Summary
Tonotopic differentiations of ion channels ensure sound processing across frequencies. Afferent input plays a critical role in differentiations. We demonstrate here in organotypic culture of chicken cochlear nucleus that expression of Kv1.1 was coupled with Ca2+ to a different degree depending on tonotopic regions, thereby differentiating the level of expression within the nucleus. In the culture, Kv1.1 was down-regulated and not differentiated tonotopically. Chronic depolarization increased Kv1.1 expression in a level-dependent manner. Moreover, the dependence was steeper at higher-frequency regions, which restored the differentiation. The depolarization increased Kv1.1 via activation of Cav1 channels, whereas basal Ca2+ level elevated similarly irrespective of tonotopic regions. Thus, the efficiency of Ca2+-dependent Kv1.1 expression would be fine-tuned in a tonotopic-region-specific manner, emphasizing the importance of neuronal tonotopic identity as well as pattern of afferent input in the tonotopic differentiation of the channel in the auditory circuit.
Subject Areas: Neuroscience, Cellular Neuroscience, Molecular Neuroscience
Graphical Abstract
Highlights
-
•
Kv1.1 expression is down-regulated in slice culture of chicken cochlear nucleus
-
•
Depolarization up-regulates Kv1.1 in a tonotopic-region-specific manner
-
•
Level of Kv1.1 expression is dependent on basal calcium concentration
-
•
Efficiency of calcium-dependent Kv1.1 expression is differentiated tonotopically
Neuroscience; Cellular Neuroscience; Molecular Neuroscience
Introduction
Precise temporal coding of sound requires proper adjustment of ion channel expression in auditory neurons (Johnston et al., 2010). Two types of voltage-gated K+ (Kv) channels, Kv1.1 and Kv3.1, play central roles in the temporal coding. These channels mediate low- and high-voltage-activated K+ currents, respectively. Kv1.1 is activated strongly at subthreshold potential and improves phasic firing by suppressing aberrant spike generation during synaptic depolarization, whereas Kv3.1 accelerates falling phase of spikes and promotes firing at high frequency (Oertel, 1999, Trussell, 1999).
Avian nucleus magnocellularis (NM), a homolog of mammalian anteroventral cochlear nucleus, is involved in the temporal coding (Sullivan and Konishi, 1984, Warchol and Dallos, 1990) and well known for rich expression of these Kv channels (Fukui and Ohmori, 2004, Parameshwaran et al., 2001, Rathouz and Trussell, 1998, Reyes et al., 1994). NM neurons are tuned to a specific frequency of sound (characteristic frequency, CF), and arranged tonotopically within the nucleus such that neurons with high CF are located rostromedially (Parks and Rubel, 1978). Moreover, they are differentiated biophysically as well as morphologically along the tonotopic axis (Fukui and Ohmori, 2004, Kuba and Ohmori, 2009, Oline et al., 2016, Wang et al., 2017). One prominent example is the differentiation of Kv1.1; the expression level of this channel increases in neurons with higher CF, which is considered crucial in adjusting neuronal excitability to the CF-specific patterns of afferent input in NM (Fukui and Ohmori, 2004). Recently, we reported that the differentiation of Kv1.1 was created because afferent input augmented the expression to a larger extent in higher-CF neurons (Akter et al., 2018), showing the importance of afferent input in setting the level of Kv1.1 expression. Intriguingly, however, the auditory threshold is higher for higher-frequency sound (Jones et al., 2006, Saunders et al., 1973), and therefore, the level of afferent input cannot solely explain the graded expression of Kv1.1 toward higher CF, raising a possibility that additional factors may contribute to the differentiation.
Thus, we explored the mechanisms of tonotopic differentiation of Kv1.1 in NM, using organotypic culture of chicken brainstem (Sanchez et al., 2011), in which neurons were totally deprived of afferent input. We found that chronic depolarization increased Kv1 current in a level-dependent manner, but the extent was larger at higher-CF regions, causing the tonotopic difference of the current in the culture. The depolarization increased Kv1 current via elevation of [Ca2+]i, whereas it elevated [Ca2+]i similarly irrespective of tonotopic regions. The results showed that the Ca2+-dependent process of Kv1.1 expression was more efficient at higher-CF regions, suggesting the importance of neuronal tonotopic identity as well as pattern of afferent input for the tonotopic differentiation of Kv1.1 in NM.
Results
Characteristics of NM Neurons in Slice Culture
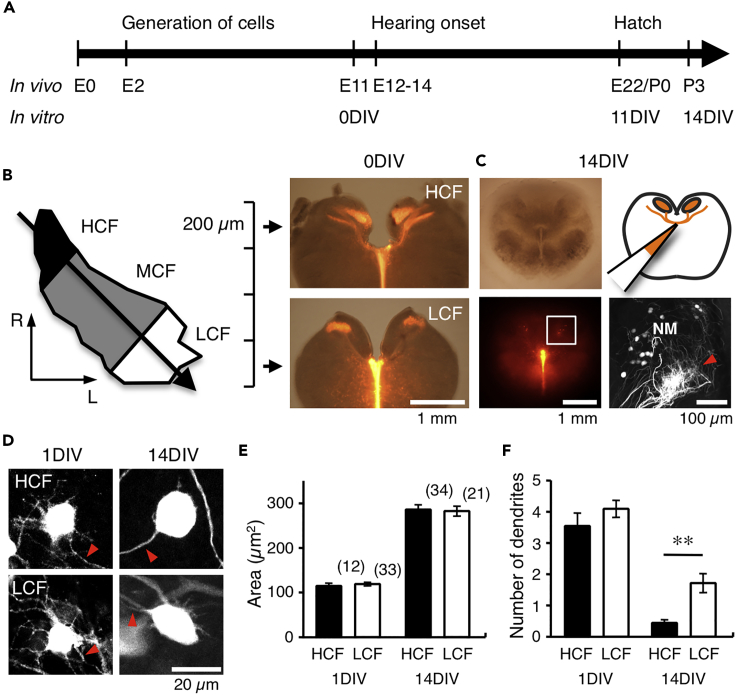
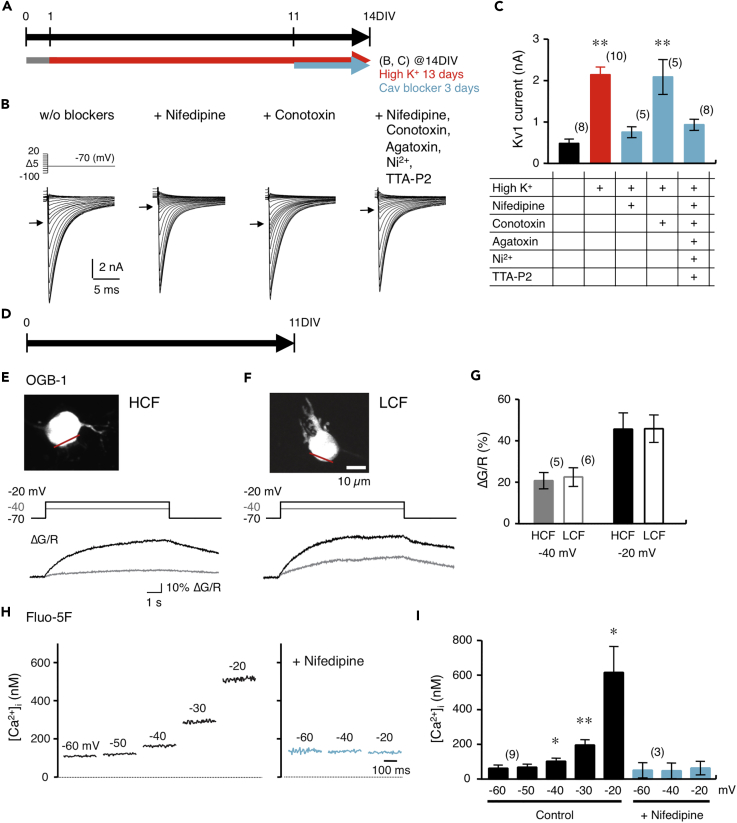
Brainstem slices were prepared from chicken embryos (embryonic day 11, E11) before hearing onset, and those including high- or low-CF neurons were cultured and used for experiments at 14 days in vitro (14DIV), corresponding to posthatch day 3 (P3) (Figures 1A and 1B). An injection of dextran tetramethylrhodamine (TMR) into the midline tract region visualized NM neurons and projection fibers from contralateral NM (Figure 1C). The morphology of NM neurons developed during the course of culture; the neurons showed multipolar shape and several dendrites at 1DIV but became spherical shape and decreased the number of dendrites by 14DIV (Figure 1D). Importantly, the loss of dendrites was more prominent in higher-CF neurons, making the neurons almost adendritic at 14DIV (Figure 1F), which resembled the morphological development in vivo (Jhaveri and Morest, 1982). Cross-sectional area of the soma increased with development and became similar between high- and low-CF neurons (Figure 1E).
Figure 1.
High- and Low-CF NM Neurons in Slice Culture
(A) Development of chicken auditory system in vivo and in vitro.
(B) NM is organized tonotopically from rostromedial to caudolateral direction. Four to five slices of 200 μm thickness were prepared from an animal at E11 after retrograde labeling of NM neurons with dextran TMR. NM in rostral one or two slices was defined as high-CF (HCF) region and that in the caudal most slice as low-CF (LCF) region.
(C) Cultured slice at 14DIV. NM was labeled with dextran TMR (upper right). Bright-field (upper left) and fluorescence (lower left) images of the slice. Square was magnified (lower right). Anterogradely labeled projections from contralateral NM (arrowhead).
(D) Retrogradely labeled NM neurons in high- (upper) and low-CF regions (lower) at 1DIV (left) and 14DIV (right). Arrowheads indicate axon.
(E and F) Cross-sectional area (E) and the number of dendrites (F) of cultured neurons. Numbers in parentheses are the number of cells. **p < 0.01.
See also Figure S1.
We also evaluated excitatory synaptic input in the cultured NM neurons at 11DIV (Figure S1A). Auditory nerve terminals are differentiated tonotopically in NM in vivo; they form end-bulb synapses on high-CF neurons and bouton synapses on low-CF neurons (Fukui and Ohmori, 2004, Köppl, 1994). Although the auditory nerve was lost in the culture, multiple VGlut2-positive glutamatergic terminals apposed on both high- and low-CF neurons (Figures S1B and S1E). The number and size of these terminals were similar between the neurons. Consistently, both neurons showed spontaneous excitatory postsynaptic currents (EPSCs) of similar amplitude and rate in the culture medium (Figures S1C, S1F, and S1H–S1J). However, the amplitude of EPSCs was mostly below 100 pA, the rate was low (6–7 Hz), and their contributions to spike generation would be small. Indeed, when spontaneous spikes were recorded under cell-attached clamp, the rate (<1 Hz) was far lower than that of spontaneous EPSPs (1–2 Hz) (Figures S1D, S1G, S1K, and S1L) and almost negligible compared with that in vivo (100–500 Hz, Fukui et al., 2006, Warchol and Dallos, 1990). Thus, the culture was preferable in evaluating the mechanism of tonotopic differentiation of Kv1.1 expression in NM under the minimal influence of synaptic and spike activities.
Membrane Properties Were Similar in Cultured High- and Low-CF Neurons
Membrane parameters of cultured NM neurons were measured at 14DIV, corresponding to P3, by injecting a rectangular current to the soma under current clamp (Table 1). The input resistance was larger and the resting potential was more positive than those in posthatch neurons in acute slices (see Akter et al., 2018), suggesting a decrease of Kv1 current in the culture. More importantly, the parameters were not different between high- and low-CF neurons. This contrasted with the clear tonotopic differences in the parameters in acute slices, such as lower input resistance and more negative resting potential in higher-CF neurons (Akter et al., 2018, Fukui and Ohmori, 2004), indicating that the tonotopic differentiation of Kv1 current would be small or absent in the culture.
Table 1.
Parameters of Membrane Property and Action Potential
| Control |
High K+ (1.5K) |
|||
|---|---|---|---|---|
| High CF (n = 18) | Low CF (n = 17) | High CF (n = 7) | Low CF (n = 10) | |
| Membrane property | ||||
| Rm (MΩ) | 62.2 ± 4.5 | 61.8 ± 4.7 | 19.0 ± 1.6a,b | 35.0 ± 2.9a |
| Vm (mV) | −59.2 ± 1.3 | −58.5 ± 1.0 | −65.9 ± 2.2b,c | −56.0 ± 1.0 |
| Cm (pF) | 40.3 ± 1.8 | 45.6 ± 2.2 | 43.9 ± 3.9 | 48.6 ± 3.9 |
| τm (ms) | 2.4 ± 0.1 | 2.8 ± 0.2 | 0.8 ± 0.1a,b | 1.7 ± 0.2a |
| No. of spike (1-nA input) | 2.0 ± 0.2d | 3.2 ± 0.5 | 1.0 ± 0.1a,b | 1.6 ± 0.4 |
| Action potential | ||||
| Th. current (nA) | 0.3 ± 0.1 | 0.2 ± 0.1 | 1.5 ± 0.2a,b | 0.3 ± 0.1 |
| Th. potential (mV) | −42.3 ± 1.4 | −45.3 ± 0.9 | −44.7 ± 3.2 | −41.7 ± 1.3c |
| Amplitude (mV) | 44.3 ± 1.4 | 47.2 ± 2.3 | 22.8 ± 1.9a,b | 49.2 ± 1.5 |
| Half-width (ms) | 0.5 ± 0.1d | 0.4 ± 0.1 | 0.3 ± 0.1a,b | 0.5 ± 0.1 |
| Max. dV/dt (V/s) | 224.2 ± 12.9 | 255.5 ± 16.8 | 148.5 ± 9.1a,b | 242.9 ± 13.7 |
| Mini. dV/dt (V/s) | −105.5 ± 7.6d | −127.8 ± 7.6 | −127.9 ± 6.8 | −129.2 ± 7.9 |
| Latency (ms) | 2.3 ± 0.1 | 2.3 ± 0.1 | 1.1 ± 0.1a,b | 2.1 ± 0.1 |
Input resistance (Rm), resting potential (Vm), membrane capacitance (Cm), and membrane time constant (τm).
p < 0.01 (between control and high-K+ condition).
p < 0.01 (between high- and low-CF neurons).
p < 0.05 (between control and high-K+ condition).
p < 0.05 (between high- and low-CF neurons).
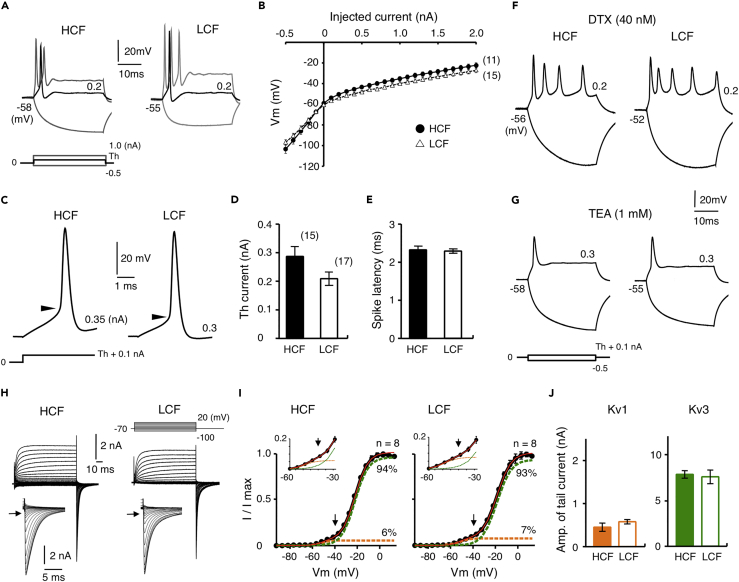
We then examined voltage responses to currents between −0.5 and 2 nA in the culture (Figure 2). Both high- and low-CF neurons showed outward-rectifying responses and generated one or a few spikes at the onset of depolarization (Figures 2A and 2B), as in acute slices (Akter et al., 2018, Fukui and Ohmori, 2004). Bath application of dendrotoxin (DTX, 40 nM, blocker of Kv1) increased the number of spikes; the number was 3.3 ± 0.4 (n = 10) (p = 3.9×10−6; compared with control, 0.9 ± 0.1, n = 15) and 3.9 ± 0.4 (n = 8) (p = 3.7×10−6; compared with control, 1.2 ± 0.2, n = 17) in high- and low-CF neurons, respectively (p = 0.29) (Figure 2F). On the other hand, tetraethylammonium (TEA, 1 mM, blocker of Kv3) increased the half-width of spikes; the increase was 158% (n = 6) (p = 8.2×10−6) and 211% (n = 4) (p = 2.9×10−10) in high- and low-CF neurons, respectively (p = 0.08) (Figure 2G), confirming the presence of Kv1 and Kv3 currents in the neurons. The level of outward rectification was similar between the high- and low-CF neurons (Figure 2B). Moreover, many spike parameters, including threshold current and spike latency, did not differ between the neurons (Table 1; Figures 2C–2E), being compatible with the idea that the tonotopic differentiation of Kv1 current was almost absent in the culture. Nevertheless, a 1-nA current generated more spikes and their falling phase was accelerated in low-CF neurons (Table 1), and therefore, small differences of Kv currents could be present between the neurons.
Figure 2.
Kv1 Current Was Not Differentiated Tonotopically in Culture
(A) Voltage responses to current injection (30 ms) between −0.5 and 2 nA with a 0.1-nA step at 14DIV. High- (left) and low-CF (right) neurons. Traces at −0.5 and 1 nA (gray) and that at threshold current (black) are shown. Membrane potential is indicated at left.
(B) Voltage-current relationship measured at pulse end.
(C) Action potentials at 0.1 nA above threshold current. Arrowheads indicate threshold potential.
(D and E) Threshold current (D) and latency (E) of spikes. Numbers in parentheses are the number of cells.
(F and G) Voltage responses in the presence of 40 nM DTX (F) or 1 mM TEA (G). Traces at −0.5 nA and 0.1 nA above threshold current are shown.
(H) Kv current recorded with voltage pulses (100 ms) between −100 and +20 mV at −70 mV. Tail current was expanded (inset). High- (left) and low-CF (right) neurons.
(I) Conductance-voltage curve of tail current. Magnified curve (inset). The curve was fitted to a double Boltzmann equation (red), and Kv1 and Kv3 components were shown in orange and green, respectively. Arrows indicate tail current at −40 mV and roughly represents Kv1 current.
(J) Kv1 (left, orange) and Kv3 (right, green) currents calculated at +20 mV.
See also Figure S2.
Kv Current Was Similar in Cultured High- and Low-CF Neurons
We compared Kv current between high- and low-CF neurons at 14DIV under voltage clamp, using a Cs+-based internal solution with voltage pulses (100 ms) between −100 and +20 mV at a holding potential of −70 mV (Figure 2H). An outward current appeared during the test pulse, and an inward tail current (inset) followed after cessation of the pulse in both neurons. The current would be mediated via Kv1 and Kv3 currents (see Kuba et al., 2015, Rathouz and Trussell, 1998). Voltage-dependence of activation of Kv current was evaluated via plotting conductance-voltage curve of the tail current and fitting it to a double Boltzmann equation (Figure 2I; Table 2). Kv1 current was identified as the low-voltage-activated component with a V1/2 of about −50 mV and Kv3 current as the high-voltage-activated component with a V1/2 of about −20 mV in both neurons. Remarkably, Kv1 current was small and similar in both neurons (0.4–0.6 nA, 6%–7% of total current) (Figures 2I and 2J), which was 3–10 times smaller than that in posthatch neurons (Akter et al., 2018). The results indicated that the expression of Kv1.1 was down-regulated in the culture and not differentiated tonotopically, being consistent with the findings in current clamp experiments (see Figures 2A–2E). On the other hand, Kv3 current was two times larger than that in posthatch neurons (7–8 nA, 93%–95% of total current) (Figures 2I and 2J; see Akter et al., 2018).
Table 2.
Parameters of Kv Current
| Control |
High K+ (1.5K) |
|||
|---|---|---|---|---|
| High CF (n = 8) | Low CF (n = 8) | High CF (n = 10) | Low CF (n = 8) | |
| Kv1 current (nA) | 0.5 ± 0.1 | 0.6 ± 0.1 | 2.1 ± 0.2a,b | 0.9 ± 0.1a |
| Kv3 current (nA) | 7.7 ± 0.4 | 7.6 ± 0.7 | 9.7 ± 0.6c | 10.1 ± 1.0c |
| Kv1 V1/2 (mV) | −51.4 ± 0.8 | −51.8 ± 0.7 | −53.4 ± 0.6c | −53.1 ± 1.0 |
| Kv3 V1/2 (mV) | −21.6 ± 0.5 | −19.4 ± 1.1 | −20.0 ± 0.7 | −18.5 ± 1.3 |
| Kv1 slope (mV) | 3.5 ± 0.3 | 4.0 ± 0.2 | 4.7 ± 0.2a | 4.7 ± 0.8 |
| Kv3 slope (mV) | 5.8 ± 0.2 | 5.9 ± 0.1 | 5.7 ± 0.1 | 6.3 ± 0.2 |
p < 0.01 (between control and high-K+ condition).
p < 0.01 (between high- and low-CF neurons).
p < 0.05 (between control and high-K+ condition).
Kv current increased during the course of culture from 5.0 ± 0.3 nA (n = 15) at 4DIV to 8.1 ± 0.4 nA (n = 8) at 14DIV, whereas Kv1 current did not change during the period; it was 0.3 ± 0.1 nA (7% of total current, n = 15) and 0.5 ± 0.1 nA (6% of total current, n = 8) for 4DIV and 14DIV, respectively (p = 0.4). This tendency was similar in neurons cultured with tetrodotoxin (TTX) (0.1 μM) (three cells), suggesting that spontaneous activities had little effects on Kv1 current in the culture. The results also indicated that Kv3 current could increase without postsynaptic depolarization, which agreed with the observations in otocysts-removed animals (Akter et al., 2018).
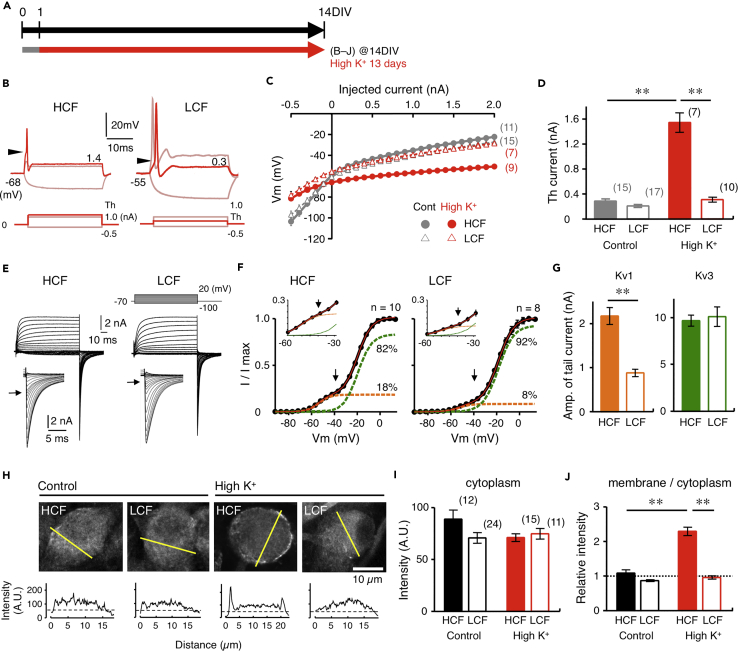
Chronic Depolarization Increased Kv1 Current More Efficiently in High-CF Neurons
Auditory input is important for the expression of Kv1.1 in NM neurons (Akter et al., 2018). Thus, we mimicked the effect of auditory input in the culture, through chronic depolarization via elevating [K+]o in the culture medium by 1.5 times (7.95 mM, 1.5K medium) for 13 days between 1DIV and 14DIV (Figure 3A). After the treatment with 1.5K medium, the resting potential became more negative (5 mV) in normal artificial cerebrospinal fluid (ACSF) and the input resistance decreased by three times in high-CF neurons (Table 1). In addition, the treatment augmented the outward rectification, shortened the spike latency by half, and increased the threshold current by five times in the neurons (Figures 3B–3D; Table 1), suggesting that the chronic depolarization increased Kv1 current in the high-CF neurons. Accordingly, the number of spikes decreased and spikes were no longer generated at 1 nA (Figure 3B). In low-CF neurons, on the other hand, input resistance decreased, but spike latency and threshold current did not change after the treatment, and most neurons still generated more than one spike at 1 nA.
Figure 3.
Chronic Depolarization Restored Tonotopic Differentiation of Kv1 Current
(A) [K+]o in culture medium was elevated by 1.5 times (7.95 mM) for 1–14DIV by adding KCl to culture medium.
(B) Voltage responses to current injection after chronic depolarization. High- (left) and low-CF (right) neurons. Traces at −0.5 and 1 nA (thin) and that at threshold current (thick) are shown. Arrowheads indicate threshold potential.
(C) Voltage-current relationship measured at pulse end. Controls were from Figure 2B (gray).
(D) Threshold current of spikes. Controls were from Figure 2D (gray). Numbers in parentheses are the number of cells.
(E) Kv current after chronic depolarization. Tail current was expanded (inset). High- (left) and low-CF (right) neurons.
(F) Conductance-voltage curve of tail current. Magnified curve (inset). The curve was fitted to a double Boltzmann equation (red), and Kv1 and Kv3 components were shown in orange and green, respectively. Arrows indicate tail current at −40 mV, and roughly represents Kv1 current.
(G) Kv1 (left, orange) and Kv3 (right, green) currents calculated at +20 mV. Note that Kv1 current became larger by 4.5 times in high-CF neurons and 1.5 times in low-CF neurons after chronic depolarization.
(H) Immunostaining of Kv1.1 at 14DIV in control and after chronic depolarization (upper). High- (left) and low-CF (right) neurons. Intensity profile was plotted along a line (yellow) across the cells (lower).
(I and J) Signal intensity at cytoplasmic region (I) and relative intensity between membranous and cytoplasmic regions (J). Note that membranous signal was augmented in high-CF neurons after the depolarization.
**p < 0.01.
The inward tail current increased in both neurons after the treatment with 1.5K medium (Figure 3E). However, the increase of Kv1 current was far larger in high-CF neurons than in low-CF neurons, creating a tonotopic difference of Kv1 current in the culture; the increase was about 5 times (2.1 ± 0.2 nA, 18% of total current) and 1.3 times (0.9 ± 0.1 nA, 8% of total current) in high- and low-CF neurons, respectively (Figures 3F and 3G; Table 2). In addition, strong immunosignals of Kv1.1 delineated the cell soma after the treatment specifically in the high-CF neurons with little changes in cytoplasmic signals (Figures 3H–3J). Importantly, the differential increase of Kv1 current occurred even when slices from different tonotopic regions were cultured on the same Millicell membrane (data not shown), suggesting that contributions of diffusible factors to the differentiation could be small. These results indicated that efficiency to drive Kv1.1 expression via depolarization would differ tonotopically in NM, being higher in high-CF neurons, which was consistent with the observations in vivo (Akter et al., 2018). Kv3 current increased slightly after the treatment, but the increase was about 1.3 times and did not differ between high- and low-CF neurons (Figure 3G; Table 2).
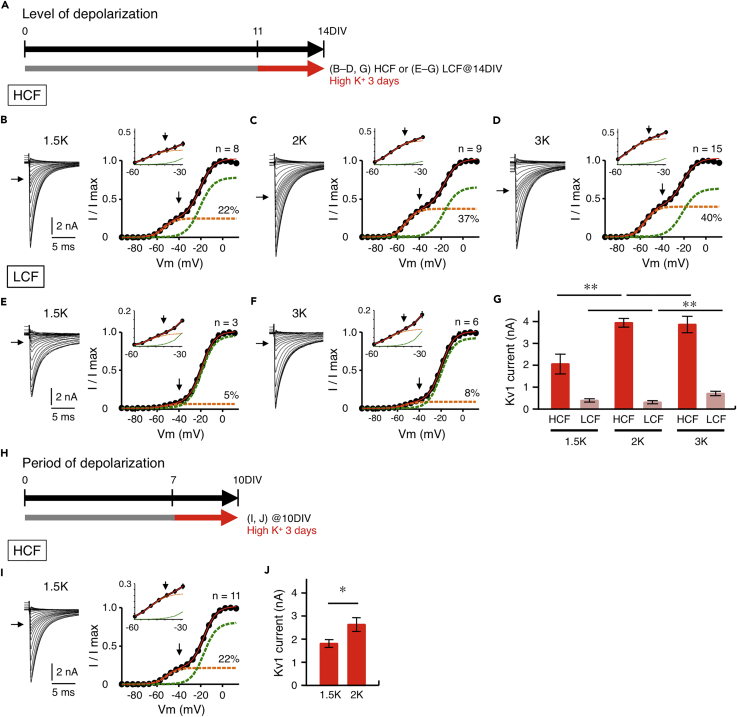
Increase of Kv1 Current Was Dependent on Level of Depolarization
We then examined the effects of the level of depolarization on the increase of Kv1 current (Figure 4A). Membrane was depolarized for 3 days between 11DIV and 14DIV, corresponding to P0–3, at which activity-dependent increase of Kv1 current occurs in vivo, via elevating [K+]o in the culture medium by 1.5 times (7.95 mM, 1.5K medium), 2 times (10.6 mM, 2K medium), or 3 times (15.9 mM, 3K medium). The level of depolarization increased upon high-K+ treatment in a manner dependent on [K+]o irrespective of tonotopic regions, and the membrane remained depolarized at 14DIV, although the depolarization decreased slightly during the high-K+ treatment particularly in high-CF neurons, likely reflecting the increase of Kv1.1 expression in the neurons (Table 3). In the high-CF neurons, the treatment with 1.5K medium increased Kv1 current by five times (2.1 ± 0.5 nA, 22% of total current) (Figures 4B and 4G), and the extent was similar to that after 13 days of treatment (1–14DIV) (see Figures 3E–3G), indicating that 3 days of depolarization was sufficient for the increase of Kv1 current, as in vivo (Akter et al., 2018). Kv1 current increased further and reached a plateau after the treatment with 2K medium (3.9 ± 0.2 nA, 37% of total current) or 3K medium (3.9 ± 0.4 nA, 40% of total current) (Figures 4C, 4D, and 4G), and the size was comparable with that in posthatch neurons (4.2 nA, 53% of total current; Akter et al., 2018). In low-CF neurons, on the other hand, Kv1 current increased slightly with 3K medium (0.7 ± 0.1 nA, 9% of total current) but with neither 1.5K (0.4 ± 0.1 nA, 5% of total current) nor 2K (0.3 ± 0.1 nA, 5% of total current) medium (Figures 4E–4G). Thus, the level of Kv1.1 expression depended on the level of depolarization in NM neurons, but the dependence was much steeper in the high-CF neurons than in the low-CF neurons.
Figure 4.
Increase of Kv1 Current Depended on Level of Depolarization but the Dependence Was Steeper in High-CF Neurons
(A) [K+]o in culture medium was elevated by 1.5–3 times for 11–14DIV in high- and low-CF neurons.
(B–D) Effects of level of depolarization on Kv current in high-CF neurons. Tail current (left) and conductance-voltage curve (right) at 1.5 times (7.95 mM, B), 2 times (10.6 mM, C), and 3 times (15.9 mM, D) of [K+]o. The curve was fitted to a double Boltzmann equation (red), and Kv1 and Kv3 components were shown in orange and green, respectively. Arrows indicate tail current at −40 mV and roughly represents Kv1 current. Magnified curve (inset).
(E and F) Effects of level of depolarization on Kv current in low-CF neurons. Tail current (left) and conductance-voltage curve (right) at 1.5 times (E) and 3 times (F) of [K+]o. [K+]o was not increased further, because the neurons could not survive in the medium.
(G) Kv1 current calculated at +20 mV at high- and low-CF regions. Note that Kv1 current increased more steeply with an elevation of [K+]o in high-CF neurons.
(H–J) Effects of period of depolarization on Kv current in high-CF neurons. [K+]o in culture medium was elevated for 7–10DIV, and recording was made at 10DIV (H). Tail current (left) and conductance-voltage curve (right) at 1.5 times of [K+]o (I). Kv1 current calculated at +20 mV (J).
*p < 0.05, **p < 0.01. See also Figures S2 and S3.
Table 3.
Effects of High-K+ Treatment on Membrane Potential
| Control | 1.5K | 2K | 3K | |
|---|---|---|---|---|
| High CF | ||||
| Onset | −53.2 ± 0.4 (n = 6) | −46.1 ± 0.7 (n = 7) | −40.4 ± 1.2 (n = 6) | −36.1 ± 1.3 (n = 6) |
| End | −55.6 ± 0.7 (n = 6)a | −52.7 ± 0.3 (n = 6)b | −45.3 ± 1.5 (n = 6)a | −39.0 ± 0.9 (n = 7) |
| Low CF | ||||
| Onset | −52.4 ± 0.7 (n = 9) | −45.6 ± 0.6 (n = 10) | −40.8 ± 0.6 (n = 7) | −35.5 ± 0.8 (n = 5) |
| End | −56.0 ± 1.7 (n = 5) | −47.1 ± 1.1 (n = 9)c | −43.1 ± 1.0 (n = 9) | −37.4 ± 0.4 (n = 8)a |
Membrane potential was measured at onset (11DIV) and end (14DIV) of 3-day treatment in the corresponding culture medium.
[K+] was 5.3 mM, 7.95, 10.6, and 15.9 mM for control, 1.5K, 2K, and 3K medium, respectively.
High- K+ treatment depolarized the membrane (p < 0.01, compared with control), and the level of depolarization depended on [K+].
p < 0.05 (between onset and end of high- K+ treatment).
p < 0.01 (between onset and end of high- K+ treatment).
p < 0.01 (between high- and low-CF neurons).
Importantly, Kv1 current increased in high-CF neurons even when [K+]o was elevated between 7DIV and 10DIV, corresponding to E18–20 (Figures 4H–4J); the amplitude was 1.8 ± 0.2 and 2.6 ± 0.3 nA (22% and 35% of total current) for 1.5K and 2K media, respectively, suggesting that the mechanism of depolarization-dependent Kv1.1 expression might be already equipped in the neurons at embryonic periods.
We also evaluated the effects of the high-K+ media on spontaneous activities in the culture (Figure S2A). When the cultures were bathed in the 1.5K medium at 11DIV, the rate of spontaneous EPSCs increased slightly but it was still low in both high- and low-CF neurons (about 10 Hz) (Figures S2B, S2D, and S2G). In addition, their amplitude decreased substantially in the medium (30–60 pA) (Figures S2B, S2D, and S2F). This occurred because large EPSCs were suppressed in the medium (see Figure S1H), presumably due to progressions of both Na+ current inactivation and K+ current activation in the presynaptic neurons. Consistently, spontaneous EPSPs and spikes were almost absent in the 1.5K medium in both neurons (<0.1 Hz) (Figures S2C, S2E, S2H, and S2I). Moreover, chronic treatment with 1.5K medium increased Kv1 current in the presence of TTX (0.1 μM), 6,7-dinitroquinoxaline-2,3-dione (DNQX) (20 μM), and D-AP5 (100 μM) in high-CF neurons (1.5 ± 0.2 nA, 19% of total current) (Figures S2J–S2L). These results indicated that the increase of Kv1 current would be primarily driven by chronic depolarization of NM neurons during the high-K+ treatment in the culture.
Intracellular Cl− level is high and ECl is depolarized in NM neurons (Hyson et al., 1995, Monsivais et al., 2000, Lu and Trussell, 2001). In addition, Cl− permeability may increase when [K+]o is elevated, affecting the level of depolarization via alterations of [Cl−]i (Babot et al., 2005). However, this possibility would be low, because 1.5K medium did not change intracellular Cl− level, when measured with a Cl− dye (MQAE, see Transparent Methods), and the effects were similar with or without a cocktail of Cl− channel blockers (Figure S3).
Elevation of [Ca2+]i Increased Kv1 Current More Efficiently in High-CF Neurons
Depolarization is expected to elevate intracellular Ca2+ level via activation of Cav channels. Various types of Cav channels are reported in NM neurons, including L-type (Cav1), P/Q-type (Cav2.1), N-type (Cav2.2), R-type (Cav2.3), and T-type (Cav3) channels (Koyano et al., 1996, Lu and Rubel, 2005). We tested the contributions of these channels to the increase of Kv1 current by depolarizing the tissues with elevated [K+]o (1.5K medium) and then by concurrently applying Cav channel blockers between 11DIV and 14DIV (Figure 5A). Nifedipine (10 μM, blocker of Cav1) or (+)-Bay-K8644 (5 μM, blocker of Cav1) occluded the increase of Kv1 current during the depolarization (0.7 ± 0.1 nA, 8% of total current for nifedipine; 0.7 ± 0.1 nA, 11% for (+)-Bay-K8644), whereas ω-conotoxin GVIA (2 μM, blocker of Cav2.2) did not affect the increase of the current (2.1 ± 0.4 nA, 19% of total current) (Figures 5B, 5C, and S4). Further application of ω-agatoxin IVA (0.2 μM, blocker of Cav2.1), NiCl2 (100 μM, blocker of Cav2.3 and Cav3), and TTA-P2 (2 μM, blocker of Cav3) did not show additional effects (0.9 ± 0.1 nA, 8% of total current). In addition, nifedipine did not affect spontaneous EPSCs in the 1.5K medium; the rate was 11.5 ± 3.2 Hz (n = 7, p = 0.84 compared with 1.5K medium). These results indicated that elevation of Ca2+ level was necessary for the increase of Kv1 current and it was mediated via postsynaptic Cav1 channels in the culture.
Figure 5.
Chronic Depolarization Elevated Basal Ca2+ Level Similarly Irrespective of Tonotopic Regions
(A–C) Effects of Cav channel blockers on increase of Kv current during depolarization in high-CF neurons. [K+]o in culture medium was elevated by 1.5 times, while Cav channel blockers were added for 11–14DIV (A). Tail current without blockers (left), after the treatment of nifedipine (10 μM, left middle), ω-conotoxin GVIA (2 μM, right middle), and cocktail of blockers (right) (B). Arrows indicate tail current at −40 mV, and roughly represents Kv1 current. Kv1 current calculated at +20 mV in high-CF neurons (C). Data in control (black) and 1.5K treatment (red) were from Figures 2J and 3G. Numbers in parentheses are the number of cells. **p < 0.01 compared with control.
(D) Neurons were cultured in normal medium, and two-photon imaging was made at 11DIV.
(E and F) Ca2+ signals in high- (E) and low-CF (F) neurons. Cells were loaded with a high-affinity indicator (OGB-1, G) and Alexa 594 (R) through a patch pipette, and line scans were made at the soma (top, red line). ΔG/R increased in both neurons (bottom), when a depolarizing voltage pulse (10 s) was applied under voltage clamp (middle).
(G) ΔG/R at corresponding voltage.
(H) [Ca2+]i measured with a low-affinity indicator (Fluo-5F) under voltage clamp.
(I) Dependence of [Ca2+]i on membrane depolarization. Values were similar between high- and low-CF neurons (p > 0.1), and data from both neurons were combined. *p < 0.05, **p < 0.01 compared with −60 mV. Note that [Ca2+]i was still within nanomolar range (about 200 nM) even at membrane potential comparable with that in 2K medium (−40 mV). The increase of [Ca2+]i was occluded by nifedipine (10 μM, light blue). Cocktail of blockers in (B) showed similar suppression of [Ca2+]i; [Ca2+]i in the cocktail was 117.3 ± 22.4 nM at −60 mV, 133.9 ± 34.5 nM at −40 mV, and 213.0 ± 70.8 nM at −20 mV (n = 5) (p > 0.1 compared with that of nifedipine at each voltage).
See also Figures S4–S6.
We then examined Ca2+ level in high- and low-CF neurons with a two-photon microscope at 11DIV (Figure 5D). Cells were loaded with a high-affinity Ca2+ indicator (OGB-1, G) and a volume marker (Alexa 594, R) through a patch pipette, and line scans were made across the cells (red lines), while a depolarizing current was applied under voltage clamp (Figures 5E–5G). Upon depolarization, the Ca2+ signal (G/R) increased in both neurons. More importantly, the increase (ΔG/R) depended on the level of depolarization. Indeed, when [Ca2+]i was measured at various holding potentials with a low-affinity Ca2+ indicator (Fluo-5F), a similar depolarization dependence of [Ca2+]i was observed and it was blocked with nifedipine or (+)-Bay-K8644 (Figures 5H, 5I, and S4). Remarkably, [Ca2+]i was rather low (below 200 nM) even at −40 mV, comparable with the membrane potential in the 2K medium. The results indicated that a slight change in the basal Ca2+ level would critically affect the expression of Kv1.1 and fine adjustment of the Ca2+ level should be the key in setting the level of Kv1.1 expression. To our surprise, however, the elevation of [Ca2+]i did not differ between the high- and low-CF neurons (Figure 5G). In addition, Cav1 mRNAs were expressed similarly at both regions (Figure S5). These findings suggested that downstream mechanisms of Ca2+ would be differentiated between the high- and low-CF neurons, contributing to the tonotopic-region-specific increase of Kv1 current during the depolarization.
[Ca2+]i in 1.5K medium was also measured at 14DIV after incubating slices for 3 days in the medium, and it was still dependent on depolarization and comparable with that measured at 11DIV in normal ACSF (Figure S6, see Figure 5I). Although it was not significant, the dependence tended to become weaker after the treatment, and this might reflect rundown or inactivation of Cav channels during the depolarization.
Discussion
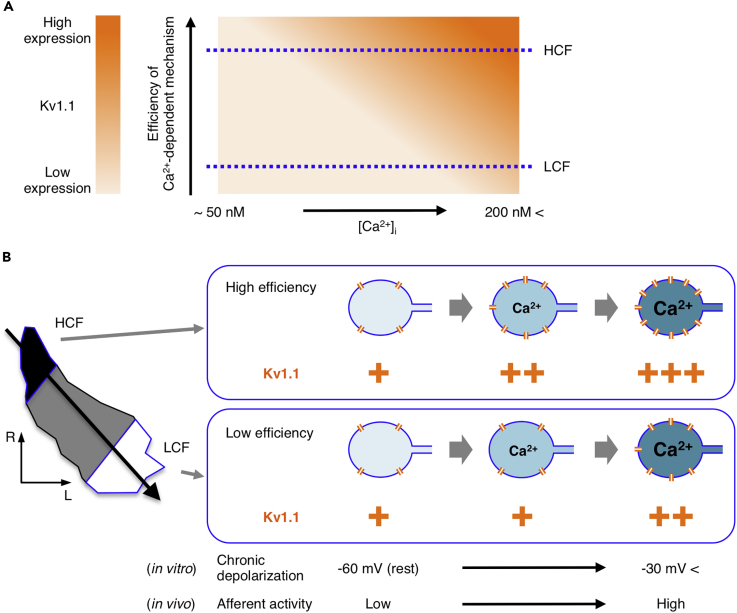
Cultured NM neurons showed a tonotopic difference of Kv1 current only when the neurons were chronically depolarized with high-K+ media. This occurred because the increase of Kv1 current was dependent on the level of depolarization, and the dependence was steeper in higher-CF neurons. Blockade of Cav1 channels suppressed the increase of Kv1 current and the differentiation. In addition, depolarization elevated [Ca2+]i similarly irrespective of tonotopic regions. These findings suggested that the Ca2+-dependent expression of Kv1.1 was more efficient in higher-CF neurons, proposing that neuronal tonotopic identity as well as pattern/level of afferent activity would be important for the tonotopic differentiation of Kv1.1 in NM (see Figure 6).
Figure 6.
Efficiency of Translating Ca2+ Level into Kv1.1 Expression Would Be Differentiated along the Tonotopic Axis in NM
(A) Level of Kv1.1 expression is regulated via an interaction between “efficiency” of Ca2+-dependent mechanism and [Ca2+]i. Note that Kv1.1 level is the same irrespective of efficiency at low [Ca2+]i, whereas the level increases more steeply at higher efficiency with an increase of [Ca2+]i. The efficiency would be higher in higher-CF neurons (dotted horizontal lines).
(B) Schematic drawing of relationship between [Ca2+]i and Kv1.1 expression in high- and low-CF neurons. Kv1.1 increased with an increase of [Ca2+]i in both neurons, but the dependence was steeper in high-CF neurons, causing higher expression of Kv1.1 in the neurons (orange). This implied that the coupling between Ca2+ and Kv1.1 expression would be determined in a tonotopic-region-specific manner, suggesting that genetic program as well as afferent activity would be crucial for the tonotopic-differentiation of Kv1.1 expression in NM. Note that the increase of [Ca2+]i was mediated via activation of Cav1 channels during chronic depolarization in the culture, whereas the increase would be driven by afferent activity via activations of AMPA receptors and/or Cav channels in vivo.
Activity-Dependence of Kv1.1 Expression Would Be Intrinsically Determined in a Tonotopic-Region-Specific Manner
In NM in vivo, Kv1 current increased efficiently with auditory input in higher-CF neurons, whereas the current remained small in low-CF neurons even after elevation of the input (Akter et al., 2018), leading to the idea that efficiency of neurons to drive Kv1.1 expression via auditory input would become higher toward the high-CF region in NM. The present study provided direct evidence to this idea. In the culture, Kv1 current was down-regulated, comparable with that in immature animals (E15), and not different tonotopically even after maturation (14DIV). These would be attributed to the fact that the cultured NM neurons were deprived of both spontaneous and sound-driven inputs from the auditory nerve, which normally occur at high rates (100–500 Hz) and cause substantial depolarization in vivo (Fukui et al., 2006, Warchol and Dallos, 1990). Indeed, chronic depolarization increased Kv1 current particularly at high-CF regions and restored the tonotopic difference of the current in the culture. Thus, the activity-dependent mechanism of Kv1.1 expression would be programmed in a tonotopic-region-specific manner, which may underlie the tonotopic difference of the current in NM. These were consistent with the recent findings that couplings between neuronal activity and gene expression programs are determined differentially in individual neurons in the brain (Hrvatin et al., 2018, Kalish et al., 2018).
Importantly, the input-dependent increase of Kv1 current was small and either suppression or elevation of auditory input had only minor effects on the current during embryonic period in vivo (Akter et al., 2018). This contrasted with the observations that the depolarization increased Kv1 current substantially in the high-CF neurons irrespective of days in culture. One possible explanation for this discrepancy is that auditory input was limited in embryos owing to attenuation of sound through the eggshell and embryonic fluid (Jones et al., 2006), whereas the neurons were depolarized efficiently with the high-K+ treatment in the culture. The results might indicate that the high efficiency to express Kv1.1 via auditory input is already equipped in NM neurons during early periods of maturation. Nevertheless, whether the period specificity for the Kv1.1 expression exists or not would require further studies in vivo.
Downstream Mechanism of Ca2+ Would Be Differentiated Tonotopically
Patterns and/or levels of afferent activities differ tonotopically in the auditory system (Fukui et al., 2006, Johnson et al., 2011, Lippe, 1995), which is considered crucial for the differentiations of auditory circuits (Kandler et al., 2009). This suggests that [Ca2+]i in postsynaptic neurons plays a pivotal role in the differentiations (Friauf and Lohmann, 1999). This was the case in the differentiation of Kv1 current in NM. In support, homeostatic increase of Kv1 current was suppressed via chronic inhibition of Cav channels in cultured medial nucleus of trapezoid body (MNTB) neurons (Tong et al., 2010) or of NMDA receptors in cultured hippocampal neurons (Lee et al., 2015). In NM neurons, Cav1 channels would be activated via depolarization during synaptic and/or spike activities and work as a source of Ca2+ entry for the differentiation. In addition, AMPA receptors could be another source of Ca2+ entry in vivo, as synaptic activity is robust in vivo and the receptors are highly permeable to Ca2+ in NM (Trussell, 1999). On the other hand, contributions of NMDA receptors would be small, because expression of the receptors declines before hatch in NM (Lu and Trussell, 2007, Tang and Carr, 2007), whereas a major increase of Kv1 current occurs after hatch (Akter et al., 2018). In cultured NM neurons, a change in basal Ca2+ level was sufficient to cause the differentiation of Kv1 current, suggesting that overall activity is important for the differentiation. However, NM neurons receive different patterns of afferent input among tonotopic regions in vivo (Fukui and Ohmori, 2004, Fukui et al., 2006), and a possibility remains that a difference in Ca2+ transient further contributes to the differentiation. This difference in Ca2+ dynamics may explain why Kv1 current in low-CF neurons remained small in the culture even after the treatment with 3K medium (0.8 nA, Figure 4), compared with that in posthatch animals (1.5 nA, Akter et al., 2018).
The striking finding in the present study was that depolarization increased [Ca2+]i similarly irrespective of tonotopic regions, indicating that the downstream mechanism of Ca2+ would be differentiated tonotopically in NM. As [Ca2+]i necessary for Kv1.1 expression was rather low of around 100–200 nM (Figure 5; see also Awatramani et al., 2005), the mechanism may have rather high sensitivity to Ca2+ in high-CF neurons. This idea was consistent with the fact that a prolonged elevation of [Ca2+]i above 250 nM promoted cell death in NM (Zirpel et al., 1998). In low-CF neurons, on the other hand, the high-K+ treatment showed only a small increase of Kv1 current, and the mechanism of Kv1.1 expression may have lower sensitivity to Ca2+. Interestingly, the expression of Ca2+-binding proteins, such as calretinin and parvalbumin, differs along the tonotopic axis in NM (Wang et al., 2017). These tonotopic variations of Ca2+-related molecules could be attributable to the fact that rhombomeres from which NM neurons arise are different depending on tonotopic regions (Cramer et al., 2000).
Kv1.1 mRNA increases toward the high-CF region in posthatch NM (Fukui and Ohmori, 2004). In addition, membranous localization of Kv1.1 protein increases in the high-CF region in vivo (Akter et al., 2018) and in vitro (Figure 3). Thus, the tonotopic difference in the mechanism of Kv1.1 expression may arise via transcriptional regulation and/or posttranscriptional modification of Ca2+-dependent molecules. As membrane trafficking of Kv1.1 is negatively regulated by ER-retention domain at the external pore region of the channel (Vacher and Trimmer, 2012), the molecules may include those that modulate the domain; matrix metalloprotease 23 could be a candidate molecule, because it binds to the domain and contributes to ER retention of Kv1.1, while it is released from the ER membrane via a Ca2+-dependent cleavage by an endoprotease, furin (Galea et al., 2014, Rangaraju et al., 2010, Salvas et al., 2005). Alternatively, the molecules could be protein kinase C and/or protein kinase A, which were reported to increase synthesis and translocation of Kv1.1 protein (Levin et al., 1995, Winklhofer et al., 2003).
Functional Implication
Afferent activity contributes to tonotopic differentiation of ion-channel expression in both birds and mammals (Kuba et al., 2010, Leao et al., 2006, Akter et al., 2018, von Hehn et al., 2004). This is indeed the case in Kv1.1; it increases Kv1 current at the high-CF region in NM of chickens (Akter et al., 2018) and at the low-CF region in MNTB of mice (Leao et al., 2006). This discrepancy in the tonotopic regions could be related to the difference in audible frequency between the animals; it is much higher in mice (2–100 kHz, Heffner and Heffner, 2007) than in chickens (0.2–4 kHz, Saunders et al., 1973). Interestingly, the frequency range, at which the activity-dependent increase of Kv1 current occurs, is similar between the animals and comparable with the frequency, where temporal information is particularly important for auditory function (∼2 kHz, Rayleigh, 1907). This may emphasize the importance of Kv1.1 in temporal coding in the auditory system (Kopp-Scheinpflug et al., 2003).
In the present study, we showed that the efficiency of translating [Ca2+]i into Kv1.1 expression was fine-tuned in a tonotopic-region-specific manner, and it was higher at the high-CF region. The high efficiency would be beneficial for the high-CF neurons to ensure sufficient Kv1.1 expression, because the auditory threshold is high for high-frequency sound and the level of afferent input may not be high in the neurons (Saunders et al., 1973), whereas the low efficiency would be important for the low-CF neurons to prevent excess increase of Kv1.1 with auditory input. Thus, the topographic differentiation of activity-dependent mechanisms could be a secure and flexible way to ensure the optimum level of Kv1.1 expression at each tonotopic region and to shape precise and reliable temporal coding across frequencies. Interestingly, development of Kv3 current progressed independently of depolarization and tonotopic regions, indicating that expression of Kv channels is regulated in a subtype-specific manner. This is reasonable because Kv3.1 promotes spike generation and would be important for embryonic neurons to promote firing during the low level of afferent input. Thus, the activity-dependent process of ion-channel expression would be strategically designed in a tonotopic-region-specific manner for each channel type, which would ensure the precise temporal coding of sound across frequencies in the brainstem auditory circuit.
Limitation of Study
We showed in NM that the efficiency of activity-dependent Kv1.1 expression was determined in a tonotopic-region-specific manner, contributing to differentiating the channel expression within the nucleus. However, the data were obtained in slice culture, which preserves structures other than neurons, and further experiments in dissociate culture would be necessary to strengthen the conclusion that tonotopic neuronal identity is indeed different within the nucleus.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by grants-in-aid from MEXT (15H04257 to H.K., 16K07345 to R.A., 16K08493 to R.Y.) and Innovative Areas “Dynamic regulation of Brain Function by Scrap & Build” (17H05742) to H.K.
Author Contributions
R.A. and H.K. designed research. R.A. and R.Y. performed research and analyzed data. R.A., R.Y., and H.K. wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Published: March 29, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.02.022.
Supplemental Information
References
- Akter N., Adachi R., Kato A., Fukaya R., Kuba H. Auditory input shapes tonotopic differentiation of Kv1.1 expression in avian cochlear nucleus during late development. J. Neurosci. 2018;38:2967–2980. doi: 10.1523/JNEUROSCI.2472-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani G.B., Price G.D., Trussell L.O. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Babot Z., Cristòfol R., Suñol C. Excitotoxic death induced by released glutamate in depolarized primary cultures of mouse cerebellar granule cells is dependent on GABAA receptors and niflumic acid-sensitive chloride channels. Eur. J. Neurosci. 2005;21:103–112. doi: 10.1111/j.1460-9568.2004.03848.x. [DOI] [PubMed] [Google Scholar]
- Cramer K.S., Fraser S.E., Rubel E.W. Embryonic origins of auditory brain-stem nuclei in the chick hindbrain. Dev. Biol. 2000;224:138–151. doi: 10.1006/dbio.2000.9779. [DOI] [PubMed] [Google Scholar]
- Friauf E., Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res. 1999;297:187–195. doi: 10.1007/s004410051346. [DOI] [PubMed] [Google Scholar]
- Fukui I., Ohmori H. Tonotopic gradients of membrane and synaptic properties for neurons of the chicken nucleus magnocellularis. J. Neurosci. 2004;24:7514–7523. doi: 10.1523/JNEUROSCI.0566-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui I., Sato T., Ohmori H. Improvement of phase information at low sound frequency in nucleus magnocellularis of the chicken. J. Neurophysiol. 2006;96:633–641. doi: 10.1152/jn.00916.2005. [DOI] [PubMed] [Google Scholar]
- Galea C.A., Nguyen H.M., George Chandy K., Smith B.J., Norton R.S. Domain structure and function of matrix metalloprotease 23 (MMP23): role in potassium channel trafficking. Cell. Mol. Life Sci. 2014;71:1191–1210. doi: 10.1007/s00018-013-1431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner H.E., Heffner R.S. Hearing ranges of laboratory animals. J. Am. Assoc. Lab. Anim. Sci. 2007;46:20–22. [PubMed] [Google Scholar]
- Hrvatin S., Hochbaum D.R., Nagy M.A., Cicconet M., Robertson K., Cheadle L., Zilionis R., Ratner A., Borges-Monroy R., Klein A.M. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci. 2018;21:120–129. doi: 10.1038/s41593-017-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyson R.L., Reyes A.D., Rubel E.W. A depolarizing inhibitory response to GABA in brainstem auditory neurons of the chick. Brain Res. 1995;677:117–126. doi: 10.1016/0006-8993(95)00130-i. [DOI] [PubMed] [Google Scholar]
- Jhaveri S., Morest D.K. Sequential alterations of neuronal architecture in nucleus magnocellularis of the developing chicken: a Golgi study. Neuroscience. 1982;7:837–853. doi: 10.1016/0306-4522(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Johnson S.L., Eckrich T., Kuhn S., Zampini V., Franz C., Ranatunga K.M., Roberts T.P., Masetto S., Knipper M., Kros C.J., Marcotti W. Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat. Neurosci. 2011;14:711–717. doi: 10.1038/nn.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J., Forsythe I.D., Kopp-Scheinpflug C. Going native: voltage-gated potassium channels controlling neuronal excitability. J. Physiol. 2010;588:3187–3200. doi: 10.1113/jphysiol.2010.191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.A., Jones S.M., Paggett K.C. Emergence of hearing in the chicken embryo. J. Neurophysiol. 2006;96:128–141. doi: 10.1152/jn.00599.2005. [DOI] [PubMed] [Google Scholar]
- Kalish B.T., Cheadle L., Hrvatin S., Nagy M.A., Rivera S., Crow M., Gillis J., Kirchner R., Greenberg M.E. Single-cell transcriptomics of the developing lateral geniculate nucleus reveals insights into circuit assembly and refinement. Proc. Natl. Acad. Sci. U S A. 2018;115:E1051–E1060. doi: 10.1073/pnas.1717871115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K., Clause A., Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat. Neurosci. 2009;12:711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C. Auditory nerve terminals in the cochlear nucleus magnocellularis: differences between low and high frequencies. J. Comp. Neurol. 1994;339:438–446. doi: 10.1002/cne.903390310. [DOI] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C., Fuchs K., Lippe W.R., Tempel B.L., Rübsamen R. Decreased temporal precision of auditory signaling in Kcna1-null mice: an electrophysiological study in vivo. J. Neurosci. 2003;23:9199–9207. doi: 10.1523/JNEUROSCI.23-27-09199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano K., Funabiki K., Ohmori H. Voltage-gated ionic currents and their roles in timing coding in auditory neurons of the nucleus magnocellularis of the chick. Neurosci. Res. 1996;26:29–45. doi: 10.1016/0168-0102(96)01071-1. [DOI] [PubMed] [Google Scholar]
- Kuba H., Ohmori H. Roles of axonal sodium channels in precise auditory time coding at nucleus magnocellularis of the chick. J. Physiol. 2009;587:87–100. doi: 10.1113/jphysiol.2008.162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H., Oichi Y., Ohmori H. Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- Kuba H., Yamada R., Ishiguro G., Adachi R. Redistribution of Kv1 and Kv7 enhances neuronal excitability during structural axon initial segment plasticity. Nat. Commun. 2015;6:8815. doi: 10.1038/ncomms9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao R.N., Sun H., Svahn K., Berntson A., Youssoufian M., Paolini A.G., Fyffe R.E., Walmsley B. Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. J. Physiol. 2006;571:563–578. doi: 10.1113/jphysiol.2005.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.Y., Royston S.E., Vest M.O., Ley D.J., Lee S., Bolton E.C., Chung H.J. N-methyl-D-aspartate receptors mediate activity-dependent down-regulation of potassium channel genes during the expression of homeostatic intrinsic plasticity. Mol. Brain. 2015;8:4. doi: 10.1186/s13041-015-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin G., Keren T., Peretz T., Chikvashvili D., Thornhill W.B., Lotan I. Regulation of RCK1 currents with a cAMP analog via enhanced protein synthesis and direct channel phosphorylation. J. Biol. Chem. 1995;270:14611–14618. doi: 10.1074/jbc.270.24.14611. [DOI] [PubMed] [Google Scholar]
- Lippe W.R. Relationship between frequency of spontaneous bursting and tonotopic position in the developing avian auditory system. Brain Res. 1995;703:205–213. doi: 10.1016/0006-8993(95)01096-3. [DOI] [PubMed] [Google Scholar]
- Lu Y., Rubel E.W. Activation of metabotropic glutamate receptors inhibits high-voltage-gated calcium channel currents of chicken nucleus magnocellularis neurons. J. Neurophysiol. 2005;93:1418–1428. doi: 10.1152/jn.00659.2004. [DOI] [PubMed] [Google Scholar]
- Lu T., Trussell L.O. Mixed excitatory and inhibitory GABA-mediated transmission in chick cochlear nucleus. J. Physiol. 2001;535:125–131. doi: 10.1111/j.1469-7793.2001.t01-1-00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Trussell L.O. Development and elimination of endbulb synapses in the chick cochlear nucleus. J. Neurosci. 2007;27:808–817. doi: 10.1523/JNEUROSCI.4871-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais P., Yang L., Rubel E.W. GABAergic inhibition in nucleus magnocellularis: implications for phase locking in the avian auditory brainstem. J. Neurosci. 2000;20:2954–2963. doi: 10.1523/JNEUROSCI.20-08-02954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D. The role of timing in the brain stem auditory nuclei of vertebrates. Annu. Rev. Physiol. 1999;61:497–519. doi: 10.1146/annurev.physiol.61.1.497. [DOI] [PubMed] [Google Scholar]
- Oline S.N., Ashida G., Burger R.M. Tonotopic optimization for temporal processing in the cochlear nucleus. J. Neurosci. 2016;36:8500–8515. doi: 10.1523/JNEUROSCI.4449-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameshwaran S., Carr C.E., Perney T.M. Expression of the Kv3.1 potassium channel in the avian auditory brainstem. J. Neurosci. 2001;21:485–494. doi: 10.1523/JNEUROSCI.21-02-00485.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks T.N., Rubel E.W. Organization and development of the brain stem auditory nuclei of the chicken: primary afferent projections. J. Comp. Neurol. 1978;180:439–448. doi: 10.1002/cne.901800303. [DOI] [PubMed] [Google Scholar]
- Rangaraju S., Khoo K.K., Feng Z.-P., Crossley G., Nugent D., Khaytin I., Chi V., Pham C., Calabresi P., Pennington M.W. Potassium channel modulation by a toxin domain in matrix metalloprotease 23. J. Biol. Chem. 2010;285:9124–9136. doi: 10.1074/jbc.M109.071266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathouz M., Trussell L. Characterization of outward currents in neurons of the avian nucleus magnocellularis. J. Neurophysiol. 1998;80:2824–2835. doi: 10.1152/jn.1998.80.6.2824. [DOI] [PubMed] [Google Scholar]
- Rayleigh L. On our perception of sound direction. Philos. Mag. 1907;13:214–232. [Google Scholar]
- Reyes A.D., Rubel E.W., Spain W.J. Membrane properties underlying the firing of neurons in the avian cochlear nucleus. J. Neurosci. 1994;14:5352–5364. doi: 10.1523/JNEUROSCI.14-09-05352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvas A., Benjannet S., Reudelhuber T.L., Chrétien M., Seidah N.G. Evidence for proprotein convertase activity in the endoplasmic reticulum/early Golgi. FEBS Lett. 2005;579:5621–5625. doi: 10.1016/j.febslet.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Sanchez J.T., Seidl A.H., Rubel E.W., Barria A. Preparation and culture of chicken auditory brainstem slices. J. Vis. Exp. 2011;49 doi: 10.3791/2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J.C., Coles R.B., Gates G.R. The development of auditory evoked responses in the cochlea and cochlear nuclei of the chick. Brain Res. 1973;63:59–74. doi: 10.1016/0006-8993(73)90076-0. [DOI] [PubMed] [Google Scholar]
- Sullivan W.E., Konishi M. Segregation of stimulus phase and intensity coding in the cochlear nucleus of the barn owl. J. Neurosci. 1984;4:1787–1799. doi: 10.1523/JNEUROSCI.04-07-01787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.Z., Carr C.E. Development of N-methyl-D-aspartate receptor subunits in avian auditory brainstem. J. Comp. Neurol. 2007;502:400–413. doi: 10.1002/cne.21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H., Steinert J.R., Robinson S.W., Chernova T., Read D.J., Oliver D.L., Forsythe I.D. Regulation of Kv channel expression and neuronal excitability in rat medial nucleus of the trapezoid body maintained in organotypic culture. J. Physiol. 2010;588:1451–1468. doi: 10.1113/jphysiol.2009.186676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L.O. Synaptic mechanisms for coding timing in auditory neurons. Annu. Rev. Physiol. 1999;61:477–496. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- Vacher H., Trimmer J.S. Trafficking mechanisms underlying neuronal voltage-gated ion channel localization at the axon initial segment. Epilepsia. 2012;53:21–31. doi: 10.1111/epi.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hehn C.A., Bhattacharjee A., Kaczmarek L.K. Loss of Kv3.1 tonotopicity and alterations in cAMP response element-binding protein signaling in central auditory neurons of hearing impaired mice. J. Neurosci. 2004;24:1936–1940. doi: 10.1523/JNEUROSCI.4554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Hong H., Brown D.H., Sanchez J.T., Wang Y. Distinct neural properties in the low-frequency region of the chicken cochlear nucleus magnocellularis. eNeuro. 2017;4 doi: 10.1523/ENEURO.0016-17.2017. e0016–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol M.E., Dallos P. Neural coding in the chick cochlear nucleus. J. Comp. Physiol. 1990;166:721–734. doi: 10.1007/BF00240021. [DOI] [PubMed] [Google Scholar]
- Winklhofer M., Matthias K., Seifert G., stocker M., Sewing S., Herget T., Steinhäuser C., Saaler-Reinhardt S. Analysis of phosphorylation-dependent modulation of Kv1.1 potassium channels. Neuropharmacology. 2003;44:829–842. doi: 10.1016/s0028-3908(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Zirpel L., Lippe W.R., Rubel E.W. Activity-dependent regulation of [Ca2+]i in avian cochlear nucleus neurons: roles of protein kinases A and C and relation to cell death. J. Neurophysiol. 1998;79:2288–2302. doi: 10.1152/jn.1998.79.5.2288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.