Abstract
Perlecan/HSPG2, a large heparan sulfate proteoglycan (HSPG), is indispensable for development of musculoskeletal tissues, where it is deposited within the pericellular matrix (PCM) surrounding chondrocytes and disappears nearly completely at the chondro-osseous junction (COJ) of developing long bones. Destruction of perlecan at the COJ converts an avascular cartilage compartment into one that permits blood vessel infiltration and osteogenesis. Mutations in perlecan are associated with chondrodysplasia with widespread musculoskeletal and joint defects. This work elucidated novel signaling roles of perlecan core protein in endochondral bone formation and chondrocyte behavior. Perlecan subdomains were tested for chondrogenic properties in ATDC5 cells, a model for early chondrogenesis. A region within domain IV of perlecan (HSPG2 IV-3) was found to promote rapid pre-chondrocyte clustering. Introduction of the mutation (R3452Q) associated with the human skeletal disorder Schwartz-Jampel Syndrome (SJS) limited HSPG2 IV-3-induced clustering. HSPG2 IV-3 activity was enhanced when thermally unfolded, likely because of increased exposure of the active motif(s). HSPG2 IV-3-induced clustering was accompanied by deactivation of key components of the focal adhesion complex, FAK and Src, with increased mRNA levels of pre-cartilage condensation markers Sox9 and N-cadherin (Cdh2), and cartilage PCM components collagen II (Col2a1) and aggrecan (Acan). HSPG2 IV-3 reduced signaling through the ERK pathway, where loss of ERK1/2 phosphorylation coincided with reduced FoxM1 protein levels and increased mRNA levels cyclin-dependent kinase inhibitor 1C (Cdkn1c) and activating transcription factor 3 (Atf3), reducing cell proliferation. These findings point to a critical role for perlecan domain IV in cartilage development through triggering chondrocyte condensation.
Keywords: perlecan, heparan sulfate, cartilage, Schwartz-Jampel Syndrome, chondrogenesis
Introduction
The heparan sulfate proteoglycan (HSPG)/HSPG2 perlecan plays a key role in endochondral bone formation. Reduced perlecan secretion, as seen in the human skeletal disorders Schwartz-Jampel syndrome (SJS, OMIM #255800) and dyssegmental dysplasias, Silverman-Handmaker (DDSH, OMIM #224410), leads to widespread skeletal defects with major failure at the epiphyseal plates (Arikawa-Hirasawa et al., 2002; Arikawa-Hirasawa, Watanabe, Takami, Hassell, & Yamada, 1999). During embryogenesis, perlecan deposition is diffuse throughout the mesenchyme, but becomes prominent in cartilage primordia, particularly at sites of endochondral ossification (French et al., 1999; Handler, Yurchenco, & Iozzo, 1997). As bone is shaped, perlecan concentrates at the epiphyseal cartilage ends (Handler et al., 1997). The highest levels of perlecan protein are at the leading edge of cartilage-bone conversion, i.e., the hypertrophic zone of the growth plate leading to the COJ (Brown et al., 2008).
The perlecan protein core consists of five individual structural domains, referred to as domains I-V, and contains both N-terminal and C-terminal glycosaminoglycan (GAG) attachment sites. Chondrogenic activity has been linked in part to the GAG chains in domain I, through modulation of growth factor signaling (French et al., 2002). The complete functions of the perlecan core protein remain to be discovered, although it clearly originated as a barrier molecule in tissues, i.e. in basement membranes, synovium, and has an ancient key role in establishing tissue layers (Farach-Carson, Warren, Harrington, & Carson, 2014; Warren et al., 2015). At the epiphyseal plate, loss of perlecan disrupts the organization of the columnar structure of the hypertrophic chondrocytes (Costell et al., 1999). The core protein links together the ECM network by binding to components such as collagen and laminin, and connects cells to their substratum through integrins (Behrens et al., 2012; Hayashi, Madri, & Yurchenco, 1992). Perlecan’s influence on cell migration is a mechanism by which it maintains growth plate organization (Nakamura, Nakamura, & Fukunaga, 2015). The perlecan C-terminal domain-derived fragment, endorepellin, and its α2β1 integrin interaction disrupt actin stress fibers and focal adhesion complexes, which then inhibit endothelial cell migration with an anti-angiogenic effect (Bix et al., 2004). Domain IV, the largest of the five domains, makes up half the protein core mass and has gained increasing interest for its role in regulating cell adhesion dynamics. Our laboratory identified a peptide sequence within domain IV that promotes cell adhesion, spreading, and cell differentiation (Farach-Carson, Brown, Lynam, Safran, & Carson, 2008). More recently, we discovered that a region within domain IV supports cell-cell adhesion and prevents cell migration (Grindel et al., 2014). Domain IV houses most of the mutations identified in SJS patients (Stum et al., 2006), raising questions about its functional involvement in SJS.
Pre-cartilage mesenchyme condensation and subsequent chondrocyte differentiation are prerequisites for skeletal formation; however, the mechanisms controlling these actions remain unclear. Bone morphogenetic proteins (BMPs), transforming growth factor β (TGFβ), and Sox9 are key players in chondrogenesis, although equally important is the local deposition of territorial matrix. Prechondrogenic mesenchymal cells form contacts with matrix that drive key signaling cascades to initiate cytoskeleton reorganization, cell migration, cell-cell adhesion, and cell condensation. These contacts typically are formed by cell surface integrins, α1β1 and α5β1 that serve as key receptors expressed during mesenchyme condensation and differentiation (Goessler et al., 2009). Integrin engagement activates focal adhesion kinase (FAK), important for pre-cartilage condensation and cartilage nodule formation (Gemba, Valbracht, Alsalameh, & Lotz, 2002). FAK then activates downstream signaling cascades including the mitogen-activated protein kinase (MAPK) pathway (Gemba et al., 2002). ERK1/2 is inactive during the prechondrogenic condensation in developing chick limb (Corson, Yamanaka, Lai, & Rossant, 2003), suggesting that MAPK/ERK signaling controls mesenchymal cell proliferation at the distal limb region. An inhibitor targeted to upstream activator MEK1/2 suppressed limb outgrowth (Eblaghie et al., 2003). Inhibition of MEK/ERK decreased expression of cell adhesion molecules (N-cadherin, fibronectin, and α5β1 integrin) important for mesenchyme condensation (Chang et al., 1998; Oh et al., 2000). Fibronectin is upregulated during chondrogenesis and is a key activator of MAPK/ERK signaling (Thant et al., 2000) during chondrogenesis, a highly dynamic process that requires both spatial and temporal regulation. Thus, uncovering novel mechanisms that control MAPK/ERK signaling are of high interest.
The mechanism by which perlecan core protein affects prechondrogenic cell condensation and subsequent differentiation is unknown. The work described here explored the chondrogenic potential of the perlecan core protein, with a focus on the function of tandem Immunoglobulin (Ig)-module comprised domain IV. Surprisingly, despite accounting for almost half of the molecule, this region remains poorly studied. This work also addressed the consequence of a common point mutation seen in Schwartz-Jampel Syndrome (SJS) that occurs in this third subdomain of domain IV (HSPG2 IV-3) with the goal of better explaining the role of this domain IV mutation and chondrodysplasia.
Results
HSPG2 IV-3 promotes cell clustering.
To examine the chondrogenic activity of the perlecan domain IV core protein, recombinant perlecan subdomains were tested using a murine chondrogenic cell line, ATDC5. Figure 1A shows a schematic diagram illustrating the location along the perlecan core protein from which the subdomains were created, as well as the site of the introduced SJS mutation in domain IV. Bioactivity was assessed immediately after cells were seeded on each substrate to identify those having the strongest and most immediate impact, with the aim of further investigating the immediate chondrogenic potential of such candidate(s). Perlecan clustering activity was determined by changes in cell morphology and ability to trigger cell-cell association. Shown below the perlecan schematic are images captured 1hr after cell seeding on perlecan or various perlecan constructs (figure 1B-E) where figure 1B shows a representative image of ATDC5 cells as a control grown as a monolayer on uncoated tissue culture plates. Full-length perlecan with intact GAGs was used as a positive control, considering its known ability to promote clustering (figure 1C). As expected, full-length perlecan with GAGs prevented early cell attachment and stimulated cell rounding and clustering. Subdomain HSPG2 IV-3 had a strong effect, producing large cell clusters resembling nodules seen in in vitro precartilage mesenchyme condensation models (figure 1D). This response was not observed with R3452Q (figure 1E). Coating with BSA, PLN IV-1, IV-2, or full-length perlecan core only (H/C digested) coated plates did not support a clustering response and cells grown on these surfaces appeared like those in figure 1B (not shown).
Figure 1. HSPG2 IV-3 initiates ATDC5 cell clustering resembling cartilage nodule formation.
Bioactivity of each subdomain was accessed in ATDC5 cells. (A) A schematic representation of the domain structure of perlecan and purified recombinant subdomains used in these studies are shown in the top panel. Domain IV was produced as three separate subdomains (i.e. IV-1, IV-2 and IV-3). A R3452Q mutation is located within the 19th Ig module of domain IV, as indicated by the arrow. (B). Representative images of ATDC5 cells cultured for 1 hr on various perlecan substrates are provided in the bottom panel (B-E). ATDC5 cell spreading occurred on the control uncoated plates. The following substrates produced a similar response to control uncoated, H/C digested intact perlecan, H/C enzyme control mix, BSA, and subdomains IV-1 and IV-2. (C) Full-length perlecan with intact GAG side chains, and (D) HSPG2 IV-3, reduced cell attachment and spreading, and promoted cell-cell adhesion. Cell-clustering was most noticeable in cells grown on HSPG2 IV-3. (E) The R3452Q mutation appears to only slightly reduce cell dispersion. Magnification of insets is shown in the bottom right corner of each image. Scale bars indicate 200 μm.
R3452Q SJS mutation diminishes cell cluster formation.
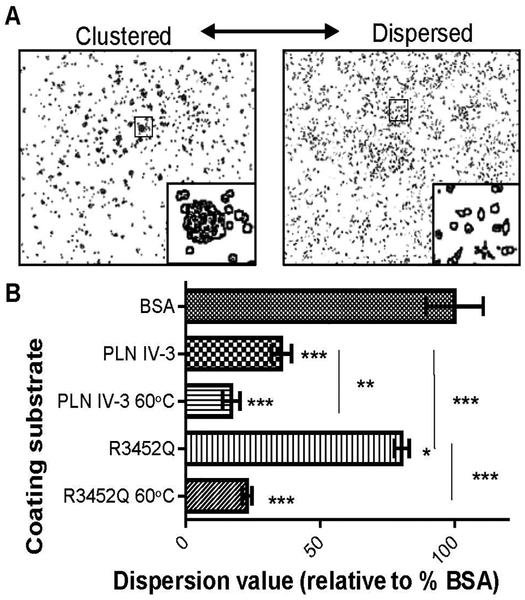
As a measure of subdomain activity, ATDC5 cell dispersion (spreading) versus clustering was quantified. Representative images for clustered and dispersed cells are shown in figure 2A. Data is normalized to percent dispersion relative to BSA-coated plates (top bar of figure 2B) and expressed as a dispersion value relative to control BSA-coated plates. HSPG2 IV-3 induced strong cell clustering, represented by low dispersion value of 35%. The introduction of the R3452Q mutation near fully reversed this effect, although, there remained a 20% reduction in cell spreading when compared to the BSA control. HSPG2 IV-3 and R3452Q were examined by far-UV CD for dissimilarities in their structural properties. However, the proteins had similar CD profiles (supplemental figure 1A and B). Furthermore, the thermal unfolding curves revealed similar melting temperatures, indicating there were no major structural changes between the two (supplemental figure 2). An intermediate conformation was observed when both proteins were heated to 60°C (indicated by black arrow in supplemental figure 1A and B). To test the activity of the intermediate conformation, as observed by CD, proteins were denatured by heating protein solutions to 60°C for 10 mins prior to coating tissue culture plates. Interestingly, denatured protein substrates (HSPG2 IV-3 60°C and R3452Q 60°C) induced a greater clustering effect than their native counterparts (figure 2B). The HSPG2 IV-3 60°C substrate decreased dispersion to 17% and the unfolded R3452Q reduced dispersion to 22% of control.
Figure 2. Thermal unfolding HSPG2 IV-3 enhances cell cluster activity{ XE “Figure 3.7 ATDC5 cell clustering affected by altered perlecan domain IV-3 substrates” }.
Quantification of ATDC5 cell dispersion on various HSPG2 IV-3 substrates. (A) Representative images used for analysis are show above. (B) Values are reported as a percentage of the total dispersion of cells cultured on BSA-coated plates. Cell dispersion was reduced on all tested substrates but to a lesser extent when cells were seeded on R3452Q. Cell clustering was highest on unfolded protein substrates (60°C), compared to their native form. Asterisks directly above SEM errors bars indicate the statistical significance relative to BSA control. All shown conditions were significantly different from the BSA control. Vertical lines represent the statistical comparison of two conditions (endpoints of each the line) with respective P-values shown on the right. P-value, *< 0.05, ***p< 0.001, ***<0.001.
HSPG2 IV-3 increased the steady state RNA levels of gene products associated with mesenchyme condensation and cartilage formation.
To assess the chondrogenic potential of HSPG2 IV-3, ATDC5 cells were subjected to a micromass culture system. ATDC5 cells, seeded at high density, formed large nodules resembling in vitro chondrogenesis. Representative images are provided in supplemental figure 3. The mRNA levels of precartilage condensation markers Sox9 (figure 3A) and Cdh2 (figure 3B), as well as the cartilage matrix components Col2a1 (figure 3C) and Acan (figure 3D) were examined in HSPG2 IV-3 induced cell clusters by QPCR. Relative mRNA levels were measured on 1 and 2 days post ATDC5 seeding. mRNA levels for all four major chondrogenic markers were elevated in cell cultures grown on HSGP2 IV-3 by day 1, relative to control uncoated conditions. By day 2, IV-3 induced mRNA levels had begun to subside, although they remained significantly different from those of control cells. Cell cluster formation was strongest on day 1 (Supplemental figure 3D). By day 2, the non-cluster forming cell population had expanded and filled the remainder of the cell culture plate. As such, the differential expression of mRNA markers was most noticeable on day 1 when the ATDC5 cell response was highest.
Figure 3. HSPG2 IV-3 increases mRNA expression of chondrogenic differentiation markers and cartilage-specific matrix molecules{ XE "Figure 3’14 PLN IV-3 controls expression of early cartilage-associated genes sox9 and cdh2" }.
mRNA levels for Sox9 (A), Cdh2 (B), Col2a1 (C) and Acan (D) were determined for ATDC5 cells cultured on HSPG2 IV-3 for one (D1) and two (D2) days. Data is normalized to Ppai and represented as a fold change relative to cells cultured on uncoated plates. P-value, *<0.05; ***<0.001.
HSPG2 IV-3 interferes with cell-substrate attachment by suppressing FAK/Src activity.
Cell spreading/clustering follows focal adhesion dynamics. Results from a cell attachment assay indicate HSPG2 IV-3 cell cluster formation begins with the disruption of cell-substrate attachment (figure 4A). Western blot analysis using phospho-specific antibodies targeted to active FAK (Tyr 397) or Src (Tyr 416) are shown in figure 4B and C, respectively, with representative blots shown below. Reduced phospho-FAK (pFAK) phospho-Src (pSrc) levels demonstrate HSPG2 IV-3’s ability to interfere with focal adhesion dynamics. Although the graphs represent active/total protein, in general, lower total FAK/Src protein levels were observed in IV-3 grown cultures.
Figure 4. IV-3 inhibits cell attachments and suppresses FAK/Src activation.
(A) Loss of cell-adhesion correlates with reduced activation of FAK (B) and Src (C). For Western blot analysis, cell lysates were collected at 24 hours post seeding. Graphs B and C show the results of densitometry quantification of active phosphorylated FAK and Src normalized with respect to their total protein levels. Representative Western blots are shown below each graph for three independent cell cultures. Average band intensities for each experimental condition are represented in the graphs above. P-value, *<0.05, **<0.01.
HSPG2 IV-3 cell clustering interferes with ERK signaling.
FAK-Src signaling through ERK plays a role in the regulation of the cell adhesion and spreading (Brown et al., 2008). For this reason, HSPG2 IV-3’s ability to alter activity of ERK, indicated by loss of phospho-ERK1/2 (pERK1/2; Thr202/Tyr204) antibody detection (figure 5A). HSPG2 IV-3 suppressed pERK1/2 by over 50%. To further investigate perlecan-mediated FAK-Src/ERK signaling, downstream events associated with kinase inhibition were examined. As a substrate, HSPG2 IV-3 reduced ATDC5 production of total FoxM1 protein by almost 50% (figure 5B). The transcription factor FoxM1 is a well-known controller of genes involved in cell cycle progression (X. Chen et al., 2013), although this is the first report of its involvement in chondrogenesis.
Figure 5. HSPG2 IV-3 inhibits ERK signaling{ XE "Figure 3’10 PLN IV-3 suppresses ERK1/2 activation” }.
Western blots analysis of activated ERK signaling and its downstream target Foxm1 in ATDC5 cells at 24 hrs. (A) ERK and pERK antibodies on ATDC5 cell lysate from cultures grown on uncoated and HSPG2 IV-3 coated plates. The graph shows results from densitometry analysis with pERK antibody normalized by total ERK. (B) FoxM1 protein levels were compared in ATDC5 cells seeded either on uncoated tissue culture plates or on a HSPG2 IV-3-coated substrate. The graph shows results from densitometry analysis with FoxM1 antibody normalized to GAPDH. Representative Western blots are shown below each graph. Cell lysates were collected at 24 hr. P-value, **<0.01, ***< 0.005.
HSPG2 IV-3 decreases cell proliferation.
QPCR was performed to analyze cell cycle inhibitors cyclin-dependent kinase inhibitor 1C (Cdkn1c) and activating transcription factor 3 (Atf3) in response to HSPG2 IV-3. Both Cdkn1c and Atf3 RNA levels increased in HSPG2 IV-3 grown cultures over the course of two days (figure 6A and B). As expected, the loss of FoxM1, and increase in Cdkn1c and Atf3 levels, coincided with decreased cell proliferation, measured by MTS assay for metabolic activity (figure 6C). This reduction in cell metabolic activity, coincided with a delay in proteoglycan synthesis, demonstrated by Alcian blue staining (Supplemental figure 4).
Figure 6. HSPG2 IV-3 upregulates mRNA expression of cell cycle inhibitor genes, coinciding with reduced cell proliferation{ XE "Figure 3’12 IV-3 controls expression of Cdkn1c and atf3 mRNA" }.
(A-B) Cdkn1c and atf3 mRNA levels were determined one (D1) and two (D2) days after ATDC5 cells were seeded. mRNA levels were normalized to ppai. Data is represented as a fold change relative to day 1 uncoated. (A) HSPG2 IV-3 increased Cdkn1c expression 2-fold at days 1 and 2. (B) Atf3 expression reached almost 2-fold higher after day 2. Cell activity was compared between ATDC5 cells grown on standard tissue culture plates or on a HSPG2 IV-3-coated substrates using MTS assay. MTS assay was conducted over the course of 48 hr. P-values, *<0.05, **<0.01; ***<0.001.
Discussion
The goals of this study were to identify regions of the perlecan core protein responsible for chondrogenic activity and to elucidate the mechanisms by which they initiate signals that support chondrocyte condensation in developing cartilage. Further, we hypothesized that a mutation in an active domain could account for the dysmorphic phenotype seen in SJS. Previous studies in our lab found that the N-terminal domain I of perlecan, with attached GAG chains has chondrogenic potential (French et al., 2002; French et al., 1999). Knowing that mutations in perlecan far downstream from domain I in domain IV are associated with chondrogenic abnormalities in humans (Arikawa-Hirasawa et al., 2002), we examined these regions of domain IV for chondrogenic activity. Recently, we observed that the most C-terminal subdomain, HSPG2 IV-3, within domain IV of perlecan, induced a cell clustering response that could parallel in vitro precartilage condensation. Clustering activity is unique to HSPG2 IV-3, because no similar activity was observed in subdomains PLN IV-1 or PLN IV-2 despite their similar arrangement of tandem Ig modules (Murdoch, Dodge, Cohen, Tuan, & Iozzo, 1992). Recently, a direct link was established between the perlecan core protein and the chondrogenesis of mesenchymal cells (Sadatsuki et al., 2017), although other studies did not find chondrogenic activity of the perlecan core protein alone (French et al., 2002; French et al., 1999). Notably, several studies showed that the perlecan core protein facilitates cell-substratum attachment rather than cell-cell adhesion (Farach-Carson et al., 2008; SundarRaj, Fite, Ledbetter, Chakravarti, & Hassell, 1995; Whitelock et al., 1999). Thus, the context in which perlecan is presented to cells varies the outcome (Grindel et al., 2014). In a previous study (French et al., 2002), we tested various murine perlecan constructs in an embryonic mesenchymal cell line without finding chondrogenic activity in either half of murine perlecan domain IV, although we note that murine perlecan lacks 7 key Ig modules found in human perlecan (Gomes, Kirn-Safran, Farach-Carson, & Carson, 2002; Noonan et al., 1991).
SJS mutations have been associated with intracellular retention and reduced secretion of perlecan into the territorial matrix, resulting from protein instability, improper folding, or defective trafficking (Iwata et al., 2015; Stum et al., 2006). The R3452Q mutation resides within the HSPG2 IV-3 subdomain and is one of the more common mutations identified across several families with SJS (Stum et al., 2006). Here, we found the R3452Q HSPG2 IV-3 supported cell spreading rather than clustering, despite having similar conformation. In thermal unfolding studies, we discovered that at 60˚C both control and mutated subdomains formed partially unfolded intermediates, and that use of heat treated constructs increased the activity of both HSPG2 IV-3 and R3452Q. We thus suspect the R3452Q mutant must have misfolded or aggregated, and by doing so masked the bioactive region. Upon further investigation of this IV-3 region and by using in silico sequence analysis and homology modeling, we determined this site doesn’t appear to impact GAG binding given there is no conserved heparin-binding motif in close proximity.
Consistent with previous findings, our data supports a role for perlecan in chondrogenesis through its regulation of the transcription factor Sox9 (Sadatsuki et al., 2017). HSPG2 IV-3-induced clustering coincided with elevated mRNA levels of Sox9, as seen during chondrogenesis in micromass cultures in vitro and in mesenchyme condensation during limb bud formation in vivo (Akiyama, Chaboissier, Martin, Schedl, & de Crombrugghe, 2002). Our cell clusters were further marked by increased mRNA levels of Cdh2, a downstream target of Sox9 and an adhesion molecule that is required to mediate key cell-cell interactions during mesenchymal cell condensation (DeLise & Tuan, 2002; Panda, Miao, Lefebvre, Hendy, & Goltzman, 2001). Futhermore, HSPG2 IV-3 increased mRNA levels of the chondrogenic markers collagen type II and aggrecan, suggesting perlecan has distinct roles at various stages of the chondrocyte process, i.e., mesenchyme condensation and cell differentiation (Shukunami et al., 1996). In contrast, we observed a reduction in total GAG content in response to HSPG2 IV-3 likely due to an overall decrease in cell metabolic activity. This may account for an overall decrease in cell number and explain why we observed lower protein levels (i.e. total FAK/Src protein) in our Western blot studies. We propose perlecan, through its most C-terminal domain IV region, initiates key signaling events that enhance cartilage development.
The ability to regulate focal adhesion dynamics suggests a key role for perlecan in precartilage mesenchyme condensation. Loss of cell substratum interactions and preference for cell-cell adhesion is correlated with HSPG2 IV-3 inhibition of FAK and Src activity (Webb et al., 2004). Interestingly, the inhibition of Src using the pharmacological compound PP2 promoted the chondrocyte phenotype (Bursell et al., 2007). Blocking phosphorylation of FAK (Tyr 397) and Src (Tyr 416) also disrupted chondrocyte cell-matrix interactions and prevented cell death from mechanical load (Jang, Buckwalter, & Martin, 2014).
Multiple signaling pathways affected by the HSPG2 IV-3 appear to disrupt focal adhesions, via the FAK/Src complex (Mitra, Hanson, & Schlaepfer, 2005), which supports the cooperative roles of FAK and MAPK in chondrocyte and bone biology. In vivo studies have shown ERK signaling regulates chondrocyte differentiation during endochondral bone formation (Z. Chen, Yue, Zhou, Greenfield, & Murakami, 2015). Interestingly, fibronectin, another major ECM component of developing cartilage, activates human articular chondrocytes through FAK and ERK (Gemba et al., 2002). Our results indicate an opposing role of HSPG2 IV-3, which inactivates ERK signaling, and may prevent chondrocyte progression to fibrocartilage. This antagonism is common in ECM molecules and is a tactic by which the ECM maintains dynamic control of fundamental cellular processes. Perlecan’s ability to affect MAPK signaling is relevant to processes involved in maintaining mature healthy cartilage. In early-stage osteoarthritis, the FAK/MAPK pathway protects cartilage from damage (Xia et al., 2015). Interestingly perlecan expression is elevated in osteoarthritis patients (Tesche & Miosge, 2004), where it has been proposed that chondrocytes increase matrix synthesis in an attempt to preserve their native avascular environment. Loss of perlecan and perlecan-associated signaling, such as occurs at the COJ in developing cartilage or in advanced osteoarthritis, may allow vascular infiltration of bone at the expense of healthy cartilage.
HSPG2 IV-3 inhibition of ERK1/2 and its negative regulation of cell proliferation led us to investigate downstream events associated with ERK signaling. We showed that the widely used prechondrocyte cell model ATDC5 expresses the transcription factor FoxM1, a well-known target of ERK and a marker for proliferating cells in developing tissues such as cartilage (Kalin, Ustiyan, & Kalinichenko, 2011). FoxM1 protein levels decreased in ATDC5 cells grown on HSPG2 IV-3-coated substrates, coinciding with the loss of ERK activity and cell dispersion. FoxM1 is found in the cytoplasm during late-G1 and S phases, but prior to entry into the G2/M phase it is activated through the MAPK cascade and becomes translocated to the nucleus (Ma et al., 2005).
Interestingly, FoxM1 expression correlates inversely with cell cycle inhibitors, including p57/Cdkn1c (Calderon et al., 2015). HSPG2-induced cell cluster formation coincided with increased transcriptional output of Cdkn1c and Atf3. Atf3, a member of the CREB protein family of transcription factors (Bursell et al., 2007), facilitates chondrocytes from exiting the cell cycle and terminally differentiating (James, Woods, Underhill, & Beier, 2006). As expected, HSPG2 led to an increase in Cdkn1c and Atf3 mRNA levels, and reduced cell proliferation. It thus seems that a key function of HSPG2 IV-3 region is to stabilize a strongly clustered, cartilage phenotype by preventing focal adhesion formation and downstream signals associated with growth, migration, or conversion to fibrocartilage or bone. Loss of such key functions may be associated with the malformations seen in SJS. Because unheated R3452Q HSPG2 IV-3 fails to induce robust clustering, we predict that this mutation attenuates the downstream signaling associated with perlecan’s promotion of chondrogenesis allowing for FAK, Src, and ERK to remain active, and markers of fibrocartilage rather than cartilage to be expressed. The observation that heating R3452Q HSPG2 IV-3 reversed this and restored pro-chondrogenic activity suggests that the defect lies in the effect of the mutation on folding rather than a loss of a key epitope needed for protein-receptor interactions.
Summary and Conclusions
This study identified a novel active chondrogenic region within domain IV of the perlecan core protein with direct relevance to cartilage development and dysplasia. We show that HSPG2 IV-3 induced cell-cell interactions reminiscent of mesenchyme stem cell condensation and elevated major markers associated with chondrocyte differentiation. Studies are ongoing to identify HSPG2/perlecan-cell surface interactions that support chondrogenesis. These study results clearly established a connection between the HSPG2 IV-3 cell cluster phenotype and loss of ERK signaling, a conventional pathway implicated in balancing cartilage and bone formation. Introduction of a mutation associated with SJS abrogated the clustering activity of HSPG2 IV-3, which may partially explain the cartilage dysmorphisms seen in patients with this mutation. Together, these results point to a key role for the perlecan core protein in chondrocyte condensation in the developing growth plate cartilage, which is disrupted in certain chondrodysplasias.
Experimental Procedures
Materials.
Supplemental table 1 list all the antibodies used for this study. Heparitinase I, II, III and chondroitinase ABC (H/C) were purchased from Sigma-Aldrich (St. Louis, MO). All other materials used were reagent grade or better.
Purification of perlecan and domain IV subdomains.
Full-length perlecan was purified from HT-29 cells, formerly called WiDR, as previously described (Grindel et al., 2014; Wijeratne et al., 2016). Where appropriate, GAG side chains were removed from full-length perlecan enzymatically using a combination of heparitinase I, II, III and chondroitinase ABC (H/C). H/C digested perlecan samples were passaged through an immunoaffinity column immobilized with perlecan domain V A74 antibody following established protocols (Whitelock et al., 1999). Perlecan domain IV, amino acids (aa) 1677–3662, was produced as three separate recombinant subdomains as previously described (Grindel et al., 2014). PLN IV-1 (aa 1677–2338), PLN IV-2 (aa 2338–3010) and HSPG2 IV-3 (aa 3011–3662) each contained 7 Ig repeats. In addition, a mutant HSPG2 IV-3 mutant subdomain associated with SJS was created by replacing the Arg at residue 3452 with a Gln (R3452Q) using site directed mutagenesis (QuikChange II XL kit, Agilent Technologies, Santa Clara, CA).
Cell culture.
ATDC5 cells, a murine teratocarcinoma-derived pre-chondrogenic cell line, were cultured as previously described (Brown et al., 2008). Cells were cultured in Dulbecco’s Modified Eagle’s Medium-Ham’s F12 (DMEM-F12; Thermo Fisher Scientific), with 10% (v/v) heat inactivated fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), and 100 U/ml penicillin/streptomycin Thermo Fisher Scientific). Cells were incubated at 37°C in a humidified 5% (v/v) CO2 atmosphere.
Coating substrates with perlecan and perlecan subdomains.
Tissue culture plates were coated with the following proteins: full-length perlecan, H/C digested perlecan, PLN IV-1, IV-2, IV-3 or R3452Q. Uncoated, bovine serum albumin (BSA)-coated, and H/C only served as controls. Tissue culture plates, 96-well (0.32 cm2) and 24 well (1.9 cm2) (Corning), were coated with 1 or 5 μg of protein diluted into 100 μl and 250 μl of DPBS, pH 7.4 (Thermo Fisher Scientific), respectively. Plates were incubated overnight at 37°C to adsorb proteins to the plate surface. For experiments that required preheating, undiluted protein solutions were heated to 60°C for 10 mins prior to being diluted in PBS.
Assessing the bioactivity of perlecan subdomains.
ATDC5 cells were seeded into various substrate-coated wells (23,500 cells/cm2 in 96-well plates) for analysis. Initial cell attachment and spreading were monitored 1 hr after seeding and images were captured with a Nikon (Melville, NY) inverted microscope in bright field mode with a 10X objective.
ATDC5 cell dispersion.
Quantification of ATDC5 cell spreading was performed as previously described in 96-well plates (Grindel et al., 2014). ATDC5 cells (23,500 cells/cm2) were seeded onto various substrates and cultured for 24 hrs imaged at 4X objective in bright field mode. Using ImageJ software (https://imagej.nih.gov/ij/) batch images were quantified for particle count, particle size, and area fraction. Cell dispersion values are equal to (particle count)/(particle size)*(area fraction). A higher value is more dispersed and a lower value is more clustered.
ATDC5 nodule formation.
To enhance the ATDC5-HSPG2 IV-3 response, we adapted our in vitro system to a large plate format with higher cell density. Cells were seeded (131,600 cells/cm2) into 24-well plates coated with 5 μg of protein. To maximize HSPG2 IV-3 clustering activity, we adopted the 60°C pretreatment as our standard for PLN-IV3 studies. As a control, ATDC5 cells were grown on uncoated plates where they remained in a monolayer and evenly dispersed throughout the plate. High density cultures were subjected to quantitative PCR and Western blot analysis.
RNA isolation and quantitative PCR.
RNA was isolated 24 and 48 hrs after seeding cells on various substrates. RNA was extracted using 1 ml TRIzol reagent (Thermo Fisher Scientific) and suspended in 50 μl nuclease-free water. DNA-free kit (Thermo Fisher Scientific) was used to degrade remaining genomic DNA in accordance with manufacturers’ directions. One μg of RNA was reverse-transcribed using cDNA Supermix (Quanta Biosciences, Gaithersburg, MD) as per the manufacturer’s protocol and amplified using a CFX96 Real Time System (BioRad). For quantitative PCR (QPCR), B-R SYBR Green Supermix for IQ (Quanta Biosciences) was used. The thermal cycling program was as follows: 95°C for 3 min, then 40 cycles of the following two steps: 95°C for 30 sec and 60°C for 15 sec. All samples were analyzed as technical triplicates. Primer sets used for qRT-PCR are listed in supplemental table 2. Reactions were normalized to Ppia as validated by (Zhai, Yao, & Wang, 2013) and relative mRNA levels were calculated using the 2−ΔΔCT method.
Western blot analysis.
Western blot analyses were performed as described (Grindel et al., 2014). After culture for 24 hr on various substrates, cell lysate was collected with RIPA buffer containing 150 mM NaCl, 1.0% (v/v) Triton-X-100, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) sodium dodecyl sulfate (SDS), 10% (v/v) glycerol, 1mM ethylenediaminetetraacetic acid (EDTA), 1mM ethylene glycol tetraacetic acid (EGTA), 50 mM Tris, pH 8.0, protease inhibitor cocktail mix (EMD Millipore) at 1:100 dilution and phosphatase inhibitor cocktail (EMD Millipore) mix at 1:100 dilution. Protein extract was denatured at 99°°C with Pierce™ lane marker reducing sample buffer (Thermo Fisher Scientific) supplemented with 2% (v/v) 2-mercaptoethanol, then separated by SDS-polyacrylamide gel electrophoresis (PAGE) as previously described (Grindel et al., 2014). GADPH was used at concentration of 1:5,000 and all other antibodies were used at 1:1,000. The secondary antibodies goat anti-rabbit or sheep anti-mouse HRP conjugated antibody (Jackson) were used at 1:50,000. Blots were exposed to X-ray film, and signal intensities were quantitated using ImageJ software. Pixel optical densitometric values were obtained for each protein band signal and normalized to respective loading control.
Cell proliferation assay.
ATDC5 cells were seeded at a density of 23,500 cells/cm2 in 96-well plates uncoated or coated with HSPG2 IV-3 for assay of proliferative activity with the use of the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega; Madison, WI). Cell metabolic activity was quantified by measuring the conversion of MTS to formazan according to the manufacturer’s instructions. MTS solution was added to each well for 1 hr, and light absorbance at 490 nm was detected by Tecan M1000 plate reader (Männedorf, Switzerland).
Cell attachment assay.
Cell adhesion assays were performed in 96-well plates in serum free conditions. Cells (23,500 cells/cm2) adhered to various substrates for 1 hr, after which wells were rinsed with PBS and cells were fixed with ice-cold 100% (v/v) methanol at −20°C. After fixation, wells were rinsed with PBS and a single bright field image was taken at the center of the well using a taken at 4X objective. The total cells in each image were counted using the ImageJ particle count function software. The software considers one cell equal to one particle (NIH, Bethesda, MD). The results were normalized such that the average number of cells bound to uncoated control plates equaled 100% attachment. Data is represented as a percentage of the total number of cells that attached to control plates
Alcian blue stain.
ATDC5 cells (131,600 cells/cm2 in 24-well plates) were seeded on normal or HSPG2 IV-3 coated plates and cultured for 14 days with media replaced every 2–3 days. ECM production, a measurement of chondrogenic activity, was evaluated by staining cells with Alcian blue, a stain for sulfated and carboxylated acid mucopolysaccharides, and sulfated and carboxylated glycoproteins. Cells were fixed in 4% (w/v) paraformaldehyde at room temperature for 5–10 minutes and stained with 1% (w/v) Alcian blue (Sigma-Aldrich) in 3% acetic acid (v/v), pH 2.5 M overnight. The following day, cells were washed in dH2O. Alcian blue-stained cell layers were extracted (from the wells) with 6 M guanidine-HCl for 6 hr at room temperature and the OD was determined at 630 nm by spectrophotometry. A total of three biological replicates were performed.
Circular dichroism.
Circular dichroic spectra were obtained as described (Costell, Mann, Yamada, & Timpl, 1997). Data was recorded with a Jasco J-815 spectropolarimeter (Easton, MD) using the SpectraManager software provided by manufacturer. Measurements were recorded in the far-UV region using a quartz cell with an optical path length of 1 mm. Temperature interval scan mode was used to profile entire spectra from 20–90°C. Thermal unfolding studies were performed using variable temperature mode at a wavelength of 202 nm. To minimized insoluble protein aggregates samples were diluted to 0.2 mg/ml (2.67 μM) in Milli-Q water. Data was processed with the Jasco spectra analysis software. Secondary structural were predicted using the on-line analysis software DichroWeb (Whitmore & Wallace, 2004). CD data represents the average value of three separate recordings.
Statistical Analyses.
For cell the cluster and dispersion assay, MTS assay, Alcian blue staining, QPCR and Western blot analyses, an unpaired student’s t-test was utilized to determine statistical significances. All experiments were performed in triplicate and repeated at least three times with biological replicates, and the means and standard error of the mean (SEM) were plotted using the statistical software GraphPad Prism 5.
Supplementary Material
Supplemental Figure 1. Far UV CD structural analysis of HSPG2 IV-3 and SJS mutant R3452Q{ XE "Figure 3.4. Far UV CD structural analysis of Dm IV-3 and SJS mutant R3452Q" }. CD spectra are plotted as wavelength versus mean residue ellipticity values. CD spectra revealed both (A) HSPG2 IV-3 and (B) R3452Q have a typically beta-sandwich-like conformation with a maximum at 202 nm and minimum at 217 nm when kept at ambient temperatures. Structural prediction software (DichroWeb) indicated the two proteins are composed of approximately 50% beta strands. HSPG2 IV-3 (A) and R3452Q (B) spectra were analyzed over a temperature range of 20 to 90?C, with measurements recorded every 10?C. HSPG2 IV-3 (A) and R3452Q mutant version (B) displayed similar changes in their spectra with increasing temperature. Both proteins partially unfolded at 60?C (black arrows) and completely lost their secondary structures at 70?C. Each data point represents an average of three separate experiments.
Supplemental Figure 2. Thermal denaturation of HSPG2 IV-3 and R3452Q monitored by CD spectroscopy{ XE "Figure 3.5 Thermal denaturation of PLN IV-3 and R3452Q monitored by CD spectroscopy" }. Protein stability was monitored by absorbance at 202 nm, corresponding to the maximum peak in figure supplemental figure 1. Data are normalized to show the folded fraction as a function of temperature for HSPG2 IV-3 (black points) and R3452Q (unfilled). CD ellipticity values are normalized to fraction folded, ranging from 1 to 0, relative to the low temperature value. Melting temperatures (Tm) were determined by fitting the curves to a Boltzmann sigmoidal equations (HSPG2 IV-3, Tm = 63.72 °C; R3452Q, Tm = 62.39 °C). Fitted curves are shown as black (HSPG2 IV-3) and dashed (R3452Q) lines.
Supplemental Figure 3. ATDC5 cells, in a high density culture, form clusters resembling cartilage nodules after 24 hr of being seeded on a purified HSPG2 IV-3 substrate{ XE "Figure 3.8 ATDC5 cells, in a high density culture, aggregate to from clusters resembling cartilage nodules after 24 hr of being seeded on a purified PLN IV-3 substrate" }. ATDC5 cells were cultured on normal tissue culture plates (A and C) or HSPG2 IV-3 coated substrate (B and D) for up to 48 hrs. ATDC5 cells spread and grew in a monolayer on uncoated plates, while those grown on a HSPG2 IV-3 substrate formed large tight clusters. Representative images were captured at 24 and 48 hrs using a 4X objective. Although not present the first day, clusters continued to develop later in regions of high cell density, as indicated by the arrow in panel D. Scale bar represents 500 μm.
Supplemental Figure 4. HSPG2 IV-3 decreases ATDC5 production of GAGs{ XE "Figure 3'11 IV-3 reduced Foxm1 protein levels and decreased cell proliferation" }. (B) ATDC5 cells were grown for 14 days either on uncoated or HSPG2 IV-3-coated plates. Alcian blue staining was used to assess GAG production during this time. Alcian blue stain was extracted and quantified by measuring the absorbance at 630 nm. P-value, *< 0.05; **< 0.005, ***<0.0001.
Acknowledgements
The authors thank Drs. Liyun Wang, Daniel Harrington and William R. Thompson for many helpful discussions. The authors benefitted greatly from the input from the Daniel D. Carson and Farach-Carson lab members. This work was supported by grants from the National Institutes of Health (R01AR054385 and P01CA098912) and the Rice University Alliances for Graduate Education and the Professoriate (0450363 to JRM). Additional funding was awarded to KMH (F31DE025179).
Abbreviations:
- COJ
chondro-osseous junction
- HSPG2
heparan sulfate proteoglycan 2
- Ig
Immunoglobulin
- PCM
pericellular matrix
- PLN IV
perlecan domain IV
- SJS
Schwartz-Jampel Syndrome
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
References
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, & de Crombrugghe B (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev, 16(21), 2813–2828. doi: 10.1101/gad.1017802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Le AH, Nishino I, Nonaka I, Ho NC, Francomano CA, . . . Yamada Y (2002). Structural and functional mutations of the perlecan gene cause Schwartz-Jampel syndrome, with myotonic myopathy and chondrodysplasia. Am J Hum Genet, 70(5), 1368–1375. doi: 10.1086/340390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, & Yamada Y (1999). Perlecan is essential for cartilage and cephalic development. Nat Genet, 23(3), 354–358. doi: 10.1038/15537 [DOI] [PubMed] [Google Scholar]
- Behrens DT, Villone D, Koch M, Brunner G, Sorokin L, Robenek H, . . . Hansen U (2012). The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J Biol Chem, 287(22), 18700–18709. doi: 10.1074/jbc.M111.336073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, . . . Iozzo RV (2004). Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through alpha2beta1 integrin. J Cell Biol, 166(1), 97–109. doi: 10.1083/jcb.200401150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Alicknavitch M, D’Souza SS, Daikoku T, Kirn-Safran CB, Marchetti D, . . . Farach-Carson MC (2008). Heparanase expression and activity influences chondrogenic and osteogenic processes during endochondral bone formation. Bone, 43(4), 689–699. doi: 10.1016/j.bone.2008.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursell L, Woods A, James CG, Pala D, Leask A, & Beier F (2007). Src kinase inhibition promotes the chondrocyte phenotype. Arthritis Res Ther, 9(5), R105. doi: 10.1186/ar2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon MJ, Ploegman AG, Bailey B, Jung DO, Navratil AM, & Ellsworth BS (2015). Loss of Foxm1 Results in Reduced Somatotrope Cell Number during Mouse Embryogenesis. PLoS One, 10(6), e0128942. doi: 10.1371/journal.pone.0128942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Oh CD, Yang MS, Kang SS, Lee YS, Sonn JK, & Chun JS (1998). Protein kinase C regulates chondrogenesis of mesenchymes via mitogen-activated protein kinase signaling. J Biol Chem, 273(30), 19213–19219. [DOI] [PubMed] [Google Scholar]
- Chen X, Muller GA, Quaas M, Fischer M, Han N, Stutchbury B, . . . Engeland K (2013). The forkhead transcription factor FOXM1 controls cell cycle-dependent gene expression through an atypical chromatin binding mechanism. Mol Cell Biol, 33(2), 227–236. doi: 10.1128/MCB.00881-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yue SX, Zhou G, Greenfield EM, & Murakami S (2015). ERK1 and ERK2 regulate chondrocyte terminal differentiation during endochondral bone formation. J Bone Miner Res, 30(5), 765–774. doi: 10.1002/jbmr.2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson LB, Yamanaka Y, Lai KM, & Rossant J (2003). Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development, 130(19), 4527–4537. doi: 10.1242/dev.00669 [DOI] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, . . . Fassler R (1999). Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol, 147(5), 1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costell M, Mann K, Yamada Y, & Timpl R (1997). Characterization of recombinant perlecan domain I and its substitution by glycosaminoglycans and oligosaccharides. Eur J Biochem, 243(1–2), 115–121. [DOI] [PubMed] [Google Scholar]
- DeLise AM, & Tuan RS (2002). Alterations in the spatiotemporal expression pattern and function of N-cadherin inhibit cellular condensation and chondrogenesis of limb mesenchymal cells in vitro. J Cell Biochem, 87(3), 342–359. doi: 10.1002/jcb.10308 [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Lunn JS, Dickinson RJ, Munsterberg AE, Sanz-Ezquerro JJ, Farrell ER, . . . Tickle C (2003). Negative feedback regulation of FGF signaling levels by Pyst1/MKP3 in chick embryos. Curr Biol, 13(12), 1009–1018. [DOI] [PubMed] [Google Scholar]
- Farach-Carson MC, Brown AJ, Lynam M, Safran JB, & Carson DD (2008). A novel peptide sequence in perlecan domain IV supports cell adhesion, spreading and FAK activation. Matrix Biol, 27(2), 150–160. doi: 10.1016/j.matbio.2007.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farach-Carson MC, Warren CR, Harrington DA, & Carson DD (2014). Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol, 34, 64–79. doi: 10.1016/j.matbio.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French MM, Gomes RR Jr., Timpl R, Hook M, Czymmek K, Farach-Carson MC, & Carson DD (2002). Chondrogenic activity of the heparan sulfate proteoglycan perlecan maps to the N-terminal domain I. J Bone Miner Res, 17(1), 48–55. doi: 10.1359/jbmr.2002.17.1.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French MM, Smith SE, Akanbi K, Sanford T, Hecht J, Farach-Carson MC, & Carson DD (1999). Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro. J Cell Biol, 145(5), 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemba T, Valbracht J, Alsalameh S, & Lotz M (2002). Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J Biol Chem, 277(2), 907–911. doi: 10.1074/jbc.M109690200 [DOI] [PubMed] [Google Scholar]
- Goessler UR, Bugert P, Bieback K, Stern-Straeter J, Bran G, Sadick H, . . . Riedel F (2009). In vitro analysis of integrin expression in stem cells from bone marrow and cord blood during chondrogenic differentiation. J Cell Mol Med, 13(6), 1175–1184. doi: 10.1111/j.1582-4934.2008.00451.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gomes R, Kirn-Safran C, Farach-Carson MC, & Carson DD (2002). Perlecan: an important component of the cartilage pericellular matrix. J Musculoskelet Neuronal Interact, 2(6), 511–516. [PMC free article] [PubMed] [Google Scholar]
- Grindel BJ, Martinez JR, Pennington CL, Muldoon M, Stave J, Chung LW, & Farach-Carson MC (2014). Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol, 36, 64–76. doi: 10.1016/j.matbio.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler M, Yurchenco PD, & Iozzo RV (1997). Developmental expression of perlecan during murine embryogenesis. Dev Dyn, 210(2), 130–145. doi: [DOI] [PubMed] [Google Scholar]
- Hayashi K, Madri JA, & Yurchenco PD (1992). Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: an adhesion modulated by glycosaminoglycan. J Cell Biol, 119(4), 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S, Ito M, Nakata T, Noguchi Y, Okuno T, Ohkawara B, . . . Ohno K (2015). A missense mutation in domain III in HSPG2 in Schwartz-Jampel syndrome compromises secretion of perlecan into the extracellular space. Neuromuscul Disord, 25(8), 667–671. doi: 10.1016/j.nmd.2015.05.002 [DOI] [PubMed] [Google Scholar]
- James CG, Woods A, Underhill TM, & Beier F (2006). The transcription factor ATF3 is upregulated during chondrocyte differentiation and represses cyclin D1 and A gene transcription. BMC Mol Biol, 7, 30. doi: 10.1186/1471-2199-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KW, Buckwalter JA, & Martin JA (2014). Inhibition of cell-matrix adhesions prevents cartilage chondrocyte death following impact injury. J Orthop Res, 32(3), 448–454. doi: 10.1002/jor.22523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin TV, Ustiyan V, & Kalinichenko VV (2011). Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell Cycle, 10(3), 396–405. doi: 10.4161/cc.10.3.14709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma RY, Tong TH, Cheung AM, Tsang AC, Leung WY, & Yao KM (2005). Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J Cell Sci, 118(Pt 4), 795–806. doi: 10.1242/jcs.01657 [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, & Schlaepfer DD (2005). Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol, 6(1), 56–68. doi: 10.1038/nrm1549 [DOI] [PubMed] [Google Scholar]
- Murdoch AD, Dodge GR, Cohen I, Tuan RS, & Iozzo RV (1992). Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Biol Chem, 267(12), 8544–8557. [PubMed] [Google Scholar]
- Nakamura R, Nakamura F, & Fukunaga S (2015). Perlecan Diversely Regulates the Migration and Proliferation of Distinct Cell Types in vitro. Cells Tissues Organs, 200(6), 374–393. doi: 10.1159/000440950 [DOI] [PubMed] [Google Scholar]
- Noonan DM, Fulle A, Valente P, Cai S, Horigan E, Sasaki M, . . . Hassell JR (1991). The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem, 266(34), 22939–22947. [PubMed] [Google Scholar]
- Oh CD, Chang SH, Yoon YM, Lee SJ, Lee YS, Kang SS, & Chun JS (2000). Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem, 275(8), 5613–5619. [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Lefebvre V, Hendy GN, & Goltzman D (2001). The transcription factor SOX9 regulates cell cycle and differentiation genes in chondrocytic CFK2 cells. J Biol Chem, 276(44), 41229–41236. doi: 10.1074/jbc.M104231200 [DOI] [PubMed] [Google Scholar]
- Sadatsuki R, Kaneko H, Kinoshita M, Futami I, Nonaka R, Culley KL, . . . Ishijima M (2017). Perlecan is required for the chondrogenic differentiation of synovial mesenchymal cells through regulation of Sox9 gene expression. J Orthop Res, 35(4), 837–846. doi: 10.1002/jor.23318 [DOI] [PubMed] [Google Scholar]
- Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, & Hiraki Y (1996). Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol, 133(2), 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stum M, Davoine CS, Vicart S, Guillot-Noel L, Topaloglu H, Carod-Artal FJ, . . . Nicole S (2006). Spectrum of HSPG2 (Perlecan) mutations in patients with Schwartz-Jampel syndrome. Hum Mutat, 27(11), 1082–1091. doi: 10.1002/humu.20388 [DOI] [PubMed] [Google Scholar]
- SundarRaj N, Fite D, Ledbetter S, Chakravarti S, & Hassell JR (1995). Perlecan is a component of cartilage matrix and promotes chondrocyte attachment. J Cell Sci, 108 ( Pt 7), 2663–2672. [DOI] [PubMed] [Google Scholar]
- Tesche F, & Miosge N (2004). Perlecan in late stages of osteoarthritis of the human knee joint. Osteoarthritis Cartilage, 12(11), 852–862. doi: 10.1016/j.joca.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Thant AA, Nawa A, Kikkawa F, Ichigotani Y, Zhang Y, Sein TT, . . . Hamaguchi M (2000). Fibronectin activates matrix metalloproteinase-9 secretion via the MEK1-MAPK and the PI3K-Akt pathways in ovarian cancer cells. Clin Exp Metastasis, 18(5), 423–428. [DOI] [PubMed] [Google Scholar]
- Warren CR, Kassir E, Spurlin J, Martinez J, Putnam NH, & Farach-Carson MC (2015). Evolution of the perlecan/HSPG2 gene and its activation in regenerating Nematostella vectensis. PLoS One, 10(4), e0124578. doi: 10.1371/journal.pone.0124578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, & Horwitz AF (2004). FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol, 6(2), 154–161. doi: 10.1038/ncb1094 [DOI] [PubMed] [Google Scholar]
- Whitelock JM, Graham LD, Melrose J, Murdoch AD, Iozzo RV, & Underwood PA (1999). Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol, 18(2), 163–178. [DOI] [PubMed] [Google Scholar]
- Whitmore L, & Wallace BA (2004). DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res, 32(Web Server issue), W668–673. doi: 10.1093/nar/gkh371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeratne SS, Martinez JR, Grindel BJ, Frey EW, Li J, Wang L, . . . Kiang CH (2016). Single molecule force measurements of perlecan/HSPG2: A key component of the osteocyte pericellular matrix. Matrix Biol, 50, 27–38. doi: 10.1016/j.matbio.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Shen S, Lin Q, Cheng K, Ren S, Gao M, & Li X (2015). Low-Intensity Pulsed Ultrasound Treatment at an Early Osteoarthritis Stage Protects Rabbit Cartilage From Damage via the Integrin/Focal Adhesion Kinase/Mitogen-Activated Protein Kinase Signaling Pathway. J Ultrasound Med, 34(11), 1991–1999. doi: 10.7863/ultra.14.10016 [DOI] [PubMed] [Google Scholar]
- Zhai Z, Yao Y, & Wang Y (2013). Importance of suitable reference gene selection for quantitative RT-PCR during ATDC5 cells chondrocyte differentiation. PLoS One, 8(5), e64786. doi: 10.1371/journal.pone.0064786 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Far UV CD structural analysis of HSPG2 IV-3 and SJS mutant R3452Q{ XE "Figure 3.4. Far UV CD structural analysis of Dm IV-3 and SJS mutant R3452Q" }. CD spectra are plotted as wavelength versus mean residue ellipticity values. CD spectra revealed both (A) HSPG2 IV-3 and (B) R3452Q have a typically beta-sandwich-like conformation with a maximum at 202 nm and minimum at 217 nm when kept at ambient temperatures. Structural prediction software (DichroWeb) indicated the two proteins are composed of approximately 50% beta strands. HSPG2 IV-3 (A) and R3452Q (B) spectra were analyzed over a temperature range of 20 to 90?C, with measurements recorded every 10?C. HSPG2 IV-3 (A) and R3452Q mutant version (B) displayed similar changes in their spectra with increasing temperature. Both proteins partially unfolded at 60?C (black arrows) and completely lost their secondary structures at 70?C. Each data point represents an average of three separate experiments.
Supplemental Figure 2. Thermal denaturation of HSPG2 IV-3 and R3452Q monitored by CD spectroscopy{ XE "Figure 3.5 Thermal denaturation of PLN IV-3 and R3452Q monitored by CD spectroscopy" }. Protein stability was monitored by absorbance at 202 nm, corresponding to the maximum peak in figure supplemental figure 1. Data are normalized to show the folded fraction as a function of temperature for HSPG2 IV-3 (black points) and R3452Q (unfilled). CD ellipticity values are normalized to fraction folded, ranging from 1 to 0, relative to the low temperature value. Melting temperatures (Tm) were determined by fitting the curves to a Boltzmann sigmoidal equations (HSPG2 IV-3, Tm = 63.72 °C; R3452Q, Tm = 62.39 °C). Fitted curves are shown as black (HSPG2 IV-3) and dashed (R3452Q) lines.
Supplemental Figure 3. ATDC5 cells, in a high density culture, form clusters resembling cartilage nodules after 24 hr of being seeded on a purified HSPG2 IV-3 substrate{ XE "Figure 3.8 ATDC5 cells, in a high density culture, aggregate to from clusters resembling cartilage nodules after 24 hr of being seeded on a purified PLN IV-3 substrate" }. ATDC5 cells were cultured on normal tissue culture plates (A and C) or HSPG2 IV-3 coated substrate (B and D) for up to 48 hrs. ATDC5 cells spread and grew in a monolayer on uncoated plates, while those grown on a HSPG2 IV-3 substrate formed large tight clusters. Representative images were captured at 24 and 48 hrs using a 4X objective. Although not present the first day, clusters continued to develop later in regions of high cell density, as indicated by the arrow in panel D. Scale bar represents 500 μm.
Supplemental Figure 4. HSPG2 IV-3 decreases ATDC5 production of GAGs{ XE "Figure 3'11 IV-3 reduced Foxm1 protein levels and decreased cell proliferation" }. (B) ATDC5 cells were grown for 14 days either on uncoated or HSPG2 IV-3-coated plates. Alcian blue staining was used to assess GAG production during this time. Alcian blue stain was extracted and quantified by measuring the absorbance at 630 nm. P-value, *< 0.05; **< 0.005, ***<0.0001.