Abstract
Excessive MMP activity may impair tendon-to-bone healing. However, little is known about the effect of joint motion on MMP activity after ACL reconstruction. The aim of this study was to determine the effect of different durations of knee immobilization on MMP activity in a mouse ACL reconstruction model using a fluorescent MMP probe which detects MMP 2, 3, 9, and 13 and near-infra red in vivo imaging. Sixty C57BL male mice underwent ACL reconstruction. Post-operatively, the animals were treated with free cage activity (Group 1), or with the use of an external fixator to restrict knee motion and weight bearing for 5 days (Group 2), 14 days (Group 3), and 28 days (Group 4). At days 3, 7, 16, 23, and 30, five mice underwent IVIS imaging. At days 3, 7, 16, and 30, histological analysis was also performed. Probe signal intensity in the whole limb peaked at day 7, followed by a decrease at day 16, and maintenance up to day 30. There was no significant difference among groups at any time point based on IVIS, but histologic localization of MMP probe signal showed significantly less activity in Group 2 and Group 3 compared to Group 4 in the bone tunnel at day 30. We demonstrated that short-term immobilization led to less MMP activity around the bone tunnel compared with prolonged immobilization. A short period of immobilization after ACL reconstruction might enhance graft-bone interface healing by mitigating excess MMP expression. These findings have implications for post-operative rehabilitation protocols following ACL reconstruction. © 2018 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res
Keywords: ACL reconstruction, graft-bone interface healing, MMP, immobilization
Successful ACL reconstruction using a tendon graft requires sufficient healing between the tendon and bone.1,2 Delayed or incomplete graft healing after ACL reconstruction may be associated with poor clinical outcomes, including recurrent knee instability, reduced performance, and lower rates of return to sport. The ultimate function of an ACL graft is dependent on secure healing at the tendon-bone interface in the bone tunnels.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteases that degrade connective tissue and remodel the extracellular matrix. MMPs play a pivotal role in tissue remodeling and healing, with a delicate balance required between matrix formation and matrix remodeling. Excessive or imbalanced MMP activity may have a detrimental effect on tissue healing. Particularly, MMP-2, −3, −9, and −13 all play important roles in tendon matrix biology. These MMPs are induced by inflammation and have been implicated in pathologic processes such as tendinopathy3 and osteoarthritis (OA).4 MMPs have been localized to muscle, adipose tissue, bone marrow, and cartilage. It was previously reported that local administration of a broad spectrum MMP inhibitor improved tendon-to-bone interface healing in a rat rotator cuff repair model and in a rabbit ACL reconstruction model, suggesting that suppression of excessive MMP activity after surgery may lead to better tendon-to-bone healing.5,6
In addition, biomechanical forces have an important effect on the tendon-to-bone healing process. While some clinical studies demonstrate that immobilization does not have an effect on anterior-posterior laxity or bone tunnel enlargement after ACL reconstruction using hamstring tendon,7,8 several animal studies have demonstrated that a period of immobilization improves tendon-to-bone healing.9–11 In prior work in our laboratory, we demonstrated that a period of immobilization following ACL reconstruction had positive effects on inflammation and bone formation along the healing tendon-bone interface compared with immediate motion after surgery.12 Our laboratory recently demonstrated that shortterm immobilization was more effective for promoting healing, when compared to both no immobilization and long-term immobilization following ACL reconstruction in a mouse model.13 However, little is known about the effect of joint motion and subsequent graft stress on MMP activity after ACL reconstruction.
Recent developments in near-infrared protease activatable probes combined with in vivo imaging (IVIS) allow in vivo evaluation of protein expression, permitting repeated measures in the same animal.14 These optical tracers are fluorescently suppressed until a linker domain is cleaved by a specific protease of interest, which then produces a robust fluorescent signal. In the current study we used MMP680 Sense probe which is specific for MMP-2, −3, −9, and −13 and gives off near-infra red fluorescence. These techniqueshave been extensively validated and used in studies of cancer15 and atherosclerosis,16 and have also proven to be useful for studies of the musculoskeletal system such as bone metabolism17 and OA.18,19 However, as far as we know, there have been no reports utilizing this methodology to investigate tendon-to-bone healing.
The aim of this study was to determine the effect of different knee immobilization periods on MMP activity in a mouse ACL reconstruction model using a fluorescent MMP probe and near-infra red in vivo imaging.
METHODS
Study Design
All study procedures were approved by Weill Cornell Institutional Animal Care and Use Committee (2016–0035). Sixty C57BL male mice (age: 12 weeks, weight: 27–30 g, Jackson Laboratory, Bar Harbor, ME) underwent ACL reconstruction as previously described.13 Post-operatively, the animals were treated with no immobilization (Group 1), or with the use of an external fixator to restrict knee motion and weight bearing on the operated side for 5 days (Group 2), 14 days (Group 3), or 28 days (Group 4), respectively (Fig. 1). The external fixator was 3D printed from rigid plastic with minimal autofluorescence and was designed to immobilize the knee in 45° of flexion as previously described (Fig. 2).20 At days 3, 7, 16, 23, and 30, five mice underwent IVIS imaging using MMP Sense 680 probe (PerkinElmer Waltham, MA). At each time point, one mouse without the probe was imaged to serve as a control. At days 3, 7, and 16, two mice with the probe and one mouse without the probe in each group were euthanized and at 30 days, five mice with the probe and one mouse without the probe in each group were euthanized for histological analysis. We did not do histological analysis at day 23 due to limited animal numbers. Five mice which did not undergo any surgical procedures served as normal controls. The mice were fed a special diet which was alfalfa-free to minimize abdominal autofluorescence compared to a normal diet (5K96, LabDiet, St. Louis, MO) beginning 7 days prior to surgery and continuing throughout the post-operative period (Supplemental Fig. S1).
Figure 1.
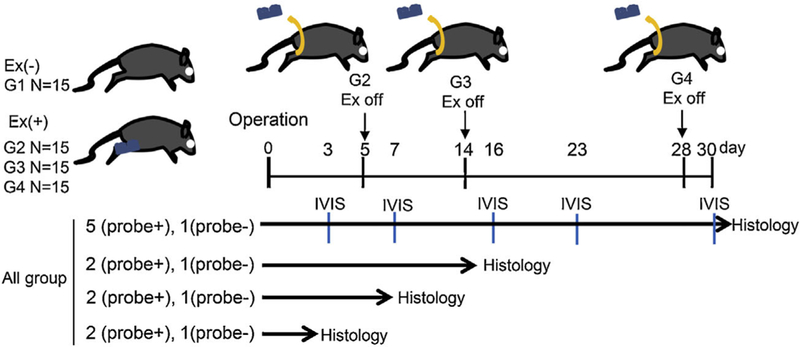
Study design. Sixty C57BL male mice (age: 12 weeks) underwent ACL reconstruction in the right knee using ipsilateral FDL tendon graft. Post-operatively the animals were treated with no immobilization (Group 1), or with the use of an external fixator to immobilize the operated knee joint for 5 days (Group 2), 14 days (Group 3), and 28 days (Group 4). At day 3, 7, 16, 23, and 30, five mice underwent IVIS imaging using MMP Sense 680 probe, which localizes MMP-2, −3, −9, and −13. One mouse without the probe was imaged at each time point to serve as control. At day 3, 7, and 16, two mice with the probe and one mouse without the probe in each group were euthanized, and at 30 days five mice with the probe and one mouse without the probe in each group were euthanized for histological analysis.
Figure 2.
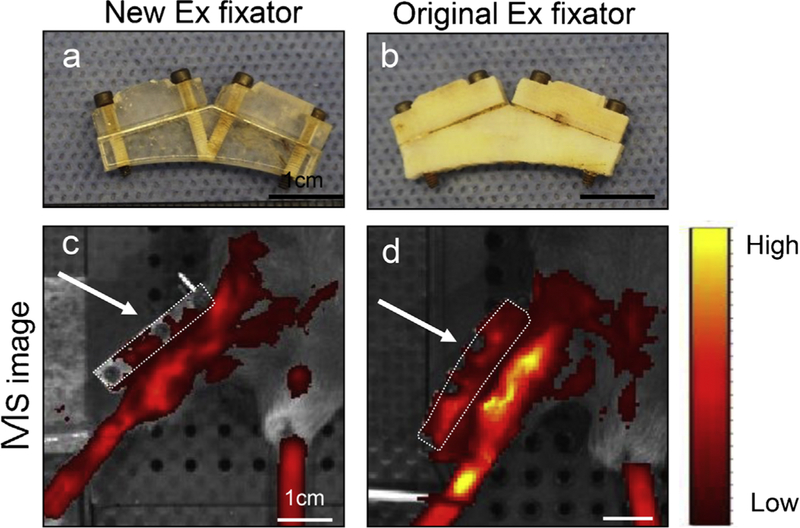
The difference in autofluorescence in external fixators. (a) New external fixator used in this study; (b) Original external fixator used in the previous our study. Scale bar = 1 cm. (c and d) IVIS images with color bar (yellow = higher). Scale bar =1 cm. White arrows and white dotted line indicate external fixator. IVIS images demonstrated that our new external fixator had lower autofluorescence.
Surgical Procedure
Anterior Cruciate Ligament Reconstruction
Anesthetic induction and maintenance were performed using 2% isoflurane in high-flow oxygen on a custom surgical table. Animals were placed in the supine position and the entire right lower extremity was prepped and draped. The flexor digitorum longus tendon (Fig. 3Aa) was harvested through two percutaneous incisions on the medial lower leg and plantar surface of the foot. A 6–0 Prolene suture (RB-2, Ethicon Medline Industries, Mundelein, IL) was tied to the proximal end (Fig. 3Ab) and a small surgical clip (SuperFine MicroClips Titanium Hemostatic Clip, Synovis Micro Co Alliance Inc., Birmingham, AL) was applied to the distal end of the graft (Fig. 3Ac). The tendon graft was harvested (Fig. 3Ad) and kept in moist gauze.
Figure 3.
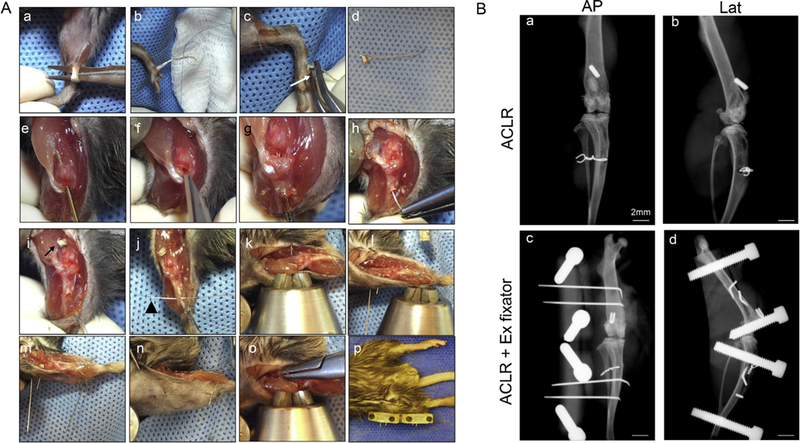
Surgical procedures and post-operative X-ray. (A) Surgical procedures of ACL reconstruction and an external fixator. (a) Harvest ipsilateral FDL tendon. (b) Tie a 6–0 prolene suture at proximal end of the tendon. (c) Apply a clip to distal end of the tendon. White arrow indicates clip. (d) Prepared graft (e) Drill bone tunnel in the femur with a 23-gauge needle. (f) Transect ACL. (g) Drill bone tunnel in the tibia with a 23-gauge needle. (h and i) Pass the graft through tunnels using the attached 6–0 prolene needle. White arrow indicates suspensory fixation utilizing the clip. (j) Fixation of the graft using a 5–0 wire. White arrow head indicates the wire. (k) Insert two 30-gauge needles in the femur. (l) Two femoral pins after percutaneous placement. (m) Insert two 30-gauge needles in the tibia. (n) The pins were placed percutaneously. (o) The medial side of the pins were bent and then pulled back flush with the medial femoral bone. (p) The external fixator was connected to the four pins. (B) X-ray. (a) AP and (b) lateral X ray after ACL reconstruction using FDL tendon. (c) AP and (d) lateral X-ray after placement of external fixator following ACL reconstruction. Scale bar = 2 mm.
A standard medial parapatellar arthrotomy was created to expose the knee. The native ACL was cut using a No. 11 blade and then the femoral tunnel was drilled using a 23-gauge needle (Fig. 3Ae, 3Af). An anterior drawer was applied to aid exposure and then a tibial tunnel was drilled in a retrograde fashion using a 23-gauge needle (0.64 mm diameter) (Fig. 3Ag). The graft was then passed through the femoral tunnel, into the knee, and out through the tibial tunnel using the attached Prolene suture (Fig. 3Ah). The clip provided suspensory fixation on the femoral side (Fig. 3Ai) and the graft was fixed on the tibial side under manual tension while the knee was extended. Tibial fixation was done using a transosseous 5–0 surgical steel monofilament wire (Ethicon) passed through the anterior tibia (Fig. 3Aj). The wire was tied over the graft just distal to the exit from the tibial tunnel on the anteromedial tibia.
External Fixator
For 45 mice in Groups 2–4, a custom 3D printed external fixator was applied to the operative extremity. Two 30-gauge pins were inserted into the femur and tibia (Fig. 3Ak and l) proximal and distal approximately 5 mm from the bone tunnels, respectively. These needles were placed through the skin and musculature of the lateral leg and thigh and advanced through the bone in a bicortical fashion (Fig. 3Am and n). The medial ends of the needles were bent and pulled back laterally so that they abutted the medial cortex (Fig. 3Ao). The arthrotomy and skin incisions were closed in layers. The fixator was mounted and attached to the 30-gauge needles using small set screws (Fig. 3Ap) Routine subcutaneous administration of buprenorphine analgesia was provided daily for the 72-h postoperative period.
X-ray
X-ray was taken with Faxitron (Faxitron Bioptics, LLC, Tucson, AZ) with exposure time of 10 s and voltage of 26 kv.
IVIS
MMPSense 680 probe (PerkinElmer, Waltham, MA) which is specific for MMP-2, −3, −9, and −13 was used in this study. At days 3, 7, 16, 23, and 30, five mice underwent IVIS imaging using MMP Sense 680 probe. One mouse in each group without the probe was imaged at each time point to serve as control.
Twenty-four hours prior to taking each image, mice were anesthetized via isoflurane inhalation, and 150 μl (2 nmol) of probe was administered to each mouse via retro-orbital vein injection.21 Images were acquired (13.1cm field of view) in the imaging system (IVIS Spectrum, PerkinElmer). Both legs were secured to the table at the ankle using a 5–0 wire and pipette tip (Supplemental Fig. S2). Image processing and quantification were performed via IVIS Living Image software (PerkinElmer). The excitation and emission filters were 675 and 700 nm, respectively, and were chosen based on the peak excitation and emission spectra of the probes (680/ 700 nm ex/em). The exposure time for each probe was 2.0 s. Spatial binning of pixels was set at the Medium option. Quantification of fluorescent intensity was performed by evaluating the mean radiant efficiency of the signal within a region of interest (ROI). Two different ROIs were examined. The first ROI was set on the entire limb from the heel to the inguinal line (Fig. 4A). The background was set to the center of the abdomen (indicated by the red circle). The second ROI was focused on the knee joint, registered to the superimposed X-ray image (Fig. 4B), with the background again based on the center of the abdomen. The mean signal intensity was calculated by subtraction of background signal intensity from the value of the ROI to account for mouse-to-mouse variation in delivery of the fluorescent probe.
Figure 4.
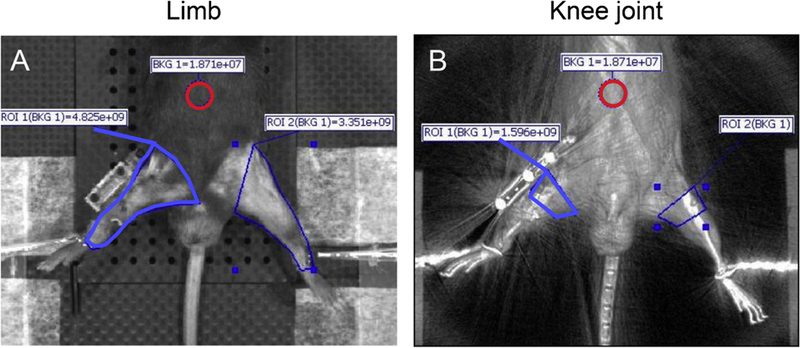
Quantitative analysis of IVIS images. Methodology of setting the region of interest (ROI) for quantifying fluorescent signals in each knee. (A) The ROI was set on the entire limb from heel to inguinal line (indicated by blue line). The background was set at the center of the abdomen (indicated by red circle). (B) The ROI was focused on the knee joint, registered to superimposed X-ray image (indicated by blue line). The background was set to the center of the abdomen (indicated by red circle).
Histological Analysis
The dissected knee joint was decalcified with 10% EDTA for 5 days following 4% paraformaldehyde fixation for 4h to preserve the fluorescent label. After decalcification, the femoral condyle was carefully dissected, cut perpendicular to the graft, and was then immediately embedded in O.C.T compound (Tissue-Tek, Sakura Fintek USA, Inc., Torrance, CA) and frozen with dry ice and 2-methylbutane. Cryosections of 12 μm thickness were prepared with a CM 3050S cryostat (LEICA, Nussloch, Germany) using a coated high profile disposable blade (Thermo science, Waltham, MA).22 The specimens were observed and photographed using fluorescent microscopy with Cy 5.5 filter (EVOS, Thermo Fisher Scientific, Waltham, MA). Each specimen was then stained with hematoxylin and eosin (HE) and photographed using light microscopy (Eclipse E800, Nikon, Melville, NY) with transmitted light at ×4 magnification. For quantitative analysis of MMP activity at the graft-bone interface and in the graft, the area of MMP probe fluorescence was measured using Image J (NIH, Bethesda, MD). Fluorescence in the green channel in the gray scale was measured on the same section as the HE image (Fig. 5A). A 0.64 mm circle (the diameter of 23G needle is 0.64 mm) was drawn in the center of the bone tunnel. The graft was manually delineated using the HE image as a reference (Fig. 5B). The threshold was set to cover the MMP probe positive areas (Fig. 5C). The area between these two delineations represented the graft-bone interface. The percent of MMP probe positive area in the graft-bone interface and graft were calculated using Image J.
Figure 5.
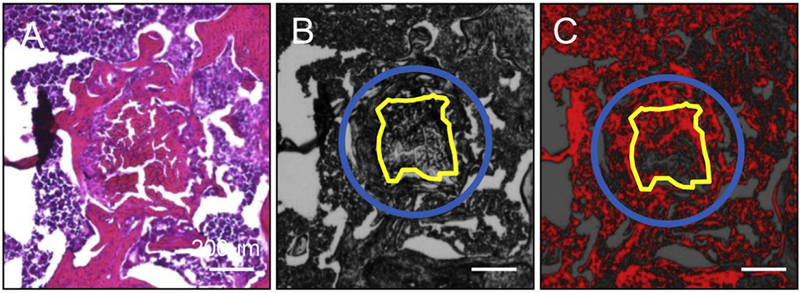
Quantitative analysis of histology images. (A) Quantification methodology of MMP probe positive area in the fluorescent microscope pictures. (a) HE picture. (b) Fluorescent gray scale image of the same section as HE. A 0.64mm circle (blue circle; the diameter of 23G needle is 0.64 mm) was drawn in the center of the bone tunnel. The graft was manually delineated (yellow line) referring to HE picture. (C) The threshold was set to cover MMP probe positive areas. The area between these two delineations represented the graft-bone interface. The percent of MMP probe positive area in the graft-bone interface and graft were calculated using Image J. Scale bar = 200 μm.
Healing of the tendon graft in the bone tunnel was evaluated using an established scoring system (Tendon Bone Tunnel Healing (TBTH) scoring system).23 We assessed three subcategories including graft degeneration, graft remodeling, and percentage of fibrous tissue at the graft-bone interface. This score ranges from 0 to 13. A higher score indicates superior healing.
Immunohistochemistry
For MMP13 immunohistochemistry, sections were blocked with 1% serum for 10 min at room temperature, and antigen retrieval was performed by incubating sections in trypsin for 15min at 37°C. Sections were then incubated with the primary antibody (MMP13 antibody, GTX100665, purified rabbit polyclonal IgG, GeneTex, Irvine, CA). A secondary antibody (ImmPRESS Anti-Rabbit Ig Kit, Vector Laboratories, Inc., Burlingame, CA) and Dako Liquid DAB+ Substrate Chromogen system (K3468, Agilent, Santa Clara, CA) were used following the immunostaining procedures provided by the manufacturer instructions. Finally, the sections were counterstained with hematoxylin.
Statistical Analysis
Statistical analysis was performed using ANOVA followed by post hoc analysis with Tukey test for multigroup comparison with GraphPad Prism version.7.03 (GraphPad Software Inc., La Jolla, CA).
RESULTS
IVIS Images
Control animals had less signal intensity than all study animals. Overall probe signal intensity peaked (yellow = higher) at day 7, followed by a decrease to day 16, and leveled out up to day 30 compared to the normal group which did not undergo surgery (Fig. 6). Fluorescent signal intensity was quantified in two different ROIs, one based on the entire lower limb (Fig. 4A), and the other on the knee joint (Fig. 4B). Similar overall results were found using these two methods. All groups showed significant differences between day 3, day 7, and normal (Fig. 6B, C). In most of the groups, signal intensity at day 7 was higher than at later time points. Despite no significant difference among groups at all time points, signal intensity of Group 1 (no external fixator) was consistently lower than the other groups.
Figure 6.
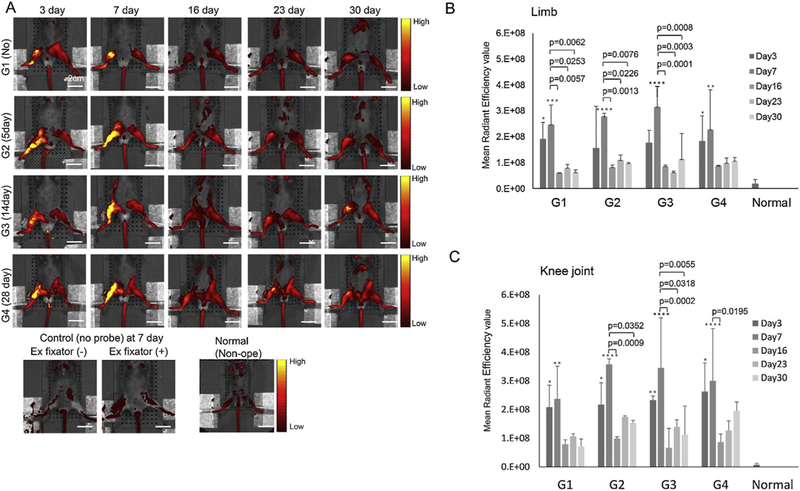
Representative IVIS images of each group from days 3 to 30. (A) Right side was operated. The control group was not injected with probe. The normal group did not undergo surgery. Color bar indicates signal intensity (yellow = higher). Scale bar = 2.0 cm. Control animals showed much less signal than all study animals. Signal intensity peaked at day 7, followed by a decrease to day 16, and maintenance up to day 30 compared with normal animals. (B) Mean radiant efficiency values of each group from MMP sense 680 probe at each time point. The ROI was set on the entire limb. (C) The ROI was focused on the knee joint. All data are presented as mean ± standard deviation (n = 5). p-value was calculated by two-way ANOVA with post hoc Tukey’s test. Comparison with normal *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. All groups showed significant difference between day 3, day 7, and normal. In most of groups signal intensity at day 7 was higher than later time points (day 16, day 23, and day 30). No significant difference was found among groups at all time points.
Histology
Histologic localization of MMP probe signal was found not only in the graft-bone interface and graft but also in the bone marrow and muscle. Control specimens had minimal signal intensity (Fig. 7A). Probe signal intensity peaked at day 7 (Fig. 7B) and day 16 (Fig. 7C) time points in all groups. At day 30 (Fig. 7D), MMP probe positive area of the graft-bone interface in Group 2 (p = 0.0327) and 3 (p = 0.0452) were less than that in Group 4 (Fig. 7E). Although the differences were not significantly different, the same trend was seen in the graft (Fig. 7F). Histologic evaluation demonstrated superior healing in Group 2 compared with Group 1 (p = 0.036) and Group 4 (p = 0.016) based on the TBTH scoring system (Fig. 7G and H). MMP13 expression was less in Groups 2 and 3 at the graft-bone interface than Groups 1 and 4 based on immuno-histochemical analysis (Fig. 8).
Figure 7.
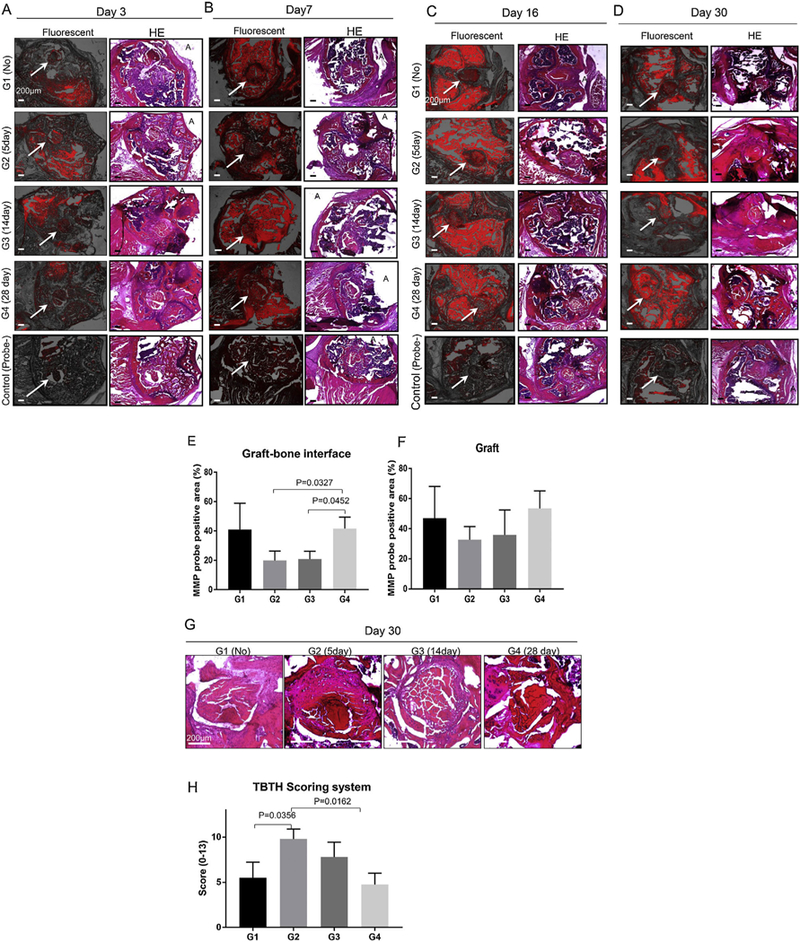
Representative transverse section of fluorescent microscope and hematoxylin eosin (HE) pictures of each group at each time point. (A) Day 3. (B) Day 7. (C) Day 16. (D) Day 30. Left column is fluorescent image (MMP 680 probe fluorescence is red). Right column is HE stained section. A indicates anterior aspect of femoral condyle. Scale bar = 200 μm. White arrows indicate bone tunnel and graft. The control group was not injected with probe. Control specimens showed much less signal than study specimens. Probe signal intensity seemed to peak at day 16 (Fig. 6C) time points in all groups. MMP probe fluorescent positive area at graft-bone interface (E) and graft at day 30 (F). All data are presented as mean ± standard deviation (n = 5). p-value was calculated by one-way ANOVA with post hoc Tukey’s test. There was a significant difference in MMP probe positive area at the graft-bone interface between Groups 2, 3, and 4 (p < 0.05). (G) Representative transverse section of magnified graft and bone tunnel pictures of each group at day 30. Scale bar = 200 μm. (H) Tendon Bone Tunnel Healing (TBTH) scoring system. Group 2 showed better score than Group 1 and Group 4 (p < 0.05).
Figure 8.
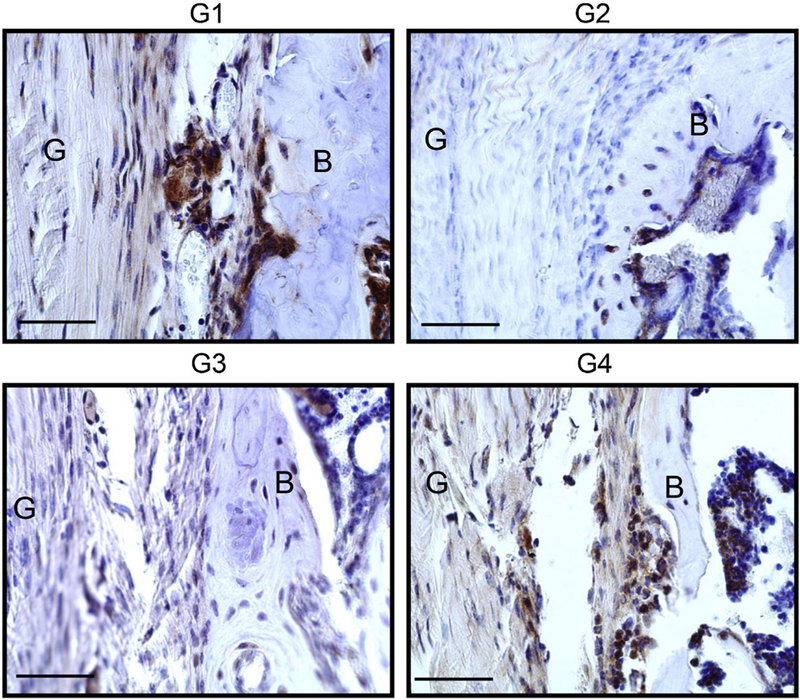
Immunohistochemistry of MMP. Representative pictures at Day 30. G: Graft B: Bone Scale bar=50μm.
DISCUSSION
In this study, we aimed to investigate how different knee immobilization periods affected MMP activity at the healing graft-bone interface. We utilized a novel mouse ACL reconstruction model and external fixator to control immobilization.20,24 In order to measure MMP expression, we used a fluorescent MMP probe and near-infra red in vivo imaging.18 Additionally, for assessing localization of MMP activity around the bone tunnel, we measured MMP probe fluorescent signal in histologic sections.
The most important findings of this study is that short-term immobilization suppressed MMP activity more than long-term immobilization and no immobilization. Histological analysis showed that MMP activity in the group with prolonged immobilization (Group 4) was higher than that in animals with shorter periods of immobilization (Groups 2 and 3). Immunohis-tochemistry of MMP 13 supported this finding. It is known that stress deprivation affects MMP activity. Prior studies demonstrate that stress deprivation increases MMP expression in tenocytes in vitro.25,26 Based on the results presented here, long-term stress deprivation of a tendon graft in a bone tunnel also increases MMP activity.
We also found that animals that resumed immediate cage activity with no immobilization (Group 1) showed higher MMP expression than animals with short periods of immobilization (Groups 2 and 3), although the differences were not statistically significant. Additionally, histological analysis demonstrated that short-term immobilization (especially Group 2) was superior to both no immobilization and long-term immobilization in terms of graft-bone healing. Similarly, prior studies in our laboratory using the same animal model found that short-term immobilization resulted in better healing based on both biomechanical and histologic criteria compared with both no immobilization and long-term immobilization.13 Recent studies demonstrated that moderate load bearing decreases MMP expression through induction of CITED2, which is transcription regulator that suppresses the expression of MMP (1 and 13) in chondrocytes.27,28 Taken together, these data indicate that short-term immobilization after ACL reconstruction might mitigate excess MMP expression and result in superior bone-to-tendon healing.
We utilized the IVIS in vivo imaging system to investigate how post-operative knee motion and mechanical loading affects MMP activity around the bone tunnel. The IVIS Spectrum System allows repeat, in vivo imaging and quantification of MMP activity in this mouse surgical model. These techniques potentially will enable further studies to investigate the molecular mechanisms of bone-to-tendon healing. However, in contrast to the histologic findings, IVIS images showed that MMP activity in Group 1 (without external fixator) tended to be less than that in other groups (with external fixator). This finding might be due to the more invasive surgery required for placement of the external fixator. The external fixator was utilized for joint immobilization, but it caused more soft tissue damage during the surgical installation due to a longer skin incision and the insertion of four pins. Previous reports found that MMP activity measured using the MMP 680 sense probe with IVIS was enhanced by inflammation.29 Therefore, the MMP activity measured by IVIS images might reflect the extent of inflammation of the entire knee joint and surrounding tissue after ACL reconstruction and placement of the external fixator, rather than just MMP activity of graft and bone tunnel.
The discrepancy between IVIS and histology is likely due to the fact that histology enabled us to specifically evaluate MMP activity of the bone tunnel and graft, while IVIS measures MMP activity not only from the bone tunnel and graft but also the surrounding tissues, muscles, and bone marrow. IVIS imaging does not appear to have adequate specificity to investigate only the graft and bone tunnel in this small animal model. A promising solution could be to overlay images of high resolution micro CT and IVIS which would provide better localization of MMP signal and thus measurement of a more specific ROI. The high variability of the IVIS system might have limited thecapability to detect relatively small differences in fluorescence levels, particularly in a surgical model. The quantification of fluorescent signals using the IVIS images exhibited large variance and a strong dependence on position of the mice on the imaging stage. In order to obviate this limitation, we created a device to maintain the lower extremity in a consistent position for each imaging session. The IVIS Spectrum system produces a circular beam of light from the excitation light source above the stage. Previous reports demonstrate that the position of the animal in the imaging system affects illumination.18 We acquired images one mouse at a time in order to minimize variability from the position of the animal relative to the imaging system. Additionally, some of the surgical materials may have significant autofluorescence that can affect the results. In order to obviate this limitation, we fabricated an external fixator using materials with the least autofluorescence after comparing several different types of metal and plastic materials. Additionally, we used a 5–0 wire for graft fixation to avoid the autofluorescence associated with 5–0 Ethibond suture, which we have used in our prior animal models.13 With these modifications, we were able to successfully track the course of MMP activity following ACL reconstruction with or without an external fixator in this mouse ACL reconstruction model. Despite these limitations, the current study provides preliminary data for further studies using IVIS for in vivo imaging.
There was another discrepancy between IVIS and histology. It seemed that MMP activity based on histology peaked at day 16 while IVIS images showed the highest signal at day 7, earlier time point. Previously we found gene expression of MMP13 and MMP14 in the graft and graft-bone interface peaked at day 14 in a mouse ACLR model with RT-PCR analysis.24 We assumed that MMP activity around the bone tunnel in this model would peak around day 14 or later based on the results presented in this study and our previous study. IVIS images would be affected by the inflammatory response to surgery, which could account for the earlier peak.
There are several advantages to using a murine model for ACL reconstruction to study bone-to-tendon healing, including the physiologic similarities with humans,30 the availability of transgenic mice to study specific molecular pathways, availability of robust biologic assays, and less demanding husbandry compared to other species. The mouse model is most suited for in vivo imaging using the IVIS system, which enabled us to measure MMP expression non-invasively on the same animal over an extended period of time. Such in vivo imaging in a larger animal model requires a much larger amount of probe and is thus not practical from an economic standpoint.
There are important limitations to this study. First, we were unable to specify which particular MMP subtype is active in bone to tendon healing since this probe localizes MMP-2, −3, −9, and −13. Only MMP13 was verified by immunohistochemistry. In this study, we selected MMP13 for immunohistochemistry since previously we found MMP 13 expression was higher than other MMPs such as MMP3 or 14 evaluated with RT-PCR analysis using a mouse ACLR model.24 Second, MMP 680 sense probe does not react to some important MMP subtypes which may play an important role in bone-to-tendon healing, such as MMP14, which is upregulated during embryogenesis in areas that develop into tendon-bone insertion sites.31 In support of the possible role of MMP-14, prior work in our laboratory found that bone marrow-derived mesenchymal stem cells genetically modified with the developmental gene MT1-MMP, which codes for MMP14, improved tendon-to-bone healing in a rat rotator cuff repair model.32 Third, to address the effects of surgical invasiveness of external fixator placement on the IVIS images, the no immobilization group (Group 1) should have undergone a sham operation with insertion and then removal of the external fixator pins. Fourth, we did not evaluate tunnel enlargement in this study. Future study will include micro CT. Lastly, we did not perform any biomechanical tests, thus we cannot conclude that lower MMP expression correlated to better graft-to-bone healing.
Despite these limitations and recognizing the need for further study, our histologic data suggest that short-term immobilization after ACL reconstruction may mitigate excess MMP expression and enhance bone-to-tendon healing. These findings have implications for post-operative rehabilitation protocols following ACL reconstruction. The combination of our established mouse ACL reconstruction model and near-infrared protease-activatable probes may be useful in further studies to elucidate the molecular mechanisms of soft tissue healing.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge with thanks the important technical assistance of Ching Tung, PhD (Professor of Chemistry in Radiology, Weill Cornell Medicine) and Myung Shin Han (Chemistry in Radiology, Weill Cornell Medicine) for EVOS microscope, and Bin He, PhD (Weill Cornell Medicine) and Jonathan Dyke, PhD (Weill Cornell Medicine) for in vivo imaging. We also recognize the valuable support of Daniel Nemirov and Brett Croen (Orthopaedic Soft Tissue Research Program, Hospital for Special Surgery) for histological analysis.
Abbreviations:
- ACL
anterior cruciate ligament
- IVIS
in vivo imaging
- MMP
matrix metalloproteinase
- OA
osteoarthritis
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Ellman MB, Sherman SL, Forsythe B, et al. 2015. Return to play following anterior cruciate ligament reconstruction. J Am Acad Orthop Surg 23:283–296. [DOI] [PubMed] [Google Scholar]
- 2.Webster KE, Feller JA, Hartnett N, et al. 2016. Comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction: a 15-year follow-up of a randomized controlled trial. Am J Sports Med 44:83–90. [DOI] [PubMed] [Google Scholar]
- 3.Pasternak B, Schepull T, Eliasson P, et al. 2010. Elevation of systemic matrix metalloproteinases 2 and 7 and tissue inhibitor of metalloproteinase 2 in patients with a history of Achilles tendon rupture: pilot study. Br J Sports Med 44:669–672. [DOI] [PubMed] [Google Scholar]
- 4.Hirata M, Kugimiya F, Fukai A, et al. 2012. C/EBPbeta and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2alpha as the inducer in chondrocytes. Hum Mol Genet 21:1111–1123. [DOI] [PubMed] [Google Scholar]
- 5.Bedi A, Kovacevic D, Hettrich C, et al. 2010. The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. J Shoulder Elbow Surg 19:384–391. [DOI] [PubMed] [Google Scholar]
- 6.Demirag B, Sarisozen B, Ozer O, et al. 2005. Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. J Bone Joint Surg Am 87:2401–2410. [DOI] [PubMed] [Google Scholar]
- 7.Tajima T, Chosa E, Kawahara K, et al. 2015. Prospective comparisons of femoral tunnel enlargement with 3 different postoperative immobilization periods after double-bundle anterior cruciate ligament reconstruction with hamstring grafts. Arthroscopy 31:651–658. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Deie M, Adachi N, et al. 2007. A prospective study of 3-day versus 2-week immobilization period after anterior cruciate ligament reconstruction. Knee 14:34–38. [DOI] [PubMed] [Google Scholar]
- 9.Sakai H, Fukui N, Kawakami A, et al. 2000. Biological fixation of the graft within bone after anterior cruciate ligament reconstruction in rabbits: effects of the duration of postoperative immobilization. J Orthop Sci 5:43–51. [DOI] [PubMed] [Google Scholar]
- 10.Thomopoulos S, Williams GR, Soslowsky LJ. 2003. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng 125:106–113. [DOI] [PubMed] [Google Scholar]
- 11.Muneta T, Yamamoto H, Takakuda K, et al. 1993. Effects of postoperative immobilization on the reconstructed anterior cruciate ligament. An experimental study in rabbits. Am J Sports Med 21:305–313. [DOI] [PubMed] [Google Scholar]
- 12.Brophy RH, Kovacevic D, Imhauser CW, et al. 2011. Effect of short-duration low-magnitude cyclic loading versus immobilization on tendon-bone healing after ACL reconstruction in a rat model. J Bone Joint Surg Am 93:381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camp CL, Lebaschi A, Cong GT, et al. 2017. Timing of postoperative mechanical loading affects healing following anterior cruciate ligament reconstruction: analysis in a murine model. J Bone Joint Surg Am 99:1382–1391. [DOI] [PubMed] [Google Scholar]
- 14.Shcherbakova DM, Verkhusha VV. 2013. Near-infrared fluorescent proteins for multicolor in vivo imaging. Nat Methods 10:751–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clapper ML, Hensley HH, Chang WC, et al. 2011. Detection of colorectal adenomas using a bioactivatable probe specific for matrix metalloproteinase activity. Neoplasia 13:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razansky D, Harlaar NJ, Hillebrands JL, et al. 2012. Multispectral optoacoustic tomography of matrix metalloproteinase activity in vulnerable human carotid plaques. Mol Imaging Biol 14:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozloff KM, Quinti L, Patntirapong S, et al. 2009. Noninvasive optical detection of cathepsin K-mediated fluorescence reveals osteoclast activity in vitro and in vivo. Bone 44:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satkunananthan PB, Anderson MJ, De Jesus NM, et al. 2014. In vivo fluorescence reflectance imaging of protease activity in a mouse model of post-traumatic osteoarthritis. Osteoarthritis Cartilage 22:1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukui T, Tenborg E, Yik JH, et al. 2015. In-vitro and in-vivo imaging of MMP activity in cartilage and joint injury. Biochem Biophys Res Commun 460:741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lebaschi A, Deng XH, Coleman NW, et al. 2017. Restriction of postoperative joint loading in a murine model of anterior cruciate ligament reconstruction: botulinum toxin paralysis and external fixation. J Knee Surg 30:687–693. [DOI] [PubMed] [Google Scholar]
- 21.Yardeni T, Eckhaus M, Morris HD, et al. 2011. Retro-orbital injections in mice. Lab Anim (NY) 40:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa Y, Muneta T, Otabe K, et al. 2016. Cartilage derived from bone marrow mesenchymal stem cells expresses lubricin in vitro and In vivo. PLoS ONE 11:e0148777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lui PP, Ho G, Lee YW, et al. 2011. Validation of a histologic scoring system for the examination of quality of tendon graft to bone tunnel healing in anterior cruciate ligament reconstruction. Anal Quant Cytol Histol 33:36–49. [PubMed] [Google Scholar]
- 24.Deng XH, Lebaschi A, Camp CL, et al. 2018. Expression of signaling molecules involved in embryonic development of the insertion site is inadequate for reformation of the native enthesis: evaluation in a novel murine ACL reconstruction model. J Bone Joint Surg Am 100:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnoczky SP, Tian T, Lavagnino M, et al. 2004. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res 22:328–333. [DOI] [PubMed] [Google Scholar]
- 26.Lavagnino M, Arnoczky SP, Tian T, et al. 2003. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect Tissue Res 44: 181–187. [DOI] [PubMed] [Google Scholar]
- 27.Leong DJ, Li YH, Gu XI, et al. 2011. Physiological loading of joints prevents cartilage degradation through CITED2. FasebJ 25:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun HB, Zhao L, Tanaka S, et al. 2012. Moderate joint loading reduces degenerative actions of matrix metalloproteinases in the articular cartilage of mouse ulnae. Connect Tissue Res 53:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barber PA, Rushforth D, Agrawal S, et al. 2012. Infrared optical imaging of matrix metalloproteinases (MMPs) up regulation following ischemia reperfusion is ameliorated by hypothermia. BMC Neurosci 13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carballo CB, Hutchinson ID, Album ZM, et al. 2018. Biomechanics and microstructural analysis of the mouse knee and ligaments. J Knee Surg 31:520–527. [DOI] [PubMed] [Google Scholar]
- 31.Holmbeck K, Bianco P, Chrysovergis K, et al. 2003. MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J Cell Biol 163:661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gulotta LV, Kovacevic D, Montgomery S, et al. 2010. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med 38: 1429–1437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


