Summary
Stem cell therapy has great prospects for brain white matter disorders, including the genetically determined disorders called leukodystrophies. We focus on the devastating leukodystrophy vanishing white matter (VWM). Patients with VWM show severe disability and early death, and treatment options are lacking. Previous studies showed successful cell replacement therapy in rodent models for myelin defects. However, proof-of-concept studies of allogeneic cell replacement in models representative of human leukodystrophies are lacking. We tested cell replacement in a mouse model representative of VWM. We transplanted different murine glial progenitor cell populations and showed improved pathological hallmarks and motor function. Improved mice showed a higher percentage of transplanted cells that differentiated into GFAP+ astrocytes, suggesting best therapeutic prospects for replacement of astroglial lineage cells. This is a proof-of-concept study for cell transplantation in VWM and suggests that glial cell replacement therapy is a promising therapeutic strategy for leukodystrophy patients.
Keywords: cell replacement therapy, astrocytes, oligodendrocytes, glial cells, white matter disorder, vanishing white matter, leukodystrophy, stem cells
Graphical Abstract
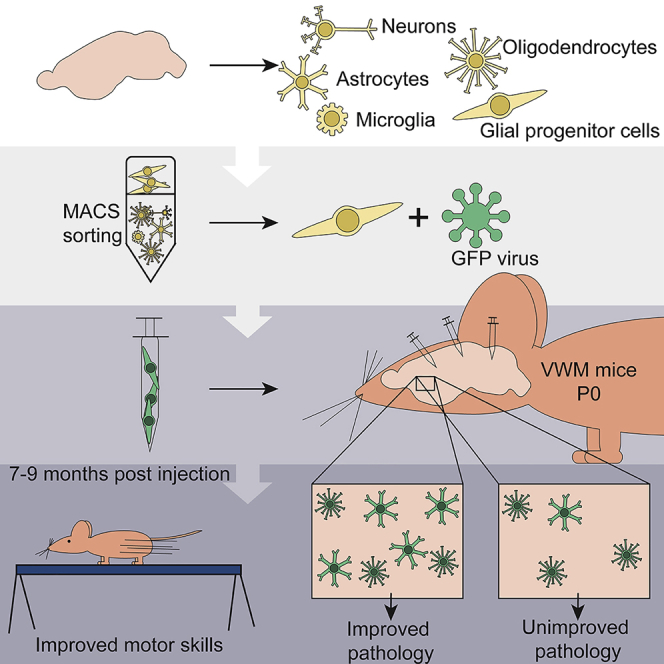
Highlights
-
•
Cell therapy improved pathology and motor skills in vanishing white matter mice
-
•
Astrocyte differentiation of donor cells was associated with recovery of VWM symptoms
Vanishing white matter (VWM) is a severe genetic white matter disorder for which no curative treatment is available. Heine and colleagues show that transplantation with glial progenitor cells can improve pathology and motor skills in VWM mice. Improvement was correlated with increased astrocyte differentiation of donor cells, showing that astrocyte targeting is essential for VWM therapy.
Introduction
Leukodystrophies are genetic disorders that affect the white matter of the brain. They form a group of many different, rare disorders, all characterized by neurological dysfunction leading to severe disability. While leukodystrophies are caused by mutations in genes expressed in different cell types, glial cells play a major role in disease pathology. Glia replacement therapy is seen as one of the promising treatment options for leukodystrophies (van der Knaap et al., 2016). The transplanted healthy glia are expected to compensate for the aberrant functioning of the host glia in the brains of leukodystrophy patients. Early studies showed myelin formation after transplantation with oligodendrocyte progenitor cells (OPCs) in myelin mutants or demyelinated lesions in rodents (Groves et al., 1993, Learish et al., 1999). Previous studies showed that transplantation of human glial progenitor cells (GPCs) in the shiverer mouse, a hypomyelinating mouse model, resulted in structurally normal myelin sheets and increased survival, with a rescue of the shiverer phenotype in about 20% of the animals (Windrem et al., 2008). Shiverer mice have a deletion of a large part of the Mbp gene, which codes for an essential myelin protein, and therefore do not produce functional myelin (Readhead and Hood, 1990). The shiverer mice are often used as a model for hypomyelinating leukodystrophies, although there are no patients known with hypomyelination due to MBP mutations. The first clinical trial transplanting human glia in patients with leukodystrophy Pelizaeus-Merzbacher disease (PMD) showed no major side effects, indicating that this treatment option is safe (Gupta et al., 2012). To allow new clinical trials with therapeutic effects, we need cell replacement studies in animal models that are representative of human leukodystrophy (Marteyn et al., 2016).
Here we present a proof of concept that glia replacement has a therapeutic effect on vanishing white matter (VWM). VWM is one of the more prevalent leukodystrophies, caused by recessive mutations in EIF2B1-5 (Leegwater et al., 2001). It is a progressive disease with most often an early-childhood onset (van der Knaap et al., 2006). The brain white matter of VWM patients is diffusely abnormal and cavitated, and shows selectively affected oligodendrocytes and astrocytes (Bugiani et al., 2013). Currently no curative treatment is available for VWM. We transplanted murine GPCs in the neonatal brain of VWM mice (Dooves et al., 2016), which recapitulate the human disease with a shortened lifespan, ataxia, and affected glia. One-third of the transplanted animals showed significant pathological improvement, which was independently confirmed by discriminant analysis. Motor skills, as assessed by the time to cross the balance beam, also showed significant improvements in VWM mice after cell transplantation.
Results
Motor Skills Were Improved after Transplantation
VWM mice (2b5ho) were transplanted with either saline or primary mouse GPCs at postnatal day 0 (P0) at six injection sites, bilaterally in the anterior and posterior corpus callosum, and in the cerebellum. GPCs were sorted based on marker expression for A2B5 (mixed GPCs; Zhang et al., 2014), GLAST (astroglial lineage; Zhang et al., 2014), or PDGFαR (OPCs; Sim et al., 2011), and labeled with GFP before injection.
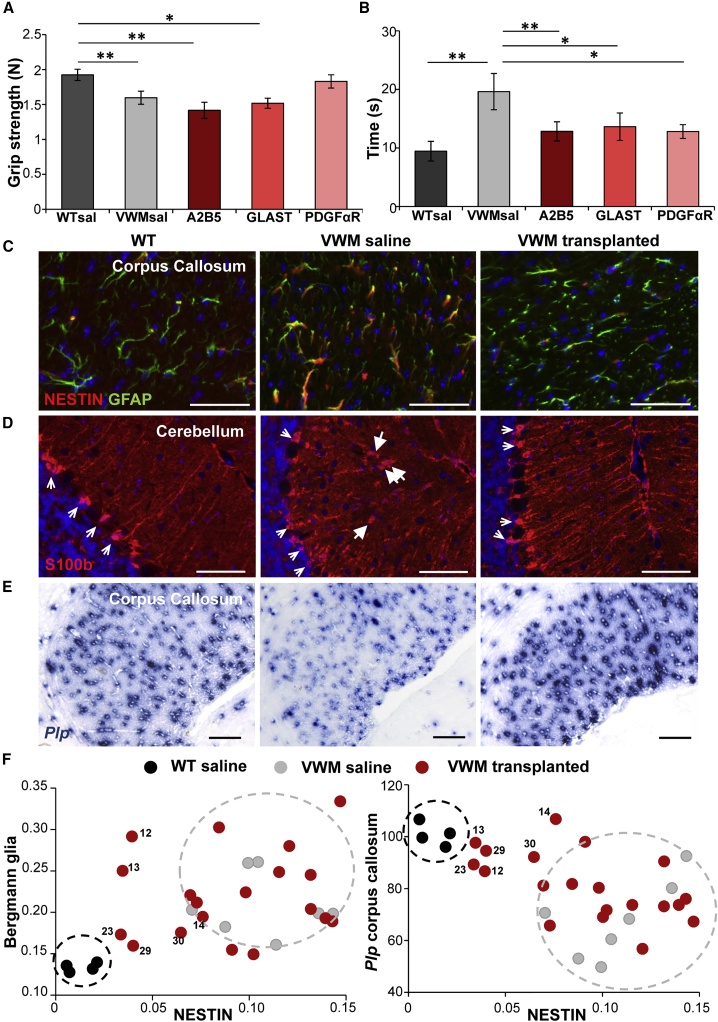
Previous studies showed that VWM mice show motor deficits (Dooves et al., 2016). We tested whether cell transplantation gave improved performance on the balance beam, grip strength, and footprint tests. No significant improvement was observed in the number of slips on the balance beam, in gait parameters, or on grip strength (Figure 1A). Interestingly, for all cell types the injected animals showed a significant improvement on the time to cross the balance beam (Figure 1B), indicating that cell transplantation led to functional improvement.
Figure 1.
Improved VWM Pathology and Motor Dysfunction after Glial Cell Transplantation
(A) Strength of hind and front paws combined as measured on a grip strength meter in Newton. VWM saline (VWMsal) mice have significantly less strength in their paws compared with WT saline (WTsal) mice. This was similar for mice injected with A2B5+ and GLAST+ GPC populations. Mice that received PDGFαR+ GPCs did not show significant differences with the VWMsal mice or WTsal mice.
(B) Time to cross the balance beam (in seconds) for all groups of mice. VWMsal mice were significantly slower than WTsal mice, but all cell-treated groups showed a significant improvement.
(C–E) Representative pictures of the VWM disease markers in WT, saline-treated, and cell-transplanted VWM mice (mouse 29) that showed improvement. (C) NESTIN and GFAP double staining in the corpus callosum. (D) S100β staining; big arrows show translocated Bergmann glia, small arrows show correctly located Bergmann glia. (E) Plp in situ hybridization.
(F) Quantification of the disease markers in all animals. Every data point represents an individual mouse. WT mice: n = 4, black; VWM saline mice: n = 7, gray; VWM transplanted mice: n = 19, red. The circles in black and gray illustrate the range of values of WT and saline VWM mice, respectively. In both plots a number of transplanted animals cluster more with the WT animals than with the saline-treated mice; these mice are indicated with numbers.
Graphs in (A) and (B) show mean of individual mice ± SEM. WTsal n = 36, VWMsal n = 31, A2B5 n = 9, GLAST n = 7, PDGFαR n = 6. ∗p < 0.05, ∗∗p < 0.01. Scale bars, 50 μm. See also Figure S1A.
Transplantation Improved the Brain Pathology in a Subset of VWM Mice
VWM mice and patients are characterized by immature and abnormal astrocytes and oligodendrocytes in the white matter of the brain. We previously established that these pathological changes can be measured with three quantitative markers: (1) an increased number of NESTIN+ astrocytes in the corpus callosum; (2) an increased number of translocated Bergmann glia in the cerebellum (from the Purkinje cell layer into the molecular layer); and (3) a decreased number of Plp-expressing oligodendrocytes in the corpus callosum and white matter of the cerebellum compared with control (Dooves et al., 2018). These VWM disease markers were assessed in the 9-month-old saline and cell-treated animals (Figures 1C–1E and S1A; Table S1). A number of cell-treated mice presented values on the pathological parameters that were more comparable with wild-type (WT) than with saline-treated VWM mice (Figures 1F and S1A; Table S1). To independently identify the improved VWM mice, we implemented a discriminant analysis. Optimal discriminant function parameters were determined using SPSS software based on scores on the pathology parameters in control groups: the saline-injected WT (WTsal) and VWM (VWMsal) mice. The discriminant function was applied to all transplanted animals and classified 6 out of 19 transplanted mice as “WT,” indicating that these mice had scores on the disease markers that are more comparable with WTsal mice than VWMsal mice (Tables 1 and S1). Surprisingly, the GPC populations showed no obvious differences in improvement on the VWM disease parameters: all groups contained improved animals (improvement in 3 out of 8 A2B5+, 1 out of 6 GLAST+, 2 out of 5 PDGFαR+ cell-injected mice). Thus cell transplantation led to improvements in the pathological hallmarks of VWM in a subset of mice, with no clear indications that a specific GPC population was better than the others.
Table 1.
Discriminant Analysis Classifies One-Third of Transplanted Animals as WT
| Mouse Number | Groupa | Genotypeb | Predicted Genotypec | Probability WTd |
|---|---|---|---|---|
| 1 | Saline | WT | WT | 1.0000 |
| 2 | Saline | WT | WT | 1.0000 |
| 3 | Saline | WT | WT | 1.0000 |
| 4 | Saline | WT | WT | 1.0000 |
| 5 | Saline | VWM | VWM | 0.0000 |
| 6 | Saline | VWM | VWM | 0.0000 |
| 7 | Saline | VWM | VWM | 0.0000 |
| 8 | Saline | VWM | VWM | 0.0000 |
| 9 | Saline | VWM | VWM | 0.0000 |
| 10 | Saline | VWM | VWM | 0.0000 |
| 11 | Saline | VWM | VWM | 0.0000 |
| 12e | A2B5 | VWM | WT | 1.0000 |
| 13e | A2B5 | VWM | WT | 1.0000 |
| 14e | A2B5 | VWM | WT | 1.0000 |
| 15 | A2B5 | VWM | VWM | 0.0000 |
| 16 | A2B5 | VWM | VWM | 0.0000 |
| 17 | A2B5 | VWM | VWM | 0.0000 |
| 18 | A2B5 | VWM | VWM | 0.0004 |
| 19 | A2B5 | VWM | VWM | 0.0003 |
| 20 | GLAST | VWM | VWM | 0.0005 |
| 21 | GLAST | VWM | VWM | 0.0000 |
| 22 | GLAST | VWM | VWM | 0.0246 |
| 23e | GLAST | VWM | WT | 0.9998 |
| 24 | GLAST | VWM | VWM | 0.0000 |
| 25 | GLAST | VWM | VWM | 0.0000 |
| 26 | PDGFαR | VWM | VWM | 0.0000 |
| 27 | PDGFαR | VWM | VWM | 0.0000 |
| 28 | PDGFαR | VWM | VWM | 0.0000 |
| 29e | PDGFαR | VWM | WT | 0.9999 |
| 30e | PDGFαR | VWM | WT | 0.9992 |
See also Table S1.
Saline = saline-injected mice; A2B5, GLAST, and PDGFαR = mice injected with primary mouse cells sorted on marker expression of the named proteins.
Genotype of mice: WT, wild-type mice; VWM, vanishing white matter mice.
Predicted genotype according to discriminant analysis.
Probability of a mouse to have a WT genotype according to the discriminant analysis.
Underlining indicates transplanted animals that were classified as WT in the discriminant analysis.
Transplanted Primary Mouse Cells Survived and Integrated into Host Tissue
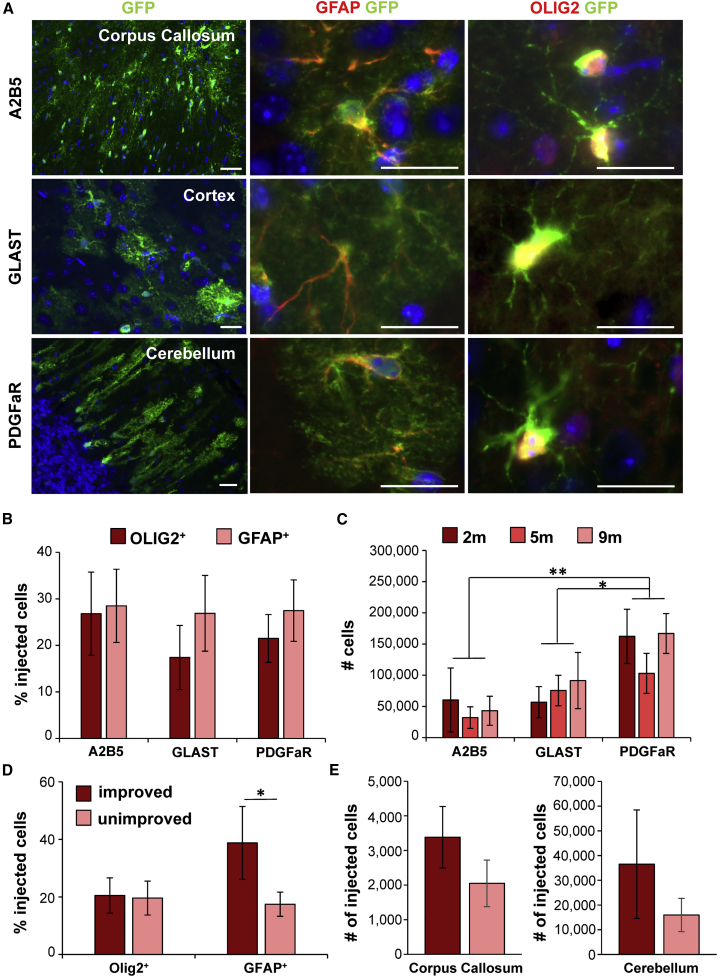
After transplantation, all GPC populations integrated into the host tissue (Figures 2A, S1B, and S1C). GFP-labeled donor cells were found in different brain areas of transplanted mice (Figure S1C). To study whether the in vivo microenvironment affected the fate of the donor cells, we analyzed the survival and cell fate of the grafted GPCs. After transplantation, all primary GPC populations differentiated into astrocytes and oligodendrocytes (Figures 2A and 2B). The A2B5+ and PDGFαR+ GPCs showed no significant changes in the percentage of OLIG2+ and GFAP+ donor cells over time. Although after injection of GLAST+ GPCs, OLIG2+ and GFAP+ cells were present at all ages, their percentages showed a decrease between 2 and 9 months (OLIG2 2 months 39% 9 months 1%; GFAP 2 months 53%, 9 months 5%). No significant changes were observed in the glial fate between the different GPC populations after transplantation (Figure 2B).
Figure 2.
All GPC Populations Show Similar Glial Fate In Vivo while Improved Mice Show Increased Astrocytic Differentiation
(A) After transplantations, all GPCs integrated into various brain areas (left) and were capable of differentiating into astrocytes (middle) and oligodendrocytes (right).
(B) Analysis of cell fate showed that cells from all GPC populations differentiated into GFAP+ astrocytes and OLIG2+ oligodendrocytes.
(C) The number of donor cells was similar in 2-, 5-, and 9-month-old mice. The survival of PDGFαR+ cells after transplantation was significantly increased compared with A2B5+ and GLAST+ cells.
(D) Improved mice had a significantly higher percentage of injected cells (GFP+) that were GFAP+ compared with unimproved mice.
(E) Improved mice showed a higher average number of donor cells present in the corpus callosum and cerebellum, albeit not significantly.
In (B) and (C), A2B5+ n = 13 mice; GLAST+ n = 10 mice; PDGFαR+ n = 17 mice. In (D) and (E), improved n = 6 mice; unimproved n = 18 mice. (B) to (E) show mean ± SEM; ∗p < 0.05, ∗∗p < 0.01. Scale bars, 20 μm. See also Figures S1B and S1C.
To analyze differences in survival, we quantified the total number of engrafted cells at 2, 5, and 9 months for each cell population. About 40% of the injected cells survived and integrated into the host tissue (Figure 2C). No significant changes in cell survival were observed over time. However, the mice that received PDGFαR+ cell injections showed a higher number of engrafted cells than mice that received A2B5+ or GLAST+ cell injections (p < 0.001; Figure 2C). Interestingly, the improved animals contained donor cell populations with a higher percentage GFAP+ cells than the unimproved animals (p = 0.04, Figure 2D). Although not significantly different, improved mice showed a higher number of integrated cells in the corpus callosum and the cerebellum, areas important for VWM pathology (Figure 2E).
To conclude, after in vivo transplantations no differences in differentiation patterns between GPC populations are observed at 2, 5, or 9 months after cell transplantation, but the PDGFαR+ GPC population showed a higher cell engraftment than the A2B5+ or GLAST+ GPC populations. In comparison with unimproved mice, the improved mice showed a higher percentage of engrafted cells that were GFAP+.
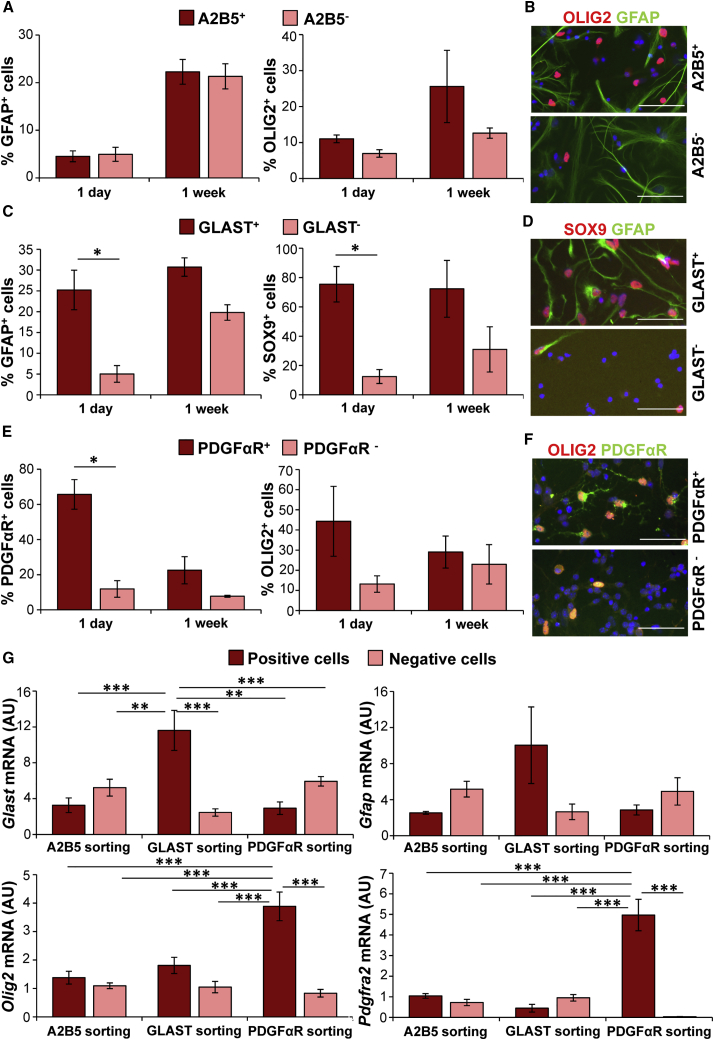
GPC Populations Showed Minor Differences after In Vitro Maintenance
We performed a more detailed analysis on whether the performed cell sorting resulted in GPC populations with different properties. Cells were plated and analyzed by immunostaining at 1 day and 1 week after sorting (Figure 3). The A2B5+ population showed a small (non-significant) increase in percentage of OLIG2+ cells compared with the A2B5− population after sorting (Figures 3A and 3B). The GLAST+ population showed a significantly increased percentage of astroglial lineage cells (GFAP+ p = 0.021 and SOX9+ p = 0.021) at 1 day after sorting (Figures 3C and 3D). At 1 week, significant differences between the GLAST+ and GLAST− population were no longer observed. The PDGFαR+ population showed a significantly increased percentage of PDGFαR+ cells (p = 0.05) at day 1 after sorting compared with the PDGFαR− population; this percentage was diminished after 1 week in culture (Figures 3E and 3F).
Figure 3.
Sorted GPCs Show Different Identity after Short-Term Maintenance In Vitro
Primary cells were sorted by MACS for marker expression of A2B5, GLAST, or PDGFαR.
(A–F) Immunostaining of cells from positive selection and cells that were not bound to microbeads (“negative”) after 1 day and 1 week in culture. (A and B) A2B5+ and A2B5− population both are positive for GFAP and OLIG2. (C and D) GLAST+ population showed a significantly higher percentage of cells that were positive for astrocyte markers GFAP and SOX9 at 1 day after sorting compared with the GLAST− population. After 1 week in culture there are no longer significant differences. (E and F) PDGFαR+ population showed a significantly higher percentage of cells that are positive for PDGFαR, and an increased number of cells positive for OLIG2, compared with the PDGFαR− population. After 1 week in culture no significant differences are observed.
(G) A qPCR analysis shows that GLAST+ population has a higher expression of Glast, and PDGFαR+ population shows a higher expression of Olig2 and Pdgfra than any of the other conditions.
Error bars represent mean ± SEM. n = 3 independent sortings for all. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (A), (C), and (E) show percentages of positive cells compared with total number of DAPI+ cells. (B), (D), and (F) show examples of immunostainings used for cell counts of cells at 1 day after plating. (G) shows 2ΔCt values of qPCR analysis in arbitrary units (AU). Scale bars, 50 μm. See also Figure S2.
In vitro qPCR analysis immediately after sorting showed that the GLAST+ population expressed a higher amount of Glast (p < 0.01) than all other cell populations (Figure 3G). The PDGFαR+ population had significantly higher expression of Olig2 (p < 0.001) and Pdgfra (p < 0.001) than all other cell populations (Figure 3G).
Earlier studies suggested that regenerative properties of transplanted cells are mediated via secretion of trophic factors (Hawryluk et al., 2012, Pluchino et al., 2009). To identify trophic factor secretion in GPC populations, we studied RNA expression levels of Ngf1, Nt3, Pdgfa, Egf, Lif, Igf1, and Tgfb immediately after sorting. All cell populations expressed all factors with only subtle differences (Figure S2). Significant differences were only observed for the GLAST+ population, which showed a significantly increased expression of Igf1 (p < 0.05; Figure S2) and a significantly decreased expression of Ngf1 (p < 0.01; Figure S2).
While cell sorting was aimed to create GPC populations with diverse differentiation potentials, in vitro analysis showed that over time all populations developed into astrocytic and oligodendrocytic lineage cells and expressed trophic factors with only minor differences.
Discussion
This study shows that neonatal transplantation with GPCs can improve motor skills and the pathology of a mouse model representative of human leukodystrophy VWM. We show that mouse glia, which lack the phylogenetic advantage of human glia, successfully integrate into the host tissue and differentiate into astrocytes and oligodendrocytes. All GPC populations engrafted well, differentiated into astrocytic and oligodendrocytic lineage cells, and were able to improve the VWM phenotype. However, mice that received PDGFaR+ cells showed the highest number of engrafted cells. The mice that showed improvements in pathology had a higher percentage of engrafted cells that differentiated into GFAP+ astrocytes, which was independent of the engrafted GPC population. This suggests that the mechanisms underlying recovery are dependent on astrocytes and that transplantation with a highly astrogenic progenitor population is important. This latter finding is in agreement with a previous study showing an important role for astrocytes in VWM pathology (Dooves et al., 2016), and further suggests that astrocyte replacement has the best therapeutic prospects for VWM.
In this study we used a discriminant analysis to classify transplanted mice into a “WT” or “VWM” phenotype. For treatments that show a large variability in their effects and with small sample sizes, this is a useful tool to identify individual mice or patients that show improvement of their symptoms, even though the means of the treatment groups are not significantly improved (Shayan et al., 2015). We showed that one-third of the transplanted mice showed pathological improvements after cell transplantation, which is consistent with previous studies that transplanted human cells in rodent models of myelin defects (Uchida et al., 2012, Windrem et al., 2008). So far glia transplantations with mouse cells (Lyczek et al., 2017) in models representative of human leukodystrophies have been more challenging. Rodent astrocytes are markedly different from human astrocytes (Oberheim et al., 2009) and lack the phylogenetic advantage that human cells have when transplanted into a rodent model. After neonatal transplantation in mice, human GPCs are able to outcompete their rodent counterparts, leading to mice with essentially a humanized white matter (Windrem et al., 2014). As human glia also do not have a phylogenetic advantage when transplanted in patients, we considered it important to prove recovery after transplanting mouse GPCs into VWM mice.
The therapeutic effects of cell transplants could be improved by changing treatment strategies. First, most leukodystrophies have a complex pathophysiology involving multiple cell types and toxic microenvironments. For example, OPC transplantation in Krabbe disease failed, probably due to the accumulation of psychosine in the microenvironment, which is toxic for mature oligodendrocytes Kuai et al. (2015). A recent study showed that transplantations with human neural precursor cells in a transgenic mouse model for PMD gave better improvements than transplantation with human OPCs, while human OPCs gave better improvements in shiverer mice (Marteyn et al., 2016). This highlights the influence of the microenvironment that is distinct to each disease and the importance of testing specific cell types in appropriate disease models. We showed that all GPC populations expressed trophic factors in comparable amounts. As trophic factors can have an influence on the brain microenvironment (Hawryluk et al., 2012), all animals could have been affected by GPC-secreted factors. The effect of neurotrophic factors on the VWM microenvironment is beyond the scope of this article but is an interesting topic for future studies.
Earlier studies showed that the VWM microenvironment has increased hyaluronan levels (Bugiani et al., 2013, Dooves et al., 2016). As hyaluronan can impede oligodendrocyte maturation (Back et al., 2005), this could be a complicating factor in cell replacement therapy. The current study may have benefited from neonatal transplantations, as no pathology or increased hyaluronan level is observed at that age. Cell transplantations in patients will likely benefit from modulation of the microenvironment by, for example, using drugs that make the microenvironment less hostile for donor cells. Second, injection of neonatal mice with high precision is challenging and induces variability due to the number of surviving cells, the injection location, and the cell fate. For the study of how transplantation parameters and a symptomatic VWM environment affect survival and integration of donor cells, transplantations at a later age could be considered.
For clinical trials we need cell populations that have therapeutic effects and are safe for use in humans. With the discovery of induced pluripotent stem cells (iPSCs) in 2006 (Takahashi and Yamanaka, 2006) and current advantages in gene correction methods (Maeder and Gersbach, 2016), it is possible to make genetically corrected patient-specific iPSC-derived glia, limiting the risk of rejection. In vitro disease modeling with patient iPSCs allows the study of how different mutations affect disease mechanisms (Nevin et al., 2017, Numasawa-Kuroiwa et al., 2014) and human-specific disease aspects. Different studies have shown that iPSC-derived GPCs have potential for white matter repair similarly to GPCs derived from fetal tissue of embryonic stem cells (Ehrlich et al., 2017, Wang et al., 2013). Recently, a clinical study using iPSC-derived cells for macular degeneration showed no tumor formation of the injected cells (Mandai et al., 2017), showing promise for the use of iPSCs in clinical applications.
To conclude, this study demonstrates the potential of cell transplantation for VWM, and shows the efficacy of allogeneic murine cell transplantation in a rodent model for a specific leukodystrophy. Although more research is needed to optimize transplantation parameters, the positive results indicate that stem cell transplantation is a promising therapeutic option for leukodystrophies such as VWM.
Experimental Procedures
Cell Injections
Cells were sorted on marker expression of A2B5, GLAST, or PDGFαR with magnetic-activated cell sorting (MACS) according to the manufacturer's protocol. WT and VWM mice were injected at postnatal day 0 (P0) with sorted cells labeled with GFP virus. At 7 months of age the motor skills of all mice were tested. Brain tissue was collected at 2, 5, and 9 months of age, and used for analysis of cell fate, cell survival, and improvement of pathology. All mouse procedures were carried out according to the guidelines of the Dutch government and approved by the Animal Approval Committee of the VU University, Amsterdam. See Supplemental Experimental Procedures for more details.
In Vitro Cell Analysis
After MACS, cells were either directly used to extract RNA or plated on 8-well chamberslides for immunostaining. RNA was analyzed by qPCR markers for cell fate and neurotrophic factors. Cells that were plated on chamberslides were fixed 1 day and 1 week after sorting. Immunostaining was used to analyze cell fate. See Supplemental Experimental Procedures for more details.
Statistical Analysis
All data were analyzed using SPSS software (IBM SPSS Statistics 20.0). All data were tested for normal distribution and appropriate statistical tests chosen. See Supplemental Experimental Procedures for more details.
Author Contributions
S.D. performed all experiments and analysis. S.K., N.B., S.B., P.S.L., and A.E.J.H. assisted in tissue slicing and immunostainings. G.J. assisted in virus production, cell culture, and genotyping of mice. S.D. and V.M.H. designed the study. S.D. and V.M.H. wrote the article with valuable contributions of M.S.v.d.K.
Acknowledgments
This study was financially supported by the NWO Spinoza grant (M.S.v.d.K.), ZonMw VIDI research grant 91712343 (V.M.H.), the ZonMw TAS IDB project 116005006 (V.M.H.), E-Rare Joint Call project 9003037601 (V.M.H., M.S.v.d.K.), and KNAW Ter Meulen Fonds (S.D.). We thank Paulien Cornelissen for technical assistance.
Published: February 21, 2019
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, two figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2019.01.018.
Supplemental Information
References
- Back S.A., Tuohy T.M., Chen H., Wallingford N., Craig A., Struve J., Luo N.L., Banine F., Liu Y., Chang A. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- Bugiani M., Postma N., Polder E., Dieleman N., Scheffer P.G., Sim F.J., van der Knaap M.S., Boor I. Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain. 2013;136:209–222. doi: 10.1093/brain/aws320. [DOI] [PubMed] [Google Scholar]
- Dooves S., Bugiani M., Postma N.L., Polder E., Land N., Horan S.T., van Deijk A.L., van de Kreeke A., Jacobs G., Vuong C. Astrocytes are central in the pathomechanisms of vanishing white matter. J. Clin. Invest. 2016;126:1512–1524. doi: 10.1172/JCI83908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooves S., Bugiani M., Wisse L.E., Abbink T.E.M., van der Knaap M.S., Heine V.M. Bergmann glia translocation: a new disease marker for vanishing white matter identifies therapeutic effects of Guanabenz treatment. Neuropathol. Appl. Neurobiol. 2018;44:391–403. doi: 10.1111/nan.12411. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Mozafari S., Glatza M., Starost L., Velychko S., Hallmann A.L., Cui Q.L., Schambach A., Kim K.P., Bachelin C. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc. Natl. Acad. Sci. U S A. 2017;114:E2243–E2252. doi: 10.1073/pnas.1614412114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A.K., Barnett S.C., Franklin R.J., Crang A.J., Mayer M., Blakemore W.F., Noble M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993;362:453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- Gupta N., Henry R.G., Strober J., Kang S.M., Lim D.A., Bucci M., Caverzasi E., Gaetano L., Mandelli M.L., Ryan T. Neural stem cell engraftment and myelination in the human brain. Sci. Transl. Med. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk G.W., Mothe A., Wang J., Wang S., Tator C., Fehlings M.G. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21:2222–2238. doi: 10.1089/scd.2011.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai X.L., Ni R.Z., Zhou G.X., Mao Z.B., Zhang J.F., Yi N., Liu Z.X., Shao N., Ni W.K., Wang Z.W. Transplantation of mouse embryonic stem-cell derived oligodendrocytes in the murine model of globoid cell leukodystrophy. Stem Cell Res. Ther. 2015;6:30. doi: 10.1186/s13287-015-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learish R.D., Brustle O., Zhang S.C., Duncan I.D. Intraventricular transplantation of oligodendrocyte progenitors into a fetal myelin mutant results in widespread formation of myelin. Ann. Neurol. 1999;46:716–722. [PubMed] [Google Scholar]
- Leegwater P.A.J., Vermeulen G., Könst A.A.M., Naidu S., Mulders J., Visser A., Kersbergen P., Mobach D., Fonds D., van Berkel C.G.M. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat. Genet. 2001;29:383–388. doi: 10.1038/ng764. [DOI] [PubMed] [Google Scholar]
- Lyczek A., Arnold A., Zhang J., Campanelli J.T., Janowski M., Bulte J.W., Walczak P. Transplanted human glial-restricted progenitors can rescue the survival of dysmyelinated mice independent of the production of mature, compact myelin. Exp. Neurol. 2017;291:74–86. doi: 10.1016/j.expneurol.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder M.L., Gersbach C.A. Genome-editing technologies for gene and cell therapy. Mol. Ther. 2016;24:430–446. doi: 10.1038/mt.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M., Watanabe A., Kurimoto Y., Hirami Y., Morinaga C., Daimon T., Fujihara M., Akimaru H., Sakai N., Shibata Y. Autologous induced stem-cell- derived retinal cells for macular degeneration. N. Engl. J. Med. 2017;376:1038–1046. doi: 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- Marteyn A., Sarrazin N., Yan J., Bachelin C., Deboux C., Santin M.D., Gressens P., Zujovic V., Baron-Van Evercooren A. Modulation of the innate immune response by human neural precursors prevails over oligodendrocyte progenitor remyelination to rescue a severe model of Pelizaeus-Merzbacher disease. Stem Cells. 2016;34:984–996. doi: 10.1002/stem.2263. [DOI] [PubMed] [Google Scholar]
- Nevin Z.S., Factor D.C., Karl R.T., Douvaras P., Laukka J., Windrem M.S., Goldman S.A., Fossati V., Hobson G.M., Tesar P.J. Modeling the mutational and phenotypic landscapes of Pelizaeus-Merzbacher disease with human iPSC-derived oligodendrocytes. Am. J. Hum. Genet. 2017;100:617–634. doi: 10.1016/j.ajhg.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasawa-Kuroiwa Y., Okada Y., Shibata S., Kishi N., Akamatsu W., Shoji M., Nakanishi A., Oyama M., Osaka H., Inoue K. Involvement of ER stress in dysmyelination of Pelizaeus-Merzbacher disease with PLP1 missense mutations shown by iPSC-derived oligodendrocytes. Stem Cell Reports. 2014;2:648–661. doi: 10.1016/j.stemcr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim N.A., Takano T., Han X., He W., Lin J.H., Wang F., Xu Q., Wyatt J.D., Pilcher W., Ojemann J.G. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S., Gritti A., Blezer E., Amadio S., Brambilla E., Borsellino G., Cossetti C., Del Carro U., Comi G., ’t Hart B. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann. Neurol. 2009;66:343–354. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- Readhead C., Hood L. The dysmyelinating mouse mutations shiverer (shi) and myelin deficient (shimld) Behav. Genet. 1990;20:213–234. doi: 10.1007/BF01067791. [DOI] [PubMed] [Google Scholar]
- Shayan Z., Mohammad Gholi Mezerji N., Shayan L., Naseri P. Prediction of depression in cancer patients with different classification criteria, linear discriminant analysis versus logistic regression. Glob. J. Health Sci. 2015;8:41–46. doi: 10.5539/gjhs.v8n7p41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim F.J., McClain C.R., Schanz S.J., Protack T.L., Windrem M.S., Goldman S.A. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nat. Biotechnol. 2011;29:934–941. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Uchida N., Chen K., Dohse M., Hansen K.D., Dean J., Buser J.R., Riddle A., Beardsley D.J., Wan Y., Gong X. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci. Transl. Med. 2012;4:155ra136. doi: 10.1126/scitranslmed.3004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap M.S., Pronk J.C., Scheper G.C. Vanishing white matter disease. Lancet Neurol. 2006;5:413–423. doi: 10.1016/S1474-4422(06)70440-9. [DOI] [PubMed] [Google Scholar]
- van der Knaap M.S., Wolf N.I., Heine V.M. Leukodystrophies: five new things. Neurol. Clin. Pract. 2016 doi: 10.1212/CPJ.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Bater J., Li X., Schanz S., Chandler-Militello D., Levine C., Maherali N., Studer L., Hochedlinger K., Windrem M. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem M.S., Schanz S.J., Guo M., Tian G.F., Washco V., Stanwoord N., Rasband M., Roy N.S., Nedergaard M., Havton L.A. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–565. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem M.S., Schanz S.J., Morrow C., Munir J., Chandler-Militello D., Wang S., Goldman S.A. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brain are chimeric for human glia. J. Neurosci. 2014;34:16153–16161. doi: 10.1523/JNEUROSCI.1510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O’Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.