Abstract
BACKGROUND:
Guidelines recommend nephrology referral for people with advanced non–dialysis-dependent chronic kidney disease, based mostly on survival benefits seen in retrospective studies of dialysis patients, which may not be generalizable to the broader population with chronic kidney disease. We aimed to examine the association between outpatient nephrology consultation and survival in adults with stage 4 chronic kidney disease.
METHODS:
We linked population-based laboratory and administrative data from 2002 to 2014 in Alberta, Canada, on adults with stage 4 chronic kidney disease (sustained estimated glomerular filtration rate ≥ 15 to < 30 mL/min/1.73 m2 for > 90 d), who had never had kidney failure and had had no outpatient nephrology encounter in the 2 years preceding study entry. Participants who had never had an outpatient nephrology visit before renal replacement treatment were considered “unexposed.” Participants who saw a nephrologist during follow-up were considered “unexposed” before the first outpatient nephrology visit and “exposed” thereafter. The primary outcome was all-cause mortality.
RESULTS:
Of the 14 382 study participants (median follow-up 2.7 yr), 64% were aged ≥ 80 years, 35% saw a nephrologist and 66% died during follow-up. Nephrology consultation was associated with lower mortality (hazard ratio [HR] 0.88, 95% confidence interval [CI] 0.82–0.93). The association was strongest in people < 70 years (HR 0.78, 95% CI, 0.65–0.92), progressively weaker with increasing age, and absent in people ≥ 90 years (HR 1.05, 95% CI 0.88–1.25).
INTERPRETATION:
The survival benefit of nephrology consultation in adults with stage 4 chronic kidney disease may be smaller than expected and appears to attenuate with increasing age. These findings should inform recommendations for nephrology referral considering the advanced age of the patient population meeting current referral criteria.
Chronic kidney disease, defined as abnormalities of kidney structure or function for more than 3 months,1 is associated with an increased risk of kidney failure and cardiovascular events.2,3 Worldwide, chronic kidney disease rose from the 27th leading cause of death in 1990 to 18th in 2010.4 Studies have reported that chronic kidney disease affects up to 15% of adults globally,5–7 and that its prevalence increases with age, being as high as 65% in people older than 80 years.8 The extent to which the estimated high prevalence of chronic kidney disease merely reflects the changing worldwide aging population is unclear.9–11 Because the condition is so common, primary care providers are responsible for caring for most people with non–dialysis-dependent chronic kidney disease. To maximize patient benefits and optimize the use of limited health resources, family physicians must decide whom to refer for nephrology care.
Guidelines1,12,13 recommend referral to a nephrologist for people with stages 4–5 chronic kidney disease, defined by estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. These recommendations are based largely on observational studies showing several benefits of nephrology referral, including a 40% reduction in mortality after initiation of dialysis in people who received longer predialysis nephrology care. However, existing evidence is of low-to-moderate quality and may be biased.14 First, most studies focused on the small, selected population of patients with chronic kidney disease who have survived long enough to develop kidney failure, and are not representative of the target population for nephrology referral:14 many candidates for referral will never start dialysis because their disease doesn’t progress, they choose not to receive dialysis, or they die before they progress to kidney failure.15 Second, previous studies looked backward from an event, such as date on which dialysis was begun, to define the optimal timing for nephrology referral, rather than defining a cohort based on information that is available to the treating physicians when they are contemplating referral. The “look-back” approach assumes that physicians know the time to kidney failure or the pace of disease progression in advance — neither is predictable. Finally, earlier studies have failed to address confounding by changes in health status over time properly, assuming that nephrology care improves the course of chronic kidney disease and other comorbid conditions. However, longer duration of care may be a surrogate for a healthier population with less aggressive disease and a better prognosis, regardless of nephrology involvement.16
We investigated the association between nephrology consultation and all-cause mortality in a population-based cohort that included adults who met recommended criteria for nephrology referral.1,12,13 We used a “look-forward” approach to study this association and applied recommended methods to address confounding in longitudinal designs.17,18 Given the increasing prevalence and uncertain clinical meaning of chronic kidney disease with advancing age,9–11,19 we also investigated whether age modifies the association between nephrology consultation and mortality to identify age-defined patient subgroups who are most likely to benefit from nephrology consultation.
Methods
Study design and study cohort
We conducted a population-based cohort study (eFigure 1, Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.181372/-/DC1), using linked administrative and laboratory data from Alberta, Canada.20 We included Alberta residents aged 18 years or older with stage 4 chronic kidney disease, defined based on at least 2 consecutive outpatient eGFR measurements of ≥ 15 and < 30 mL/min/1.73 m2 (estimated using the Chronic Kidney Disease Epidemiology Collaboration equation),21 made over a period longer than 90 days, between July 30, 2002, and Mar. 31, 2014.1,10,19 We used the first eGFR after the 90-day qualifying period to define study entry. We applied the criteria of range of eGFR values ≥ 15 and < 30 mL/min/1.73 m2 and duration for more than 90 days to minimize the inclusion of people with acute kidney injury or unstable clinical conditions, and maximize the inclusion of people with sustained stage 4 chronic kidney disease — those who were eligible for referral in an outpatient setting and were facing a referral decision.1 The study end date was Mar. 31, 2015, allowing for at least 1 year of follow-up for all study participants. We excluded people who received renal replacement therapy before study entry (nephrologists provide renal replacement therapies in Canada), or had an outpatient eGFR measurement < 15 mL/min/1.73 m2 before study entry (the reasons and outcomes of nephrology referral in stage 5 chronic kidney disease may differ from those of stage 4), or who saw a nephrologist in an outpatient setting in the 2 years preceding study entry (they were not facing a referral decision).
Exposure
We identified the first outpatient nephrology visit after study entry, based on provider specialty and location of visit recorded in the Alberta Health Physician Claims database. We defined participants as “unexposed” if they never received an outpatient nephrology visit during follow-up, or the first outpatient nephrology visit during follow-up occurred after they had started renal replacement therapy. We defined participants as “unexposed” before the first outpatient nephrology visit and “exposed” thereafter, regardless of whether they continued to see a nephrologist or not.
Outcome
We ascertained all-cause mortality using a linkage to data from Alberta Vital Statistics.20 We followed participants from study entry until the date of death or censoring, which was the earliest of outmigration from the province or study end.
Covariates
Baseline covariates
We considered demographics, health system factors, kidney health measures, overall health status and drugs dispensed as potential confounders (see Appendix 1 for details).
Time-varying covariates
We considered laboratory covariates (eGFR and albuminuria), the occurrence of cardiovascular and cerebrovascular events (congestive heart failure, myocardial infarction, stroke and transient ischemic attack) and measures of functional status (long-term care) and illness severity (length of hospital stay) as time-varying confounders (Appendix 1).
Statistical analysis
Descriptive analyses
We described baseline characteristics in “unexposed” and “exposed” groups. To characterize patterns of nephrology consultation, we summarized the cumulative incidence, accounting for the competing risk of death, across baseline age categories (< 70, 70–79, 80–89 and ≥ 90 yr); we also studied the influence of key baseline conditions (congestive heart failure, myocardial infarction, stroke, dementia and cancer) on the cumulative incidence of nephrology consultation, across the same age categories. We used the Gray test to compare cumulative incidence functions of nephrology consultation and death for different baseline age groups.22 We described overall crude mortality using the Kaplan–Meier method.
Association between nephrology consultation and mortality
We used a time-varying definition of the exposure to address immortal time bias.23 In all analyses, we used methods addressing time-varying confounding potentially affected by previous exposure (i.e., nephrology consultation).17 For example, eGFR changes over time may influence the referral decision and mortality (confounding), but may also be affected by nephrology care (mediation). To address both immortal time bias and time-varying confounding without removing indirect effects, we used the sequential Cox approach in main analyses (see Appendix 1 for details).18
Effect-measure modification
We assessed whether the association between nephrology consultation and mortality differed across age categories and across groups defined by timing from study entry to the first outpatient nephrology visit (≤ 1, > 1 and ≤ 2, > 2 yr). We tested interactions using likelihood ratio tests. After formal statistical testing, we reformulated the same 2 models using dummies to summarize the hazard ratios (HRs) associated with nephrology consultation across the categories defining each modifier.
Sensitivity analyses
We assessed the robustness of main analyses using marginal structural Cox models17,24 (another method to address time-varying confounding in longitudinal studies). We also repeated sequential Cox regression in propensity score–matched pairs of observations within each “mini-trial” (eTables 1–2, Appendix 1). To decrease the influence of functional changes in eGFR, we repeated all analyses by using a less stringent definition of stage 4 chronic kidney disease, based on moving average eGFR ≥ 15 and < 30 mL/min/1.73 m2 consistently for more than 90 days25 (see Appendix 1 for details).
Other considerations
We verified the proportional-hazards assumption using graphical methods based on residuals. We used R (version 3.5.0) and Stata (version 14) for all analyses. We used a 2-sided p value of ≤ 0.05 for statistical significance.
Ethics approval
This study was approved by the Conjoint Health Research Ethics Board of the University of Calgary, with a waiver of patient consent.
Results
Baseline characteristics
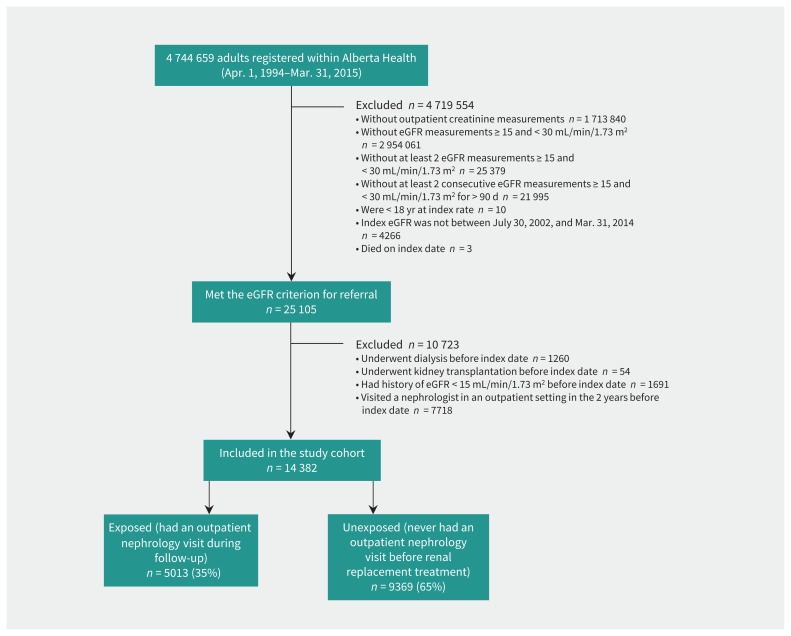
Of the 14 382 study participants (Figure 1), 64% were aged 80 years or older (mean age 81 ± 10 yr) and 35% saw a nephrologist during follow-up (median time-to-visit 7.9 mo from study entry; interquartile range 2.5–20.8 mo). Exposure groups differed in baseline demographics and key laboratory and clinical characteristics (Table 1). Patients who saw a nephrologist were younger, more likely to be male, and to have more rapid kidney function deterioration, severe albuminuria, outpatient nephrology encounters more than 2 years before study entry, and diabetes. They were less likely to have a history of atrial fibrillation, congestive heart failure, chronic pulmonary disease, dementia, stroke or transient ischemic attack, long-term care, or prolonged hospital stay. They were more likely to have been dispensed angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and statins prior to nephrology consultation.
Figure 1:
Derivation of study cohort. Note: eGFR = estimated glomerular filtration rate.
Table 1:
Characteristics of participants at study entry
| Characteristics | Exposed* no. (%)† n = 5013 |
Unexposed* no. (%)† n = 9369 |
Standardized difference‡ |
|---|---|---|---|
| Demographics | |||
| Age, yr, mean ± SD | 75.7 ± 10.7 | 84.5 ± 8.5 | −0.91 |
| Median, yr (IQR) | 77.7 (70.1–83.1) | 85.6 (80.3–90.2) | 0.14 |
| 18–44 yr | 78 (1.6) | 20 (0.2) | 0.41 |
| 45–69 yr | 682 (13.6) | 246 (2.6) | 0.53 |
| 70–79 yr | 2253 (44.9) | 1962 (20.9) | −0.31 |
| 80–89 yr | 1774 (35.4) | 4728 (50.5) | −0.62 |
| ≥ 90 yr | 226 (4.5) | 2413 (25.8) | 0.14 |
| Men | 2219 (44.3) | 3175 (33.9) | 0.21 |
| First Nations | 126 (2.5) | 90 (1.0) | 0.12 |
| Rural residence | 801 (16.0) | 1589 (17.0) | −0.03 |
| Health system factors | |||
| Period of study entry | |||
| 2002–2004 | 1165 (23.2) | 2126 (22.7) | 0.01 |
| 2005–2009 | 2100 (41.9) | 3763 (40.2) | 0.04 |
| 2010–2014 | 1748 (34.9) | 3480 (37.1) | −0.05 |
| Primary care network attachment | 2436 (48.6) | 4974 (53.1) | −0.09 |
| Renal factors | |||
| Index eGFR, mL/min/1.73 m2, median (IQR) | 24.9 (21.5–27.4) | 25.5 (22.4–27.8) | −0.15 |
| 25–29 | 2449 (48.9) | 5120 (54.6) | −0.12 |
| 20–24 | 1722 (34.4) | 3068 (32.7) | 0.03 |
| 15–19 | 842 (16.8) | 1181 (12.6) | 0.12 |
| Disease duration, d, median (IQR) | 144 (108–230) | 166 (112–280) | −0.17 |
| 91–180 | 3194 (63.7) | 5151 (55.0) | 0.18 |
| 181–365 | 1268 (25.3) | 2758 (29.4) | −0.09 |
| 366–730 | 451 (9.0) | 1141 (12.2) | −0.10 |
| > 730 | 100 (2.0) | 319 (3.4) | −0.09 |
| eGFR trajectory, mL/min/1.73 m2/yr | |||
| Improve > 5 | 779 (15.5) | 1551 (16.6) | −0.03 |
| Improve or decline ≤ 5 | 2356 (47.0) | 4922 (52.5) | −0.11 |
| Decline > 5 to ≤ 10 | 804 (16.0) | 1321 (14.1) | 0.05 |
| Decline > 10 | 1074 (21.4) | 1575 (16.8) | 0.12 |
| Prior eGFR, mL/min/1.73 m2 | |||
| ≥ 60 | 90 (1.8) | 152 (1.6) | 0.01 |
| ≥ 30 to < 60 | 3876 (77.3) | 7294 (77.9) | 0.01 |
| Unmeasured | 1047 (20.9) | 1923 (20.5) | −0.01 |
| Albuminuria | |||
| Normal or mild | 2360 (47.1) | 5436 (58.0) | −0.22 |
| Moderate | 1139 (22.7) | 1738 (18.6) | 0.10 |
| Severe | 1450 (28.9) | 1203 (12.8) | 0.40 |
| Unmeasured | 64 (1.3) | 992 (10.6) | −0.40 |
| Outpatient nephrology visit > 2 years before study entry | 814 (16.2) | 825 (8.8) | 0.23 |
| Comorbidities | |||
| Alcohol misuse | 176 (3.5) | 289 (3.1) | 0.02 |
| Atrial fibrillation | 1034 (20.6) | 3019 (32.2) | −0.27 |
| Cancer, lymphoma | 52 (1.0) | 105 (1.1) | −0.01 |
| Cancer, metastatic | 85 (1.7) | 266 (2.8) | −0.08 |
| Cancer, nonmetastatic (breast, cervical, colorectal, lung, prostate) | 398 (7.9) | 853 (9.1) | −0.04 |
| Congestive heart failure | 1596 (31.8) | 4571 (48.8) | −0.35 |
| Chronic pain | 370 (7.4) | 647 (6.9) | 0.02 |
| Chronic pulmonary disease | 1592 (31.8) | 3523 (37.6) | −0.12 |
| Cirrhosis | 32 (0.6) | 56 (0.6) | 0.01 |
| Dementia | 284 (5.7) | 2199 (23.5) | −0.52 |
| Depression | 506 (10.1) | 1202 (12.8) | −0.09 |
| Diabetes | 2520 (50.3) | 3568 (38.1) | 0.25 |
| Hypertension | 4617 (92.1) | 8628 (92.1) | 0.00 |
| Myocardial infarction | 600 (12.0) | 1251 (13.4) | −0.04 |
| Peripheral vascular disease | 371 (7.4) | 672 (7.2) | 0.01 |
| Stroke or transient ischemic attack | 1156 (23.1) | 2919 (31.2) | −0.18 |
| Long-term care | 369 (7.4) | 2247 (24.0) | −0.47 |
| Hospital stay within 1 year before study entry, d | |||
| 0 | 3354 (66.9) | 5677 (60.6) | 0.13 |
| 1–7 | 630 (12.6) | 1154 (12.3) | 0.01 |
| 8–14 | 379 (7.6) | 702 (7.5) | 0.00 |
| 15–28 | 299 (6.0) | 716 (7.6) | −0.07 |
| > 28 | 351 (7.0) | 1120 (12.0) | −0.17 |
| Drugs dispensed | |||
| ACEIs or ARBs | |||
| No | 878 (17.5) | 2782 (29.7) | −0.29 |
| Yes | 3831 (76.4) | 6487 (69.2) | 0.16 |
| Data missing | 304 (6.1) | 100 (1.1) | 0.27 |
| Statins | |||
| No | 2268 (45.2) | 6085 (64.9) | −0.40 |
| Yes | 2400 (47.9) | 3168 (33.8) | 0.29 |
| Data missing | 345 (6.9) | 116 (1.2) | 0.29 |
| NSAIDs | |||
| No | 3502 (69.9) | 7399 (79.0) | −0.21 |
| Yes | 1103 (22.0) | 1837 (19.6) | 0.06 |
| Data missing | 408 (8.1) | 133 (1.4) | 0.32 |
Note: ACEIs = angiotensin-converting enzyme inhibitors, ARBs = angiotensin receptor blockers, eGFR = estimated glomerular filtration rate, IQR = interquartile range, NSAIDs = nonsteroidal anti-inflammatory drugs, SD = standard deviation.
Exposed: patients who had an outpatient nephrology visit during follow-up; unexposed: patients who never had an outpatient nephrology visit before renal replacement treatment.
Unless stated otherwise.
Standardized differences > 0.1 are considered clinically important.
Probability of nephrology consultation
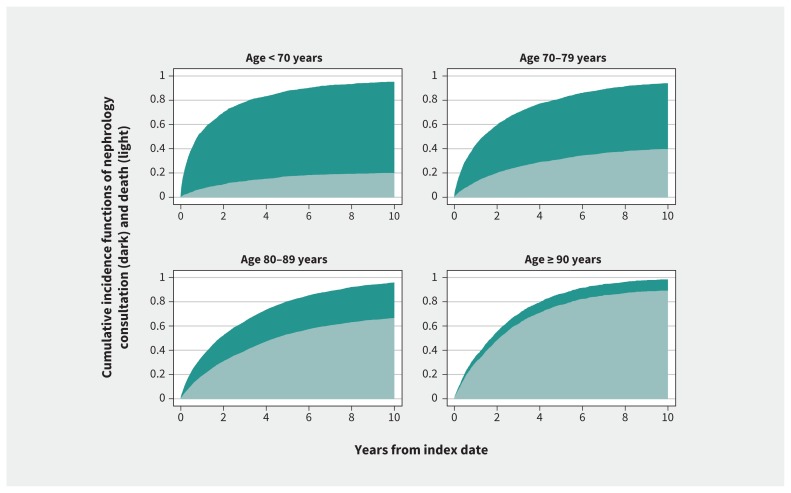
With increasing age, participants were less likely to see a nephrologist and more likely over time to die (p < 0.001) (Figure 2). The incidence of nephrology consultation across age groups also differed by baseline comorbidity (p < 0.001) (eFigure 2, Appendix 1). For example, in people younger than 80 years, a history of congestive heart failure, cancer or dementia was associated with a reduced probability of nephrology consultation. History of myocardial infarction or stroke did not modify the relationship between age and nephrology consultation.
Figure 2:
Probabilities of nephrology consultation and death by age.
Crude mortality
Over a median follow-up of 2.7 years (interquartile range 1.3–4.9), 66% participants died. The overall crude mortality was 21% within the first year of follow-up and about 19% per year in subsequent years (Table 2).
Table 2:
Overall crude mortality by follow-up times
| Follow-up time, yr | No. of deaths | Person-years | Mortality rate per 100 person-years (95% CI) |
|---|---|---|---|
| 0–1 | 2744 | 12 916 | 21 (20–22) |
| 1–2 | 1915 | 10 106 | 19 (18–20) |
| 2–3 | 1348 | 7586 | 18 (17–19) |
| 3–4 | 1065 | 5663 | 19 (18–20) |
| 4–5 | 817 | 4155 | 20 (18–21) |
| > 5 | 1668 | 8864 | 19 (18–20) |
| Total | 9557 | 49 289 | 19 (19–20) |
Note: CI = confidence interval.
Association between nephrology consultation and mortality
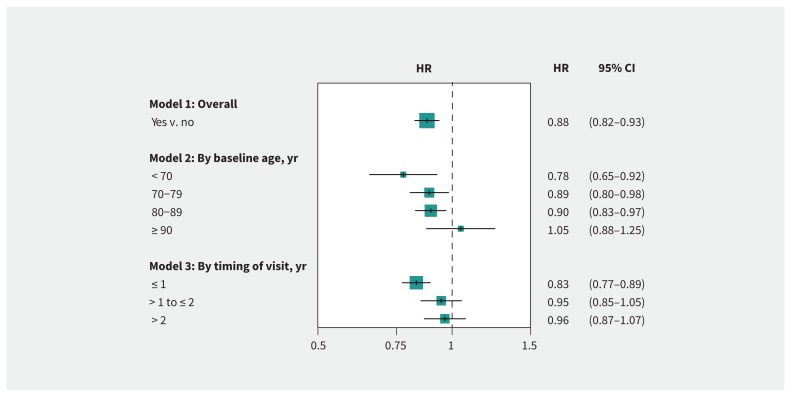
Overall, nephrology consultation was associated with 12% lower mortality compared with no consultation (HR 0.88; 95% confidence interval [CI] 0.82–0.93). The association varied by baseline age, being strongest in people younger than 70 years, weaker in those aged 70–89 years, and nonsignificant for those aged 90 years or older (pinteraction 0.006). The association was larger in people who saw a nephrologist within 1 year of study entry and nonsignificant in those seen more than 1 year after study entry (pinteraction 0.004) (Figure 3). Results were similar in sensitivity analyses (eFigure 3, Appendix 1).
Figure 3:
Association between nephrology consultation and mortality. Hazard ratios (HRs) and 95% confidence intervals (CIs) are from the sequential Cox modelling. See Appendix 1 (Supplemental Methods and eTable 3) for covariates adjusted for in overall analysis (Model 1) and their HRs (95% CIs).
Alternative definition of stage 4 chronic kidney disease
In the sensitivity analyses using moving average eGFR to study the impact of less stringent nephrology referral criteria, we included 20 459 participants. Compared with the main cohort, we observed a similar pattern of nephrology consultation, overall mortality rate and association between nephrology consultation and mortality overall, by baseline age, and by timing of visit ( eTables 4–5, eFigures 4–6, Appendix 1).
Interpretation
In this population-based study of adults with stage 4 chronic kidney disease, we found that the potential survival benefit of nephrology consultation may be smaller than previously described and appeared weaker with increasing patient age. Importantly, we found that as many as two-thirds of people meeting the nephrology referral criterion of stage 4 chronic kidney disease are 80 years of age or older, and two-thirds of study participants died during follow-up.
Age-related prognostic factors and competing demands for management of comorbidities in older adults may partly explain the small survival benefit of nephrology care and a progressively weaker association with older age. Further, stage 4 chronic kidney disease in older adults may be a result of a physiologic decline in eGFR as opposed to a progressive disease.26 These considerations raise the question of the appropriateness of applying universal eGFR-based criteria to define chronic kidney disease and recommend nephrology referral, irrespective of age.10 We also observed that people who did see a nephrologist and those who did not differed in many important clinical characteristics, which may substantially influence the decision to refer for nephrology care and its timing, as well as clinical outcomes. These are inherently difficult to control for using observational designs. Although potential benefits of nephrology care may be larger in people who can see a nephrologist within 1 year or less of meeting the referral criterion, it is also possible that healthier people are more likely to see a nephrologist earlier.
Many clinical practice guidelines recommend referral to nephrology care for people with stage 4 chronic kidney disease for early identification and management of modifiable risk factors for progression of the condition and comorbidities, and for education, planning and preparation for renal replacement therapy or conservative management of kidney failure.1,12,13 However, existing guidelines are based largely on indirect evidence from people treated with dialysis, a small and selected population of patients who are no longer facing a referral decision.14 These studies have reported considerable benefits associated with nephrology referral, including a 40% lower mortality rate, irrespective of patients’ age and comorbidities.14 Yet, the risk of bias in studies restricted to people who survived long enough to begin dialysis therapy may be substantial, considering the high mortality rate observed in our study. There is a lack of trials targeting the broader patient population in whom existing guidelines recommend referral. Two randomized controlled trials (RCTs) compared either intensive multidisciplinary case-management intervention to usual care provided by generalists,27 or nurse-coordinated care (including a nephrologist) to usual care coordinated by a generalist.28 Compared with our population-based study, participants in these 2 trials were younger and had less severe chronic kidney disease. Neither of these trials was powered to detect a potentially small survival benefit. Although nephrology referral may improve many different outcomes, available evidence from trials did not confirm those potential benefits.27,28
Few observational studies have examined the association between nephrology care and mortality in people with non–dialysis-dependent chronic kidney disease. Existing studies provided conflicting results, ranging from a small survival benefit29 to no association.30,31 Heterogeneous findings may be due to differences in participant characteristics (e.g., veterans or younger participants)29–31 or definitions of chronic kidney disease (e.g., varying eGFR thresholds and numbers of eGFR measurements). All existing studies used a “look-back” approach to assess the presence or absence of nephrology care (e.g., any previous contact with a nephrologist,31 1 visit in the past year,30 or using a 1-year landmark period).29 None addressed time-varying confounding. In our population-based, longitudinal design, we applied recommended definitions for stage 4 chronic kidney disease,1 including alternative methods,25 based on laboratory data captured from blood tests done within a defined geographical area. We assessed the impact of nephrology care on mortality in a framework that mirrors clinical practice and accounted for the influence of important baseline and time-varying health information.
Considering the global burden of chronic kidney disease,5–8 guideline recommendations regarding nephrology referral1,12,13 and current health resource constraints,32 our study is important from a patient, care provider and health system perspective. Appropriate nephrology referral can improve outcomes, but unnecessary referral may cause overtreatment without compensatory benefits or reduce access to nephrology services for those more in need. Ideally, the effects of nephrology referral should be tested in a properly designed RCT comparing the effects of nephrology referral on outcomes that are important to patients, including mortality. To devise a trial with mortality as outcome, our study raises some practical considerations related to the choice of the study population and statistical power. Nephrology consultation may confer a small survival benefit requiring a large study size, ranging from 3000 participants for a study power of 80% and type I error of 0.05 to 5000 for a study power of 90% and type I error of 0.01. At least 50 people with stage 4 chronic kidney disease would need to be referred to a nephrologist to prevent 1 death per year, assuming that all people referred to a nephrologist see a nephrologist, a 10% survival benefit of nephrology referral and 20% yearly mortality rate in the nonreferred group. Previous experience27,28 suggests that doing such a trial involving multi-faceted and complex pharmaceutical, educational, dietary and lifestyle interventions is difficult. Before an RCT is conducted in people with chronic kidney disease, further studies are needed to better clarify which patients should be referred, which providers should deliver interventions of proven benefit, and what outcomes are most important to patients, in a robust framework, as free of bias as possible. Although the findings from this study should not deter any treating physicians from considering nephrology referral when eGFR is < 30 mL/min/1.73 m2 (stages 4–5 chronic kidney disease),1,12,13 there are uncertainties as to when and in whom this recommendation should be implemented in clinical practice.
Limitations
Despite our best effort to control for important forms of bias using recommended methods for causal inference,17,18 residual confounding cannot be addressed in nonexperimental designs. As a result, we do not know how close our effect estimates are to the true effects of nephrology consultation, and in which direction unmeasured confounding may have biased the results. Second, we used outpatient nephrology visits to define the exposure, as opposed to nephrology referral. Not all nephrology referrals are necessarily followed by a nephrologist consultation, owing to patient-, physician- or system-related factors. Our “per-visit” analysis may have overestimated the potential survival benefit of an “intent-to-refer” approach. Only an RCT can estimate the true effects of a referral decision. Third, we evaluated mortality because it is an objective outcome that is important to patients. However, it is possible that referral might lead to benefit for other clinically important outcomes that our study did not capture. Fourth, our study population had universal access to specialist care, and thus our findings may not be generalizable to health systems without universal access in which availability and frequency of serum creatinine tests and referral decisions may differ. Finally, our findings may also not be generalizable to conditions for which nephrology referral is indicated, including acute kidney injury, chronic kidney disease with refractory hypertension, electrolyte abnormalities or hereditary kidney diseases.1
Conclusion
The survival benefit of nephrology consultation in adults with stage 4 chronic kidney disease may be smaller than expected and appears to attenuate with increasing age. These findings are relevant to referral practices considering that most patients meeting referral criteria are aged 80 years or older.
Acknowledgements
The authors would like to thank Mr. Jianguo Zhang, Mr. Zhihai Ma, and Mr. James Wick for their assistance with identifying or extracting some data for this study.
Footnotes
Competing interests: Robert Quinn has a patent issued, for a Dialysis Measurement Analysis and Reporting system — a Web-based data collection system to measure performance in dialysis programs. Mohammed Karim reports grants from the BC SUPPORT Unit, Michael Smith Foundation for Health Research and Natural Sciences and Engineering Research Council of Canada, and personal fees from Biogen Inc., outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Ping Liu, Robert Quinn and Pietro Ravani conceived of the work. Ping Liu and Pietro Ravani contributed to the extraction, analysis, and interpretation of data. Mohammed Karim supervised the analysis plan. Ping Liu and Pietro Ravani drafted the manuscript, and all authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Ping Liu is supported by postdoctoral fellowships from the Canadian Institutes of Health Research, the Cumming School of Medicine of the University of Calgary and the Libin Cardiovascular Institute of Alberta.
Disclaimer: This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions are those of the researchers and do not represent the views of the Government of Alberta. Neither the Government of Alberta nor Alberta Health express any opinion in relation to this study.
References
- 1.Chapter 5: Referral to specialists and models of care. Kidney Int Suppl 2011;2013:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013;382:339–52. [DOI] [PubMed] [Google Scholar]
- 3.Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet 2017;389:1238–52. [DOI] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora P, Vasa P, Brenner D, et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ 2013;185:E417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health 2016;4:e307–19. [DOI] [PubMed] [Google Scholar]
- 7.2017 USRDC annual data report: Volume 1 — CKD in the United States. In: Chapter 1: CKD in the general population. Ann Arbor (MI): USRDS Coordinating Center; 2017. Available: www.usrds.org/2017/view/v1_01.aspx (accessed 2018 June 11). [Google Scholar]
- 8.Murphy D, McCulloch CE, Lin F, et al. Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 2016;165:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spence D. Bad medicine: chronic kidney disease. BMJ 2010;340:c3188. [DOI] [PubMed] [Google Scholar]
- 10.Glassock R, Delanaye P, El Nahas M. An age-calibrated classification of chronic kidney disease. JAMA 2015;314:559–60. [DOI] [PubMed] [Google Scholar]
- 11.Ellam T, Twohig H, Khwaja A. Chronic kidney disease in elderly people: disease or disease label? BMJ 2016;352:h6559. [DOI] [PubMed] [Google Scholar]
- 12.Carville S, Wonderling D, Stevens PGuideline Development Group. Early identification and management of chronic kidney disease in adults: summary of updated NICE guidance. BMJ 2014;349:g4507. [DOI] [PubMed] [Google Scholar]
- 13.Levin A, Hemmelgarn B, Culleton B, et al. Canadian Society of Nephrology. Guidelines for the management of chronic kidney disease. CMAJ 2008;179: 1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smart NA, Dieberg G, Ladhani M, et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev 2014;(6):CD007333. [DOI] [PubMed] [Google Scholar]
- 15.Shardlow A, McIntyre NJ, Fluck RJ, et al. Chronic kidney disease in primary care: outcomes after five years in a prospective cohort study. PLoS Med 2016;13: e1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu P, Quinn RR, Oliver MJ, et al. Association between duration of predialysis care and mortality after dialysis start. Clin J Am Soc Nephrol 2018;13:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- 18.Gran JM, Røysland K, Wolbers M, et al. A sequential Cox approach for estimating the causal effect of treatment in the presence of time-dependent confounding applied to data from the Swiss HIV Cohort Study. Stat Med 2010;29:2757–68. [DOI] [PubMed] [Google Scholar]
- 19.Moynihan R, Glassock R, Doust J. Chronic kidney disease controversy: how expanding definitions are unnecessarily labelling many people as diseased. BMJ 2013;347:f4298. [DOI] [PubMed] [Google Scholar]
- 20.Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol 2009;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate [published erratum in Ann Intern Med 2011;155:408]. Ann Intern Med 2009; 150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–54. [Google Scholar]
- 23.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 24.Xiao Y, Abrahamowicz M, Moodie EE. Accuracy of conventional and marginal structural Cox model estimators: a simulation study. Int J Biostat 2010;6:13. [DOI] [PubMed] [Google Scholar]
- 25.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death [published erratum in Ann Intern Med 2011;171:1919]. Arch Intern Med 2011;171:226–33. [DOI] [PubMed] [Google Scholar]
- 26.Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol 2017;13:104–14. [DOI] [PubMed] [Google Scholar]
- 27.Harris LE, Luft FC, Rudy DW, et al. Effects of multidisciplinary case management in patients with chronic renal insufficiency. Am J Med 1998;105:464–71. [DOI] [PubMed] [Google Scholar]
- 28.Barrett BJ, Garg AX, Goeree R, et al. A nurse-coordinated model of care versus usual care for stage 3/4 chronic kidney disease in the community: a randomized controlled trial. Clin J Am Soc Nephrol 2011;6:1241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung E, Chang TI, Chertow GM, et al. Receipt of nephrology care and clinical outcomes among veterans with advanced CKD. Am J Kidney Dis 2017;70: 705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saab G, Chen SC, Li S, et al. KEEP Investigators. Association of physician care with mortality in Kidney Early Evaluation Program (KEEP) participants. Am J Kidney Dis 2012;59(Suppl 2):S34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricardo AC, Roy JA, Tao K, et al. CRIC Study Investigators. Influence of nephrologist care on management and outcomes in adults with chronic kidney disease. J Gen Intern Med 2016;31:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bello AK, Levin A, Tonelli M, et al. Assessment of global kidney health care status. JAMA 2017;317:1864–81. [DOI] [PMC free article] [PubMed] [Google Scholar]