Life history explains most contrasts in functional traits and climatic niches in subtropical grasses from China, and differentiation between annual and perennial species is greater within C3 than C4 grasses.
Keywords: C4 photosynthesis, climatic niche, functional traits, hydraulic conductance, leaf and stem anatomy, phylogeny, Poaceae, seasonality
Abstract
Life history and photosynthetic type both affect the economics of leaf physiological function. Annual plants have lower tissue densities and resource-use efficiencies than perennials, while C4 photosynthesis, facilitated in grasses by specific changes in leaf anatomy, improves photosynthetic efficiency and water-use efficiency, especially in hot climates. This study aimed to determine whether C4 photosynthesis affects differences in functional traits between annual and perennial species. We measured 26 traits and characterised niche descriptors for 42 grasses from subtropical China. Differences in the majority of traits were explained by life history. The ranges of annual species (particularly C4 annuals) extended to regions with greater temperature seasonality and lower precipitation, and annuals had less-negative turgor-loss points, higher specific leaf areas, and lower water-use efficiencies, stomatal conductances, and leaf areas per stem area than perennials. Photosynthetic type largely affected leaf physiology as expected, but interacted with life history in determining specific traits. Leaf hydraulic conductance was intermediate in perennials, highest in C4-annuals, and lowest in C3-annuals. Densities of stomata and stem vessels were similar across C3-perennials and C4 species, but stomatal densities were lower and stem vessel densities higher in C3-annuals. Phylogenetic principal component analysis confirmed that in this subtropical environment life history is the predominant axis separating species, and annuals and perennials were more different within C3 than C4 grasses. The interplay between life history and photosynthetic type may be an overlooked factor in shaping the physiological ecology of grasses.
Introduction
Leaf physiology and the economics of leaf resource use, including water use, are key constraints on plant performance and ecological strategies. Annual and perennial life histories are linked with a ‘fast–slow’ leaf economic spectrum, in which short life spans are linked with fast resource acquisition and less efficient resource use (Wright et al., 2004; Reich, 2014; Díaz et al., 2016). Leaf economics also differ between C3 and C4 plants. C4 photosynthesis increases photosynthetic efficiency and is commonly associated with changes in vascular spacing (Sage, 2004; Christin and Osborne, 2014), which should impact the relative costs of leaf construction (Niinemets et al., 2007). C4 photosynthesis has had dramatic effects on the macroevolution and macroecology of plants (Ehleringer et al., 1997; Sage, 2004; Edwards et al., 2010; Griffith et al., 2015), but the functional consequences of C4 photosynthesis have commonly been treated as independent of the unique features of species and lineages that utilise them (Edwards et al., 2007). Plant lineages such as the grasses, that show multiple independent origins of C4 photosynthesis, provide opportunities to address the impact that photosynthetic type has had on the physiological performance and ecological strategies exploited by plants while accounting for lineage-specific differences (Edwards et al., 2010).
Plant physiologists and ecologists have been fascinated by the physiological contrast between C3 and C4 plants since the discovery of C4 photosynthesis in the mid-20th century (Slack and Hatch, 1967; Osmond et al., 1982; Pearcy and Ehleringer, 1984; Ehleringer and Monson, 1993; Ehleringer et al., 1997; Sage et al., 2012). In circumstances where higher temperatures and/or low CO2 availability limit photosynthesis by exacerbating inefficiencies associated with photorespiration, C4 photosynthesis improves the rate and efficiency of net CO2 assimilation (A) compared with C3 photosynthesis (Ehleringer and Pearcy, 1983; Sage et al., 2012). The evolution of C4 grasses has therefore been linked with physiological advantages under low inter-glacial atmospheric CO2 concentrations (Ehleringer et al., 1997; Christin et al., 2008), higher leaf temperatures (Ehleringer et al., 1997), and in drier or more open habitats with higher irradiance and vapour pressure deficits (VPD) (Osborne and Freckleton, 2009; Edwards and Smith, 2010). However, recent research taking advantage of the multiple evolutionary origins of C4 photosynthesis in the grass family has demonstrated that ecological and physiological differences are attributable not only to photosynthetic type, but also to differences among lineages (Edwards and Still, 2008; Edwards and Smith, 2010; Taylor et al., 2010; Liu and Osborne, 2015). A key insight is that the outcomes of eco-physiological comparisons between C3 and C4 grasses in temperate ecosystems are confounded with phylogeny (Edwards et al., 2007). The dominant C3 grasses in temperate ecosystems arise from the Pooideae subfamily, which is phylogenetically independent of C4 Poaceae and linked with preferences for cooler habitats compared with other C3 lineages in the grass family (Edwards and Still, 2008; Edwards and Smith, 2010; Vigeland et al., 2013). Studies that focus on variation in subtropical species and communities are therefore crucial. They have potential to improve our understanding of the ecological factors underpinning the nearly 25% of terrestrial primary productivity contributed by C4 grasses (Still et al., 2003), to help predict the impacts of high-yielding C4 bioenergy crops (Heaton et al., 2008), and to facilitate attempts to engineer a C4 biochemistry into key C3 crop species (von Caemmerer et al., 2012).
Because C4 photosynthesis fundamentally affects the physiological trade-off between CO2 assimilation and water loss through stomata, it has been suggested repeatedly that shifts in plant hydraulics and water use associated with C4 evolutionary origins have influenced the ecology of C4 species (Edwards and Still, 2008; Osborne and Freckleton, 2009; Edwards and Smith, 2010). Evidence from dicot C4 species suggests that increased CO2 assimilation relative to water loss facilitates diversification in ecological strategies. C4 plants either support greater leaf area for a given stem water supply, or for an equivalent leaf area develop higher-density, lower-conductance stem tissues that are more resistant to hydraulic failure (Kocacinar and Sage, 2003). However, while improvements in the efficiency of CO2 assimilation mean that C4 photosynthesis can support novel hydraulic strategies, C4 photosynthesis in grasses is also associated with the constraint of Kranz anatomy. Kranz anatomy increases the ratio of bundle-sheath/mesophyll tissue (BS:MC) and is linked with decreased inter-vein distances (IVD) (Ueno et al., 2006; Christin et al., 2013; Griffiths et al., 2013; Lundgren et al., 2014). Such leaf-level anatomical differences have been linked with differences in the ecology of C3 and C4 grass species. In phylogenetically controlled comparisons, the evolution of Kranz anatomy and lower anatomical capacity for stomatal conductance to water (gwmax) have been shown to match the distribution of C4 grasses in drier habitats than C3 grasses (Taylor et al., 2012; Griffiths et al., 2013). Unfortunately, studies of leaf hydraulic performance in C3 and C4 grasses have either compared a limited number of species (Taylor et al., 2018) or, because they were representative of a temperate community, included mostly Pooid C3 species that are phylogenetically distant from C4 grasses (Ocheltree et al., 2014). Because leaves can contribute as much as 90% of total plant hydraulic resistance (Sack and Holbrook, 2006) and anatomical differences associated with Kranz anatomy may have significant effects on leaf hydraulic properties (Buckley et al., 2015) and construction costs (Niinemets et al., 2007), characterising leaf functional traits associated with hydraulic performance in subtropical grasses should provide key insights to the ecological importance of C4 photosynthesis.
Like C4 photosynthesis, life history is linked with effects on suites of structural and physiological functional traits (Reich, 2014). It is generally expected that, consistent with the economics of leaf construction and resource use (Grime and Hunt, 1975; Wright and Westoby, 2002), the shorter life spans of annual plants will be linked with lower tissue densities, higher photosynthetic rates, greater allocation to leaf mass and area, and higher relative growth rate than for longer lived perennials (Grime and Hunt, 1975; Garnier et al., 1997). Among herbaceous species, which include the majority of grasses, annual growth strategies are commonly linked with specific adaptations to habitat, for example through escape from drought (Volaire et al., 1998) or competition (Grime, 2006). By improving CO2 assimilation efficiency, C4 photosynthesis may decrease the relative importance of trade-offs between rapid resource acquisition and resource-use efficiency, and/or support novel ecological strategies linked with changes in growth rate or differential allocation of resources (Long, 1999). There has therefore been ongoing debate about whether C4 vegetation is intrinsically more productive (e.g. Osmond et al., 1982; Ehleringer et al., 1997) or exhibits greater niche specialisation (Sage et al., 2011). Recent evidence has supported diversification of functional strategies and expansion by C4 populations into a broader range of habitats compared with C3 sister groups (Lundgren et al., 2014; Atkinson et al., 2016). Efficiencies associated with C4 photosynthesis may, therefore, support diversification in ecological strategies while buffering against the potential costs of constrained leaf anatomy.
In this study, we quantified 26 functional traits for leaves and stems of 42 Poaceae species that grow together in subtropical China, and collected geographic data for climate proxies associated with the global distributions of these species. Our first objective was to assess the relative influences of life history (annual/perennial) and photosynthetic type (C3/C4) on different functional traits. We expected that (1) annual grasses would show functional traits linked with high-turnover, low-efficiency strategies, for example greater specific leaf areas and water transport capacities but decreased water-use efficiency compared with perennial grasses; and that (2) C4 grasses would show high intrinsic water-use efficiency, increased investment in vasculature, and decreased variability in structural properties among species. In addition, because increased resource-use efficiency might compensate for construction costs, we hypothesised that (3) C4 photosynthesis would be linked with decreased amounts of functional trait differentiation between annual and perennial species.
Our second objective was to determine whether the habitat preferences of annual and perennial grasses are influenced by photosynthetic type. We expected that differences in habitat characteristics between C3 annual and perennial species would be greater than differences between C4 annual and perennial species.
Materials and methods
Species and growth conditions
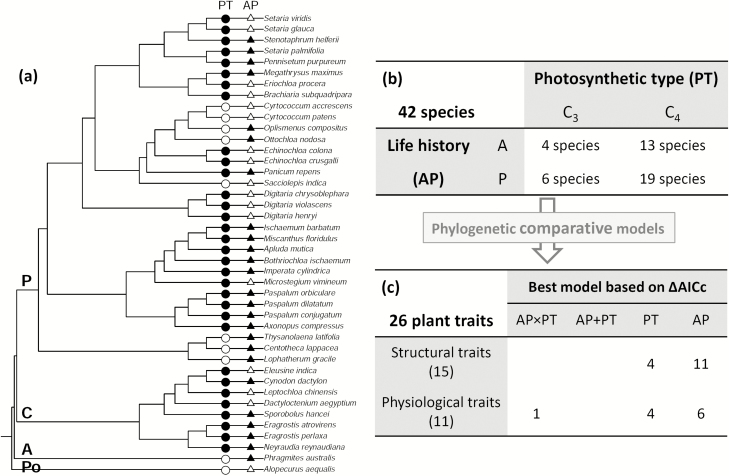
Experiments were carried out at the South China Botanical Garden, Guangzhou, China (23°11´N, 113°21´E, 100 m altitude). In 2013, 60 native grass species were surveyed widely in different habitats in Guangdong province (mountains, roadsides, farmlands, etc.), transplanted into a greenhouse, and identified at the South China Plants Identification Center, allowing assignment of photosynthetic types according to published literature (Sage et al., 1999; Grass Phylogeny Working Group II, 2012) (see Supplementary Table S1 at JXB online). Seeds were harvested in 2013 and plants for experiments were germinated in an incubator in April 2014. After excluding 18 species with low germination rates or that had many similar congeners, 42 species were retained: four C3-annuals, six C3-perennials, 13 C4-annuals, and 19 C4-perennials (Fig. 1). This mixture of species was representative of the native flora; in this subtropical monsoon climatic region, there are 316 Poaceae species excluding woody bamboos, of which 9% are C3-annuals, 16% C3-perennials, 26% C4-annuals, and 50% C4-perennials (South China Botanical Garden, 2009).
Fig. 1.
Classification of grass species, data framework, and summary of best-fit models for interspecific variation in functional traits. (a) Cladogram of the 42 grass species from subtropical China. Bold letters near the branch nodes indicate the subfamilies: Panicoideae (P), Chloridoideae (C), Arundinoideae (A), and Pooideae (Po). Symbols indicate the photosynthetic type (PT: C3, open circles; C4, black circles) and life history (AP: annual, open triangles; perennial, black triangles). (b) Breakdown of species numbers based on categorisations of PT and AP. (c) Breakdown of best-fit models for plant functional traits, indicating the modelled factors and the number of traits for which each model provided the strongest fit based on Table 2.
Seedlings were transferred into 4-l pots filled with 1:1 compost (pH 5.6–7, Penglong Gardening Company, Guangdong, China) and sieved topsoil, and were grown in an open-ended, plastic-covered greenhouse until measurements were made in July 2014. The 42 species were replicated in five randomised blocks. All plants were watered daily. During the experimental period, the mean daytime temperature in the greenhouse was 35±3 °C, relative humidity 50±10%, and sunny-day irradiance ~1000 μmol m–2 s–1, whilst at night the mean temperature was 26±2 °C and relative humidity 96±4%, similar to local ambient conditions (day, 32±4 °C, 73±15%, 1200 μmol m–2 s–1; night, 28±2 °C, 86±10%) (as determined using an ECH2O Utility, Decagon Devices Inc. WA, USA).
Functional traits
We determined values for 26 functional traits (Table 1, Supplementary Table S2).
Table 1.
Abbreviations, units, and classifications for the 26 functional traits and six climatic niche descriptors of the 42 species used in this study.
| Abbreviation and unit | Trait classification | |
| Plant functional traits | ||
| Plant height | H (cm) | Structural |
| Leaf area | LA (cm2) | Structural |
| Leaf thickness | LT (µm) | Structural |
| Specific leaf area | SLA (cm2 g–1) | Structural |
| Leaf dry matter content | LDMC (%) | Structural |
| Leaf interveinal distance | IVD (µm) | Structural |
| Maximum diameter of leaf vein bundles | Dlvb (µm) | Structural |
| Leaf to stem area ratio | A L/AS (cm2 mm–2) | Structural |
| Stomatal size | sts (µm2) | Structural |
| Stomatal density | std (mm–2) | Structural |
| Stem cross-section area | SS (mm2) | Structural |
| Diameter of stem vessels | Dsv (µm) | Structural |
| Stem vessel density | SVD (mm–2) | Structural |
| Stem density | SD (g cm–3) | Structural |
| Leaf carbon content | LC (%) | Structural |
| Leaf hydraulic conductance | K leaf (mmol m–2 s–1 MPa–1) | Physiological |
| Stem vessel area specific hydraulic conductivity | K S (kg m–1 s–1 MPa–1) | Physiological |
| Leaf specific hydraulic conductivity | K L (10–4 kg m–1 s–1 MPa–1) | Physiological |
| Predawn leaf water potential | Ψ pre (MPa) | Physiological |
| Midday leaf water potential | Ψ mid (MPa) | Physiological |
| Leaf turgor loss point | Ψ tlp (MPa) | Physiological |
| Photosynthetic rate | A (µmol m–2 s–1) | Physiological |
| Stomatal conductance | g s (mol m–2 s–1) | Physiological |
| Maximum stomatal conductance to water vapour | g wmax (mol m–2 s–1) | Physiological |
| Intrinsic water use efficiency | WUEi (µmol mol–1) | Physiological |
| Leaf δ13C | Leaf δ13C (‰) | Physiological |
| Niche descriptors | ||
| Mean annual precipitation for each species | MAP (mm) | — |
| Mean annual temperature for each species | MAT (°C) | — |
| Seasonality of annual precipitation | Ps | — |
| Seasonality of annual temperature | Ts | — |
| Mean tree cover in local habitats | Tree cover (%) | — |
| Mean wet days per year in local habitats | Wet days per year | — |
See Supplementary Table S2 for the original data.
Plant height (H) was measured for five mature plants using a ruler. Epidermal peels from both leaf surfaces of three mature fresh leaves per plant were used to determine stomatal traits [guard cell length (gl) and width (gw); stomatal size (sts) and stomatal density (std)]. Because seven of the 42 species were hypostomatous, gl, gw, sts, and std were compared only for the abaxial surface, but values from both surfaces were combined to predict theoretical maximal stomatal conductance to water (gwmax; see Supplementary Protocol S1). Hand-cut cross-sections from three leaves and stems per plant were used to determine leaf thickness (LT), leaf inter-vein distance (IVD), diameter of major leaf-vein bundles (Dlvb), stem cross-sectional size (SS), stem vessel density (SVD), and mean diameter of stem vessels (Dsv). Measurements were made using an upright microscope (Optec, Chongqing Optec Instrument Co. Ltd, China) equipped with a digital camera and a computerised image analysis system (OPTPro2012 version 4.0, Optec software).
Leaf hydraulic conductance (Kleaf) was measured for the youngest mature leaf per plant, using the high-pressure method (HPM) of Postaire et al. (2010) with slight modifications. Leaf blades were excised near the sheath and submerged into a reservoir of degassed and filtered water inside the pressure chamber (Plant Moisture Systems, Corvallis, Oregon, USA), which was used to drive the water through the leaf. Kleaf was calculated as (ΔW2−ΔW1)/[leaf area×time×(Ψ2−Ψ1)], where ΔWi are masses of flow solution collected from the cut surface of the leaf onto pre-weighed tissue papers over 60-s periods. The flow solution was collected first at the balancing pressure for the leaf (Ψ1), then after a pressure increase of ~0.5 MPa (Ψ2) (Postaire et al., 2010). Prior to collecting the flow solution, rates of flow were allowed to equilibrate for 5 min at Ψ1 and Ψ2. Leaves were scanned and leaf areas were measured using the ImageJ software (Schneider et al., 2012). The total area of the blade was recorded as single leaf area (LA), and the area submerged in the water was determined separately and used to normalise hydraulic conductance. A comparison of the HPM with the evaporative flux method (EFM) (Scoffoni et al., 2016) showed statistically similar results for six grass species, indicating that the HPM provided a reliable estimate of Kleaf for grass species (see Supplementary Protocol S2, Figs S1, S2, Table S3).
Hydraulic conductivity (Kh) was measured using culm segments with two nodes (~4–30 cm in length depending on species) cut from mature stems and stripped of leaves including sheaths. Culm segments were re-cut underwater, submerged in a tube of degassed and filtered water inside the pressure chamber, then flushed at 0.1 MPa for 5 min to remove air embolisms. Subsequently, the mass of water that flowed through the segments in a 20-s period was determined by collecting the water onto pre-weighed tissues at an initial pressure (Ψ1, ΔW1), then after an increase in pressure of ~0.1–0.4 MPa (Ψ2, ΔW2). Kh was calculated as [(ΔW2–ΔW1)×stem length]/[time×(Ψ2−Ψ1)], and was normalised to stem vessel area (KS=Kh/ASV) or total leaf area distal to the segment (KL=Kh/AL): ASV was calculated as stem cross-section area (AS)×stem vessel area proportion (VP, see Supplementary Protocol S1), and leaf area (AL) was determined by scanning. AL and AS were also used to derive a leaf area to stem cross-section area ratio (AL/AS).
Leaves and culms used for hydraulic measurements were dried (65 °C for 72 h) and their masses were determined. Specific leaf area (SLA) for each stem was calculated as the ratio of total leaf area to leaf dry mass. Stem density (SD) was determined as dry mass/volume of the segments, using water displacement to measure volumes. Finally, dried leaves were ground and leaf carbon content (LC) and carbon isotope discrimination (δ13C) were determined using an isotope-ratio mass spectrometer (Delta V advantage; Thermo Fisher Scientific, MA, USA) at the Chinese Academy of Forestry’s Stable Isotope Laboratory.
Leaf pressure–volume curves were determined using the bench drying method after rehydration (Tyree and Hammel, 1972) (Supplementary Protocol S1). Relationships between Ψleaf and relative water content [(fresh mass−dry mass)/(saturated mass−dry mass)] were analysed to determine the water potential at the turgor-loss point (Ψtlp) according to classic models (Schulte and Hinckley, 1985). Dry and saturated masses of leaves were used to determine leaf dry matter content (LDMC) as dry mass/saturated mass.
Net CO2 assimilation (A) and stomatal conductance to water (gs), which were used to calculate intrinsic water-use efficiency (WUEi=A/gs), were obtained using survey measurements on sunny mornings. Each experimental block of 42 plants took 3 d to measure with an open leaf gas-exchange system (LI-6400XT, LI-COR, Lincoln, NE, USA), which was equipped with a CO2 Injector (6400–01) and a Red/Blue LED Light Source (6400-02B). During measurements, photosynthetic photon flux density was 1800 μmol m−2 s−1, leaf chamber CO2 concentration was 380 μmol mol−1, and chamber relative humidity 50–70%. The block temperature was not controlled. Measurements were collected after the cuvette had equilibrated for 5 min and values were averaged for two youngest mature leaves from randomly chosen tillers for each plant. Measurements of leaf water potentials from each plant, both pre-dawn (Ψpre) and at midday (Ψmid), were collected on the same day as gas exchange measurements.
Niche descriptors
We obtained environmental data using geo-referenced species records from the Global Biodiversity Information Facility (GBIF) collected through GrassPortal (www.grassportal.org). Averages of mean annual temperature (MAT, 1961–1990), mean annual precipitation (MAP, 1961–1990), wet days per year (1961–1990), and tree cover percentage (1992–1993) of habitats were calculated across all geo-referenced localities for each species. Because annuals have distinct growth seasons compared with perennials, we also obtained seasonality data for temperature and precipitation from the WorldClim dataset (http://www.worldclim.org), using extract in R (version 3.0.3) (www.r-project.org) package raster (Hijmans and Van Etten, 2013).
Data analysis
We used statistical techniques that control for estimated phylogenetic covariance (Supplementary Protocol S1), because phylogenetic lineage and photosynthetic type act in concert to shape the ecology of the Poaceae (Edwards et al., 2010).
To address coordination among traits and niche descriptors, we carried out a phylogenetic principal component analysis (PPCA) (Felsenstein, 1985) using the phyl.pca function in the R package phytools (a comparison of PPCA with outcomes of linear discriminant and canonical correlation analyses is provided in Supplementary Protocol S3). Data were log-transformed to fulfil the requirement of normal distribution, and if the original values were negative (Ψtlp, Ψpre, Ψmid, and δ13C) absolute values were used. In addition to a pooled analysis we used PPCA to separately analyse 15 ‘structural’ and 11 ‘physiological’ traits. Traits fixed during development were classified as structural, e.g. stomatal density and vessel diameter, whereas traits that continuously respond to variation in environmental factors were classed as physiological, e.g. stomatal conductance (Table 1).
Because we were interested in contemporary patterns of interspecific trait variation, we modelled comparisons among species mean values using phylogenetic generalised least-squares (PGLS; function pgls in the R package caper). PGLS performs well irrespective of the degree of phylogenetic signal, making it ideal for comparisons across large numbers of traits that differ in their associations with phylogeny (Revell, 2010). We used maximum likelihood to estimate Pagel’s λ (Pagel, 1999), which assumes a Brownian motion model of trait evolution and which we modelled across a phylogenetic tree extracted from a super tree of Poaceae (Edwards et al., 2010) (Supplementary Protocol S1). For each trait and niche descriptor, and for principal components that explained ≥20% of total variance, we compared four nested linear models: life history (annual/perennial, AP) and photosynthetic type (C3 and C4 species, PT) were tested independently, additively (AP+PT), and incorporating an interaction (AP×PT).
Because the large number of comparisons and the lack of balance in the number of species in each category (Fig. 1) limited the reliability of P-values for model comparisons and post hoc tests, we compared models using an information theoretic framework (Anderson, 2007). To evaluate explanatory power, we used model probability:
ΔAICc are differences in corrected Akaike information criterion scores (AICc), between alternative models (AP×PT, AP+PT, PT, and AP), that use the model with the minimum AICc as a reference (Anderson, 2007). The numerator is equivalent to the likelihood of the model of interest (model i), and the denominator is the sum of likelihoods for all R (=4) models. Model probabilities were compared using evidence ratios (wi/wj), where wi is the probability of the focal model and wj is the probability of a comparator model. Higher evidence ratios indicate greater relative support for focal models, and comparisons between the best model (minimum AICc) and the second-best model (the second-lowest AICc) are indicated specifically by wmin/w2 (Table 2).
Table 2.
Comparisons of phylogenetic generalised least-squares (PGLS) models that estimate the effects of photosynthetic type (PT; C3 and C4) and/or life history (AP; annual and perennial) on (a) plant functional traits (n=42 species), (b) principal component scores for trait variation, (c) niche descriptors (n=34 species, due to the lack of climate data for eight species), and (d) principal component scores for niche variation.
| 1ΔAICc | 2Model probability (wi) | 3Evidence Ratio (wmin/w2) | Best model | λ for best model | |||||||
| AP×PT | AP+PT | PT | AP | AP×PT | AP+PT | PT | AP | ||||
| (a) Plant functional traits | |||||||||||
| IVD (µm) | 6.89 | 3.39 | 0.00 | 14.05 | 0.026 | 0.151 | 0.822 | 0.001 | 5.45 | PT | 0.00 |
| SD (g cm–3) | 6.51 | 3.38 | 4.97 | 0.00 | 0.030 | 0.141 | 0.064 | 0.765 | 5.42 | AP | 0.00 |
| –Ψtlp (MPa) | 6.90 | 3.38 | 13.20 | 0.00 | 0.026 | 0.152 | 0.001 | 0.821 | 5.42 | AP | 0.00 |
| Leaf δ13C (‰) | 5.62 | 3.35 | 0.00 | 109.00 | 0.048 | 0.150 | 0.802 | 0.000 | 5.34 | PT | 0.62 |
| K leaf (mmol m–2 s–1 MPa–1) | 0.00 | 5.40 | 3.34 | 4.07 | 0.721 | 0.048 | 0.136 | 0.094 | 5.31 | AP×PT | 0.24 |
| –Ψpre (MPa) | 5.95 | 3.31 | 0.00 | 6.32 | 0.040 | 0.149 | 0.778 | 0.033 | 5.23 | PT | 0.00 |
| SLA (cm2 g–1) | 6.72 | 3.28 | 13.32 | 0.00 | 0.028 | 0.158 | 0.001 | 0.813 | 5.16 | AP | 0.52 |
| Dsv (µm) | 6.33 | 3.14 | 3.88 | 0.00 | 0.030 | 0.149 | 0.103 | 0.717 | 4.81 | AP | 0.00 |
| g s (mol m–2 s–1) | 6.37 | 2.86 | 2.87 | 0.00 | 0.027 | 0.158 | 0.157 | 0.658 | 4.18 | AP | 0.36 |
| Dlvb (µm) | 4.84 | 2.74 | 4.60 | 0.00 | 0.062 | 0.176 | 0.069 | 0.693 | 3.94 | AP | 0.00 |
| sts (µm2) | 5.06 | 3.37 | 0.00 | 2.70 | 0.052 | 0.122 | 0.656 | 0.170 | 3.86 | PT | 0.20 |
| H (cm) | 5.03 | 2.30 | 7.09 | 0.00 | 0.057 | 0.222 | 0.020 | 0.701 | 3.16 | AP | 0.00 |
| LDMC (%) | 3.25 | 2.25 | 11.15 | 0.00 | 0.129 | 0.213 | 0.002 | 0.656 | 3.08 | AP | 0.08 |
| A L/AS (cm2 mm–2) | 5.27 | 2.09 | 8.89 | 0.00 | 0.050 | 0.245 | 0.008 | 0.697 | 2.84 | AP | 0.00 |
| LT (µm) | 6.26 | 3.36 | 2.02 | 0.00 | 0.027 | 0.117 | 0.228 | 0.627 | 2.75 | AP | 0.00 |
| g wmax (mol m–2 s–1) | 5.43 | 2.77 | 1.76 | 0.00 | 0.038 | 0.145 | 0.240 | 0.578 | 2.41 | AP | 0.57 |
| LA (cm2) | 2.98 | 1.69 | 6.63 | 0.00 | 0.133 | 0.254 | 0.021 | 0.591 | 2.33 | AP | 0.17 |
| SS (mm2) | 1.48 | 1.16 | 4.26 | 0.00 | 0.221 | 0.260 | 0.055 | 0.464 | 1.79 | AP | 0.00 |
| WUEi (µmol mol–1) | 4.35 | 0.80 | 0.00 | 28.38 | 0.064 | 0.376 | 0.561 | 0.000 | 1.49 | PT | 0.45 |
| LC (%) | 6.87 | 3.38 | 0.00 | 0.69 | 0.017 | 0.096 | 0.519 | 0.368 | 1.41 | PT | 0.00 |
| std (mm–2) | 0.68 | 1.96 | 0.00 | 1.06 | 0.266 | 0.140 | 0.374 | 0.220 | 1.40 | PT | 0.08 |
| K S (kg m–1 s–1 MPa–1) | 5.80 | 3.02 | 0.68 | 0.00 | 0.028 | 0.111 | 0.358 | 0.503 | 1.40 | AP | 0.00 |
| A (µmol m–2 s–1) | 4.13 | 0.59 | 0.00 | 26.72 | 0.068 | 0.398 | 0.534 | 0.000 | 1.34 | PT | 0.00 |
| –Ψmid (MPa) | 4.61 | 3.17 | 0.36 | 0.00 | 0.047 | 0.096 | 0.390 | 0.467 | 1.20 | AP | 0.00 |
| SVD (mm–2) | 0.10 | 1.80 | 2.43 | 0.00 | 0.358 | 0.153 | 0.112 | 0.377 | 1.05 | AP | 0.00 |
| K L (10–4 kg m–1 s–1 MPa–1) | 6.35 | 3.31 | 0.07 | 0.00 | 0.019 | 0.087 | 0.439 | 0.455 | 1.04 | AP | 0.51 |
| (b) Principal component scores for trait variation | |||||||||||
| PC2 of physiological traits | 6.33 | 3.39 | 8.41 | 0.00 | 0.034 | 0.148 | 0.012 | 0.806 | 5.45 | AP | 0.44 |
| PC1 of physiological traits | 3.08 | 2.04 | 0.00 | 62.47 | 0.136 | 0.229 | 0.635 | 0.000 | 2.77 | PT | 0.00 |
| PC1 of structural traits | 1.84 | 1.91 | 14.53 | 0.00 | 0.223 | 0.216 | 0.000 | 0.561 | 2.51 | AP | 0.00 |
| PC1 of all traits | 0.00 | 0.18 | 13.14 | 2.04 | 0.439 | 0.402 | 0.001 | 0.158 | 1.09 | AP×PT | 0.00 |
| (c) Niche descriptors | |||||||||||
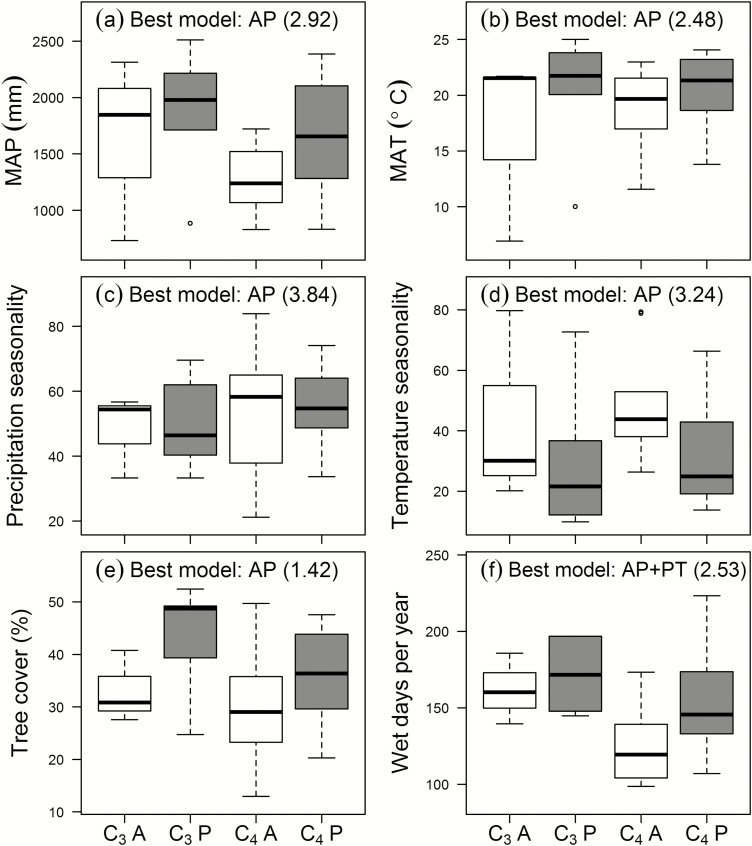
| Precipitation seasonality | 6.61 | 3.15 | 2.69 | 0.00 | 0.024 | 0.138 | 0.173 | 0.665 | 3.84 | AP | 0.50 |
| Temperature seasonality | 6.03 | 2.35 | 5.45 | 0.00 | 0.034 | 0.217 | 0.046 | 0.703 | 3.24 | AP | 0.00 |
| MAP (mm) | 5.74 | 2.14 | 2.91 | 0.00 | 0.035 | 0.210 | 0.143 | 0.612 | 2.92 | AP | 0.00 |
| Wet days per year | 2.71 | 0.00 | 1.86 | 2.14 | 0.129 | 0.501 | 0.198 | 0.172 | 2.53 | AP+PT | 0.00 |
| MAT (°C) | 7.01 | 3.39 | 1.82 | 0.00 | 0.019 | 0.114 | 0.249 | 0.619 | 2.48 | AP | 0.88 |
| Tree cover (%) | 4.24 | 0.70 | 1.57 | 0.00 | 0.053 | 0.309 | 0.200 | 0.438 | 1.42 | AP | 0.00 |
| (d) Principal component scores for niche variation | |||||||||||
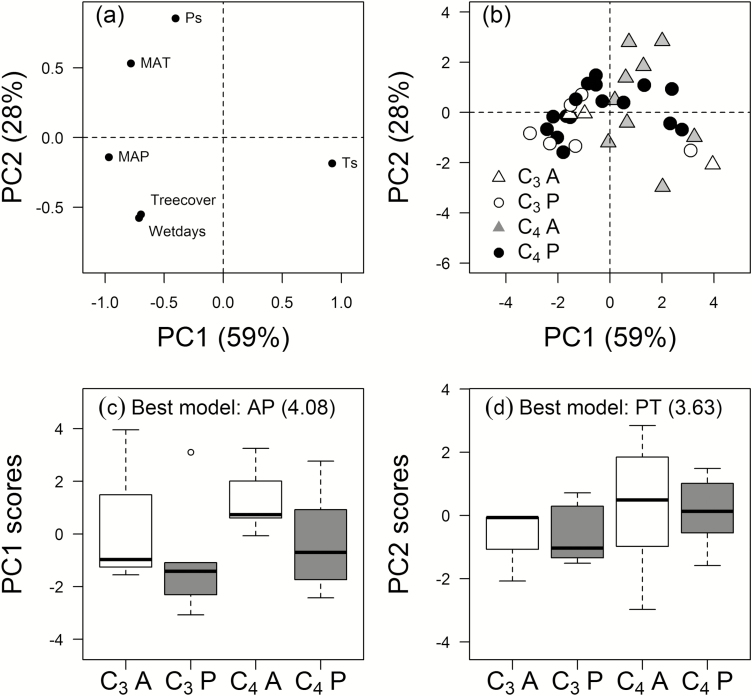
| PC1 of six niche descriptors | 6.10 | 2.81 | 4.94 | 0.00 | 0.034 | 0.178 | 0.061 | 0.726 | 4.08 | AP | 0.00 |
| PC2 of six niche descriptors | 6.70 | 3.05 | 0.00 | 2.58 | 0.023 | 0.143 | 0.654 | 0.180 | 3.63 | PT | 0.66 |
Traits and principal components are ranked by the power to identify a single ‘best’ model (evidence ratio, wmin/w2) and dashed lines separate models at evidence ratio >5 and >2.5. Models are compared using differences in the corrected Akaike Information Criterion (ΔAICc) and their probability within the four models (wi).
1 Bold type highlights models with ΔAICc<3.22 (wmin/w2≈5.00).
2 Bold type highlights models with wi>0.60.
3 Among the four wi values, the highest, which is associated with the minimum AICc, is defined as wmin, the second highest is used as w2.
Results
Impact of life history and photosynthetic type on functional traits
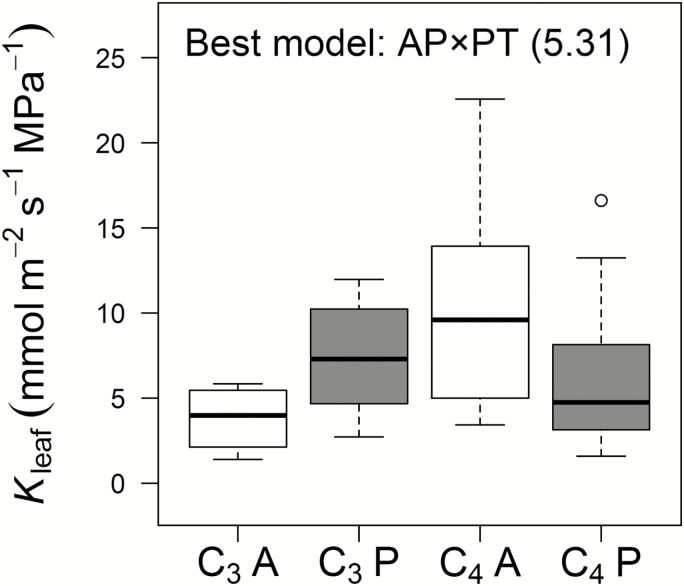
Of the 26 traits, seven (Kleaf, SD, Ψtlp, SLA, IVD, δ13C, and Ψpre) showed support for ‘best’ models with wmin/w2>5 (Table 2a). Of these, Kleaf was best modelled by AP×PT, with the highest values from C4 annuals and the lowest from C3 annuals, whilst perennials of both photosynthetic types had similar, intermediate values (Fig. 2). SD, Ψtlp, and SLA were all clearly determined by AP; and IVD, δ13C, and Ψpre depended on PT (Table 2a). Best-fitting models for a further eight traits showed evidence ratios in the range 2.5–5. Of these, seven traits (Dsv, gs, Dlvb, H, LDMC, AL/AS, LT) were best modelled as dependent on AP, and one, sts, was best modelled as depending on PT (Table 2a). In combination, traits for which best-fit models had wmin/w2>2.5 showed the following characteristics. Annuals were shorter and had higher gs. Their leaves were thinner, with smaller vascular bundles (Dlvb), higher SLA, lower LDMC, and less negative Ψtlp than perennials. The stems of annuals were less dense (SD), with narrower vessels (Dsv), and supported relatively small leaf areas (AL/AS) (Table 3a). Meanwhile, C4 species showed smaller IVD and sts, and less negative δ13C and Ψpre than C3 species (Table 3b).
Fig. 2.
Interaction between life history (AP: annual, open boxes; perennial, grey boxes) and photosynthetic type (PT: C3, left; C4, right) affecting leaf hydraulic conductance (Kleaf) of the 42 grass species. The box-plots show quartiles for each trait with extreme values as circles. Sample sizes for C3-A, C3-P, C4-A, and C4-P were 4, 6, 13 and 19, respectively. The best-fit model and its evidence ratio are shown.
Table 3.
Plant functional traits for which (a) life history or (b) photosynthetic type is supported as the sole explanatory factor with an evidence ratio >2.5.
| (a) | Annual (17 species) | Perennial (25 species) |
| SD (g cm –3) | 122.7±11.08 | 144.3±12.04 |
| Ψ tlp (MPa) | –1.2±0.04 | –1.5±0.04 |
| SLA (cm 2 g –1) | 425.4±19.43 | 321.7±16.56 |
| Dsv (µm) | 22.6±1.22 | 28.1±1.92 |
| g s (mol m–2 s–1) | 0.5±0.05 | 0.4±0.03 |
| Dlvb (µm) | 70.9±6.86 | 92.4±6.94 |
| H (cm) | 48.2±4.91 | 100.2±13.49 |
| LDMC (%) | 20.4±0.66 | 25.4±0.95 |
| A L/AS (cm2 mm–2) | 23.6±3.24 | 43.5±5.05 |
| LT (µm) | 139.0±5.41 | 157.8±8.33 |
| (b) | C3 (10 species) | C4 (32 species) |
| IVD (µm) | 314.8±44.68 | 173.4±7.52 |
| Leaf δ 13 C (‰) | –29.6±0.23 | –13.9±0.15 |
| Ψ pre (MPa) | –0.09±0.012 | –0.07±0.004 |
| sts (µm2) | 209.1±24.89 | 156.6±9.72 |
Data are means (±s.e.m.), and bold text highlights traits with evidence ratios >5.
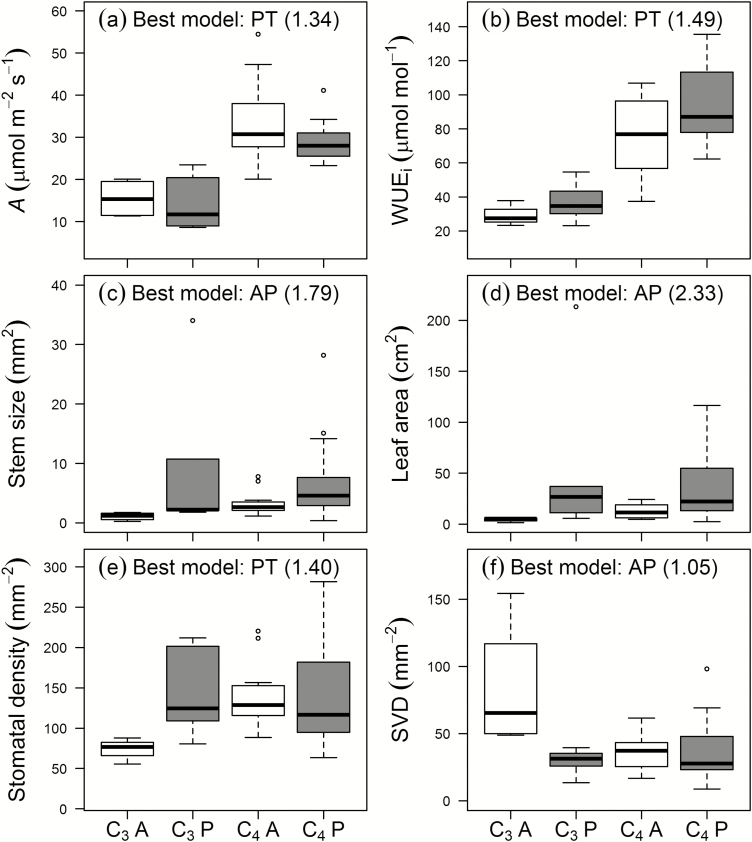
Consistent with the moderate support for many of the best models, there was also support for AP+PT models for all traits (ΔAICc ≤3.39; wmin/wAP+PT ≤5.45) except Kleaf (wAP×PT/wAP+PT=5) (Table 3). The greatest statistical support for AP+PT effects was found for A (wAP+PT=0.4), WUEi (wAP+PT=0.38), SS (wAP+PT=0.26), and LA (wAP+PT=0.25; Fig 3a–d). For these four traits, the ‘best’ models were single-factor (AP or PT) but evidence ratios for these were relatively low (wmin/w2≤2.33). For A and WUEi the best model was PT, and while C4 species had greater A and WUEi, A was slightly higher and WUEi lower within annuals than within perennials (Fig. 3a, b). For SS and LA, AP was the best model; annuals were clearly much smaller, and both leaves and stems tended to be smaller within C3 species (Fig. 3c, d).
Fig. 3.
Functional traits for which AP×PT or AP+PT had similar explanatory powers compared with the best-fitting single-factor models (AP: annual, open boxes; perennial, grey boxes; or PT: C3, left; C4, right). The box-plots show quartiles for each trait with extreme values as circles. Sample sizes for C3-A, C3-P, C4-A, and C4-P were 4, 6, 13 and 19, respectively. The best-fit models and evidence ratios are shown.
Importantly, in addition to strong evidence for AP×PT affecting Kleaf, AP×PT models fitted better than AP+PT models for two traits: std (wAP×PT/wAP+PT=1.9) and SVD (wAP×PT/wAP+PT=2.34). These two traits had single-factor best models with evidence ratios (wmin/w2) ≤1.4 (Table 2: std, PT; SVD, AP), and were similar among C3-perennials, C4-annuals, and C4-perennials, but C3-annuals showed lower std (Fig. 3e) and higher SVD (Fig. 3f).
Of the remaining traits, gwmax, Ψmid, KS, and KL were explained best by AP, but without a clear difference in explanatory power compared with PT, and the best model for LC was PT, but AP had similar explanatory power (Table 2a). For these five traits, lower evidence ratios for best-fitting, single-factor models and lack of support for AP+PT or AP×PT as secondary models (that would explain the low power of the primary models) meant that there was no convincing evidence for a strong fit by any of the four alternative models (Supplementary Fig. S3).
In summary, the majority of traits were most clearly linked with life history; however, in addition to expected contrasts between C3 and C4 photosynthetic types our data showed that differences between annual and perennial grasses in Kleaf, SVD, and std depended on photosynthetic type.
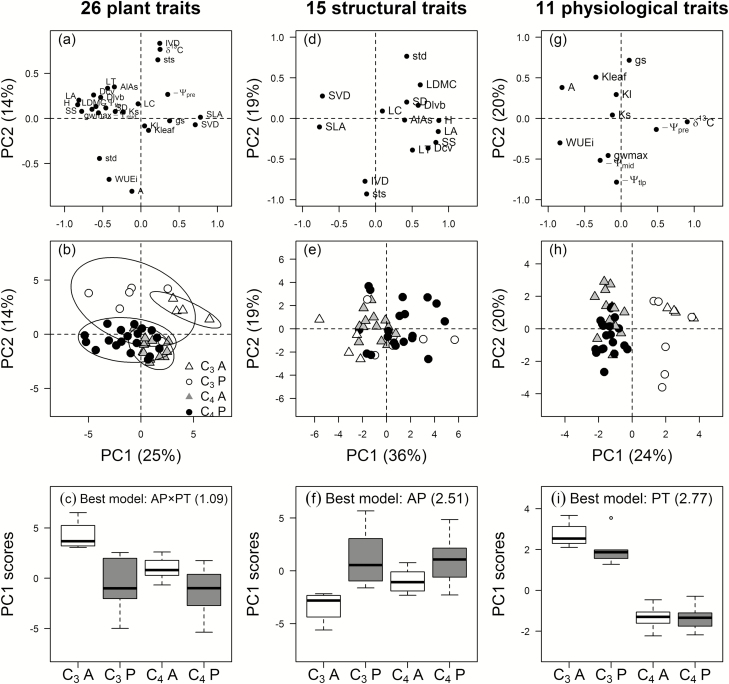
Impact of life history and photosynthetic type on trait coordination: PPCA
For the full set of 26 functional traits, the first two PCs explained 25% and 14% of total variation, respectively (Fig. 4a, b). PC1 separated annuals and perennials, whereas PC2 separated C3 and C4 species. Separation of AP along PC1 was consistent with differences between larger perennial species (H, LA, and SS; negative association) with low SLA and SVD, and smaller annual species with higher SLA and SVD. Importantly, although PC1 scores for annuals were higher than for perennials within both photosynthetic types, C3-perennials, C4-annuals, and C4-perennials showed similar scores, while C3-annuals were clearly distinguished from the other three groups by higher scores (Fig. 4c). This pattern was supported by the best-fitting AP×PT model for PC1, and although an AP+PT model could explain PC1 almost as well as AP×PT (ΔAICc=0.18, wmin/w2=1.09), PT or AP alone were much poorer models for PC1 (wi/wj>2.5; Table 2b). Although models were not compared because of the relatively small proportion of variance explained, PC2 for the full set of functional traits clearly separated species on the basis of PT (Fig. 4b): greater IVD and sts, combined with more negative δ13C (C3 traits), were separated from higher A, higher WUEi, and greater std (C4 traits; Fig. 4a).
Fig. 4.
Phylogenetic principal component analysis (PPCA) for the 26 functional traits (a–c) of 42 C3 and C4 grasses with annual or perennial life histories. Traits were secondarily classified as (d–f) 15 structural traits or (g–i) 11 physiological traits, with the first two principal components (PCs) and species scores reported and analysed. (a, d, g) PC loadings of the 26 traits; (b, e, h) scores for 42 species in four groups: C3-A, C3 annuals; C3-P, C3 perennials; C4-A, C4 annuals; C4-P, C4 perennials; and (c, f, i) box-plots of PC1 species scores. Percentages of variance explained by PCs are shown in the axis labels. Trait abbreviations are given in Table 1.
PPCA of the 15 structural traits explained 36% and 19% of variation with the first two PCs (Fig. 4d, e). Scores for SLA, SVD, and size-related traits again showed strong opposite associations along PC1, separating annuals and perennials (Fig. 4d–f). Graphically, this life history axis was, again, linked with greater separation in structure between annual and perennial C3 species compared with their C4 relatives (Fig 4f; Table 2b). However, although there was some support for AP+PT and AP×PT as alternative models with similar power (ΔAICc≤1.91, wi~0.22; Table 2b) the best model for PC1 was AP alone (wmin/w2=2.5; Table 2b). Although PC2 for the structural traits explained <20% of total variance and was not modelled, it was most strongly associated with IVD (negative scores) and a trade-off between sts (negative scores) and std (positive scores). It was therefore surprising that C3 and C4 species did not separate along PC2 (Fig. 4e, f).
Among the 11 physiological traits, the first two PCs explained less variation but PC2 was slightly more important than for structural traits (PC1, 24%; PC2, 20%; Fig. 4g–i). In contrast with structural traits, PC1 for physiological traits clearly distinguished C3 and C4 species (wi=0.635; Table 2b). C4 grasses had higher A and WUEi, and C3 grasses greater IVD and more negative δ13C (Fig. 4g–i). As for structural traits, there was moderate support for the primary model of PC1 that separated species by PT (wmin/w2=2.77), associated with greater differences in score between annual and perennial C3 species (Fig. 4i). However, unlike PC1 for the structural traits, the AP+PT model (wi=0.229) was clearly a better secondary fit to scores along PC1 than the AP×PT model (wi=0.136; Table 2b). Along PC2, AP was strongly supported as the best-fitting model (ΔAICc≥3.39; wmin/w2=5.45; Fig. 4h; Table 2b). Annual species had higher A, gs, and Kleaf, together with less negative Ψtlp and Ψmid, and, surprisingly, lower gwmax (Fig. 3g).
In summary, PPCA analysis showed that variation in structural traits was strongly aligned with differences in life history, and that even among physiological traits almost as much variation was explained by life history (20%, PC2) as by photosynthetic type (24%, PC1). Importantly, when all 26 traits were considered, differentiation of annual and perennial species along the primary axis of variation depended on photosynthetic type: there were greater differences between annual and perennial C3 species than between annual and perennial C4 species.
Impact of life history and photosynthetic type on niche descriptors
Tree cover, MAT, MAP, and seasonality of temperature and precipitation were all best explained by AP, with support increasing respectively from weak to moderate (wmin/w2: 1.42–3.84; Table 2c). In both photosynthetic types, annual species were linked with lower MAP and MAT, decreased tree cover, and increased seasonality of temperature and precipitation (although differences were weaker for seasonality of precipitation; Fig. 5a–e). AP×PT was a weak model of all niche descriptors (wi≤0.129), but AP+PT received moderate support as the best model for wet days per year (wi=0.5; wmin/w2=2.53), and was ranked second for tree cover (wi=0.31), MAP (wi=0.21), and temperature seasonality (wi=0.22) (Table 2c). Greater numbers of wet days were characteristic of habitats for both C3 and perennial species (Fig. 5f) and, in addition to differences between annuals and perennials, C3 grasses were associated with greater tree cover and MAP, and lower temperature seasonality. Moderate support for PT as an alternative model for tree cover (wi=0.2) was due to slightly higher mean values among C3 species (Fig. 5).
Fig. 5.
Niche descriptors for 34 grass species sampled in subtropical China, grouped by life history (AP: annual, open boxes; perennial, grey boxes) and photosynthetic type (PT: C3, left; C4, right). The box-plots show quartiles for each trait with extreme values as circles. Sample sizes for C3-A, C3-P, C4-A, and C4-P were 3, 6, 9 and 16, respectively. The best-fit models and evidence ratios are shown. MAP, mean annual precipitation; MAT, mean annual temperature; seasonality is the coefficient of variation of monthly values. Only 34 of the 42 study species were included in this analysis because climatic data were not available for the other eight.
Characterisation of niche spaces: PPCA
The six niche descriptors were far more effectively summarised by PPCA than were the larger array of functional traits: the first two PCs explained 87% of total variation in niche descriptors (PC1, 59%; PC2, 28%; Fig. 6a, b). PC1 was best characterised (wmin/w2=4.08, wi=0.73; Table 2) as separating annual and perennial species by seasonality of temperature, MAP, and MAT (Fig. 6a): annual species were associated with increased seasonality of temperature, and decreased MAP and MAT. PC2 was best modelled as dependent on PT (wmin/w2=3.63, wi=0.65; Table 2d) with a relatively large correction for phylogenetic covariance (λ=0.66). Along PC2, C4 species were associated with greater MAT and seasonality of precipitation, and decreased tree cover and wet days per year (Fig. 6b–d).
Fig. 6.
Phylogenetic principal component analysis (PPCA) for six niche descriptors of 42 C3 and C4 grasses with annual or perennial life histories. (a) Principal component (PC) loadings of the six niche descriptors; (b) scores for 42 species in four groups: C3-A, C3 annuals; C3-P, C3 perennials; C4-A, C4 annuals; C4-P, C4 perennials; and (c, d) box-plots of PC1 and PC2 species scores. Percentages of variance explained by PCs are shown in the axis labels. Trait abbreviations are given in Table 1.
Influence of phylogeny on trait distributions
For most of the 26 functional traits and four associated PCs, phylogenetic signal had weak effects on the best-fitting models (λ≤0.2 for 22 of the 30 models; Table 2a, b). The highest λ value for a best-fitting model was 0.62 for δ13C when accounting for PT (Table 2; λ for all models are provided in Supplementary Table S4 ).
For the individual niche descriptors, both seasonality of precipitation (λ=0.50) and MAT (λ=0.88) showed moderate to strong phylogenetic signals, in each case after accounting for PT as the best-fitting model (other niche descriptors showed λ≈0 for their best-fitting models; Table 2). Consistent with these results, PC2 for the niche descriptors, which was defined by a contrast between greater seasonality of precipitation and MAT versus greater tree cover and numbers of wet days, also showed a phylogenetic signal after accounting for PT as a primary effect (λ=0.66, Table 2).
Discussion
The majority of variation in the 26 functional traits and six niche descriptors of the grasses we studied was best characterised by differences in life history: at the local scale, therefore, life history is a key factor shaping the functional ecology of subtropical grasses, and is more important for trait differentiation than photosynthetic type. Annual grasses were smaller, with higher SLA, denser stems, less-negative Ψtlp, and higher gs. Photosynthetic type had important effects on physiological traits and affected how several traits linked with hydraulic function differed between annual and perennial species. Kleaf, a trait at the nexus of leaf structure and function (Sack and Holbrook, 2006), was lower in C3 annuals and higher in C4 annuals than in perennial species of either photosynthetic type. C3-annuals also had greater stem vessel and lower stomatal densities than C4 annuals or perennials of both photosynthetic types. We found no strong evidence for interactions between annual/perennial life history (AP) and photosynthetic type (PT) affecting niche descriptors. Annual grasses had clear preferences for drier, less shaded, and more seasonal habitats, and C4 species, especially annuals, were found to inhabit locations with higher temperatures, low tree cover, and lower, more variable rainfall.
Interplay between life history and photosynthetic type
High-turnover, resource-inefficient annual strategies are likely to be economic only where resources are sufficiently limited that larger, more competitive plants capable of greater resource capture are excluded (Grime and Hunt, 1975; Reich, 2014). Compatible with this, we found that annuals exploited drier habitats with less tree cover that were more seasonal and cooler on an annual basis than those of their perennial relatives.
Notably, of the four classes of grass species we investigated, C4-annuals tended to inhabit the driest locations, and our findings suggest that C3 and C4 annuals differ in key traits that may influence resistance to loss of hydraulic function. C3-annuals were characterised by low stomatal densities, high stem vessel densities, and low Kleaf, while C4 annuals showed higher Kleaf compared with perennial species. Moreover, because differences in gs were relatively small, Kleaf/gs (supply versus demand) was also greatest in C4-annuals and smallest in C3-annuals. Higher Kleaf/gs should decrease stomatal sensitivity to VPD (Brodribb and Holbrook, 2003; Osborne and Sack, 2012; Ocheltree et al., 2014), so this result supports greater impacts of drought and high VPD conditions on gas exchange of C3 than C4 annuals. Meanwhile, the high stem vessel densities, low stomatal densities, and low Kleaf of C3 annuals further suggest a hydraulic system tuned to combat hydraulic failure by minimising vulnerability. We were, however, surprised to find that C3 and C4 perennials showed substantial overlap in Kleaf.
In C4 plants, smaller IVD is linked with improved photosynthetic efficiency, especially quantum yield (Ogle, 2003), and might increase Kleaf relative to demand from transpiration (Osborne and Sack, 2012). For annual species, differences in Kleaf were consistent with smaller IVD in C4 species resulting in higher Kleaf. However, consistent with a study that compared temperate species (Ocheltree et al., 2014), C3 and C4 perennials in our study had similar Kleaf. Going forwards, it will be important to determine the consequences for leaf hydraulic function of differences among grass lineages (Liu and Osborne, 2015), of structural differences that underpin leaf size and thickness, of lateral vessels that affect vein length per area (Ueno et al., 2006; Sack and Scoffoni, 2013), and of bundle-sheath tissues (Griffiths et al., 2013).
Independent effects of life history and photosynthetic type
Life history was the single factor that best explained functional trait variation in our experiment. As expected, annual grasses tended to be shorter, with thinner leaves and stems (Garnier et al., 1997) and low AL/AS. The thinner leaves of annuals also had smaller vascular bundles and tended to show higher gs, lower WUEi, and less-negative Ψtlp. These traits, in particular higher gs and lower WUEi, support our hypothesis of high-demand hydraulic systems in annuals. Less-negative Ψtlp suggests a tendency towards greater vulnerability of leaf performance to declining water status, but may also be associated with rapid leaf wilting that might counteract lower WUEi in high-irradiance environments. Wilting can improve leaf level WUE by decreasing interception of irradiance, and hence transpiration (Turner and Begg, 1981).
Expected differences between C3 and C4 grasses were clearly represented in our dataset. Along with less-negative δ13C (Farquhar et al., 1989) and smaller IVD (Sage, 2004; Ueno et al., 2006; Lundgren et al., 2014), C4 grasses had higher A and WUEi (Pearcy and Ehleringer, 1984; Taylor et al., 2010;, 2011). However, some of our results are at variance with those of previous studies. For example, previous studies of eudicots have shown that KS and/or KL can be lower among C4 species, which may reduce vulnerability to hydraulic failure (Kocacinar and Sage, 2004). We found no good evidence for differences in KS or KL based on photosynthetic type. In our results, AP was marginally better than PT as an explanation for differences in KS and KL, but there was little support for systematic differences due to AP or PT in either of these traits. In addition, while we confirmed that smaller, more closely spaced stomata were broadly characteristic of C4 species, this was not associated with a shift in gwmax, as has been reported previously (Taylor et al., 2012). Two features of our study might explain the lack of a PT effect on gwmax. First, the C4 genus Aristida was not represented in the subtropical grass flora we studied; species of Aristida are characteristic of dry habitats and were a key group showing low gwmax in Taylor et al. (2012). Second, our experimental design increased the representation of annual species compared with previous studies, and we found that low std was a particular feature of C3 annuals. This may explain why AP was a marginally better explanation of gwmax than PT. Previous evidence has suggested links between gwmax and habitat water availability (Taylor et al., 2012), so lower gwmax within C3 annuals in this subtropical environment is consistent with the other lines of evidence from our experiment that suggest they are commonly exposed to water stress.
Niche descriptors of subtropical grasses
For all of the niche descriptors there was some evidence that PT had independent effects on species niche preferences: after accounting for AP differences, the ranges of C4 species extended into drier, more seasonal locations than those of C3 species. Our results therefore supported the broad hypothesis that C4 photosynthesis often provides advantages in drier, more open habitats (Osborne and Freckleton, 2009; Edwards and Smith, 2010). By contrast, we found that AP was always a stronger explanation for temperature preferences than PT: annuals were linked with lower average MAT and increased seasonality of temperature. Preferences for MAT were also linked with the strongest phylogenetic signals in our dataset, consistent with previous studies (Edwards and Still, 2008; Edwards and Smith, 2010; Liu and Osborne, 2015). Our results therefore support previous suggestions that thermal constraints are less important than tree cover and rainfall in determining C3/C4 distributions in the subtropics (Edwards and Still, 2008; Edwards and Smith, 2010). However, we chose to investigate a subtropical community to avoid strong effects of deeper divergences and global diversity within the Poaceae that affect studies in temperate communities (Edwards and Still, 2008; Edwards and Smith, 2010). As a consequence, the C3 subfamilies Pooideae and Arundinoideae were each represented by a single species, and the subfamilies, Bambusoideae, Ehrhartoideae, Aristidoideae, Micrairoideae, and Danthonioideae were not represented at all. Thus, the relatively small impact of phylogeny compared with life history and photosynthetic type that we observed is likely to be particular to the subtropical species assemblage. Impacts of phylogeny will be greater when comparisons are made in communities that include species with diverse climate preferences, or when phylogenetic diversity among Poaceae is more broadly represented.
Conclusions
For the subtropical grass species that we studied, life history was the predominant explanation of differences in most functional traits and niche descriptors. As we expected, annual grasses showed functional traits related with high-turnover and low-efficiency strategies. Annual grasses, in particular C4-annuals, also tended to be distributed in drier and more seasonal habitats than perennial grasses. A particularly novel finding was that functional trait contrasts between annual and perennial species interacted with photosynthetic type. Specifically, trait variation between annual and perennial grasses was greater among C3 than C4 species. Hydraulic traits, in particular Kleaf, were central to this finding. These results suggest that interactions with life history are a key factor to be considered when trying to establish the impacts of photosynthetic type or phylogeny on species functional ecology.
Supplementary Data
Supplementary data are available at JXB online.
Protocol S1. Supplementary methods for determining functional traits.
Protocol S2. Comparison of the evaporative flux method and high-pressure method for determining Kleaf.
Protocol S3. Comparison of phylogenetic principal component analysis with linear discriminant analysis and canonical correlation analysis for data in this study.
Table S1. Phylogenetic clades, species names, and groups of the 42 species used in this study.
Table S2. Values for the 26 functional traits and six climatic niche descriptors of the 42 species used in this study.
Table S3. Raw data used to compare the evaporative flux method and high-pressure method for determining Kleaf.
Table S4. Pagel’s λ for phylogenetic generalised least-squares models to analyse the effects of photosynthetic type and life history on plant traits, principal component scores, and niche descriptors.
Fig. S1. Comparisons between the evaporative flux method and high-pressure method to determine Kleaf.
Fig. S2. Images of leaf cross-sections of four typical species used in this study to determine Kleaf.
Fig. S3. Five functional traits for which the photosynthetic type and life history models had similar explanatory power.
Acknowledgements
We are grateful to Prof. Howard Griffiths, Dr Adam Roddy, and anonymous reviewers for their insightful and constructive suggestions on earlier versions of this article. We thank Dr Ruijiang Wang and Dr Qing Liu of the South China Plants Identification Center for their assistance in species identification. We also thank Xiaorong Liu and Lei Hua for their technical assistance during the lab and field work. This work was funded by the National Natural Science Foundation of China (31670411, 31300334), the Pearl River S&T Nova Program of Guangzhou (201806010083), and the State Scholarship Fund of China Scholarship Council (201804910141). The authors have no conflicts of interest to declare.
References
- Anderson DR. 2007. Model based inference in the life sciences: a primer on evidence. New York: Springer Science & Business Media. [Google Scholar]
- Atkinson RR, Mockford EJ, Bennett C, Christin PA, Spriggs EL, Freckleton RP, Thompson K, Rees M, Osborne CP. 2016. C4 photosynthesis boosts growth by altering physiology, allocation and size. Nature Plants 2, 16038. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM. 2003. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiology 132, 2166–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, John GP, Scoffoni C, Sack L. 2015. How does leaf anatomy influence water transport outside the xylem? Plant Physiology 168, 1616–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Besnard G, Samaritani E, Duvall MR, Hodkinson TR, Savolainen V, Salamin N. 2008. Oligocene CO2 decline promoted C4 photosynthesis in grasses. Current Biology 18, 37–43. [DOI] [PubMed] [Google Scholar]
- Christin PA, Osborne CP. 2014. The evolutionary ecology of C4 plants. New Phytologist 204, 765–781. [DOI] [PubMed] [Google Scholar]
- Christin PA, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ. 2013. Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proceedings of the National Academy of Sciences, USA 110, 1381–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S, Kattge J, Cornelissen JH, et al. . 2016. The global spectrum of plant form and function. Nature 529, 167–171. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Osborne CP, Stromberg CAE, Smith SA, Consortium CG. 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587–591. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Smith SA. 2010. Phylogenetic analyses reveal the shady history of C4 grasses. Proceedings of the National Academy of Sciences, USA 107, 2532–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Still CJ. 2008. Climate, phylogeny and the ecological distribution of C4 grasses. Ecology Letters 11, 266–276. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Still CJ, Donoghue MJ. 2007. The relevance of phylogeny to studies of global change. Trends in Ecology & Evolution 22, 243–249. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112, 285–299. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Monson RK. 1993. Evolutionary and ecological aspects of photosynthetic pathway variation. Annual Review of Ecology and Systematics 24, 411–439. [Google Scholar]
- Ehleringer J, Pearcy RW. 1983. Variation in quantum yield for CO2 uptake among C3 and C4 plants. Plant Physiology 73, 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40, 503–537. [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. American Naturalist 125, 1–15. [DOI] [PubMed] [Google Scholar]
- Garnier E, Cordonnier P, Guillerm JL, Sonié L. 1997. Specific leaf area and leaf nitrogen concentration in annual and perennial grass species growing in Mediterranean old-fields. Oecologia 111, 490–498. [DOI] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group II. 2012. New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytologist 193, 304–312. [DOI] [PubMed] [Google Scholar]
- Griffith DM, Anderson TM, Osborne CP, Strömberg CA, Forrestel EJ, Still CJ. 2015. Biogeographically distinct controls on C3 and C4 grass distributions: merging community and physiological ecology. Global Ecology and Biogeography 24, 304–313. [Google Scholar]
- Griffiths H, Weller G, Toy LF, Dennis RJ. 2013. You’re so vein: bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant, Cell & Environment 36, 249–261. [DOI] [PubMed] [Google Scholar]
- Grime JP. 2006. Plant strategies, vegetation processes, and ecosystem properties. Chichester, UK: John Wiley & Sons. [Google Scholar]
- Grime JP, Hunt R. 1975. Relative growth-rate: its range and adaptive significance in a local flora. The Journal of Ecology 63, 393–422. [Google Scholar]
- Heaton EA, Flavell RB, Mascia PN, Thomas SR, Dohleman FG, Long SP. 2008. Herbaceous energy crop development: recent progress and future prospects. Current Opinion in Biotechnology 19, 202–209. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Van Etten J. 2013. raster: geographic data analysis and modeling. R package version 2.1-49.http://CRAN.R-project.org/package=raster.
- Kocacinar F, Sage RF. 2003. Photosynthetic pathway alters xylem structure and hydraulic function in herbaceous plants. Plant, Cell & Environment 26, 2015–2026. [DOI] [PubMed] [Google Scholar]
- Kocacinar F, Sage RF. 2004. Photosynthetic pathway alters hydraulic structure and function in woody plants. Oecologia 139, 214–223. [DOI] [PubMed] [Google Scholar]
- Liu H, Osborne CP. 2015. Water relations traits of C4 grasses depend on phylogenetic lineage, photosynthetic pathway, and habitat water availability. Journal of Experimental Botany 66, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP. 1999. Environmental responses. In: Sage RF, Monson RK, eds. C4 plant biology. San Diego: Academic Press, 215–249. [Google Scholar]
- Lundgren MR, Osborne CP, Christin PA. 2014. Deconstructing Kranz anatomy to understand C4 evolution. Journal of Experimental Botany 65, 3357–3369. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Portsmuth A, Tobias M. 2007. Leaf shape and venation pattern alter the support investments within leaf lamina in temperate species: a neglected source of leaf physiological differentiation? Functional Ecology 21, 28–40. [Google Scholar]
- Ocheltree TW, Nippert JB, Prasad PV. 2014. Stomatal responses to changes in vapor pressure deficit reflect tissue-specific differences in hydraulic conductance. Plant, Cell & Environment 37, 132–139. [DOI] [PubMed] [Google Scholar]
- Ogle K. 2003. Implications of interveinal distance for quantum yield in C4 grasses: a modeling and meta-analysis. Oecologia 136, 532–542. [DOI] [PubMed] [Google Scholar]
- Osborne CP, Freckleton RP. 2009. Ecological selection pressures for C4 photosynthesis in the grasses. Proceedings of the Royal Society B: Biological Sciences 276, 1753–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Sack L. 2012. Evolution of C4 plants: a new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367, 583–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond C, Winter K, Ziegler H. 1982. Functional significance of different pathways of CO2 fixation in photosynthesis. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, eds. Physiological plant ecology II. Encyclopedia of plant physiology, vol. 12/B. Berlin, Heidelberg: Springer, 479–547. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884. [DOI] [PubMed] [Google Scholar]
- Pearcy R, Ehleringer J. 1984. Comparative ecophysiology of C3 and C4 plants. Plant, Cell & Environment 7, 1–13. [Google Scholar]
- Postaire O, Tournaire-Roux C, Grondin A, Boursiac Y, Morillon R, Schäffner AR, Maurel C. 2010. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiology 152, 1418–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102, 275–301. [Google Scholar]
- Revell LJ. 2010. Phylogenetic signal and linear regression on species data. Methods in Ecology and Evolution 1, 319–329. [Google Scholar]
- Sack L, Holbrook NM. 2006. Leaf hydraulics. Annual Review of Plant Biology 57, 361–381. [DOI] [PubMed] [Google Scholar]
- Sack L, Scoffoni C. 2013. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytologist 198, 983–1000. [DOI] [PubMed] [Google Scholar]
- Sage RF. 2004. The evolution of C4 photosynthesis. New Phytologist 161, 341–370. [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin PA, Edwards EJ. 2011. The C4 plant lineages of planet Earth. Journal of Experimental Botany 62, 3155–3169. [DOI] [PubMed] [Google Scholar]
- Sage RF, Li M, Monson RK. 1999. The taxonomic distribution of C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 plant biology. San Diego: Academic Press, 551–584. [Google Scholar]
- Sage RF, Sage TL, Kocacinar F. 2012. Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PJ, Hinckley TM. 1985. A comparison of pressure–volume curve data analysis techniques. Journal of Experimental Botany 36, 1590–1602. [Google Scholar]
- Scoffoni C, Chatelet DS, Pasquet-Kok J, Rawls M, Donoghue MJ, Edwards EJ, Sack L. 2016. Hydraulic basis for the evolution of photosynthetic productivity. Nature Plants 2, 16072. [DOI] [PubMed] [Google Scholar]
- Slack CR, Hatch MD. 1967. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. The Biochemical Journal 103, 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South China Botanical Garden. 2009. Flora of Guangdong. Guangzhou, China: Guangdong Science and Technology Press. [Google Scholar]
- Still CJ, Berry JA, Collatz GJ, DeFries RS. 2003. Global distribution of C3 and C4 vegetation: carbon cycle implications. Global Biogeochemical Cycles 17, 6-1–6-14. [Google Scholar]
- Taylor SH, Aspinwall MJ, Blackman CJ, Choat B, Tissue DT, Ghannoum O. 2018. CO2 availability influences hydraulic function of C3 and C4 grass leaves. Journal of Experimental Botany 69, 2731–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SH, Franks PJ, Hulme SP, Spriggs E, Christin PA, Edwards EJ, Woodward FI, Osborne CP. 2012. Photosynthetic pathway and ecological adaptation explain stomatal trait diversity amongst grasses. New Phytologist 193, 387–396. [DOI] [PubMed] [Google Scholar]
- Taylor SH, Hulme SP, Rees M, Ripley BS, Woodward FI, Osborne CP. 2010. Ecophysiological traits in C3 and C4 grasses: a phylogenetically controlled screening experiment. New Phytologist 185, 780–791. [DOI] [PubMed] [Google Scholar]
- Taylor SH, Ripley BS, Woodward FI, Osborne CP. 2011. Drought limitation of photosynthesis differs between C3 and C4 grass species in a comparative experiment. Plant, Cell & Environment 34, 65–75. [DOI] [PubMed] [Google Scholar]
- Turner NC, Begg JE. 1981. Plant–water relations and adaptation to stress. Plant and Soil 58, 97–131. [Google Scholar]
- Tyree M, Hammel H. 1972. The measurement of the turgor pressure and the water relations of plants by the pressure-bomb technique. Journal of Experimental Botany 23, 267–282. [Google Scholar]
- Ueno O, Kawano Y, Wakayama M, Takeda T. 2006. Leaf vascular systems in C3 and C4 grasses: a two-dimensional analysis. Annals of Botany 97, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeland MD, Spannagl M, Asp T, Paina C, Rudi H, Rognli OA, Fjellheim S, Sandve SR. 2013. Evidence for adaptive evolution of low-temperature stress response genes in a Pooideae grass ancestor. New Phytologist 199, 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volaire F, Thomas H, Lelievre F. 1998. Survival and recovery of perennial forage grasses under prolonged Mediterranean drought. I. Growth, death, water relations and solute content in herbage and stubble. New Phytologist 140, 439–449. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Quick WP, Furbank RT. 2012. The development of C4 rice: current progress and future challenges. Science 336, 1671–1672. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Westoby M. 2002. Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytologist 155, 403–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.