Abstract
Polygonum tinctorium Lour. (family Polygonaceae), known as indigo plant, has been useful as a medicinal or edible plant abundant in polyphenolic compounds. We have recently shown that flavonol O-glycosides with 3,5,4′-trihydroxy-6,7-methylenedioxyflavone (TMF) are predominant flavonoids in indigo leaves. However, no study has been performed regarding changes in the levels of flavonoid species during the germination and growth of indigo plant. Here, we attempted to determine the individual constituents of flavonol O-glycosides and the changes in their contents of the seeds, sprouts, and aerial parts. These results revealed that only the seeds predominantly contained flavonol O-(acetyl)-rhamnosides with quercetin or kaempferol as an aglycone. During the development of the sprouts and aerial parts, flavonol O-glycosides with TMF as an aglycone became mainly detectable and accounted for 79.4% and 74.9% of total flavonol O-glycosides from the extracts of aerial parts harvested in 2016 and 2017, respectively. Of the plant organs tested, the aerial parts exhibited the highest antioxidant activities concomitant with greatly increased levels of total polyphenols. Thus, we were able to conduct the identification and quantification of flavonol O-glycosides from the seeds, sprouts, and aerial parts of indigo plant and to evaluate antioxidant activities of their extracts. Taken together, our findings clearly provide the evidence that the aerial parts of indigo plant are a rich source of flavonol O-glycosides with TMF and exhibit much higher antioxidant activities, indicating the usefulness for the application to food and nutraceutical purposes.
Keywords: Pharmaceutical science, Food technology, Food science, Analytical chemistry, Food analysis
1. Introduction
Polygonum tinctorium Lour. (family Polygonaceae), known as indigo plant, is an annual herb native to Southeast Asia, which has been utilized as a well-known source for the pigment of blue indigo since ancient times. On the other hand, indigo plant has been valuable as a medicinal plant for the preparation of traditional herbal medicine for the purpose of expecting detoxifying, antipyretic, and antiphlogistic effects (Iwaki and Kurimoto, 2002). For example, the extracts of the seeds and leaves of indigo plant were described to be effective in the detoxification and reduction of fever in Japan (Murakami, 1985). As well, the extracts of indigo leaves were shown to have therapeutic effects on stomatitis, chapped lips, and swelling in the body. In some regions of Japan, indigo leaves is useful as a food raw material to garnish certain traditional Japanese foods (Murakami, 1985). In Korea also, indigo plant is utilized as a traditional folk medicine called Naju JjoK for antipyretic and detoxifying purposes (Han et al., 2014).
Earlier studies described a variety of biologically active substances isolated from indigo plant, such as gallic acid and caffeic acid serving as antioxidants (Kimoto et al., 1999), indirbin showing anti-allergic activity (Kunikata et al., 2000), tryptanthrin with anti-cancer (Koya-Miyata et al., 2001) and anti-inflammatory effects (Micallef et al., 2002), and 3,5,4′-trihydroxy-6,7-methylenedioxyflavone (TMF)-3-O-β-D-glucopyranoside having an anti-coagulating action of blood (Kohda et al., 1990). Moreover, tryptanthrin, 6-methoxykaempferol, kaempferol, and TMF were reported to serve as effective anti-bacterial agents against Helicobacter pylori (Hashimoto et al., 1999). More recently, we have identified different types of flavonol O-glycosides with quercetin, kaempferol, isorhamnetin, or TMF as an aglycone from indigo leaves (Kimura et al., 2015). Of these, flavonol O-glycosides with TMF occurring as predominant components can inhibit 3-hydroxy-3-methylglutaryl-CoA reductase, the rate-limiting enzyme of cholesterol biosynthesis, in a dose-dependent manner (Kimura et al., 2015). In addition, our laboratory has shown that free TMF generated from acidic cleavage of flavonol O-glycosides with TMF as an aglycone exerts more potent anti-inflammatory activity than the unhydrolyzed compounds (Tokuyama-Nakai et al., 2018). The free TMF can be detectable in the blood circulation in mice after oral administration of the fraction of flavonol O-glycosides with TMF as an aglycone from indigo leaves.
Thus, flavonol O-glycosides with TMF are major flavonoids characteristic of indigo leaves. It is still unknown whether these flavonoids are already present in the seeds or biosynthesized at the growing stages. Earlier, Jang et al. (2012) described antioxidant activities of the extracts from the seeds, immature leaves, and mature leaves of indigo plant. However, they did not perform the identification of the polyphenolic compounds in various tissues of indigo plant. Until now, no reports have been available regarding the identification and quantification of flavonol O-glycosides in the seeds and sprouts as far as we know. Therefore, this study was undertaken to identify flavonol O-glycosides with different aglycones by ultra-performance liquid chromatography-electrospray ionization-time-of-flight/mass spectrometryE (UPLC-ESI-TOF/MSE) and to quantify the individual components by reverse-phase high-performance liquid chromatography (HPLC) using the 80% methanol extracts from the seeds, sprouts, and aerial parts of indigo plant. We also attempted to evaluate antioxidant activities of the extracts from those tissues by the methods of hydrophilic oxygen radical absorbance capacity (H-ORAC) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity.
2. Materials and methods
2.1. Materials
The seeds of P. tinctorium were purchased from Takii Seed (Kyoto, Japan) in 2016 and 2017, which were deposited as the voucher specimens of No. 16001 and No. 17001, respectively, in the Department of Research and Development, Kotobuki Seika in Japan. The plants were grown in a field of Sakaiminato, Tottori in Japan as described previously (Kimura et al., 2015). The sprouts were harvested on April 18, 2016 and on April 14, 2017 as the voucher specimens of No. 16002 and No. 17002 after the seeds were planted on April 2, 2016 and on March 29, 2017, respectively. The aerial parts including leaves and stems were then harvested on July 20, 2016 and on July 3, 2017 as the voucher specimens of No. 16003 and No. 17003, respectively. With comparative aims, the fruits of Cucumis sativus (cucumber), the green leaves of Lactuca sativa L. (lettuce), and Spinacia oleracea L. (spinach) were analyzed in the same manner. The edible plants were obtained from Yonago Seika (Yonago, Japan).
Quercetin-3-O-β-D-glucuronide, quercetin-3-O-α-L-rhamnoside, kaempferol-3-O-α-L-rhamnoside, DPPH, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), sodium fluorescein, the Folin-Ciocalteu reagent, and acetonitrile for HPLC were obtained from Sigma-Aldrich (St Louis, MO, USA). Kaempferol-3-O-β-D-glucopyranoside and isorhamnetin-3-O-β-D-glucopyranoside were products of Extrasynthese (Lyon, France). Quercetin, kaempferol, hesperetin, 2,2′-azobis(2-amidinopropane)dihydrochloride (AAPH), and other reagents were purchased from Fujifilm Wako Pure Chemical (Osaka, Japan). Diaion HP-20 for column chromatography was supplied by Nippon Rensui (Tokyo, Japan). The Acquity UPLC BEH C18 column was obtained from Nihon Waters (Tokyo, Japan). The YMC-Pack ODS-AM column for HPLC is a product of YMC Co. (Kyoto, Japan). The Alltec C18 Maxiclean cartridge column was obtained from Grace (Columbia, MD, USA). The Advantec filter paper No. 2 was purchased from Advantec (Tokyo, Japan), and the Kiriyama funnel filter paper No. 4 was from Kiriyama Glass Co. (Tokyo, Japan). The Torast disc with 0.22-μm membrane filter was the product of Shimadzu GLC (Tokyo, Japan). All other chemicals were of analytical grade unless otherwise stated.
2.2. Extraction and fractionation of flavonoids from indigo plant for analysis by UPLC-ESI-TOF/MSE
The seeds, sprouts, and aerial parts with leaves and stems of indigo plant were separately harvested in 2016 and 2017 and stored at -20 °C before the extraction and fractionation of flavonoids. The fresh samples were ground into small pieces in a mortar using a pestle. The respective materials were separately mixed with 100 ml of 80% methanol in water, and then sonicated for 20 min at 20 °C in a Bransonic sonicator (Emerson Japan, Tokyo, Japan). After the filtration through an Advantec filter No. 2, the extracts were evaporated to dryness at 40 °C under reduced pressure by a rotatory evaporator. The dried materials were dissolved in a small portion of water and applied to column chromatography on a Diaion HP-20 column (200 mm × 30 mm i.d.). The column was washed with 500 ml of water and followed by the elution with 400 ml of methanol. The resulting eluates were evaporated into dryness and kept in a dessicator overnight in vacuo. The fractions of flavonoids were recovered as dry matter masses of 42.1 mg, 30 mg, and 600 mg from fresh weights of 5.8 g, 1.4 g, and 16.8 g corresponding to the seeds, sprouts, and aerial parts of indigo plant in 2016, respectively. The described method was used for qualitative identification of flavonoids. The quantitative method was separately described in the section of 2.5 for samples from 2016 and 2017.
2.3. Analysis of flavonoids by UPLC-ESI-TOF/MSE
Prepared fractions of flavonoids from seeds, sprouts, and aerial parts of indigo plant in Section 2.2, were separately dissolved in a mixture of 0.1% formic acid/acetonitrile (90:10, v/v) as analytes. The analysis of flavonoids was performed by UPLC-ESI-TOF/MSE on a Waters Synapt G2 High Definition Mass Spectrometry system following on-line separation by an Acquity UPLC system equipped with a photodiode array (PDA) detector as described previously (Kimura et al., 2015; Tokuyama-Nakai et al., 2018). An Acquity UPLC BEH C18 column (100 mm × 2.1 mm i.d., 1.7-μm particle) was employed for the separation of flavonoids. Injection volumes of the analytes from seeds, sprouts, and aerial parts were 3, 1, and 1 μl, respectively. The UPLC column was eluted at a flow rate of 0.3 ml/min at 40 °C for a total of 10 min with a mobile phase of 0.1% formic acid/acetonitrile in a linear gradient system from 90:10 (v/v) to 50:50 (v/v). The peaks were detected by a PDA detector by monitoring the absorbance at 340 nm. The MSE analysis was conducted for the detection of molecular and product ions in a negative-ion mode using different collision energies (Gonzales et al., 2014; Kimura et al., 2015; Tokuyama-Nakai et al., 2018). The analytical conditions used here are: capillary voltage, 2.5 kV; sampling cone voltage, 50 V; extraction cone voltage, 4 V; source temperature, 150 °C; desolvation temperature, 500 °C; cone gas flow, 50 L/h; desolvation gas flow, 1000 L/h; scan time, 0.5 s; trap collision energy [low energy], 6 V; and ramp trap collision energy [high energy], 6–45 V. Transfer collision energy and ramp transfer collision energy were switched off.
2.4. Alkaline hydrolysis of flavonoids
The extract of indigo seeds (1 mg dry weight) was prepared as described in the section of 2.2. The resulting materials were dissolved in 0.5 ml of 50% methanol and mixed with 0.5 ml of 20% potassium hydroxide, after which the reaction was allowed to react overnight at 25 °C. Following the neutralization of the reaction mixture with HCl, the solution was applied into a Alltec C18 Maxiclean cartridge column. The cartridge column was washed with 5 ml of distilled water and followed by the elution of the hydrolysis products with 5 ml of 100% methanol. The eluted materials were analyzed by UPLC-ESI-TOF/MSE as describe in the section of 2.3.
2.5. Quantitative analysis of flavonol O-glycosides by HPLC
The seeds, sprouts, and aerial parts of indigo leaves were harvested as described in the section of 2.2 and used for the preparation of dried materials by lyophilization. The dry materials ground in a mortar using a pestle from the seeds (900 mg dry weight (DW)), sprouts (100 mg DW), or aerial parts (100 mg DW) were individually mixed with 10 ml of 80% methanol and 200 μl of dimethyl sulfoxide containing 200 μg of hesperetin as an internal standard. The mixture was vortexed vigorously for 30 sec and then sonicated for 20 min at 20 °C in a Bransonic sonicator (Emerson Japan, Tokyo, Japan) for the extraction of flavonoids. The resulting supernatant was collected after centrifugation at 1500 × g for 5 min. The same extraction procedure was repeated again by mixing the pellets with 10 ml of 80% methanol. The combined supernatants were filtrated through a Kiriyama funnel filter paper No. 4, after which the extracts were filled up to 20 ml with 80% methanol. The 1-ml aliquot of the filled extracts was evaporated into dryness and redissolved in 1 ml of a mixture of 0.1% formic acid/acetonitrile (95:5, v/v). A portion of the extracts was applied to the analysis by reverse-phase HPLC following filtration through a membrane filter with a pore size of 0.22 μm. The HPLC analysis was performed using a YMC-Pack ODS-AM column (150 mm × 3 mm i.d., 5-μm particle size) on a Shimadzu LC-2010A system equipped with a Chromatopac C-R8A recorder. The column was eluted with a flow rate of 0.4 ml/min at 40 °C for a total of 40 min with a mobile phase of 0.1% formic acid/acetonitrile in a linear gradient system from 95:5 (v/v) to 60:40 (v/v). The quantified value was expressed as hesperetin equivalent (HesE) (μg HesE/g DW).
2.6. Assay of total polyphenols and antioxidant activities
The seeds, sprouts, and aerial parts of indigo plant were harvested as described in the section of 2.2 and used for the separate preparations of those freeze-dried materials. The resulting dry materials were ground and applied for the extraction of antioxidants including flavonoids with a mixture of acetone/water/acetic acid (70:29.5:0.5, v/v) (AWA) as reported previously (Prior et al., 2003; Kimura et al., 2017). The AWA extracts of different tissues were used for the assays of total polyphenols and antioxidant activities as below.
Total polyphenols were quantified by the method of Folin-Ciocalteu using gallic acid as a standard (Julkunen-Tiitto, 1985). The data were expressed as gallic acid equivalents (GAE) (mg GAE/g DW) in the fractions of flavonoids from different organs. Antioxidant activity against reactive oxygen species was determined by H-ORAC as described earlier (Kimura et al., 2017). This assay is based on the evaluation of the protective effect of samples to be tested on the decrease in the amount of fluorescein caused by the generation of a peroxy radical with AAPH. The H-ORAC values were expressed as Trolox equivalents (TE) (μmol TE/g DW) in the fraction of flavonoids from different organs. Radical scavenging activity was assessed using DPPH according to the previous method (Kimura et al., 2017). The DPPH radical scavenging activities of individual fractions were expressed as μmol TE/g DW.
2.7. Statistical analysis
Data were shown as means ± S.E.M. of three independent experiments. Comparison of multiple data was carried out by one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test. The values of p < 0.05 were considered to be statistically significant.
3. Results and discussion
3.1. Identification of flavonol O-glycosides from seeds, sprouts, and aerial parts of indigo plant
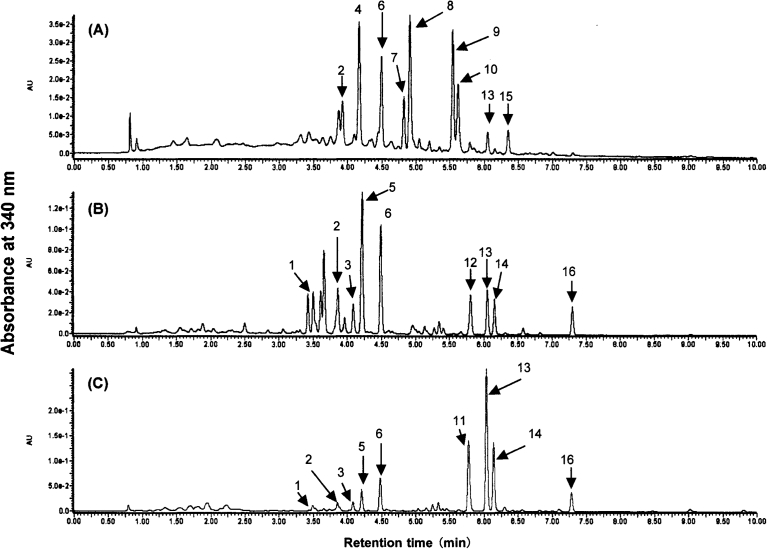
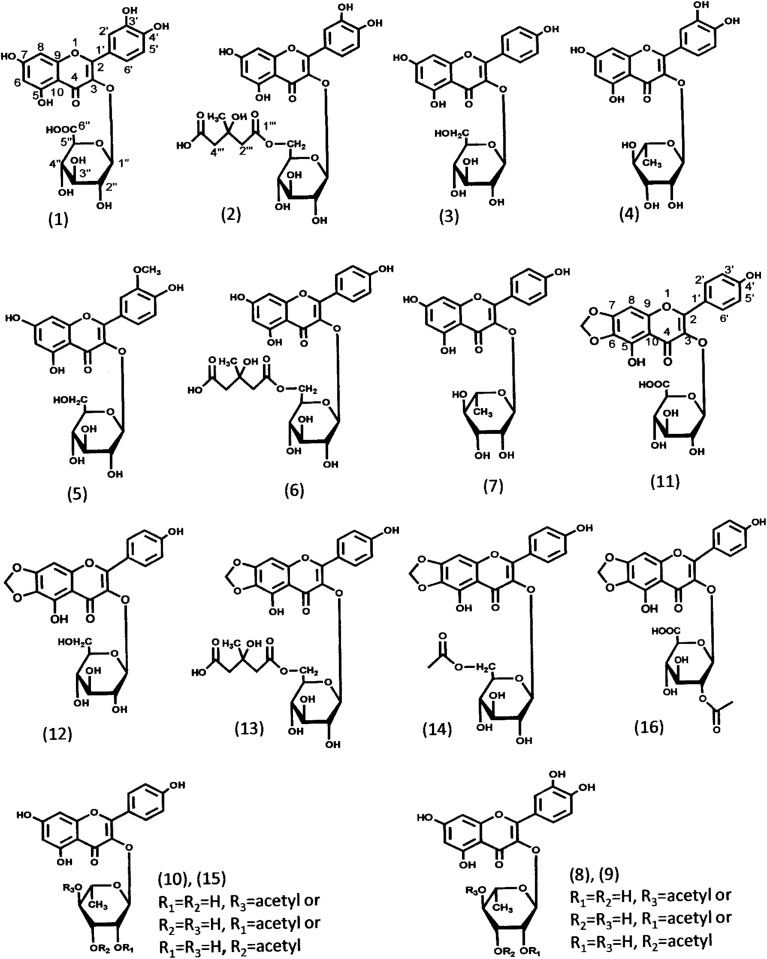
The fractions containing flavonoids were obtained by column chromatography on Diaion HP-20 following the extraction of polyphenolic compounds with 80% methanol from the seeds, sprouts, and aerial parts of indigo plant in 2016 and 2017. The analysis of these fractions by UPLC-ESI-TOF/MSE revealed almost similar chromatographic profiles between the same organs from 2016 and 2017 (Fig. 1 and Table 1). We have recently isolated compounds 1, 2, 3, 5, 6, 11, 13, 14, and 16 from the extract of indigo leaves and determined their chemical structures by studying the spectroscopic properties of UPLC-ESI-TOF/MSE, 1H-nuclear magnetic resonance (NMR), 13C-NMR, two-dimensional NMR, Fourier-transform infrared spectroscopy, and ultraviolet spectrum as well as chemical methods (Kimura et al., 2015) (Fig. 2).
Fig. 1.
UPLC analysis of flavonoids extracted with 80% methanol from seeds (A), sprouts (B), and aerial parts (C) of indigo plant harvested in 2016. Chromatographic profiles were recorded by monitoring the absorbance at 340 nm. Peak numbers represent flavonol O-glycosides.
Table 1.
Identification of flavonol O-glycosides from seeds, sprouts, and aerial parts of indigo plant by UPLC-ESI-TOF/MSE and UV spectra.
| Compound no. | Compound name | m/z [M-H]− | m/z Product ion | Wavelength for UV maximum (nm) |
|---|---|---|---|---|
| 1 | Quercetin-3-O-β-D-glucuronide | 477.06 | 301.04 [M-H-GluA]− | 255, 265sh, 351 |
| 2 | Quercerin-3-O-[6″-O-(3-hydroxy-3-methylglutaryl) -β-D-glucopyranoside] | 607.13 | 301.03 [M-H-HMG-Glu]− | 254, 265sh, 351 |
| 3 | Kaempferol-3-O-β-D-glucopyranoside (astragalin) | 447.05 | 285.02 [M-H-Glu]− | 265, 344 |
| 4 | Quercetin-3-O-α-L-rhamnoside | 447.10 | 301.03 [M-H-Rha]− | 254, 265sh, 348 |
| 5 | Isorhamnetin-3-O-β-D-glucopyranoside | 477.06 | 315.03 [M-H-Glu]− | 269, 337 |
| 6 | Kaempferol-3-O-[6"-O-(3-hydroxy-3-methylglutaryl) -β-D-glucopyranoside] | 591.09 | 447.06 [M-H-HMG]−, 285.02 [M-H-HMG-Glu]− | 265, 347 |
| 7 | Kaempferol-3-O-α-L-rhamnoside | 431.10 | 285.04 [M-H-Rha]− | 265, 344 |
| 8 | Quercetin-3-O-[x″-O-(acetyl)-α-L-rhamnoside] | 489.10 | 447.1 [M-H-acetyl]−, 301.03 [M-H-acetyl-Rha]− | 253, 265sh, 347 |
| 9 | Quercetin-3-O-[x″-O-(acetyl)-α-L-rhamnoside] | 489.10 | 447.1 [M-H-acetyl]−, 301.03 [M-H-acetyl-Rha]− | 254, 265sh, 347 |
| 10 | Kaempferol-3-O-[x″-O-(acetyl)-α-L-rhamnoside] | 473.10 | 285.04 [M-H-acetyl-Rha]− | 264, 339 |
| 11 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-β-D-glucopyranoside | 475.10 | 313.03 [M-H-Glu]− | 277, 339 |
| 12 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-β-D-glucuronide | 489.07 | 313.03 [M-H-GluA]− | 277, 340 |
| 13 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-[6″-O-(3-hydroxy-3-methylglutaryl)-β-D-glucopyranodside] | 619.13 | 475.08 [M-H- HMG]−, 313.03 [M-H-HMG-Glu]− | 277, 340 |
| 14 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-[6″-O-(acetyl)-β-D-glucopyranoside] | 517.09 | 475.08 [M-H- acetyl]−, 313.04 [M-H-HMG-acetyl]− | 277, 340 |
| 15 | Kaempferol-3-O-[x″-O-(acetyl)-α-L-rhamnoside] | 473.10 | 285.04 [M-H-acetyl-Rha]− | 264, 344 |
| 16 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-[2″-O-(acetyl)-β-D-glucuronide] | 531.07 | 313.03 [M-H-CH3COOH-GluA]− | 278, 338 |
Abbreviations: Glu, glucose; Rha, rhamnose; GluA, glucuronic acid; HMG, 3-hydroxy-3-methylglutary; X, binding site unknown; N.D., not detect; sh, shoulder.
Fig. 2.
Structural formulas of flavonol O-glycosides from seeds, sprouts, and aerial parts of indigo plant. Numbers and names of identified compounds are shown in Table 1.
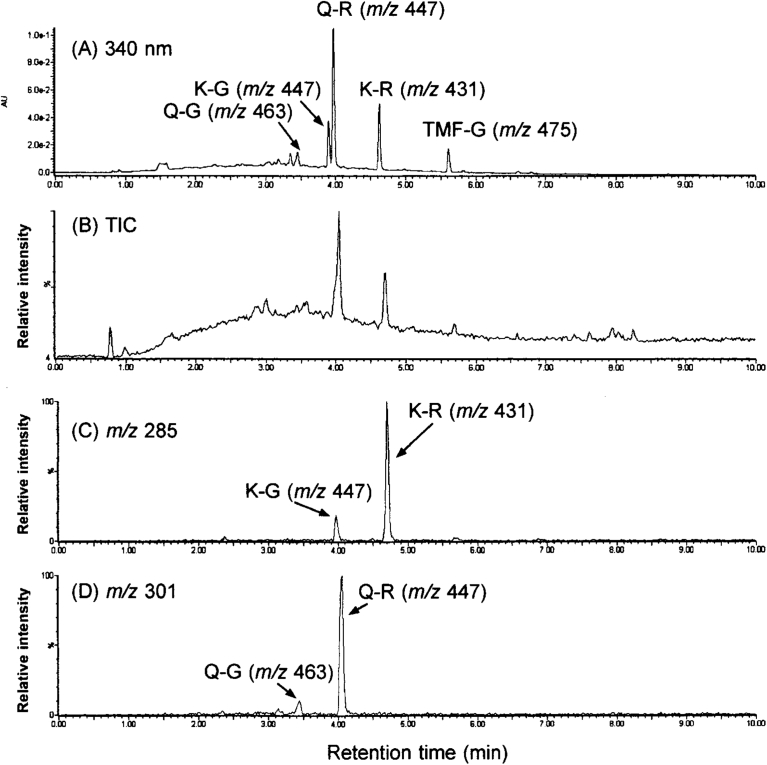
The extract of the seeds exhibited characteristic peaks including compounds 2, 4, 6, 7, 8, 9, 10, 13, and 15 (Fig. 1A). When the extract was subjected to alkaline hydrolysis of flavonoids, those peaks of 2, 6, 8, 9, 10, 13, and 15 were not detectable. Instead, compounds 2 and 6 gave the hydrolysis products of quercetin-3-O-β-D-glucopyranoside and kaempferol-3-O-β-D-glucopyranoside (3), respectively (Fig. 3). In addition, quercetin-3-O-α-L-rhamnoside (4) was obtained from compounds 8 and 9 while kaempferol-3-O-α-L-rhamnoside (7) was generated from compounds 10 and 15 by their alkaline hydrolysis. Moreover, the alkaline treatment of the compound 13 was found to yield 3,5,4′-trihydroxy-6,7-methylenendioxyflavone-3-O-β-D-glucopyranoside (11).
Fig. 3.
UPLC–ESI-TOF/MSE analysis of flavonol O-glycosides after alkaline hydrolysis of total extracts from indigo seeds. Chromatograms were recorded by monitoring the absorbance at 340 nm on a PDA (A) along with MS data on total ions (B) and selective ions at m/z 285 (C) and m/z 301 (D) of mass spectra in the negative-ion mode. Abbreviations: TIC, total ion chromatogram; Q-G, quercetin-3-O-β-D-glucopyranoside; K-G, kaempferol-3-O-β-D-glucopyranoside; Q-R, quercetin-3-O-α-L-rhamnoside; K-R, kempferol-3-O-α-L-rhamnoside; TMF-G, 3,5,4′-trihydroxy-6,7-methylenendioxyflavone-3-O-β-D-glucopyranoside.
We also confirmed that the peaks of 4 and 7 were consistent with those of commercially available authentic compounds corresponding to quercetin-3-O-α-L-rhamnoside and kempferol-3-O-α-L-rhamnoside, respectively (Mikulic-Petkovsek et al., 2012; Olszewska and Wolbis, 2001). On the basis of the data on the UPLC-ESI-TOF/MSE, compounds 8 and 9 are identified as isomers of quercetin-3-O-[x''-O-(acetyl)-α-L-rhamnoside] (Mikulic-Petkovsek et al., 2012; Qin et al., 2017). As well, compounds 10 and 15 were determined to be isomers of kaempferol-3-O-[x''-O-(acetyl)-α-L-rhamnoside] (Díaz et al., 2008). These findings indicate that the predominant flavonoid species from the seeds of indigo plant are flavonol O-(acetyl)-rhamnosides with quercetin or kaempferol as an aglycone (Fig. 2).
On the other hand, the UPLC-ESI-TOF-MSE analysis of the extracts from the sprouts of indigo plant allowed us to detect the predominant peaks of 5 and 6, which were identified as isorhamnetin-3-O-β-D-glucopyranoside (Kimura et al., 2015) and kaempferol-3-O-[6″-O-(3-hydroxy-3-methylglutaryl)-β-D-glucopyranoside] (Iwashita et al., 2004; Kimura et al., 2015)., respectively (Fig. 1B). We also recognized the characteristic peaks of 12, 14 and 16 present in the sprouts, which were not detectable in the seeds. These compounds were found to be flavonol O-glycosides with TMF as an aglycone as described previously in indigo leaves (Fig. 2, Table 1) (Kimura et al., 2015). Interestingly, compound 12 determined as 3,5,4′-trihydroxy-6,7-methylenendioxyflavone-3-O-β-D-glucuronide was detected specifically in the extract of the sprouts. UV analysis revealed that compound 12 had absorbance maxima at 277 nm and 340 nm characteristic of TMF as an aglycone (Kimura et al., 2015). When 3,5,4′-trihydroxy-6,7- methylenedioxyflavone-3-O-[2″-O-(acetyl)-β-D-glucuronide] (16) were isolated from indigo leaves and subjected to alkaline hydrolysis, the product gave the molecular ion of m/z 489.07 and a fragment ion of m/z 313.03 reflecting TMF, which are consistent with the data of the compound 12. As far as we know, compound 12 unique to the sprouts has not been reported previously in other plants.
In the extract from the aerial parts including the leaves and stems of indigo plant, compounds 11, 13, 14, and 16 were predominantly detectable and identified as flavonol O-glycosides with TMF as an aglycone (Figs. 1C and 2, and Table 1) (Kimura et al., 2015). However, compound 12, one of flavonol O-glycosides with TMF, was not detectable in the extract of the aerial parts although it is uniquely found in that of the sprouts. Our laboratory has recently reported that the major flavonoids from indigo leaves are flavonol O-glycosides with TMF as an aglycone (Kimura et al., 2015). By contrast, a more recent study of us has shown that these major products with TMF are not recognized in the extract of the stems, which contains only compounds 1 and 2 (Tokuyama-Nakai et al., 2018). Thus, indigo leaves are considered to be mainly responsible for the production of flavonol O-glycosides with TMF as an aglycone detected in the extract of the aerial parts of indigo plant. The commonly detectable compounds during the growing stages of the seeds, sprouts, and aerial parts were found to be compounds 2, 6, and 13, all of which had a 3-hydroxy-3-methylglutaryl group in the glycosides of different flavonols (Fig. 2).
3.2. Quantification of flavonol O-glycosides from seeds, sprouts, and aerial parts
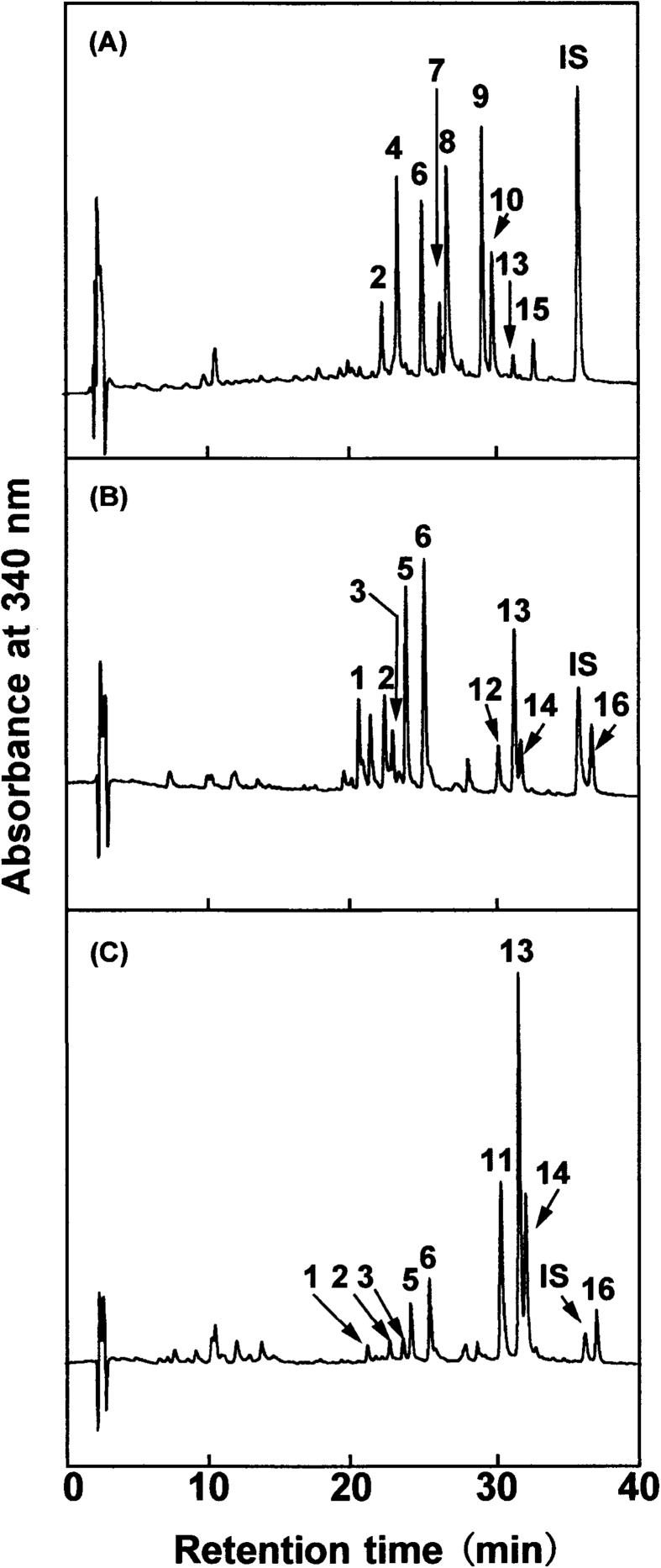
The 80% methanol extracts from the seeds, sprouts, and aerial parts of indigo plant in 2016 and 2017 were applied to the quantification of flavonol O-glycosides by reverse-phase HPLC using hesperetin as an internal standard (Fig. 4). The analysis gave similar results between the samples from 2016 and 2017. The levels of compounds 11, 13, and 14 increased substantially, reaching to the amounts higher than those of sprouts by over 10-fold (Table 2). Flavonol O-glycosides with TMF were found to comprise 79.4% of the related compounds from the aerial parts in 2016 and 74.9% of them in 2017. Earlier, 3.5,4′-trihydroxy-6,7-methylenedioxyflavone-3-O-β-D-glucopyranoside was described to have anti-coagulant activity (Kohda et al., 1990). Our recent study has reported that all the isolated compounds of flavonol O-glycosides with TMF as an aglycone including compounds 11, 13, 14, and 16, and free TMF exhibit the inhibitory activity against 3-hydroxy-3-methylglutaryl-CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis, in a dose-dependent manner (Kimura et al., 2015). Moreover, we have just recently shown that free TMF is more effective to exert anti-inflammatory activity than flavonol O-glycosides with TMF as an aglycone as determined by the suppressed synthesis of NO and prostaglandin E2 in cultured macrophage cells stimulated with lipopolysaccharide (Tokuyama-Nakai et al., 2018). Since the aerial parts of indigo plant, namely the leaves, are very rich in flavonol O-glycosides with TMF as an aglycone, these bioactive compounds are regarded as promising sources for the application to food and pharmaceutical purposes.
Fig. 4.
Quantitative HPLC analysis of flavonoids extracted with 80% methanol from seeds (A), sprouts (B), and aerial parts (C) of indigo plant harvested in 2016. The chromatographic profiles were recorded by monitoring the absorbance at 340 nm. The peak numbers represent flavonol O-glycosides. IS, internal standard (hesperetin).
Table 2.
Quantitative HPLC analysis of flavonol O-glycosides from seeds, sprouts and aerial parts of indigo plant.
| Compound no. | Compound name | Amount (mg HesE/g DW) |
|||||
|---|---|---|---|---|---|---|---|
| Seed |
Sprout |
Aerial part |
|||||
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | ||
| 1 | Quercetin-3-O-β-D-glucuronide | N.D. | N.D. | 0.501 ± 0.061 | 1.49 ± 0.03 | 1.76 ± 0.03 | 1.91 ± 0.02 |
| 2 | Quercerin-3-O-[6″-O-(3-hydroxy-3-methylglutaryl)-β-D-glucopyranoside] | 0.0454 ± 0.0064 | 0.0286 ± 0.0008 | 1.41 ± 0.14 | 2.01 ± 0.07 | 1.70 ± 0.04 | 2.57 ± 0.07 |
| 3 | Kaempferol-3-O-β-D-glucopyranoside (astragalin) | N.D. | N.D. | 0.349 ± 0.075 | 1.38 ± 0.12 | 1.78 ± 0.04 | 1.20 ± 0.01 |
| 4 | Quercetin-3-O-α-L-rhamnoside | 0.129 ± 0.008 | 0.0922 ± 0.0005 | N.D. | N.D. | N.D. | N.D. |
| 5 | Isorhamnetin-3-O-β-D-glucopyranoside | N.D. | N.D. | 2.79 ± 0.33 | 8.01 ± 0.23 | 4.16 ± 0.09 | 4.01 ± 0.03 |
| 6 | Kaempferol-3-O-[6″-O-(3-hydroxy-3-methylglutaryl)-β-D-glucopyranoside] | 0.0960 ± 0.0067 | 0.0541 ± 0.0013 | 3.83 ± 0.35 | 5.71 ± 0.16 | 6.62 ± 0.61 | 7.19 ± 0.06 |
| 7 | Kaempferol-3-O-α-L-rhamnoside | 0.0415 ± 0.0023 | 0.0295 ± 0.0005 | N.D. | N.D. | N.D. | N.D. |
| 8 | Quercetin-3-O-[x"-O-(acetyl)-α-L-rhamnoside] | 0.152 ± 0.021 | 0.0838 ± 0.0014 | N.D. | N.D. | N.D. | N.D. |
| 9 | Quercetin-3-O-[x"-O-(acetyl)-α-L-rhamnoside] | 0.131 ± 0.015 | 0.0747 ± 0.0030 | N.D. | N.D. | N.D. | N.D. |
| 10 | Kaempferol-3-O-[x"-O-(acetyl)-α-L-rhamnoside] | 0.0742 ± 0.0058 | 0.0444 ± 0.0020 | N.D. | N.D. | N.D. | N.D. |
| 11 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-β-D-glucopyranoside | N.D. | N.D. | N.D. | N.D. | 14.6 ± 0.8 | 9.04 ± 0.20 |
| 12 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-β-D-glucuronide | N.D. | N.D. | 0.897 ± 0.104 | 1.50 ± 0.03 | N.D. | N.D. |
| 13 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-[6″-O-(3-hydroxy-3-methylglutaryl)-β-D-glucopyranodside] | 0.0143 ± 0.0012 | 0.00934 ± 0.00015 | 2.52 ± 0.27 | 2.69 ± 0.02 | 27.4 ± 1.3 | 20.7 ± 0.3 |
| 14 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-[6″-O-(acetyl)-β-D-glucopyranoside] | N.D. | N.D. | 0.884 ± 0.086 | 0.902 ± 0.016 | 15.1 ± 0.6 | 15.4 ± 0.3 |
| 15 | Kaempferol-3-O-[x"-O-(acetyl)-α-L-rhamnoside] | 0.0233 ± 0.0013 | 0.0155 ± 0.0003 | N.D. | N.D. | N.D. | N.D. |
| 16 | 3,5,4′-Trihydroxy-6,7-methylenedioxyflavone-3-O-[2″-O-(acetyl)-β-D-glucuronide] | N.D. | N.D. | 1.36 ± 0.14 | 1.57 ± 0.03 | 4.60 ± 0.11 | 5.10 ± 0.08 |
Abbreviations: X, binding site unknown; N.D., not detectable. Data represent the mean ± S.E.M. (n = 3).
3.3. Contents of total polyphenols and antioxidant activities of the extracts from seeds, sprouts, and aerial parts
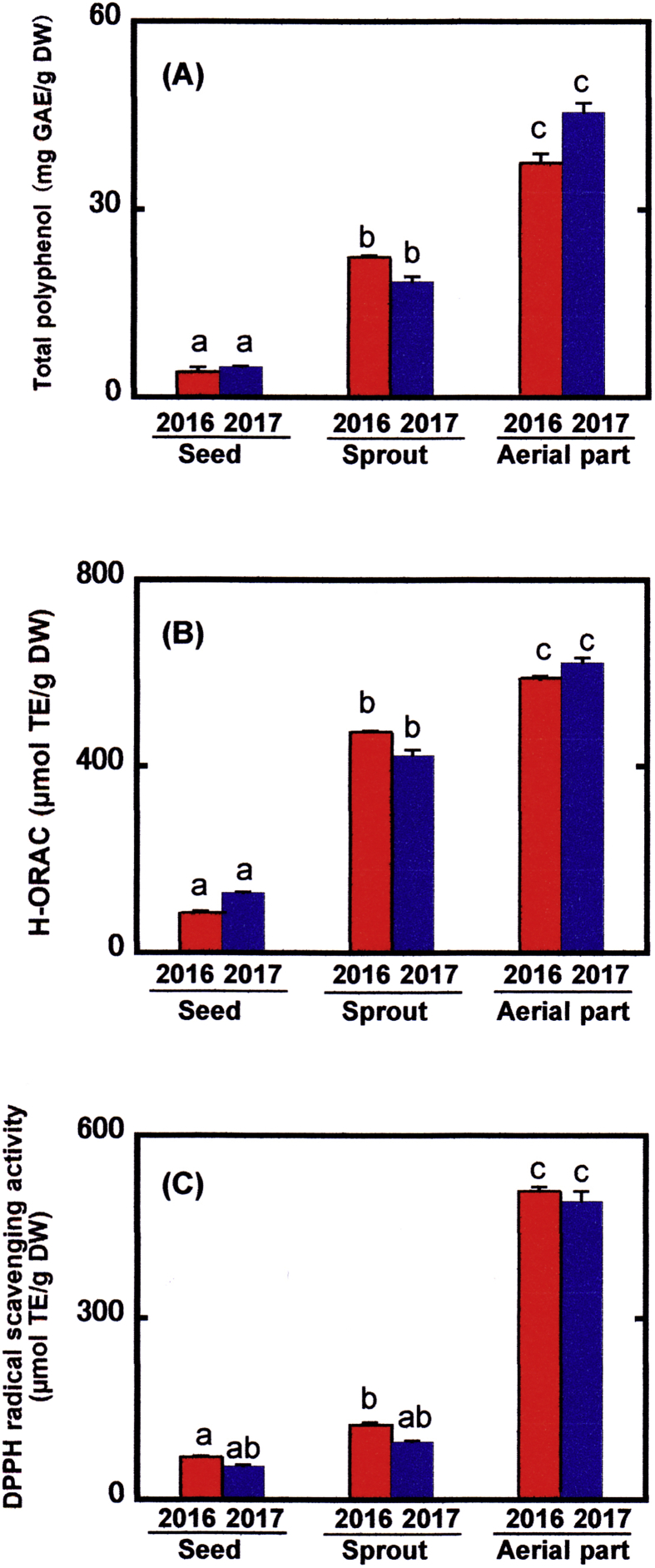
To efficiently extract flavonoids, the AWA mixture (Prior et al., 2003) was used for the extraction of hydrophilic flavonol O-glycosides from the seeds, sprouts, and aerial parts of indigo plant. The resulting extracts were subjected to the determination of total polyphenols and antioxidant activities by the methods of H-ORAC and DPPH radical scavenging activity (Fig. 5). Both extracts of the aerial parts obtained in 2016 and 2017 exhibited much higher levels of total polyphenols (Fig. 5A), and the H-ORAC (Fig. 5B) and DPPH (Fig. 5C) values than those of the seeds and sprouts. Flavonol O-glycosides with different aglycones could be responsible for the amounts of total polyphenols on the basis of the quantified levels of them. The H-ORAC method is known to evaluate the ability of antioxidants to transfer a hydrogen atom to reduce a peroxy radical. On the other hand, The DPPH radical scavenging activity is based on an electron transfer-based assay. Thus, it is not surprising that the changes in the H-ORAC and DPPH values are not consistent between the different samples because the antioxidant mechanisms are different between those methods (Prior et al., 2005).
Fig. 5.
Total polyphenol contents and antioxidant activities of seeds, sprouts, and aerial parts of indigo plant harvested in 2016 and 2017. Total polyphenol content (A), H-ORAC (B), and DPPH radical scavenging activity (C) were determined as described in the section of 2.6 under Materials and methods. Data represent means ± S.E.M. of three independent experiments. The different letters (a, b, c) indicate the values that differ significantly (p < 0.05).
More recently, we have shown that individual flavonol O-glycosides with quercetin or kaempferol from the seeds of Japanese horse chestnut have much higher H-ORAC values than their respective DPPH values due to the covalent attachment of glycosides to the hydroxyl moiety at C3 of the C ring in those flavonols (Kimura et al., 2017). Instead, the linkage of glycosides at the C3 position suppresses the DPPH radical scavenging activities of free flavonols. In the present study, all the flavonoid O-glycosides from different organs of indigo plant were found to comprise various glycosides through the binding to the hydroxyl group at C3 of the C ring of flavonols including TMF, quercetin, and kaempferol. Taking these findings into consideration, we suggest that flavonol O-glycosides would be main components of polyphenolic compounds in the sprouts. On the other hand, the ratios of the H-ORAC values to the DPPH values of the individual extracts from the seeds and aerial parts were relatively lower those of the extracts from the sprouts, implying the contribution of radical scavenging activities of other antioxidants such as proanthocyanidins from the aerial parts as described earlier in the seed shells of Japanese horse chestnut (Kimura et al., 2017).
Earlier studies described the determination of the polyphenolic contents and antioxidant activities using the extracts from the seeds and leaves of indigo plant with methanol (Jang et al., 2012) or water (Kim et al., 2012) although the identification and quantification of the individual components were not performed. According to their results, higher values of polyphenolic levels and antioxidant activities were recorded in the order of mature leaves>immature leaves>seeds. These findings are similar to our results in the order of aerial parts>sprouts>seeds using the AWA extracts. Our current results showed that the H-ORAC values of the aerial parts of indigo plant in 2016 and 2017 were 587 ± 7 and 623 ± 10 μmol TE/g DW, respectively. In addition, the DPPH values of those tissues were found to be 509 ± 6 and 492 ± 17 μmol TE/g DW. These values were compared with those of edible plants including spinach leaves, cucumber fruits, and lettuce leaves. In our study, the edible parts of spinach, cucumber, and lettuce exhibited the H-ORAC values of 222 ± 16, 40.2 ± 0.9, and 43.1 ± 0.5 μmol TE/g DW, and the DPPH values of 23.0 ± 2.3, 11.9 ± 5.3, and 12.1 ± 3.7 μmol TE/g DW, respectively. On the basis of the comparison between these data, it is obvious that the aerial parts of indigo plant have much more potent antioxidant activities than those common edible plants. Taken together, the high content of flavonol O-glycosides with TMF as an aglycone in the aerial parts of indigo plant make these tissues a promising rich source of potent antioxidants to be used in food additives, dietary supplements and other nutraceutical purposes.
4. Conclusion
Little is known about the difference in the species and levels of polyphenolic compounds from the seeds, sprouts, and aerial parts of indigo plant. This study enabled us to identify the flavonoid species in those organs at different growth stages by UPLC-ESI-TOF-MSE coupled with PDA detection as well as chemical methods. They were found to be flavonol O-glycosides with different structures of alycones and glycosides. The quantification of individual components by HPLC revealed that flavonol O-glycosides with TMF as an aglycone became predominantly detectable in the aerial parts at much higher levels than in the seeds and sprouts. As well, the AWA extracts from the aerial parts exhibited significantly higher antioxidant activities as determined by the assays of H-ORAC and DPPH radical scavenging activity due to more abundant levels of total polyphenols. Our results are informative in understanding the structural characterization and changes in the contents of flavonol O-glycosides responsible for the antioxidant activities in different organs of indigo plant.
Declarations
Author contribution statement
Hideto Kimura: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kazushige Yokota: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shota Tokuyama-Nakai: Performed the experiments; Wrote the paper.
Yu Hirabayashi, Tomoe Ishihara: Performed the experiments.
Mitsuo Jisaka: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Dr. Yuko Sugimoto at Applied Biotechnology, Food Research Institute Local Independent Administrative Institution Tottori Institute Industrial Technology, Sakaiminato, Tottori for instructing the assay of H-ORAC.
References
- Díaz J.G., Carmona A.J., Paz P.P., Herz W. Acylated flavonol glycosides from Delphinium staphisagria. Phytochem. Lett. 2008;1:125–129. [Google Scholar]
- Gonzales G.B., Raes K., Coelus S., Struijs K., Smagghe G., Camp J.V. Ultra (high)-pressure liquid chromatography-electrospray ionization-time of flight-ion mobility-high definition mass spectrometry for the rapid identification and structural characterization of flavonoid glycosides from cauliflower waste. J. Chromatogr. A. 2014;1323:39–48. doi: 10.1016/j.chroma.2013.10.077. [DOI] [PubMed] [Google Scholar]
- Han N.R., Kang S.W., Moon P.D., Jang J.B., Kim H.M., Jeong H.J. Genuine traditional Korean medicine, Naju Jjok (Chung-Dae, Polygonum tinctorium) improves 2,4-dinitrofluorobenzene-induced atopic dermatitis-like lesional skin. Phytomedicine. 2014;21(4):453–460. doi: 10.1016/j.phymed.2013.09.021. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Aga H., Chaen H., Fukuda S., Kurimoto M. Isolation and identification of anti-Helicobacter pylori compounds from Polygonum tinctorium Lour. Nat Med. 1999;53(1):27–31. [Google Scholar]
- Iwaki K., Kurimoto M. Cancer preventive effects of the indigo plant, Polygonum tinctorium. Recent Res. Develop. Cancer. 2002;4:429–437. [Google Scholar]
- Iwashina T., Omori Y., Kitajima J., Akiyama S., Suzuki T., Ohba H. Flavonoids in translucent bracts of the Himalayan Rheum nobile (Polygonaceae) as ultraviolet shields. J. Plant Res. 2004;117:101–107. doi: 10.1007/s10265-003-0134-2. [DOI] [PubMed] [Google Scholar]
- Jang H.G., Heo B.G., Park Y.S., Namiesnik J., Barasch D., Katrich E., Vearasilp K., Trakhtenberg S., Gorinstein S. Chemical composition, antioxidant and anticancer effects of the seeds and leaves of indigo (Polygonum tinctorium Ait) plant. Appl. Biochem. Biotechnol. 2012;167(7):1986–2004. doi: 10.1007/s12010-012-9723-7. [DOI] [PubMed] [Google Scholar]
- Julkunen-Tiitto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985;33(2):213–217. [Google Scholar]
- Kim K.S., Hwang W.G., Jang H.G., Heo B.G., Suhaj M., Leontowicz H., Leontowicz M., Jastzebsli Z., Tashma Z., Gorinstein S. Assessment of Indigo (Polygonum tinctorium Ait.) water extracts’ bioactive compounds, and their antioxidant and antiproliferative activities. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2012;46:500–510. [Google Scholar]
- Kimoto T., Koya S., Hino K., Yamamoto Y., Aga H., Hashimoto T., Masaki N., Hanaya T., Micallef M.J., Iwaki K., Ishihara T., Ushio S., Aga M., Kunikata T., Arai S., Ikeda M., Fukuda S., Kurimoto M. Protection by Indigo plant (Polygonum tinctorium Lour.) against renal oxidative damage in mice treated with ferric nitrilotriacetate. Nat Med. 1999;53(6):291–296. [Google Scholar]
- Kimura H., Ogawa S., Ishihara T., Maruoka M., Tokuyama-Nakai S., Jisaka M., Yokota K. Antioxidant activities and structural characterization of flavonol O-glycosides from seeds of Japanese horse chestnut (Aesculus turbinata BLUME) Food Chem. 2017;228:348–355. doi: 10.1016/j.foodchem.2017.01.084. [DOI] [PubMed] [Google Scholar]
- Kimura H., Tokuyama S., Ishihara T., Ogawa S., Yokota K. Identification of new flavonol O-glycosides from indigo (Polygonum tinctorium Lour) leaves and their inhibitory activity against 3-hydroxy-3-methylglutaryl-CoA reductase. J. Pharm. Biomed. Anal. 2015;108:102–112. doi: 10.1016/j.jpba.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Kohda H., Niwa A., Nakamoto Y., Takeda O. Flavonoid glucosides from Polygonum tintorium. Chem. Pharm. Bull. 1990;38(2):523–524. [Google Scholar]
- Koya-Miyata S., Kimoto T., Micallef M.J., Hino K., Taniguchi M., Ushio S., Iwaki K., Ikeda M., Kurimoto M. Prevention of azoxymethane-induced intestinal tumors by a crude ethyl acetate-extract and tryptanthrin extracted from Polygonum tinctorium Lour. Anticancer Res. 2001;21:3295–3300. [PubMed] [Google Scholar]
- Kunikata T., Takefuji T., Aga H., Iwaki K., Ikeda M., Kurimoto M. Indirbin inhibits inflammatory reactions in delayed-type hypersensitivity. Eur. J. Pharmacol. 2000;410:93–100. doi: 10.1016/s0014-2999(00)00879-7. [DOI] [PubMed] [Google Scholar]
- Micallef M.J., Iwaki K., Ishihara T., Ushio S., Aga M., Kunikata T., Koya-Miyata S., Kimoto T., Ikeda M., Hino K., Kurimoto M. The natural plant product tryptanthrin ameliorates dextran sodium sulfate-induced colitis in mice. Int. Immunopharmacol. 2002;2:565–578. doi: 10.1016/s1567-5769(01)00206-5. [DOI] [PubMed] [Google Scholar]
- Mikulic-Petkovsek M., Slatnar A., Stampar F., Veberic R. HPLC-MSn idetification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012;135(4):2138–2146. doi: 10.1016/j.foodchem.2012.06.115. [DOI] [PubMed] [Google Scholar]
- Murakami K. Tokushima Shinbun; Tokushima, Japan: 1985. Tokushima-ken Yakuso Zukan, No. 1; pp. 132–133. [Google Scholar]
- Olszewska M., Wolbis M. Flavonoids from the flowers of Prunus spinosa L. Acta Pol. Pharm. 2001;58:367–372. [PubMed] [Google Scholar]
- Prior R.L., Hoang H., Gu L., Wu L., Wu X., Bacchiocca M., Howard L., Hampsch-Woodill M., Huang D., Ou B., Jacob R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003;51(11):3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Qin Y., Gao B., Shi H., Cao J., Yin C., Lu W., Yu L., Cheng Z., Cheng Z. Characterization of flavonol mono-, di-, tri- and tetra-O-glycosides by ultra-performance liquid chromatography-electrospray ionization-quadrupole time-of-flight mass spectrometry and its application for identification of flavonol glycosides in Viola tianschanica. J. Pharm. Biomed. Anal. 2017;142:113–124. doi: 10.1016/j.jpba.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Tokuyama-Nakai S., Kimura H., Ishihara T., Jisaka M., Yokota K. In vitro anti-inflammatory and antioxidant activities of 3,5,4'-trihydroxy-6,7- methylenedioxyflavone-O-glycosides and their aglycone from leaves of Polygonum tinctorium Lour. Appl. Biochem. Biotechnol. 2018;184(2):414–431. doi: 10.1007/s12010-017-2555-8. [DOI] [PubMed] [Google Scholar]