Abstract
Introduction
Clinical trials involving patients with Alzheimer's disease (AD) continue to try to identify disease-modifying treatments. Although trials are designed to meet regulatory and registration requirements, many do not measure outcomes of the disease most relevant to key stakeholders.
Methods
A systematic review sought research that elicited information from people with AD, their caregivers, and health-care professionals on which outcomes of the disease were important. Studies published in any language between 2008 and 2017 were included.
Results
Participants in 34 studies described 32 outcomes of AD. These included clinical (memory, mental health), practical (ability to undertake activities of daily living, access to health information), and personal (desire for patient autonomy, maintenance of identity) outcomes of the disease.
Discussion
Evidence elicited directly from the people most affected by AD reveals a range of disease outcomes that are relevant to them but are not commonly captured in clinical trials of new treatments.
Keywords: Alzheimer's Disease, Mild cognitive impairment, Outcomes, Systematic review, Qualitative, Quantitative, Quality of life, Memory, Activities of daily living, Autonomy, Burden, Patients, Caregivers, Healthcare professionals
1. Background
Dementia has a substantial global impact, affecting over 46 million people in 2015 and costing an estimated US $818 billion [1]. Alzheimer's disease (AD) is the most common cause of dementia, accounting for 50-70% of cases [2], [3]. AD is typically characterized by impairments in memory, executive functions, and activities of daily living (ADLs), but the range and impact of symptoms and outcomes across the disease spectrum are diverse. The underlying causes of AD and effective treatments for the disease remain elusive [3], [4].
Clinical trials involving patients with AD continue to try to identify disease-modifying treatments. However, although such trials may meet regulatory and registration requirements, they may not provide convincing evidence of relevance to patients, caregivers, or health-care professionals. Some trials are criticized for using inappropriate or inadequately sensitive endpoints [4], [5], and it is often unclear how much stakeholder input, other than that of regulators, is applied in the selection of trial endpoints [5].
Helping key stakeholders to understand which AD outcomes are most relevant and what constitutes a meaningful delay in disease progression could help researchers develop and evaluate relevant, effective treatments and improve health services and care [6]. This systematic review, conducted on behalf of the international consortium Real World Outcomes Across the AD Spectrum for Better Care (ROADMAP; https://roadmap-alzheimer.org/), aimed to collate all available evidence about prioritization of AD outcomes and criteria for meaningful disease progression from the perspective of patients, caregivers, and health-care professionals. ROADMAP partners will seek to match these to “real-world evidence” sources, including disease registries, population-based cohort studies, and electronic health records documenting routine patient care.
We sought evidence from studies covering a spectrum from prodromal AD and mild cognitive impairment (MCI) to confirmed AD dementia [7]. We sought research studies that elicited information from stakeholders, addressing the following research questions:
-
1.
Which outcomes of AD across the spectrum are prioritized by patients, caregivers, and health-care professionals?
-
2.
What do these stakeholders consider a meaningful delay in progression of AD across the spectrum?
2. Methods
The systematic review protocol is available in the PROSPERO database (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=75722).
2.1. Search strategy
We developed a search strategy for the MEDLINE database, balanced for sensitivity and specificity, and adapted it for use in Embase, CINAHL, and PsycINFO (Table S1). We developed groups of search terms for each research question using input from ROADMAP partners. We combined terms related to AD across the disease spectrum, stakeholder groups and study methods, with terms related to outcomes and priority (for research question 1) and separately with terms related to a meaningful delay of disease (for research question 2). We combined all searches to remove duplicates. We searched for gray literature using “Alzheimer” in combination with “outcome” or “progression”, applying date limits 2008-2017 on relevant websites (Table S1).
We included relevant studies regardless of language by arranging translation into English by colleagues. We sought additional relevant studies through manual searches of key articles' citation lists and checking relevant conference abstracts for full publications.
2.2. Study screening, quality appraisal, and selection
We established specific inclusion and exclusion criteria to guide the selection of relevant studies for inclusion and screened the titles and abstracts of all retrieved citations (Table 1). Two members of the research team independently screened the first half of the retrieved citations, with 95% agreement on decisions to include/exclude (Cohen's kappa, 0.53 [0.42-0.64]). A discussion of discrepancies with a third member of the team revealed that most disagreements were due to the appraiser including potentially relevant articles that did not meet all inclusion criteria. The risk of missing relevant evidence was considered to be low; hence, one research team member screened the remaining half of the citations.
Table 1.
Inclusion and exclusion criteria
| Included all relevant studies which |
|
| |
| |
| |
| Excluded all relevant studies which |
|
| |
| |
| |
|
Abbreviation: AD, Alzheimer's disease.
Two members of the team independently used published tools with our inclusion and exclusion criteria to appraise the full text of articles that passed screening for relevance and quality. Checklists from the Clinical Appraisal Skills Program were used to appraise qualitative studies (http://www.casp-uk.net/casp-tools-checklists) and the National Institute of Health for quantitative studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). Discrepancies were discussed by the team and resolved through consensus. Through iterative discussion, we established a minimum quality threshold for inclusion, agreeing to exclude studies with incomplete descriptions of recruitment or analysis or unclear reporting of results which did not allow appraisal of their rigor.
2.3. Data extraction
For each included study, one member of the research team completed data extraction, noting the research approach, recruitment methodology, participant stakeholder demographics, disease stage, data analysis and synthesis methods, research findings, and conclusions (Table S2). At least one additional member of the team verified the extracted data.
2.4. Data synthesis process
We extracted quotations and other study findings that referred to outcomes or meaningful delay of AD across the disease spectrum. We sought feedback from consortium colleagues to group specific outcomes into overarching domains (e.g., memory within the cognition domain) (Table 2). Because many outcomes were multifaceted and some overlapped domains, we placed each outcome where it fitted best according to clinical nosology (e.g., grouping behavioral, mental, and neuropsychiatric outcomes as used in international classification systems [8]) and previous ROADMAP activities [9].
Table 2.
Domains and outcomes: the number of included studies in which each outcome was considered important and the number of countries involved in those studies
| Overarching domain | Outcome | Definition | No. of studies in which the outcome was considered important by: |
The overall no. of studies in which the outcome was considered important (range: 1-11) | No. of countries represented in relevant studies (range: 1-10) | ||
|---|---|---|---|---|---|---|---|
| Patients | Caregivers | HPs | |||||
| Cognition | Memory/slowing of forgetfulness | Recalling names, events, and dates; general forgetfulness; slowing of memory loss | 5 | 6 | 1 | 10 | 6 |
| Language and communication | Verbal and written communication, such as verbal fluency and object naming | 3 | 3 | - | 6 | 3 | |
| General cognitive health | Cognitive functioning without explicitly referring to a specific cognitive function | 1 | 1 | 1 | 2 | 2 | |
| Judgment and insight | Ability to retain an intuitive understanding of oneself and of the disease process | 2 | 1 | - | 2 | 1 | |
| Executive functions | Planning, multitasking, and focused concentration | 2 | - | - | 2 | 2 | |
| Functioning and dependency | Activities of daily living (ADLs) | Competent and independent ability to complete instrumental (cooking meals, housekeeping, managing finances) and basic (using the toilet, eating meals, dressing, self-hygiene) ADLs | 3 | 7 | 2 | 10 | 7 |
| Driving | Legal implications and issues surrounding surrendering the patient's license | 1 | 1 | 1 | 2 | 2 | |
| Maintaining hobbies | Continued ability to partake in preferred leisure activities and hobbies | 3 | 1 | - | 3 | 3 | |
| Eating behaviors | Appetite or frequency of eating | - | 2 | - | 2 | 3 | |
| Patients' independence and autonomy | The ability to function as an autonomous individual, both physically and psychologically | 7 | 4 | 1 | 10 | 7 | |
| Social engagement | Socialization and social support | 2 | 1 | - | 3 | 3 | |
| Physical health and mobility | Physical health, fitness, and mobility | 2 | 1 | - | 3 | 3 | |
| Behavioral and neuropsychiatric | Mental health | Changes in affect and irritation, symptoms of anxiety, depression, and reality distortion | 3 | 5 | 1 | 10 | 6 |
| Maintaining identity or personality | Personality traits, knowledge, or emotional bonds with others | 4 | 4 | 1 | 9 | 7 | |
| Challenging and distressing behaviors | Verbal or physical aggression, anger, and injurious behaviors | - | 5 | 1 | 6 | 4 | |
| Apathy | General engagement with their environment and an interest, motivation, or enthusiasm for everyday life | 1 | 3 | - | 3 | 1 | |
| Sleep patterns | Patterns or the duration and frequency of sleep | - | 2 | 1 | 3 | 2 | |
| Self-efficacy | Patients' belief and confidence in their abilities | - | 1 | - | 1 | 1 | |
| Patient length and the quality of life | Patient quality of life | Living with dignity, leading a fulfilling life, and an overall sense of satisfaction with life | 2 | 3 | 1 | 6 | 7 |
| Length of patient life | Longevity and staying healthy for as long as possible | 1 | - | - | 1 | 1 | |
| Caregiver-oriented outcomes | Caregiver burden | The burden associated with care, including a loss of social life, time spent caring, stress, mental and physical impact, and giving up work or study | 1 | 8 | 1 | 9 | 8 |
| Family participation in care | Family members drifting apart since diagnosis of the illness, an unequal share of caregiving duties, and the importance/positives of involving family in the caregiving process | - | 5 | 2 | 6 | 8 | |
| Caregiver social support | Need for social support as a caregiver, the reported benefits of providing support to fellow caregivers via shared understanding, barriers to seeking social support, the importance of seeking social support from family members, and information regarding the support services that caregivers use | - | 5 | 1 | 6 | 8 | |
| Spouses' “duty” to care | Belief that it is the “duty” of the spousal caregiver to provide care to their ill partner because of the marital bond | 1 | 2 | 1 | 3 | 2 | |
| Quality of patient/caregiver relationship | Strain placed on marital and parental relationships | 2 | 2 | - | 4 | 3 | |
| Caregiver quality of life | Changes to lifestyle, freedom, physical burden, and emotional impact that affect life as a whole | - | 2 | - | 2 | 6 | |
| Health, social care, and treatment-related outcomes | Health services and disease information | Disease information provided at various health services, availability and relevance of provided information, quality of communication between health-care professionals, patients, and caregivers | 5 | 8 | 2 | 11 | 10 |
| Stability of symptoms and general symptom control | Treatment expectations or controlling symptoms at a level that enables functionality | 1 | 4 | 1 | 5 | 7 | |
| Delaying entry into institutional care | Stay in their own home for as long as possible | 1 | 1 | 2 | 4 | 3 | |
| Medication side effects | The importance of limiting the side effects | 1 | 2 | - | 2 | 6 | |
| Certainty of diagnosis | Accuracy of diagnosis | 2 | 1 | - | 2 | 2 | |
| Social issues | Stigma | Anticipated, perceived, or actual labeling or stereotyping because of the AD process, alongside the emotional, psychological, and societal effects of experiencing such stigmatization | 1 | 3 | - | 4 | 3 |
Abbreviations: AD, Alzheimer's disease; HPs, health-care professionals.
To allow integration of evidence emerging from studies using a range of different methodologies, we established a framework for deciding which of the outcomes discussed within each study should be considered important from the perspective of the relevant stakeholder group. In studies where outcomes were directly ranked for priority, the top 50% of outcomes were included. In studies where outcomes were surveyed for importance but not ranked in the order of priority, outcomes that were deemed important by over 50% of participants were included. In studies adopting qualitative methods, outcomes that were grouped into themes by authors or that recurred frequently in participant quotations were included.
3. Results
Of the 3772 citations identified in the deduplicated searches, we excluded 3653 at title/abstract screening and a further 92 after full-text screening (Fig. 1). In addition to the 27 studies that passed full-text review and quality appraisal, we further found seven studies meeting all inclusion criteria through citation searching key articles and follow up of relevant conference abstracts. No additional studies were found through the gray literature search.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart showing citation numbers in each stage of the screening process. Abbreviations: AD, Alzheimer's disease; MCI, mild cognitive impairment.
3.1. Study characteristics
The 34 included studies (representing 32 distinct research projects) were conducted in 13 countries (Fig. 2). Individual studies involved between four and 1116 participants. Twenty studies recruited patients with MCI or AD dementia, 23 recruited caregivers, and six recruited health-care professionals (the majority of whom were generalist or specialist physicians and community or nursing home nurses; details are shown in Table 3). No studies included the views of patients living with preclinical or prodromal AD or with AD at the severe end of the spectrum.
Fig. 2.
Map showing the number of studies recruiting participants from countries around the world (some studies included participants from more than one country and so are enumerated on the map more than once; see Table 3). Darker shading implies a larger number of studies within a country, as shown on the scale beneath the map.
Table 3.
Summary of characteristics and results of included studies
| First author; location (language) (reference) | Participant numbers according to stakeholder type and AD/MCI patient status | Methods of data collection and analysis | Findings |
|
|---|---|---|---|---|
| Research question 1: reported outcomes | Research question 2: definition of a meaningful delay | |||
| Andersen; Canada (English) [10] | Mild AD patients: n = 4 AD caregivers: n = 4 AD HPs: n = 11 (nurses, physicians, pharmacists) |
Qualitative: semistructured interviews, 30-60 minutes, thematic analysis | AD patients: memory, general cognitive health AD caregivers: stability of symptoms and general symptom control AD HPs: stability of symptoms and general symptom control, delaying entry into care, patient social engagement, patient QoL, ADL, apathy |
AD patients: positive results of treatment indicated by slowed rate of memory loss or improvement in cognitive function AD caregivers: positive results indicated by stabilized symptom/halting of deterioration AD HPs: positive results indicated by stabilization of symptoms to defer requirement to leave home, retention of ability to be socially engaged, improvement in cognitive or physical function |
| Barrios; USA (English) [11] | MCI patients: n = 16 MCI caregivers: n = 33 (study 1), n = 16 (study 2) |
Quantitative: ranking of 12 outcomes from 1 (most important) to 12 (least important) | MCI patients: before the intervention, MCI patients ranked patient depression as significantly less important (mean rank 7.9) than the MCI caregivers (mean rank 4.2; P < .01) MCI caregivers: ADL, patient self-efficacy, patient mental health, caregiver QoL, patient QoL; Note: caregiver burden and caregiver depression were ranked as the outcomes with the least priority by the caregivers. |
|
| Beard; USA (English) [12] | MCI/mild AD patients: n = 17 MCI/AD caregivers: n = 68 |
Qualitative: 14 focus groups conducted throughout the USA using a common interview guide, grounded theory | MCI/AD patients: patients' mental health, independence and autonomy, social engagement, physical health and mobility, judgment, and insight MCI/AD caregivers: patient independence and autonomy, memory |
|
| Blieszner; USA (English) [13] | MCI caregivers: n = 86 | Mixed methods: individual, face-to-face interviews, thematic analysis | MCI caregivers: patients' apathy, patients' sleep patterns, caregiver burden, quality of patient-caregiver relationship, family participation in care, health services, and disease information | |
| Bronner; Germany (English) [14] | Mild AD patients: n = 5 AD caregivers: n = 6 AD HPs: n = 13 (physicians, social engagement workers, legal guardians, nurses, paid caregiver) |
Qualitative: individual, face-to-face semistructured interviews, categorical content analysis | AD patients: patient independence and autonomy, spouses' “duty” to care, health services, and disease information AD caregivers: memory, family participation in care AD HPs: ADL, patients' independence and autonomy, driving, patients' mental health, maintaining identity or personality, spouses' “duty” to care, caregiver burden, health services and disease information, caregiver social support, delaying entry into care |
|
| Cheng; China (English) [15] | AD caregivers: n = 57 (for patients with mild to moderate AD) | Qualitative: tape recorded diaries, thematic analysis | AD caregivers: ADL, eating behaviors, stigma, spouses' “duty” to care, caregiver social support, access to health services and disease information, patient QoL, caregiver burden | |
| Dai; China (English) [16] | MCI caregivers: n = 13 | Qualitative: individual, in-depth interviews, grounded theory | MCI caregivers: general cognitive health, patient independence and autonomy, patient mental health, stigma, health services, and disease information | |
| Dean; UK (English) (a) [17] (b) [18] |
MCI patients: n = 23 Study (b) added MCI caregivers: n = 20 |
Qualitative: individual, in-depth semistructured interviews, thematic analysis | MCI patients: memory, language and communication, maintaining hobbies, patient social engagement, patients' mental health, maintaining identity or personality, caregiver social support, stigma, certainty of diagnosis, access to health services and disease information; (b) MCI caregivers: health services and disease information, stigma, caregiver social support | |
| Frank; UK, US, Spain (English) [19] | Mild to moderate AD patients: n = 18 AD caregivers: n = 46 |
Qualitative: focus groups with caregivers and patients, thematic analysis | AD patients: ADL, patient independence, and autonomy AD caregivers: memory, caregiver social support |
|
| Gelman; USA (English) [20] | AD caregivers: n = 10 | Qualitative: counseling sessions were conducted, categorical content analysis | AD caregivers: memory, patient sleep patterns, spouses' “duty” to care, caregiver burden, family participation in care, health services, and disease information | |
| Gordon; USA (English) [21] | MCI patients: n = 25 | Qualitative: mixture of focus groups and individual meetings, thematic analysis | MCI patients: memory, language and communication, executive functions | |
| Hauber; USA and Germany (English) [22] | AD caregivers: n = 400 (USA) and 403 (Germany) | Quantitative: 15 best-worst scaling questions that correspond to 10 activities from the Disability Assessment for Dementia | AD caregivers: ADL, eating behaviors | |
| Hulko; Canada (English) [23] | Mild to severe AD patients: n = 4 (not possible to differentiate by AD stage) | Qualitative: participant observation sessions, in-home interviews, and focus groups, grounded theory | AD patients: patients' independence and autonomy, quality of patient-caregiver relationship, health services, and disease information | |
| Jones; France, Germany, Italy, Spain, UK (English) [24] | AD caregivers: n = 250 (50 from each location) AD HPs: n = 500 (100 from each location), half specialists (e.g., neurologist/neuropsychiatrist) and half generalists. |
Quantitative: surveys consisting of a series of attitudinal statements requiring a response on a Likert scale | AD caregivers: caregiver burden, caregiver QoL, family participation in care AD HPs: caregiver QoL, health services and disease information, family participation in care |
|
| Joosten-Weyn; Netherlands (English) [25] | MCI patients: n = 8 | Qualitative: individual interviews, grounded theory | MCI patients: executive functions, physical health and mobility, patient independence and autonomy, patient mental health, maintaining identity or personality | |
| Kunneman; Netherlands (English) [26] | MCI patients: n = 1; mild AD: n = 2; AD: n = 3 MCI/AD caregivers: n = 6 |
Mixed methods: focus groups, content analysis | MCI/AD patients: certainty of diagnosis, health services, and disease information MCI/AD caregivers: certainty of diagnosis |
|
| Kurz; Brazil, Canada, France, Germany, Spain, USA (English) [27] | Mild to moderate AD patients: n = 502 (∼100 from each of USA, France, Germany, Spain, Brazil) AD caregivers: n = 614 (as above, ∼100 from Canada) |
Quantitative: survey | AD patients: stability of symptoms and general symptom control, medication side effects, health services and disease information, patient QoL AD caregivers: caregiver social support, medication side effects, health services, and disease information |
|
| Lenardt; Brazil (Portuguese) [28] | AD caregivers: n = 14 (patients with mild to moderate AD) | Qualitative: semistructured interviews, taxonomic analysis | AD caregivers: ADL, challenging and distressing behaviors, caregiver burden, maintaining identity or personality | |
| Lu; USA (English) [29] | MCI caregivers: n = 10 | Qualitative: open-ended interviews, interpretive phenomenological analysis | MCI caregivers: language and communication, patient independence and autonomy, challenging and distressing behaviors, maintaining identity or personality, caregiver burden, quality of patient/caregiver relationship | |
| Lu; USA (English) [30] | MCI patients: n = 9 (7 male) MCI caregivers: n = 9 |
Qualitative: focus groups, content analysis | MCI patients: ADL, patients' independence and autonomy MCI caregivers: language and communication, ADL, patients' mental health |
|
| MacRae; Canada (English) (a) [31] (b) [32] |
Mild AD patients: n = 8, (b) added one further patient with AD (total n = 9) | Qualitative: in-depth, semistructured interviews, thematic analysis | AD patients: memory, patient independence and autonomy, delaying entry into care, maintaining identity or personality, stigma, length of patient life, patient QoL, length of patient life | |
| Malthouse; UK (English) [33] | Mild AD patients: n = 5 AD caregivers: n = 5 |
Qualitative: open-ended interviews, thematic analysis | AD patients: patients' independence and autonomy AD caregivers: physical health and mobility, patients' mental health |
|
| Naumann; Germany (German) [34] | AD caregivers: n = 35 | Quantitative: 25 outcomes (referred to as ‘benefit aspects’) were ranked for priority. Average scores for each item were calculated. | AD caregivers: language and communication, delaying entry into care, challenging and distressing behaviors, stability of symptoms and general symptom control, patient QoL | |
| Oremus; Canada (English) [35] | AD caregivers: n = 216 (patients with mild (81%) or moderate (19%) AD | Quantitative: questionnaire, regression analysis | AD caregivers: medication side effects | |
| Pavarini; Brazil (Portuguese) [36] | AD caregivers: n = 14 (patients with probable, mild AD) | Qualitative: interviews, categorical content analysis | AD caregivers: memory, ADL, caregiver burden, health services and disease information, patients' social engagement, patients' mental health, patients' challenging and distressing behaviors, family participation in care | |
| Rockwood; Canada (English) [37] | AD HPs: number and specialty not stated Mild to moderate AD patients: n = 99 AD caregivers: n = 99 |
Quantitative: clinical assessment using ADAS-Cog at 8-week intervals over 24 weeks compared to changes on other assessments (PGAS, CGAS, CIBIC+) measured by clinician interview | At group-level analysis, a 4-point improvement was significantly related to improvements on other assessments. Worsening scores were nonsignificantly related to clinical changes. At individual level, there was substantial variability, with around half misclassified; often when ADAS-Cog detected no change, clinically meaningful effects could be detected. | |
| Ropacki; USA (English) [38] | MCI patients: n = 25 MCI caregivers: n = 25 |
Quantitative: focus groups, categorical content analysis | MCI patients: memory, language and communication, judgment and insight, ADL, maintaining hobbies, driving, patient apathy, patient sleep patterns, patient mental health, caregiver burden, maintaining identity and personality MCI caregivers: memory, judgment and insight, ADL, maintaining hobbies, driving, patient apathy, patient sleep patterns, challenging and distressing behaviors, maintaining identity or personality, caregiver burden |
|
| Schrag; UK (English) [39] | Mild AD patients: n = 181 AD HPs: number and specialty not stated, all based within neuroimaging initiative sites |
Quantitative: ADAS-Cog compared to clinician-assessed memory and nonmemory cognitive function using FAQ and CDRS | ADAS-Cog scores among those with a clinically relevant change at 6 months were between 3.1 and 3.8. Scores in those without a clinical change were between 1.9 and 2.0. Minimally, clinically relevant changes determined to be 3 points. | |
| Smith; Australia (English) [40] | AD patients: n = 5 AD caregivers: n = 6 |
Qualitative: in-depth, semistructured interviews, thematic analysis | AD patients: stability of symptoms and general symptom control | |
| Smith; Canada (English) [41] | AD caregivers: n = 17 (16 patients with early to moderate AD, 1 with moderate to severe AD) | Qualitative: in-depth narrative interviews conducted, grounded theory | AD caregivers: maintaining identity or personality, stability of symptoms, and general symptom control | |
| Sorensen; Denmark (English) [42] | Patients with mild AD: n = 11 | Qualitative: semistructured interviews, grounded theory | AD patients: maintaining hobbies, maintaining identity or personality, quality of patient-caregiver relationship | |
| Yektatalab; Iran (English) [33] | AD HPs: n = 14, clinical and social caregivers for residents in a nursing home | Qualitative: interviews with open-ended questions, descriptive content analysis | AD HPs: challenging and distressing behaviors, family participation in care | |
Abbreviations: AD, Alzheimer's disease; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive Subscale; ADL, activities of daily living; CDRS, Clinical Dementia Rating Scale; CIBIC+, Clinician Interview-Based Impression of Change plus caregiver input; CGAS, clinician Goal-Attainment Scaling; FAQ, functional activities questionnaire; HPs, health-care professionals; MCI, mild cognitive impairment; PGAS, patient/carer Goal-Attainment Scaling; QoL, quality of life.
Qualitative methods were used in 23 studies [10], [12], [14], [15], [16], [17], [18], [19], [20], [21], [23], [25], [28], [29], [30], [31], [32], [33], [36], [40], [41], [42], [43], nine used quantitative methods [11], [22], [24], [27], [34], [35], [37], [38], [39], and two used mixed methods [13], [26] (Table 3). Six studies included explicit prioritization of outcomes through ranking or survey responses from stakeholders [11], [22], [24], [27], [34], [38]; the rest included relevant material in the form of quotations and themes from interviews or focus groups that was used to infer the importance of outcomes from stakeholder perspectives [10], [12], [21], [25], [26], [28], [29], [30], [31], [32], [33], [35], [37], [39], [40], [41], [42], [43].
Only three of the 34 included studies provided any evidence on stakeholders' views about criteria for a meaningful delay in disease progression [10], [37], [39]. Evidence was sought on either the duration of such a delay or the symptoms delayed in their onset or worsening, which would be considered meaningful. These studies gathered data via stakeholder interviews and clinical assessments of cognition.
3.2. Outcomes of AD across the spectrum
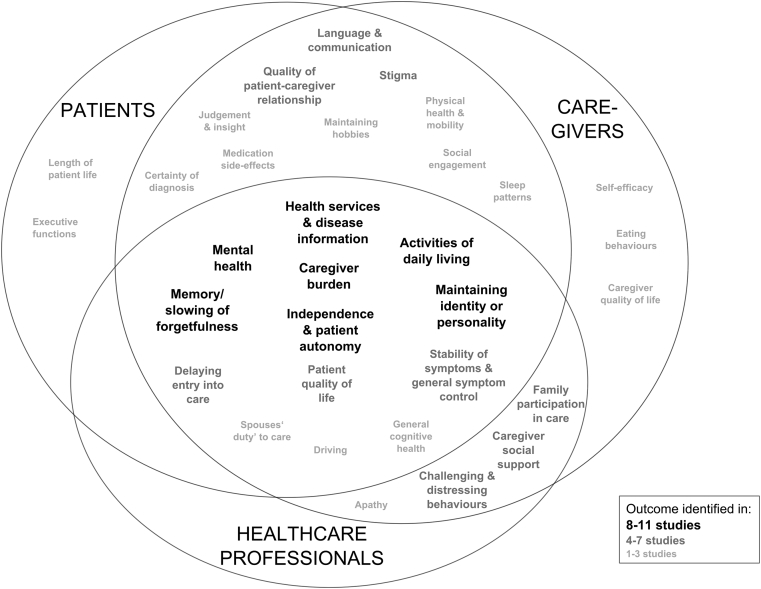
Table 2 lists 32 outcomes across seven domains which emerged from the included studies, indicating which stakeholder(s) considered them important, the number of studies in which the outcome was described by each stakeholder group, and the number of countries from which participants were recruited. Fig. 3 shows the overlap by the stakeholder group.
Fig. 3.
Overlap of outcomes according to the stakeholder group which raised their importance and the number of studies in which they appeared.
The most consistent evidence across the stakeholder groups is summarized in the following section, including illustrative quotations and other relevant findings.
3.2.1. Outcomes raised by all stakeholder groups
Eight outcomes, spanning all seven overarching domains, were considered important by all stakeholder groups. They emerged from between six and eleven studies, from a range of evidence types (Table 2).
The impact and importance of memory decline was considered in relation to MCI and AD. This encompassed recalling names and dates and a general sense of forgetfulness. “…it gets … frustrating to not be able to finish a conversation without at least having one instance where I don't remember a certain item, a name or place” (patient with MCI) [21]. “I was losing my mother—she was forgetting people, how to do the most basic things… forgetting who she was” (familial caregiver of AD patient) [20]. The ten studies [10], [12], [14], [18], [19], [20], [21], [36], [38] reporting this were conducted in North and South America and Europe.
ADLs and loss of functioning included completion of routine activities, such as independently managing cooking, finances, and self-hygiene. Evidence for significance in relation to MCI and AD came from ten studies [10], [11], [14], [15], [19], [22], [28], [30], [36], [38] conducted in North and South America, Europe, and Asia. “Improve ADLs so far as you know... buttoning up a shirt or closing a zipper” (physician regarding expectations of treatment) [10]. “A big issue is car driving…. we have to fight that they give up…. call the police” (professional describing main topics emerging after early AD diagnosis) [14].
The importance of maintaining patient independency and autonomy was discussed in relation to MCI and AD in ten studies [12], [14], [16], [19], [23], [25], [29], [30], [31], [33] from North America, Europe, and Asia. This concept went beyond functional capacity to the ability to self-govern, preserving both physical and psychological autonomy. “The issue is [they] want to maintain [their] autonomy. [They] don't want to be patronised” (health-care professional for AD patients) [14].
The impact of mental health issues, such as anxiety, depression, and reality distortion, was mentioned in relation to MCI and AD, with evidence from ten studies [12], [12], [14], [16], [18], [25], [30], [33], [36], [38] conducted in North and South America, Europe, and Asia. When asked which key areas should be targeted in an intervention for MCI, spousal caregivers believed that targeting depression was important [30].
The importance of maintaining patient quality of life (QoL) (encompassing the concepts of living a fulfilled and dignified life) was highlighted in relation to MCI and AD; evidence came from six studies [10], [11], [15], [27], [31], [34] conducted in North and South America, Europe, and Asia. In one study, caregivers of patients with MCI ranked patient QoL as the most important outcome from a predetermined list of twelve outcomes [11].
Maintenance of patients' identity and personality was discussed in relation to MCI and AD in nine studies [14], [18], [25], [28], [29], [31], [38], [41], [42] carried out in North and South America and Europe. This related to the preservation of personality traits, knowledge, and emotional bonds with others. “My best friend [husband with MCI] has gone. He is part of me, but he is no longer the same person… I really miss him” [29].
The impact of the disease through caregiver burden was discussed in relation to MCI and AD in nine studies [13], [14], [15], [20], [24], [28], [29], [36], [38] carried out in North and South America, Europe, and Asia. “I quit my job, my house, to take care of her. I do not go to the cinema, I do not go out for a walk, I do not go to the shopping mall, I do not go to the hairdresser” (caregiver of AD patient) [28].
The importance of health services and disease information (and its absence) was discussed by all stakeholder groups in relation to MCI and AD in eleven studies [13], [14], [15], [16], [17], [20], [23], [24], [26], [27], [36] from North and South America, Europe, and Asia. “If there is something I don't understand, I will go on [the computer] and look it up… I just want to be able, [if] something happens, [to] take care of him as far as [possible] in every situation …” (caregiver of an AD patient) [13].
3.2.2. Outcomes raised by both patients and caregivers
Patients and caregivers mentioned the importance of retaining language and communication functions in relation to MCI and AD. This included cognitive aspects of verbal and written communication, such as verbal fluency and object naming. Evidence came from six studies [18], [21], [29], [30], [34], [38] conducted in North America and Europe, including one study in which familial caregivers of AD patients ranked “improvement to communication abilities” as the fourth most important of a predetermined list of 25 outcomes [34].
Patients and caregivers dealing with MCI and AD discussed the importance of maintaining a quality patient-caregiver relationship. Evidence emerged from four studies [13], [23], [29], [42] conducted in North America, Europe, and Asia. “My husband gets angry with me when I can't remember things we have decided to do. He talks a lot about it. Sometimes I think that it is worse for him than for me” (a patient with AD) [42].
The impact of experiencing stigma due to AD emerged in four studies [15], [16], [18], [31] conducted in North America, Europe, and Asia. Stakeholders described anticipated, perceived, and overt stereotyping because of AD. “We haven't discussed this, but I get the feeling that [my husband] wouldn't like that, because there is a certain amount of stigma attached to dementia. So no, I haven't” (wife of an AD patient when asked about seeking social support) [18].
3.2.3. Outcomes mentioned by both health-care professionals and caregivers
Both health-care professionals and caregivers discussed the difficulty of dealing with challenging and distressing behaviors in relation to MCI and AD, such as verbal or physical aggression. Evidence came from six studies [28], [29], [34], [36], [38], [43] conducted in North and South America, Europe, and Asia. “If you don't have patience here, you won't last for even 2 months because of patients' yelling and their aggression” (a paid caregiver of AD patients) [43].
The benefits of caregiver social support and the challenges of accessing it were discussed in relation to MCI and AD, with evidence from six studies [14], [15], [17], [18], [19], [27] from North and South America, Europe, and Asia. “I started to realize that I should get someone to talk to when feeling helpless sometimes. I felt better after letting everything out from within” (a caregiver of AD patients) [15].
Patients dealing with MCI and AD described the importance of maintaining family participation in care throughout the disease process to preserve relationships and spread duties. This emerged in six studies [13], [14], [20], [24], [36], [43] conducted in North and South America, Europe, and Asia. “He (the patient) loved his sister so much, and now she won't even call to see how he's doing. She's angry that I keep insisting there's something wrong, and that he ‘took my side’ because he hasn't called her. But of course he can't call her. This is tearing the family apart.” (a caregiver of an AD patient) [20].
Outcomes discussed less frequently are described in Table 2 and Fig. 3.
3.3. Meaningful delay
Only three included studies [10], [37], [39] reported relevant statements or data relating to changes in outcomes of the disease over time, and none comprehensively defined what constituted a meaningful delay in disease progression from stakeholders' perspectives. One study involving patients with mild AD described changes in symptomatology without specific reference to time periods (e.g., slowing memory deterioration and retaining ability to undertake ADLs) [10] (details are shown in Table 3). Two studies involving patients with mild to moderate AD referred to disease progression in terms of cutoff points on the AD Assessment Scale-Cognitive describing 3- [39] or 4-point changes [37] as clinically meaningful. Authors of both these studies emphasized that although these changes may be significant when analyzed at a group level, there was a substantial variation among individuals and their experienced symptoms at any given score on the scale.
4. Discussion
Our systematic review provides a framework of real-world outcomes which represents the voices of stakeholders personally impacted by AD across the spectrum of disease, adding a different perspective to the previous work based on AD trial outcomes and their measures [44], [45]. Important outcomes included clinical aspects of the disease, e.g., memory and mental health, and social aspects, such as the devastating impact of caregiver burden. Other frequently observed important outcomes reflect practical challenges such as accessing AD information or the ability to complete ADLs alongside personal aspects such as maintenance of patient autonomy, identity, and QoL.
Our review identified the importance of several outcomes consistent with those typically assessed in clinical trials, such as cognition and ADLs. However, we revealed the importance of several additional outcomes that are infrequently assessed in clinical trial settings, including preservation of the patient's personality or the accessibility of health services and disease information. These concepts may be captured by patient reported outcome and experience measures [46], which can be used to describe and evaluate the effectiveness of treatments and quality of care, respectively, and their use may enhance patient and carer engagement with—and so recruitment to—clinical trials.
Stakeholders prioritized outcomes with tangible and directly observable effects on the daily lives of patients and caregivers. Disease biomarkers (e.g., those based on tau or amyloid, structural neuroimaging measures, or combination of these) are now incorporated into research diagnostic criteria and therefore commonly used to assess interventions in clinical trials [2]. These were not mentioned by stakeholders, although the desire for certainty of diagnosis was identified in two studies. It is possible that clinical biomarker-based outcomes were not flagged as being important because of the sparsity of data from health-care professionals (six of the 34 included studies), the lack of data from people with prodromal or severe AD, and/or the relatively new and tentative nature of the evidence linking biomarkers to clinical symptomatology.
Although we identified a substantial body of research reporting on the importance of various outcomes to stakeholders, no reliable conclusions relating to the definition of a meaningful delay in AD progression could be drawn. The available evidence highlighted the difficulty of using scales to determine clinically relevant or meaningful change because these are not accurate or predictive of symptoms at the individual level, suggesting the need for further investigation of this issue.
4.1. Strengths and limitations
This review benefitted from the multidisciplinary team of researchers and experts across Europe who conducted the work and contributed at key decision-making stages.
The inclusion of studies published in multiple languages in different countries (although few from developing countries) improves the generalizability of the evidence, and its methodological heterogeneity adds confidence by incorporating outcomes that emerged from a range of study types. This combined evidence facilitates a complete understanding of stakeholder perspectives.
Although our search strategy was relatively sensitive, it may not have captured all relevant material. We used the term “patient” with condition terms to identify individuals with AD across the spectrum, so relevant studies may have been missed which used terms such as “person” or “subject”. We found no studies that addressed the opinions of patients or corresponding stakeholders at the severe end of the AD spectrum. Given the time-intensive and complex nature of qualitative work and the requirement of appropriate consent procedures, it would be practically and ethically difficult to conduct such research. We also did not capture the opinions of former caregivers of people with AD. Both groups represent important missing voices in the evidence presented. The interpretation of research evidence into outcomes and their categorization into domains may have obscured some relevant concepts. For example, language and communication may have been captured by the broader “cognition” domain from the perspective of health-care professionals.
5. Conclusions
Our systematic review provides evidence about the outcomes of MCI and mild to moderate AD dementia which are of importance to patients, caregivers, and health-care professionals. It demonstrates that although current clinical trials typically assess some of these, others are rarely included as outcomes. Involving these varied and nuanced AD outcomes in the trial design would be a substantial challenge, but as they reflect distinct, important aspects of the experience and burden of the disease, they could help ensure that successful treatments or evaluation of the quality of care is better focused on aspects of AD most important to the people affected by it.
As there was limited evidence to define a “meaningful delay in AD disease progression” from any of the stakeholders' perspectives, further research is essential to explore this important issue at different stages of the AD spectrum. AD outcomes of importance in preclinical and severe stages of the disease should also be explored.
Our findings will help future researchers meet the challenge of designing and undertaking optimized clinical trials with the greatest potential to provide treatments for AD which mitigate its most devastating effects at a personal and social level.
Research in context.
-
1.
Systematic review: The authors searched four databases (Medline, Embase, CINAHL, and PsycINFO) and online sources of gray literature for research published in any language which elicited the views of stakeholders to identify clinical and nonclinical outcomes of Alzheimer's disease which were important to them.
-
2.
Interpretation: There was consistent evidence across stakeholder groups, countries where studies were based, and study methodologies. Overall, 32 outcomes encompassed by seven broad domains were uncovered, reflecting impacts across the spectrum of the disease. There was more evidence for the importance of patient memory, activities of daily living, quality of life, independence and autonomy, mental health, maintaining identity and personality, caregiver burden, and access to health services and disease information.
-
3.
Future directions: Understanding which real-world outcomes of Alzheimer's disease are most relevant to patients, caregivers, and health-care professionals should help guide future research to develop relevant, effective treatments and improve health services.
Acknowledgment
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under the grant agreement no.: 116020 (“ROADMAP”). This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation program and EFPIA.
Footnotes
Conflicts of interest: A.G. is a partner of Quantify Research, providing consultancy services to pharmaceutical companies and other private and public organizations and institutions. A.G.'s contribution to ROADMAP was on behalf of Roche Pharmaceuticals. C.B. (Alzheimer Europe) received an Honoraria from ProLog Wissen GmbH, Cologne, Germany, for a cognitive training program.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dadm.2018.12.003.
Supplementary data
References
- 1.Prince M.J. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. Alzheimer's Dis Int. 2015:1–87. [Google Scholar]
- 2.Winblad B., Amouyel P., Andrieu S., Ballard C., Brayne C., Brodaty H. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 3.Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 4.Posner H., Curiel R., Edgar C., Hendrix S., Liu E., Loewenstein D.A. Outcomes Assessment in Clinical Trials of Alzheimer's Disease and its Precursors: Readying for Short-term and Long-term Clinical Trial Needs. Innov Clin Neurosci. 2017;14:22–29. [PMC free article] [PubMed] [Google Scholar]
- 5.Cano S.J., Posner H.B., Moline M.L., Hurt S.W., Swartz J., Hsu T. The ADAS-cog in Alzheimer's disease clinical trials: psychometric evaluation of the sum and its parts. J Neurol Neurosurg Psychiatry. 2010;81:1363–1368. doi: 10.1136/jnnp.2009.204008. [DOI] [PubMed] [Google Scholar]
- 6.Makady A., de Boer A., Hillege H., Klungel O., Goettsch W. What Is Real-World Data? A Review of Definitions Based on Literature and Stakeholder Interviews. Value Health. 2017;20:858–865. doi: 10.1016/j.jval.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's Demen. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization ICD-10 online versions. http://www.who.int/classifications/icd/icdonlineversions/en/ Available at: Accessed January 10, 2019.
- 9.Ly A. ROADMAP WP2 partners. D2.1 First list of priority Real World Evidence relevant outcomes for AD 2017. https://roadmap-alzheimer.org/wp-content/uploads/2017/04/116020_ROADMAP_D2.1_First-list-of-priority-RWE-outcomes-for-AD.pdf Available at: Accessed January 10, 2019.
- 10.Andersen E., Silvius J., Slaughter S., Dalziel W., Drummond N. Lay and professional expectations of cholinesterase inhibitor treatment in the early stage of Alzheimer's disease. Dementia. 2008;7:545–558. [Google Scholar]
- 11.Barrios P.G., Gonzalez R.P., Hanna S.M., Lunde A.M., Fields J.A., Locke D.E.C. Priority of Treatment Outcomes for Caregivers and Patients with Mild Cognitive Impairment: Preliminary Analyses. Neurol Ther. 2016;5:183–192. doi: 10.1007/s40120-016-0049-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beard R.L., Fetterman D.J., Wu B., Bryant L. The two voices of Alzheimer's: Attitudes toward brain health by diagnosed individuals and support persons. Gerontologist. 2009;49:S40–S49. doi: 10.1093/geront/gnp083. [DOI] [PubMed] [Google Scholar]
- 13.Blieszner R., Roberto K.A. Care partner responses to the onset of mild cognitive impairment. Gerontologist. 2010;50:11–22. doi: 10.1093/geront/gnp068. [DOI] [PubMed] [Google Scholar]
- 14.Bronner K., Perneczky R., McCabe R., Kurz A., Hamann J. Which medical and social decision topics are important after early diagnosis of Alzheimer's Disease from the perspectives of people with Alzheimer's Disease, spouses and professionals? BMC Res Notes. 2016;9:149. doi: 10.1186/s13104-016-1960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng S.-T., Mak E.R.M., Lau R.W.L., Ng N.S.S., Lam L.C.W. Voices of Alzheimer Caregivers on Positive Aspects of Caregiving. Gerontologist. 2016;56:451–460. doi: 10.1093/geront/gnu118. [DOI] [PubMed] [Google Scholar]
- 16.Dai B., Mao Z., Mei J., Levkoff S., Wang H., Pacheco M. Caregivers in China: knowledge of mild cognitive impairment. PLoS One [Electronic Resource] 2013;8:e53928. doi: 10.1371/journal.pone.0053928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dean K., Walker Z., Churchman D., Wilcock G., Jenkinson C. The development and validation of a quality of life measure for people with mild cognitive impairment (the MCQ) Value in Health. 2014;16:A550. doi: 10.1017/S1041610213002251. [DOI] [PubMed] [Google Scholar]
- 18.Dean K., Jenkinson C., Wilcock G., Walker Z. Exploring the experiences of people with mild cognitive impairment and their caregivers with particular reference to healthcare - a qualitative study. Int Psychogeriatrics. 2014;26:475–485. doi: 10.1017/S104161021300207X. [DOI] [PubMed] [Google Scholar]
- 19.Frank L., Howard K., Jones R., Lacey L., Leibman C., Lleo A. A qualitative assessment of the concept of dependence in Alzheimers disease. Am J Alzheimer's Dis other Demen. 2010;25:239–247. doi: 10.1177/1533317509356690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelman C.R. 'La Lucha': the experiences of Latino family caregivers of patients with Alzheimer's disease. Clin Gerontologist. 2010;33:181–193. [Google Scholar]
- 21.Gordon M.F., Lenderking W.R., Duhig A., Chandler J., Lundy J.J., Miller D.S. Development of a patient-reported outcome instrument to assess complex activities of daily living and interpersonal functioning in persons with mild cognitive impairment: The qualitative research phase. Alzheimer's Demen. 2016;12:10. doi: 10.1016/j.jalz.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Hauber A.B., Mohamed A.F., Johnson F.R., Cook M., Arrighi H.M., Zhang J. Understanding the relative importance of preserving functional abilities in Alzheimer's disease in the United States and Germany. Qual Life Res. 2014;23:1813–1821. doi: 10.1007/s11136-013-0620-5. [DOI] [PubMed] [Google Scholar]
- 23.Hulko W. From ‘not a big deal’ to ‘hellish’: Experiences of older people with dementia. J Aging Stud. 2009;23:14. [Google Scholar]
- 24.Jones R.W., Mackell J., Berthet K., Knox S. Assessing attitudes and behaviours surrounding Alzheimer's disease in Europe: key findings of the Important Perspectives on Alzheimer's Care and Treatment (IMPACT) survey. J Nutr Health Aging. 2010;14:525–530. doi: 10.1007/s12603-010-0263-y. [DOI] [PubMed] [Google Scholar]
- 25.Joosten-Weyn Banningh L., Vernooij-Dassen M., Rikkert M.O., Teunisse J.P. Mild cognitive impairment: coping with an uncertain label. Int J Geriatr Psychiatry. 2008;23:148–154. doi: 10.1002/gps.1855. [DOI] [PubMed] [Google Scholar]
- 26.Kunneman M., Pel-Littel R., Bouwman F.H., Gillissen F., Schoonenboom N.S.M., Claus J.J. Patients' and caregivers' views on conversations and shared decision making in diagnostic testing for Alzheimer's disease: The ABIDE project. Alzheimer's Demen Translational Res Clin Interventions. 2017;3:314–322. doi: 10.1016/j.trci.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurz A., Schulz M., Reed P., Wortmann M., Rodrigo J., von Lutzau Hohlbein H. Personal perspectives of persons with Alzheimer's disease and their carers: A global survey. Alzheimer's Demen. 2008;4:345–352. doi: 10.1016/j.jalz.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Lenardt M.H., da Silva S.C., Willig M.H., Seima M.D. Elderly with Alzheimer's disease: the care and the knowledge of the familial caregiver. Revista Mineira de Enfermagem. 2010;14:301–307. [Google Scholar]
- 29.Lu Y.Y., J.E. H. Experience and Perspectives of Caregivers of Spouse with Mild Cognitive Impairment. Curr Alzheimer Res. 2009;6:14. doi: 10.2174/156720509788929309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y.Y., Haase J.E. Content validity and acceptability of the daily enhancement of meaningful activity program: intervention for mild cognitive impairment patient-spouse dyads. J Neurosci Nurs. 2011;43:317–328. doi: 10.1097/JNN.0b013e318234e9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacRae H. ‘Making the best you can of it’: living with early-stage Alzheimer's disease. Sociol Health Illness. 2008;30:17. doi: 10.1111/j.1467-9566.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 32.MacRae H. Managing Identity While Living With Alzheimer's Disease. Qual Health Res. 2010;20:13. doi: 10.1177/1049732309354280. [DOI] [PubMed] [Google Scholar]
- 33.Malthouse R., Fox F. Exploring experiences of physical activity among people with Alzheimer's disease and their spouse carers: a qualitative study. Physiotherapy. 2014;100:169–175. doi: 10.1016/j.physio.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Naumann J., Grimm C., Rychlik R., Brunner H. Benefit of therapies in Alzheimer's disease - The perspective of family nursing. Gesundheitsokonomie und Qualitatsmanagement. 2011;16:160–165. [German] [Google Scholar]
- 35.Oremus M., Tarride J.E., Pullenayegum E., Clayton N., Mugford G., Godwin M. Caregivers' willingness-to-pay for Alzheimer's disease medications in Canada. Dementia. 2015;14:63–79. doi: 10.1177/1471301213490709. [DOI] [PubMed] [Google Scholar]
- 36.Pavarini S.C.I., de Melo L.C., Silva V.M., Orlandi Fd, de Mendiondo M.S.Z., Filizola C.L.A. Caring for elders with Alzheimer's disease: experiences of family caregivers. Rev Eletr Enf. 2008;10:11. [Google Scholar]
- 37.Rockwood K., Fay S., Gorman M. The ADAS-cog and clinically meaningful change in the VISTA clinical trial of galantamine for Alzheimer's disease. Int J Geriatr Psychiatry. 2010;25:191–201. doi: 10.1002/gps.2319. [DOI] [PubMed] [Google Scholar]
- 38.Ropacki M.T., Hannesdottir K., Hendrix S., Gordon M.F., Stephenson D., Coons S.J. Clinically Meaningful Outcomes in Early Alzheimer Disease: A Consortia-Driven Approach to Identifying What Matters to Patients. Ther Innovation Regul Sci. 2017;51:380–390. doi: 10.1177/2168479016689712. [DOI] [PubMed] [Google Scholar]
- 39.Schrag A., Schott J.M., Alzheimer's Disease Neuroimaging I What is the clinically relevant change on the ADAS-Cog? J Neurol Neurosurg Psychiatry. 2012;83:171–173. doi: 10.1136/jnnp-2011-300881. [DOI] [PubMed] [Google Scholar]
- 40.Smith B., Chur-Hansen A., Neale A., Symon J. Quality of life and cholinesterase inhibitors: a qualitative study of patients with Alzheimer's disease and their carers. Australas Psychiatry. 2008;16:433–437. doi: 10.1080/10398560802375990. [DOI] [PubMed] [Google Scholar]
- 41.Smith A., Kobayashi K., Chappell N., Hoxsey D. The controversial promises of cholinesterase inhibitors for Alzheimer's disease and related dementias: A qualitative study of caregivers' experiences. J Aging Stud. 2011;25:397–406. [Google Scholar]
- 42.Sørensen L., Waldorff F., Waldemar G. Coping with mild Alzheimer's disease. Dementia (14713012) 2008;7:287–299. [Google Scholar]
- 43.Yektatalab S., Sharif F., Kaveh M.H., Khoshknab M.F., Petramfar P. Living with and Caring for Patients with Alzheimer's Disease in Nursing Homes. J Caring Sci. 2013;2:9. doi: 10.5681/jcs.2013.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison J., Noel-Storr A., Demeyere N., Reynish E., Quinn T. Outcomes measures in a decade of dementia and mild cognitive impairment trials. Alzheimers Res Ther. 2016;8:48. doi: 10.1186/s13195-016-0216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webster L., Groskreutz D., Grinbergs-Saull A., Howard R., O'Brien J., Mountain G. Development of a core outcome set for disease modification trials in mild to moderate dementia: a systematic review, patient and public consultation and consensus recommendations. Health Technol Assess. 2017;21 doi: 10.3310/hta21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothrock N., Kaiser K., Cella D. Developing a Valid Patient-Reported Outcome Measure. Clin Pharmacol Ther. 2011;90:6. doi: 10.1038/clpt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.