Abstract
Deep brain stimulation (DBS) implanted in different basal ganglia nuclei regulates the dysfunctional neuronal circuits and improves symptoms in movement disorders. However, the understanding of the neurophysiological mechanism of DBS is at an early stage. Transcranial magnetic stimulation (TMS) can be used safely in movement disorder patients with DBS, and can shed light on how DBS works. DBS at a therapeutic setting normalizes the abnormal motor cortical excitability measured with motor evoked potentials (MEP) produced by primary motor cortical TMS. Abnormal intracortical circuits in the motor cortex tested with paired-pulse TMS paradigm also show normalization with DBS. These changes are accompanied with improvements in symptoms after chronic DBS. Single-pulse DBS produces cortical evoked potentials recorded by electroencephalography at specific latencies and modulates motor cortical excitability at certain time intervals measured with MEP. Combination of basal ganglia DBS with motor cortical TMS at stimulus intervals consistent with the latency of cortical evoked potentials delivered in a repetitive mode produces plastic changes in the primary motor cortex. TMS can be used to examine the effects of open and closed loop DBS. Patterned DBS and TMS delivered in a repetitive mode may be developed as a new therapeutic method for movement disorder patients.
Keywords: Basal ganglia, cortical circuit, cortical plasticity, deep brain stimulation, movement disorder, transcranial magnetic stimulation
1. Introduction
Movement disorders are characterized by hyperkinetic or hypokinetic movements. Abnormal neuronal activities in the cortex and other regions related to disturbances in the basal ganglia nuclei is a major cause of many movement disorders (Lang and Lozano, 1998a; Lozano and Lipsman, 2013). Modulation of disordered circuits within basal ganglia-thalamo-cortical pathways is a critical therapeutic strategy in various movement disorders. Although a pharmacological approach to correct disordered circuits is useful, potential exposure of all neurons in the brain to a drug may make the therapy less attractive in some patient populations (Lang and Lozano, 1998b). Pinpointing the dysfunctional neuronal circuits and regulating their activity with deep brain stimulation (DBS) after surgical placement may be a better option for patients who have failed conventional pharmacological therapy (Benabid et al., 1991; Deuschl et al., 2006b; Kupsch et al., 2006; Lozano and Lipsman, 2013). There is level 1 evidence for subthalamic nucleus (STN) and internal globus pallidus (GPi) DBS as treatment of motor symptoms in Parkinson’s disease (Rughani et al., 2018) and level 3 evidence for ventralis intermedius nucleus of thalamus DBS as treatment of essential tremor patients (Zesiewicz et al., 2011). There is also level 1 evidence with large, randomized controlled trials for GPi DBS as treatment of generalized and segmental dystonia patients (Kupsch et al., 2006; Volkmann et al., 2014).
Despite the clinical evidence of treatment efficacy in some movement disorders, the understanding of the neurophysiological basis for the mechanisms of action of DBS is still at an early stage (Fasano et al., 2017; Kringelbach et al., 2007; Lozano et al., 2002; Lozano and Lipsman, 2013; Udupa and Chen, 2015). Although animal studies are powerful and successful in determining certain DBS targets and examination of its underlying mechanisms in some movement disorders (Bergman et al., 1990; Hamani and Temel, 2012), no animal model is currently able to recreate all aspects of a particular movement disorder. One basic idea in clinical neurophysiology for solving this difficulty is to study the physiological effects of DBS in human patients by overcoming the technical barriers of both invasive and non-invasive recordings in humans to reliably record from dysfunctional brain circuits.
Transcranial magnetic stimulation (TMS) is a noninvasive and painless technique to stimulate the intact human brain (Hallett, 2000, 2007). When TMS is applied to the primary motor cortex, it activates the corticospinal neurons and generates motor evoked potential (MEP) in the target muscles. TMS is safe in movement disorders patients with DBS implanted in different targets in the basal ganglia (Phielipp et al., 2017; Udupa and Chen, 2015). The motor portions of basal ganglia circuits project their outputs to motor related cortical areas through the thalamus (Alexander et al., 1986; DeLong and Wichmann, 2007; Nambu, 2007). Therefore, investigation of changes in basal ganglia circuits and motor cortical circuits caused by DBS using various TMS paradigms may provide insights on the mechanisms of DBS in movement disorders. Repetitive TMS, which involves application of trains of regularly repeating TMS pulses, modulates cortical plasticity and produces long-term potentiation or depression-like effects in the cortex (Hallett, 2000, 2007). Combination of DBS and TMS applied in repetitive mode is a novel technique to identify plastic changes in the cortex in movement disorders. In addition, DBS may produce plastic changes directly and this may be related to the therapeutic effects of DBS. In this article, we review TMS studies with various designs and paradigms that investigated the physiological effects of DBS in movement disorders patients with DBS implanted in different targets including STN, GPi and ventralis intermedius nucleus of thalamus (Fig. 1).
Figure 1. Experimental design for testing the effects of deep brain stimulation on primary motor cortex using transcranial magnetic stimulation.
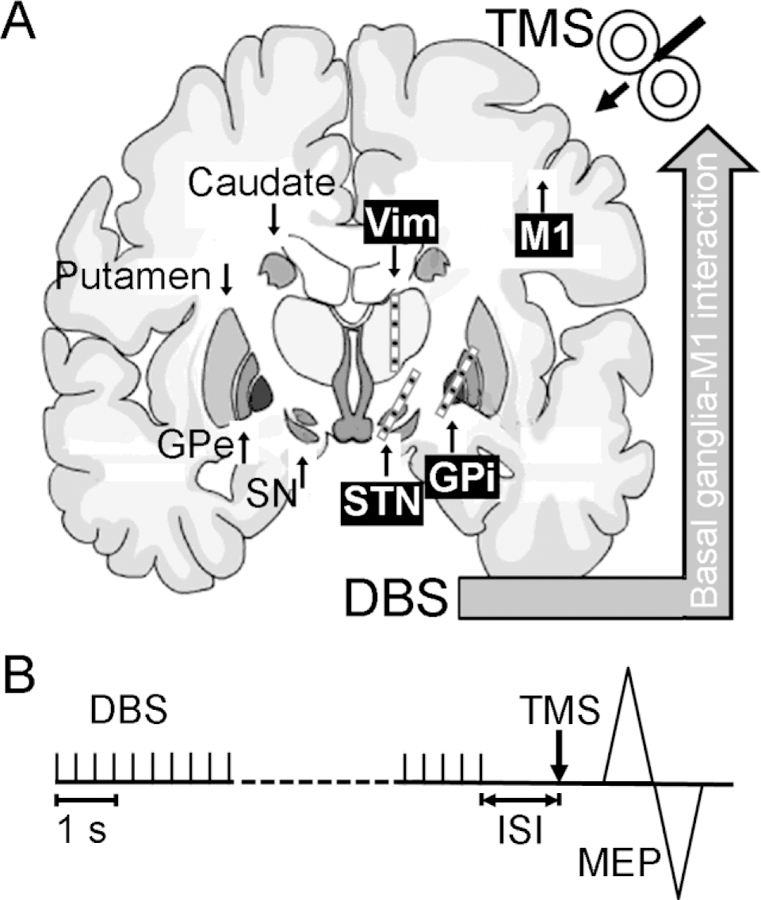
(A) The effects of DBS on the M1 have been tested using TMS with various experimental paradigms in movement disorders patients with DBS implanted in different basal ganglia nuclei. Common DBS targets for movement disorders including STN, GPi and Vim are labeled on the right side of the brain. The DBS electrodes (typically have four contacts in total) are usually implanted with the middle contacts located in the target nuclei. Other important basal ganglia nuclei including SN, GPe, putamen and caudate are labeled on the left side of the brain. TMS is applied to the M1 ipsilateral to the basal ganglia nucleus being tested. (B) Experimental setup. In many studies, DBS on the studied side was set at low frequency (< 10 Hz) and that on the contralateral side was turned off. TMS was triggered with DBS artifact at certain ISI. The interactions between basal ganglia nucleus and M1 were tested by measuring the changes in MEP amplitudes. DBS = deep brain stimulation, GPe = external globus pallidus, GPi = internal globus pallidus, ISI = interstimulus interval, M1 = primary motor cortex, MEP = motor evoked potential, SN = substantia nigra, STN = subthalamic nucleus, TMS = transcranial magnetic stimulation, Vim = ventralis intermedius nucleus of thalamus.
2. Physiological effects with deep brain stimulation at therapeutic setting
The primary motor cortex plays an important role in voluntary movement and is the primary target of the output of the motor portions of the basal ganglia (Alexander et al., 1986; DeLong and Wichmann, 2007; Udupa and Chen, 2015). Therefore, comparing motor cortical excitability and cortical circuits in patients with movement disorders in the DBS on and off states is a way to elucidate the mechanism of action of DBS in these diseases (Fig. 1A)(Hallett, 2000; Lozano et al., 2002).
2.1. Changes in motor cortical excitability
Since DBS disturbs the disordered firing and pathological oscillatory activity in the target basal ganglia nuclei, it likely changes the excitability of primary motor cortex indirectly through multiple synaptic transfers and corrects abnormal signals to the motor cortex. Therefore, the normalization of MEP induced by TMS may be detected in the DBS on compared to off state (Lozano et al., 2002; Udupa and Chen, 2015). STN and GPi are the two most common target nuclei for DBS in patients with Parkinson’s disease and dystonia (Lang, 2000; Lozano et al., 2002; Lozano and Lipsman, 2013). DBS of the ventralis intermedius nucleus of thalamus (an area receiving cerebellar inputs) is an effective treatment for essential tremor (Deuschl et al., 2011). Studies with single-pulse TMS have revealed that DBS on these targets are associated with changes in motor cortical excitability.
MEP threshold refers to the lowest TMS intensity to produce a small but reliable MEP in the target muscle. MEP threshold reflects the excitability and local density of a central core of corticospinal neurons with the highest sensitivity to TMS (Hallett, 2000, 2007). MEP threshold either at rest or during tonic contraction of the target muscle did not change with DBS. The phenomenon was consistent across different diseases with DBS implanted in various basal ganglia nuclei, including STN (Cunic et al., 2002; Wessel et al., 2016) and GPi (Chen et al., 2001) in Parkinson’s disease, GPi in dystonia (Ruge et al., 2011a; Ruge et al., 2011b) and motor thalamus in essential tremor patients (Molnar et al., 2005; Molnar et al., 2004). The results are consistent with the anatomy of basal ganglia circuits that all these nuclei for DBS target project to the motor cortex through motor thalamus. On the other hand, it is likely that the effect of DBS is not limited to the motor cortical neurons with the highest sensitivity to stimulation and is manifested differently in other neuronal groups with higher firing threshold as changes in MEP amplitude with higher TMS intensity were more complex. MEP amplitude from suprathreshold TMS was increased in Parkinson’s disease patients with STN and GPi DBS stimulation turned off compared to healthy controls (Chen et al., 2001; Cunic et al., 2002; Wessel et al., 2016). The finding is similar to that in Parkinson’s disease patients without DBS (Ni et al., 2013). As DBS is generally performed in moderate to advanced Parkinson’s disease patients, it could be inferred that motor cortical output is increased even at an early stage (in patients without DBS) and the increased motor cortical excitability involves cortical neurons with high firing threshold or further from the center core (as MEP threshold did not change in these patients). The widely accepted model of Parkinson’s disease pathophysiology suggests that the loss of dopaminergic neurons in substantia nigra pars compacta reduces the activities in the direct pathway and increases activities in the indirect pathway (Kravitz et al., 2010; Lang and Lozano, 1998b; Nambu, 2008). This produces exaggerated inhibition from GPi to the motor thalamus and may lead to compensatory mechanisms to increase motor cortical excitability. Surprisingly, neither STN nor GPi DBS normalizes the increased MEP in advanced Parkinson’s disease patients (Chen et al., 2001; Cunic et al., 2002; Wessel et al., 2016), suggesting that the compensatory mechanisms may not be simply replaced by the effects of STN or GPi DBS although motor symptoms are largely improved with DBS. MEP amplitude in dystonia patients did not change after GPi DBS surgery even after the DBS was turned on for 6 months (Ruge et al., 2011b). However, switching off the stimulator might lead to a transient reduction in the slope of MEP recruitment curve (Kuhn et al., 2003). Increased MEP amplitude with high but not with low TMS intensity compared to healthy controls was found in essential tremor patients when motor thalamus DBS was turned on, suggesting that DBS has facilitatory effects in the target area to activate the excitatory thalamocortical projection to motor cortical neurons located in deeper layers (Molnar et al., 2005).
2.2. Changes in local intracortical circuits
In addition to the effects on motor cortical excitability, DBS also modulates the local intracortical circuits in the primary motor cortex. This can be tested with well-established paired-pulse TMS paradigms with which the MEP induced by a test pulse to the primary motor cortex is conditioned by a preceding conditioning pulse at different stimulus parameters (e.g., stimulus location, intensity, interstimulus interval, etc.)(Hallett, 2007; Ni et al., 2011).
Short (Kujirai et al., 1993) and long interval intracortical inhibition (Wassermann et al., 1996) are the most commonly tested intracortical circuits, mediated by gamma-aminobutryic-acid type A and B receptors, respectively (Ziemann, 2004). Short interval intracortical inhibition was decreased in Parkinson’s disease patients. The reduced inhibition was only partially normalized by dopaminergic medication (MacKinnon et al., 2005a; Ni et al., 2013; Ridding et al., 1995). This is likely because short interval intracortical inhibition is contaminated by short interval intracortical facilitation as the stimulus intensities (around motor threshold for the first pulse) and interstimulus intervals (1–5 ms) used to measure these two cortical circuits overlap (Hallett, 2007; Ni et al., 2011). Indeed, our previous study has demonstrated increased short interval intracortical facilitation in Parkinson’s disease and the exaggerated facilitation can be normalized by dopaminergic medications. On the other hand, the reduced cortical inhibition in Parkinson’s diseases measured with stimulus parameters outside the range for potential contamination of cortical facilitation was only partially normalized by dopaminergic medications (Ni et al., 2013). Similar impairment in cortical inhibition was observed in more advanced patients with STN and GPi DBS when the DBS was turned off (Fig 2A and 2B)(Chen et al., 2001; Cunic et al., 2002). STN DBS normalized the decreased inhibition at the DBS on state (Cunic et al., 2002), suggesting that the effect of DBS and that of dopaminergic medication are different and DBS does not simply compensate for dopamine deficit in basal ganglia circuits. Clinically ineffective STN DBS at half the stimulation intensity used to produce clinical benefit did not normalize the reduced inhibition, suggesting that the restoration of motor cortical inhibition may be related to improvement of motor symptoms. Short interval intracortical inhibition did not change with GPi DBS (Fig. 2C). The different physiological effects between GPi DBS and STN DBS may be related to different therapeutic effects of the two types of DBS. Intracortical facilitation did not change with STN or GPi DBS. Long interval intracortical inhibition either in the rest or active state (measured as changes in cortical silent period) did not change with STN DBS (Cunic et al., 2002). On the other hand, GPi DBS shortened the prolonged cortical silent period in Parkinson’s disease patients (Chen et al., 2001).
Figure 2. Effects on motor cortical circuits with deep brain stimulation.
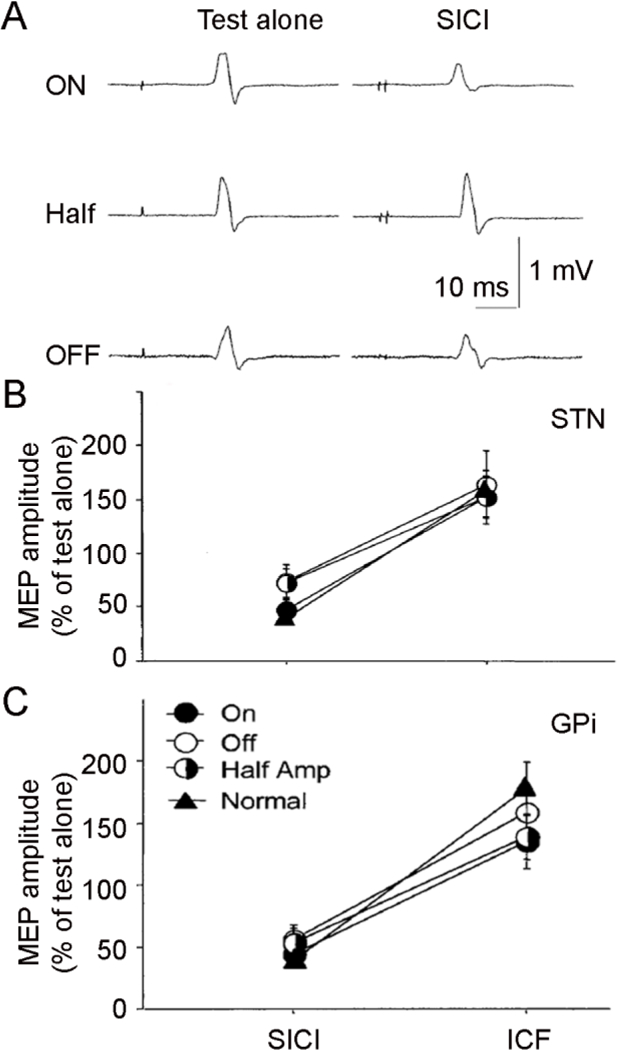
Effects on SICI (tested with interstimulus interval of 2 ms) and ICF (interval of 10 ms) in Parkinson’s disease patients with STN and GPi DBS. (A) Examples of single trials for SICI from one patient with STN DBS. The test pulse produced MEP of about 1 mV in all three conditions: DBS ON (with clinical setting), Half ON (half voltage of that used in clinical setting) and DBS OFF. SICI was stronger in the ON condition compared to the Half and OFF conditions. (B) and (C) Group analysis (mean ± standard deviation) for SICI and ICF with STN (B) and GPi DBS (C). The ordinate indicates MEP amplitude induced by paired-pulse TMS normalized as a percentage of that with test stimulus alone. Values below 100% represent inhibition and those above 100% represent facilitation. Filled circles represent ON state, half-filled circles represent Half state, open circles represent OFF state, and filled triangles represent age-matched healthy controls. STN but not GPi DBS restored impaired SICI in Parkinson’s disease. ICF did not change with either STN or GPi DBS. DBS = deep brain stimulation, GPi = internal globus pallidus, ICF = intracortical facilitation, MEP = motor evoked potential, SICI = short interval intracortical inhibition, STN = subthalamic nucleus, TMS = transcranial magnetic stimulation. Modified from Chen et al. (2001) and Cunic et al. (2002).
Short interval intracortical inhibition is reduced in dystonia. GPi DBS normalized the impaired inhibition in dystonia patients (Ruge et al., 2011a; Ruge et al., 2011b). The effects persisted even when the DBS was turned off for two days in the patients with chronic (longer than 6 years) DBS implantation (Ruge et al., 2011a), suggesting that cortical plastic change occurred in addition to the restoration of cortical inhibition after long-term treatment with DBS.
Cerebellar inhibition refers to the effect that a TMS pulse delivered to the cerebellum inhibits the MEP produced by a motor cortical TMS 4–7 ms later, reflecting the excitability of the cerebello-thalamo-cortical pathway (Hallett, 2007; Ugawa et al., 1995). The inhibition was preserved in mild essential tremor patients (Pinto et al., 2003), but was absent in patients with DBS of ventralis intermedius thalamus in the off stimulation state (Molnar et al., 2004). The lack of cerebellar inhibition in the DBS patients at a more advance stage may be due to the progression of disease or microlesion effect of the thalamus caused by DBS surgery. On the other hand, motor thalamic DBS restored the lost inhibition in essential tremor patients. The restoration of inhibition occurred to a lesser extent even when DBS was at half on state (Molnar et al., 2004). Therefore, thalamic DBS may functionally change the excitability of inhibitory neurons and normalize the activity along cerebello-thalamo-cortical pathways in the patients. However, recruitment of additional circuits by DBS in the disynaptic cerebellar-cortical pathway at or near the ventralis intermedius thalamus may also be possible. The findings are consistent with the hypothesis that DBS activates rather than inhibits the target area. Changes in motor cortical excitability and cortical circuit in movement disorders patients with DBS tested by TMS are summarized in Table 1.
Table 1.
Changes in motor cortical excitability and cortical circuits with deep brain stimulation
| Measurements | Parkinson’s disease STN | Parkinson’s disease GPi | Dystonia GPi | |||
|---|---|---|---|---|---|---|
| OFF | ON | OFF | ON | OFF | ON | |
| MEP threshold | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| MEP amplitude | ↑ | ↑ | ↑ | ↑ | ↓a | ↔a |
| SICI | ↓ | Normalized | ↓ | ↓ | ↓ | Normalized |
| ICF | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| LICI | ↔ | ↔ | ↑b | Normalizedb | Not tested | Not tested |
Abbreviations
GPi = internal globus pallidus, ICF = intracortical facilitation, LICI = long interval intracortical inhibition, MEP = motor evoked potential, SICI = short interval intracortical inhibition, STN = subthalamic nucleus, ↑ = increase, ↓ = decrease, ↔ = no change.
All changes are based on the comparison between patients (Parkinson’s disease patients with STN and GPi deep brain stimulation and dystonia patients with GPi stimulation) and healthy controls.
ON refers to the state that deep brain stimulation was turned on with the clinical setting and OFF refers to the state that the stimulation was turned off.
Normalized refers to the effect that deep brain stimulation normalized the abnormality in the patients when the stimulation was turned off.
Notes:
MEP amplitude in dystonia patients with GPi deep brain stimulation does not change even after the stimulation has been turned on for 6 months. However, switching off the stimulator may lead to a transient reduction in the slope of MEP recruitment curve.
Tested with cortical silent period in the active state with voluntary muscle contraction.
3. Physiological effects of single-pulse deep brain stimulation on cortical circuits
The therapeutic premise of DBS in movement disorders is that the high frequency stimulation regulates the dysfunctional output from local neural circuits due to the underlying diseases. High frequency DBS with clinical benefit leads to augmented synchrony of neuronal activities in the basal ganglia and is different from the effects of single-pulse DBS (Lozano and Lipsman, 2013; Nambu, 2008; Udupa and Chen, 2015). In addition, high frequency DBS may lead to the loss of specificity in the stimulated area and change the pattern of neuronal activation (Nambu, 2008; Oswal et al., 2013). Nevertheless, testing the physiological effects produced by a single-pulse DBS in the basal ganglia nuclei and other brain areas may provide important clues to understand the mechanism of action of DBS. In particular, electroencephalography records cortical activity with scalp electrodes instantaneously and non-invasively (Buzsaki and Draguhn, 2004; Llinas, 1988). Activation of basal ganglia nuclei by single-pulse DBS may be transmitted to cortical areas through synaptic activations along the pathways between the stimulated nuclei and cortex (Mink, 1996; Nambu, 2007; Udupa and Chen, 2015), which manifest as cortical evoked potentials recorded with electroencephalography. The connectivity between a basal ganglia nucleus and the primary motor cortex and the nature of connectivity along the pathway can be further studied by investigating the effect of a single-pulse DBS on the corresponding target nucleus using a paired-pulse TMS paradigm (Fig. 1, motor cortical TMS conditioned by DBS).
3.1. Single-pulse subthalamic nucleus stimulation
Cortical evoked potentials with a single-pulse STN DBS have been recorded in Parkinson’s disease patients. An early study reported a negative peak of evoked potential at a short latency (2–8 ms)(Ashby et al., 2001). Subsequent studies further identified a positive peak of evoked potential at a later latency (18–25 ms) in the same patient population (Fig. 3A)(Kuriakose et al., 2010; MacKinnon et al., 2005b). These electroencephalographic studies demonstrated that the STN is connected with cortical areas. Furthermore, it was reported that a single-pulse STN stimulation facilitated the MEP induced by TMS applied to the ipsilateral motor cortex with a posterior-anterior directed current at short interstimulus intervals of 3–4 ms during the postoperative period when the DBS lead was externalized (Hanajima et al., 2004). The intervals were consistent with the latencies of cortical evoked potentials. A later study in patients with chronically implanted DBS further demonstrated that the MEP facilitation after STN stimulation occurred for TMS with posterior-anterior and anterior-posterior current directions but not for TMS with latero-medial current direction. In addition, the MEP facilitation occurred at the interstimulus intervals coincident with the latencies of both the early and late evoked potentials (Fig. 4A)(Kuriakose et al., 2010). An antidromic activation of the hyperdirect pathway from the cortex to STN is likely responsible for the early phase of cortical activity with STN stimulation while the later phase may be mediated by synaptic activation through the indirect pathway with transfer at the motor thalamus (Kuriakose et al., 2010). In addition, as posterior-anteriorly and anterior-posteriorly directed currents predominantly activate facilitatory interneurons in the motor cortex and produce indirect waves whereas latero-medially directed current preferentially activates the corticospinal neurons and produces the direct wave (Di Lazzaro et al., 2001; Di Lazzaro et al., 2008; Sakai et al., 1997), the facilitatory effect produced by STN DBS is likely mediated by synaptic inputs to the cortical facilitatory interneurons rather than activation of subcortical structures.
Figure 3. Cortical evoked potential from deep brain stimulation.
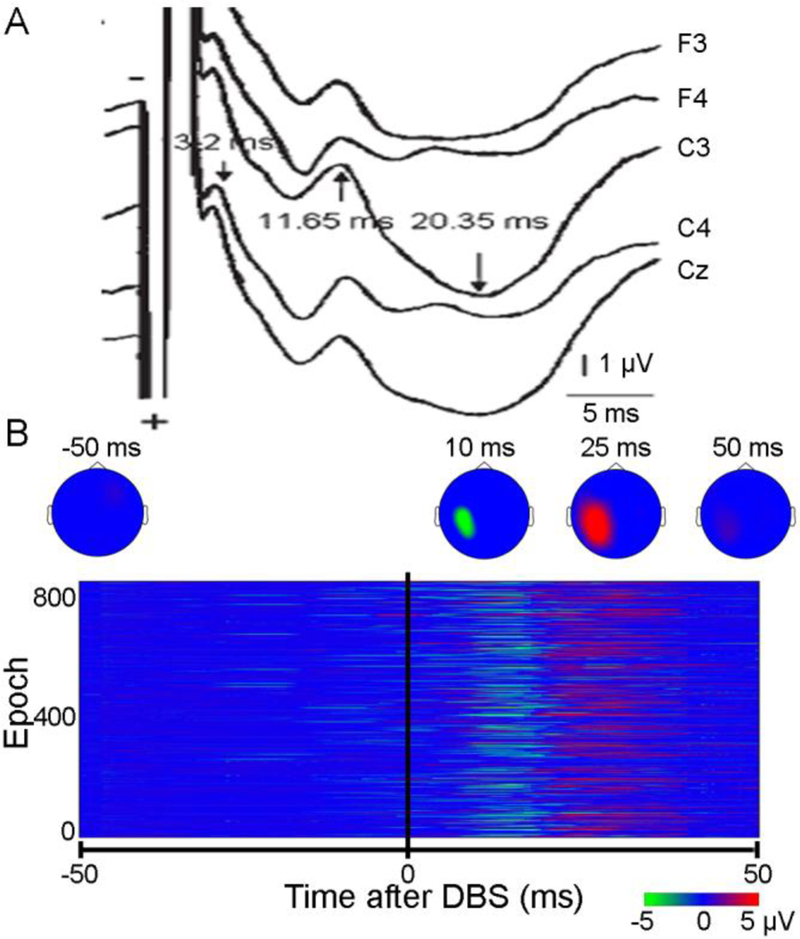
(A) Cortical evoked potential recorded with electroencephalography from different electrodes induced by single-pulse STN DBS in a Parkinson’s disease patient. (B) Cortical evoked potential with heat map and epochs of recordings induced by single-pulse GPi DBS in a cervical dystonia patient. The time of DBS delivery was time 0. The maximum amplitude of DBS evoked potentials was observed in electrodes over the primary motor cortical area in both patients. Multiple peaks of evoked potentials with different latencies and different polarities were recorded in both patients. DBS = deep brain stimulation, GPi = internal globus pallidus, STN = subthalamic nucleus. Modified from Kuriakose et al. (2010) and Ni et al. (2018).
Figure 4. Time course of the effects of deep brain stimulation on motor cortical excitability.
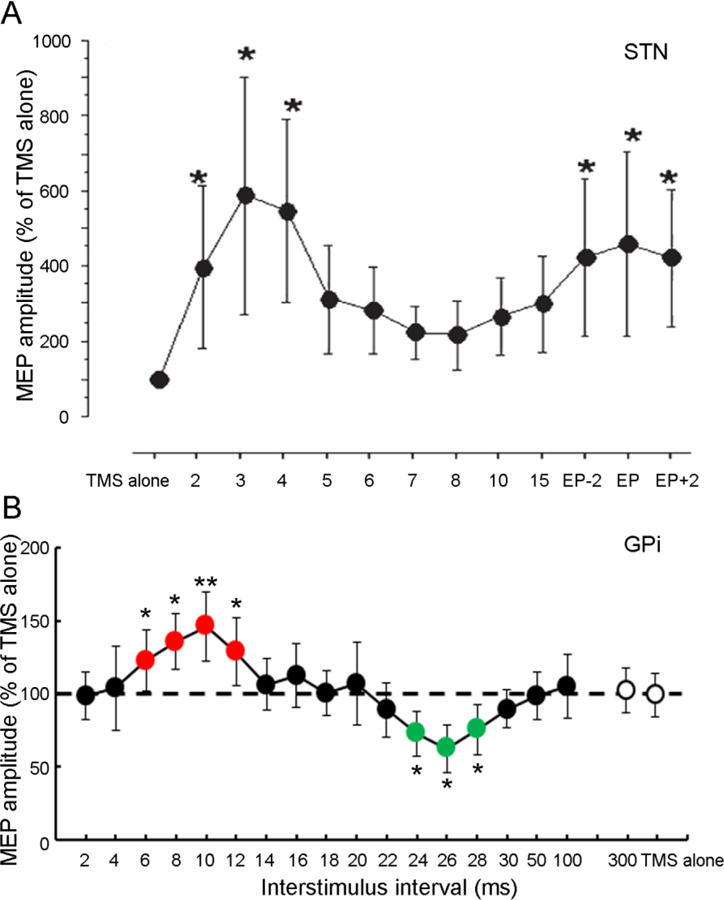
Time course of the effects of single-pulse STN DBS in a Parkinson’s disease patient (A) and GPi DBS in a cervical dystonia patient (B) on MEP amplitude produced by TMS with anterior-posterior current direction. The abscissa indicates the interstimulus intervals between DBS and TMS. The ordinate indicates MEP amplitudes induced by TMS and DBS (normalized as a percentage of that with TMS alone). MEP facilitation occurred at early latency of 2 to 5 ms and a later latency (around EP latency, ~23 ms) after STN DBS (A). In dystonia patient with GPi DBS (B), MEP facilitation occurred at interstimulus interval of ~10 ms and MEP inhibition at ~25 ms. * P<0.05, ** P<0.01, post hoc t-test, comparing MEP induced by DBS and TMS to that with TMS alone. Error bars represent standard deviations. DBS = deep brain stimulation, EP = evoked potential, GPi = internal globus pallidus, MEP = motor evoked potential, STN = subthalamic nucleus, TMS = transcranial magnetic stimulation. Modified from Kuriakose et al. (2010) and Ni et al. (2018)
3.2. Single-pulse internal globus pallidal stimulation
The physiological effects of a single-pulse GPi DBS was tested in cervical dystonia patients (Ni et al., 2018). GPi stimulation produced two peaks of cortical evoked potentials in the ipsilateral primary motor cortical area with peak latencies of ~10 ms (negative) and ~25 ms (positive)(Fig. 3B). The physiological property of these two peaks of cortical potential was further tested with a paired-pulse TMS paradigm. MEP was facilitated by GPi DBS delivered at ~10 ms before motor cortical TMS and was inhibited by the GPi stimulation at ~25 ms before the motor cortical TMS (Fig. 4B). If the antidromic activation of the hyperdirect pathway (from the cortex to STN) is responsible for the early phase of cortical evoked potential with STN stimulation (2–8 ms)(Kuriakose et al., 2010), activation along the similar pathway from GPi to cortex passing through STN may explain the slightly longer latency of early phase of cortical activation produced by GPi stimulation (~10 ms)(Ni et al., 2018). The later phase may be mediated by synaptic activation through thalamus. These pathways underlying the early and later phases of cortical activation were also consistent with the findings from studies using tractography with diffusion magnetic resonance imaging (Milardi et al., 2015) and coherence between basal ganglia and cortical areas studied with local field potential (Neumann et al., 2015; Williams et al., 2002). The different phases of cortical activation likely contribute to the balance between inhibition and facilitation for the net outcome from basal ganglia to the cortex.
It should be noted that no single mechanism has emerged to account for the effects of DBS in different nuclei or even the same nucleus in different disease models. The complex nature of the connections between the basal ganglia nuclei (GPi and STN) and cortex were demonstrated by several findings from our studies. First, the polarities for the early and later phases of cortical activation with GPi DBS are opposite while those with STN DBS are the same. Second, both early and later phases of cortical activation produced by GPi DBS have longer latency than those by STN DBS which cannot be fully explained by the antidromic activation for the early phase and orthodromic activation for the later phase. In addition, activation of multiple neural elements and co-release of different transmitters by DBS cannot be readily tested with current technology available for use in human.
4. Modulation of motor cortical plasticity with deep brain stimulation
Plasticity is one of the basic physiological properties of the human brain. Abnormal motor cortical plasticity occurs in movement disorders such as Parkinson’s disease (Lang and Lozano, 1998b) and dystonia (Hallett, 1998; Quartarone et al., 2006). Animal studies and microelectrode recordings in patients during DBS surgery have shown impaired synaptic plasticity in basal ganglia circuits in movement disorders (Calabresi et al., 2007; Lozano and Lipsman, 2013). Neuronal activation and inhibition produced by the electrical field around DBS in the target area (Kringelbach et al., 2007; Lozano et al., 2002) determines the immediate effects in improving the symptoms of movement disorders. In contrast, gradual improvements in clinical features in Parkinson’s disease and dystonia patients with DBS suggest that modulation of motor cortical plasticity is related to the mechanisms of action of DBS.
4.1. Normalization of motor cortical plasticity with deep brain stimulation
Previous studies reported that motor cortical plasticity in Parkinson’s disease patients can be modulated by repetitive TMS and this may be related to the improvements in motor symptoms with repetitive TMS (Elahi et al., 2009; Fregni et al., 2005; Zanjani et al., 2015). Paired associative stimulation with median nerve stimulation paired with TMS is a special form of repetitive TMS and can be used to test motor cortical plasticity in Parkinson’s disease. Paired associative stimulation at interstimulus interval of 21–25 ms produced spike-timing dependent, long-term potentiation-like effect in the primary motor cortex (Hallett, 2007; Stefan et al., 2000). The plasticity effect was decreased in patients with Parkinson’s disease in off medication state. Furthermore, the impaired cortical plasticity was restored by dopaminergic medication in patients without levodopa induced dyskinesia but not in patients with dyskinesia (Morgante et al., 2006; Ueki et al., 2006). The findings support the notion that abnormal synaptic plasticity is involved in the pathophysiology of levodopa induced dyskinesia (Calabresi et al., 2007; Lang and Lozano, 1998b). In advanced Parkinson’s disease patients with levodopa induced dyskinesia treated with STN DBS, cortical plasticity induced by paired associative stimulation was restored with both medication and DBS on conditions but not in other conditions with either DBS or medication off. The findings suggest that STN DBS together with dopaminergic medications restores cortical plasticity in Parkinson’s disease and this may be one of the mechanisms of how DBS produces clinical benefit (Kim et al., 2015).
Cortical plasticity tested with paired associative stimulation is exaggerated in dystonia patients (Quartarone et al., 2003; Quartarone et al., 2006). On the other hand, the response to the paired associative stimulation was absent in dystonia patients treated with GPi DBS one month after surgery. The cortical plasticity was rebuilt gradually and returned to the same level as healthy controls six months after surgery (Ruge et al., 2011b). In addition, the response to paired associative stimulation in dystonia patients treated with GPi DBS was highly variable. Interestingly, patients who had higher level of cortical plasticity in the DBS on state also had better retention of clinical benefits when DBS was turned off for two days (Ruge et al., 2011a), suggesting that modulation of cortical plasticity is related to the mechanism of GPi DBS in dystonia.
4.2. Motor cortical plasticity induced by paired deep brain stimulation and motor cortical stimulation
Since the clinical benefit of DBS in movement disorders is often associated with correction of abnormal cortical plasticity (discussed above), modulation of motor cortical plasticity is likely related to improvements in motor symptoms with DBS. Examining how single-pulse DBS modulates cortical plasticity is a fundamental step in determining the effects of DBS on cortical plasticity. Particularly, a paired associative stimulation paradigm combining single-pulse DBS with motor cortical TMS based on the connection between basal ganglia nuclei and primary motor cortex was used to investigate this question (Fig. 1B and Fig. 5A). Repetitive pairing of STN DBS with motor cortical TMS in Parkinson’s disease patients induced a long-term potentiation-like effect at interstimulus intervals of ~3 ms and ~23 ms. The results suggest that STN DBS can modulate cortical excitability with spike-timing dependent plasticity. Importantly, the two effective intervals (~ 3 and 23 ms) were consistent with the latencies of early and later phases of cortical evoked potentials induced by the single-pulse STN stimulation (Fig. 5B)(Udupa et al., 2016). A further study in cervical dystonia patients with GPi DBS tested similar long-term potentiation-like effect when DBS was paired with motor cortical TMS at intervals of ~10 ms and 25 ms, and the intervals were also coincident with the latencies when cortical excitability was modulated by single-pulse GPi DBS (Fig. 5C)(Ni et al., 2018).
Figure 5. Induction of motor cortical plasticity by pairing deep brain stimulation with transcranial magnetic stimulation.
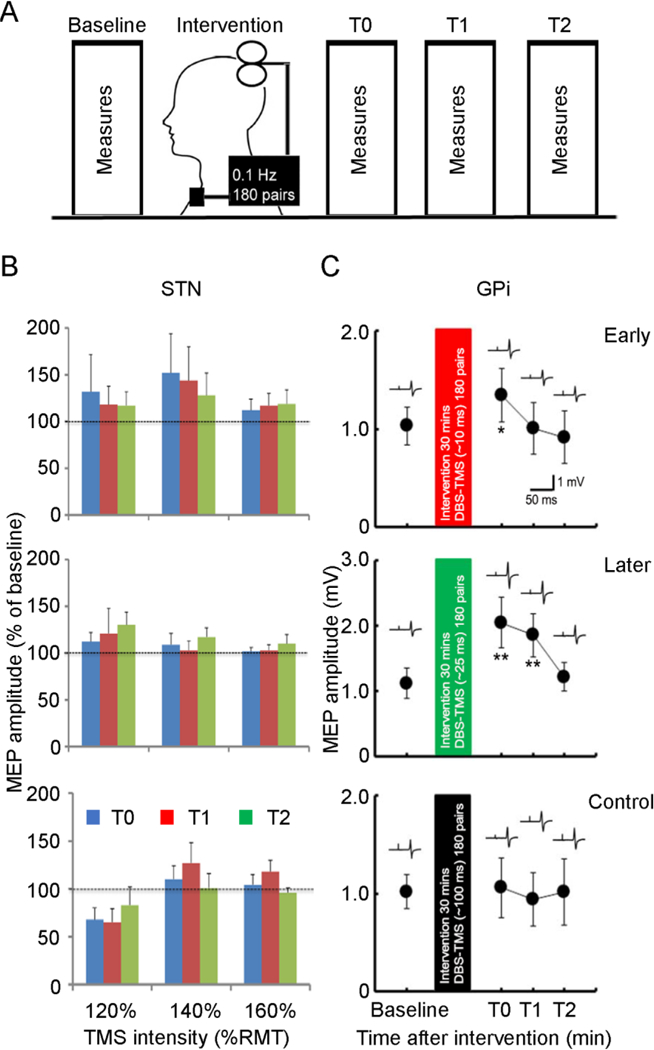
(A) Experimental setup. Motor cortical plasticity was tested by a paired associative stimulation paradigm. Interventional protocols with paired associative stimulation were applied with 180 pairs of DBS and TMS at 0.1 Hz for 30 min. Three different interstimulus intervals (early, later and a control interval) were tested. Measurements were performed before (baseline) and at three different time points after the interventional protocol (T0 immediately, T1 about 30 minutes, T2 about 60 minutes after the intervention). (B) and (C) Group analysis (mean ± standard deviation) for the effect on motor cortical plasticity in Parkinson’s disease patients with STN DBS (B) and in cervical dystonia patients with GPi DBS (C). (B) Interstimulus intervals of 3 ms (early), 23 ms (later) and 167 ms (control) were tested in patients with STN DBS. MEP recruitment curve were measured with TMS intensity set at 120%, 140%, and 160% of RMT. The abscissa indicates TMS intensities. The ordinate indicates MEP amplitudes normalized as a percentage of that before intervention (baseline). Blue columns represent time T0, red columns represent time T1, green columns represent time T2. Analysis of variance revealed a trend toward significance for the interaction between interventional protocol and TMS intensity (P=0.07). (C) Interstimulus intervals of ~10 ms (early), ~25 ms (later) and 100 ms (control) were tested in patients with GPi DBS. The abscissa indicates the time after interventional protocol. The ordinate indicates MEP amplitude. Example of recordings shows MEP measured at the corresponding time points. Paired associative stimulation with interstimulus interval of ~25 ms produced MEP facilitation for longer than 30 minutes (note the different ordinate for this panel) and that with interval of ~10 ms produced MEP facilitation at the time point immediately after the intervention. MEP after the paired associative stimulation with interstimulus interval of 100 ms did not change. *P<0.05, ** P<0.01, post hoc t-test, comparing MEP after paired associative stimulation to that at baseline. DBS = deep brain stimulation, GPi = internal globus pallidus, MEP = motor evoked potential, RMT = resting motor threshold, STN = subthalamic nucleus, TMS = transcranial magnetic stimulation. Modified from Udupa et al. (2016) and Ni et al. (2018).
4.3. Technical difficulties of combining deep brain stimulation with transcranial magnetic stimulation
The ability to induce cortical plastic changes by repeatedly pairing DBS and motor cortical TMS in patients with chronic DBS has the potential to normalize the abnormal cortical plasticity in the underlying diseases (Follett et al., 2010; Udupa and Chen, 2015). However, a concern was that DBS was delivered at 3 Hz due to a technical limitation of the DBS devices (Fig. 1B). With the pairing of DBS and TMS occurring once every 10 s in our studies, only one in every 30 DBS pulses was paired with TMS and this could have limited the efficacy of plasticity induction. More rapid paring of DBS and TMS pulses could be tested in future studies. In addition, the possibility of subthreshold activation of the corticospinal tract with DBS cannot be completely excluded although well-designed control experiments were performed (Ni et al., 2018). Furthermore, TMS employs a magnetic field to stimulate the underlying brain tissue. The strong magnetic field can damage electrical devices such as a DBS pulse generator when the TMS coil is placed close to these devices, although no serious complication or damage to DBS devices has been reported (Kumar et al., 1999; Shimojima et al., 2010). TMS has been observed to shut off the DBS pulse generator, likely due to induction of antidromic current in the cable connected to the pulse generator. Therefore, the studies reviewed in this paper tested the hemisphere contralateral to the pulse generator to avoid stimulation near the cable to minimize the antidromic current.
5. Open questions and future directions
5.1. Novel patterns of deep brain stimulation
Open loop stimulation at a specific frequency, voltage and pulse width is currently used for DBS and has considerable success in the treatment of movement disorders. A programming process with many clinic visits is required to establish the DBS settings to optimize clinical benefit (Bronstein et al., 2011; Deuschl et al., 2006a). Development of closed loop DBS with adaptive stimulation parameters is an attractive option to improve therapeutic efficacy and limit side effects while preserving battery life (Little et al., 2013; Rosin et al., 2011). Although oscillatory signals in specific frequency bands (particularly beta frequency) recorded with electroencephalography or local field potential are being tested as pathophysiological signals to drive adaptive DBS (de Hemptinne et al., 2015; Little et al., 2013; Priori et al., 2013), the relatively poor stability of the pathophysiological signals and the difficulties in extracting them have limited the development of this technique. On the other hand, changes in MEP and other TMS measurements (e.g. intracortical circuits) in movement disorders reviewed in previous sections have high potential to help the programming of DBS. However, it is not yet clear how these measurements may be used as future options to produce feedback signals to modulate DBS output in a closed loop system (Lozano et al., 2002; Udupa and Chen, 2015). This may be facilitated by the development of a simplified, portable TMS and recording device that can be used in DBS clinics or even by patients at home to automatically adjust DBS parameters.
5.2. Combination of deep brain stimulation and cortical stimulation
Recent advances in technology in signal processing (e.g., artifact filtering) allowed the recordings of DBS (Ni et al., 2018) and TMS (Rogasch and Fitzgerald, 2013) evoked potentials with electroencephalography. In addition to the corticospinal activation measurable with MEP, variation in TMS induced evoked potential in other cortical areas caused by DBS on different target nuclei provides information of how basal ganglia nuclei connect with and affect these cortical areas. In this regard, a recent study reported that the global power and high alpha oscillation of the TMS evoked cortical activity was enhanced by STN DBS at therapeutic setting in Parkinson’s disease (Casula et al., 2017). Future studies testing the long-term changes of the TMS evoked potentials in non-motor areas are of particular interests as DBS is associated with beneficial effects on non-motor symptoms in movement disorder patients (Dafsari et al., 2018; Follett et al., 2010). Furthermore, studies investigating the effect of single-pulse DBS on TMS evoked potentials in non-motor cortical areas using the reviewed paired-pulse paradigm (Fig. 1B, pairing single-pulse DBS with TMS) may help explore the pathophysiological mechanisms about the development of non-motor symptoms during the disease progression.
Repetitive TMS using established protocols such as paired associative stimulation and theta burst stimulation induce long-term potentiation and depression-like effects. A large number of studies in the past decade have shown that TMS and other noninvasive brain stimulation techniques are potentially effective treatments for movement disorders (Elahi et al., 2009; Quartarone et al., 2006; Zanjani et al., 2015). The finding that paired associative stimulation combining DBS with TMS induced cortical plasticity opened a new avenue for therapeutic treatment to use two techniques simultaneously (Ni et al., 2018; Udupa et al., 2016). However, DBS and repetitive TMS with clinical benefit are currently delivered at completely different frequencies. Interestingly, a recent study in rodents raised the possibility for application of noninvasive DBS by delivering multiple stimulations at different frequencies and locations from the scalp (Grossman et al., 2017). In addition, delivery of combination of different types of stimulation with closed loop patterns during the specific phases of pathological oscillations may further promote the efficacy of therapeutic stimulation (de Hemptinne et al., 2015; Little et al., 2013).
5.3. Future functional studies with deep brain stimulation
Although technical innovation in DBS devices with modification of pulse shape, stimulation patterns, current steering, capability of recording local field potential and closed-loop stimulation are being developed, translation of these ideas to the clinic still requires further theoretical advances with understanding of functional connectivity between the basal ganglia nuclei and cortical areas. While traditional TMS studies examined the effects of DBS on the motor cortex (MEP, intracortical circuits, etc.), the existence of a hyperdirect pathway may provide the opportunity to test the pathophysiological mechanisms in movement disorders in the opposite direction (Chu et al., 2017; Mink, 1996; Nambu, 2007; Nambu et al., 2002). Specifically, activation of cortical neurons with TMS may be transmitted to basal ganglia nuclei through the hyperdirect pathway. Recordings of local field potentials with DBS electrodes at the target basal ganglia nuclei may help understand the cortico-basal ganglia connections and how the connections are related to the physiological effect of DBS. While DBS is increasingly used to treat movement disorders patients, treatment strategies including medication also improved the clinical state of the patient (Alexander et al., 1986; DeLong and Wichmann, 2007; Lozano et al., 2002; Lozano and Lipsman, 2013). The surgical and pharmacological treatments likely act by different physiological mechanisms and these multiple mechanisms may interact with each other. The interactions among different treatments for movement disorders could be studied in patients treated with DBS.
6. Conclusions
Despite progress in surgical procedures with sophisticated implantation techniques, the lack of full understanding of the mechanisms of DBS may limit its further clinical applications. Multidisciplinary neuroscience studies have revealed that the effects of DBS can be related to different mechanisms including functional changes with neuronal activation or inhibition, neurotransmitter release and long-term plastic changes in the target area and remote areas (Lozano et al., 2002; Lozano and Lipsman, 2013; Udupa and Chen, 2015). TMS is safe for movement disorders patients with DBS and is a powerful technique to evaluate the physiological effects of DBS. Future studies harnessing the combined use of TMS and DBS in patients with movement disorders may lead to novel treatment strategies for these patients.
Highlights.
Deep brain stimulation normalizes abnormal cortical excitability and circuits in movement disorders.
Single-pulse deep brain stimulation modulates the motor cortical circuits.
Repetitive deep brain stimulation with motor cortical stimulation produces cortical plastic changes.
Acknowledgements
Dr. Zhen Ni and Dr. Mark Hallett are supported by the National Institute of Neurological Disorders and Stroke (NINDS) Intramural Program. Dr, Robert Chen is supported by the Canadian Institutes of Health Research (FDN 154292) and National Institutes of Health (NS106822).
Abbreviations
- DBS
deep brain stimulation
- GPi
internal globus pallidus
- MEP
motor evoked potential
- STN
subthalamic nucleus
- TMS
transcranial magnetic stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander GE, Delong MR, Strick PL Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- Ashby P, Paradiso G, Saint-Cyr J, Chen R, Lang A, Lozano A Potentials evoked at the scalp by stimulation near the human subthalamic nucleus. Clin Neurophysiol 2001;112:431–437. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollack P, Gervason C, Hoffmann D, Gao DM, Hommel M et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate nucleus of the thalamus. Lancet 1991;337:403–406. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 1990;249:1436–1438. [DOI] [PubMed] [Google Scholar]
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 2011;68:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A Neuronal oscillations in cortical networks. Science 2004;304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 2007;30:211–219. [DOI] [PubMed] [Google Scholar]
- Casula EP, Stampanoni Bassi M, Pellicciari MC, Ponzo V, Veniero D, Peppe A et al. Subthalamic stimulation and levodopa modulate cortical reactivity in Parkinson’s patients. Park Relat Disord 2017;34:31–37. [DOI] [PubMed] [Google Scholar]
- Chen R, Garg RR, Lozano AM, Lang AE Effects of internal globus pallidus stimulation on motor cortex excitability. Neurology 2001;56:716–723. [DOI] [PubMed] [Google Scholar]
- Chu HY, McIver EL, Kovaleski RF, Atherton JF, Bevan MD Loss of Hyperdirect Pathway Cortico-Subthalamic Inputs Following Degeneration of Midbrain Dopamine Neurons. Neuron 2017;95:1306–1318 e1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunic D, Roshan L, Khan FI, Lozano AM, Lang AE, Chen R Effects of subthalamic nucleus stimulation on motor cortex excitability in Parkinson’s disease. Neurology 2002;58:1665–1672. [DOI] [PubMed] [Google Scholar]
- Dafsari HS, Silverdale M, Strack M, Rizos A, Ashkan K, Mahlstedt P et al. Nonmotor symptoms evolution during 24 months of bilateral subthalamic stimulation in Parkinson’s disease. Mov Disord 2018;33:421–430. [DOI] [PubMed] [Google Scholar]
- de Hemptinne C, Swann NC, Ostrem JL, Ryapolova-Webb ES, San Luciano M, Galifianakis NB et al. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat Neurosci 2015;18:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T Circuits and circuit disorders of the basal ganglia. Arch Neurol 2007;64:20–24. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Herzog J, Kleiner-Fisman G, Kubu C, Lozano AM, Lyons KE et al. Deep brain stimulation: postoperative issues. Mov Disord 2006a;21 Suppl 14:S219–237. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Hellriegel H, Elble R Treatment of patients with essential tremor. Lancet Neurol 2011;10:148–161. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 2006b;355:896–908. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P et al. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res 2001;138:268–273. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ziemann U, Lemon RN State of the art: Physiology of transcranial motor cortex stimulation. Brain Stimul 2008;1:345–362. [DOI] [PubMed] [Google Scholar]
- Elahi B, Elahi B, Chen R Effect of transcranial magnetic stimulation on Parkinson motor function-- systematic review of controlled clinical trials. Mov Disord 2009;24:357–363. [DOI] [PubMed] [Google Scholar]
- Fasano A, Lozano AM, Cubo E New neurosurgical approaches for tremor and Parkinson’s disease. Curr Opin Neurol 2017;30:435–446. [DOI] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med 2010;362:2077–2091. [DOI] [PubMed] [Google Scholar]
- Fregni F, Simon DK, Wu A, Pascual-Leone A Non-invasive brain stimulation for Parkinson’s disease: a systematic review and meta-analysis of the literature. J Neurol Neurosurg Psychiatry 2005;76:1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N, Bono D, Dedic N, Kodandaramaiah SB, Rudenko A, Suk HJ et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017;169:1029–1041.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M The neurophysiology of dystonia. Arch Neurol 1998;55:601–603. [DOI] [PubMed] [Google Scholar]
- Hallett M Transcranial magnetic stimulation and the human brain. Nature 2000;406:147–150. [DOI] [PubMed] [Google Scholar]
- Hallett M Transcranial magnetic stimulation: a primer. Neuron 2007;55:187–199. [DOI] [PubMed] [Google Scholar]
- Hamani C, Temel Y Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med 2012;4:142rv8. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ashby P, Lozano AM, Lang AE, Chen R Single pulse stimulation of the human subthalamic nucleus facilitates the motor cortex at short intervals. J Neurophysiol 2004;92:1937–1943. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Udupa K, Ni Z, Moro E, Gunraj C, Mazzella F et al. Effects of subthalamic nucleus stimulation on motor cortex plasticity in Parkinson disease. Neurology 2015;85:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010;466:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ Translational principles of deep brain stimulation. Nat Rev Neurosci 2007;8:623–635. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Meyer BU, Trottenberg T, Brandt SA, Schneider GH, Kupsch A Modulation of motor cortex excitability by pallidal stimulation in patients with severe dystonia. Neurology 2003;60:768–774. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Chen R, Ashby P Safety of transcranial magnetic stimulation in patients with implanted deep brain stimulators. Mov Disord 1999;14:157–158. [DOI] [PubMed] [Google Scholar]
- Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006;355:1978–1990. [DOI] [PubMed] [Google Scholar]
- Kuriakose R, Saha U, Castillo G, Udupa K, Ni Z, Gunraj C et al. The nature and time course of cortical activation following subthalamic stimulation in Parkinson’s disease. Cereb Cortex 2010;20:1926–1936. [DOI] [PubMed] [Google Scholar]
- Lang AE Surgery for Parkinson disease: A critical evaluation of the state of the art. Arch Neurol 2000;57:1118–1125. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM Parkinson’s disease. First of two parts. N Engl J Med 1998a;339:1044–1053. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM Parkinson’s disease. Second of two parts. N Engl J Med 1998b;339:1130–1143. [DOI] [PubMed] [Google Scholar]
- Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 2013;74:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 1988;242:1654–1664. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Dostrovsky J, Chen R, Ashby A Deep brain stimulation for Parkinson’s disease: disrupting the disruption. Lancet Neurol 2002;1:225–231. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 2013;77:406–424. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Gilley EA, Weis-McNulty A, Simuni T Pathways mediating abnormal intracortical inhibition in Parkinson’s disease. Ann Neurol 2005a;58:516–524. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Webb RM, Silberstein P, Tisch S, Asselman P, Limousin P et al. Stimulation through electrodes implanted near the subthalamic nucleus activates projections to motor areas of cerebral cortex in patients with Parkinson’s disease. Eur J Neurosci 2005b;21:1394–1402. [DOI] [PubMed] [Google Scholar]
- Milardi D, Gaeta M, Marino S, Arrigo A, Vaccarino G, Mormina E et al. Basal ganglia network by constrained spherical deconvolution: a possible cortico-pallidal pathway? Mov Disord 2015;30:342–349. [DOI] [PubMed] [Google Scholar]
- Mink JW The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 1996;50:381–425. [DOI] [PubMed] [Google Scholar]
- Molnar GF, Sailer A, Gunraj CA, Cunic DI, Lang AE, Lozano AM et al. Changes in cortical excitability with thalamic deep brain stimulation. Neurology 2005;64:1913–1919. [DOI] [PubMed] [Google Scholar]
- Molnar GF, Sailer A, Gunraj CA, Lang AE, Lozano AM, Chen R Thalamic deep brain stimulation activates the cerebellothalamocortical pathway. Neurology 2004;63:907–909. [DOI] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain 2006;129:1059–1069. [DOI] [PubMed] [Google Scholar]
- Nambu A Globus pallidus internal segment. Prog Brain Res 2007;160:135–150. [DOI] [PubMed] [Google Scholar]
- Nambu A Seven problems on the basal ganglia. Curr Opin Neurobiol 2008;18:595–604. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 2002;43:111–117. [DOI] [PubMed] [Google Scholar]
- Neumann WJ, Jha A, Bock A, Huebl J, Horn A, Schneider GH et al. Cortico-pallidal oscillatory connectivity in patients with dystonia. Brain 2015;138:1894–1906. [DOI] [PubMed] [Google Scholar]
- Ni Z, Bahl N, Gunraj CA, Mazzella F, Chen R Increased motor cortical facilitation and decreased inhibition in Parkinson disease. Neurology 2013;80:1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Kim SJ, Phielipp N, Ghosh S, Udupa K, Gunraj CA et al. Pallidal deep brain stimulation modulates cortical excitability and plasticity. Ann Neurol 2018;83:352–362. [DOI] [PubMed] [Google Scholar]
- Ni Z, Müller-Dahlhaus F, Chen R, Ziemann U Triple-pulse TMS to study interactions between neural circuits in human cortex. Brain Stimul 2011;4:281–293. [DOI] [PubMed] [Google Scholar]
- Oswal A, Brown P, Litvak V Synchronized neural oscillations and the pathophysiology of Parkinson’s disease. Curr Opin Neurol 2013;26:662–670. [DOI] [PubMed] [Google Scholar]
- Phielipp NM, Saha U, Sankar T, Yugeta A, Chen R Safety of repetitive transcranial magnetic stimulation in patients with implanted cortical electrodes. An ex-vivo study and report of a case. Clin Neurophysiol 2017;128:1109–1115. [DOI] [PubMed] [Google Scholar]
- Pinto AD, Lang AE, Chen R The cerebellothalamocortical pathway in essential tremor. Neurology 2003;60:1985–1987. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Rossi L, Marceglia S Adaptive deep brain stimulation (aDBS) controlled by local field potential oscillations. Exp Neurol 2013;245:77–86. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A et al. Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain 2003;126:2586–2596. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Siebner HR, Rothwell JC Task-specific hand dystonia: can too much plasticity be bad for you? Trends Neurosci 2006;29:192–199. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol 1995;37:181–188. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Fitzgerald PB Assessing cortical network properties using TMS-EEG. Hum Brain Mapp 2013;34:1652–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin B, Slovik M, Mitelman R, Rivlin-Etzion M, Haber SN, Israel Z et al. Closed-loop deep brain stimulation is superior in ameliorating parkinsonism. Neuron 2011;72:370–384. [DOI] [PubMed] [Google Scholar]
- Ruge D, Cif L, Limousin P, Gonzalez V, Vasques X, Hariz MI et al. Shaping reversibility? Long-term deep brain stimulation in dystonia: the relationship between effects on electrophysiology and clinical symptoms. Brain 2011a;134:2106–2115. [DOI] [PubMed] [Google Scholar]
- Ruge D, Tisch S, Hariz MI, Zrinzo L, Bhatia KP, Quinn NP et al. Deep brain stimulation effects in dystonia: time course of electrophysiological changes in early treatment. Mov Disord 2011b;26:1913–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rughani A, Schwalb JM, Sidiropoulos C, Pilitsis J, Ramirez-Zamora A, Sweet JA et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Subthalamic Nucleus and Globus Pallidus Internus Deep Brain Stimulation for the Treatment of Patients With Parkinson’s Disease: Executive Summary. Neurosurgery 2018;82:753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res 1997;113:24–32. [DOI] [PubMed] [Google Scholar]
- Shimojima Y, Morita H, Nishikawa N, Kodaira M, Hashimoto T, Ikeda S The safety of transcranial magnetic stimulation with deep brain stimulation instruments. Park Relat Disord 2010;16:127–131. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 2000;123:572–584. [DOI] [PubMed] [Google Scholar]
- Udupa K, Bahl N, Ni Z, Gunraj C, Mazzella F, Moro E et al. Cortical Plasticity Induction by Pairing Subthalamic Nucleus Deep-Brain Stimulation and Primary Motor Cortical Transcranial Magnetic Stimulation in Parkinson’s Disease. J Neurosci 2016;36:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udupa K, Chen R The mechanisms of action of deep brain stimulation and ideas for the future development. Prog Neurobiol 2015;133:27–49. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A et al. Altered plasticity of the human motor cortex in Parkinson’s disease. Ann Neurol 2006;59:60–71. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I Magnetic stimulation over the cerebellum in humans. Ann Neurol 1995;37:703–713. [DOI] [PubMed] [Google Scholar]
- Volkmann J, Mueller J, Deuschl G, Kuhn AA, Krauss JK, Poewe W et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol 2014;13:875–884. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE et al. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscle. Exp Brain Res 1996;109:158–163. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Ghahremani A, Udupa K, Saha U, Kalia SK, Hodaie M et al. Stop-related subthalamic beta activity indexes global motor suppression in Parkinson’s disease. Mov Disord 2016;31:1846–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Tijssen M, Van Bruggen G, Bosch A, Insola A, Di Lazzaro V et al. Dopamine-dependent changes in the functional connectivity between basal ganglia and cerebral cortex in humans. Brain 2002;125:1558–1569. [DOI] [PubMed] [Google Scholar]
- Zanjani A, Zakzanis KK, Daskalakis ZJ, Chen R Repetitive transcranial magnetic stimulation of the primary motor cortex in the treatment of motor signs in Parkinson’s disease: A quantitative review of the literature. Mov Disord 2015;30:750–758. [DOI] [PubMed] [Google Scholar]
- Zesiewicz TA, Elble RJ, Louis ED, Gronseth GS, Ondo WG, Dewey RB Jr. et al. Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards subcommittee of the American Academy of Neurology. Neurology 2011;77:1752–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U TMS and drugs. Clin Neurophysiol 2004;115:1717–1729. [DOI] [PubMed] [Google Scholar]


