyvitamin D3 levels and vitamin D receptor variants in melanoma patients from the Mediterranean area of Barcelona. BMC Med Genet. 2013;14:26. doi: 10.1186/1471-2350-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sinnberg T, Levesque MP, Krochmann J, Cheng PF, Ikenberg K, Meraz-Torres F, Niessner H, Garbe C, Busch C. Wnt-signaling enhances neural crest migration of melanoma cells and induces an invasive phenotype. Mol Cancer. 2018;17:59. doi: 10.1186/s12943-018-0773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Piotrowska A, Wierzbicka J, Nadkarni S, Brown G, Kutner A, Żmijewski MA. Antiproliferative Activity of Double Point Modified Analogs of 1,25-Dihydroxyvitamin D2 Against Human Malignant Melanoma Cell Lines. Int J Mol Sci. 2016;17:17. doi: 10.3390/ijms17010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wasiewicz T, Piotrowska A, Wierzbicka J, Slominski AT, Zmijewski MA. Antiproliferative Activity of Non-Calcemic Vitamin D Analogs on Human Melanoma Lines in Relation to VDR and PDIA3 Receptors. Int J Mol Sci. 2018;19:19. doi: 10.3390/ijms19092583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Field S, Newton-Bishop JA. Melanoma and vitamin D. Mol Oncol. 2011;5:197–214. doi: 10.1016/j.molonc.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szyszka P, Zmijewski MA, Slominski AT. New vitamin D analogs as potential therapeutics in melanoma. Expert Rev Anticancer Ther. 2012;12:585–599. doi: 10.1586/era.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Al-Qatati A, Aliwaini S. Combined pitavastatin and dacarbazine treatment activates apoptosis and autophagy resulting in synergistic cytotoxicity in melanoma cells. Oncol Lett. 2017;14:7993–7999. doi: 10.3892/ol.2017.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Del Bello B, Toscano M, Moretti D, Maellaro E. Cisplatin-induced apoptosis inhibits autophagy, which acts as a pro-survival mechanism in human melanoma cells. PLoS One. 2013;8:e57236. doi: 10.1371/journal.pone.0057236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kissel CK, Schadendorf D, Röckmann H. The altered apoptotic pathways in cisplatin and etoposide-resistant melanoma cells are drug specific. Melanoma Res. 2006;16:527–535. doi: 10.1097/CMR.0b013e3280103a7c. [DOI] [PubMed] [Google Scholar]
- 87.Wangpaichitr M, Wu C, Li YY, Nguyen DJM, Kandemir H, Shah S, Chen S, Feun LG, Prince JS, Kuo MT, et al. Exploiting ROS and metabolic differences to kill cisplatin resistant lung cancer. Oncotarget. 2017;8:49275–49292. doi: 10.18632/oncotarget.17568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldstein I, Rivlin N, Shoshana OY, Ezra O, Madar S, Goldfinger N, Rotter V. Chemotherapeutic agents induce the expression and activity of their clearing enzyme CYP3A4 by activating p53. Carcinogenesis. 2013;34:190–198. doi: 10.1093/carcin/bgs318. [DOI] [PubMed] [Google Scholar]
- 89.Ortiz de Montellano PR. Cytochrome P450-activated prodrugs. Future Med Chem. 2013;5:213–228. doi: 10.4155/fmc.12.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu Y, Cederbaum AI. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol Sci. 2006;89:515–523. doi: 10.1093/toxsci/kfj031. [DOI] [PubMed] [Google Scholar]
- 91.Wierzbicka J, Piotrowska A, Zmijewski MA. The renaissance of vitamin D. Acta Biochim Pol. 2014;61:679–686. doi: 10.18388/abp.2014_1830. [DOI] [PubMed] [Google Scholar]
- 92.Box NF, Vukmer TO, Terzian T. Targeting p53 in melanoma. Pigment Cell Melanoma Res. 2014;27:8–10. doi: 10.1111/pcmr.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slominski AT, Kim TK, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, Li W, Tuckey RC, Jetten AM. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as ‘biased’ agonists on VDR and inverse agonists on RORα and RORγ. J Steroid Biochem Mol Biol. 2017;173:42–56. doi: 10.1016/j.jsbmb.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, Wang J, Postlethwaite A, Li W, Tuckey RC, et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014;28:2775–2789. doi: 10.1096/fj.13-242040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin Z, Marepally SR, Goh ESY, Cheng CYS, Janjetovic Z, Kim TK, Miller DD, Postlethwaite AE, Slominski AT, Tuckey RC, et al. Investigation of 20S-hydroxyvitamin D3 analogs and their 1α-OH derivatives as potent vitamin D receptor agonists with anti-inflammatory activities. Sci Rep. 2018;8:1478. doi: 10.1038/s41598-018-19183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin Z, Chen H, Belorusova AY, Bollinger JC, Tang EKY, Janjetovic Z, Kim TK, Wu Z, Miller DD, Slominski AT, et al. 1α,20S-Dihydroxyvitamin D3 Interacts with Vitamin D Receptor: Crystal Structure and Route of Chemical Synthesis. Sci Rep. 2017;7:10193. doi: 10.1038/s41598-017-10917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Slominski AT, Kim TK, Janjetovic Z, Brożyna AA, Żmijewski MA, Xu H, Sutter TR, Tuckey RC, Jetten AM, Crossman DK. Differential and Overlapping Effects of 20,23(OH)2 D3 Epidermal Keratinocytes: Identification of AhR as an Alternative and 1,25(OH)2D3 on Gene Expression in Human Receptor for 20,23(OH)2D3. Int J Mol Sci. 2018;19:19. doi: 10.3390/ijms19103072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, Tang EK, Miller D, Li W, Slominski AT. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol. 2012;361:143–152. doi: 10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barutello G, Rolih V, Arigoni M, Tarone L, Conti L, Quaglino E, Buracco P, Cavallo F, Riccardo F. Strengths and Weaknesses of Pre-Clinical Models for Human Melanoma Treatment: Dawn of Dogs’ Revolution for Immunotherapy. Int J Mol Sci. 2018;19:19. doi: 10.3390/ijms19030799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuzu OF, Nguyen FD, Noory MA, Sharma A. Current State of Animal (Mouse) Modeling in Melanoma Research. Cancer Growth Metastasis. 2015;8(Suppl 1):81–94. doi: 10.4137/CGM.S21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoffman RM. Patient-Derived Orthotopic Xenograft (PDOX) Models of Melanoma. Int J Mol Sci. 2017;18:18. doi: 10.3390/ijms18091875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawaguchi K, Han Q, Li S, Tan Y, Igarashi K, Miyake K, Kiyuna T, Miyake M, Chemielwski B, Nelson SD, et al. Intra-tumor L-methionine level highly correlates with tumor size in both pancreatic cancer and melanoma patient-derived orthotopic xenograft (PDOX) nude-mouse models. Oncotarget. 2018;9:11119–11125. doi: 10.18632/oncotarget.24264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kawaguchi K, Igarashi K, Li S, Han Q, Tan Y, Miyake K, Kiyuna T, Miyake M, Murakami T, Chmielowski B, et al. Recombinant methioninase (rMETase) is an effective therapeutic for BRAF-V600E-negative as well as -positive melanoma in patient-derived orthotopic xenograft (PDOX) mouse models. Oncotarget. 2017;9:915–923. doi: 10.18632/oncotarget.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.