Abstract
Objectives
Several studies have documented the HPV genotypes in the Senegalese general population. The objective was to explore the HPV genotype distribution in Senegalese FSWs in order to assess the potential relevance of currently-available vaccines.
Methods
Vaginal swabs samples collected as part of the National Integrated Biological and Behavioral Survey in 14 regions throughout the country were randomly selected for HPV testing using bead-based multiplex genotyping (TS-MPG).
Results
Among the 436 FSW samples analyzed, the overall HPV prevalence was 79.8% (N = 348), with 70.1% (N = 244) cases presenting as multiple infections. High Risk HPV genotypes affecting at least 10% of FSWs included in order of decreasing frequency: 52, 16, 35, 51, 33, 31, 18, and 45. Sixty-seven (15.4%) FSWs were HIV positive and they were significantly more affected by HPV (94% vs 77%; p < 0.01) than seronegative FSWs as well as infections with multiple genotype.
Conclusion
The present study indicates that FSW in Senegal experience a high burden of HPV infection with a high frequency of coinfection with HIV and multiple HPV genotypes. Public health interventions for this key population should include an earlier cervical dysplasia/cancer detection and preventative measures such as vaccination programs that must consider the HPV genotype distribution.
Keywords: Human Papillomavirus, Human immunodeficiency virus female sex worker, Senegal
1. Introduction
Cervical cancer is the second most common cancer for women living in developing regions, and it is the second leading cause of cancer-related deaths in sub-Saharan Africa [1]. The link between cervical cancer and Human Papillomavirus (HPV) has been well established, with HPV being documented as the cause of almost all cases of cervical epithelial cell dysplasia and cervical cancer [2], [3] HPV is the most common sexually transmitted virus worldwide, and HPV infection among all women is highest in sub-Saharan Africa [4], [5]. This is most notable in Eastern and Western Africa, specifically [6].
Many genotypes of HPV exist and they have been classified based upon their relationship with cervical cancer into High Risk (HR) (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59), possible or probable high-risk high-risk (pHR) (26, 53, 66, 67, 68, 70, 73, and 82), and low-risk (LR) (6, 11, 42, 43, and 44) genotype groups [7]. However, it is well recognized that HPV 16 and 18 are associated with approximately two thirds of all invasive cervical carcinomas [2], [7], [8]. A major clinical risk factor contributing to infection with any HPV genotype is a higher number of lifetime sexual partners [5], [9]. It has been established as well that this risk factor facilitates co-infection with multiple types of HPV, other sexual transmitted infection (STI) agents [9], [10], [11] including co-infection with Human Immunodeficiency Virus (HIV) [12].
In Senegal, a country located in West Africa, cervical cancer is the most frequently occurring form of cancer, with estimated incidence and mortality rates of 37.8 and 29 per 100,000 women-year, respectively [13]. Several studies in Senegalese women have shown that HIV infection decreased HPV clearance [14] was associated with an increased risk of high grade or invasive cervical cancer ([15] and was associated with harboring multiple infections of HPV-16 [16]. However, no data has yet been published describing HPV status in Female Sex Workers (FSW) Laboratoire of Bacteriolog for women older than 21 years named registered FSW. The registration status refers to whether or not the individual has gone through the processes of legally noting their work as a seller of sex, including all governmentally-required sexual health screenings. This process is legally required by all FSWs in Senegal, though not all women choose to register [17], [18]. This key population is highly affected by HIV at rates nearly 10 times higher than that reported among women in the general population [19], [20], [21], and is therefore likely to be disproportionately affected by HPV as well. The goal of this study was to document the epidemiology of HPV in FSW, to describe the HPV genotypes, and to clarify the association of HIV and HPV in Senegalese FSW.
2. Methods
2.1. Sampling approach for target population
FSW were recruited during the 2010 National Integrated Biological and Behavioral Survey conducted in all 14 regions of Senegal. Recruitment sites were selected based on the target population's attendance frequency (e.g. bars, brothels, and certain streets) and population sizes were estimated via cluster sampling. First of all, an exhaustive identification of all the sites by region where FSW can be found was done. After that identification and based on the total number of FSWs needed to be recruited by region, a random site selection was made to identify the primary units (or cluster). Within each cluster, the secondary units (targets or FSWs) were randomly selected from the eligible persons present at the time of the survey. Recruitment is stopped as soon as the cluster target is reached or when there are no targets (FSW) eligible to investigate in the dedicated time. All women over the age of 15 who acknowledged ever having commercial sex experience were invited to participate. Consent was first obtained prior to the collection of behavioral data and then again before the collection of biological samples. Biological samples were later randomly selected for further HPV testing.
2.2. Biological samples collection
Blood samples (10 mL from each consenting individual) were collected in EDTA tubes and were tested for HIV using a 4th generation ELISA (Murex HIV-1.2, Abbott laboratoires), ImmunoComb® II HIV 1 & 2 (Orgenics Ltd, Inverness). HIV BLOT 2.2 (MP Diagnostics) testing served as the ultimate determinant in discordant serological analyses.
Vaginal discharges were collected with 2 swabs in Cobas PCR media tubes (Roche Diagnostics) by auto vaginal swab sampling.
2.3. DNA extraction
HPV DNA extraction was performed using the QIAamp blood mini kit (Qiagen, Hilden, Germany) according to manufacturer's instructions. Extraction was carried out at the Bacteriology-Virology laboratory of Aristide le Dantec University Teaching Hospital (Dakar, Senegal).
2.4. HPV DNA genotyping
HPV genotyping was perform at the International Agency of Research Cancer (IARC) at Lyon (France) using type-specific PCR bead-based multiplex genotyping (E7-MPG) assays that combine multiplex polymerase chain reaction (PCR) and bead-based Luminex technology (Luminex Corp., Austin, TX, USA), as described elsewhere [22], [23]. The multiplex type-specific PCR method uses specific primers for the detection of 12 HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59), 7 pHR HPV types (HPV 26, 53, 66, 68 a and b, 70, 73, 82), and two low-risk (LR)-HPV types (HPV 6 and 11). Two primers for the amplification of the β-globin gene were included to provide a positive control for the quality of the DNA in the sample.
Following multiplex PCR amplification, 10 μl of each reaction mixture was analyzed by multiplex HPV genotyping (MPG) using Luminex technology (Luminex Corporation, Austin, TX) as described previously [22], [23]. Briefly, PCR products were denatured and hybridized to the bead-coupled probes in 96-well plates. Unhybridized DNA was removed after transfer into wash plates with filter bottoms. Subsequently, biotinylated PCR products were stained by Strep-PE conjugate. After further washing steps, the beads were analyzed in the Luminex reader, which contains two lasers to identify the bead set by the internal bead color and to quantify the reporter fluorescence on the bead. The results are expressed as the median fluorescence intensity (MFI) of at least 100 beads per bead set. For each probe, the MFI values obtained when no PCR product was added to the hybridization mixture were considered as background values. The cutoff was computed by adding 5 MFI to 1.1× the median background value as described by Schmitt et al. [23] This multiplex PCR protocol is highly sensitive, with the ability to detect only 10 copies of the viral genome [24]. All of the Luminex bead probes were designed within the region of no conserved sequences amplified by the type-specific E7 primers to decrease the risk of cross-reactivity. The specificity of the assay has been evaluated using picked bacterial colonies harboring the genome of the respective virus. No cross-reactivity has been observed [22], [23].
2.5. Statistical analysis
Statistical analyses were performed using SPSS version 16.0 (SPSS Inc. Chicaco, Illinois, USA) and Epi Info 7 (Centers for Disease Control and Prevention, Atlanta, Georgia USA). An alpha of 0.05 was used. Pearson's Exact Test (Asymptomatic 2-sided P value), the Kruskal-Wallis H Test, and Regression Modeling were used during analyses [25].
3. Results
Socio demographic characteristics of FSW tested for HPV are presented in Table 1. Among a total of 436 FSW, 94.5% (410/434) were non-married and 40% were not educated. The mean age was 34 years old ranged from 15 to 62, and regarding the HIV status, there was statistically no difference in socio demographic characteristic, except for legal registration status due to that all seropositive FSW were no registered.
Table 1.
FSW socio-demographic characteristics.
| All | HIV+ | HIV- | p | |
|---|---|---|---|---|
| *Age | 35 (26–42) | 36 (30–45) | 34 (26–42) | 0.0586 |
| Education Level | ||||
| None | 40.1% (175/436) | 43.3% (29/67) | 39.6% (146/369) | 0.5896 |
| Primary | 40.4% (176/436) | 43.3% (29/67) | 39.8% (147/369) | 0.5917 |
| Secondary/Above | 19.5% (85/436) | 13.4% (9/67) | 20.6% (76/369) | 0.2396 |
| Marital Status | ||||
| Single | 27.2% (118/434) | 17.9% (12/67) | 28.9% (106/367) | 0.0731 |
| Married | 5.5% (24/434) | 4.5% (3/67) | 5.7% (21/367) | 0.3650 |
| Divorced/Widowed | 67.3% (292/434) | 77.6% (52/67) | 65.4% (240/367) | 0.0649 |
| Sex Work Specific | ||||
| Legal Registration Status | 57.8% (252/436) | 0% (0/67) | 68.3% (252/369) | <0.01 |
| *Years of Sex Work | 3(0–6) | 6(3–11) | 6(2–10) | 0.3915 |
Median (IQR).
The overall prevalence of HPV infection was 79.8% (348/436) while HIV prevalence was 15.4% (67/436). According to HIV infection, seropositive FSW were globally significantly more affected by HPV (94% vs 77%; p < 0.01) and for any risk group (91% vs 69.1%, 56.7% vs 41.5%, and 19.4% vs 9.4% for HR-HPV, pHR-HPV, and LR-HPV, respectively). No differences in HPV infection prevalence existed across age groups (p = 0.4032).
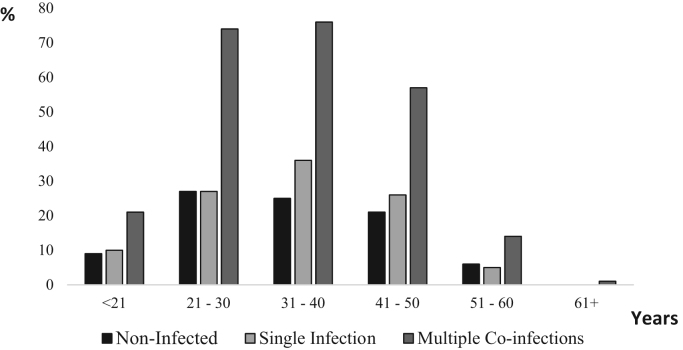
Among HPV infected FSW, 29.9% (104/348) were infected by a single genotype and 70.1% (244/348) presented multiple HPV genotype infection, up to 13. Fig. 1 shows the difference in number (non-infected, single and multiple) of infecting HPV genotypes for each age group. HPV infection was more frequent between 21 and 40 years old. In addition, infection with multiple HPV genotypes was more prevalent than single infection for any age group but no difference was observed in number of infecting genotypes between age groups (p = 0.7567).
Fig. 1.
HPV Infection in Senegalese FSW according to age The proportion of FSW within each age range was presented according to their status regarding HPV infection. The proportion of FSW not infected, infected by a single HPV genotype and infected by multiple HPV genotypes are presented in black (Non-infected), in light grey (Single infected) and in hard grey (Multiple co-infections), respectively. Within each age range, the majority of FSWs harbored multiple HPV genotypes.
HPV genotype frequencies are displayed in Table 2. HR HPV types accounted for the majority of HPV infections with 72.5% of all FSW presented a HR HPV genotype. HPV-52 was the most prevalent HPV genotype, detected in 32.6% of all FSW. HPV-16 was the second-most common HPV type, affecting 19.7% of all FSW tested. HPV genotypes affecting at least 10% of the study population included eight HR HPV genotypes (HPV-16, -18, -31, -33, -35, -45, -51, -52, -66, and -68) and one pHR-HPV genotypes (HPV-53). According to HIV infection, HPV genotypes significantly more prevalent in seropositive FSW included four HR HPV (HPV-16, 35, 45, and 52), 2 pHR-HPV (HPv-70 and -73), and one LR HPV (HPV-6).
Table 2.
Infection frequencies for HR-, pHR-, and LR-HPV types in Senegalese FSWs.
| Genotype | All N = 436 | HIV negative (N = 369) | HIV positive (N = 67) | P |
|---|---|---|---|---|
| HPV positive | 348 (79.8%) | 285 (77.2%) | 63 (94%) | <0.01 |
| HR | 316 (72.5%) | 255 (69.1%) | 61 (91%) | <0.01 |
| pHR | 191 (43.8%) | 153 (41.5%) | 38 (56.7%) | <0.05 |
| LR | 41 (9.4%) | 28 (7.6%) | 13 (19.4%) | <0.01 |
| HR-HPV | ||||
| *16 | 86 (19.7%) | 64 (17.3%) | 22 (32.8%) | <0.01 |
| *18 | 52 (11.9%) | 42 (11.4%) | 10 (14.9%) | 0.4139 |
| *31 | 54 (12.4%) | 43 (11.7%) | 11 (16.4%) | 0.3120 |
| *33 | 56 (12.8%) | 43 (11.7%) | 13 (19.4%) | 0.1096 |
| *35 | 70 (16.1%) | 52 (14.1%) | 18 (26.9%) | <0.05 |
| 39 | 28 (6.4%) | 21 (5.7%) | 7 (10.5%) | 0.1712 |
| *45 | 50 (11.5%) | 35 (9.5%) | 15 (22.4%) | <0.01 |
| *51 | 68 (15.6%) | 53 (14.4%) | 15 (22.4%) | 0.1015 |
| *52 | 142 (32.6%) | 105 (28.5%) | 37 (55.2%) | <0.01 |
| 56 | 42 (9.6%) | 32 (8.7%) | 10 (14.9%) | 0.1171 |
| 58 | 27 (6.2%) | 20 (5.4%) | 7 (10.5%) | 0.1615 |
| 59 | 18 (4.1%) | 13 (3.5%) | 5 (7.5%) | 0.1724 |
| pHR-HPV | ||||
| 26 | 7 (1.6%) | 7 (1.9%) | 0 | 0.6017 |
| *53 | 73 (16.7%) | 58 (15.7%) | 15 (22.4%) | 0.2118 |
| *66 | 74 (17.0%) | 60 (16.3%) | 14 (20.9%) | 0.3767 |
| *68 | 80 (18.4%) | 64 (17.4%) | 16 (23.9%) | 0.17 |
| 70 | 19 (4.4%) | 13 (3.5%) | 6 (9%) | 0.0552 |
| 73 | 36 (8.3%) | 23 (6.2%) | 13 (19.4%) | <0.01 |
| 82 | 3 (0.7%) | 2 (0.5%) | 1 (1.5%) | 0.3946 |
| LR-HPV | ||||
| 6 | 34 (7.8%) | 23 (6.2%) | 11 (16.4%) | <0.05 |
| 11 | 7 (1.6%) | 5 (1.4%) | 2 (3%) | 0.2934 |
HPV: Human Papillomavirus.
HR: high-risk HPV types, pHR: possible or probable high-risk high-risk HPV types, LR: low-risk HPV types.
In grey, HPV genotypes significantly associated with HIV seropositivity.
Affects at least 10% of the entire study population.
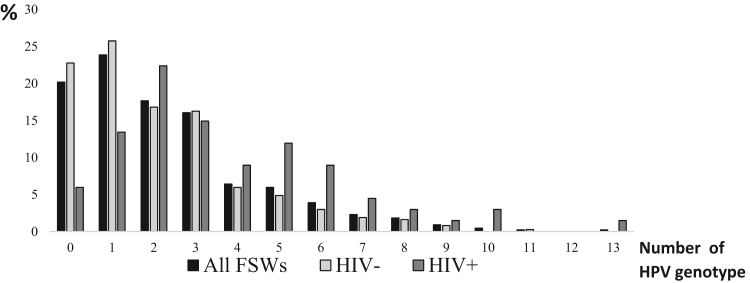
Fig. 2 shows the number of HPV genotypes present as a function of HIV serostatus. The total number of infecting HPV genotypes ranged from 0 to 11 for seronegative FSW and from 0 to 13 for seropositive FSW. In addition, HIV serostatus was also associated with multiplicity of HPV genotype infection: HIV negative FSW harbored a median (IQR) of 2 (1−3) HPV genotypes vs 3 (2–5) in HIV positive FSW (p < 0.01).
Fig. 2.
HPV multi infection in Senegalese FSWs according to HIV serostatus The proportion of FSW was presented according to the number of HPV genotypes identified from cervical auto-swabs. The number of HPV genotyped varied between 0 and 13. The global proportion of FSW, HIV seronegative FSW and HIV seropositive FSW are presented in black (All FSWs), in light grey (HIV-) and in hard grey (HIV+), respectively.
4. Discussion
In this study, the current population of FSW had an extremely high prevalence of HPV infection and is also highly affected by HIV as reported worldwide in many other studies [27], [28]. These results confirm previous studies regarding HIV prevalence in FSW in Senegal [19], [28] and point out their extreme vulnerability to HPV comparing to general population. Indeed, the HPV prevalence is almost four times higher than that reported previously in Senegalese women in the general population (79.8% vs 23%) [29]. Other studies have also noted the similar disparities regarding HPV burden between the general female population and FSW [30], [31], [32] and this discrepancy further predicts a disproportionate burden of cervical cancer diseases among FSW.
For all age groups, a majority of FSW presented multiple HPV infections (2 or more HPV types). Compared to a previous study, multiple infections in FSW are more prevalent than in general senegalese female population [29]. These multiple infections are certainly be due to a high level of exposure with a significantly high number of sexual partners, leading to multiple independent infections, and to a lower use of condom during sexual practices as previously reported [9], [26], [30].
In addition, an association between HPV and HIV has been found with a higher prevalence of HPV as well as a higher proportion of multiple HPV genotypes in HIV seropositive FSW, compared to HIV seronegative FSW. These results were a real concern for FSW health care as similar studies shown that HIV decrease the clearance of HR-HPV infections [14], [33], [34], [35] and its association with multiple HR-HPV genotypes increased risk of cervical abnormalities [15], [36].
According to genotypes, it is well known that HPV-16 and HPV-18 are the two most frequently implicated in cervical cancer [37]. However, HPV-16 and 18 are not the most commonly occurring genotypes in the present study population. HPV-52 was most prevalent in the global FSW followed by HPV-16 and -35 as well as in the group of HIV infected FSW. Eleven HPV genotypes infected at least 10% of FSW including eight HR HPV types, which demonstrates the diversity of HPV infections.
Previous study in Senegalese female population found similarly that HPV-16 and 18 were only the sixth and tenth most prevalent in carriage, respectively after HPV-31 the most prevalent followed by 52, 53, 66, and 45 [29]. In another study regarding HIV and HPV in the capital city, HPV 58, 52, and 16 were the most prevalent for all women included in analyses, followed by 18, 35, and 45 for HIV positive women and 33, 31, and 18 for HIV negative women [16].
In Senegal also, it has been demonstrated that HPV-16 and 18 account for approximately 70% in cervical cancer lesions [38], [39]. However, the remaining 30% of cervical cancer is related to other HR or pHR genotypes that are prevalent in Senegalese FSW, even if it is not really known which particular HPV type will persist and develop into cervical cancer. Therefore, it is important to mitigate cancer risk by reducing the burden of all pHR and HR genotypes with prevention measurements like vaccine. Six of these other genotypes are targeted by the new 9vHPV (FDA), which indicates that this vaccine may be suitable for use in this population.
Vaccination programs have high potential efficacy if given prior to infection and current recommendations support also vaccine efficacy if given to patients 26 years old or younger [40], [41]. Although FSW in this study are typically older than the recommended vaccination age, recent studies have shown vaccine efficacy for women up to age 55 [42], [43]. However the impact of this vaccination for FSW could be mitigated due to their high level of HPV infection. Furthermore, more than 40% of FSW were not registered and consequently were not regularly followed up for STI screening and some non-registered FSWs do not consider themselves as sex workers in need of care if they only work occasionally [44]. According to these findings, HPV vaccine prevention scale up in young girls become an urgent need.
5. Conclusion
On the whole, this study confirm the high vulnerability of FSW for HPV with multiple genotype infections. Therefore, public health interventions should associated cervical cancer screening and young girl vaccination program that need to take into account these genotypes distribution. Furthermore, due to their high vulnerability to HIV and its defect association with HPV, prevention should also continue to incorporate condom distribution, screening for STIs and treatment programs for HIV in order to reduce morbidity and mortality associated with these two viruses.
Authors’ contributions
Halimatou Diop-Ndiaye and Tarik Gheit performed DNA extraction and HPV testing. Halimatou Diop, Kaylin Beiter and Tarik Gheit performed statistical analysis of the data and wrote the manuscript. Coumba Touré-Kane and Bakary Sylla supervised the project. All authors provided feedback and helped shape the research project.
Acknowledgments
KB received support from the United States Fulbright Student Research Program.
Acknowledgments
Declarations
Ethics approval and consent to participate.
Consent for publication
If applicable.
Availability of data and materials
Data generated during this study are included in the published article.
Competing interest
The authors declare that they have no competing interests.
Funding
This study was funded by the Laboratory of Bacteriology-Virology at CHU Aristide Le Dantec, Dakar SENEGAL and the International Agency for Research on Cancer, Lyon, FRANCE.
The funding body has no role in the design of the study, collection, analysis, and interpretation of data and in writing the manuscript.
Declarations of interest
None.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018 doi: 10.3322/caac.21492. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 2.Bosch F.X., Lorincz A., Muñoz N., Meijer C.J., Shah K.V. The causal relation between human Papillomavirus and cervical cancer. J. Clin. Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Martel C., Plummer M., Vignat J., Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de sanjosé S., Diaz M., Castellsague X., Clifford G., Bruni L., Muñoz N., Bosch F.X. Worldwide prevalence and genotype distribution of cervical human Papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect. Dis. 2007;7:453–459. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 5.Bruni L., Diaz M., Castellsagué X., Ferrer E., Bosch F.X., de Sanjosé S. Cervical human Papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010;202:1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 6.De Vuyst H., Alemany L., Lacey C., Chibwesha C.J., Sahasrabuddhe V., Banura C., Denny L., Parham G.P. The burden of human Papillomavirus infections and related diseases in sub-Saharan Africa. Vaccine. 2013;31(Suppl 5):F32–F46. doi: 10.1016/j.vaccine.2012.07.092. (Review) (092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouvard V., Baan R., Straif K., Grosse Y., Secretan B., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., Cogliano V., WHO International Agency for Research on Cancer Monograph Working Group A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 8.Brianti P., De Flammineis E., Mercuri S.R. Review of HPV-related diseases and cancers. New Microbiol. 2017;40:80–85. [PubMed] [Google Scholar]
- 9.Chaturvedi A.K., Katki H.A., Hildesheim A., Rodríguez A.C., Quint W., Schiffman M., Van Doorn L.J., Porras C., Wacholder S., Gonzalez P., Sherman M.E., Herrero R., Group C.V.T. Human Papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J. Infect. Dis. 2011;203:910–920. doi: 10.1093/infdis/jiq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trottier H., Mahmud S., Costa M.C., Sobrinho J.P., Duarte-Franco E., Rohan T.E., Ferenczy A., Villa L.L., Franco E.L. Human Papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol. Biomark. Prev. 2006;15:1274–1280. doi: 10.1158/1055-9965.EPI-06-0129. [DOI] [PubMed] [Google Scholar]
- 11.Pista A., Oliveira A., Verdasca N., Ribeiro F. Single and multiple human Papillomavirus infections in cervical abnormalities in Portuguese women. Clin. Microbiol. Infect. 2011;17:941–946. doi: 10.1111/j.1469-0691.2010.03387.x. [DOI] [PubMed] [Google Scholar]
- 12.Auvert B., Marais D., Lissouba P., Zarca K., Ramjee G., Williamson A.L. High-risk human papillomavirs is associated with HIV acquisition among South African female sex workers. Infect. Dis. Obstet. Gynocol. 2011;2011:692012. doi: 10.1155/2011/692012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GLOBOCAN. 〈http://gco.iarc.fr/today/data/factsheets/populations/686-senegal-fact-sheets.pdf〉.
- 14.Rowhani-Rahbar A., Hawes S.E., Sow P.S., Toure P., Feng Q., Dem A., Dembele B., Critchlow C.W., N'Doye I., Kiviat N.B. The impact of HIV status and type on the clearance of human Papillomavirus infection among Senegalese women. J. Infect. Dis. 2007;196:887–894. doi: 10.1086/520883. [DOI] [PubMed] [Google Scholar]
- 15.Hawes S.E., Critchlow C.W., Sow P.S., Touré P., N'Doye I., Diop A., Kuypers J.M., Kasse A.A., Kiviat N.B. Incident high-grade squamous intraepithelial lesions in Senegalese women with and without human immunodeficiency virus type 1 (HIV-1) and HIV-2. J. Natl. Cancer Inst. 2006;98:100–109. doi: 10.1093/jnci/djj010. [DOI] [PubMed] [Google Scholar]
- 16.Hanisch R.A., Sow P.S., Toure M., Dem A., Dembele B., Toure P., Winer R.L., Hughes J.P., Gottlieb G.S., Feng Q., Kiviat N.B., Hawes S.E., University of Washington-Dakar HIV and Cervical Cancer Study Group Influence of HIV-1 and/or HIV-2 infection and CD4 count on cervical HPV DNA detection in women from Senegal, West Africa. J. Clin. Virol. 2013;58:696–702. doi: 10.1016/j.jcv.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.do Espirito Santo M.E., Etheredge G.D. And then I became a prostitute… some aspects of prostitution and brothel prostitutes in Dakar, Senegal. Soc. Sci. J. 2004;41:137–146. [Google Scholar]
- 18.Homaifar N., Wasik S.Z. Interviews with Senegalese commercial sex trade workers and implications for social programming. Health Care Women Int. 2004;26:118–133. doi: 10.1080/07399330590905576. [DOI] [PubMed] [Google Scholar]
- 19.Meda I., Ndoye S., M'Boup A., Wade S., Ndiaye C., Niang F., Sarr I., Diop Caraël M. Low and stable HIV infection rates in Senegal: natural course of the epidemic or evidence for success of prevention? AIDS. 1999;13:1397–1405. doi: 10.1097/00002030-199907300-00018. [DOI] [PubMed] [Google Scholar]
- 20.Wang C., Hawes S.E., Gaye A., Sow P.S., Ndoye I., Manhart L.E., Wald A., Critchlow C.W., Kiviat N.B. HIV prevalence, previous HIV testing, and condom use with clients and regular partners among Senegalese commercial sex workers. Sex. Transm. Infect. 2007;88:534–540. doi: 10.1136/sti.2007.027151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kane C.T., Diawara S., Ndiaye H.D., Diallo P.A., Wade A.S., Diallo A.G., Belec L., Mboup S. Concentrated and linked epidemics of both HSV-2 and HIV-1/HIV-2 infections in Senegal: public health impacts of the spread of HIV. Int. J. STD AIDS. 2009;20:793–796. doi: 10.1258/ijsa.2008.008414. [DOI] [PubMed] [Google Scholar]
- 22.Gheit T., Landi S., Gemignani F., Snijders P.J., Vaccarella S., Franceschi S., Canzian F., Tommasino M. Development of a sensitive and specific assay combining multiplex PCR and DNA microarray primer extension to detect high-risk mucosal human Papillomavirus types. J. Clin. Microbiol. 2006;44(6):2025–2031. doi: 10.1128/JCM.02305-05. (Jun) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt M., Dondog B., Waterboer T., Pawlita M., Tommasino M., Gheit T. Abundance of multiple high-risk human Papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J. Clin. Microbiol. 2010;48:143–149. doi: 10.1128/JCM.00991-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gheit T., Billoud G., de Koning M.N., Gemignani F., Forslund O., Sylla B.S., Vaccarella S., Franceschi S., Landi S., Quint W.G., Canzian F., Tommasino M. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect Betapapillomavirus types. J. Clin. Microbiol. 2007;45(8):2537–2544. doi: 10.1128/JCM.00747-07. (Aug) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald J. Sparky House Publishing; Baltimore, MD, USA: 2009. Handbook of Biological Statistics. [Google Scholar]
- 26.Soohoo M., Blas M., Byraiah G., Carcamo C., Brown B. Cervical HPV infection in female sex workers: a global perspective. Open AIDS J. 2013;7:58–66. doi: 10.2174/1874613601307010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baral S., Beyrer C., Muessig K K., Poteat T., Wirtz A.L., Decker M.R., Sherman S.G., Kerrigan D. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:538–549. doi: 10.1016/S1473-3099(12)70066-X. [DOI] [PubMed] [Google Scholar]
- 28.Laurent C., Seck K., Coumba N., Kane T., Samb N., Wade A., Liégeois F., Mboup S., Ndoye I., Delaporte E. Prevalence of HIV and other sexually transmitted infections and risk behaviors in unregistered sex workers in Dakar, Senegal. AIDS. 2003;17:1811–1816. doi: 10.1097/00002030-200308150-00010. [DOI] [PubMed] [Google Scholar]
- 29.Mbaye El.H.S., Gheit T., Dem A., McKay-Chopin S., Toure-Kane N.C., Mboup S., Tommasino M., Sylla B.S., Boye C.S. Human Papillomavirus infection in women in four regions of Senegal. J. Med. Virol. 2014;86:248–256. doi: 10.1002/jmv.23719. [DOI] [PubMed] [Google Scholar]
- 30.Chandeying V., Garland S.M., Tabrizi S.N. Prevalence and typing of human papilloma virus (HPV) among female sex workers and outpatient women in southern Thailand. Sex. Health. 2006;3:11–14. doi: 10.1071/sh05019. [DOI] [PubMed] [Google Scholar]
- 31.González C., Torres M., Canals J., Fernández E., Belda J., Ortiz M., Del Amo J. Higher incidence and persistence of high-risk human Papillomavirus infection in female sex workers compared with women attending family planning. Int. J. Infect. Dis. 2011;15:e688–e694. doi: 10.1016/j.ijid.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 32.Jia H., Wang X., Long Z., Li L. Human Papillomavirus infection and cervical dysplasia in female sew workers in Northeast China: an observational study. BMC Public Health. 2015;23:15. doi: 10.1186/s12889-015-2066-x. (695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon S., van den Broeck D., Rossi R., Ogbe E., Mabeya H. Multiple HPV infections in female sex workers in Western Kenya: implications for prophylactic vaccines within this sub population. Infect. Agent Cancer. 2017;12:2–8. doi: 10.1186/s13027-016-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adler D., Wallace M., Bennie T., Abar B., Sadeghi R., Meiring T., Williamson A.L., Bekker L.G. High risk human Papillomavirus persistence among HIV-infected young women in South Africa. Int. J. Infect. Dis. 2015;33:219–221. doi: 10.1016/j.ijid.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adebamowo S.N., Famooto A., Dareng E.O., Olawande O., Olaniyan O., Offiong R., Adebamowo C.A. Clearance of type-specific, low-risk, and high-risk cervical human Papillomavirus infections in HIV-negative and HIV-positive women. J. Glob. Oncol. 2018;4:1–12. doi: 10.1200/JGO.17.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adler D.H., Wallace M., Bennie T., Abar B., Meiring T.L., Williamson A.L., Bekker L.G. Cumulative impact of HIV and multiple concurrent human Papillomavirus infections on the risk of cervical dysplasia. Adv. Virol. 2016:7310894. doi: 10.1155/2016/7310894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosch F.X., Burchell A.N., Schiffman M., Giuliano A.R., de Sanjose S., Bruni L., Tortolero-Luna G., Kjaer S.K., Muñoz N. Epidemiology and natural history of human Papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26S:K1–K16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 38.Ndiaye C., Alemany L., Ndiaye N., Kamaté B., Diop Y., Odida M., Banjo K., Tous S., Klaustermeier J.E., Clavero O., Castellsagué X., Bosch F.X., Trottier H., de Sanjosé S. Human Papillomavirus distribution in invasive cervical carcinoma in sub-Saharan Africa: could HIV explain the differences? Trop. Med. Int. Health. 2012;17:1432–1440. doi: 10.1111/tmi.12004. [DOI] [PubMed] [Google Scholar]
- 39.H. Diop, J.D. Coulibaly, M. Rakoto-Andrianarivelo, R. Njoum, N. Berthet, M. Diop, HPV genotypes in high-grade intraepithelial neoplasia lesions and invasive cancers in the african consortium on cervical cancer control research (COFAC-Col), in Proceedings of the 11th International Conference on Cancer in Africa, AORTIC.
- 40.Food and Drug Administration, Gardasil 9. United States Government. 2014, updated 2017. 〈https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm426445.htm〉.
- 41.Brown B., Blas M., Cabral A., Carcamo C., Gravitt P., Halsey N. Randomized trial of HPV4 vaccine assessing the response to HPV4 vaccine in two schedules among Peruvian female sex workers. Vaccine. 2012;30:2309–2314. doi: 10.1016/j.vaccine.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Einstein M.H., Takacs P., Chatterjee A., Sperling R.S., Chakhtoura N., Blatter M.M., Lalezari J., David M.P., Lin L., Struyf F., Dubin G. HPV-010 study group. Comparison of long-term immunogenicity and safety of human Papillomavirus (HPV)-16/18 AS04 adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a phase III randomized trial. Hum. Vaccines Immunother. 2014;10:3435–3445. doi: 10.4161/hv.36121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz T.F., Galaj A., Spaczynski M., Wysocki J., Kaufmann A.M., Poncelet S., Suryakiran P.V., Folschweiller N., Thomas F., Lin L., Struyf F. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer Med. 2017;6:2723–2731. doi: 10.1002/cam4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tucker G.M. The invisible challenge to HIV/AIDS prevention: clandestine prostitution in Senegal. J. Int. Women's. Stud. 2012;13:19–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated during this study are included in the published article.