Abstract
BACKGROUND: Type VI collagen (COL6) is associated with several pro-tumorigenic events. COL6 is primarily composed of three alpha-chains (a1-a3) forming a specialized microfibrillar network to support tissue architecture. COL6 homeostasis is lost in the tumor due to increased COL6 synthesis by activated fibroblast and altered proteolytic degradation by matrix metalloproteases (MMPs). Consequently, pathology-specific COL6 fragments are released to the circulation. This study evaluates four COL6 fragments measured in serum as potential biomarkers for cancer. METHODS: C6Ma1 (MMP-generated neo-epitope on the a1 chain), C6Ma3 (MMP-generated neo-epitope on the a3 chain), PRO-C6 (C-terminal of the a3 chain) and IC-6 (internal epitope on the a1 chain) were measured by ELISA in serum from patients with various stage 1–4 cancer indications (n = 4–11 per indication, total n = 65) and healthy controls (n = 13). RESULTS: C6Ma1 and C6Ma3 were significantly elevated in most cancer types compared to controls; PRO-C6 and IC6 were not. No significant differences were seen according to age, gender and TNM stage. Comparing cancer patients to controls, the AUROC was 0.90 (P < .0001), 0.87 (P < .0001), 0.59 (P = .311) and 0.53 (P = .747) for C6Ma1, C6Ma3, PRO-C6 and IC-6, respectively. Only C6M and C6Ma3 correlated significantly (Spearman, r = 0.74, P < .0001). CONCLUSIONS: MMP-generated COL6 fragments (C6Ma1, C6Ma3) were elevated in serum from cancer patients compared to controls and had promising diagnostic accuracy. This supports that MMP-mediated COL6 remodeling is important in tumorigenesis and indicate cancer biomarker potential of quantifying COL-6 fragments in serum. Future studies should determine biological and clinical applicability of the COL-6 serum biomarkers in relation to cancer.
Introduction
Loss of tissue microarchitecture is a prominent feature of cancer driven by alterations in the composition and quality of the extracellular matrix (ECM) [1]. The major components of the ECM are the collagens of which 28 different molecules have been described [2]. Some of these collagens are good and some are bad [3]. In particular type VI collagen (COL6) is interesting in the context of cancer [4]. The expression of COL6 is altered in several solid tumor types including pancreatic cancer, breast cancer, colon cancer, melanomas, ovarian cancer, lung cancer and salivary cancer where it associate with proliferative signaling, invasion and metastasis, resisting of cell death, induction of angiogenesis, tumor inflammation and response to chemo- and radiotherapy [4], [5], [6], [7], [8], [9], [10], [11], [12], [13].
COL6 is a beaded filament collagen found in close proximity of the basement membrane that take part in maintaining epithelial- and endothelial-cell polarity [14], [15]. Fibroblasts, tumor cells hematopoietic cells and chondrocytes can also bind to COL6 [16], [17], [18]. COL6 remodeling is linked to tumor progression mediated by an accumulation of activated cancer associated fibroblast and increased matrix metalloprotease (MMP) activity [4], [19], [20], [21].
COL6 is primarily composed of three different alpha chains, COL6a1, COL6a2 and COL6a3 [22]. The three alpha chains form heterotrimeric tetramers within the cell. These tetramers are subsequently secreted into the extracellular surroundings where they associate into microfibrils. Several sub-domains in the N- and C-terminal of COL6 are involved in the triple helix and microfibril formation. The so-called C1 subdomain assists in the triple helix formation, the N5 subdomain of the a3 chain is suggested to be involved in the assembly of microfibrils and the C5 subdomain of the a3 chain is important for mature microfibril formation and is immediately cleaved off after secretion [23], [24], [25], [26]. Interestingly, part of the C5 domain of the a3 chain is also known as endothrophin, a molecule with potent pro-tumorigenic signaling capacity [5], [9], [10], [11].
While the importance of COL6 in cancer is well established less is known regarding the COL6 biomarker potential. Due to the loss of COL6 homeostasis in the tumor, pathologically relevant COL6 fragments may be generated and released into the circulation. These specific COL6 fragment could potentially be used as cancer biomarkers in the clinical setting.
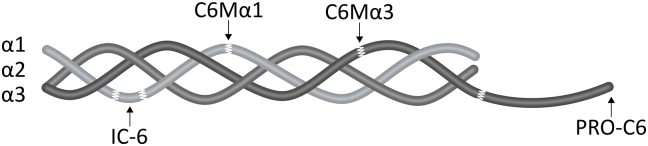
The aim of this study was to evaluate the potential of four biomarkers targeting unique peptide-epitopes on COL6 in serum from patients with various solid tumors in comparison with healthy controls. Moreover, the aim was to analyze the association between turnover of different COL6-chains (COL6a1 and COL6a3) in the same patient. An overview of the four biomarkers and their specific target is given in Figure 1 and Table 1.
Figure 1.
Schematic illustration of s type VI collagen (COL6) tetramer consisting of a1, a2, and a3 heterotrimers and with illustrations of the COL6 biomarker targets (C6Ma1, C6Ma3, IC-6 and PRO-C6) measured in serum. Details of the biomarkers can be found in Table 1.
Table 1.
Type VI collagen (COL6) serum biomarker overview
| Name | Description | Amino acid sequence |
|---|---|---|
| C6Ma1 | MMP-generated epitope on a1 chain | 573’.YRGPEGPQGP’581 |
| C6Ma3 | MMP-generated epitope on a3 chain | 2279’GPKGGIGNRG.’2288 |
| PRO-C6 | C-terminal of a3 chain (endothrophin) | 3168’KPGVISVMGT’3177 |
| IC-6 | Internal epitope on a1 chain | 216’ADWGQSRDAEEAISQ’230 |
Methods
Study Subjects
Serum samples from cancer patients (n = 65) were supplied by Asterand (Detroit, MI, USA). Serum samples from the healthy controls (n = 13) were supplied by Valley BioMedical (Winchester, VA, USA). Samples were collected after informed consent and approval by the local Ethics Committee and in compliance with the Helsinki Declaration of 1975. All serum samples from cancer patients were collected prior to resection. According to the suppliers, samples were collected, processed and stored in a similar fashion. All analyses were performed blindly. An overview of the included subjects is shown in Table 2.
Table 2.
Subject characteristics.
| Group | No. of patients | Stage |
Gender, |
Age, years, |
||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Unk. | % female | median (range) | ||
| Breast cancer | 8 | - | 6 | 2 | - | - | 88% | 57 (51–68) |
| Colon cancer | 7 | - | 3 | 4 | - | - | 86% | 63 (49–72) |
| Gastric cancer | 7 | 3 | 1 | 2 | 1 | - | 43% | 65 (58–81)⁎ |
| Malignant melanoma | 4 | 1 | 2 | 1 | - | - | 25% | 58 (43–64) |
| NSCLC | 10 | 4 | 2 | 3 | - | 1 | 30% | 58 (47–80) |
| SCLC | 7 | 1 | 2 | 3 | 1 | - | 29% | 58 (46–82) |
| Ovarian cancer | 8 | 1 | 2 | 5 | - | - | 100% | 57 (37–74) |
| Pancreas cancer | 4 | 1 | - | 1 | - | 2 | 75% | 66.5 (56–69) |
| Prostate cancer | 10 | 1 | 9 | - | - | - | 0% | 63 (57–72)⁎ |
| Healthy controls | 13 | - | - | - | - | - | 23% | 53 (39–63) |
Significantly different (P < .05) from healthy controls (Kruskal-Wallis test adjusted with Dunn's test for comparing each cancer group to the healthy controls). NSCLC: Non-small cell lung cancer; SCLC: Small cell lung cancer.
Measurements of COL-6 Fragments in Serum
Four different COL-6 protein fragments (biomarkers) were measured in serum by well-characterized and validated competitive ELISAs, according to manufacturer's instructions (Nordic Bioscience, Herlev, Denmark). The specific C6Ma1, C6Ma3 and IC-6 target epitopes were identified by mass spec analysis (Nordic Bioscience, Herlev, Denmark) and PRO-C6 was identified by sequence analysis of the C-terminal of Col6a3 according to Uniprot database [27]. In brief, the biomarkers ware assayed as follows: a 96-well streptavidin-plates were coated with biotinylated synthetic target-peptide dissolved in assay buffer. The plate then incubated for 30 minutes at 20°C. The plate was washed five times in 20 mM Tris, 50 mM NaCl, pH 7.2 (wash-buffer). Then, 20 μL of peptide calibrator or sample was added to the appropriate wells followed immediately by the addition of either 100 μL of a Horse Radish Peroxidase (HRP)-conjugated target-specific monoclonal antibody or 100 μL of an unconjugated target-specific monoclonal antibody. The plate was incubated for 1 hour at 20°C or overnight at 4°C, depending on the assay and was then washed five times in wash-buffer. For the assays using unconjugated target-specific monoclonal antibodies, 100 μL of HRP-conjugated secondary IgG antibody (Thermo Scientific, Waltham, MA, USA; cat. #31437) diluted in assay buffer was added and the plate incubated for an additional hour and then washed five times in wash-buffer. Finally, 100 μL Tetramethylbenzidine (Kem-En-Tec Diagnostics, Taastrup, Denmark) was added and the plates incubated for 15 minutes at 20°C in darkness before adding 100 μL stopping solution 1% H2SO4 to stop the reaction. The optical density (OD) of each well was measured at 450 nm with 650 nm as reference.
Statistical Analysis
Biomarker levels measured in serum from controls were compared to individual patient groups by a Kruskal-Wallis test adjusted for multiple comparisons with Dunn's test. Tumor stages (1 + 2 vs 3 + 4) and gender (not shown) were compared by the Mann–Whitney test. Diagnostic accuracy (controls vs ‘all cancers’) was evaluated by calculating the area under the receiver operating characteristics (AUROC) curve for each biomarker. The correlation between the individual biomarkers and biomarkers and age (not shown) were evaluated by Spearman's correlation coefficient (r). Statistical analyses were performed using MedCalc Statistical Software v.12 (Ostend, Belgium). Results were considered statistically significant if P < .05.
Results
Specific Fragments of COL6a1 and COL6a3 are Elevated in Serum from Patients with Various Cancer Indications Compared to Healthy Controls
Serum levels of four different COL6 fragments (see Table 1/Figure 1 for details) were measured by ELISA in healthy controls and in patients with various solid tumor types. To exclude that the biomarker levels were driven by age or gender, the four COL6 fragments were correlated with age and evaluated according to gender (data not shown). No association was found between the COL6 fragments and age or gender.
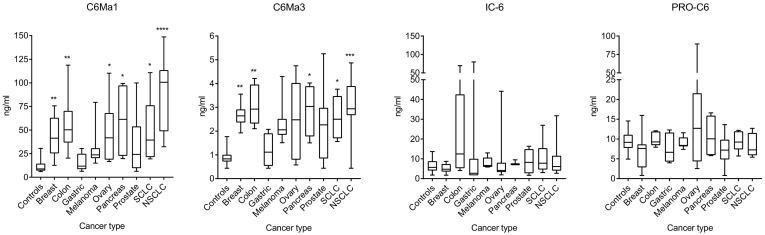
As shown in Figure 2, overall the two MMP-generated fragments (C6Ma1 and C6Ma3) were significantly elevated in most of the different cancer indications compared to controls. In contrast, the two other COL6 fragments (IC-6 and PRO-C6) were not statistically different from controls, however few patients presented with high biomarker levels (e.g. colon cancer for IC-6 and ovarian cancer for PRO-C6). In detail, C6Ma1 was significantly elevated in patients with cancer of the breast, colon, ovary, pancreas and lung (SCLC and NSCLC) compared to controls. No significant difference was seen for patients with gastric cancer, malignant melanoma and prostate cancer, albeit the median levels were higher in both melanoma and prostate cancer patients. Similar to C6Ma1, C6Ma3 was significantly elevated in patients with cancer of the breast, colon, pancreas and lung (SCLC and NSCLC) compared to controls. No significant difference was seen for patients with gastric cancer, ovarian cancer, malignant melanoma and prostate cancer, albeit median levels were higher in both melanoma, ovarian and prostate cancer patients. For both C6Ma1 and C6Ma3 the patient-to-patient variation is relatively high and in absolute numbers (ng/ml) C6Ma1 was more abundant in serum than C6Ma3, both in healthy controls and in cancer patients. IC-6 and PRO-C6 showed similar absolute abundancy.
Figure 2.
Serum levels (ng/ml) of the four different type VI collagen (COL6) fragments (see Table 1 for details) in healthy controls and in patients with various solid tumor types. Biomarker levels from controls were compared to individual patient groups by a Kruskal-Wallis test adjusted for multiple comparisons with Dunn's test. *P < .05, **P < .01, ***P < .001, ****P < .0001. SCLC: small cell lung cancer; NSCLC: non-small cell lung cancer.
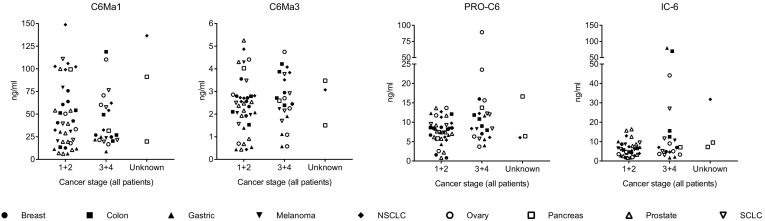
Next, the levels of the four COL6 fragments were compared according to disease stage (TNM stage), by dividing the cancer patients into early stage (1 + 2) or late stage (3 + 4) cancer at time of diagnosis. No significant difference was detected between early and late stage for any of the markers (Figure 3). Still, there was a trend towards elevated levels in late stage for PRO-C6 (P = .072) and IC-6 (P = .177), whereas C6Ma1 (P = .45) and C6Ma3 (P = .79).
Figure 3.
Serum levels (ng/ml) of four different type VI collagen (COL6) fragments measured in patients with various solid tumor types according to early (1 + 2) vs late (3 + 4) stage of disease as well as three patients with unknown stage. Each tumor indication has been given its own symbol. Biomarker levels were compared by the Mann–Whitney test. No significant differences could be detected. SCLC: small cell lung cancer; NSCLC: non-small cell lung cancer.
MMP-Generated COL-6 Fragments (C6Ma1, C6Ma3) Correlate when Measured in Serum
All four COL6 fragments were compared pairwise and evaluated by Spearman correlation coefficient, r. The analyses included both the healthy control and the cancer patients. Only the two MMP-generated fragments C6Ma1 and C6Ma3 were highly correlated (r: 0.739, P < .0001) when compared pairwise (Table 3). A trend was also seen towards a weak correlation between biomarkers of the same alpha-chain (C6Ma3 vs PRO-C6, r: 0.216, P = .058 and C6Ma1 vs IC-6, r: 0.207, P = .058).
Table 3.
Correlations between the four COL6 serum biomarkers.
| Biomarkers | Spearman, r | P |
|---|---|---|
| C6Ma1 vs C6Ma3 | 0.739 | <.0001 |
| C6Ma3 vs PRO-C6 | 0.216 | .058 |
| C6Ma1 vs IC-6 | 0.207 | .088 |
| C6Ma3 vs IC-6 | 0.163 | .179 |
| IC-6 vs PRO-C6 | 0.123 | .313 |
| C6Ma1 vs PRO-C6 | 0.042 | .715 |
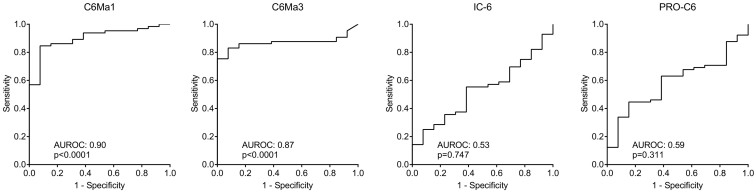
MMP-Generated COL-6 Fragments can Discriminate Between Cancer Patients and Healthy Controls (Diagnostic Power)
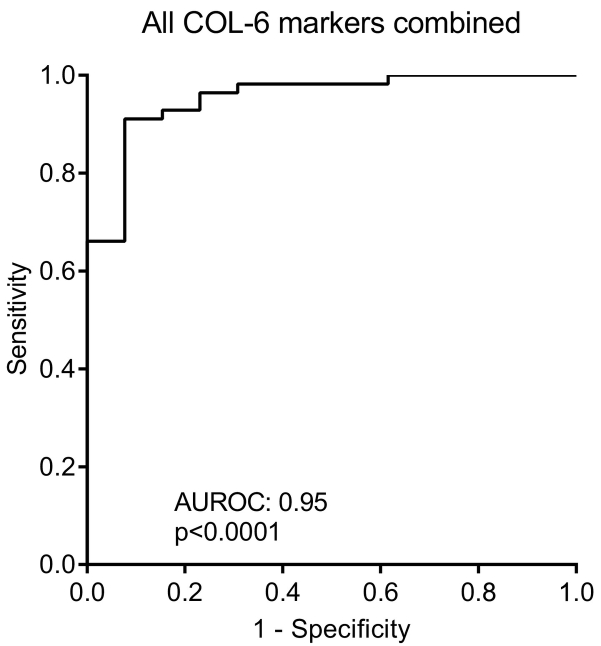
To evaluate the diagnostic power of the COL6 fragments the AUROC was calculated. As shown in Figure 4, the diagnostic power of C6Ma1 and C6Ma3 was highly significant with an AUROC of 0.90 (P < .0001) and of 0.87 (P < .0001), respectively when comparing healthy control to patients with cancer. In contrast, neither IC-6 nor PRO-C6 had any diagnostic power with an AUROC of 0.59 (P = .311) and 0.53 (P = .747), respectively. As the biomarkers did correlate with r: 0.739, P < .0001 to the best (Table 3), the additive effect on the diagnostic power was investigated by combining all four markers. When combining all biomarkers, a diagnostic power of 0.95 (P < .0001) was achieved (Figure 5), indicating that the combination of all biomarkers leads to almost complete discrimination between healthy controls and cancer patients.
Figure 4.
Diagnostic power evaluated by receiver operating characteristics (ROC) curve analysis for the ability of the four serum COL-6 fragments to separate healthy controls from patients with cancer. AUROC, area under the ROC curve.
Figure 5.
Diagnostic power evaluated by receiver operating characteristics (ROC) curve analysis for the ability of the four serum COL-6 fragments combined to separate healthy controls from patients with cancer. AUROC, area under the ROC curve.
Discussion
While the importance of COL6 in cancer is well established, much less is known regarding the biomarker potential. In the present study four specific COL6 fragments were measured in serum as potential biomarkers for cancer. Interestingly, MMP-generated COL6 fragments (C6Ma1, C6Ma3) were the best biomarkers of the four COL-6 fragments evaluated. Both C6Ma1 and C6Ma3 were significantly elevated across several different cancer indications when compared to controls, had a relatively large patient-to-patient variation within each patient-group and showed promising diagnostic accuracy. Altogether, this supports that quantifying MMP-generated COL6 remodeling/degradation have biomarker potential in cancer. Furthermore, the significant correlation between C6Ma1 and C6Ma3 indicates that both the COL6a1 and COL6a3 chains are degraded in the same patients as part of tumorigenesis. As both markers were elevated in all cancer stages, they may potentially be applied in patients with early, as well as late stage of cancer. The fact the MMP-generated collagen fragments have biomarker potential in cancer is no surprise. It has previously been established from the same cohort, and other cohorts of cancer patients, that fragments of MMP-degraded type I collagen, MMP-degraded type III collagen and MMP-degraded type IV collagen all are elevated in cancer compared to healthy controls [28], [29], [30], [31], [32], [33], [34].
To our knowledge, only few other studies have evaluated the clinical significance of measuring COL6 in serum from cancer patients. COL6a3 fragments have been found upregulated in the circulation of patients with colorectal cancer compared to healthy controls [35]. Likewise, one study focused on quantifying COL6a3 in serum from patients with pancreatic ductal adenocarcinomas and found higher levels in cases compared to controls [36]. Another study evaluated levels of COL6a1/a2 chains in serum from patients with malignant melanoma and reached similar conclusions [37]. This is however the first study to show cancer serum biomarker potential of (MMP-degraded) COL6 in serum across several cancer indications.
While this is the first study to show a link between MMP-degraded COL6 and different cancer types, several other non-oncology studies have evaluated the biomarker potential of MMP-degraded COL6. MMP-degraded COL6 has been found to be increased in liver fibrosis [38], to correlate with muscle mass and anabolic response during training in young men [39] and to associate with exacerbations [40], predict lung function changes [41] and predict mortality [42] in chronic obstructive pulmonary disease (COPD). C6Ma1 has also been found relevant for idiopathic pulmonary fibrosis (IPF), with longitudinal changes being significantly higher in patients with progressive disease [43].
Taken together, these findings support that MMP-mediated COL6 remodeling is an important component of tumorigenesis, but also that it may reflect a more common fibrosis-related pathological event. For instance, it has been reported that C6Ma1 is also been found in patients with IPF and COPD, which clearly limits the relevance of this marker for lung cancer diagnosis [40], [41], [42], [43]. Still, MMP's are well characterized and important players in both fibrosis and cancer. Interestingly, more attention is drawn towards tumor fibrosis (desmoplasia) due to the association with a poor prognosis and lack of response to treatment in cancer patients with a severe desmoplastic reaction [44], [45]. Clearly, novel tools are needed to quantify the desmoplastic reaction in oncology, and future studies should evaluate C6Ma1 and C6Ma3 in this context. As the present study only evaluated the turnover of COL6a1 and COL6a3, future studies should evaluate the turnover of COL6a2 which has also been linked to MMP activity and hence tumorigenesis [46].
The COL6 fragments IC-6 and PRO-C6 have also been evaluated in other diseases. IC-6 has been shown to correlate with muscle mass in young subjects [39]. PRO-C6 has been more extensively studied. From a biomarker perspective serum PRO-C6 has been found associated with chronic and diabetic kidney disease [47], [48]. Likewise, in type 2 diabetic patients PRO-C6 was found to predict response to a PPAR agonist treatment [49]. PRO-C6 has also shown prognostic value in COPD [42]. Interestingly, a preliminary study has shown that high pre-treatment levels of PRO-C6 predicts outcome in patients with metastatic colorectal cancer when treated with chemotherapy [50]. These findings indicate that PRO-C6 could be prognostic in cancer, which is also supported by the borderline significant association between cancer stage and PRO-C6 levels (P = .072, when comparing early vs late stage patients). As mentioned, PRO-C6 targets the signaling molecule endothrophin. The protease(s) responsible for generating endothrophin is currently unknown, although in one study incorrectly folded type VI collagen was found in MMP-11 deficient mice [51]. Thus, studies are warranted to evaluate PRO-C6/endothrophin in the oncology setting.
While the present study is limited by a relatively small size, the quantification of COL6 remodeling seems promising. As biomarker levels are different in the analyzed tumor types, it is conceivable that the diagnostic power of C6Ma1 and C6Ma3 is driven by specific tumor types showing the highest biomarker levels (e.g. pancreatic tumors, NSCLC) and further investigations are required to elucidate how specific tumor types are more associated with COL-6 remodeling. To fully analyze the diagnostic/prognostic applicability of the COL-6 fragments used here, more patients, other types of cancer and diseases with a similar pathology (e.g. fibrosis) should be evaluated as well. The cross-sectional nature of the study is also a limiting factor in the present study, and a larger longitudinal study is therefore needed to understand the full potential of these biomarkers.
In conclusion, MMP-generated COL6 fragments (C6Ma1, C6Ma3) were elevated in serum from cancer patients compared to controls and had promising diagnostic accuracy. This supports that MMP-mediated COL6 remodeling is an important component of tumorigenesis and future studies are needed to determine biological and clinical applicability of quantifying various COL6 fragments in serum in relation to cancer.
Acknowledgments
Acknowledgements
We acknowledge the Danish Research Foundation for supporting the biomarker measurements in this study and Aisha Wardak for her contribution to handling samples measurements.
Conflict of Interest
Nicholas Willumsen, Cecilie Bager and Morten A Karsdal are employed at Nordic Bioscience involved in biomarker discovery and assay development.
Research Data for this Article
Research data for this article is available upon reasonable request to the corresponding author.
References
- 1.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karsdal M. Academic Press; 2016. Biochemistry of collagens: structure, function and biomarkers. [Google Scholar]
- 3.Karsdal MA, Nielsen SH, Leeming DJ, Langholm LL, Nielsen MJ, Manon-Jensen T, Siebuhr A, Gudmann NS, Rønnow S, Sand JM. The good and the bad collagens of fibrosis – Their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43–56. doi: 10.1016/j.addr.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Chen P, Cescon M, Bonaldo P. Collagen VI in cancer and its biological mechanisms. Trends Mol Med. 2013;19(7):410–417. doi: 10.1016/j.molmed.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Scherer PE. Endotrophin – linking obesity with aggressive tumor growth. Oncotarget. 2012;33(12):1487–1488. doi: 10.18632/oncotarget.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnoor M, Cullen P, Lorkowski J, Stolle K, Robenek H, Troyer D, Rauterberg J, Lorkowski S. Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J Immunol. 2008;180(8):5707–5719. doi: 10.4049/jimmunol.180.8.5707. [DOI] [PubMed] [Google Scholar]
- 7.Wright A, Li Y-H, Zhu C, Woodruff W, Coulter H. The differential effect of endothelial cell factors on in vitro motility of malignant and non-malignant cells. Ann Biomed Eng. 2008;36(6):958–969. doi: 10.1007/s10439-008-9489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han J, Daniel JC. Biosynthesis of type VI collagen by glioblastoma cells and possible function in cell invasion of three-dimensional matrices. Connect Tissue Res. 1995;31(2):161–170. doi: 10.3109/03008209509028404. [DOI] [PubMed] [Google Scholar]
- 9.Sherman-Baust CA, Weeraratna AT, Rangel LBA, Pizer ES, Cho KR, Schwartz DR, Shock T, Morin PJ. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell. 2003;3(4):377–386. doi: 10.1016/s1535-6108(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 10.Varma RR, Hector SM, Clark K, Greco WR, Hawthorn L, Pendyala L. Gene expression profiling of a clonal isolate of oxaliplatin-resistant ovarian carcinoma cell line A2780/C10. Oncol Rep. 2005;14(4):925–932. [PubMed] [Google Scholar]
- 11.Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115(5):1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voiles L, Lewis DE, Han L, Lupov IP, Lin T-L, Robertson MJ, Petrache I, Chang H-C. Overexpression of type VI collagen in neoplastic lung tissues. Oncol Rep. 2014;32(5):1897–1904. doi: 10.3892/or.2014.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angenendt L, Mikesch J-H, Görlich D, Busch A, Arnhold I, Rudack C, Hartmann W, Wardelmann E, Berdel WE, Stenner M. Stromal collagen type VI associates with features of malignancy and predicts poor prognosis in salivary gland cancer. Cell Oncol. 2018;41(5):517–525. doi: 10.1007/s13402-018-0389-1. [DOI] [PubMed] [Google Scholar]
- 14.Kuo HJ, Maslen CL, Keene DR, Glanville RW. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem. 1997;272(42):26522–26529. doi: 10.1074/jbc.272.42.26522. [DOI] [PubMed] [Google Scholar]
- 15.Cescon M, Gattazzo F, Chen P, Bonaldo P. Collagen VI at a glance. J Cell Sci. 2015;128(19):3525–3531. doi: 10.1242/jcs.169748. [DOI] [PubMed] [Google Scholar]
- 16.Aumailley M, Mann K, von der Mark H, Timpl R. Cell attachment properties of collagen type VI and Arg-Gly-Asp dependent binding to its alpha 2(VI) and alpha 3(VI) chains. Exp Cell Res. 1989;181(2):463–474. doi: 10.1016/0014-4827(89)90103-1. [DOI] [PubMed] [Google Scholar]
- 17.Doane KJ, Yang G, Birk DE. Corneal cell-matrix interactions: Type VI collagen promotes adhesion and spreading of corneal fibroblasts. Exp Cell Res. 1992;200(2):490–499. doi: 10.1016/0014-4827(92)90200-r. [DOI] [PubMed] [Google Scholar]
- 18.Klein G, Muller CA, Tillet E, Chu M-L, Timpl R. Collagen type VI in the human bone marrow microenvironment: A strong cytoadhesive component. Blood. 1995;86(5):1740–1748. [PubMed] [Google Scholar]
- 19.LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. 2018;11(4) doi: 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 22.Baldock C, Sherratt MJ, Shuttleworth CA, Kielty CM. The supramolecular organization of collagen VI microfibrils. J Mol Biol. 2003;330(2):297–307. doi: 10.1016/s0022-2836(03)00585-0. [DOI] [PubMed] [Google Scholar]
- 23.Ball SG, Baldock C, Kielty CM, Shuttleworth CA. The Role of the C1 and C2 A-domains in Type VI Collagen Assembly. J Biol Chem. 2001;276(10):7422–7430. doi: 10.1074/jbc.M002816200. [DOI] [PubMed] [Google Scholar]
- 24.Lamandé SR, Mörgelin M, Adams NE, Selan C, Allen JM. The C5 domain of the collagen VI alpha3(VI) chain is critical for extracellular microfibril formation and is present in the extracellular matrix of cultured cells. J Biol Chem. 2006;281(24):16607–16614. doi: 10.1074/jbc.M510192200. [DOI] [PubMed] [Google Scholar]
- 25.Aigner T, Hambach L, Söder S, Schlötzer-Schrehardt U, Pöschl E. The C5 domain of Col6a3 is cleaved off from the Col6 fibrils immediately after secretion. Biochem Biophys Res Commun. 2002;290(2):743–748. doi: 10.1006/bbrc.2001.6227. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald J, Mörgelin M, Selan C, Wiberg C, Keene DR, Lamandé SR, Bateman JF. The N-terminal N5 subdomain of the α3(VI) chain is important for collagen VI microfibril formation. J Biol Chem. 2001;276(1):187–193. doi: 10.1074/jbc.M008173200. [DOI] [PubMed] [Google Scholar]
- 27.Magrane M, Uniprot Consortium UniProt Knowledgebase: a hub of integrated protein data. Database (Oxford) 2011;2011 doi: 10.1093/database/bar009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willumsen N, Bager CL, Leeming DJ, Smith V, Christiansen C, Karsdal MA, Dornan D, Bay-Jensen A-C. Serum biomarkers reflecting specific tumor tissue remodeling processes are valuable diagnostic tools for lung cancer. Cancer Med. 2014;3(5):1136–1145. doi: 10.1002/cam4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willumsen N, Bager CL, Leeming DJ, Smith V, Karsdal MA, Dornan D, Bay-Jensen A-C. Extracellular matrix specific protein fingerprints measured in serum can separate pancreatic cancer patients from healthy controls. BMC Cancer. 2013;13:554. doi: 10.1186/1471-2407-13-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bager CL, Willumsen N, Leeming DJ, Smith V, Karsdal MA, Dornan D, Bay-Jensen AC. Collagen degradation products measured in serum can separate ovarian and breast cancer patients from healthy controls: A preliminary study. Cancer Biomark. 2015:783–788. doi: 10.3233/CBM-150520. [DOI] [PubMed] [Google Scholar]
- 31.Kehlet SN, Sanz-Pamplona R, Brix S, Leeming DJ, Karsdal MA, Moreno V. Excessive collagen turnover products are released during colorectal cancer progression and elevated in serum from metastatic colorectal cancer patients. Sci Rep. 2016;6(May):30599. doi: 10.1038/srep30599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipton A, Leitzel K, Ali SM, Polimera HV, Nagabhairu V, Marks E, Richardson AE, Krecko L, Ali A, Koestler W. High turnover of extracellular matrix reflected by specific protein fragments measured in serum is associated with poor outcomes in two metastatic breast cancer cohorts. Int J Cancer. 2018;143(11):3027–3034. doi: 10.1002/ijc.31627. [DOI] [PubMed] [Google Scholar]
- 33.Bager CL, Willumsen N, Kehlet SN, Hansen HB, Bay-Jensen A-C, Leeming DJ, Dragsbæk K, Neergaard JS, Christiansen C, Høgdall E. Remodeling of the Tumor Microenvironment Predicts Increased Risk of Cancer in Postmenopausal Women: The Prospective Epidemiologic Risk Factor (PERF I) Study. Cancer Epidemiol Biomarkers Prev. 2016;25(9):1348–1355. doi: 10.1158/1055-9965.EPI-16-0127. [DOI] [PubMed] [Google Scholar]
- 34.Willumsen N, Bager CL, Kehlet SN, Dragsbaek K, Neergaard JS, Hansen HB, Bay-Jensen A-C, Leeming DJ, Lipton A, Christiansen C. Excessive matrix metalloprotease-mediated degradation of interstitial tissue (type I collagen) independently predicts short-term survival in an observational study of postmenopausal women diagnosed with cancer. Oncotarget. 2017;5(0):52501–52510. doi: 10.18632/oncotarget.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao J, Fang C-Y, Chen S-X, Wang X-Q, Cui S-J, Liu X-H, Jiang Y-H, Wang J, Zhang Y, Yang P-Y. Stroma derived COL6A3 is a potential prognosis marker of colorectal carcinoma revealed by quantitative proteomics. Oncotarget. 2015;6(30):29929–29946. doi: 10.18632/oncotarget.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang CY, Wang J, Axell-House D, Soni P, Chu M-L, Chipitsyna G, Sarosiek K, Sendecki J, Hyslop T, Al-Zoubi M. Clinical Significance of Serum COL6A3 in Pancreatic Ductal Adenocarcinoma. J Gastrointest Surg. 2014;18(1):7–15. doi: 10.1007/s11605-013-2326-y. [DOI] [PubMed] [Google Scholar]
- 37.Burchardt ER, Hein R, Bosserhoff AK. Laminin, hyaluronan, tenascin-C and type VI collagen levels in sera from patients with malignant melanoma. Clin Exp Dermatol. 2003;28(5):515–520. doi: 10.1046/j.1365-2230.2003.01326.x. [DOI] [PubMed] [Google Scholar]
- 38.Veidal SS, Karsdal MA, Vassiliadis E, Nawrocki A, Larsen MR, Nguyen QHT, Hägglund P, Luo Y, Zheng Q, Vainer B. MMP mediated degradation of type VI collagen is highly associated with liver Fibrosis - Identification and validation of a novel biochemical marker assay. PLoS One. 2011;6(9):1–9. doi: 10.1371/journal.pone.0024753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nedergaard A, Sun S, Karsdal MA, Henriksen K, Kjær M, Lou Y, He Y, Zheng Q, Suetta C. Type VI collagen turnover-related peptides-novel serological biomarkers of muscle mass and anabolic response to loading in young men. J Cachexia Sarcopenia Muscle. 2013;4(4):267–275. doi: 10.1007/s13539-013-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sand JMB, Knox AJ, Lange P, Sun S, Kristensen JH, Leeming DJ, Karsdal MA, Bolton CE, Johnson SR. Accelerated extracellular matrix turnover during exacerbations of COPD. Respir Res. 2015;16(1):69. doi: 10.1186/s12931-015-0225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leeming DJ, Byrjalsen I, Sand JMB, Bihlet AR, Lange P, Thal-Singer R, Miller BE, Karsdal MA, Vestbo J, Vestbo J. Biomarkers of collagen turnover are related to annual change in FEV1 in patients with chronic obstructive pulmonary disease within the ECLIPSE study. BMC Pulm Med. 2017;17(1):164. doi: 10.1186/s12890-017-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sand JMB, Leeming DJ, Byrjalsen I, Bihlet AR, Lange P, Tal-Singer R, Miller BE, Karsdal MA, Vestbo J. High levels of biomarkers of collagen remodeling are associated with increased mortality in COPD - results from the ECLIPSE study. Respir Res. 2016;17(1):125. doi: 10.1186/s12931-016-0440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell A-M, Braybrooke R, Molyneaux PL, McKeever TM, Wells AU, Flynn A. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med. 2015;2600(15):1–11. doi: 10.1016/S2213-2600(15)00048-X. [DOI] [PubMed] [Google Scholar]
- 44.DeClerck YA. Desmoplasia: a response or a niche? Cancer Discov. 2012;2(9):772–774. doi: 10.1158/2159-8290.CD-12-0348. [DOI] [PubMed] [Google Scholar]
- 45.Cannon A, Thompson C, Hall BR, Jain M, Kumar S, Batra SK. Desmoplasia in pancreatic ductal adenocarcinoma: insight into pathological function and therapeutic potential. Genes Cancer. 2018;9(3–4):78–86. doi: 10.18632/genesandcancer.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freise C, Erben U, Muche M, Farndale R, Zeitz M, Somasundaram R, Ruehl M. The alpha 2 chain of collagen type VI sequesters latent proforms of matrix-metalloproteinases and modulates their activation and activity. Matrix Biol. 2009;28(8):480–489. doi: 10.1016/j.matbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen DGK, Fenton A, Jesky M, Ferro C, Boor P, Tepel M, Karsdal MA, Genovese F, Cockwell P. Urinary endotrophin predicts disease progression in patients with chronic kidney disease. Sci Rep. 2017;7(1):17328. doi: 10.1038/s41598-017-17470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fenton A, Jesky MD, Ferro CJ, Sørensen J, Karsdal MA, Cockwell P, Genovese F. Serum endotrophin, a type VI collagen cleavage product, is associated with increased mortality in chronic kidney disease. PLoS One. 2017;12(4):1–14. doi: 10.1371/journal.pone.0175200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karsdal MA, Henriksen K, Genovese F, Leeming DJ, Nielsen MJ, Riis BJ, Christiansen C, Byrjalsen I, Schuppan D. Serum endotrophin identifies optimal responders to PPARγ agonists in type 2 diabetes. Diabetologia. 2017;60(1):50–59. doi: 10.1007/s00125-016-4094-1. [DOI] [PubMed] [Google Scholar]
- 50.Kehlet SN, Høye A, Boisen MK, Johansen JS, Karsdal MA, Willumsen N, Erler J. Prognostic evaluation of a new class of liquid biopsy biomarkers in patients with metastatic colorectal cancer: Using the tumor microenvironment as a source of protein biomarkers. J Clin Oncol. 2018;36(15_suppl):3588. [Google Scholar]
- 51.Motrescu ER, Blaise S, Etique N, Messaddeq N, Chenard M-P, Stoll I, Tomasetto C, Rio M-C. Matrix metalloproteinase-11/stromelysin-3 exhibits collagenolytic function against collagen VI under normal and malignant conditions. Oncogene. 2008;27(49):6347–6355. doi: 10.1038/onc.2008.218. [DOI] [PubMed] [Google Scholar]