Abstract
Objectives:
Quantitative ultrasound (QUS), including grayscale level analysis (GLA) and quantitative backscatter analysis (QBA), and electrical impedance myography (EIM) have been proposed as biomarkers in Duchenne muscular dystrophy (DMD). However, the relationship between these methods has not been assessed.
Methods:
QUS values (including GLA and QBA) and several EIM measures were recorded from six muscles in 36 DMD and 29 healthy boys between ages 5 and 13 years at baseline, 6-months, and 12-months.
Results:
In the DMD boys, a moderate correlation was noted between QUS and EIM parameters, with the strongest correlations being identified for averaged muscle values. Of the individual muscles, biceps brachii and deltoid showed the strongest correlations. For example, in biceps, the QBA/EIM correlation coefficient (Spearman rho) was ≥0.70 (p<0.01). Importantly, changes in QUS values over 12 months also correlated moderately with changes in EIM parameters and EIM/QBA rho values mostly varied between −0.53 and −0.70 (p≤0.02). No significant correlations were identified in the healthy boys.
Conclusions:
A moderate correlation of QUS with EIM in DMD boys suggests that the two technologies provide related data but are sensitive to different pathological features of muscle.
Significance:
The use of both technologies jointly in assessing DMD progression and response to therapy should be considered.
Keywords: Electrical impedance myography, Quantitative ultrasound, Duchenne muscular dystrophy, Outcome measures
1. Introduction
Clinical outcome measures, such as the 6-minute walk test (6MWT) and North Star Ambulatory Assessment (NSAA), are commonly used in Duchenne muscular dystrophy (DMD) clinical trials (Mayhew et al., 2007; McDonald et al., 2010; Mazzone et al., 2011). Dystrophin expression in myofibers (Mendell et al., 2013) and muscle magnetic resonance imaging (MRI) can provide more objective measures, but have their own limitations (Bonati et al., 2015; Wary et al., 2015; Willcocks et al., 2016). Recently, quantitative ultrasound (QUS) methods, such as quantitative backscatter analysis (QBA), grayscale level analysis (GLA), along with electrical impedance myography (EIM), have emerged as potential biomarkers in DMD. Moreover, both ultrasound and EIM appear to be sensitive to disease progression (Rutkove et al., 2014, 2017b; Zaidman et al., 2017).
QUS measures muscle echo intensity, which increases with greater muscle fat and fibrosis. The degree of echo intensity correlates with measures of strength and function and likely reflects disease progression over time (Pillen et al., 2008; Rutkove et al., 2014; Shklyar et al., 2015; Zaidman et al., 2017). QBA derives from analysis of the amplitudes of the reflected ultrasound echoes. GLA derives from measurement of the displayed grayscale pixel level (Pillen et al., 2008, 2009; Zaidman et al., 2017). On the other hand, EIM measures the passive electrical characteristics of muscles; resistance (difficulty in passing current through the tissue, which generally increases with disease progression), reactance (the capacitive effects of the cell membranes, which generally decreases with disease progression), and phase (the geometric relationship between reactance and resistance, which also decreases with disease progression). EIM is sensitive to the muscle’s microscopic structure and composition, including changes to myocyte fiber size, inflammation, edema, and connective tissue and fat deposition (Rutkove et al., 2017a; Sanchez and Rutkove 2017a). Initially, single frequency values were used as the sole EIM parameter, but multifrequency outputs were noted to provide a richer portrait of muscle health. A two-frequency phase ratio (100 kHz/300 kHz) has been shown to be more sensitive and less affected by subcutaneous fat (Geisbush et al., 2015; Schwartz et al., 2015).
QUS and EIM detect alterations in muscle pathology in DMD (Figure 1). However, the relationship between these approaches has not been thoroughly assessed. We have previously noted the nearly significant correlation between GLA values and 50 kHz phase EIM parameters in DMD patients in a cross-sectional analysis (Rutkove et al., 2014). In this study, we extend work on the recently completed Quantitative ultrasound and EIM in DMD (QED) study (Zaidman et al., 2017; Rutkove et al., 2017b), by exploring the relationship between QUS values (QBA and GLA) and multiple EIM parameters in DMD patients over a one-year period. Given that, both QUS and EIM correlate with muscle health in DMD, it is anticipated that they would have some degree of correlation with one another; however, an in-depth and longitudinal analysis will help us to better understand this relationship.
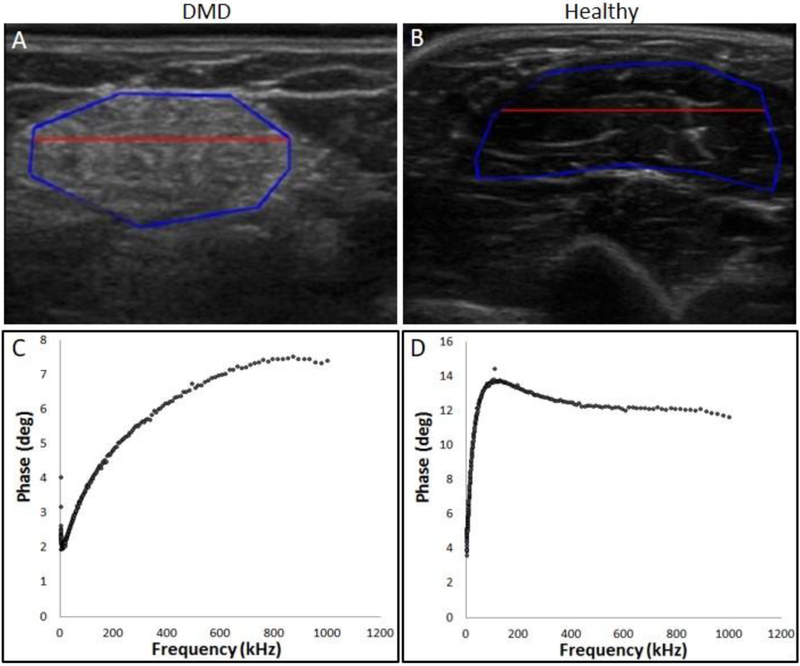
Figure 1:
Top Panel: Echo-intensity is higher in ultrasound image (GLA) of biceps brachii muscles from a 12-year-old boy with DMD (A) compared to a same-aged healthy control (B). The area within the blue line demarcates the region of interest and area above the red line is the most superficial one third. Bottom Panel: Example of multifrequency impedance spectra of biceps brachii from the same patient (C) and the healthy control (D).
2. Materials and Methods
2.1. Subjects
Thirty-six boys with DMD and 29 age-similar healthy volunteers, aged 2-to-14 years, were recruited from the neuromuscular clinic at the Boston Children’s Hospital for the QED study (Zaidman et al., 2017; Rutkove et al., 2017b). All boys with DMD were either probands or had genetic confirmation. DMD boys enrolled in a therapeutic clinical trial and boys with significant other medical comorbidities were excluded. DMD boys were enrolled and followed regardless of corticosteroid use. The Boston Children’s Hospital Institutional Review Board approved the protocol.
2.2. QUS and EIM measurements
Details of GLA, QBA, and EIM parameters acquisition have been described previously (Rutkove et al., 2014, 2017b; Zaidman et al., 2017). Briefly, six muscles from the dominant side, including deltoid, biceps brachii, anterior forearm compartment (a composite of all the muscles in this region), rectus femoris, tibialis anterior (TA), and medial gastrocnemius were measured transversely relative to the long axis of each muscle at multiple time points for both DMD boys and healthy controls.
Ultrasound images were obtained using the Terason t3000 system (Teratech, Burlington, MA) with a 10 MHz probe at predefined locations (Rutkove et al., 2017b). The same gain, depth, focal points, and transducer frequency were used for all measurements. All subjects were seated with the knee bent at 90° and the arm extended at mid-chest height with the elbow straight and supported by the examiner or a pillow during the ultrasound measurement. All measurements were performed at predefined locations (Shklyar et al, 2015) (Supplementary table 1). All images were converted to JPEG files and analyzed using Matlab® (MathWorks, Inc, Natick, MA) to obtain the brightness of the region of interest (ROI), measured as median grayscale level (GLA). The ROI was defined as a region of fixed dimensions (130 pixels × 64 pixels) directly below the subcutaneous fat layer and above the bone. For QBA, raw backscatter intensity values were derived from the ultrasound data and used to create images using software provided by Teratech Inc, the manufacturer of the equipment. Thus, the backscatter data is free of any standard post-processing alterations applied to create the standard ultrasound image, which was used for GLA analysis (Shklyar et al, 2015). An identical ROI was placed on each image for measurement of the GLA and QBA. We measured the median backscatter value (in decibels) from the most superficial one-third of the ROI of the muscle.
EIM measurements were obtained with the Imp SFB7 (Impedimed, Inc, Sydney, Australia) using a custom hand-held array, with three different probe sizes being used depending on the child’s size. The array dimensions were: small, 4 × 1.5 cm; medium, 5 × 2 cm and; large: 7 × 2.5 cm. Along with 50 kHz phase (EIM-50 kHz), a two-frequency phase ratio of 100 kHz and 300 kHz (EIM 100/300) and a least-squares fit of the multifrequency EIM data between 100 and 500 kHz were used (EIM-slope).
The measurements were not influenced by significant outliers and in most of our previous work we have reported six muscles averaged values only (Shklyar et al., 2015; Schwartz et al., 2015), but in this study, we also have looked into individual muscles.
Spearman rank-order correlation method was used for correlation analysis. Data processing was performed by R software version 3.4.3. We also performed a Benjamini-Hochberg false discovery rate procedure, which controls for expected proportion of false discoveries relative to the total discoveries, to control for multiple testing across all the correlations for the DMD boys (Benjamini and Hochberg 1995).
3. Results
Mean (range) age of DMD boys was 7.3 years (2.2–13.1 years); for the healthy boys, it was 7.1 years (2.0–14.6 years).
Among the DMD patients, when an average of all muscles was considered, a significant correlation was noted at baseline, 6-months, and 12-months between GLA, QBA values, and nearly all EIM parameters (Table 1, Supplementary table 2). Group of proximal muscles and upper extremity muscles also showed a consistently significant correlation between these parameters at all time points. At baseline, correlation coefficients were mild to moderate, but at the 6 and 12-month timepoints, the correlation coefficient was ≥ 0.6 for most measures (> 80%). Correlations between QBA and EIM parameters in the distal muscles group were significant 67% of the time. However, the correlation between the parameters in the lower extremity muscles group reached significance far less frequently.
Table 1:
Correlation of QBA with the EIM parameters of muscle groups of DMD patients.
| EIM-50 kHz Rho (p-value) |
EIM 100/300 Rho (p-value) |
EIM-slope Rho (p-value) |
||
|---|---|---|---|---|
| Muscle groups | Time point | Correlation with QBA | ||
| Six-Muscles | Baseline (n=34) | −0.66 (<0.01) | −0.41 (0.02) | 0.29 (0.09) |
| 6 months (n=22) | −0.64 (<0.01) | −0.61 (<0.01) | 0.67 (<0.01) | |
| 12 months (n=20) | −0.58 (<0.01) | −0.65 (<0.01) | 0.65 (<0.01) | |
| Proximal | Baseline (n=33) | −0.66 (<0.01) | −0.57 (<0.01) | 0.47 (<0.01) |
| 6 months (n=22) | −0.69 (<0.01) | −0.72 (<0.01) | 0.76 (<0.01) | |
| 12 months (n=20) | −0.59 (<0.01) | −0.64 (<0.01) | 0.64 (<0.01) | |
| Distal | Baseline (n=34) | −0.63 (<0.01) | −0.32 (0.06) | 0.25 (0.16) |
| 6 months (n=22) | −0.59 (<0.01) | −0.53 (0.01) | 0.56 (<0.01) | |
| 12 months (n=20) | −0.42 (0.07) | −0.57 (0.01) | 0.62 (<0.01) | |
| Upper | Baseline (n=33) | −0.71 (<0.01) | −0.45 (<0.01) | 0.40 (0.02) |
| 6 months (n=20) | −0.82 (<0.01) | −0.62 (<0.01) | 0.59 (<0.01) | |
| 12 months (n=20) | −0.60 (<0.01) | −0.66 (<0.01) | 0.68 (<0.01) | |
| Lower | Baseline (n=34) | −0.52 (<0.01) | −0.23 (0.20) | 0.15 (0.40) |
| 6 months (n=22) | −0.60 (<0.01) | −0.55 (<0.01) | 0.56 (<0.01) | |
| 12 months (n=20) | −0.33 (0.16) | −0.36 (0.12) | 0.39 (0.08) | |
At the individual muscle level, biceps, deltoid, and gastrocnemius showed stronger correlation between QBA and EIM parameters compared to other muscles examined. The correlation was consistently significant for all EIM parameters in biceps brachii and deltoid. Particularly strong correlations were noted in biceps with EIM 100/300 ratio and EIM-slope at 12-months; (spearman rho>0.70 and p-values < 0.01). Correlation reached statistical significance in gastrocnemius about 80% of time. Consistent correlation was noted in forearm muscles only with EIM-50 kHz. For rectus femoris, statistical significance was noted at 6 months with all EIM parameters. No consistent correlation pattern was noted in tibialis anterior (TA) with any of the EIM parameters (Supplementary Table 3).
GLA values showed a similar trend. The correlation was consistently significant between GLA values and all EIM parameters in biceps brachii and deltoid. Statistically significant correlation was noted about 44% to 55% of time in all other muscles except TA (Supplementary Table 3). Correlation coefficients between QBA and EIM parameters were generally higher than coefficients between GLA and EIM parameters. Rho values were significantly higher for EIM-50 kHz/QBA than EIM-50 kHz/GLA when individual muscles were considered (p-value <0.01).
No consistent correlation pattern was noted between the QUS and EIM parameters in healthy subjects (Supplementary Table 4). Correlation coefficients predominantly ranged between 0 to 0.25 more than 85% of time.
We correlated changes in QBA and GLA values over one year (12 months – baseline; ΔQBA and ΔGLA, respectively) with the ΔEIM parameters over the same period. When looking at groups of muscles, significant correlations were found (Table 2, Supplementary table 5). Overall, the correlation between ΔQBA values with ΔEIM parameters was more consistent, when compared to the to ΔGLA and ΔEIM correlation. However, at the single muscle level there were no consistent correlations between QUS and EIM except in gastrocnemius (Supplementary Table 6). Also, as anticipated, in the healthy subjects, no such consistent correlation was noted between ΔQBA to ΔEIM or ΔGLA to ΔEIM parameters (Supplementary Table 7).
Table 2:
Correlation between ΔEIM and ΔQBA (12 months-0 months) in DMD patients.
| Correlations with QBA | |||
|---|---|---|---|
| Muscle Groups | EIM 50 kHz | EIM 100/300 | EIM-slope |
| Rho (p-value) | Rho (p-value) | Rho (p-value) | |
| Six-muscles | −0.54 (0.02) | −0.63 (<0.01) | 0.57 (0.01) |
| Proximal | −0.32 (0.19) | −0.56 (0.01) | 0.55 (0.01) |
| Distal | −0.57 (0.01) | −0.69 (<0.01) | 0.70 (<0.01) |
| Upper | −0.57 (0.01) | −0.53 (0.02) | 0.42 (0.07) |
| Lower | −0.63 (<0.01) | −0.70 (<0.01) | 0.68 (<0.01) |
Given that multiple comparisons were performed, we used the Benjamini-Hochberg method to correct for false discovery rate and only 11.2% of the total number of statistically significant results became non-significant following the correction (marked in the tables as p-value#).
4. Discussion
We found that GLA and QBA values correlate with EIM parameters in DMD patients, with some individual muscles showing stronger correlation than the others. The correlation was consistently stronger in biceps, deltoids, and in averaged upper extremity muscles than in the lower extremities. Overall, correlation coefficients were higher between QBA-EIM correlations than between GLA-EIM correlations. We also noted that ΔQUS from baseline to 12-months correlated with ΔEIM parameters from baseline to 12-months when groups of muscles were considered together, but not at the individual muscle level.
Alterations in QUS parameters in disease are derived from images and they are easy to understand. On the other hand, EIM measures are more conceptual with the obtained data expressed only as numerical values, not in images. By showing that correlations are present, it supports that EIM technique is measuring meaningful changes in muscle.
It is worth noting that EIM-50 kHz and EIM 100/300 negatively correlated with the QUS parameters. This stems from the simple fact that EIM phase generally reduces with disease progression whereas QUS values usually increases with disease progression (Zaidman et al., 2017; Rutkove et al., 2017b). On the other hand, EIM-Slope, which is derived from the multifrequency spectral characteristics of the EIM data, increases with disease progression and thus correlated positively with the QUS parameters.
The first cross-sectional study employing both QUS and EIM simultaneously in DMD showed that QUS and EIM could differentiate DMD from healthy muscle and that most measures correlated with the NSAA (Rutkove et al., 2014). More recently, longitudinal studies have shown that QUS and EIM can detect DMD disease progression and are possibly more sensitive than commonly used functional measures in early identification of muscle deterioration (Zaidman et al., 2017; Rutkove et al., 2017b). EIM also captured the therapeutic effect of corticosteroid in DMD patients (Rutkove et al., 2017b). However, our understanding of the relationship between these two technologies is limited. As mentioned earlier, the only previous analysis on a cross-sectional study between the averaged 50-kHz EIM phase value and GLA value from the group of six-muscles showed moderate correlation (rho −0.4) and near significance (p-value was 0.054) (Rutkove et al., 2014). In the current study, we have considered the relationship of these technologies in considerably greater detail evaluating both cross-sectional and longitudinal change.
The mechanism of pathological change in DMD leads directly to the alterations observed in both ultrasound and EIM. As DMD progresses, healthy muscle tissue is gradually replaced by fat and connective tissue. The interspersion of muscle fibers with fat and fibrosis results in numerous tissue/sound velocity transitions that reflect ultrasound signal back to the transducer, registering as increased echo intensity. These changes also result in increased signal scattering helping to produce the “ground glass” appearance that is so characteristic of DMD ultrasound images (Pillen et al., 2016; Zaidman and Alfen 2016). EIM is similarly affected by these compositional changes (connective tissue and fat deposition), but EIM also is impacted by other features, including myofiber size, structure, and even intracellular health (Shiffman et al., 2013). Muscle fiber orientation also has a substantial impact on the EIM data conferring directional dependence to the data, so-called anisotropy (Sanchez and Rutkove 2017b; Garmirian et al., 2009). Anisotropy also becomes reduced in myopathic conditions and shows substantial reduction in DMD in particular (Rutkove et al., 2016). Thus, EIM reflects alterations in both muscle composition and microstructure (Tarulli et al., 2009; Sanchez and Rutkove 2017b).
In addition to differences in the biophysical underpinnings of both techniques, a number of other factors undoubtedly contributed to our results and the fact that the correlation coefficients were no higher than about 0.70 in the DMD patients. Despite good reproducibility of both QUS and EIM (Zaidman et al., 2017; Rutkove et al., 2017), some variation will be inevitable when aiming to place the ultrasound and EIM transducers on identical location on each muscle. Moreover, QUS, by design evaluates a relatively narrow cross-section of muscle whereas EIM measures a larger volume. Hence there are actual differences in the specific region being evaluated. Similarly, the depth of measurement may vary. By choosing to measure only the top third of the muscle with ultrasound, we were likely very much overlapping with the region measured by EIM. Nevertheless, electrical current also likely descends deeper into the muscle impacting results. Similarly, differences in the gross size and shape of a given muscle, and also the thickness of subcutaneous fat tissue overlying the muscle may impact EIM data more significantly than ultrasound further contributing to our findings of only modest correlation (Sanchez and Rutkove 2017b; Rutkove et al., 2016, 2017a).
An important second and new finding is the significant correlation of EIM and QUS change over time. Interestingly, we found significant correlations between the parameters in muscle groups, but not in individual muscles. A simple explanation for this inconsistency is that each measurement has a certain amount of noise associated with it. By taking differences between two time points, that effect is amplified. This noise may produce sufficient variation so as to obscure the correlation. However, by averaging data across muscles, the impact of noise is lessened, and the true correlation emerges.
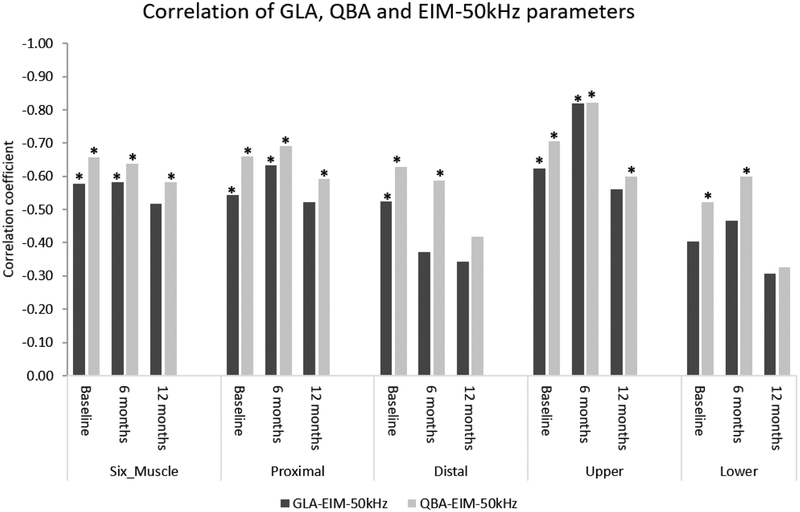
In general, in the DMD boys, the correlation coefficients for QBA values and EIM parameters were higher when compared to the correlation coefficients between GLA values and EIM parameters (Figure 2). This is likely because QBA contains a relatively large amount of data, which might become lost or skewed when proprietary algorithms compress the original values into the 256 GLAs used for imaging display. However, just because QBA correlates better to EIM than GLA does not imply that QBA is more sensitive to change the GLA. In fact, the differences between QBA and GLA correlations with EIM were generally small and one longitudinal study assessing both QUS modalities has identified very similar degrees of change (Zaidman et al., 2017).
Figure 2:
Graphical representation of the correlation coefficients between GLA, QBA, and EIM-50 kHz parameters in averaged muscle groups in DMD patients. Overall, the rho between QBA and EIM-50 kHz was higher than the rho between GLA and EIM-50 kHz.
We noted a stronger correlation between the QUS and EIM parameters in the upper extremity muscles than the lower extremity muscles. There is no obvious explanation for this finding, except that it is possible that correlations were easier to observe in less-affected upper extremity muscles than in typically more effected lower extremity muscles, especially in older boys.
We did not find any correlation between the QUS and EIM parameters among healthy subjects. This is consistent with the previous result (Rutkove et al., 2014). In general, in a group of healthy individuals, both QUS and EIM values across a group of healthy muscles will be similar. In the absence of significant variation in values and a narrow dynamic range, correlation between the technologies is expected to be poor and the result was not surprising (Rutkove et al., 2014). Moreover, EIM values would be expected to increase with increasing age (reflecting large myofiber size), whereas QUS data should remain relatively unchanged (Kapur et al., 2018).
There are several limitations to this study worth highlighting. First, both technologies, in their current state, are somewhat limited. Ultrasound technology has not been specifically optimized for muscle echointensity quantification and EIM methodologies are still very much in the process of refinement and development. Thus, the specific values obtained need to be interpreted cautiously. Second, we did not attempt to identify or remove those boys on corticosteroids during this study, which may have added a second confounder to our analyses, although we would anticipate that the therapeutic benefit of steroids would impact both EIM and QUS signals. Third, some patients were lost to follow up. Variation in sample size across time points may have impacted the correlation analyses to some extent. Finally, and most importantly, we only have used simple correlation analysis. Use of complex regression models, incorporating other features, including the subject age, height, and weight, may have provided a somewhat richer analysis. Nevertheless, the use of simple correlation analysis perhaps provides a more straightforward conceptual interpretation of the data than with such a multivariate approach.
In conclusion, this study supports that both QUS and EIM techniques provide related but complementary data on muscle condition in DMD, including change over time. Moreover, this study showed that certain parameters are probably more closely related to each other. These data support the use of either technology in any future DMD clinical therapeutic trials. It also raises the possibility of using both methodologies in a combined fashion, potentially providing still greater power to detect a treatment effect. Given the need for assessment tools that are sensitive to change and that do not rely on patient effort and mood, the use of these easily applied, user-friendly approaches, either individually or together, should be considered.
Supplementary Material
Highlights.
Electrical impedance myography and quantitative ultrasound are potential biomarkers in Duchenne muscular dystrophy (DMD).
They provide related but complementary information on muscle health.
Their combined use may be more valuable for assessing disease progression in DMD.
Acknowledgments
This study was funded in its entirety by NIH grant NIAMS R01AR060850.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
S.B.R. has equity in, and serves as a consultant and scientific advisor to, Myolex Inc, a company that designs impedance devices for clinical and research use; he is also a member of the company’s Board of Directors. The company also has an option to license patented impedance technology of which S.B.R. is named as an inventor. This study, however, did not employ any relevant company or patented technology. Other authors have no competing interests.
References
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57: 289–300. [Google Scholar]
- Bonati U, Hafner P, Schadelin S, Schmid M, Naduvilekoot Devasia A, Schroeder J, et al. Quantitative muscle MRI: A powerful surrogate outcome measure in Duchenne muscular dystrophy. Neuromuscul Disord 2015; 25: 679–685. doi: 10.1016/j.nmd.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Garmirian LP, Chin AB, Rutkove SB. Discriminating neurogenic from myopathic disease via measurement of muscle anisotropy. Muscle Nerve 2009; 39: 16–24. doi: 10.1002/mus.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbush TR, Visyak N, Madabusi L, Rutkove SB, Darras BT. Inter-session reliability of electrical impedance myography in children in a clinical trial setting. Clin Neurophysiol 2015; 126(9): 1790–1776. doi: 10.1016/j.clinph.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur K, Taylor RS, Qi K, Nagy JA, Li J, Sanchez B, et al. Predicting myofiber size with electrical impedance myography: A study in immature mice, Muscle Nerve 2018. February 24. doi: 10.1002/mus.26111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew JE, Florence JM, Mayhew TP, Henricson EK, Leshner RT, McCarter RJ, et al. Reliable surrogate outcome measures in multicenter clinical trials of Duchenne muscular dystrophy. Muscle Nerve 2007; 35: 36–42. Doi: 10.1002/mus.20654. [DOI] [PubMed] [Google Scholar]
- Mazzone E, Vasco G, Sormani MP, Torrente Y, Berardinelli A, Messina S, et al. Functional changes in Duchenne muscular dystrophy: A 12-month longitudinal cohort study. Neurology 2011; 77: 250–256. doi: 10.1212/WNL.0b013e318225ab2e. [DOI] [PubMed] [Google Scholar]
- McDonald CM, Henricson EK, Han JJ, Abresch RT, Nicorici A, Elfring GL, et al. The 6-minute walk test as a new outcome measure in Duchenne muscular dystrophy. Muscle Nerve 2010; 41: 500–510. doi: 10.1002/mus.21544. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol 2013; 74: 637–647. doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- Pillen S, Arts IM, Zwarts MJ. Muscle ultrasound in neuromuscular disorders. Muscle Nerve 2008; 37: 679–693. doi: 10.1002/mus.21015. [DOI] [PubMed] [Google Scholar]
- Pillen S, Tak RO, Zwarts MJ, Lammens MM, Verrijp KN, Arts IM, et al. Skeletal muscle ultrasound: Correlation between fibrous tissue and echo intensity. Ultrasound Med Biol 2009; 35: 443–446. doi: 10.1016/j.ultrasmedbio.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Pillen S, Boon A, Alfen NV. Muscle ultrasound In: Masdeu JC, Gonzalez RG, editors. Handb Clin Neurol, Neuroimaging, Part II, Vol. 136 (3rd series), Netherlands: Elsevier; 2016. p. 843–853. doi: 10.1016/B978-0-444-53486-6.00042-9. [DOI] [PubMed] [Google Scholar]
- Rutkove SB, Geisbush TR, Mijailovic A, Shklyar I, Pasternak A, Visyak N, et al. Cross-sectional evaluation of electrical impedance myography and quantitative ultrasound for the assessment of Duchenne muscular dystrophy in a clinical trial setting. Pediatr Neurol 2014; 51: 88–92. doi: 10.1016/j.pediatrneurol.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkove SB, Wu JS, Zaidman C, Kapur K, Yim S, Pasternak A, et al. Loss of electrical anisotropy is an unrecognized feature of dystrophic muscle that may serve as a convenient index of disease status. Clin Neurophysiol 2016; 127: 3546–51. doi: 10.1016/j.clinph.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkove SB, Pacheck A, Sanchez B. Sensitivity distribution simulations of surface electrode configurations for electrical impedance myography. Muscle Nerve 2017; 56(5): 887–895. doi: 10.1002/mus.25561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkove SB, Kapur K, Zaidman CM, Wu JS, Pasternak A, Madabusi L, et al. Electrical impedance myography for assessent of Duchenne muscular dystrophy. Ann Neurol 2017; 81(5): 622–632. doi: 10.1002/ana.24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez B, Rutkove SB. Electrical Impedance Myography and Its Applications in Neuromuscular Disorders. Neurotherapeutics 2017; 14(1): 107–118. doi: 10.1007/s13311-016-0491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez B, Rutkove SB. Present Uses, Future Applications, and Technical Underpinnings of Electrical Impedance Myography. Curr Neurol Neurosci Rep 2017; 17(11): 86. doi: 10.1007/s11910-017-0793-3. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Geisbush TR, Mijailovic A, Pasternak A, Darras BT, Rutkove SB. Optimizing electrical impedance myography measurements by using a multifrequency ratio: a study in Duchenne muscular dystrophy. Clin Neurophysiol 2015; 126: 202–208. doi: 10.1016/j.clinph.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman CA, Rutkove SB. Circuit modeling of the electrical impedance: I. Neuromuscular disease. Physiol Meas 2013; 34(2): 203–221. doi: 10.1088/0967-3334/34/2/203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklyar I, Geisbush TR, Mijialovic AS, Pasternak A, Darras BT, Wu JS, et al. Quantitative muscle ultrasound in Duchenne Muscular Dystrophy: A comparison of techniques. Muscle Nerve 2015; 51(2): 207–213. doi: 10.1002/mus.24296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarulli AW, Duggal N, Esper GJ, Garmirian LP, Fogerson PM, Lin CH, et al. Electrical impedance myography in the assessment of disuse atrophy. Arch Phys Med Rehabil 2009; 90(10): 1806–10. doi: 10.1016/j.apmr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wary C, Azzabou N, Giraudeau C, Le Louër J, Montus M, Voit T, et al. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed 2015; 28: 1150–1162. doi: 10.1002/nbm.3352. [DOI] [PubMed] [Google Scholar]
- Willcocks RJ, Rooney WD, Triplett WT, Forbes SC, Lott DJ, Senesac CR, et al. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large Duchenne muscular dystrophy cohort. Ann Neurol 2016; 79: 535–547. doi: 10.1002/ana.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidman CM, Alfen NV. Ultrasound in the Assessment of Myopathic Disorders. J Clin Neurophysiol 2016; 33(2): 103–111. doi: 10.1097/WNP.0000000000000245. [DOI] [PubMed] [Google Scholar]
- Zaidman CM, Wu JS, Kapur K, Pasternak A, Madabusi L, Yim S et al. Quantitative muscle ultrasound detects disease progression in Duchenne muscular dystrophy. Ann Neurol 2017; 81(5): 633–640. doi: 10.1002/ana.24904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.