Abstract
Objective:
The purpose of this study was to evaluate pre- and postseason measures of body composition, skeletal muscle, and blood parameters/liver lipid in wildland firefighters (WLFF) over the fire season.
Methods:
Alaskan WLFF (N = 27) crews were evaluated pre- and postwildfire season, which included 63 ± 10 operational days. Body composition, thigh muscle area, and liver lipid were quantified using dual-energy radiograph absorptiometry and magnetic resonance imaging, respectively. Blood metabolic and lipid panels were also collected and analyzed.
Results:
Total body, fat, and visceral fat mass increased from pre- to postseason (P < 0.05). Total cholesterol, LDL, and total globulin also increased (P < 0.05). There was a trend (P = 0.06) towards an increase in intrahepatic lipid.
Conclusions:
The observed maladaptive changes in adipose tissue, blood lipids, and hepatic function may reflect adaptations/consequences to occupational demands/conditions and warrant evaluation of appropriate countermeasures.
Keywords: body composition, cholesterol, intrahepatic lipid, metabolic, wildland firefighter
Approximately 6 million wildfires have burned almost 10% of the United States in the last 60 years.1 Above average temperatures throughout the Pacific Northwest combined with population growth have complicated the suppression of wildfires. Since 2002, 6% of four western states have been burned by wildfires that were primarily not only caused by lightning, but also influenced by human activity.2 The climate-related events have a multifactorial impact on human health with workers in agriculture, construction, transportation, and firefighters being particularly vulnerable to occupational health risks.3
Wildland firefighters (WLFF) are positioned at a challenging nexus that balances the protection of life, resources, and property against risks to their own health.4 These relationships are complicated by work conditions that can contribute to periods of negative energy balance and variations in dietary intake.4 Although several studies have provided data across a range of individuals that suggest work rates analogous to 3 times that of resting metabolic status,5,6 work output varies substantially between training, ingress, and egress.7 Variations in daily work rate and nutrient availability may contribute to oscillations in energy balance.7–9 In fact, a recent meta-regression from nine separate studies suggested that extreme circumstances may foster a negative energy balance beyond the physiological capacity to maintain skeletal muscle, resulting in decreased physical performance.10 Given the importance of skeletal muscle in this scenario, evaluations of seasonal changes in skeletal muscle in WLFF using the precision of magnetic resonance imaging (MRI) would be beneficial.11
Wildland firefighting may be often comingled with sleep deprivation that increases the risk of injury and metabolic disease.12 Even though physical performance does not seem affected by sleep deprivation during simulated wildland firefighting,13 detrimental alterations in acute inflammatory responses have been described.11 The number of days “on fire” and the severity of the fires can contribute to additional challenges in this regard as well.14 Physiological strain, negative energy balance, and sleep deprivation may not only increase the acute health risks,15,16 but sleep duration of less than 5 hours has been linked to a 50% increase in the incidence of metabolic syndrome.17 The Environmental Protection Agency has also suggested that exposure to wildland fire smoke may pose significant health risks, but the overall impact on physiological and/or cardiovascular indices in WLFF has not been well described in a field setting.18 Therefore, the objective of the present study was to examine seasonal alterations in body composition, cross-sectional area of the upper thigh muscles (XT), intrahepatic lipid (IHL), and blood lipids in Alaska WLFF over the summer season of 2017.
METHODS
Following approval by the University of Alaska Fairbanks (UAF) Institutional Review Board, WLFF were recruited from the Chena, Midnight Sun, and North Star Hot Shot crews, and the UAF wildland fire crew (overall n = 27, 25 M, 2 F, 27 ± 1 years). Study participants completed two visits that included measurement of body composition, XT, IHL, and blood lipids that corresponded with pre- and postfire season during late June through early September of 2017. Following the fire season, participants returned to the laboratory within 72 hours of their return to Fairbanks, AK. According to Clinical Laboratory Improvement Amendments from the Federal Food and Drug Administration, blood sampling and analysis were completed by LabCorp, Inc.
We utilized dual-energy radiograph absorptiometry scans (General Electric iDXA, Boston, MA) to measure fat mass (FM) and lean tissue mass (LTM). The XT and IHL measurements were imaged using a Toshiba Excelart/Vantage 1.5 T MRI/spectroscopy system (Canon, Õtawara, Tochigi, Japan). Axial and coronal T1-weighted images were acquired using a Field Echo sequence (TR = 172 ms, TE = 4ms), and axial T2 images were acquired with a Fast Spin Echo sequence (TR = 3700ms, TE = 90 ms). Seven of the axial T1-weighted images (FOV = 30 × 30 cm, matrix 256 × 256, NAQ = 2) were selected based on the half-way point of the femur or between the superior border of the patella and the greater trochanter during each visit. These images were analyzed using Osirix software (Pixmeo, Bernex, Switzerland).
For the measurement of IHL, participants were placed in a prone, headfirst position in the whole body coil of the MRI system. Axial and coronal T1-weighted images were acquired using a Field Echo sequence (TR = 172 ms, TE = 4 ms), and axial T2 images were acquired with a Fast Spin Echo sequence (TR = 3700 ms, TE = 90 ms, FOV = 30 × 50 cm). Spectra were acquired on a 3 × 3 × 2 cm voxel using a PRESS sequence (TR = 2000 ms, TE = 136, NAQ = 256).
Raw data from the spectra including an unsuppressed water reference were converted to ascii format using a custom script, before analysis using JMRUI. All spectra were Fourier transformed, phased and referenced (1.4 ppm for lipid spectra, 4.8 ppm for water reference). The results from both the lipids spectra and water reference spectra were then used to calculate a lipid-to-water ratio.
Statistical Analysis
Data were analyzed using Microsoft Excel, iDXA, Osirix, and Prism 5 software. Regular paired t-tests were utilized to compare differences in pre- and post-season data. Statistics were considered significant with a P value of less than 0.05. Data are presented as means ± SD.
RESULTS
Clinical characteristics: We enrolled 27 participants who spent 63 ± 10 days on wildfire assignments. Ten participants failed to complete all data collection for their blood panels and molecular imaging due to limited postseason availability and/or crew dispersion.
Body/Tissue composition (n = 27): Total body mass, total FM, arm FM, leg FM, and visceral FM increased from pre- to postseason (P < 0.05) (Table 1). There were no changes in total LTM, arm LTM, and leg LTM (P > 0.05) (Table 1).
TABLE 1.
Body Composition and MRI-Derived Data
| Pre | Post | |
| Weight | 78.7 ± 12.8 | 79.7 ± 12.3* |
| Body mass index, kg/m2 | 23.9 ± 2.7 | 24.5 ± 2.7* |
| Total fat mass, kg | 12.4 ± 5.2 | 13.9 ± 4.9* |
| Total lean mass, kg | 62.8 ± 9.1 | 63.1 ± 8.7 |
| Lean to fat ratio | 5.9 ± 2.5 | 5.0 ± 1.4 |
| Arm—fat, kg | 1.4 ± 0.6 | 1.5 ± 0.6 |
| Arms—lean, kg | 8.5 ± 1.4 | 8.6 ± 1.5 |
| Legs—fat, kg | 4.0 ± 1.8 | 4.8 ± 1.7 |
| Legs—lean, kg | 21.7 ± 3.6 | 21.9 ± 3.7 |
| Torso—fat, kg | 6.1 ± 3.0 | 6.7 ± 2.9 |
| Torso—lean, kg | 29.0 ± 4.6 | 29.2 ± 3.7 |
| Relative skeletal muscle index, kg/m2 | 9.3 ± 1.1 | 9.3 ± 1.1 |
| Visceral fat, kg | 318 ± 47 | 419 ± 48* |
| Upper thigh muscle, cm3 | 152.2 ± 24.6 | 151.8 ± 21.2 |
Data are presented as means ± SD.
*Significant difference (P < 0.05).
Molecular imaging (n = 17): There were no changes in XT from pre- to postseason (P > 0.05) (Table 1). There was a strong trend (P = 0.06) supporting an increase in IHL (0.143 ± 0.0040 to 0.236 ± 0.0050 lipid/water from pre- to postseason, respectively (Figure 1).
FIGURE 1.
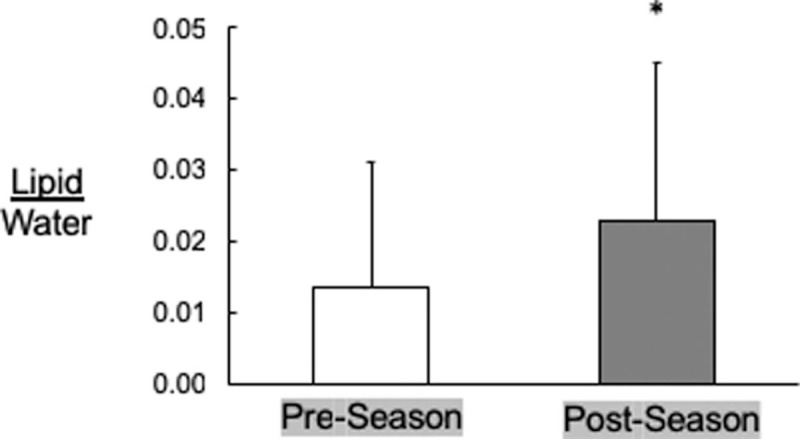
Pre- and postseason intrahepatic lipid as measured by magnetic resonance imaging/spectroscopy (n = 17). ∗P = 0.06. Data = mean ± SD.
Blood parameters (n = 17): There was an increase in total cholesterol and LDL-cholesterol from pre- to postseason (P < 0.05) (Table 2). VLDL-cholesterol and HDL-cholesterol remained unchanged (P > 0.05). There were no changes in glucose, blood urea nitrogen, creatinine, estimated glomerular filtration rate, creatinine ratio, sodium, potassium chloride, carbon dioxide, and calcium (P > 0.05) (Table 2). There were no changes in albumin, bilirubin, alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase (P > 0.05), but there was an increase in globulin and a decrease in albumin/globulin ratio and direct bilirubin (P < 0.05) (Table 2).
TABLE 2.
Lipid, Metabolic, and Hepatic Blood Panels
| Pre | Post | |
| Total cholesterol | 161.8 ± 28.1 | 179.9 ± 33.5* |
| LDL-cholesterol | 85.7 ± 22.5 | 100.3 ± 31.5* |
| VLDL-cholesterol | 18.4 ± 10.7 | 21.8 ± 8.1 |
| HDL-choleseterol | 54.7 ± 18.9 | 57.5 ± 15.7 |
| Triglycerides, mg/dL | 91.8 ± 53.5 | 109.6 ± 41.2 |
| Glucose, mg/dL | 87.0 ± 5.7 | 89.3 ± 7.2 |
| Blood urea nitrogen, mg/dL | 15.5 ± 5.7 | 16.1 ± 4.1 |
| Creatinine, mg/dL | 0.9 ± 0.2 | 1.0 ± 0.1 |
| Estimated glomerular filtration rate, mL/min/1.73 | 109.4 ± 14.2 | 106.9 ± 12.6 |
| Blood urea nitrogen/creatinine ratio | 16.8 ± 3.7 | 16.9 ± 3.4 |
| Serum sodium, mmol/L | 140.8 ± 1.3 | 134.9 ± 23.1 |
| Serum potassium, mmol/L | 4.4 ± 0.3 | 4.5 ± 0.3 |
| Total carbon dioxide, mmol/L | 24.5 ± 1.7 | 24.5 ± 1.5 |
| Serum calcium, mg/dL | 9.5 ± 0.3 | 9.5 ± 0.3 |
| Serum total protein, g/dL | 6.8 ± 0.3 | 6.9 ± 0.3 |
| Serum albumin, g/dL | 4.6 ± 0.2 | 4.5 ± 0.2 |
| Total globulin, g/dL | 2.3 ± 0.3 | 2.3 ± 0.3* |
| Albumin/globulin ratio | 2.1 ± 0.2 | 2.0 ± 0.3* |
| Total bilirubin, mg/dL | 0.6 ± 0.3 | 0.5 ± 0.3 |
| Alanine aminotransferase, IU/L | 27.2 ± 12.3 | 28.0 ± 12.6 |
| Aspartate aminotransferase, IU/L | 31.8 ± 12.4 | 26.1 ± 5.8 |
| Alkaline phosphatase, IU/L | 65.8 ± 15.0 | 64.3 ± 15.4 |
| Direct bilirubin, mg/dL | 0.15 ± 0.07 | 0.13 ± 0.06* |
Data are presented as means ± SD.
*Significant difference (P < 0.05).
DISCUSSION
The main finding of this study was that WLFF experienced a decline in indices of metabolic and cardiovascular health over the course of the fire season. Evidence for this assertion has been provided by an increase in total plasma cholesterol, LDL-cholesterol, VLDL-cholesterol, and an increment in IHL. Perturbations in hepatic function were also evidenced by an increase in globulin and a decrease in albumin/globulin ratio and direct bilirubin. The deterioration in the metabolic biomarkers occurred despite the preservation of skeletal muscle as indicated by no changes in LTM or XT. In many ways, these data may seem surprising given the arduous work previously reported in WLFF on assignment.4–6 Despite potentially challenging circumstances where sustained negative caloric balance might increase the risk of muscle loss,10 the retention of skeletal muscle in our research participants may have been positively influenced by the anabolic influence of activity on protein synthesis.19 Alterations in dietary intake, stress responses, smoke exposure, and/or a potential detraining effect may be responsible for the observed dysregulation of lipid metabolism.17,18
Previous studies in WLFF have demonstrated levels of total energy expenditure (TEE) that reach 2719 to 6260 kcal/d.6 Energy expenditure from physical activity (EEA) alone may reach as high as 3900 kcal/d after subtracting basal metabolic rate and dietary induced thermogenesis.6 Even though TEE or total energy intake (TEI) was not measured in the present study, this cohort typically demonstrates high rates of EEA across diverse assignments.5–7 In another study recently performed in our laboratory in backcountry hunters over the course of 12 unsupported days, a similar level of energy expenditure and negative energy balance led to dramatic improvements in metabolic health.20 These results suggest that the level of physical exertion demonstrated in previous field studies involving substantial EEA should lead to favorable changes in metabolic outcomes.
There have been considerable efforts directed towards ensuring adequate nutrient provisions for WLLF. For example, an “eat on the move” strategy has been suggested to help optimize physical performance, reaction time, and mood status.21 Using a combination of video dietary intake documentation and an estimation of energy expenditure via wearable technology at different times during the day, recent studies have suggested higher levels of energy expenditure during the entry to the project fire (ingress phase) than the exit from the project fire (ie, egress phase) of the operation in Canadian WLFF.22 These findings were generally consistent with previous work suggesting chronic levels of negative energy balance.4–6 It is somewhat unexpected that our participants actually gained weight, FM, and visceral FM in conjunction with detrimental alterations in blood and tissue lipid. Without the existence of a negative energy balance required for improvements in lipid metabolism,23 the perturbation in lipid metabolism would be anticipated. We suggest that the findings in our present study may be potentially indicative of reduced physical exertion and/or increased access to nutrients overall and/or during the egress element of the season. These findings should not be generalized to all WLFF as the conditions and occupational requirements may vary widely depending on multiple circumstances.
The influence of smoke exposure may have also contributed to the increased risk of metabolic abnormalities with numerous chemicals of potential concern identified in a 2004 report.24 Short- and long-term exposure to ambient air pollutants such NO2, O3, and PM2.5 have been linked to a reduction in insulin sensitivity and an increase in atherogenic blood lipids.25 Although occupational risk factors cannot be singled out, maladaptive changes may be influenced by smoke-induced proinflammatory and/or oxidative stress responses in combination with chronic stress, insufficient sleep, and/or a high-fat diet.26 The influence of sleep deprivation alone may negate the protective role of sleep against chronic elevations in cortisol that have been linked to adverse health outcomes.26 The decrease in direct bilirubin is also consistent with oxidative stress and chronic inflammation.27 Overall, the combined influence of environmental and nutritive factors has been linked to elevations in visceral adipose tissue and the accumulation of IHL,28,29 and complicate the interpretation of these data.
Previous studies have demonstrated that wildland fire suppression represents work that requires 2 to 3 times more calories that many individuals consume over the course of a day. Our data demonstrate an occupational physical activity paradox where the benefits of exercise on lipid metabolism and hepatic function30 may be negatively overshadowed by alterations in nutrient delivery, smoke exposure, chronic stress, and insufficient sleep.17 In addition, high levels of smoke exposure may be particularly detrimental to human health.31 The interplay between these variables present a continuum with regard to metabolic health instead of a “one size fits all” paradigm. Based on the findings of the present study, we suggest that additional investigative efforts are needed to evaluate the influence of four primary factors: (1) physical exertion, (2) diet, (3) stress and sleep deprivation, and (4) smoke exposure on metabolic health, and how these factors may differ depending on assignment and factors mentioned earlier.
Acknowledgments
The authors wish to recognize Doug Mackey, Thomas Greiling, Tylan Martin, and Josh Turnbow for their assistance in scheduling participants for this study, and would also like to thank Mr. Jim Kimball, ATC and Jeff Zuckerman, MD for their professional consultation on this project. Lastly, the authors would like to express their sincere appreciation to the Alaska wildland firefighters who volunteered their time and effort for this study.
Footnotes
Clinical Significance: Over the course of a 5-month season, 63±5 days on wildfire assignments were incurred. In this seasonal exposure model, combined operational stressors linked to wildland fire suppression promoted an increase in fat mass deleterious changes in lipid metabolism that may ultimately increase the risk of metabolic disease.
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under grant number P20GM103395 and the United States Forest Service (USFS), Missoula Technology and Development Center. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH and/or the USFS.
The authors report no conflicts of interest.
REFERENCES
- 1.“Total Wildland Fires and Acres” (1960–2014), NIFC. Available at: https://www.nifc.gov/fireInfo/fireInfo_stats_totalFires.html Accessed March 9, 2018. [Google Scholar]
- 2.Theobald DM, Romme WH. Expansion of the US wildland-urban interface. Landsc Urban Plan 2007; 83:34–354. [Google Scholar]
- 3.Kiefer M, Rodriguez-Guzmán J, Watson J, van Wendel de Joode B, Mergler D, Soares da Silva A. Worker health and safety and climate change in the Americas: issues and research needs. Ver Panam Salud Publica 2016; 40:192–197. [PMC free article] [PubMed] [Google Scholar]
- 4.Britton C, Lynch CF, Ramirez M, Torner J, Buresh C, Peek-Asa C. Epidemiology of injuries to wildland firefigthers. Am J Emerg Med 2013; 31:339–345. [DOI] [PubMed] [Google Scholar]
- 5.Cuddy JS, Sol JA, Hailes WS, Ruby BC. Work patterns dictate energy demands and thermal strain during wildland firefighting. Wilderness Environ Med 2015; 26:221–226. [DOI] [PubMed] [Google Scholar]
- 6.Ruby BC, Shriver T, Zderic TW, Sharkey BJ, Burks C, Tysk S. Total energy expenditure during arduous wildfire suppression. Med Sci Sports Exerc 2002; 34:1048–1054. [DOI] [PubMed] [Google Scholar]
- 7.Sol JA, Ruby BC, Gaskill SE, Dumke CL, Domitrovich JW. Metabolic demand of hiking in wildland firefighting. Wilderness Environ Med 2018; 29:304–314. [DOI] [PubMed] [Google Scholar]
- 8.Ruby BC, Schoeller DA, Sharkey BJ, Burks C, Tysk S. Water turnover and changes in body composition during arduous wildlife suppression. Med Sci Sports Exerc 2003; 35:1760–1765. [DOI] [PubMed] [Google Scholar]
- 9.Cuddy JS, Gaskill SE, Sharkey BJ, Harger SG, Ruby BC. Supplemental feedings increase self-selected work output during wildlife suppression. Med Sci Sports Exerc 2007; 39:1004–1012. [DOI] [PubMed] [Google Scholar]
- 10.Murphy NE, Carrigan CT, Philip KJ, Pasiakos SM, Margolis LM. Threshold of energy deficit and lower-body performance declines in military personnel: a meta-regression. Sports Med 2018; 48:2169–2178. [DOI] [PubMed] [Google Scholar]
- 11.Deyle GD. The role of MRI in musculoskeletal practice: a clinical perspective. J Man Manipu Ther 2011; 19:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGillis Z, Dorman SC, Robertson A, et al. Sleep quantity and quality of Ontario wildland firefigthers across a low-hazard fire season. J Occup Environ Med 2017; 59:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent GE, Aisbett B, Larsen B, Ridgers ND, Snow R, Ferguson SA. The impact of heat exposure and sleep restriction on firefighter's work performance and physiology during simulated wildfire suppression. Int J Environ Res Public Health 2017; 14:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent GE, Aisbett B, Hall SJ, Ferguson SA. Fighting fire and fatigue: sleep quantity and quality during multi-day wildlife suppression. Ergonomics 2016; 59:932–940. [DOI] [PubMed] [Google Scholar]
- 15.O’Hara R, Henry A, Serres J, Russel D, Locke R. Operational stressors on physical performance in special operators and countermeasures to improve performance: a review of the literature. J Spec Oper Med 2014; 14:67–78. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Marroyou JA, López-Satue J, Pernía R, et al. Physiological work demands of Spanish wildland firefighters during wildfire suppression. Int Arch Occup Environ Health 2012; 85:221–228. [DOI] [PubMed] [Google Scholar]
- 17.Iftikhar IH, Donley MA, Mindel J, Pleister A, Soriano S, Magalang UJ. Sleep duration and metabolic syndrome. An updated dose-risk metaanalysis. Ann Am Thorac Soc 2015; 12:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cascio WE. Wildland fire smoke and human health. Sci Total Environ 2018; 624:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita S, Rasmussen BB, Cadenas JG, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 2007; 56:1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coker RH, Coker MS, Bartlett L, et al. The energy requirements and metabolic benefits of wilderness hunting in Alaska. Physiol Rep 2018; 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montain SJ, Baker-Fulco CJ, Niro PJ, Reinert AR, Cuddy JS, Ruby BC. Efficacy of eat-on-move ration for sustaining physical activity, reaction time and mood. Med Sci Sports Exerc 2008; 40:1970–1976. [DOI] [PubMed] [Google Scholar]
- 22.Robertson AH, Lariviére C, Leduc CR, et al. Novel tools in determining the physiological demands and nutritional practices of Ontario FireRangers during fire deployments. PLoS One 2017; 12:e0169390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenkilde M, Rygaard L, Nordby P, Nielsen LB, Stallknecht B. Exercise and weight loss effects on cardiovascular risk factors in overweight men. J Appl Physiol 2018; 125:901–908. [DOI] [PubMed] [Google Scholar]
- 24.Booze TF, Reinhardt TE, Quirling SJ, Ottmar RD. A screening-level assessment of the health risk of chronic smoke exposure for wildland firefighters. J Occup Environ Hyg 2004; 1:296–305. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Salam MT, Toledo-Corral C, et al. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care 2016; 39:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolkow A, Aisbett B, Reynolds J, Ferguson SA, Main LC. Relationships between inflammatory cytokine and cortisol response in firefighters exposed to simulated wildlife suppression work and sleep restriction. Physiol Rep 2015; 3:e12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Djoussé L, Levy D, Cupples LA, Evans JC, D’Agostino RB, Ellison RC. Total serum bilirubin and risk of cardiovascular disease in the Framingham Offspring Study. Am J Cardiol 2001; 7:1196–1200. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 2012; 61:3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Non-alcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001; 120:1183–1192. [DOI] [PubMed] [Google Scholar]
- 30.Brouwers B, Hesselink MKC, Schrauwen P, Schrauwen-Hinderling VB. Effects of exercise training on intrahepatic lipid content. Diabetologia 2016; 59:2068–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reisen F, Hansen D, Meyer CP. Exposure to bushfire smoke during prescribed burns and wildfires: firefighters’ exposure risk and options. Environ Int 2011; 37:314–321. [DOI] [PubMed] [Google Scholar]


