Abstract
Objective:
Cancer-associated fibroblasts (CAFs) play an important role in the progression of pancreatic ductal adenocarcinoma (PDAC) by promoting tumor cell migration and drug resistance. We determined the impact of CAFs on PDAC cancer stem cells (CSCs).
Methods:
Fibroblast cell lines from patients’ tumors were co-cultured with PDAC cells and examined for clonogenic growth and self-renewal using colony forming assays and migration in vitro. Changes in the frequency of CSCs was determined by flow cytometry. The effect of integrin-Focal Adhesion Kinase (FAK) signaling on CAF-mediated clonogenic growth was evaluated using shRNAs against β1 integrin and FAK as well as a small molecule FAK inhibitor.
Results:
Cancer-associated fibroblasts enhanced PDAC clonogenic growth, self-renewal, and migration that was associated with an increase in the frequency of CSCs. These fibroblast cells were activated by PDAC cells and increased collagen synthesis resulting in FAK activation in PDAC cells. Knock-down of β1-Integrin and FAK or the inhibition of FAK kinase activity in PDAC cells abrogated the impact of CAFs on clonogenic growth.
Conclusion:
Therefore, CAFs enhance PDAC clonogenic growth, self-renewal, and the frequency of CSCs through type I collagen production that enhances integrin-FAK signaling in PDAC cells.
Keywords: pancreatic cancer, cancer stem cell, tumor microenvironment, reactive stroma, cancer-associated fibroblast
Introduction
Cancer stem cells (CSCs) have been prospectively identified in many diseases based on their increased tumor-initiating capacity, self-renewal potential, and the ability to phenotypically recapitulate the original tumor in the ectopic setting.1–3 In PDAC, CSCs are highly migratory and found in metastatic lesions suggesting an additional role in disease progression.4 A number of cell-intrinsic pathways regulating PDAC CSCs have been identified,5 but the impact of extrinsic cell factors are not well understood.
The tumor microenvironment (TME) in pancreatic ductal adenocarcinoma (PDAC) is characterized by a dense desmoplastic reaction composed of stromal, immune, and endothelial cells as well as extracellular matrix (ECM) proteins. Changes in the TME are associated with poor clinical outcomes, and the activation of cancer-associated fibroblasts (CAFs) can impair intratumoral drug delivery by inducing fibrosis and facilitate metastatic disease by promoting the epithelial-mesenchymal transition (EMT) and tumor cell migration.6–8 The reactive stroma has been found to impact stem cell functional properties in breast, lung, and colon carcinoma,9–11 and we examined the impact of CAFs on PDAC CSCs.
We co-cultured CAFs and PDAC cells and found that direct cell to cell interactions enhanced tumor cell clonogenic growth, self-renewal, and migratory potential that was associated with an increased frequency of aldehyde dehydrogenase (ALDH) expressing PDAC CSCs. Type I collagen has been shown to increase PDAC tumor-initiating potential and self-renewal by signaling through β1-Integrin and Focal Adhesion Kinase (FAK),12 and we found that the inhibition of integrin-FAK signaling in PDAC cells partially abolished the impact of CAFs on these cells. In addition, PDAC cells enhanced the expression of type I collagen in CAFs. Therefore, the interaction between CAFs and PDAC cells may establish a positive feedback mechanism promoting stem cell features.
MATERIALS AND METHODS
Cell Lines and Xenografts
Primary cancer-associated fibroblasts (CAFs) were isolated from surgically resected primary tumors as previously described.13 Normal human lung fibroblasts (nHLFs), Capan-1, and BxPC-3 cell lines were purchased from the American Type Culture Collection (Manassas, Va) and cultured in DMEM containing 10% fetal bovine serum (Sigma, St. Louis, Mo), 1% penicillin/streptomycin, and 2mM L-glutamine. Pancreatic cancer cells and CAFs were co-cultured at a 1:1 ratio for 7 or 14 days then washed with phosphate buffered saline (PBS), trypsinized, flow-sorted, and re-suspended in medium containing 10% FBS. Treatment with the FAK-inhibitor PF573228 (500nM; Sigma) was carried out for 7 days. The low passage patient-derived xenograft (PDX) JH102 has been previously described.14,15 Conditioned media from CAFs was collected after 7 days of culture and used at a final concentration of 50%.
Plasmids
pLVX-IRES-mCherry and pLVX-IRES-zsGreen lentiviral vectors were purchased from Clontech Laboratories (Mountain View, Calif), and stable lines were generated following FACS sorting. Short hairpin RNA (shRNA) constructs against FAK (shFAK), or a scrambled control sequence was cloned into the Tet-pLKO-puro lentiviral vector (Addgene, Watertown, Mass).12 The shRNA constructs against β1-Integrin were cloned into the pLKO-puro vector. Cell lines expressing shRNA against FAK or β1-Integrin were selected using puromycin (Thermo Fisher Scientific, Waltham, Mass).
Clonogenic Assay
Tumor colony formation in methylcellulose was carried out as previously described.14,15
Quantitative Real-time PCR Analysis
Total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) and reverse-transcribed with SuperScript III reverse transcriptase (Invitrogen, Carlsbad, Calif). Quantitative RT-PCR was carried out using TaqMan probes (Applied Biosystems, Foster City, Calif) for GAPDH (Hs99999905_m1), COL1A1 (Hs00164004_m1), SNAI1(Hs00195591_m1), SNAI2 (Hs00161904_m1), ZEB1 (Hs01566407_m1), ZEB2 (Hs00207691_m1), or CDH2 (Hs00983056_m1).
Flow Cytometry
Aldefluor staining was performed according to the manufacturer’s protocol (Stemcell Technologies, Vancouver, Canada), and flow cytometry was carried out as previously described.14,15 For intracellular staining, cells were fixed with 2% formalin for 10 minutes, followed by permeabilization with 1% NP40 for 10 minutes at room temperature. Cells were stained with rabbit monoclonal anti-pY397-FAK (Invitrogen), monoclonal alpha-Smooth Muscle Actin (M0851, DAKO-Agilent, Santa Clara, Calif), or rabbit polyclonal anti-FAK (Cell Signaling, Danvers, Mass) antibodies followed by the monoclonal anti-rabbit-APC antibody (Invitrogen).
Migration Assay
Cell migration assay was carried out as previously described.14,15
Statistics
Statistical differences between two groups were analyzed using the two-tailed, unpaired Student t-test (Version- Prism 6, GraphPad Prism Software, Inc.. La Jolla, Calif). P values < 0.05 were considered significant.
RESULTS
Cancer-associated Fibroblasts Enhance the Clonogenic Growth Potential of PDAC
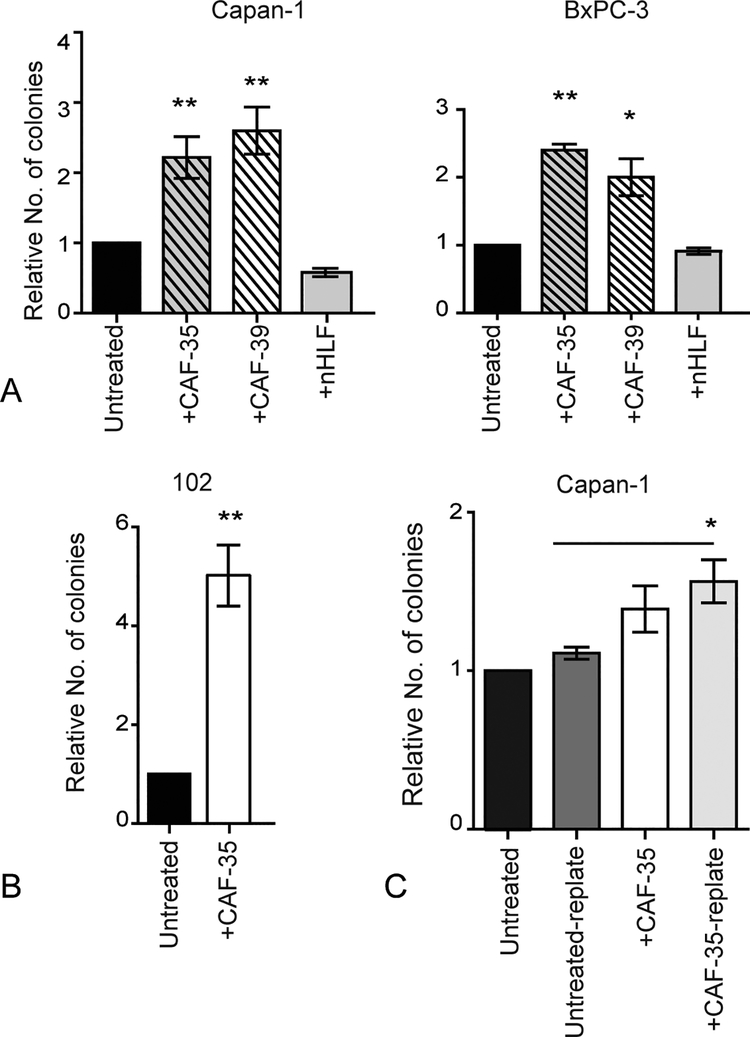
Cancer-associated fibroblasts are a major cellular component of the reactive stroma and contribute to PDAC progression by secreting ECM proteins and cytokines.16 To determine their impact on clonogenic tumor growth, CAF cell lines established from primary surgical specimens and stably expressing mCherry or zsGreen fluorescent proteins were co-cultured with PDAC cells.13 Tumor cells were then isolated by FACS and plated in methylcellulose to quantify clonogenic growth potential (Supplemental Fig. 1A). Compared to Capan-1 and BxPC-3 cells cultured alone or with nHLFs, CAFs derived from multiple primary tumors significantly increased tumor colony formation by 1.3–2.5 fold (Fig. 1A; P ≤ 0.05). Similarly, PDAC cells isolated from a low passage PDX (JH102) formed significantly more colonies when co-cultured with CAFs (Fig. 1B; P ≤ 0.001). This enhanced clonogenic growth potential was cell-contact dependent as tumor cell colony formation was not significantly impacted by CAF-conditioned media (Supplemental Fig. 1B). Notably, increased colony formation was not due to changes in the proliferation (data not shown) of PDAC cells. The maintenance of clonogenic growth over time is required for disease relapse, and tumor colonies were harvested and serially replated to quantify the impact of CAFs on PDAC self-renewal. Although cells were not further exposed to CAFs, secondary colony formation by Capan-1 cells remained significantly increased > 1.3 fold (Fig. 1C; P ≤ 0.05). Therefore, CAFs enhance PDAC clonogenic growth and self-renewal.
FIGURE 1.
Cancer-associated fibroblasts enhance the clonogenic growth potential of PDAC. A, Colony formation by PDAC cell lines (Capan-1, and BxPC-3) and B, a PDX (JH102) cells following co-culture with CAFs or nHLFs for 7 days. Data represent the mean and SD of 4 experiments. *P < 0.05, **P < 0.005. C, Primary and secondary colony formation by Capan-1 cells cultured with or without CAFs for seven days. Data represent the mean and SD of 4 experiments. *P < 0.05.
Cancer-associated Fibroblasts Induce EMT and Facilitate PDAC Migration
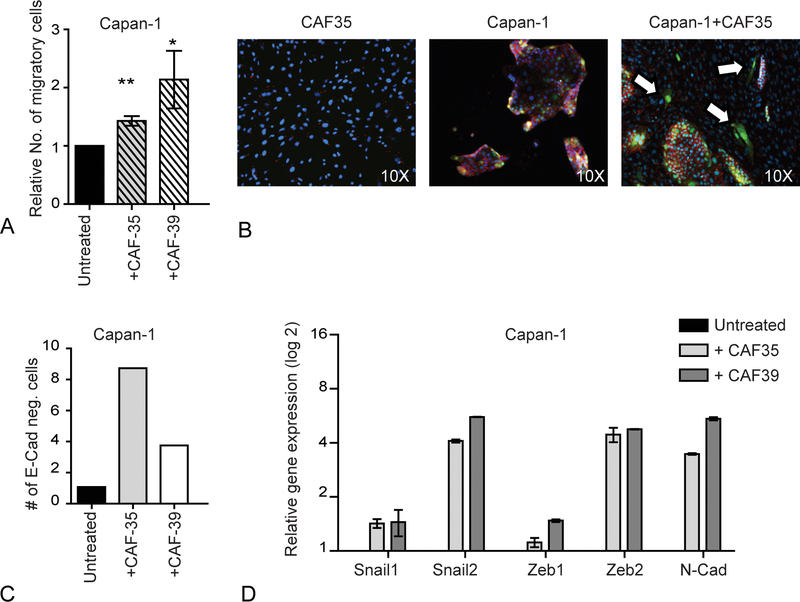
The migratory potential of CSCs is increased compared to bulk tumor cells and suggests a role in metastatic PDAC progression.4 Following co-culture with CAFs, the migration of PDAC cells was significantly increased by 1.3–2.1 fold (Fig. 2A, Supplemental Fig. 2A; P ≤ 0.05) similar to other reports in PDAC and other cancers.17–20 In contrast, the treatment of PDAC cells with CAF-conditioned medium did not affect migration (Supplemental Fig. 2B) suggesting that direct interaction with CAFs was required. In various cancers, including PDAC, EMT contributes to metastasis by promoting cell invasion and migration.21–28 Cancer stem cells may express features suggestive of EMT,29 and we found that the co-culture of Capan-1 cells with CAFs decreased E-cadherin expression and increased the expression of genes such as Snail, Slug, Zeb1, Zeb2, and N-cadherin that are associated with EMT (Figs. 2B–D). Therefore, CAFs promote PDAC cell migration by inducing EMT.
FIGURE 2.
Cancer-associated fibroblasts induce EMT and facilitate PDAC migration. A, In vitro migration of Capan-1 cells following culture with CAF-conditioned or control media for 7 days. Data represent the mean and SD of 4 experiments. *P < 0.05, **P < 0.001, ***P < 0.0001. B, E-cadherin staining (red) of Capan-1 cells (GFP) following co-culture with CAFs for 7 days. Arrows indicate E-cadherin negative Capan-1 cells. C, The frequency of E-cadherin negative Capan-1 cells following culture with or without CAFs detected by flow cytometry. D, Relative mRNA expression of EMT associated genes in sorted Capan-1 cells following co-culture with CAFs. Data represent the mean and SD of 3 experiments.
Cancer-associated Fibroblasts Enhance the Frequency of ALDH+ PDAC CSCs
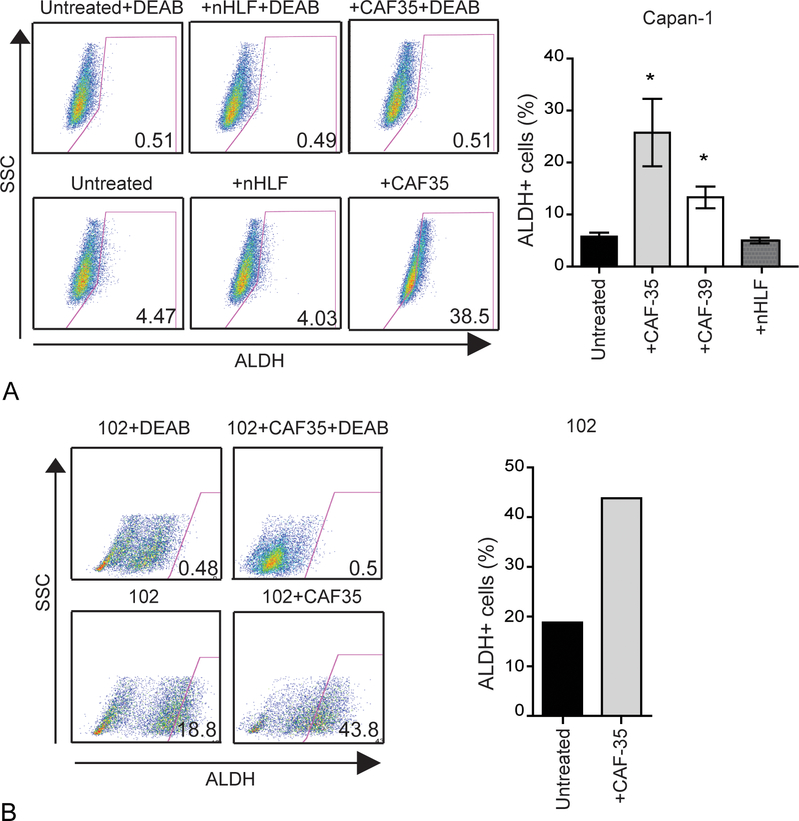
We previously demonstrated that highly clonogenic PDAC CSCs express aldehyde dehydrogenase (ALDH) activity.4 Since co-cultures enhanced PDAC clonogenic growth and migration, the impact of CAFs on the frequency of ALDH+ CSCs was examined. In Capan-1 and BxPC-3 cells, the frequency of ALDH+ cells significantly increased by 1.6–8 fold following co-cultures with CAFs compared to PDAC cells cultured alone or with nHLFs (Fig. 3A, Supplemental Fig.3A; P ≤ 0.05). Similarly, the frequency of ALDH+ CSCs was increased by 2.3 fold in cells from the JH102 PDX when cultured with CAFs (Fig. 3C). Therefore, CAFs enhance the frequency of ALDH+ PDAC CSCs.
FIGURE 3.
Cancer-associated fibroblasts enhance the frequency of ALDH+ PDAC CSCs. A, ALDH expression by Capan-1 cells following 7 days of co-culture with CAFs. Cells treated with DEAB were used as negative control for ALDH staining. Data represent the mean and SD of 3 experiments. *P < 0.05, *** P < 0.0001. B, The frequency of ALDH+ cells was analyzed in patient-derived low passage PDX (102) following co-culture with CAF35.
Co-cultured CAFs Induce Integrin-FAK Signaling in PDAC Cells
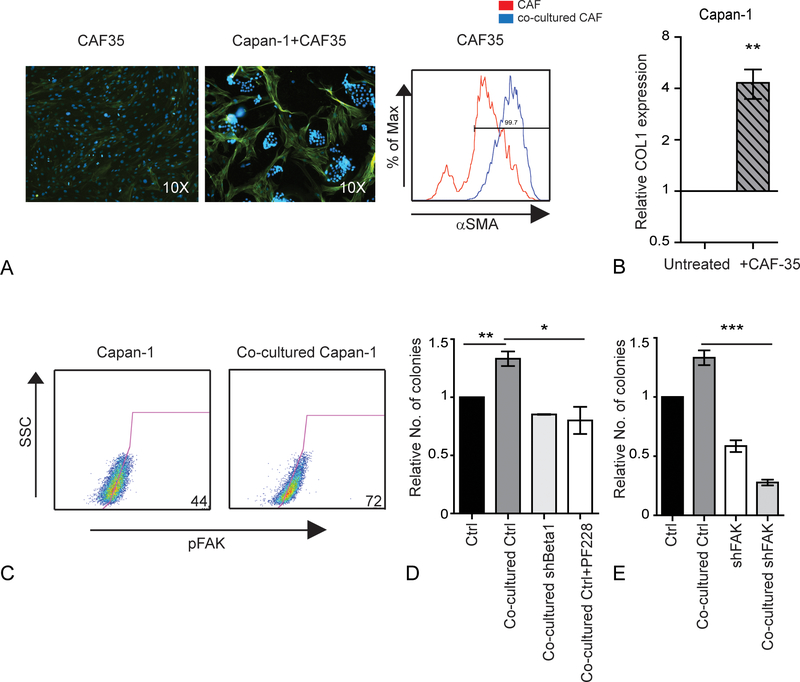
Activated CAFs express alpha-smooth muscle actin (αSMA) and several ECM proteins, and their presence in primary PDAC tumors is associated with the poor outcomes.16,30 Following co-culture with PDAC cells, the expression of αSMA was increased (Fig. 4A, Supplemental Fig.4A) suggesting that their activation was induced by tumor cells. In addition, CAFs co-cultured with PDAC cells expressed significantly higher levels of type I collagen (Fig 4B; P = 0.0002). We previously demonstrated that type I collagen specifically increases tumor initiation and self-renewal potential by signaling through β1-Integrin and activating FAK.12 Following co-culture with CAFs, FAK activation, determined by phosphorylation at residue 397 (pY397), was increased in Capan-1 cells (Fig 4C). To determine the role of β1-Integrin and FAK in the interaction between tumor and stromal cells, Capan-1 cells with shRNA against β1-Integrin were co-cultured with CAFs and evaluated for clonogenic growth. Compared to cells with a control shRNA construct, the loss of β1-Integrin expression abrogated the enhanced clonogenic growth of CAFs (Fig. 4D; P ≤ 0.05). Similarly, treatment with a small molecule FAK inhibitor (PF573228) inhibited the enhanced clonogenic growth induced by CAFs. In our previous study, we also found that the loss of FAK expression inhibited CSCs to a greater extent than FAK kinase inhibition likely due to the loss of its role as a cellular scaffold.12 Similarly, FAK knock-down significantly inhibited tumor colony formation even in the presence of CAFs (Fig 4E; P = 0.002, Supplemental Fig. 4B). Therefore, both the kinase and non-kinase activities of FAK impact the interactions between CAFs and PDAC cells.
FIGURE 4.
Cancer-associated fibroblasts express type I collagen and enhance integrin-FAK signaling in PDAC. A, αSMA expression of CAFs following co-culture with or without Capan-1 cells for 7 days. Left: immunostaining of αSMA (green) and nucleus-Hoechst 33342 (blue). Right: αSMA expression by CAF35 cells before and after co-cultured with Capan-1 cells. B, Relative mRNA expression of type I collagen by CAF35 cells following co-culture with Capan-1 cells. C, The frequency of pY397-FAK+ Capan-1 cells following co-culture with CAFs. D, Colony formation by Capan-1 cells expressing a scrambled control (Ctrl) or β1 integrin (shBeta1) shRNA vectors or treated with PF573228 following co-culture with CAFs. Data represent the mean and SD of 3 experiments. **P < 0.001. E, Colony formation by Capan-1 cells expressing a scrambled control (Ctrl) or FAK (shFAK) shRNA following co-culture with CAFs. Data represent the mean and SD of 3 experiments. *P < 0.05; **P < 0.001, ***P < 0.0001.
DISCUSSION
In normal systems, the surrounding microenvironment regulates stem cell fate decisions that include proliferation, self-renewal, and differentiation. In many diseases, specific components of the TME, including specific cellular components, such as mesenchymal stem cells, stromal cells, immune cells, and endothelial cells have been found to regulate CSC self-renewal and differentiation.31 In some cancers, direct cell contact or the production of cytokines by non-tumor cells within the TME can promote stem cell functional properties and the expansion of CSCs.10,32–34 In PDAC, CAFs are a distinct part of the TME, and we found that these cells enhance the clonogenic growth and self-renewal of PDAC cells. Pancreatic CSCs are relatively more invasive and migratory than non-CSCs implicating a role in metastatic tumor dissemination, and previous studies have demonstrated that CAFs increase PDAC cell migration.35,36 We found that CAFs increased the migratory potential of PDAC cells as well as the frequency of ALDH+ cells suggesting that this enhancement may be in part due to increased CSCs.
The activation of CAFs leads to the production of several ECM proteins that contribute to the desmoplastic reaction in PDAC. We previously demonstrated that type I collagen enhances stem cell properties in PDAC by signaling through β1-Integrin and activating FAK.12 In other diseases, ECM molecules produced by CAFs have also been found to induce stem cell properties.12,37 Here we demonstrate that CAFs are activated by PDAC cells to express type I collagen that subsequently activates FAK in PDAC CSCs. Therefore, it is possible that the bidirectional interactions between CAFs and PDAC cells ultimately lead to disease relapse and progression by stimulating CSCs.
Fibrosis produced by the reactive stroma may play an important role in PDAC therapeutic resistance by restricting adequate intratumoral levels of chemotherapeutic agents.38 Attempts to clinically target the stromal cellular compartment by inhibiting the Hedgehog signaling pathway have not been successful.39 Furthermore, inhibiting αSMA resulted in more aggressive tumors in transgenic mice models, suggesting that the stroma may be protective in restricting tumor dissemination.40,41 We found that the loss of β1-Integrin or FAK activity in PDAC cells inhibited the impact of CAFs on clonogenic growth. Therefore, strategies to block the effects of the TME, such as FAK inhibitors,12,42 rather than CAFs themselves may represent a novel therapeutic approach.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ada Tam, Hao Zhang and Lee Blosser of the Johns Hopkins Core Flow Cytometry facilities for their assistance with flow cytometry and cell sorting.
Grant support
Support was provided by The Pancreatic Cancer Action Network-AACR 10-70-25-RASH (ZAR), The V Foundation (ZAR), NIH R01CA150142 (WM), R01CA193887 (WM), and The Lustgarten Foundation (WM).
Footnotes
Conflict of Interest
Asma Begum, Ross McMillan, Yu-Tai Chang, Vesselin Penchev, NV Rajeshkumar, Anirban Maitra, Christopher Wolfgang, Zeshaan A. Rasheed, and William Matsui do not have competing financial interests.
REFERENCES
- 1.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. [DOI] [PubMed] [Google Scholar]
- 3.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. [DOI] [PubMed] [Google Scholar]
- 4.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CJ, Li C, Simeone DM. Human pancreatic cancer stem cells: implications for how we treat pancreatic cancer. Transl Oncol. 2008;1:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabashima-Niibe A, Higuchi H, Takaishi H, et al. Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci. 2013;104:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HO, Mullins SR, Franco-Barraza J, et al. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WJ, Ho CC, Chang YL, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun. 2014;5:3472. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Ginestier C, Ou SJ, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeulen L, De Sousa EMF, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. [DOI] [PubMed] [Google Scholar]
- 12.Begum A, Ewachiw T, Jung C, et al. The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS One. 2017;12:e0180181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter K, Omura N, Hong SM, et al. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol Ther. 2008;7:882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jimeno A, Feldmann G, Suarez-Gauthier A, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasheed Z, Wang Q, Matsui W. Isolation of stem cells from human pancreatic cancer xenografts. J Vis Exp. 2010. pii: 2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soon PS, Kim E, Pon CK, et al. Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocr Relat Cancer. 2013;20:1–12. [DOI] [PubMed] [Google Scholar]
- 18.Lebret SC, Newgreen DF, Thompson EW, et al. Induction of epithelial to mesenchymal transition in PMC42-LA human breast carcinoma cells by carcinoma-associated fibroblast secreted factors. Breast Cancer Res. 2007;9:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, Lan L, Jiang YG, et al. Epithelial-mesenchymal transition and migration of prostate cancer stem cells is driven by cancer-associated fibroblasts in an HIF-1alpha/beta-catenin-dependent pathway. Mol Cells. 2013;36:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan T, Chen S, Chen X, et al. Prometastatic mechanisms of CAF-mediated EMT regulation in pancreatic cancer cells. Int J Oncol. 2017;50:121–128. [DOI] [PubMed] [Google Scholar]
- 21.Trimboli AJ, Fukino K, de Bruin A, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. [DOI] [PubMed] [Google Scholar]
- 22.Adam L, Zhong M, Choi W, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15:5060–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim MA, Lee HS, Lee HE, et al. Prognostic importance of epithelial-mesenchymal transition-related protein expression in gastric carcinoma. Histopathology. 2009;54:442–451. [DOI] [PubMed] [Google Scholar]
- 24.Frank DK, Szymkowiak B, Josifovska-Chopra O, et al. Single-cell microinjection of cytochrome c can result in gap junction-mediated apoptotic cell death of bystander cells in head and neck cancer. Head Neck. 2005;27:794–800. [DOI] [PubMed] [Google Scholar]
- 25.Krebs AM, Mitschke J, Lasierra Losada M, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19:518–529. [DOI] [PubMed] [Google Scholar]
- 26.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. [DOI] [PubMed] [Google Scholar]
- 27.Comijn J, Berx G, Vermassen P, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. [DOI] [PubMed] [Google Scholar]
- 28.Chaffer CL, San Juan BP, Lim E, et al. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. [DOI] [PubMed] [Google Scholar]
- 29.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang LM, Silva MA, D’Costa Z, et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:4183–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabarcas SM, Mathews LA, Farrar WL. The cancer stem cell niche--there goes the neighborhood? Int J Cancer. 2011;129:2315–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanno T, Lim Y, Wang Q, et al. Growth differentiating factor 15 enhances the tumor-initiating and self-renewal potential of multiple myeloma cells. Blood. 2014;123:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waghray M, Yalamanchili M, Dziubinski M, et al. GM-CSF Mediates Mesenchymal-Epithelial Cross-talk in Pancreatic Cancer. Cancer Discov. 2016;6:886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erdogan B, Ao M, White LM, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216:3799–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirakawa T, Yashiro M, Doi Y, et al. Pancreatic Fibroblasts Stimulate the Motility of Pancreatic Cancer Cells through IGF1/IGF1R Signaling under Hypoxia. PLoS One. 2016;11:e0159912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shek FW, Benyon RC, Walker FM, et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catenacci DV, Junttila MR, Karrison T, et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33:4284–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melstrom LG, Salazar MD, Diamond DJ. The pancreatic cancer microenvironment: A true double agent. J Surg Oncol. 2017;116:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.