Abstract
Uterine serous adenocarcinoma is a rare but highly malignant form of endometrial cancer, comprising over 50% of recurrences and deaths from endometrial cancer. We report a case of a 68-year old woman with recurrent uterine serous adenocarcinoma who underwent molecular testing and genetic sequencing of her tumor. She was found to have focal amplification of ERBB2 confirmed by amplification and overexpression of HER2/neu via fluorescence in situ hybridization and immunohistochemistry. Given the identification of this potential target and progression of disease, trastuzumab was added to the patient's chemotherapy regimen with ultimate complete response.
Keywords: Uterine papillary serous carcinoma; Trastuzumab; Precision medicine; Genetic sequencing; HER2/neu; Uterine serous adenocarcinoma, uterine serous carcinoma
Highlights
-
•
68-year old woman with recurrent uterine serous adenocarcinoma who underwent molecular testing of her tumor.
-
•
Amplification of ERBB2 and overexpression of HER2/neu was confirmed.
-
•
Trastuzumab was added to the patient's therapy with complete response.
-
•
Targeted therapies may be advantageous in patients with limited therapeutic options.
1. Introduction
Uterine serous adenocarcinoma is a rare but highly aggressive form of endometrial cancer, accounting for 5 to 10% of all endometrial carcinomas and over 50% of recurrences and deaths (Hamilton et al., 2006; Slomovitz et al., 2003). Uterine serous adenocarcinoma is known for its tendency to invade myometrium and lymphatics. Involvement of the peritoneum, such as omental caking, and parenchymal involvement of abdominal organs is also common even when myometrial invasion is minimal or absent, similar to the pathologic course of epithelial ovarian carcinoma (Slomovitz et al., 2003; Fader et al., 2009; Goff et al., 1994).
Given the tendency for spread and relapse with worse survival rates compared to endometrioid endometrial cancer, the Society for Gynecologic Oncology (SGO) recommends all patients with uterine serous adenocarcinoma undergo comprehensive surgical staging with subsequent adjuvant platinum and taxane-based chemotherapy (Boruta et al., 2009). Numerous studies have demonstrated improved progression-free survival with adjuvant chemotherapy but there is little data to aid in the selection of patients who would potentially benefit from systemic therapy (Fader et al., 2009; Hamilton et al., 2005)
Molecular targeted therapies are becoming an increasingly prominent new area of research in treatment of endometrial cancer within an emerging clinical framework of “Precision Medicine”. The goal of these therapies include blocking specific pathways related to carcinogenesis and tumor growth rather than merely interfering with all rapidly growing cells. With the emergence of next generation sequencing (NGS), genomic sequencing of cancer DNA to identify potential therapeutic targets is now a reality for many patients with uterine serous adenocarcinoma. We present the case of a successful application of precision medicine in a patient with recurrent uterine serous adenocarcinoma.
2. Case
A 68-year-old woman who presented with postmenopausal vaginal bleeding and was diagnosed with serous adenocarcinoma (FIGO grade 3) of the endometrium with areas suggestive of malignant mixed mullerian tumor (MMMT) was referred to our department for further management. The preoperative evaluation revealed no evidence of metastatic disease. The patient underwent a robotic-assisted total hysterectomy, bilateral salpingoophorectomy, pelvic and para-aortic lymph node dissection and omentectomy. Surgical pathology confirmed serous adenocarcinoma of the endometrium with minor (less than 5%) component of MMMT invading 1 cm of a 4 cm thick myometrium and diffusely covering the entire endometrial cavity extending to the lower uterine segment, with pelvic washings positive for adenocarcinoma. No cervical, serosal or adnexal involvement or capillary-lymphatic space invasion was identified, and all lymph nodes were negative for malignancy, consistent with stage IA. Six months after her initial diagnosis, the patient successfully completed six cycles of adjuvant paclitaxel and carboplatin, as well as vaginal brachytherapy. There was no evidence of disease on physical exam or post-therapy computed tomography (CT) scan.
Thirteen months after completing adjuvant chemotherapy, the patient underwent a surveillance fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT scan which demonstrated new retroperitoneal and supraclavicular lymphadenopathy (Fig. 1). Biopsy of the supraclavicular node confirmed recurrence of her uterine serous adenocarcinoma. She received an additional three cycles of carboplatin and paclitaxel resulting in complete resolution of FDG-avidity within the retroperitoneal and left supraclavicular adenopathy. After a disease-free interval of six months, a surveillance CT scan showed recurrent retroperitoneal lymphadenopathy. She received another 9 cycles of carboplatin and paclitaxel. Following an initial partial response, she ultimately developed a slight increase in size and metabolic activity of the para-aortic lymph node metastases (Fig. 2).
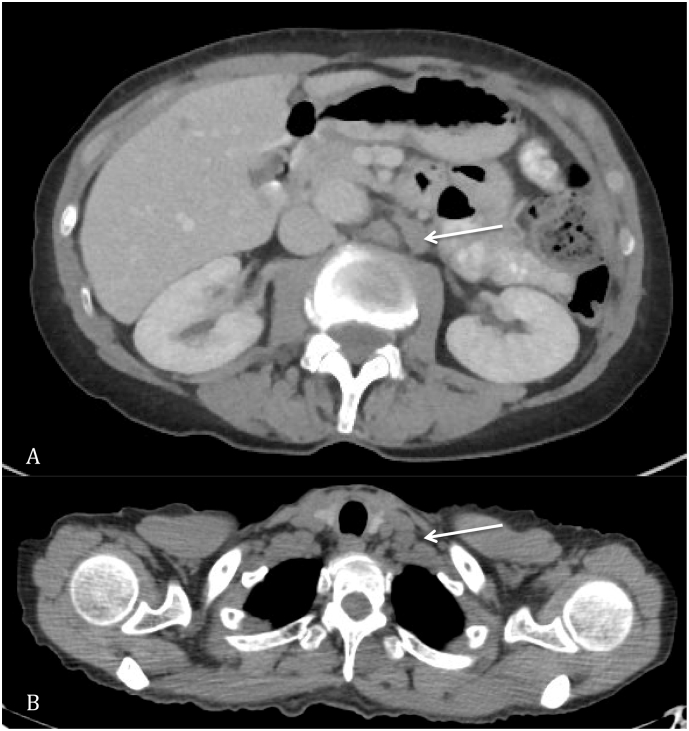
Fig. 1.
A. New retroperitoneal lymphadenopathy on surveillance computed tomography scan. B. New enlarged supraclavicular lymph node measuring 1.3 × 1.2 cm.
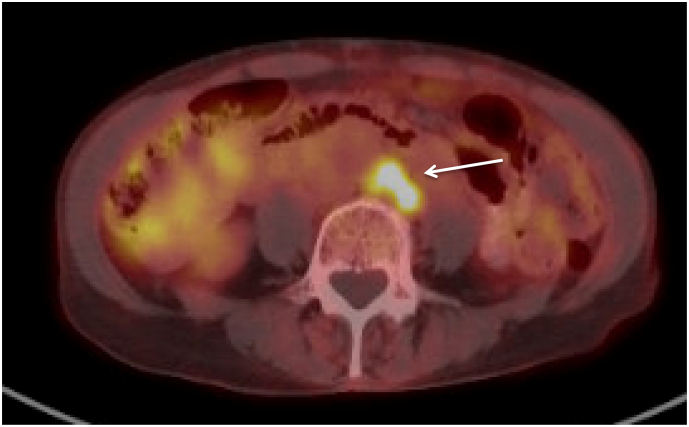
Fig. 2.
Positron emission tomography scan with a cluster of para-aortic lymph nodes spanning 1.2 × 1.7 cm associated with increased FDG avidity with SUV 5.5.
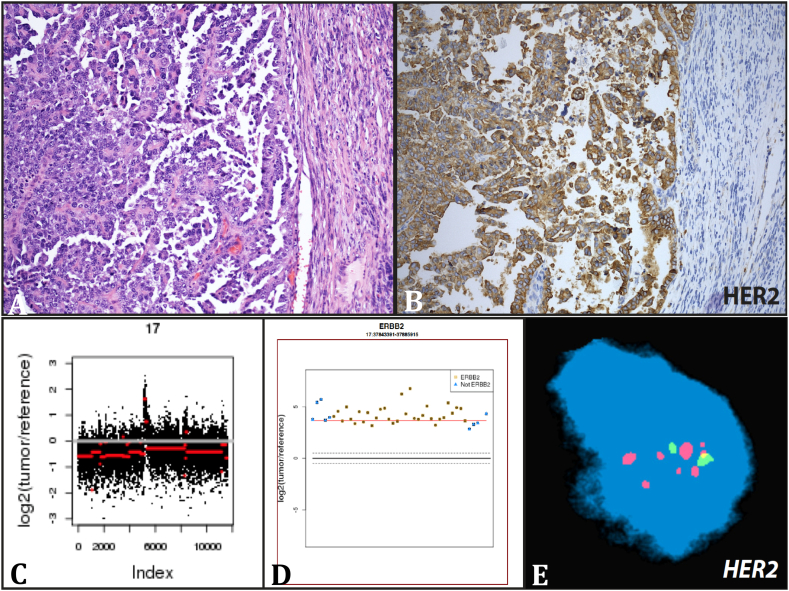
During treatment for this second recurrence, the patient enrolled in an IRB approved protocol (IRB# 1305013903) for molecular testing. Her primary (uterine) tumor (Fig. 3A) and germline DNA (from peripheral blood mononuclear cells) were sequenced and analyzed using EXaCT-1 (Beltran et al., 2015; Rennert et al., 2016), a New York State-approved whole-exome sequencing assay. Two somatic alterations involving clinically relevant genes and 75 alterations in cancer associated genes were identified. Within the clinically relevant genes, focal amplifications of FGFR1 and ERBB2 were identified (Fig. 3C and D). Both amplification and overexpression of the HER2/neu receptor were confirmed via fluorescence in situ hybridization and immunohistochemistry, respectively (Fig. 3B and E).
Fig. 3.
Uterine serous adenocarcinoma with HER2 amplification. A representative histology image of patient's hysterectomy specimen showing classic features of high-grade serous carcinoma with well-formed papillae lined by pleomorphic tumor cells with prominent nucleoli (A). Whole-exome sequencing analysis revealed HER2 amplification as illustrated in the copy number alteration plots (C and D) of chromosome 17 and HER2 gene, respectively. Validation assays included immunohistochemistry (B) and fluorescence in situ hybridization (E).
Given the identification of a potential therapeutic target (ERBB2) and her recent progression of disease, the decision was made to add trastuzumab to the patient's chemotherapy regimen. On day one, an 8 mg/kg loading dose of trastuzumab was administered over a 90 min period in addition to paclitaxel and carboplatin. Beginning on day twenty-one, she received 6 mg/kg of trastuzumab every three weeks. A PET/CT after cycle 6 of triple therapy showed stabilization of her disease. The patient continued this regimen for a total of 24 cycles with partial response on serial PET/CT scans. The decision was made to then continue trastuzumab as a single agent, and after three months on trastuzumab monotherapy, repeat PET/CT showed stable disease with no evidence of new metastatic disease in the chest, abdomen or pelvis (Fig. 4). The patient continued single agent trastuzumab for 12 cycles without toxicity and follow-up PET/MRI scan showed complete response and resolution of the prior adenopathy. To date, she has received 20 cycles of single agent trastuzumab with continued clinical remission and no toxicity.
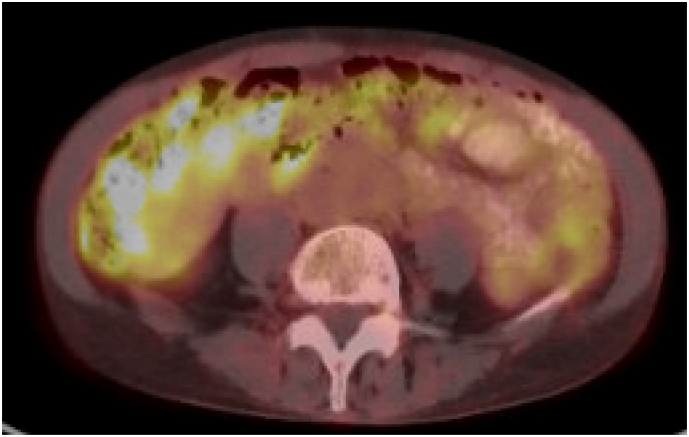
Fig. 4.
Follow-up positron emission tomography scan with no FDG-avid uptake in retroperitoneal or para-aortic lymph nodes and no evidence of new metastatic disease in the chest, abdomen or pelvis after administration of single-agent trastuzumab.
3. Discussion
Precision medicine uses next-generation sequencing technology and biomarkers to tailor therapy to the individual patient's tumor. This approach is particularly attractive for rare tumors where evidence-based treatment options are limited and molecular information may identify actionable targets. Further, given the rapid speed at which novel genomic markers are being discovered, whole exome sequencing is advantageous in identifying alterations that could be classified as actionable in the future (Beltran et al., 2015). In this case, the identification of a focal amplification of ERBB2 and subsequent confirmation of overexpression of the HER2/neu receptor was critical in guiding the decision to treat this patient with trastuzumab.
HER2/neu, also known as ERBB2, is an epidermal growth factor receptor within the family of transmembrane tyrosine kinases. Upon heterodimerization with other HER ligands, phosphorylation of the dimer's intracellular domain leads to constitutive activation of cellular pathways involved in proliferation, migration, survival and differentiation, especially via AKT phosphorylation in the PI3K/AKT pathway. When HER2/neu overexpression is present in cancer cells, increased proliferation and decreased apoptosis occurs (Buza et al., 2014). HER2/neu overexpression has been associated with a variety of cancer types, including breast, ovarian, endometrial, gastric, bladder and cervical cancers (Yan et al., 2014). Overexpression of HER2/neu in uterine serous adenocarcinoma has been examined in prior studies, with reported immunohistochemistry (IHC) positivity rates ranging from 10 to 62% (Buza et al., 2014; Slomovitz et al., 2004; Singh et al., 2008; Santin et al., 2005; Mentrikoski and Stoler, 2014), indicating that many of these tumors may be responsive to trastuzumab. Fluorescence in situ hybridization (FISH) amplification has been observed in a fraction (17 to 42%) of those found to be positive on IHC (Buza et al., 2014; Slomovitz et al., 2004; Santin et al., 2005).
Introduced in 1998, trastuzumab is a monoclonal antibody that specifically target cells expressing HER2/neu, leading to an immune-mediated response resulting in internalization and downregulation of the HER2 receptor (Bange et al., 2001). Trastuzumab may work through a variety of mechanisms, including activating the tumor suppressor p27, decreasing activation of AKT, suppressing angiogenesis, or by inducing antibody-dependent cell-mediated cytotoxicity to specifically target and kill cancer cells (Kute, 2004). Due to all of these mechanisms, trastuzumab has demonstrated effectiveness in HER2/neu positive breast cancer, gastric cancer, and other types as well.
Trastuzumab was investigated as a single therapy for HER2/neu positive endometrial carcinoma in a phase II clinical trial (Gynecologic Oncology Group protocol 181B), but did not demonstrate sufficient activity to warrant further study (Fleming et al., 2010). Despite this, successful treatment of advanced endometrial cancer overexpressing HER2/neu with trastuzumab has been noted in previous case studies (Santin et al., 2008; Villella et al., 2006). Additionally, a remarkable response of recurrent uterine serous adenocarcinoma to the antibody-drug-conjugate trastuzumab-emansine has been reported (Santin et al., 2017). The dramatic response in our case provides further indication that trastuzumab be revisited as therapy against endometrial cancers overexpressing HER2/neu as a single agent, or in combination with other agents. To our knowledge, there is currently no clinical trial investigating single-agent trastuzumab specifically for treatment of advanced endometrial carcinoma overexpressing HER2/neu. However, the use of trastuzumab as a dual therapy with pertuzumab for advanced solid tumors with HER2/neu amplification, mutation, or overexpression is currently being investigated in the My Pathway trial (NCT02091141).
As demonstrated in this case, advances in genomics provide a means to select patients for targeted therapies based on their tumor's underlying molecular alterations aiming to reduce drug toxicity and improve efficacy. The present case demonstrates the effective use of next-generation sequencing of cancer DNA to identify an actionable target for a patient with limited therapeutic options.
Author contributions
Kelsey Musselman – First author.
Shannon Glynn – Second author.
Juan Miguel Mosquera – Third author, compiled Fig. 3.
Olivier Elemento - Director of the Englander Institute of Precision Medicine.
Andrea Sboner – Computational contributor.
Himisha Beltran – Principal investigator.
Kevin Holcomb – Principal investigator.
Conflict of interest
The authors report no conflict of interest.
References
- Bange J., Zwick E., Ullrich A. Molecular targets for breast cancer therapy and prevention. Nat. Med. 2001;7(5):548–552. doi: 10.1038/87872. [DOI] [PubMed] [Google Scholar]
- Beltran H., Eng K., Mosquera J.M. Whole-exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol. 2015;1(4):466–474. doi: 10.1001/jamaoncol.2015.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruta D.M., Gehrig P., Fader A.N. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol. Oncol. 2009;115:142–153. doi: 10.1016/j.ygyno.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Buza N., Roque D., Santin A.D. HER2/neu in endometrial cancer: a promising therapeutic target with diagnostic challenges. Arch. Pathol. Lab. Med. 2014;138(3):343–350. doi: 10.5858/arpa.2012-0416-RA. [DOI] [PubMed] [Google Scholar]
- Fader A.N., Starks D., Gehrig P.A. An updated clinicopathologic study of early-stage uterine papillary serous carcinoma (UPSC) Gynecol. Oncol. 2009;115(2):244–248. doi: 10.1016/j.ygyno.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Fleming G.F., Sill M.W., Darcy K.M. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a gynecologic oncology group study. Gynecol. Oncol. 2010;116(1):15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B.A., Kato D., Schmidt R.A. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol. Oncol. 1994;54(3):264–268. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- Hamilton C.A., Liou W., Osann K. Impact of adjuvant therapy on survival of patients with early-stage uterine papillary serous carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2005;63(3):839–844. doi: 10.1016/j.ijrobp.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Hamilton C.A., Cheung M., Osann K. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br. J. Cancer. 2006;94(5):642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kute TLCWMBBWHBKMTVJ Development of Herceptin resistance in breast cancer cells. Cytometry. 2004;57A(2):86–93. doi: 10.1002/cyto.a.10095. [DOI] [PubMed] [Google Scholar]
- Mentrikoski M.J., Stoler M. HER2 immunohistochemistry significantly overestimates HER2 amplification in uterine papillary serous carcinomas. Am. J. Surg. Pathol. 2014;38(6):844–851. doi: 10.1097/PAS.0000000000000182. [DOI] [PubMed] [Google Scholar]
- Rennert H., Eng K., Zhang T. Development and validation of a whole-exome sequencing test for simultaneous detection of point mutations, indels and copy-number alterations for precision cancer care. NPJ Genom. Med. 2016;1 doi: 10.1038/npjgenmed.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santin A.D1., Bellone S., Van Stedum S., Bushen W., De Las Casas L.E., Korourian S., Tian E., Roman J.J., Burnett A., Pecorelli S. Determination of HER2/neu status in uterine serous papillary carcinoma: comparative analysis of immunohistochemistry and fluorescence in situ hybridization. Gynecol. Oncol. 2005;98(1):24–30. doi: 10.1016/j.ygyno.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Santin A.D., Bellone S., Roman J.J., McKenney J.K., Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Int. J. Gynaecol. Obstet. 2008;102(2):128–131. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Santin A.D., Bellone S., Buza N., Schwartz P.E. Regression of metastatic, radiation/chemotherapy-resistant uterine serous carcinoma overexpressing HER2/neu with trastuzumab emtansine (TDM-1) Gynecol. Oncol. Rep. 2017;19:10–12. doi: 10.1016/j.gore.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Smith C., Cheetham G. Serous carcinoma of the uterus-determination of HER-2/neu status using immunohistochemistry, chromogenic in situ hybridization, and quantitative polymerase chain reaction techniques: its significance and clinical correlation. Int. J. Gynecol. Cancer. 2008;18(6):1344–1351. doi: 10.1111/j.1525-1438.2007.01181.x. [DOI] [PubMed] [Google Scholar]
- Slomovitz B.M., Burke T., Eifel P.J. Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol. Oncol. 2003;91(3):463–469. doi: 10.1016/j.ygyno.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Slomovitz B.M., Broaddus R., Burke T.W. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J. Clin. Oncol. 2004;22(15):3126–3132. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- Villella J.A., Cohen S., Smith D.H., Hibshoosh H., Hershman D. HER-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. Int. J. Gynecol. Cancer. 2006;16(5):1897–1902. doi: 10.1111/j.1525-1438.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- Yan M., Parker B.A., Schwab R., Kurzrock R. HER2 aberrations in cancer: implications for therapy. Cancer Treat. Rev. 2014;40(6):770–780. doi: 10.1016/j.ctrv.2014.02.008. [DOI] [PubMed] [Google Scholar]