To the Editor: The article by Kantoff et al1 described the phase II study, TBC-PRO-002, a randomized, double-blind, controlled study to evaluate the safety and efficacy of PROSTVAC-VF/TRICOM in combination with granulocyte-macrophage colony-stimulating factor in patients with androgen-independent adenocarcinoma of the prostate. This study was industry sponsored, with a collaborative research and development agreement with the National Cancer Institute (NCI). The initial sponsor, Therion Biologics, managed the study through treatment, primary end point evaluation, and 1 year of overall survival (OS) follow-up. The results were negative for progression-free survival, the primary end point. Therion went out of business in August 2006, and oversight of the PROSTVAC program reverted to the NCI. In 2008, Bavarian Nordic (BN) and the NCI established a new collaborative research and development agreement to develop PROSTVAC.
BN completed OS data collection despite the negative progression-free survival results. Long-term follow-up (LTFU) results suggested improved OS for patients in the PROSTVAC-VF group compared with patients in the control group, which justified conducting a phase III study that is now fully accrued. Anticipating the planned PROSTVAC biologics license application, BN undertook a source data verification of the phase II study. Reported data (dated July 24, 2009) consisted of the final locked SAS (SAS Institute, Cary, NC) data sets sent to BN, including data collected in the study’s open-label extension phase (Therion’s original 2006 data). The clinical study report (CSR) also included data from the LTFU case report form (CRF); data collected by BN via e-mail and telephone calls from study sites; and Social Security Death Index (SSDI) data collected via a public database. BN has discovered that a subset of the LTFU OS data derived from the SSDI was not confirmed by study sites because some of them were unable to reopen the study or chose not to participate.
Former BN staff made best efforts in preparing the 2009 study CSR; however, current responsible parties at BN have determined that a revised OS analysis is warranted. OS data reported in this 2016 CSR addendum consist of CRF data from the original 2006 database; data recorded on the LTFU CRF; e-mail and telephone contact between BN and study sites; and SSDI data collected by BN via a public database and confirmed by the sites via telephone in 2008.
For this addendum, if primary analysis data did not include a patient’s survival status, date of death, and/or last-known-alive date, OS was derived using last known survival status from the original data. If the patient’s date of death or last-known-alive date included the month and year, but not the day, the fifteenth day of the month was ascribed. In summary, this reanalysis only included data confirmed by the sites as captured on the original CRFs or the LTFU CRFs completed by the sites, and SSDI data collected by BN via a public database and confirmed by the sites via e-mail and telephone.
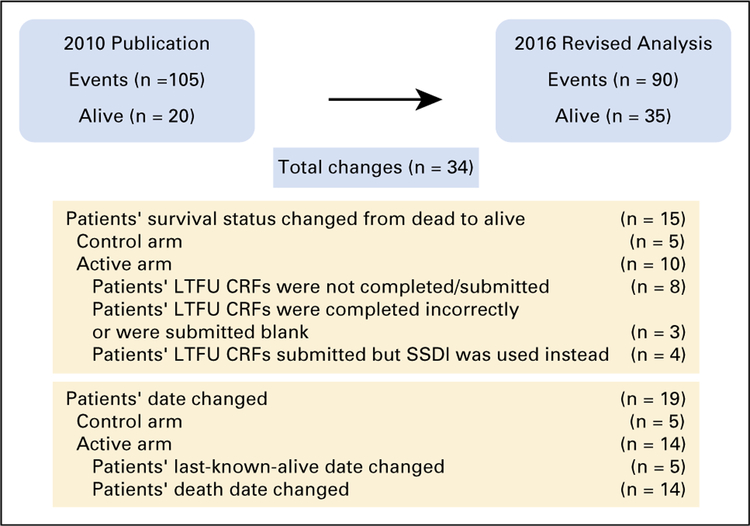
There were 34 changes made to the 2016 CSR addendum from the 2009 CSR, on the basis of lack of documented confirmation by study sites (Appendix Fig A1, online only), including:
Fifteen patients reported as deceased in 2009 are now reported as alive at that time.
The death or last-known-alive date for 19 patients was changed in accordance with the convention of fifteenth day of the month.
In the control arm, five patients’ status changed from dead to alive, and five patients’ death dates were changed.
In the active arm, 10 patients’ status changed from dead to alive, nine patients’ death dates were changed, and five patients’ last-known-alive dates were changed.
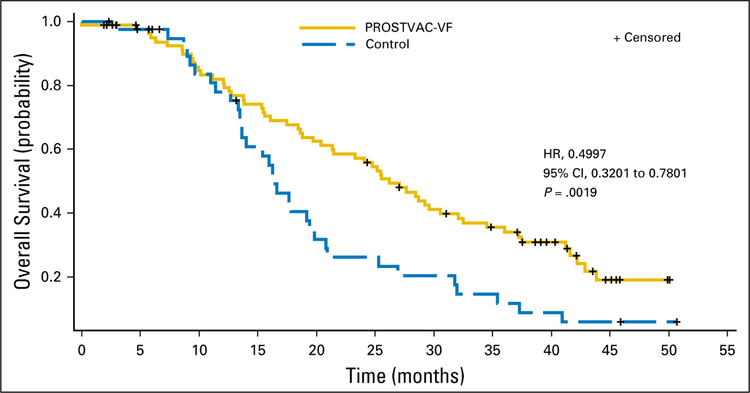
For these 34 changes, BN relied on the original 2006 database for last-known-alive dates and confirmed patient status dates verified by the sites in 2008. Revised and original OS results for the stratified intention-to-treat population are listed in Table 1. Revised median OS for the stratified intention-to-treat population (Fig 1) was significantly longer for the PROSTVAC-VF group than for the control group (empty vector; 26.2 v 16.3 months, respectively; stratified hazard ratio, 0.4997; 95% CI, 0.3201 to 0.7801; stratified log-rank P = .0019). The revised OS results are consistent with the original results in the 2009 CSR submitted to the US Food and Drug Administration and published in Journal of Clinical Oncology.1 Any numerical differences between the revised and original OS results change neither the outcome nor the conclusions of this study. We believe that the OS analysis provided in this addendum is a more conservative but preferred analysis for reporting purposes because all data used in the analysis have been verified by the clinical sites.
Table 1.
Overall Survival: 2016 Revised Analysis and 2010 Publication—Stratified for the ITT Population
| Variable | Revised OS Analysis | Original OS Analysis | ||
|---|---|---|---|---|
| PROSTVAC-VF (n = 84) | Control (n = 41) | PROSTVAC-VF (n = 84) | Control (n = 41) | |
| No. (%) of patients | ||||
| Died (event) | 57 (67.9) | 33 (80.5) | 67 (79.8) | 38 (92.7) |
| Censored | 27 (32.1) | 8 (19.5) | 17 (20.2) | 3 (7.3) |
| OS, months* | ||||
| Median (95% CI) | 26.2 (20.4 to 32.1) | 16.3 (13.7 to 19.8) | 25.1 (NR) | 17.7 (NR) |
| Hazard ratio (95% CI)† | 0.4997 (0.3201 to 0.7801) | 0.581 (0.384 to 0.880) | ||
| P‡ | .0019 | .0095 | ||
NOTE. Patient 046–0030 (assigned to the PROSTVAC-VF group), who died as a result of a completed suicide before receiving any study treatment, was treated as an event, with OS of 0 months.
Abbreviations: ITT, intent-to-treat; NR, not reported; OS, overall survival.
Overall survival in months was calculated as (the date of death/censored − date of randomization + 1)/30.4375.
Hazard ratio was from a Cox proportional hazards model stratified by bisphosphonate use (yes or no).
P value was from a stratified log-rank test stratified by bisphosphonate use (yes or no).
Fig 1.
Revised overall survival for the stratified intention-to-treat population. HR, hazard ratio.
Appendix
Fig Al.
Summary of changes in patient status and dates between the 2009 clinical study report and the 2016 clinical study report addendum. CRF, case report form; LTFU, long-term follow-up; SSDI, Social Security Death Index.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript.
Philip W. Kantoff
Stock or Other Ownership: Bellicum Pharmaceuticals, Blend Therapeutics, Metamark Genetics, Placon
Consulting or Advisory Role: Bavarian Nordic, Janssen Pharmaceuticals, Millennium Pharmaceuticals, MorphoSys, Pfizer, Astellas Pharma, Bellicum Pharmaceuticals, BIND Biosciences, Blend Therapeutics, Endocyte, Metamark Genetics, Medivation, Merck, MTG Biotherapeutics, OncoCellMDX, OncoGeneX, Sotio, Sanofi, Tokai Pharmaceuticals, Bayer, Genentech, Cristal Therapeutics, Ipsen, Omnitura Therapeutics, GTx
Research Funding: Medivation (Inst), Sanofi (Inst), OncoGeneX (Inst), Aragon Pharmaceuticals (Inst), Amgen (Inst), Astellas Pharma (Inst), Bayer (Inst), Bavarian Nordic (Inst), Dendreon (Inst), Exelixis (Inst), Janssen Pharmaceuticals (Inst)
Expert Testimony: Sanofi, Janssen Pharmaceuticals
Travel, Accommodations, Expenses: Sanofi, Janssen Pharmaceuticals, BIND Biosciences, Bavarian Nordic, Millennium
James L. Gulley
No relationship to disclose
Cesar Pico-Navarro Employment: Bavarian Nordic
Contributor Information
Philip W. Kantoff, Memorial Sloan Kettering Cancer Center, New York, NY
James L. Gulley, National Cancer Institute, Bethesda, MD
Cesar Pico-Navarro, Bavarian Nordic, Redwood City, CA.
REFERENCES
- 1.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. : Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 28: 1099–1105, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]