Abstract
Cutaneous burn injury is one of the most devastating injuries one can obtain with tissue damage extending beyond the skin wound to distal organs, including the gastrointestinal tract, liver, and lungs. Multiple organ failure is a leading cause of death after burn injury resulting in excessive systemic and localized inflammation directly contributing to end organ damage. We postulated that the gut-liver-lung inflammatory axis underscores multiple organ failure in the context of burn injury and is hyper-activated when ethanol intoxication precedes burn. Mesenchymal stem cells (MSCs) are regenerative and anti-inflammatory and MSC treatment has been shown to be beneficial in several immune disorders and injury models. Our objective was to determine whether intravenous infusion of exogenous bone marrow-derived MSCs could reduce post-burn and intoxication pulmonary, hepatic, and systemic inflammation. Vehicle or ethanol (1.6 g/kg) treated mice were subjected to sham or 15% total body surface area scald burn. One hour post-injury, mice were given 5×105 CFSE-labeled MSCs or phosphate buffered saline intravenously (i.v.) and euthanized 24h later. We assessed circulating biomarkers of inflammation and liver damage, measured cytokine and chemokine production and quantified apoptosis in lung and liver tissue. Compared to intoxicated and burned mice, those treated with MSCs had less cellularity, limited apoptosis, and a slight reduction in the pro-inflammatory cytokine interleukin-6 (IL-6) and the neutrophil chemokine, KC (CXCL1) in lung tissue. MSCs treatment had more dramatic anti-inflammatory effects on systemic and hepatic inflammation, as serum IL-6 levels were diminished by 43%, il6 and kc expression in liver tissue were markedly reduced, as were biomarkers of liver damage, aspartate transaminase (AST) and alanine transaminase (AST), compared with intoxicated and burned mice. Taken together, our results suggest intravenous MSCs treatment can diminish systemic inflammation, lessen hepatic damage, and decrease liver and lung apoptosis and inflammation, indicating MSCs as a novel therapy for restoring homeostasis of multiple organ systems in intoxicated burn patients.
Keywords: alcohol, burn injury, lung, liver, apoptosis, mesenchymal stem cells
Introduction
In the United States, people are typically binge drinkers rather than chronic alcoholics [1]. Binge drinking, defined by the number of drinks consumed within a 2 hour window (4 for women, 5 for men) or a blood alcohol concentration of 0.08%, is a common drinking pattern among trauma patients, including those with burn injuries [2, 3]. In fact, nearly half all burn patients are intoxicated at the time of injury [4, 5]. Intoxication at the time of burn injury results in adverse effects in multiple major organ systems, including intestinal, hepatic and pulmonary damage [6-8]. As a result, respiratory failure is one of the leading causes of death after burn [9]. This combined insult results in longer hospital stays and greater fluid resuscitation and mechanical ventilation requirements, leading to increased risk for pulmonary complications, infections, and mortality [4, 10, 11]. Using a murine model of ethanol intoxication followed by a moderate size burn injury, our laboratory and others demonstrated that ethanol intoxication exacerbates inflammatory responses in multiple organ systems, including the lungs, liver, and gut after burn, similar to clinical observations.
Our laboratory has proposed a working model in which the gut-liver-lung axis is a major contributing factor underlying multi-organ failure after burn injury [12]. We and others have shown that burn injury reduces intestinal epithelial cell barrier integrity which is exacerbated when ethanol intoxication precedes injury [13-15]. After the combined insult, we found that bacteria and bacterial products, including lipopolysaccharide (LPS), are released and translocated to distal sites, including mesenteric lymph nodes [14]. The degree of intestinal damage is directly proportional to bacterial overgrowth in the intestine after injury [15]. LPS and other bacterial products trigger the hepatic acute phase and inflammatory response, contributing to the high levels of circulating interleukin-6 (IL-6) observed after burn in both preclinical models and burn patients [12, 16, 17] [18-20]. Elevated plasma levels of IL-6 in burn patients have been correlated with increased morbidity and mortality [21] and with sepsis severity [22]. These excessive circulating levels of IL-6 then contribute to pulmonary inflammation, as IL-6 knockout mice and mice treated with anti-IL-6 antibody had reduced pulmonary inflammation and leukocyte infiltration after intoxication and burn [23]. After the combined insult, the lungs display characteristics of acute respiratory distress syndrome, including alveolar wall thickening, neutrophil accumulation and heightened IL-6 levels, relative to either intoxication or burn alone [12, 23-27]. The amplification of pulmonary inflammation parallels impaired respiratory parameters and reduced survival [28]. Additionally, abnormal breathing patterns are correlated with increased neutrophil quantity and impaired lung function, and are exacerbated by intoxication [27, 28]. Taken together, these studies suggest that managing the hyperactivation of the gut-liver-lung inflammatory axis after remote injury will likely limit morbidity and mortality in intoxicated burn patients.
Bone marrow and tissue resident mesenchymal stem cells (MSCs) have the ability to suppress immune cell responses and have been beneficial in clinical trials for treatment of various inflammatory disorders and injury models, including Crohn’s disease [29], graft-versus-host disease [30, 31], ischemia [32], pulmonary fibrosis [33, 34], liver fibrosis/cirrhosis [35, 36], sepsis (reviewed in [37]), and ARDS [38] and in preclinical animal models, including a porcine model of acute lung injury [39], an ovine model of bacterial pneumonia [40], and in rat models of LPS endotoxemia and burn injury [41]. MSCs modulation of acute inflammation is partially due to their ability to influence macrophage phenotype [42]. MSC-macrophage interaction and the release of paracrine soluble factors by MSCs reduce lung inflammation and polarize macrophages into an anti-inflammatory phenotype [42-46]. Resident alveolar macrophages modulate pulmonary inflammation during both the onset and resolution stages of the inflammatory response [47-49]. Hence, factors that elicit an anti-inflammatory alveolar macrophage phenotype may therefore promote pulmonary homeostatic restoration after injury. The ability of MSCs to diminish lung inflammation through direct and indirect mechanisms suggests MSCs would be particularly advantageous in attenuating the excessive pulmonary inflammation observed after intoxication and injury.
The purpose of this study was to determine whether the intravenous infusion of exogenous bone marrow-derived MSCs could restore homeostasis of the liver-lung inflammatory axis by diminishing tissue damage and restoring hepatic and pulmonary homeostasis. Since intoxication at the time of injury results in greater pulmonary complications and mortality rates than burn injury alone [28], we chose to examine the effect of MSC treatment in intoxicated, burn-injured mice. In vitro-expanded MSCs were given intravenously 1 hour after injury. We assessed circulating biomarkers of hepatic damage and inflammation, measured cytokine/chemokine production, and quantified apoptosis in lung and liver tissue. Our results suggest intravenous MSCs treatment can attenuate systemic inflammation, reduce hepatic damage, and diminish liver and lung apoptosis and inflammation, establishing MSCs as a novel therapeutic agent for restoring the liver-lung axis to homeostasis in intoxicated burn patients.
Materials and Methods
Mice
Male (C57BL/6) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used at 8-10 weeks old. Mice were housed in sterile micro-isolator cages under specific pathogen-free conditions in the Loyola University Medical Center Comparative Medicine facility for a minimum of 1 week prior to experimentation. All experiments were conducted in accordance with the Institutional Animal Care and Use Committee. Mice weighing between 22 to 27 g were used in these studies.
Mesenchymal stem cell culture
Gibco® Mouse (C57BL/6) bone marrow-derived MSCs (Life Technologies, Grand Island, NY) were expanded in vitro per manufacturer protocol. Briefly, MSC growth medium consisted of 10% FBS and 10mg/ml gentamicin in MEMα Medium with GlutaMAX™-I, ribonucleosides and deoxyribonucleosides. MSCs were plated at a density of 5,000 cells per cm2 in T75 flasks and incubated at 37°C, 5% CO2 and 90% humidity. Medium was changed every 2 days until cultures were 70-80% confluent. MSCs were detached from flasks using pre-warmed trypsin, fluorescently labeled with carboxyfluoresceindiacetate, succinimidyl ester (CFSE) using the manufacturer protocol (Life Technologies), and resuspended in sterile Dulbecco’s phosphate buffered saline (PBS) [50]. Passage 4 cells were used in all experiments.
Murine model of binge ethanol and burn injury
A murine model of single dose binge ethanol intoxication and burn injury was employed using oral gavage, as described previously [13, 51, 52]. Animals were given 400 μl of 10% (v/v) ethanol solution (1.6 g/kg) or water control by gavage at a dose designed to elevate the blood alcohol concentration to 150 mg/dL at 30 min after ethanol exposure [53]. Thirty minutes following the ethanol exposure, the mice were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine) and their dorsum shaved. The mice were placed in a plastic template exposing 15% of the total body surface area and subjected to a scald injury in a 92-95°C water bath or a sham injury in room-temperature water [51]. The scald injury results in an insensate, full-thickness burn [54]. The mice were then resuscitated with 1.0 ml saline and allowed to recover on warming pads. All experiments were performed between 8 and 9 am to avoid confounding factors related to circadian rhythms. One hour after injury, intoxicated and burn-injured animals either received an intravenous tail vein injection of 5×105 CFSE-labeled MSCs or PBS as a control in 200ul [55]. Animals were euthanized at 24 h. MSC treatment did not alter mouse weight (data not shown).
Flow cytometry analysis of CFSE+ mesenchymal stem cells
The upper left lung lobe was removed and cut into small pieces with a razor blade. The lung tissue was then transferred to a C-tube (Miltenyi Biotec, Auburn, CA) and processed using digestion buffer containing 1mg/ml of Collagenase D and 0.1 mg/ml DNase I (Roche, Indianapolis, IN) in HBSS and a GentleMACS dissociator (Miltenyi Biotec), according to manufacturer’s instructions [28]. The homogenates were then filtered through 70 um nylon cell strainers to obtain a single cell suspension. Red blood cells were lysed using ACK lysis buffer (Life Technologies). Cells were counted using trypan blue to exclude dead cells. To assess mesenchymal stem cells, 1×106 lung cells were first incubated with anti-CD16/32 (clone 93, eBioscience, San Diego, CA) to block unspecific binding to the Fcy II/III receptor. Cells were then incubated with rat anti-mouse F4/80 APC (clone BM8, eBioscience), CD11b eFluor 450 (clone M1/70, eBioscience), CD11c APC-eFluor 780 (clone N418, eBioscience), and Siglec-F PE-CF594 (clone E50-2440, BD Biosciences, San Jose, CA). Antibody incubation was carried out for 30 minutes at 4°C. Cells were washed and fixed as described [56, 57]. Samples were run on a BD Fortessa cytometer (BD Biosciences). Data analysis was performed using Flow Jo FCS analysis software (Tree Star Inc., Ashland, OR).
Histopathologic examination of the lungs
The upper right lobe of the lung was inflated with 10% formalin and fixed overnight, as described previously [26], embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). Sections were evaluated using light microscopy (Zeiss AxioVert, Zeiss, Thorndale, CA) and histology photographs were taken at 400x magnification.
Terminal deoxynucleotidyl transferase-dUTP nick end labeling (TUNEL) staining
Lung and liver tissue were fixed in 10% formalin overnight, embedded in paraffin, and sectioned, as described [26]. Click-it® Plus in situ terminal deoxynucleotidyl transferase-dUTP nick end labeling (TUNEL) Alexa Fluor 488 assay was performed, according to manufacturer guidelines (Life Technologies). Lung and liver sections were evaluated using fluorescent microscopy. TUNEL+ cells were counted in a blinded fashion in 10 individual high power fields. Two independent experiments were performed, n=7-12 per group, total. Data are presented as the average number of TUNEL+ cells per 200x field ± SEM.
Cytokine and chemokine analysis of lung homogenates and serum
The right middle lung lobe was snap-frozen in liquid nitrogen and then homogenized in 1 ml of BioPlex cell lysis buffer according to manufacturer’s protocol (BioRad, Hercules, CA). The homogenates were filtered and analyzed for cytokine (IL-6) and chemokine (KC) production using an enzyme-linked immunosorbent assay (ELISA). The results were normalized to total protein using the BioRad protein assay (BioRad) [23, 25]. Serum aliquots were used to measure IL-6 by ELISA (BD Biosciences). Results from three independent experiments were pooled and are presented as mean cytokine level per milligram of protein (pg/mg total protein) for homogenates or pg/milliliter for serum. n= 10-14 per group
Quantitative RT-PCR of liver gene expression
RNA was extracted from liver tissue using the RNeasy Mini Kit (Qiagen, Germantown, MD) and converted to cDNA using the iScript™ cDNA Synthesis Kit (BioRad, Hercules, CA), following manufacturer guidelines. Quantitative RT-PCR was performed using TaqMan Gene Expression Assays (ThermoFisher Scientific, Waltham, MA). Results were analyzed using the ΔΔCt algorithm[14] with GAPDH as the endogenous control. Data are presented as mean fold change ± SEM relative to sham-injured, vehicle treated controls. n = 2-6 per group. Representative data from two independent experiments are shown.
Serum aminotransferase measurements
Blood was collected via cardiac puncture and the serum was isolated and stored at −80°C. Serum aliquots were used to measure liver alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels using a DRI-CHEM 7000 (HESKA, Loveland, CO). Representative data from three independent experiments are shown. n=3-4 per group, per experiment. Data are presented as mean unit/liter (U/L) ± SEM.
Statistical analysis
Statistical comparisons were made between the sham vehicle, burn ethanol + PBS, and burn ethanol + MSC treatment groups. One-way analysis of variance (ANOVA) was used with Tukey’s post-hoc test and values were considered statistically significant when p < 0.05. Data is reported as mean values ± the standard error of the mean (SEM). Data is representative of two independent experiments, unless otherwise stated. N = 3-6 animals per group in each experiment.
Results
Mesenchymal stem cells are present the lungs 24 hours after administration
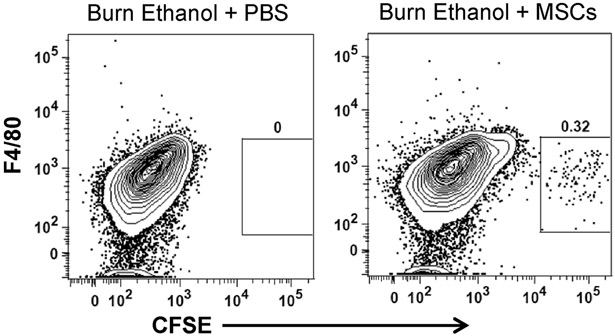
MSCs were labeled with CFSE and administered to mice an hour after burn injury. At 24 hours, CFSE+ MSCs were identified in the lungs of intoxicated and burn injured mice using flow cytometry. Lung cells were negatively selected for alveolar macrophage/eosinophil marker Siglec-F and granulocyte/neutrophil marker CD11b (data not shown), and analyzed for CFSE+ cells compared to macrophage/monocyte marker F4/80 (Fig. 1). Additionally, CFSE+ cells were negative for dendritic cell marker CD11c (data not shown). Our results indicated there were approximately 100 CFSE+ MSCs recovered per 3.5 × 105 total lung cells. Lack of Siglec-F, CD11b, F4/80 and CD11c expression supports the identification of individual CFSE+ cells and not CFSE+ cells engulfed by macrophage/monocytes, granulocytes, or dendritic cells. These data confirm that MSCs are in a position to attenuate pulmonary inflammation by their ability to localize in the lungs and remain there at least 24 hours after infusion.
Fig 1.
Flow cytometry of MSCs in dissociated lung tissue. Representative flow cytometry plots of each treatment group. CFSE+ MSCs were identified in dissociated lung tissue from intoxicated and injured mice at 24 h using flow cytometry. Total dissociated lung cells were negatively selected for Siglec-F and CD11b (data not shown), followed by F4/80− CFSE+ cells (box) in burn ethanol + PBS and burn ethanol + MSCs treatment groups.
Mesenchymal stem cell treatment improves lung histopathology after burn and intoxication
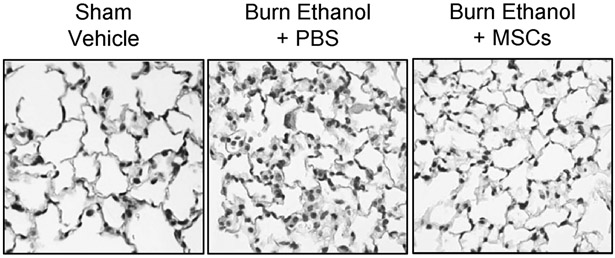
We previously demonstrated that ethanol intoxication prior to burn leads to increased neutrophil infiltration and cellular edema [23-26, 28]. Here, we performed similar histochemical analyses of sectioned lung tissue and observed that ethanol intoxication prior to burn injury results in increased cellularity and pulmonary congestion in the distal airway at 24 hours, which was reduced by MSC administration (Fig. 2). No changes in liver histology were observed at this time point (data not shown).
Fig 2.
Histological assessment of pulmonary inflammation. Lungs were sectioned and stained with H&E and assessed for cellular infiltration. Representative sections from each treatment group are shown at 400x.
Mesenchymal stem cell treatment decreases pulmonary cytokine and chemokine levels
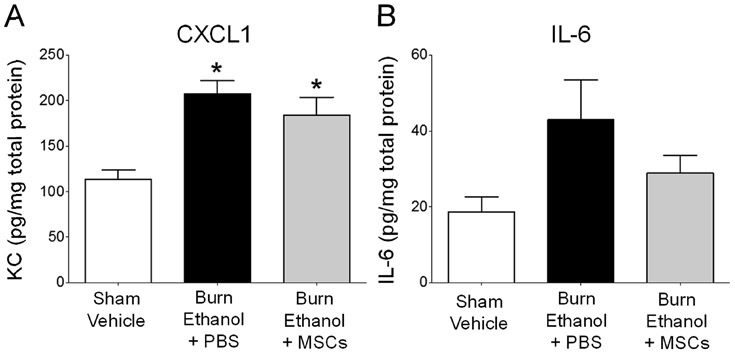
Since we observed lower lung cellularity with MSC treatment, we next examined levels of the pulmonary neutrophil chemoattractant, CXCL1, and the pro-inflammatory cytokine, IL-6. Consistent with previous studies [24-26, 28], there was an 83% rise in CXCL1 in lung tissue of intoxicated mice subjected to burn injury, in comparison to control animals (Fig. 3A) (p<0.05). MSC treatment marginally limited CXCL1 levels observed in intoxicated, burned mice by 11% (Fig. 3A). We observed a similar pattern with IL-6, as levels were increased by 78% after intoxication and burn, and slightly reduced by 13% after MSC treatment, though not statistically significant (Fig. 3B). These data support our observation that MSC treatment reduces leukocyte infiltration into lung tissue, though only modestly reducing pulmonary inflammation.
Fig 3.
Pulmonary neutrophil and chemokine levels. Lung homogenates were analyzed for levels of A) CXCL1 (KC) and B) IL-6. *p<0.05 versus sham vehicle by One Way ANOVA with Tukey’s post multiple comparison post test. Data are presented as mean pg/mg protein ± SEM. Data combined from 2 independent experiments.
Mesenchymal stem cell treatment lowers post-burn and intoxication serum levels of IL-6 and aminotransferases
Dramatic increases in circulating IL-6 levels have been detected with intoxication and injury, relative to either insult alone [18] and a high serum level of IL-6 in burn injured patients has been correlated with morbidity and mortality [21]. Here, we also found that intoxication and burn injury elevated serum levels of this cytokine from 81.1 ± 57.9 pg/ml in control mice to 709.9 ±123.7 pg/ml in intoxicated burned mice (p<0.05 compared to sham vehicle), and that treating intoxicated, burned animals with MSCs reduced serum IL-6 by 43% to 404.1 ± 108.0 (Table 1). In addition, we also measured serum biomarkers of hepatic damage, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), since the liver is a primary source of circulating IL-6 after intoxication and injury [58]. Consistent with previous reports [12, 13, 17], intoxication and burn injury elevated serum ALT to 217.0 ± 38.2 U/L (p < 0.05) and serum AST to 1035.8 ± 209.0 U/L (p < 0.05), relative to control animal AST (24.3 ± 2.9 U/L) and ALT (145.3 ± 88.2 U/L) levels. Though not statistically significant compared to intoxication and injury, MSC treatment after intoxication and injury trended towards a moderate reduction in ALT levels by 20% to 174.0 ± 50.3 U/L and AST levels by 21% to 815.5 ± 229.2 U/L, to levels not statistically different from controls (Table 1).
Table 1:
Serum cytokine and aminotransferase levels
| Sham Vehicle | Burn Ethanol + PBS | BBurn Ethanol + MSCs | |
|---|---|---|---|
| Serum | |||
| IL-6 | 81.1 ± 57.9 | 709.9 ± 123.7* | 404.1 ± 108.0 |
| ALT | 24.3 ± 2.9 | 217.0 ± 38.2* | 174.0 ± 50.3 |
| AST | 145.3 ± 88.2 | 1035.8 ± 209.0* | 815.5 ± 229.2 |
Serum cytokine and aminotransferase levels. Serum was analyzed for IL-6, AST, and ALT levels. Serum IL-6 levels are presented as mean picograms/ milliliter ± SEM. AST and ALT are presented as the mean units/liter ± SEM.
p<0.05 versus sham vehicle by One-way ANOVA with Tukey’s multiple comparison post-test
Mesenchymal stem cell treatment attenuates hepatic il6 and cxcl1 expression
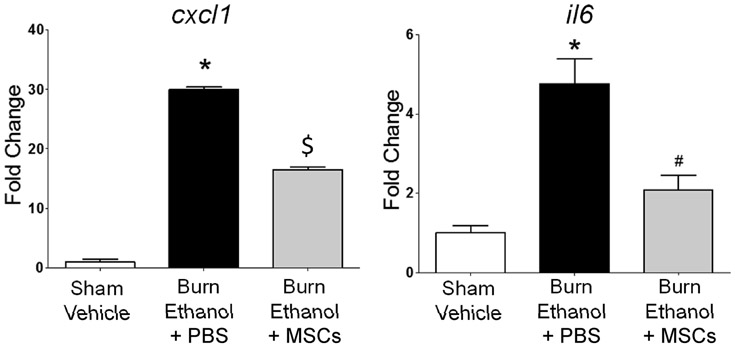
Since biomarkers of liver damage were moderately reduced by MSC treatment after burn injury and ethanol intoxication, we next measured gene expression levels of the pro-inflammatory immunomodulators, cxcl1 and il6, using quantitative RTPCR. Compared to sham-injured, vehicle-treated controls, we observed a dramatic 29-fold increase in liver cxcl1 expression after burn and intoxication (p<0.05 compared to sham vehicle). However, when intoxicated and injured mice were treated with MSCs, cxcl1 did not increase as dramatically, as expression was 17-fold above controls (p<0.05 compared to sham vehicle; p<0.05 compared to burned, intoxicated mice) (Fig. 4A). Similarly, there was a 4.7-fold increase in il6 expression when intoxication preceded burn (p<0.05 compared to control), which was significantly reduced to 2.1-fold above control levels (p<0.05 compared to burn, ethanol treated mice) (Fig. 4B).
Fig. 4.
Gene expression levels of liver cytokines and chemokines. Quantitative RTPCR was performed to measure expression levels of A) cxcl1 and B) il6 in liver tissue. Results were analyzed using the ΔΔCt algorithm[14] with GAPDH as the endogenous control. Data are representative of two independent experiments and presented as mean fold change ± SEM relative to sham-injured, vehicle treated controls. n = 2-6 per group; *p<0.05 compared to sham vehicle, #p<0.05 compared to burn ethanol + PBS, and $p<0.05 compared to all other groups by One-way ANOVA with Tukey’s multiple comparison test.
Mesenchymal stem cell treatment diminishes liver and lung apoptosis
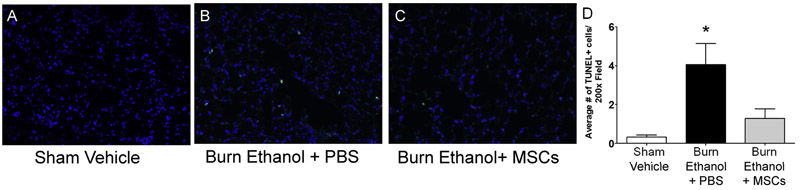
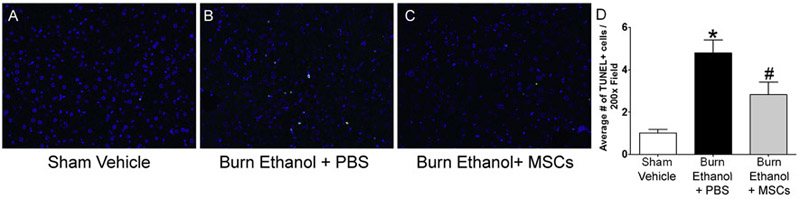
Cutaneous burn injury causes distal organ damage often leading to cell death. We previously reported alveolar macrophage apoptosis in our mouse model of 15% TBSA burn injury and ethanol intoxication [27] and others reported pulmonary microvascular endothelial cell apoptosis in a 30% TBSA rat burn model [59]. In addition, mitochondrial dysfunction, endoplasmic reticulum stress, and hepatocyte apoptosis have been observed in a rat 60%TBSA burn model [60]. Therefore, we next examined cellular apoptosis in liver and lung tissue sections to determine if MSC treatment could limit post-burn and intoxication-driven programmed cell death. Using TUNEL immunofluorescence staining, we quantified the number of apoptotic cells in lung tissue. As previously reported by our laboratory [27] intoxication prior to burn elevates the number of TUNEL+ cells. In this study, animals given ethanol prior to burn had 11.5-fold more apoptotic cells (4.1 ± 1.1 TUNEL+ cells per 200x field) compared to vehicle-treated, sham-injured mice (0.3 ± 0.1 TUNEL+ cells per 200x field) (p<0.05). MSC treatment after intoxication and burn lowers the number of apoptotic cells to 2.9-fold above sham (1.3 ± 0.5 TUNEL+ cells per 200x field), which was not statistically different from other groups. (Fig. 5 A-D) To determine if stem cell treatment decreased liver apoptosis in our mouse ethanol intoxication and burn injury model, we performed TUNEL immunofluorescence staining in liver tissue sections. Intoxication prior to burn injury yielded in a 3.7-fold increase in the number of apoptotic liver cells (4.8 ± 0.6 TUNEL+ cells per 200x field), compared to controls (1.0 ± 0.2 TUNEL+ cells per 200x field) (p<0.05). MSC treatment after intoxication and burn reduced the number of TUNEL+ cells to 2.8 ± 0.6 per 200x field, which was statistically lower than the number of TUNEL+ cells observed in intoxicated, injured mice without MSC treatment (p<0.05) but not different when compared to controls. (Fig. 6 A-D) Together, these results demonstrate MSC treatment diminishes liver and lung apoptosis when ethanol intoxication precedes burn.
Fig. 5.
Quantification of lung apoptosis. Representative images of TUNEL+ cells in A) sham vehicle, B) burn ethanol + PBS, and C) burn ethanol + MSC treated mice are shown, where green indicates TUNEL+ cells and blue indicates DAPI nuclear stain. D) Quantification of TUNEL+ cells. Representative data from two independent experiments are shown, n=4-6 per group, per experiment. Data are presented as the average number of TUNEL+ cells in one 200x high power field. *p<0.05 compared sham-injured, vehicle treated controls, by One-way ANOVA with Tukey’s multiple comparison post-test.
Fig. 6.
Quantification of liver apoptosis. Representative images of TUNEL+ cells in A) sham vehicle, B) burn ethanol + PBS, and C) burn ethanol + MSC treated mice are shown, where green indicates TUNEL+ cells and blue indicates DAPI nuclear stain. D) Quantification of TUNEL+ cells. Data from two independent experiments were combined, n=7-12 per group. Data are presented as the average number of TUNEL+ cells in one 200x high power field. *p<0.05 compared sham-injured, vehicle treated controls, # p<0.05 compared burn-injured, intoxicated mice by One-way ANOVA with Tukey’s multiple comparison post-test.
Discussion
The data presented herein demonstrate that intravenous administration of exogenous MSCs can moderate systemic, lung, and liver inflammation in the context of burn injury with antecedent ethanol intoxication. We observed reduced cellularity, pro-inflammatory cytokine and chemokine production, and apoptosis in the lungs of MSC-treated animals. In addition, circulating biomarkers of liver damage (AST and ALT) were diminished after treatment, which corresponded with lower hepatic apoptosis and inflammatory gene transcription, including the neutrophil chemokine, KC (CXCL1) and IL-6, a key pro-inflammatory cytokine driving multiple organ damage after intoxication and injury.
MSCs anti-inflammatory properties are derived from several mechanisms. Mathias et al. demonstrated that short-term localization of exogenous human MSCs (hMSCs) within the lungs was sufficient to inhibit allergic inflammation. Through clodronate liposome depletion of alveolar macrophages it was established that the anti-inflammatory effect of hMSCs was dependent on the indirect role of the alveolar macrophage in interleukin-10 (IL-10) production [61]. hMSCs can also attenuate allergic inflammation and improve lung function through transforming growth factor β1 (TGFβ1) signaling and promoting an anti-inflammatory alveolar macrophage phenotype [62]. Due to their large size, several studies suggest MSCs become trapped within the pulmonary vasculature with only a fraction of these cells passing through and localizing in other organs [55, 63, 64]. The resulting reduction in capillary flow allows MSCs to secrete anti-inflammatory mediators, including tumor necrosis factor-α induced protein 6, into the lung niche, as well as into the bloodstream, to reduce local inflammation and at the primary site of injury [65]. MSCs secretion of anti-inflammatory mediators, such as IL-10 and TGFβ1, can also down-regulate IL-6 production in alveolar macrophages [62]. While we did not see increased lung IL-10 after MSC treatment in intoxicated, burn-injured mice, we did see a reduction in localized and systemic pro-inflammatory cytokines. It is possible that the secretion of anti-inflammatory mediators by MSCs into the circulation diminished inflammation at the burn site, which in turn, lowers IL-6 release from the wound bed, thereby decreasing systemic IL-6 levels and inflammation. Additionally, MSCs entrapment within the lung capillary bed may induce an anti-inflammatory alveolar macrophage phenotype and help to restore lung homeostasis.
MSCs also elicit anti-inflammatory effects through activation of multiple signaling cascades, inhibition of apoptosis, and enhancement of cellular proliferation. Bone marrow derived MSCs restored renal, hepatic, and pulmonary parameters in a LPS-induced rat sepsis model by attenuation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), signal transducer and activator or transcription-3 (Stat3) and p38-mitogen-activated protein kinases (MAPK) signaling pathways [66]. These signaling pathways have all been implicated as important regulators of inflammatory responses in our mouse model of burn and intoxication. We have previously demonstrated that blockade of p38-MAPK signaling via pharmacologic inhibition (SB203580) reduced hepatic damage, pulmonary inflammation, and alveolar wall thickening after ethanol and burn [17] and that attenuation of IL-6 signaling, through anti-IL-6 antibody and in IL-6 knockout mice, limited pulmonary STAT3 phosphorylation after the combined insult [23]. In addition, we showed that Toll-like receptor (TLR)-4 activation, an upstream activator of NF-κB nuclear translocation, is critical for pulmonary inflammation after intoxication and burn injury [25]. MSCs have also been shown to reduce Kupffer cell apoptosis in a mouse transplant model [67] and lung apoptosis in a LPS-induced rat acute lung injury model. [68], and decrease acute-liver injury related apoptosis in a D-galactosamine and LPS-injury rat model [69]. MSCs directly promote tissue repair in animal models of acute liver failure by inducing renal-tubule epithelial cell proliferation [70]. In this study, we observed a reduction in both liver and lung apoptosis after MSC treatment in mice given ethanol prior to burn.
Several studies have used experimental MSC therapy in burn injury, though most utilized either topical application or injection of MSCs near the site of injury [41, 71-73]. Xue et al. demonstrated the plasticity of MSCs to differentiate into tissue-specific cells and to promote accelerated wound healing, while others have shown an increase in neoangiogenesis and a decrease in cellular infiltration at the wound site [73, 74]. Yagi et al. investigated the therapeutic effectiveness of intramuscular MSC transplantation in rats given a 30% TBSA burn injury, and observed reduced inflammatory cell infiltration, cell death, and inflammatory cytokine levels in multiple organs, including kidney, liver, and lungs, 48 hours after injury [41]. Recently, Oh et al. performed an in vivo mouse experiment where they tracked fluorescently labeled MSCs injected intravenously to the burn injury site using bioluminescence imaging. MSCs appeared localized in the lungs at 24 hours and in the burn lesion after 4 days. A similar bioluminescent MSC trafficking approach showed that MSCs are able to localize to bone fracture injury sites and promote healing but that a large percentage of MSCs are present in the lungs 1 day after transfer and localize to the bone fracture site by 3 to 14 days after administration [75-77]. Of note, intoxication at the time of bone fracture did not inhibit the localization of MSCs to the site of injury [78], suggesting intoxication does not directly affect exogenous MSCs migration through the vascular system. In the current study, we observed MSCs in the lungs of intoxicated burned mice 24 hours after injection, but histological examination of the liver and skin burn margin did not reveal CFSE+ cells in either of these organs (data not shown).
Burn injury increases capillary permeability and causes tissue edema, underscoring the need for fluid resuscitation [79]. Intoxicated burn patients require more fluids compared to those who did not consume alcohol [4]. Our laboratory reported that intoxication heightens post-burn dehydration, even after fluid resuscitation, while also causing a shift in fluid compartments, resulting in greater ischemic end-organ damage [80]. The increase in capillary permeability and loss of fluid from the vascular space may highly influence the fate of infused MSCs. The large size of MSCs may not only trap them within the lung capillary bed, but also retain them within constrained vasculature of intoxicated and burn-injured mice. It is also possible the migration aptitude of the Gibco® MSCs differs from freshly isolated primary bone marrow-derived MSCs. However, published studies have confirmed the immunosuppressive ability of this cell line [81]. Overall, we can conclude that even in the presence of acute dehydration and ischemia, a portion of MSCs are able to localize to the lungs and potentially help mediate pulmonary inflammation and apoptosis, as well as liver and systemic IL-6 and biomarkers of liver damage.
Taken together, these data demonstrate that intravenous infusion of MSCs after burn and ethanol intoxication attenuates circulating, hepatic, and lung inflammation, and further supports the central role of the gut-liver-lung axis in controlling the cytokine storm after injury. Future studies to determine the effectiveness of MSCs in reducing multiple organ failure and sepsis after burn are warranted.
Highlights.
Burn injury and ethanol intoxication leads to excessive inflammation
Mesenchymal stem cell treatment (MSC) attenuates inflammation after burn and intoxication
Circulating biomarkers of liver damage after burn and intoxication are decreased by MSCs
MSC treatment reduces lung and liver inflammation and apoptosis after injury
Acknowledgements
Research in this publication was supported by NIH R01 GM115257 (EJK), R21 AA023193 (EJK), F31 AA022566 (JAS) and the Marian and Ralph C. Falk Medical Research Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Mary Brown and Jessica Palmer for help with animal procedures and tissue processing, Patricia Simms for technical assistance in experiments involving flow cytometry, and Dr. John Callaci for assistance with mesenchymal stem cells.
Research funding: NIH R01 GM115257 (EJK), R21 AA023193 (EJK), F31 AA022566 (JAS), and the Marian and Ralph C Falk Medical Research Trust.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Naimi TS, et al. , Binge drinking among US adults. JAMA, 2003. 289(1): p. 70–5. [DOI] [PubMed] [Google Scholar]
- 2.Savola O, Niemela O, and Hillbom M, Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol Alcohol, 2005. 40(4): p. 269–73. [DOI] [PubMed] [Google Scholar]
- 3.Brezel BS, Kassenbrock JM, and Stein JM, Burns in substance abusers and in neurologically and mentally impaired patients. J Burn Care Rehabil, 1988. 9(2): p. 169–71. [DOI] [PubMed] [Google Scholar]
- 4.Silver GM, et al. , Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J Burn Care Res, 2008. 29(5): p. 784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grobmyer SR, et al. , Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J Burn Care Rehabil, 1996. 17(6 Pt 1): p. 532–9. [DOI] [PubMed] [Google Scholar]
- 6.Choudhry MA and Chaudry IH, Alcohol, burn injury, and the intestine. J Emerg Trauma Shock, 2008. 1(2): p. 81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeschke MG, The hepatic response to thermal injury: is the liver important for postburn outcomes? Mol Med, 2009. 15(9-10): p. 337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruitt BA Jr., Erickson DR, and Morris A, Progressive pulmonary insufficiency and other pulmonary complications of thermal injury. J Trauma, 1975. 15(5): p. 369–79. [PubMed] [Google Scholar]
- 9.Williams FN, et al. , The leading causes of death after burn injury in a single pediatric burn center. Crit Care, 2009. 13(6): p. R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raff T, Germann G, and Barthold U, Factors influencing the early prediction of outcome from burns. Acta Chir Plast, 1996. 38(4): p. 122–7. [PubMed] [Google Scholar]
- 11.Davis CS, et al. , Implications of alcohol intoxication at the time of burn and smoke inhalation injury: an epidemiologic and clinical analysis. J Burn Care Res, 2013. 34(1): p. 120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen MM, et al. , An alteration of the gut-liver axis drives pulmonary inflammation after intoxication and burn injury in mice. Am J Physiol Gastrointest Liver Physiol, 2014. 307(7): p. G711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MM, et al. , Intoxication by intraperitoneal injection or oral gavage equally potentiates postburn organ damage and inflammation. Mediators Inflamm, 2013. 2013: p. 971481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zahs A, et al. , Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. Am J Physiol Gastrointest Liver Physiol, 2012. 303(6): p. G705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavanaugh MJ, et al. , Effect of acute alcohol ingestion prior to burn injury on intestinal bacterial growth and barrier function. Burns, 2005. 31(3): p. 290–6. [DOI] [PubMed] [Google Scholar]
- 16.Emanuele NV, et al. , Ethanol potentiates the acute fatty infiltration of liver caused by burn injury: prevention by insulin treatment. J Burn Care Res, 2009. 30(3): p. 482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MM, et al. , Kupffer Cell p38 Mitogen-Activated Protein Kinase Signaling Drives Postburn Hepatic Damage and Pulmonary Inflammation When Alcohol Intoxication Precedes Burn Injury. Crit Care Med, 2016. 44(10): p. e973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faunce DE, Gregory MS, and Kovacs EJ, Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock, 1998. 10(2): p. 135–40. [DOI] [PubMed] [Google Scholar]
- 19.Yeh FL, et al. , Changes in circulating levels of interleukin 6 in burned patients. Burns, 1999. 25(2): p. 131–6. [DOI] [PubMed] [Google Scholar]
- 20.Ueyama M, et al. , Marked increase in plasma interleukin-6 in burn patients. J Lab Clin Med, 1992. 120(5): p. 693–8. [PubMed] [Google Scholar]
- 21.Meduri GU, et al. , Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest, 1995. 107(4): p. 1062–73. [DOI] [PubMed] [Google Scholar]
- 22.Pileri D, et al. , Concentrations of cytokines IL-6 and IL-10 in plasma of burn patients: their relationship to sepsis and outcome. Ann Burns Fire Disasters, 2008. 21(4): p. 182–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Chen MM, et al. , Pulmonary inflammation after ethanol exposure and burn injury is attenuated in the absence of IL-6. Alcohol, 2013. 47(3): p. 223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bird MD, et al. , Decreased pulmonary inflammation after ethanol exposure and burn injury in intercellular adhesion molecule-1 knockout mice. J Burn Care Res, 2010. 31(4): p. 652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bird MD, et al. , Decreased pulmonary inflammation following ethanol and burn injury in mice deficient in TLR4 but not TLR2 signaling. Alcohol Clin Exp Res, 2010. 34(10): p. 1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel PJ, et al. , Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. Am J Respir Cell Mol Biol, 1999. 20(6): p. 1229–37. [DOI] [PubMed] [Google Scholar]
- 27.Shults JA, et al. , Ethanol intoxication prolongs post-burn pulmonary inflammation: role of alveolar macrophages. J Leukoc Biol, 2016. 100(5): p. 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shults JA, et al. , Impaired respiratory function and heightened pulmonary inflammation in episodic binge ethanol intoxication and burn injury. Alcohol, 2015. 49(7): p. 713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Olmo D, et al. , A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum, 2005. 48(7): p. 1416–23. [DOI] [PubMed] [Google Scholar]
- 30.Le Blanc K, et al. , Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet, 2008. 371(9624): p. 1579–86. [DOI] [PubMed] [Google Scholar]
- 31.Le Blanc K, et al. , Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet, 2004. 363(9419): p. 1439–41. [DOI] [PubMed] [Google Scholar]
- 32.Amann B, et al. , Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant, 2009. 18(3): p. 371–80. [DOI] [PubMed] [Google Scholar]
- 33.Glassberg MK, et al. , Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest, 2017. 151(5): p. 971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antunes MA, et al. , Mesenchymal stem cell trials for pulmonary diseases. J Cell Biochem, 2014. 115(6): p. 1023–32. [DOI] [PubMed] [Google Scholar]
- 35.Mohamadnejad M, et al. , Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med, 2007. 10(4): p. 459–66. [PubMed] [Google Scholar]
- 36.Takami T, Terai S, and Sakaida I, Advanced therapies using autologous bone marrow cells for chronic liver disease. Discov Med, 2012. 14(74): p. 7–12. [PubMed] [Google Scholar]
- 37.Johnson CL, Soeder Y, and Dahlke MH, Concise Review: Mesenchymal Stromal Cell-Based Approaches for the Treatment of Acute Respiratory Distress and Sepsis Syndromes. Stem Cells Transl Med, 2017. 6(4): p. 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng G, et al. , Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res, 2014. 15: p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moodley Y, et al. , Human mesenchymal stem cells attenuate early damage in a ventilated pig model of acute lung injury. Stem Cell Res, 2016. 17(1): p. 25–31. [DOI] [PubMed] [Google Scholar]
- 40.Asmussen S, et al. , Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax, 2014. 69(9): p. 819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yagi H, et al. , Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant, 2010. 19(6): p. 823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J and Hematti P, Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol, 2009. 37(12): p. 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maggini J, et al. , Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One, 2010. 5(2): p. e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemeth K, et al. , Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med, 2009. 15(1): p. 42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dayan V, et al. , Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol, 2011. 106(6): p. 1299–310. [DOI] [PubMed] [Google Scholar]
- 46.Aggarwal S and Pittenger MF, Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 2005. 105(4): p. 1815–22. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal NR, King LS, and D'Alessio FR, Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol, 2014. 306(8): p. L709–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herold S, Mayer K, and Lohmeyer J, Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol, 2011. 2: p. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porcheray F, et al. , Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol, 2005. 142(3): p. 481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weir C, et al. , Mesenchymal stem cells: isolation, characterisation and in vivo fluorescent dye tracking. Heart Lung Circ, 2008. 17(5): p. 395–403. [DOI] [PubMed] [Google Scholar]
- 51.Faunce DE, Gregory MS, and Kovacs EJ, Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J Leukoc Biol, 1997. 62(6): p. 733–40. [DOI] [PubMed] [Google Scholar]
- 52.Messingham KA, et al. , Cellular immunity after ethanol exposure and burn injury: dose and time dependence. Alcohol, 2000. 22(1): p. 35–44. [DOI] [PubMed] [Google Scholar]
- 53.Murdoch EL, et al. , Effects of ethanol on pulmonary inflammation in postburn intratracheal infection. J Burn Care Res, 2008. 29(2): p. 323–30. [DOI] [PubMed] [Google Scholar]
- 54.Faunce DE, et al. , Neutrophil chemokine production in the skin following scald injury. Burns, 1999. 25(5): p. 403–10. [DOI] [PubMed] [Google Scholar]
- 55.Eggenhofer E, et al. , Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol, 2012. 3: p. 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boehmer ED, et al. , Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol, 2004. 75(2): p. 342–9. [DOI] [PubMed] [Google Scholar]
- 57.Murdoch EL, et al. , Prolonged chemokine expression and excessive neutrophil infiltration in the lungs of burn-injured mice exposed to ethanol and pulmonary infection. Shock, 2011. 35(4): p. 403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bankey PE, et al. , Interleukin-6 production after thermal injury: evidence for nonmacrophage sources in the lung and liver. Surgery, 1995. 118(2): p. 431–8; discussion 438-9. [DOI] [PubMed] [Google Scholar]
- 59.Bai X, et al. , SIRT1 protects rat lung tissue against severe burn-induced remote ALI by attenuating the apoptosis of PMVECs via p38 MAPK signaling. Sci Rep, 2015. 5: p. 10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeschke MG, et al. , Insulin protects against hepatic damage postburn. Mol Med, 2011. 17(5-6): p. 516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathias LJ, et al. , Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. J Immunol, 2013. 191(12): p. 5914–24. [DOI] [PubMed] [Google Scholar]
- 62.Song X, et al. , Mesenchymal stem cells alleviate experimental asthma by inducing polarization of alveolar macrophages. Inflammation, 2015. 38(2): p. 485–92. [DOI] [PubMed] [Google Scholar]
- 63.Fischer UM, et al. , Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev, 2009. 18(5): p. 683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schrepfer S, et al. , Stem cell transplantation: the lung barrier. Transplant Proc, 2007. 39(2): p. 573–6. [DOI] [PubMed] [Google Scholar]
- 65.Lee RH, et al. , Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell, 2009. 5(1): p. 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zaki OS, et al. , Bone Marrow Mesenchymal Stem Cells Combat Lipopolysaccharide-Induced Sepsis in Rats via Amendment of P38-MAPK Signaling Cascade. Inflammation, 2018. 41(2): p. 541–554. [DOI] [PubMed] [Google Scholar]
- 67.Tian Y, et al. , Mesenchymal stem cells improve mouse non-heart-beating liver graft survival by inhibiting Kupffer cell apoptosis via TLR4-ERK1/2-Fas/FasL-caspase3 pathway regulation. Stem Cell Res Ther, 2016. 7(1): p. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Y, et al. , Protective effect of bone marrow derived mesenchymal stem cells in lipopolysaccharide-induced acute lung injury mediated by claudin-4 in a rat model. Am J Transl Res, 2016. 8(9): p. 3769–3779. [PMC free article] [PubMed] [Google Scholar]
- 69.Cai Y, et al. , Bone marrow-derived mesenchymal stem cells inhibits hepatocyte apoptosis after acute liver injury. Int J Clin Exp Pathol, 2015. 8(1): p. 107–16. [PMC free article] [PubMed] [Google Scholar]
- 70.Togel F, et al. , Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol, 2007. 292(5): p. F1626–35. [DOI] [PubMed] [Google Scholar]
- 71.Xue L, et al. , Effects of human bone marrow mesenchymal stem cells on burn injury healing in a mouse model. Int J Clin Exp Pathol, 2013. 6(7): p. 1327–36. [PMC free article] [PubMed] [Google Scholar]
- 72.Huang L and Burd A, An update review of stem cell applications in burns and wound care. Indian J Plast Surg, 2012. 45(2): p. 229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rasulov MF, et al. , First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med, 2005. 139(1): p. 141–4. [DOI] [PubMed] [Google Scholar]
- 74.Rasulov MF, et al. , Cell transplantation inhibits inflammatory reaction and stimulates repair processes in burn wound. Bull Exp Biol Med, 2006. 142(1): p. 112–5. [DOI] [PubMed] [Google Scholar]
- 75.Granero-Molto F, et al. , Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells, 2009. 27(8): p. 1887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang JW, et al. , Biodistribution and in vivo efficacy of genetically modified human mesenchymal stem cells systemically transplanted into a mouse bone fracture model. Arch Pharm Res, 2013. 36(8): p. 1013–22. [DOI] [PubMed] [Google Scholar]
- 77.Lee SW, et al. , Stem cell-mediated accelerated bone healing observed with in vivo molecular and small animal imaging technologies in a model of skeletal injury. J Orthop Res, 2009. 27(3): p. 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Obermeyer TS, et al. , Mesenchymal stem cells facilitate fracture repair in an alcohol-induced impaired healing model. J Orthop Trauma, 2012. 26(12): p. 712–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demling RH, The burn edema process: current concepts. J Burn Care Rehabil, 2005. 26(3): p. 207–27. [PubMed] [Google Scholar]
- 80.Chen MM, et al. , Alcohol potentiates postburn remote organ damage through shifts in fluid compartments mediated by bradykinin. Shock, 2015. 43(1): p. 80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Djouad F, et al. , Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood, 2003. 102(10): p. 3837–44. [DOI] [PubMed] [Google Scholar]