Abstract
Telomerase reverse transcriptase (TERT) promoter mutations are commonly found in malignant melanomas but rare in melanocytic nevi. To assess its potential diagnostic utility for the distinction of melanoma from nevus, we determined the TERT promoter mutation status of 86 primary melanomas, 72 melanocytic nevi and 40 diagnostically problematic melanocytic proliferations. Of the 86 melanomas, 67 (77.9%) were TERT-positive, defined as harboring a hotspot TERT promoter mutation at positions -124C>T, -124_125CC>TT, -138_139CC>TT or -146C>T. Of the 72 nevi, only one (1.4%) was TERT-positive. Of the 40 diagnostically uncertain melanocytic proliferations, two (5.0%) were TERT-positive. TERT-positivity as a test for melanoma versus nevus had an accuracy of 87.3% (95% CI, 81.1–92.1), a sensitivity of 77.9% (95% CI, 68.9–85.4), a specificity of 98.6% (95% CI, 95.8–100), a positive predictive value of 98.5% (95% CI, 95.6–100) and a negative predictive value of 78.9% (95% CI, 72.6–85.4). Our results indicate that hotspot TERT promoter mutation status may be a useful ancillary parameter for the diagnosis of melanoma. In particular, the high specificity of these mutations for melanoma indicates the presence of a TERT promoter mutation in a melanocytic neoplasm associated with diagnostic controversy or uncertainty should increase concern for a melanoma.
Keywords: melanoma, nevi, TERT promoter mutation, diagnosis, diagnostic biomarkers, telomerase, solar elastosis
Introduction
Highly-recurrent somatic C→T mutations in the promoter of the catalytic reverse transcriptase subunit of the telomerase (TERT) gene, the ribonucleoprotein complex that maintains telomere length, have been reported in up to 67% of primary cutaneous melanomas (1–9). The majority are C to T transitions that occur at positions -124 or -146 upstream from the transcription start site. Mutations at these positions create identical 11-bp nucleotide stretches that contain a consensus-binding site for E-twenty-six (ETS) transcription factors in the ternary complex factor (TCF) subfamily. Other hotspot TERT promoter mutations reported in melanomas that create ETS/TCF binding sites occur at positions -57A>C, -124_125CC>TT or -138_139CC>TT from the start site (2, 4, 5, 7, 10). Heidenreich et al. found a statistically significant increase in TERT mRNA levels as measured by real-time quantitative-polymerase chain reaction (PCR) in melanomas with -124C>T, -146C>T, -124_125CC>TT or -138_139CC>TT mutations when compared to melanomas without TERT promoter mutations or skin (4). TERT promoter mutations at -124C>T and -146C>T increased transcriptional activity in luciferase reporter assays (1, 11). Presence of TERT promoter mutation has been associated with worse survival from melanoma (8, 12). This effect was modified by a common polymorphism rs2853669 within the TERT promoter that disrupts a preexisting non-canonical ETS2 site in the proximal region of the TERT promoter immediately adjacent to an E-box (12). Further, TERT promoter mutations in spitzoid melanocytic neoplasms were reported to predict aggressive clinical behavior (13).
In contrast to malignant melanoma, TERT promoter mutations are rare in melanocytic nevi. However, information on TERT promoter status in nevi is limited, since few nevi have been examined in this regard (2, 14, 15). Horn et al. screened 25 melanocytic nevi and found only one carried a TERT promoter mutation at position -101, which did not create an ETS/TCF motif (2). Vinagre et al. did not detect TERT promoter mutations in nine melanocytic nevi tested (14). Requena et al. found that none of 15 Spitz/Reed nevi carried TERT promoter mutations; whereas, two of nine atypical spitzoid tumors contained TERT promoter mutations, one with a -138_139CC>TT and another a -124C>T alteration (15).
Due to the frequent presence of TERT promoter mutations in cutaneous melanomas but rarity in nevi, these mutations may be used as ancillary evidence to support a diagnosis of melanoma. To explore their potential diagnostic value for the distinction of nevi from melanoma, we analyzed for TERT promoter mutations in a set of primary melanomas, nevi and diagnostically problematic melanocytic proliferations.
Methods and Materials
Specimen Selection and Interobserver Review
Formalin fixed, paraffin-embedded specimens were obtained from the University of North Carolina (UNC) and University of Rochester (UR) pathology archives based on their original diagnosis (cutaneous primary invasive melanoma, nevus or diagnostically uncertain melanocytic proliferation) abstracted from pathology reports and diagnosed between 2001 and 2012. The Institutional Review Boards at UNC and UR approved the study. Melanomas were chosen to span 7th edition American Joint Committee on Cancer (AJCC) tumor stages (16) and include common and less common histopathological subtypes. Nevi were chosen to include a variety of histopathological subtypes. Also, melanocytic tumors of uncertain diagnoses were selected, for which pathologists were unsure as to whether they were benign or malignant and/or unable to reach a consensus diagnosis. Age, sex, race, anatomic site and outcome data were abstracted from the medical record.
A hematoxylin and eosin (H&E)-stained slide of each specimen was initially reviewed by one dermatopathologist to assign diagnosis, classify histopathological subtype and score standard histopathological features. This reviewer also encircled the melanocytic tissue areas on the H&E slides for use as guides in manual microdissection. Two additional dermatopathologists reviewed the same series of specimens using H&E-stained slides or high-resolution Aperio images and assigned diagnoses of melanoma, nevus or uncertain. For the classification of the melanocytic tumors of this study as melanoma or nevus, complete consensus between the pathology report and the three dermatopathologist reviewers was required for all lesions with event-free follow-up. Incomplete consensus (one pathologist may not have been fully certain of the diagnosis) did not exclude a lesion as melanoma if the majority of dermatopathologists interpreted the lesion as melanoma and subsequent clinical follow-up (visceral metastasis and/or death) provided unequivocal evidence of the malignancy of the lesion. Melanocytic proliferations were classified as uncertain if the pathologist issuing the original report was not sure whether the lesion was benign or malignant or if among the subsequent reviewers at least one dermatopathologist had a discordant opinion from the others. After the TERT promoter mutation results were available, the one nevus and two uncertain specimens with hotspot TERT promoter mutations were examined by two additional dermatopathologists (KJB, PBG) to re-assess the prior histopathological classification.
TERT Promoter Mutation Analysis
Five μm-thick tissue sections were cut from each tissue block and mounted on uncoated glass slides. Specimens were manually microdissected using H&E slides as guides and DNAs extracted as described (17, 18). Samples were screened for TERT promoter mutation by sequencing of a 270-base pair amplicon of the TERT promoter that encompasses the main target region for mutations. This region was amplified using M13 tagged primers (primer F: 5′-GCCGGGCTCCCAGTGGATTCG; primer R: 5′-GCTTCCCACGTGCGCAGCAGGAC), as previously described, to amplify from -292 to -23 bps from the start site within the promoter region of the TERT gene (19). Several melanoma cell lines and tissues had been pre-screened to identify positive and negative controls. DNAs were sequenced in the UNC DNA Sequencing Core Facility from the purified 270 bp TERT PCR product using cycle sequencing with fluorescently labeled Big Dye terminators (Applied Biosystems (ABI), Foster City CA) on an ABI 3730 DNA Analyzer. To confirm mutations and eliminate mutational artifacts, we repeated sequencing of a separately amplified aliquot of DNA.
Statistical Analysis
TERT-positivity was defined as harboring a hotspot TERT promoter mutation at positions -124C>T, -124_125CC>TT, -138_139CC>TT or -146C>T. Diagnostic accuracy was defined as the sum of TERT-positive melanoma samples and TERT-negative nevus samples divided by total number of all samples. Sensitivity was defined as number of TERT-positive melanoma samples (true positive) divided by total number of all melanoma samples (all positive). Specificity was defined as number of TERT-negative nevus samples (true negative) divided by total number of all nevus samples (all negative). Positive predictive value (PPV) was number of TERT-positive melanoma samples (true positive) divided by number of all TERT-positive samples (predicted positive). Negative predictive value (NPV) was number of TERT-negative nevus samples (true negative) divided by number of all TERT-negative samples (predicted negative). Fisher’s exact tests were used to examine associations of TERT-positivity with clinicopathologic features. All significance tests were two-sided.
Results
Characteristics of Sample Set
An initial set of 93 melanomas, 80 nevi and 30 diagnostically uncertain melanocytic proliferations were selected. After review of these lesions by additional dermatopathologists as described above, the diagnoses remained the same for 89 melanomas and 73 benign nevi, but 11 lesions designated as nevi (n=7) or melanoma (n=4) on the original pathology report were reclassified as uncertain based on lack of consensus. The assigned classification of the specimens after interobserver review was 89 melanomas, 73 nevi and 41 uncertain. However, three melanomas, one nevus and one uncertain specimen failed TERT promoter mutation screening and were excluded from the subsequent analyses. The final sample set successfully screened for TERT promoter mutation included 86 melanomas, 72 nevi and 40 diagnostically uncertain melanocytic proliferations (Table 1).
Table 1.
Characteristics of 198 melanocytic specimens analyzed for TERT promoter mutations and classified for diagnosis (melanoma, nevus or diagnostically uncertain melanocytic proliferation) after interobserver review
| Characteristic | Primary Melanomas (n=86) | Nevi (n=72) | Uncertain* (n=40) |
|---|---|---|---|
| Number (%) | Number (%) | Number (%) | |
| Laboratory processing of unstained FFPE tissue sections | |||
| University of North Carolina Pathology Laboratories | 81 (94.2) | 66 (91.7) | 40 (100) |
| University of Rochester Pathology Laboratories | 5 (5.8) | 6 (8.3) | – |
| Sex | |||
| Male | 55 (64.0) | 35 (48.6) | 16 (40.0) |
| Female | 31 (36.1) | 37 (51.4) | 24 (60.0) |
| Age at diagnosis of mole or primary melanoma, yrs | |||
| < 65 | 44 (51.2) | 66 (91.7) | 35 (87.5) |
| ≥ 65 | 42 (48.8) | 6 (8.3) | 5 (12.5) |
| Race | |||
| Whites of European origin | 77 (89.5) | 50 (69.4) | 24 (60.0) |
| Other/unknown | 9 (10.5) | 22 (30.6) | 16 (40.0) |
| Histologic subtype of primary melanoma | |||
| Superficial spreading | 43 (50.0) | – | – |
| Nodular | 12 (14.0) | – | – |
| Lentigo maligna | 16 (18.6) | – | – |
| Acral lentiginous | 6 (7.0) | – | – |
| Other/unclassified† | 9 (10.5) | – | – |
| Anatomic site of mole or primary melanoma | |||
| Head/neck | 28 (32.6) | 19 (26.4) | 4 (10.0) |
| Trunk | 27 (31.4) | 37 (51.4) | 23 (57.5) |
| Upper extremities | 16 (18.6) | 8 (11.1) | 2 (5.0) |
| Lower extremities | 15 (17.4) | 8 (11.1) | 11 (27.5) |
| Solar Elastosis adjacent to the melanocytic lesion | |||
| Absent | 21 (24.4) | 43 (59.7) | 34 (85.0) |
| Present | 59 (68.6) | 9 (12.5) | 5 (12.5) |
| Indeterminate | 6 (7.0) | 20 (27.8) | 1 (2.5) |
| Contiguous nevus | |||
| Absent | 75 (87.2) | – | – |
| Present | 11 (12.8) | – | – |
| Melanocytic nevus type | |||
| Intradermal | – | 17 (23.6) | – |
| Common acquired | – | 9 (12.5) | – |
| Congenital pattern | – | 14 (19.4) | – |
| Dysplastic | – | 14 (19.4) | – |
| Spitz | – | 9 (12.5) | – |
| Other‡ | – | 9 (12.5) | – |
| Breslow thickness of primary melanoma, mm | |||
| Median (range) | 1.74 (0.37–17.00) | – | – |
| 0.01 to 2.00 | 45 (52.3) | – | – |
| > 2.00 | 41 (47.7) | – | – |
| Ulceration of primary melanoma | |||
| Absent | 52 (60.5) | – | – |
| Present | 33 (38.4) | – | – |
| Indeterminate | 1 (1.2) | – | – |
| Mitoses of primary melanoma | |||
| Absent | 17 (19.8) | – | – |
| Present | 69 (80.2) | – | – |
| AJCC tumor stage at diagnosis (8th edition) | |||
| 1a/1b/2a | 38 (44.2) | – | – |
| 2b/3a/3b/4a/4b | 48 (55.8) | – | – |
| AJCC overall stage at diagnosis (8th edition) | |||
| IA/IB | 34 (39.5) | – | – |
| IIA/IIB/IIC | 19 (22.1) | – | – |
| IIIA/IIIB/IIIC | 25 (29.1) | – | – |
| IV | 3 (3.5) | – | – |
| Unknown | 5 (5.8) | – | – |
| Tumor infiltrating lymphocyte (TIL) grade | |||
| Absent | 20 (23.3) | – | – |
| Present | 65 (75.6) | – | – |
| Indeterminate | 1 (1.2) | – | – |
| Pigment of the melanocytic lesion | |||
| Absent | 16 (18.6) | 12 (16.7) | 7 (17.5) |
| Present | 70 (81.4) | 60 (83.3) | 33 (82.5) |
| Regression | |||
| Absent | 70 (81.4) | – | – |
| Present | 16 (18.6) | – | – |
Melanocytic proliferations were considered diagnostically uncertain if there was interobserver disagreement between any of 3 dermatopathology readers or the pathology report diagnosis of nevus vs. melanoma or one of the dermatopathogists or pathology report described the specimen as having uncertain diagnosis.
Other types of melanoma include nevoid (n=2), desmoplastic (n=1), spindle cell (n=1), spitzoid (n=1) and unclassified (n=4). All of these were TERT-positive except two of the unclassified melanomas.
Other includes cellular blue nevus (n=2), combined intradermal or sclerotic blue nevus, not cellular (n=1), combined nevus with compound congenital pattern and deep penetrating nevus (n=2), pigmented spindle cell nevus (n=2) and proliferative nodule in congenital pattern nevus (n=2).
The 86 melanomas were from 85 patients; one patient had two synchronous primary melanomas, both of which were included in the study. Of the 86 primary melanomas screened for TERT promoter mutation, 64.0% were from males, 48.8% from patients 65 years or older and 89.5% from whites of European origin (Table 1). The melanomas had a median Breslow thickness of 1.74 mm (range of 0.37–17.00) and 50% were superficial spreading melanomas. Of the 72 nevi, 48.6% were from males, 8.3% from patients 65 years or older and 69% from whites of European origin. Of the 40 uncertain specimens, 40% were from males, 12.5% from patients 65 years or older and 60% from whites of European origin.
Frequencies of TERT Promoter Mutations
Of the 86 successfully analyzed primary invasive melanomas (Table 2), 67 (77.9%) harbored a TERT promoter mutation at one of the known hotspot sites -124C>T, -124_125CC>TT, -138_139CC>TT or -146C>T, and these mutations were mutually exclusive of each other. Of these, two melanomas with a hotspot mutation also harbored another less common TERT promoter mutation creating an ETS/TCF site (-124C>T;-103C>T (n=1) and -124C>T;-148C>T (n=1)). Four (4.7%) melanomas without hotspot mutations harbored other TERT promoter mutations that create ETS/TCF sites (-103C>T, -105_106CC>TT or -148C>T). Also, two melanomas (2.3%) had a TERT promoter mutation that did not form an ETS/TCF site (‘non-ETS’ mutation), and thirteen (15.2%) had no TERT promoter mutation. Examples of a TERT-positive and a TERT-negative melanoma are illustrated in Figures 1 and 2, respectively.
Table 2.
TERT promoter mutation status in 86 primary melanomas, 72 nevi, and 40 diagnostically uncertain melanocytic proliferations
| TERT promoter mutation* | Primary Melanomas (n=86) | Nevi (n=72) | Uncertain (n=40) |
|---|---|---|---|
| Number (%) | Number (%) | Number (%) | |
| Hotspot mutations creating ETS/TCF sites† | n=67 (77.9%) | n=1 (1.4%) | n=2 (5.0%) |
| -124C>T‡ | 29 (33.7) | 1 (1.4) | 1 (2.5) |
| -124_125CC>TT | 1 (1.2) | ||
| -138_139CC>TT | 4 (4.7) | ||
| -146C>T§ | 33 (38.4) | 1 (2.5) | |
| Less common mutations creating ETS/TCF sites¶ | n=4 (4.7%) | n=1 (2.5%) | |
| -103C>T|| | 1 (1.2) | ||
| -105_106CC>TT | 1 (1.2) | ||
| -148C>T** | 2 (2.3) | ||
| -156C>T†† | 1 (2.5) | ||
| Mutations not creating an ETS/TCF site | n=2 (2.3%) | n=7 (9.7%) | n=2 (5.0%) |
| -85C>T;-154C>T | 1 (2.5) | ||
| -106C>T;-117C>T;-149C>T | 1 (1.4) | ||
| -107C>T | 1 (1.2) | ||
| -116C>T;-179C>T | 1 (1.4) | ||
| -125C>T | 1 (1.4) | ||
| -149C>T | 2 (2.8) | ||
| -149C>T;-127C>T | 1 (1.4) | ||
| -149C>T;-160C>T | 1 (2.5) | ||
| -151C>T | 1 (1.2) | ||
| -161C>T | 1 (1.4) | ||
| No mutations | 13 (15.2) | 64 (88.9) | 35 (87.5) |
Bolded mutations create de novo ETS/TCF binding sites.
Mutations create confirmed functional ETS/TCF binding sites.
Six melanomas with -124C>T mutations had additional mutations: -124C>T;-101C>T (n=2), -124C>T;-103C>T (n=1), -124C>T;-126C>T (n=1), -124C>T;-131C>T;-166C>T (n=1) and -124C>T;-148C>T (n=1).
Twelve melanoma with -146C>T mutations had additional mutations: -146C>T;-107C>T;-117C>T (n=1), -146C>T;-149C>T;-153G>A (n=1), -146C>T;-165C>G (n=1), -146C>T;-116C>T (n=1), -146C>T;-125C>T (n=2), -146C>T;-126C>T;-195C>A (n=1), -146C>T;-127C>T (n=1), -146C>T;-149C>T (n=1), -146C>T;-150C>T (n-1), -146C>T;-150C>T;-166C>T (n=1) and -146C>T;-101C>T;-165C>T (n=1).
Mutations create ETS/TCF sites but less common and not confirmed to be functional.
The melanoma with the -103C>T mutation also had a -160C>T mutation.
One of the melanomas with a -148C>T mutation also had the following mutations: -161C>T;-175C>T;-187C>T;-242C>T
The uncertain sample with the -156C>T mutation also had a -149C>T mutation.
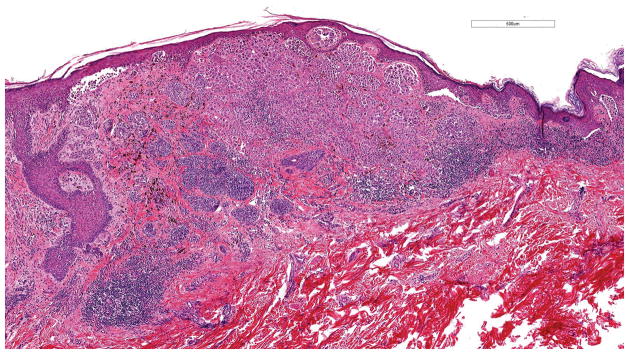
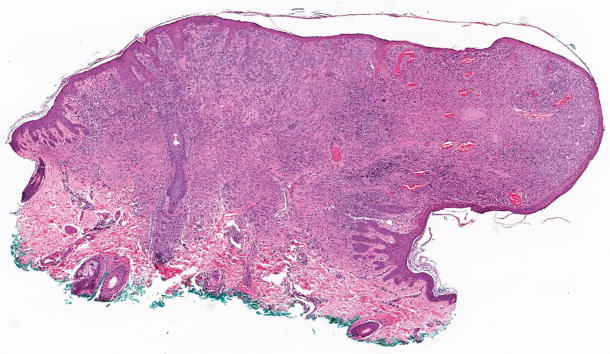
Figure 1.
Superficial spreading malignant melanoma, measuring 1.6 mm in Breslow thickness, without ulceration. This melanoma harbored a hotspot -124C>T TERT promoter mutation (hematoxylin and eosin; 4.9x magnification).
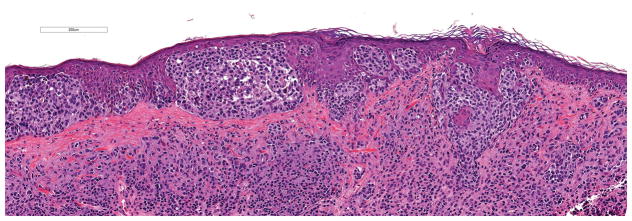
Figure 2.
Lentigo maligna melanoma, 3.0 mm Breslow thickness, non-ulcerated. No TERT promoter mutation was identified (hematoxylin and eosin; 13x magnification).
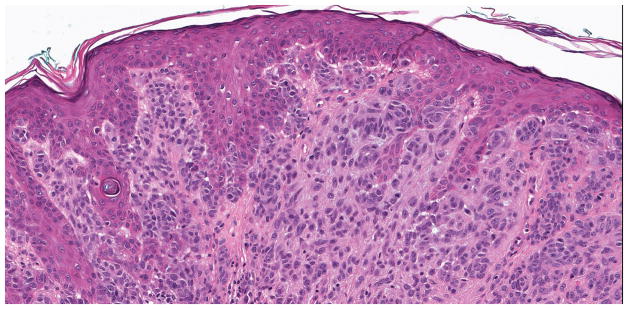
Of the 72 nevi, only one intradermal nevus (1.4%) from a 41 year old male, shown in Figure 3, had a hotspot -124C>T TERT promoter mutation. This patient had the lesion shaved with incomplete removal and was alive 64 months after diagnosis without evidence of melanoma. After the TERT promoter mutation results were available, this -124C>T TERT-positive nevus was examined by two additional dermatopathologists (KJB, PBG) who agreed with the diagnosis of nevus. Of the other 71 nevi, seven (9.7%) had a non-ETS mutation and 64 (88.9%) had no TERT promoter mutations.
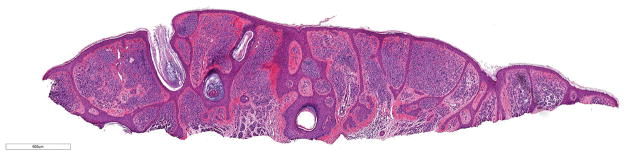
Figure 3.
A, B Benign, predominantly intradermal melanocytic nevus with a congenital pattern. This nevus was found to harbor a hotspot -124C>T TERT promoter mutation. No mitotic figures were present (hematoxylin and eosin; 3.8x and 40x magnification, respectively).
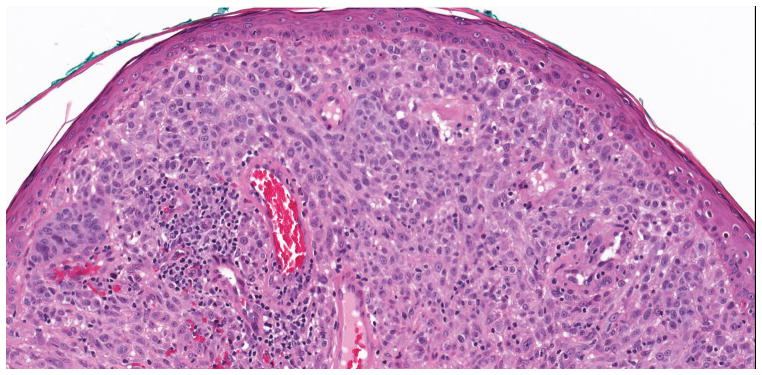
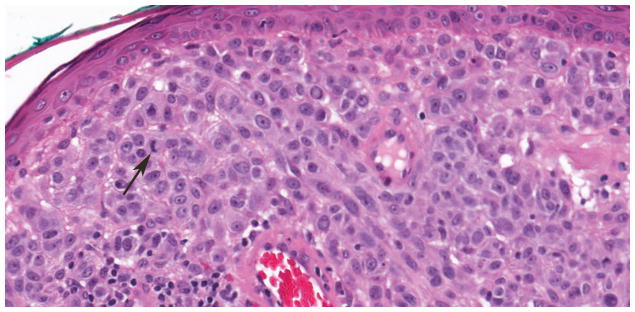
Of the 40 diagnostically uncertain specimens, two (5.0%) harbored hotspot TERT promoter mutations at -124C>T or -146C>T, one (2.5%) harbored a -156C>T mutation creating an ETS/TCF site, two (5.0%) had a non-ETS mutation and 35 (87.5%) had no TERT promoter mutation. The uncertain specimen that harbored a TERT promoter mutation at -146C>T is illustrated in Figure 4. This specimen was signed out as ‘melanoma but viewed by multiple pathologists with differing opinions’. On interobserver review, it was read as melanoma by two reviewers and uncertain by one. The patient had 37 months of follow-up with no evidence of disease. The other tumor with a hotspot TERT promoter mutation (-124C>T) that was placed in this study in the diagnostically uncertain group was signed out with a differential diagnosis of atypical compound dysplastic nevus versus thin invasive melanoma. On interobserver review, it was interpreted as nevus by two reviewers and classified as uncertain by one. Morphologically, it showed a compound melanocytic neoplasm with features of a dysplastic nevus and with severe atypia of the intraepidermal component. The patient died of causes unrelated to melanoma after 47.4 months of follow-up.
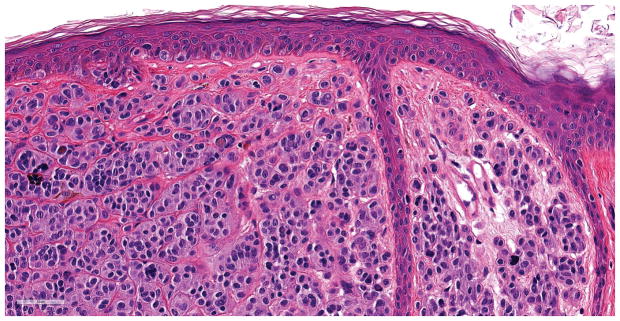
Figure 4.
Compound melanocytic neoplasm with severe architectural and cytological atypia. This indeterminate case was found to harbor a -124C>T TERT promoter mutation. A. A compound melanocytic neoplasm fills and expands the papillary dermis forming a domed shaped lesion (hematoxylin and eosin; 31x). B. The junctional component of the tumor has discrete nesting of melanocytes without confluence or pagetoid spread of cells (hematoxylin and eosin; 200x). C. Areas within the dermal component have expansive groupings of epithelioid melanocytes with vesicular chromatin patterns and prominent nucleoli, and there are lymphocytes present (hematoxylin and eosin; 200x). D. Mitotic figures (arrow) were rarely found in the dermal component of the melanocytic tumor (hematoxylin and eosin; 400x).
Diagnostic Performance Parameters
For determination of diagnostic parameters of TERT-positivity as a test for melanoma versus nevus (Table 3), we defined TERT-positive as harboring a hotspot mutation at position -124C>T, -124_125CC>TT, -138_139CC>TT or -146C>T. Using this definition, we found a diagnostic accuracy of 87.3% (95% CI, 81.1–92.1), a sensitivity of 77.9% (95% CI, 68.9–85.4), a specificity of 98.6% (95% CI, 95.8–100), a PPV of 98.5% (95% CI, 95.6–100) and a NPV of 78.9% (72.6–85.4).
Table 3.
TERT- positivity: diagnostic accuracy, sensitivity, specificity, and positive predictive value for melanoma among 86 melanomas and 72 nevi
| TERT-positivity* | Diagnostic Accuracy % (95% CI) |
Sensitivity Analysis | Specificity Analysis | PPV % (95% CI) |
NPV % (95% CI) |
||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Sensitivity % (95% CI) |
Number | Specificity % (95% CI) |
Number | ||||||
|
|
|
||||||||
| True Positive | False Negative | True Negative | False Positive | ||||||
|
|
|
||||||||
| Positives have a hotspot mutation | 87.3 (81.1–92.1) | 77.9 (68.9–85.4) | 67 | 19 | 98.6 (95.8–100) | 71 | 1 | 98.5 (95.6–100) | 78.9 (72.6–85.4) |
Abbreviations: CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
Defined as harboring a hotspot TERT promoter mutation: -124C>T, -124_125CC>TT, -138_139CC>TT or -146C>T.
Association with Clinicopathological Features
TERT-positivity in melanomas was associated with age at diagnosis, race, histologic type, anatomic site and solar elastosis (all P < 0.05) (Table 4). The frequency of TERT-positivity for melanomas was higher for patients age ≥ 65 (90.5%) than those < 65 years (65.9%) and for whites of European origin (81.8%) than those of other or unknown race (44.4%). TERT-positivity was similarly high in superficial spreading (79.1%), nodular (83.3%), lentigo maligna (87.5%) and other/unclassified (89.9%) melanomas but acral lentiginous melanoma (ALM) had a lower frequency (16.7%). In the other/unclassified category, two nevoid, one desmoplastic, one spindle cell melanoma, one spitzoid and three of four unclassified melanomas were TERT-positive. For melanoma sites, the frequency was highest for upper extremities (100%), followed by the head/neck (85.7%) and then trunk (77.8%) and lowest for lower extremities (40.0%). The frequency was higher when solar elastosis was present (84.8%) than absent (57.1%). There was no association of TERT-positivity with gender, presence of a contiguous nevus, Breslow thickness, ulceration, mitoses, AJCC tumor stage and overall stage at diagnosis (8th edition) (20), tumor infiltrating lymphocyte grade, regression, histologic pigment or presence of the rs2853669 single nucleotide polymorphism in the TERT promoter.
Table 4.
Relationship of TERT-positivity to clinicopathologic features in primary melanomas (n = 86)*
| Clinicopathologic Feature | TERT-negative (n=19) | TERT-positive (n=67) | P-value† |
|---|---|---|---|
| Number (%) | Number (%) | ||
| Sex | |||
| Male | 13 (23.6) | 42 (76.4) | 0.79 |
| Female | 6 (19.4) | 25 (80.7) | |
| Age, years | |||
| < 65 | 15 (34.1) | 29 (65.9) | 0.005 |
| ≥ 65 | 4 (9.5) | 38 (90.5) | |
| Race | |||
| Whites of European origin | 14 (18.2) | 63 (81.8) | 0.02 |
| Other/Unknown | 5 (55.6) | 4 (44.4) | |
| Histologic subtype | |||
| Superficial Spreading | 9 (20.9) | 34 (79.1) | 0.01 |
| Nodular | 2 (16.7) | 10 (83.3) | |
| Lentigo maligna | 2 (12.5) | 14 (87.5) | |
| Acral lentiginous | 5 (83.3) | 1 (16.7) | |
| Other/unclassified‡ | 1 (11.1) | 8 (89.9) | |
| Site | |||
| Head/neck | 4 (14.3) | 24 (85.7) | <.001 |
| Trunk | 6 (22.2) | 21 (77.8) | |
| Upper extremities | 0 | 16 (100.0) | |
| Lower extremities | 9 (60.0) | 6 (40.0) | |
| Solar elastosis | |||
| Absent | 9 (42.9) | 12 (57.1) | 0.03 |
| Present | 9 (15.3) | 50 (84.8) | |
| Indeterminant | 1 (16.7) | 5 (83.3) | |
| Contiguous nevus | |||
| Absent | 17 (22.7) | 58 (77.3) | 1.00 |
| Present | 2 (18.2) | 9 (81.8) | |
| Breslow thickness (mm) | |||
| 0.01 to 2.00 | 8 (17.8) | 37 (82.2) | 0.44 |
| > 2.00 | 11 (26.8) | 30 (73.2) | |
| Ulceration | |||
| Absent | 12 (23.1) | 40 (76.9) | 0.85 |
| Present | 7 (21.2) | 26 (78.8) | |
| Indeterminant | 0 | 1 (100.0) | |
| Mitoses | |||
| Absent | 3 (17.7) | 14 (82.4) | 0.75 |
| Present | 16 (23.2) | 53 (76.8) | |
| AJCC tumor stage at diagnosis (8th edition) | |||
| T1a/T1b/T2a | 7 (18.4) | 31 (81.6) | 0.60 |
| T2b/T3a/T3b/T4a/T4b | 12 (25.0) | 36 (75.0) | |
| AJCC overall stage at diagnosis (8th edition) | |||
| IA/IB | 5 (14.7) | 29 (85.3) | 0.48 |
| IIA/IIB/IIC | 5 (26.3) | 14 (73.7) | |
| IIIA/IIIB/IIIC | 6 (24.0) | 19 (76.0) | |
| IV | 1 (33.3) | 2 (66.7) | |
| Unknown | 2 (40.0) | 3 (60.0) | |
| Tumor infiltrating lymphocyte grade | |||
| Absent | 5 (25.0) | 15 (75.0) | 0.82 |
| Present | 14 (21.5) | 51 (78.5) | |
| Indeterminant | 0 | 1 (100) | |
| Pigment | |||
| Absent | 3 (18.8) | 13 (81.3) | 1.00 |
| Present | 16 (22.9) | 54 (77.1) | |
| Regression | |||
| Absent | 18 (25.7) | 52 (74.3) | 0.11 |
| Present | 1 (6.3) | 15 (93.8) | |
| rs2853669 | |||
| Absent | 10 (20.4) | 39 (79.6) | 0.50 |
| Present | 8 (22.9) | 27 (77.1) | |
| Indeterminant | 1 (50) | 1 (50) | |
Definitions: AJCC, American Joint Committee on Cancer
TERT-positivity is defined as harboring a hotspot TERT promoter mutation: -124C>T, -124_125CC>TT, -138_139CC>TT or -146C>T.
P-values were derived from the Fisher’s exact test. Bold type indicates P-values < .05 (two-sided).
Other/unclassified types of melanoma include nevoid (n=2), desmoplastic (n=1), spindle cell (n=1), spitzoid (n=1) and unclassified (n=4).
Discussion
We found a diagnostic accuracy of 87.3% (95%, 81.1–92.1) for melanoma versus nevus based on TERT-positivity defined as harboring a hotspot TERT promoter mutation. The overall sensitivity of TERT-positivity for melanoma was 77.9%, although our data indicate it is lower for certain subgroups, including those age < 65 years (65.9%), of other or unknown race (44.4%), with lesions on the lower extremity lesions (40%) or without adjacent solar elastosis (57.1%) but most clearly for those with ALM (16.7%). Most notably, the specificity of TERT-positivity for melanoma versus nevus was 98.6%, with only one of 72 nevi harboring a hotspot mutation. Only two of 40 (5%) uncertain specimens were TERT-positive, indicating that the uncertain group may be enriched with benign nevi. Our data cannot fully address which uncertain specimens are melanomas, as 22% of melanomas did not harbor hotspot TERT promoter mutations. Nevertheless, because our data indicate that TERT-positivity is rare in melanocytic nevi, melanocytic neoplasms with an uncertain diagnosis, in which the possibility of melanoma is being seriously considered because of worrisome histopathological findings, the detection of hotspot TERT promoter mutations should be regarded as evidence that increases the probability that the lesion may in fact be a melanoma. Accordingly, a positive test result in a lesion suspected to be a melanoma may provide a rationale for surgical management according to melanoma guidelines (21).
Multiple authors have reported hotspot TERT promoter mutations in melanomas (1–8). Although not included in our main analyses for TERT-positivity, we also found in melanomas but not nevi a few less common TERT promoter mutations (-103C>T, -105_106CC>TT, -148C>T) that form de novo ETS/TCF sites. To our knowledge, these mutations have not been studied functionally and only the -105_106CC>TT mutation has been previously reported in a melanoma (4). We also found a -156C>T mutation that forms an ETS/TCF site in an uncertain specimen and know of a previous report of a -156C>T mutation in a cutaneous melanoma (4).
We found TERT-positivity was associated with increased age at diagnosis similar to other studies (4, 12, 22, 23). We also found TERT-positivity occurred more frequently in melanomas from whites of European ancestry compared to other/unknown races. Notably Bai et al. found a low rate of TERT promoter mutations in melanomas in the Asian population (24). We found only 16.7% of ALMs harbored TERT promoter mutations. The relatively low frequency of TERT promoter mutations in the ALMs is consistent with many reports on this issue in the literature (4, 5, 7, 8, 12, 24–26), although a recent report found a higher frequency of TERT promoter mutations of 35% (27). Besides promoter mutations, TERT activity may also increase in ALMs through TERT copy gains, methylation, or translocations (27–29).
Similarly, to Heidenreich et al. (4), we found TERT promoter mutations were associated with melanomas arising on sun-exposed anatomic sites (defined as presence of solar elastosis in our study). We did not find associations of TERT promoter mutation with Breslow thickness, ulceration, tumor stage or mitotic rate, similar to some studies (9, 30) but unlike others (4, 7, 8, 12, 23). The differences in results between studies may be influenced by the tumor stages examined and sensitivity of the assay utilized for TERT promoter mutation detection because, as discussed by Lade-Keller (9), early melanomas may have subclonal populations with TERT promoter mutations requiring higher assay sensitivity for detection. We found no association of TERT promoter mutation in melanomas with regression, unlike studies where negative (31) or positive (32) correlations were reported. Unlike Ofner et al. (10) but similar to Nagore et al. (12), we found no significant association of TERT promoter mutation with the carrier status of the common single-nucleotide polymorphism rs2853669.
To our knowledge, this is the first study to report diagnostic parameters for TERT-positivity as a test for melanoma versus nevus and the largest series of nevi investigated to date. Inclusion of melanomas of different tumor stages and histologic subtypes and a variety of nevus subtypes are strengths of the study. We are not aware of another report in the literature or illustration of a relatively banal nevus with a TERT promoter mutation and follow-up. Another strength is the interobserver review by several dermatopathologists to classify samples. The specimens underwent rigorous TERT promoter mutational analysis with inclusion of the less common TERT promoter mutations in the reporting. The inclusion of uncertain specimens provides information on whether TERT promoter mutation can be found among samples that are difficult to classify.
Weaknesses of the study include its retrospective design, which can lead to sample selection bias. Also predictive values (PPV and NPV) are largely dependent on disease prevalence in the examined population, and, thus, may not be transferable to another population (33). We included relatively few specimens from non-white patients, and race was missing for some specimens. Further, the definition of TERT-positivity affects the diagnostic results and could possibly be expanded to include other non-hotspot mutations that formed a de novo ETS/TCF site. However, we have insufficient information to consider these non-hotspot mutations as diagnostic. Further, we did not consider transcription factor sites disrupted by TERT promoter mutations that may be important for melanomagenesis and possibly for diagnosis. Last, the assay utilized for TERT promoter mutation detection likely affects the diagnostic sensitivity, and each assay utilized will need technical validation.
In conclusion, our results indicate that hotspot TERT promoter mutations may be useful for the distinction of melanoma from nevus. From a practical standpoint, we propose that there may be a rationale for testing melanocytic tumors with ambiguous features, including microscopic findings that raise concern for possible melanoma. The high PPV of TERT-positivity for melanoma in this sample set (98.5%) indicates that a positive test in a melanocytic tumor with worrisome histopathological findings increases the probability that the tumor is a melanoma. A positive test, then, may provide evidence for treating the melanocytic lesion according to melanoma guidelines (21). However, the NPV of TERT-positivity for melanoma in our study was 78.9%; thus, lack of TERT positivity in an uncertain melanocytic lesion does not rule out melanoma, and, if atypical clinical or histopathological findings are present, treatment based on the level of concern should be initiated, i.e. judiciously either a conservative excision or treatment as melanoma. To provide additional clarity, we do not propose TERT promoter mutation testing for banal-appearing nevi or for nevi with unusual features that do not raise concern for possible melanoma. We also do not propose TERT promoter mutation testing for histologically unequivocal melanomas, as testing would not change the treatment.
However, a greater number of melanocytic lesions must be studied to determine more robustly the diagnostic value of the assay. Examination of TERT promoter mutations in diagnostically uncertain melanocytic proliferations warrants additional study among patients for whom long-term melanoma-specific survival is available, allowing a more objective classification and evaluation of the diagnostic and potential prognostic value of a melanocytic tumor’s TERT promoter status.
Acknowledgments
This work was funded by the National Cancer Institute grants R21CA134368, R33CA160138, R03CA199487 to K Conway and NE Thomas, P01CA206980 to M Berwick and NE Thomas, and P30CA016086 to HS Earp. This work was also supported by the Lineberger Comprehensive Cancer Center Bioinformatics Core. We would like to acknowledge Pamela A. Groben MD, Professor, Departments of Dermatology and Pathology and Laboratory Medicine, for participating in the pathology interobserver review.
Abbreviations
- CI
confidence interval
- NPV
negative predictive value
- PPV
positive predictive value
Footnotes
Disclosure/conflict of interest
The authors declare no conflicts of interest.
Funding sources: This work was funded by the National Cancer Institute grants R21CA134368, R33CA160138, R03CA199487 to K Conway and NE Thomas, P01CA206980 to M Berwick and NE Thomas, and P30CA016086 to HS Earp. This work was also supported by the Lineberger Comprehensive Cancer Center Bioinformatics Core.
References
- 1.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339(6122):957–9. doi: 10.1126/science.1229259. Epub 2013/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–61. doi: 10.1126/science.1230062. Epub 2013/01/26. [DOI] [PubMed] [Google Scholar]
- 3.Egberts F, Kruger S, Behrens HM, et al. Melanomas of unknown primary frequently harbor TERT-promoter mutations. Melanoma Res. 2014;24(2):131–6. doi: 10.1097/CMR.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich B, Nagore E, Rachakonda PS, et al. Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma. Nat Commun. 2014;5:3401. doi: 10.1038/ncomms4401. Epub 2014/02/27. [DOI] [PubMed] [Google Scholar]
- 5.Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545(7653):175–80. doi: 10.1038/nature22071. Epub 2017/05/04. [DOI] [PubMed] [Google Scholar]
- 6.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–13. doi: 10.1038/nm.4333. Epub 2017/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Populo H, Boaventura P, Vinagre J, et al. TERT Promoter Mutations in Skin Cancer: The Effects of Sun Exposure and X-Irradiation. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.163. [DOI] [PubMed] [Google Scholar]
- 8.Griewank KG, Murali R, Puig-Butille JA, et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lade-Keller J, Yuusufi S, Riber-Hansen R, Steiniche T, Stougaard M. Telomerase reverse transcriptase promoter mutations and solar elastosis in cutaneous melanoma. Melanoma Res. 2018 doi: 10.1097/CMR.0000000000000446. Epub 2018/03/24. [DOI] [PubMed] [Google Scholar]
- 10.Ofner R, Ritter C, Heidenreich B, et al. Distribution of TERT promoter mutations in primary and metastatic melanomas in Austrian patients. J Cancer Res Clin Oncol. 2017;143(4):613–7. doi: 10.1007/s00432-016-2322-1. Epub 2016/12/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang DS, Wang Z, He XJ, et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur J Cancer. 2015;51(8):969–76. doi: 10.1016/j.ejca.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagore E, Heidenreich B, Rachakonda S, et al. TERT promoter mutations in melanoma survival. Int J Cancer. 2016;139(1):75–84. doi: 10.1002/ijc.30042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Barnhill RL, Dummer R, et al. TERT Promoter Mutations Are Predictive of Aggressive Clinical Behavior in Patients with Spitzoid Melanocytic Neoplasms. Sci Rep. 2015;5:11200. doi: 10.1038/srep11200. Epub 2015/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinagre J, Almeida A, Populo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 15.Requena C, Heidenreich B, Kumar R, Nagore E. TERT promoter mutations are not always associated with poor prognosis in atypical spitzoid tumors. Pigment Cell Melanoma Res. 2017;30(2):265–8. doi: 10.1111/pcmr.12565. Epub 2016/12/09. [DOI] [PubMed] [Google Scholar]
- 16.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199–206. doi: 10.1200/JCO.2009.23.4799. Epub 2009/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas NE, Alexander A, Edmiston SN, et al. Tandem BRAF mutations in primary invasive melanomas. J Invest Dermatol. 2004;122(5):1245–50. doi: 10.1111/j.0022-202X.2004.22523.x. Epub 2004/05/14. [DOI] [PubMed] [Google Scholar]
- 18.Thomas NE, Edmiston SN, Alexander A, et al. Number of nevi and early-life ambient UV exposure are associated with BRAF-mutant melanoma. Cancer Epidemiol Biomarkers Prev. 2007;16(5):991–7. doi: 10.1158/1055-9965.EPI-06-1038. Epub 2007/05/18. [DOI] [PubMed] [Google Scholar]
- 19.Scott GA, Laughlin TS, Rothberg PG. Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod Pathol. 2014;27(4):516–23. doi: 10.1038/modpathol.2013.167. [DOI] [PubMed] [Google Scholar]
- 20.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–92. doi: 10.3322/caac.21409. Epub 2017/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sladden MJ, Nieweg OE, Howle J, Coventry BJ, Thompson JF. Updated evidence-based clinical practice guidelines for the diagnosis and management of melanoma: definitive excision margins for primary cutaneous melanoma. Med J Aust. 2018;208(3):137–42. doi: 10.5694/mja17.00278. Epub 2018/02/14. [DOI] [PubMed] [Google Scholar]
- 22.Nagore E, Heidenreich B, Requena C, et al. TERT promoter mutations associate with fast-growing melanoma. Pigment Cell Melanoma Res. 2016;29(2):236–8. doi: 10.1111/pcmr.12441. Epub 2015/11/18. [DOI] [PubMed] [Google Scholar]
- 23.Egberts F, Bohne AS, Kruger S, et al. Varying Mutational Alterations in Multiple Primary Melanomas. J Mol Diagn. 2016;18(1):75–83. doi: 10.1016/j.jmoldx.2015.07.010. Epub 2015/11/27. [DOI] [PubMed] [Google Scholar]
- 24.Bai X, Kong Y, Chi Z, et al. MAPK Pathway and TERT Promoter Gene Mutation Pattern and Its Prognostic Value in Melanoma Patients: A Retrospective Study of 2,793 Cases. Clin Cancer Res. 2017;23(20):6120–7. doi: 10.1158/1078-0432.CCR-17-0980. Epub 2017/07/20. [DOI] [PubMed] [Google Scholar]
- 25.Liau JY, Tsai JH, Jeng YM, Chu CY, Kuo KT, Liang CW. TERT promoter mutation is uncommon in acral lentiginous melanoma. J Cutan Pathol. 2014 doi: 10.1111/cup.12323. [DOI] [PubMed] [Google Scholar]
- 26.de Vazquez VL, Vicente AL, Carloni A, et al. Molecular profiling, including TERT promoter mutations, of acral lentiginous melanomas. Melanoma Res. 2016;26(2):93–9. doi: 10.1097/CMR.0000000000000222. Epub 2015/12/29. [DOI] [PubMed] [Google Scholar]
- 27.Liang WS, Hendricks W, Kiefer J, et al. Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome Res. 2017;27(4):524–32. doi: 10.1101/gr.213348.116. Epub 2017/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–47. doi: 10.1056/NEJMoa050092. Epub 2005/11/18. [DOI] [PubMed] [Google Scholar]
- 29.Diaz A, Puig-Butille JA, Munoz C, et al. TERT gene amplification is associated with poor outcome in acral lentiginous melanoma. J Am Acad Dermatol. 2014;71(4):839–41. doi: 10.1016/j.jaad.2014.05.035. Epub 2014/09/16. [DOI] [PubMed] [Google Scholar]
- 30.Ekedahl H, Lauss M, Olsson H, et al. High TERT promoter mutation frequency in non-acral cutaneous metastatic melanoma. Pigment Cell Melanoma Res. 2016;29(5):598–600. doi: 10.1111/pcmr.12500. Epub 2016/06/16. [DOI] [PubMed] [Google Scholar]
- 31.de Unamuno Bustos B, Murria Estal R, Perez Simo G, et al. Lack of TERT promoter mutations in melanomas with extensive regression. J Am Acad Dermatol. 2016;74(3):570–2. doi: 10.1016/j.jaad.2015.10.003. Epub 2016/02/20. [DOI] [PubMed] [Google Scholar]
- 32.Macerola E, Loggini B, Giannini R, et al. Coexistence of TERT promoter and BRAF mutations in cutaneous melanoma is associated with more clinicopathological features of aggressiveness. Virchows Arch. 2015;467(2):177–84. doi: 10.1007/s00428-015-1784-x. [DOI] [PubMed] [Google Scholar]
- 33.Simundic AM. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC. 2009;19(4):203–11. Epub 2009/01/01. [PMC free article] [PubMed] [Google Scholar]