Abstract
Diffusion-weighted magnetic resonance imaging (DWI) has been reported to be useful for the assessment of lung cancer staging. It is uncertain whether DWI is more accurate for the response evaluation of chemotherapy and/or radiotherapy compared to computed tomography (CT). The purpose of this study is to compare the response evaluation of DWI for chemotherapy and/or radiotherapy to recurrent tumors of lung cancer with that of CT which is a standard tool in RECIST (Response Evaluation Criteria in Solid Tumours). Forty-one patients who agreed to this project and had CT scan and DWI examinations within a month of each other every six months for at least 2 years after pulmonary resection of primary lung cancer were enrolled in this study. Of the patients, 24 patients had metastases or recurrences, and CT and DWI were performed for assessment of the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions. They were followed up for at least two years after the relapse. The response evaluation by CT using RECIST were PR in five patients, SD in two, and PD in the remaining 17 patients. On the other hand, the response evaluation by DWI were CR in four patients, PR in two patients, SD in one, and PD in the remaining 17 patients. Follow-up studies revealed the response evaluation by DWI were correct. Functional evaluation of DWI is better than that of CT for the response evaluation of chemotherapy and/or radiotherapy to recurrent tumors of lung cancer.
Introduction
Diffusion-weighted magnetic resonance imaging (DWI) makes use of the random, translational motion, or so-called Brownian movement, of water molecules in biologic tissue [1]. DWI has primarily been used in brain imaging, mainly for the assessment of acute ischemic stroke, demyelinating diseases and intracranial tumors [2]. Recently, researchers have been utilizing DWI to detect the restricted diffusion of water molecules in the body. It has been reported that DWI can be useful for differential diagnosis of pulmonary nodules and masses, and assessment of N factor, M factor, and stage of lung cancer [3], [4], [5], [6]. The diagnostic ability of DWI is same or more than that of FDG-PET/ CT for the assessment of pulmonary nodules and masses [7], [8]. The diagnostic ability of magnetic resonance imaging (MRI) to diagnose cancer has developed and two articles of meta-analysis reported that DWI was effective for the evaluation of N factor of lung cancer [9], [10]. Meta-analysis of MRI by Peerlings et al. [9] revealed a high diagnostic capability and that the sensitivity was 0.87, the specificity 0.88 for nodal assessment in the non-small cell lung cancer.
Furthermore, DWI may be useful for the response evaluation of chemotherapy and/or radiotherapy to recurrent or metastatic tumors of lung cancer. Although RECIST (Response Evaluation Criteria in Solid Tumours) is an international reproducible high evaluation method for the response evaluation of chemotherapy and/or radiotherapy to neoplasm [11], RECIST criteria have limitations, particularly in assessing the activity of newer cancer therapies that stabilize the disease [12]. RECIST has several problems: (1) Only maximum diameters of tumors are used for analysis: (2) There are no metabolic information, nor functional information; (3) volume of neoplasm was not include: (4) CT number or cavity lesions are not included: (5) Part-solid nodule are evaluated under lung window. On the other hand, MRI, with its lack of ionizing radiation, high soft tissue contrast and good spatial resolution, is a useful application for tumor detection and staging of malignancies and could overcome the limits of fluoro-2-deoxy-glucose positron emission tomography-computed tomography (FDG-PET/CT) [13].
In this paper, we compared the response evaluation of DWI for chemotherapy and/or radiotherapy to the recurrent tumors of lung cancer with that of CT.
Materials and Methods
The study was conducted in a double-blind manner: the surgeons who performed the operations had no influence on the radiological decisions of CT or MRI examinations. The study protocol for examining DWI and CT for the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer was approved by the ethical committee of Kanazawa Medical University (the approval number: No.189). Patients included in the study were adults (above 20 years) who agreed to this project and whose informed consent could be obtained. Patients excluded in this study were adults who had metal or pacemakers in their bodies or tattoos on their skin because of contraindication in MRI examinations. After discussing the risks and benefits of the examinations with their surgeons, we obtained written informed consents for CT and MRI examinations from each patient.
TNM classification and the lymph node stations of lung cancer were classified according to the definition of UICC 7 [14]. Two radiological specialists who were experienced in evaluating CT and MR images and blinded to the clinical data and unaware of the pathological or surgical findings interpreted CT and MRI data.
The response evaluation of chemotherapy and/or radiotherapy to lung cancer was performed based on RECIST1.1 criteria. The response evaluation as assessed by MRI with DWI was classified as follows; complete response (CR) was defined as the disappearance of all tumor foci in DWI; a partial response (PR) was at least a 30% decrease in the tumor diameter in DWI; progressive disease (PD) was at least a 20% increase in the sum of all tumor diameters from the smallest tumor size in DWI; and stable disease (SD) was neither a PR nor PD in DWI. Patients with CR or PR on MRI were considered to be responders, and the patients with SD or PD were considered to be non-responders.
In the first evaluation session, the radiological specialists assessed CT data independently. In the second evaluation session, the specialists assessed MRI data independently. In the third evaluation session, a consensus was reached if there were any differences in opinion.
Magnetic Resonance Imaging (MRI)
All MR images were obtained with a 1.5 T superconducting magnetic scanner (Magnetom Avanto; Siemens, Erlangen, Germany) with two anterior six-channel body phased-array coils and two posterior spinal clusters (six-channels each). The conventional MR images consisted of a coronal T1-weighted spin-echo sequence (repetition time [TR] ms/echo time [TE] ms/excitations, 720/20/1) and coronal and axial T2-weighted fast spin-echo sequences (6700/130/1). DWI using a single-shot echo-planar technique were performed under SPAIR (spectral attenuated inversion recovery) with respiratory triggered scan with the following parameter: TR/TE/flip angle, 3000-4500/65/90; diffusion gradient encoding in three orthogonal directions; b value = 0 and 800 s/mm2; receiver bandwidth, 2,442 Hz/Px; voxel size, 2.7×2.7×6.0mm; field of view, 350 mm; matrix size, 128×128; section thickness, 6 mm; section gap, 0 mm; and number of excitations. Whole-body DWI (WB-DWI) was performed using single-shot echo planar imaging (EPI) sequence with phased-array coils including head, neck and body matrix coils. The sequences employed were T1-weighted spin echo sequences. Its total examination time was about 30 minutes. After image reconstruction, a 2-dimensional (2D) round or elliptical region of interest (ROI) was drawn on the lesion which was detected visually on the apparent diffusion coefficient (ADC) map with reference to T1-weighted or CT image. Areas with necrosis were excluded from the ADC measurement. The optimal cutoff value (OCV) of ADC for diagnosing malignancy in DWI was determined to be 1.70×10-3mm2/sec using the receiver operating characteristics (ROC) curve as previously reported [6]. Metastatic lesions with an ADC value of the same or less than the OCV was defined as positive. Lesions with ADC value of more than the OCV or those that could not be detected on DWI were defined as negative.
Chest CT, abdominal CT, brain MRI or FDG-PET/CT were performed for the evaluation. FDG-PET/CT was performed with a dedicated PET camera (SIEMENS Biograph Sensation 16, Erlangen Germany). All patients fasted for 6 hours before the scanning. The OCV of maximum standardized uptake value (SUVmax) for diagnosing malignancy in FDG-PET/CT was determined to be 4.45 using the ROC curve as previously reported [6].
Statistics
Statistical analysis was performed using StatView for Windows (Version 5.0; SAS Institute Inc. Cary, NC, USA). The data is expressed as the mean±standard deviation. The sensitivity, specificity, and accuracy of CT versus DWI for the response evaluation of chemotherapy and/or radiotherapy by using McNemar test. A P value of <.05 was considered statistically significant.
Results
Forty-one patients who agreed to this project and had CT scan and DWI examinations within a month of each other every six months for at least 2 years after pulmonary resection for primary lung cancer were enrolled in this study in the period from June 2012 to August 2016. There were no patients who left the study. The post-operative follow-up revealed the relapse or the enlarging of the tumors in 24 patients, whereas 17 patients had no evidence of relapse. All 24 patients who relapsed underwent chemotherapy and/or radiotherapy (Table 1): chemotherapy was added to the treatment of 11 patients, chemoradiotherapy in 11 and radiotherapy in 2 patients. There were 12 adenocarcinomas, 9 squamous cell carcinomas, 1 large cell neuroendocrine carcinoma (LCNEC), 1 adenosquamous carcinoma and 1 carcinoid. Seventeen patients were male and 7 were female. Their mean age was 71 years old (range 55 to 85). There were 6 pathological Stage IA (pStage IA), 7 pStage IB, 1 pStageIIA, 5 pStage IIB, 5 pStage IIIA. These patients were followed for at least two years after their CT and DWI examinations.
Table 1.
Patient characteristics
| Sex | Male | 17 |
| Female | 7 | |
| Cell type | Adenocarcinoma | 12 |
| Squamous cell carcinoma | 9 | |
| Adenosquamous carcinoma | 1 | |
| Large cell neuroendocrine carcinoma | 1 | |
| Carcinoid | 1 | |
| Pathological stage | IA | 6 |
| I B | 7 | |
| II A | 1 | |
| II B | 5 | |
| IIIA | 5 |
The response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer are presented in Figure 1, Figure 2, Figure 3.
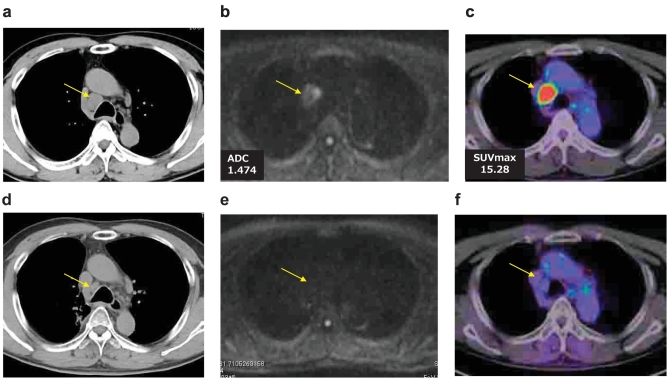
Figure 1.
Case 1.
A 66-year-old male who was underwent a right upper lobectomy and dissection of lymph nodes for pulmonary adenocarcinoma (pStage IB), Growth of lymph node station #4R was detected by CT (a), DWI (b) and FDG-PET/CT (c). It was diagnosed as the recurrence by EBUS-TBNA. Response evaluation by CT(d), DWI (e) or FDG-PET/CT(f) after chemoradiotherapy, CT showed PR, but DWI showed CR with no diffusion of the #4R lymph node. FDG-PET/CT also showed CR with little FDG accumulation of the lymph node by the treatment.
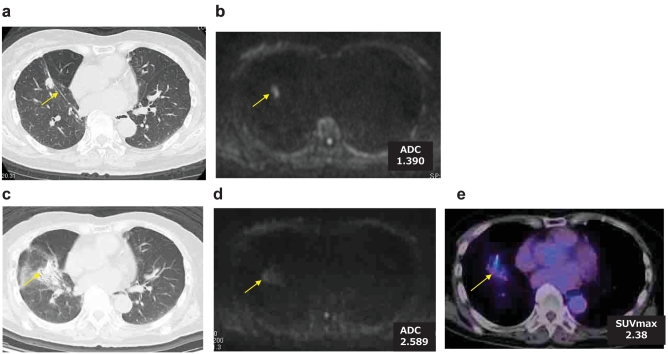
Figure 2.
Case 2.
A 76-year-old female who was underwent a right upper lobectomy and dissection of lymph nodes for pulmonary adenocarcinoma. Later partial resection of right lower lobe was performed for second primary adenocarcinoma. Local recurrence was detected by CT(a) and DWI (b). Radiation was added to the local recurrence. Although CT (c) showed PR, DWI (d) showed CR. FDG-PET/CT(e) showed moderate accumulation of FDG. Follow-up study showed CR.
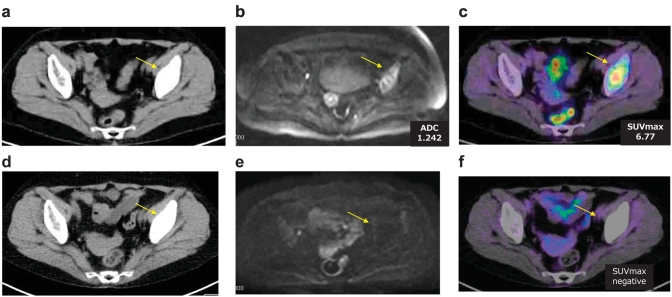
Figure 3.
Case 3.
A 72-year-old female who underwent a right lower lobectomy and dissection of lymph nodes for pulmonary adenocarcinoma (pStage IIB). Metastasis to left ilium had detected as a osteoplastic change by CT(a), as a diffusion decrease by DWI(b) and an FDG accumulation by FDG-PET/CT(C). Pathological examination by percutaneous biopsy of the left ilium lesion revealed bone metastasis from the lung cancer. The patient was treated with Gefitinib. Response evaluation by CT(d) showed SD, but DWI(e) showed CR with no diffusion decrease, and also FDG-PET/CT(f) showed CR with little FDG accumulation of the lesion.
Case 1. (Figure 1)
A 66-year-old male who underwent a right upper lobectomy and dissection of lymph nodes for pulmonary adenocarcinoma (pStage IB), Growth of lymph node station #4R was detected by CT, DWI and FDG-PET/CT. It was diagnosed as the recurrence by EBUS-TBNA (Endobronchial ultrasound-guided transbronchial needle aspiration). Radiochemotherapy (RT 50Gy + CBDCA + PTX) was given to the patient. Response evaluation by CT was revealed as partial response (PR) because of tumor size reduction, but DWI or FDG-PET/CT revealed complete response (CR) because DWI showed CR with no diffusion of the #4R lymph node and PET-CT also showed little FDG accumulation of the lymph node. The recurrent lesion had not relapsed at the 2-year follow-up.
Case 2. (Figure 2)
A 76-year-old female who underwent a right upper lobectomy and dissection of lymph nodes for pulmonary adenocarcinoma. Later partial resection of right lower lobe was performed for a second primary adenocarcinoma. Local recurrence was detected by CT and DWI. Radiation was added to the local recurrence. Although CT showed PR after the radiation, DWI showed CR because of no diffusion on the local recurrence. PET-CT showed a moderate accumulation of FDG. Follow-up study finally showed CR.
Case 3. (Figure 3)
A 72-year-old female who underwent a right lower lobectomy and dissection of lymph nodes for pulmonary adenocarcinoma (pStage IIB). Metastasis to the left ilium was detected as a osteoplastic change by CT, as a diffusion decrease by DWI and an FDG accumulation by FDG-PET/CT. Pathological examination by percutaneous biopsy of the left ilium lesion revealed bone metastasis from the lung cancer. The patient was treated with Gefitinib. Response evaluation by CT showed stable disease(SD), but DWI showed CR with no diffusion of the lesion, and also PET-CT showed CR with little FDG accumulation of the lesion. The recurrence had not relapsed at the 2-year follow-up.
The response evaluation by CT using RECIST were PR in five patients, SD in two, and PD in the remaining 17 patients (Table 2). For the response evaluation by DWI, metastatic lesions with no diffusion in DWI or ADC value of more than 1.70×10-3mm2/sec after chemotherapy/radiotherapy were judged as CR. The response evaluation by DWI were CR in four patients, PR in two, SD in one, and PD in the remaining 17 patients (Table 2). All these 4 patients had had CR classification by response evaluation of DWI after chemotherapy/radiotherapy was determined PR in 3 patients and SD in a patient in CT. A follow-up study two years after chemotherapy/radiotherapy revealed response evaluation by DWI were correct. There were no patients who left the study, and there were ten patients who died during the postsurgical follow-up period, and three patients died due to lung cancer within two years.
Table 2.
Response evaluation to the therapy for recurrence or metastasis of lung cancer
| by DWI |
Total cases | |||||
|---|---|---|---|---|---|---|
| CR | PR | SD | PD | |||
| by CT | CR | |||||
| PR | 3 | 2 | 5 | |||
| SD | 1 | 1 | 2 | |||
| PD | 17 | 17 | ||||
| Total cases | 4 | 2 | 1 | 17 | 24 | |
The sensitivity, specificity, and accuracy of CT versus DWI for the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer by using McNemar test (Table 3, Table 4, Table 5). The sensitivity 100% (20/20) of DWI for the response evaluation of chemotherapy and/or radiotherapy was same as 100% (20/20) of CT. The specificity 100% (4/4) of DWI for the response evaluation of chemotherapy and/or radiotherapy was likely to be better than 0% (0/4) of CT, but there is no difference (P = .067). The accuracy 100% (24/24) of DWI or the response evaluation of chemotherapy and/or radiotherapy was likely to be better than 83% (20/24) of CT, but there is no difference (P = .067).
Table 3.
Comparison of sensitivity of CT versus DWI for the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer by using McNemar test
| CT |
Total |
|||
|---|---|---|---|---|
| True-positive | False-negative | |||
| DWI | True-positive | 20 | 0 | 20 |
| False-negative | 0 | 0 | 0 | |
| Total | 20 | 0 | 20 | |
Ν.S.
Table 4.
Comparison of specificity of CT versus DWI for the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer by using McNemar test
| CT |
Total |
|||
|---|---|---|---|---|
| True-negative | False-positive | |||
| DWI | True-negative | 0 | 4 | 4 |
| False-positive | 0 | 0 | 0 | |
| Total | 0 | 4 | 4 | |
P=.067.
Table 5.
Comparison of accuracy of CT versus DWI for the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer by using McNemar test
| CT |
Total |
|||
|---|---|---|---|---|
| Correct | Incorrect | |||
| DWI | Correct | 20 | 4 | 24 |
| Incorrect | 0 | 0 | 0 | |
| Total | 20 | 4 | 24 | |
P=.067.
Discussion
This preliminary report showed that DWI was more accurate than CT for the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer. Although CT scan showed inflammatory shadow or remaining swelling of lymph nodes after treatment, DWI may be able to judge if there is no residual cancer by no decreased diffusion in the lesion. In DWI, recurrent bone lesions were represented as an area with an ADC of 1.70 × 10-3mm2/sec or lower. DWI is useful for not only quantitative evaluation but also qualitative evaluation which is not determined by tumor size for the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer. DWI has an advantage of functional information of neoplasm [15]. Whole body (WB)-MRI seems to be a valid alternative method compared to FDG-PET/CT in oncology [16]. Anatomic imaging alone using standard WHO, RECIST, and RECIST 1.1 criteria have limitations, particularly in assessing the response evaluation of cancer therapies where the volume of cancers was stabilized.
DWI and FDG-PET/CT show similar diagnostic accuracy for predicting pathological complete response to neoadjuvant chemotherapy in breast cancer patients [17]. The combined use of DWI and FDG-PET/CT has the potential to improve specificity in predicting a pathological complete response [17]. New functional imaging techniques, such as PET, DWI and dynamic contrast-enhanced MRI (DCE-MRI) may potentially improve the assessment of treatment response [18]. Adding DWI to T2 weighted imaging (T2WI) is helpful for detecting viable tumors after neoadjuvant chemoradiotherapy compared with T2WI alone or FDG-PET/CT in patients with locally advanced rectal cancer [19].
DWI may have better potential than FDG-PET/CT for prediction of tumor response to therapy in NSCLC patients before chemoradiotherapy [20]. The results of DWI in combination with WB-MRI were comparable with those of integrated FDG-PET/CT [21]. ADC in DWI seems to be a promising tool for monitoring the early response to or predicting prognosis after chemotherapy of non-small cell lung cancer (NSCLC) [22], [23]. High pretreatment mean ADC of colorectal hepatic metastatic lesions were predictive of poor response to chemotherapy [24]. Most published studies showed a significant correlation between changes in ADC values and treatment response, and an increase in ADC was associated with response in most cases [25].
MRIs have several benefits. MRI involves no contrast agents and requires less time for the examination. Furthermore, MRI has no radiation exposure and is preferable for the examinations of children who would react negatively to radiation [26]. On the other hand, there are some negative points with MRIs. An MRI examination is prohibited for people who have devices such as pacemakers or tattoos on the skin. An MRI examination is loud which is uncomfortable for patients.
DWI has two limitations. First, one of benign lesions which showed restricted diffusion and lower ADC values in DWI is a pulmonary abscess with histopathological necrosis. Abscesses and thrombi impede the diffusivity of water molecules because they have a hyperviscous nature [27], [28]. The heavily impeded water mobility of pus may be caused by its high cellularity and viscosity, and shows the low ADC values [29]. These articles on the properties of abscesses and thrombi can explain false-positive results in DWI for some benign pulmonary nodules and masses with abscesses. Second, in DWI mucinous carcinomas were usually hypointense and showed higher ADC values, which could be misjudged as benign lesions in DWI [8]. Mucinous carcinomas had lower DWI signal intensity and higher ADC values than tubular adenocarcinoma in the ano-rectal region, because mucinous carcinomas had lower cellularity than tubular adenocarcinomas [30].
This study has several limitations. This study was performed at a single institution and dealt with a small number of patients. We could not get consent forms from all the patients who developed recurrent or metastatic lesions after pulmonary resection for lung cancer in the period from June 2012 to August 2016. So our sample size is limited. The demographics of our sample could have been more balanced, but we could not get more female subjects.
We believe that further research into the potential of DWI may result in a newer more powerful tool for response evaluation of chemotherapy and/or radiotherapy to various kinds of neoplasms.
In conclusion, the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer were judged using DWI as well as CT. By examining decreased diffusion, DWI is proved to be able to evaluate qualitative evaluation which is not determined by tumor size.
Acknowledgments
We are grateful to Mr. Keiya Hirata of the MRI Center, Kanazawa Medical University, for technical assistance. This study were supported partly by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Grant number: 16K10694) and by 2017 Grant-in-Aid of the Magnetic Health Science Foundation, Japan.
References
- 1.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 2.Tien RD, Felsberg GJ, Friedman H, Brown M, MacFall J. MR imaging of high-grade cerebral gliomas. Value of diffusion-weighted echoplanar plus sequences. AJR. 1994;62:671–677. doi: 10.2214/ajr.162.3.8109520. [DOI] [PubMed] [Google Scholar]
- 3.Li B, Li Q, Chen C, Guan Y, Liu S. A systematic review and meta-analysis of the accuracy of diffusion-weighted MRI in the detection of malignant pulmonary nodules and masses. Acad Radiol. 2014;21:21–29. doi: 10.1016/j.acra.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Shen G, Jia Z, Deng H. Apparent diffusion coefficient values of diffusion-weighted imaging for distinguishing focal pulmonary lesions and characterizing the subtype of lung cancer: a meta-analysis. Eur Radiol. 2016;26:556–566. doi: 10.1007/s00330-015-3840-y. [DOI] [PubMed] [Google Scholar]
- 5.Usuda K, Zhao XT, Sagawa M, Matoba M, Kuginuki Y, Taniguchi M, Ueda Y, Sakuma T. Diffusion-weighted imaging is superior to positron emission tomography in the detection and nodal assessment of lung cancers. Ann Thorac Surg. 2011;91:1689–1695. doi: 10.1016/j.athoracsur.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, Matoba M, Kuginuki Y, Taniguchi M, Ueda Y. Advantages of diffusion-weighted imaging over positron emission tomography-computed tomography in assessment of hilar and mediastinal lymph node in lung cancer. Ann Surg Oncol. 2013;20:1676–1683. doi: 10.1245/s10434-012-2799-z. [DOI] [PubMed] [Google Scholar]
- 7.Mori T, Nomori H, Ikeda K, Kawanaka K, Shiraishi S, Katahira K, Yamashita Y. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. J Thorac Oncol. 2008;3:358–364. doi: 10.1097/JTO.0b013e318168d9ed. [DOI] [PubMed] [Google Scholar]
- 8.Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, Maeda S, Matoba M, Kuginuki Y, Taniguchi M. Diagnostic performance of diffusion weighted imaging of malignant and benign pulmonary nodules and masses: comparison with positron emission tomography. Asian Pac J Cancer Prev. 2014;15:4629–4635. doi: 10.7314/apjcp.2014.15.11.4629. [DOI] [PubMed] [Google Scholar]
- 9.Peerlings J, Troost EG, Nelemans PJ, Cobben DC, de Boer JC, Hoffmann AL, Beets-Tan RG. The diagnostic value of MR Imaging in determining the lymph node status of patients with non-small cell lung cancer: A meta-analysis. Radiology. 2016;281:86–98. doi: 10.1148/radiol.2016151631. [DOI] [PubMed] [Google Scholar]
- 10.Shen G, Hu S, Deng H, Kuang A. Performance of DWI in the nodal characterization and assessment of lung cancer: A meta-analysis. Am J Roentgenol. 2016;206:283–290. doi: 10.2214/AJR.15.15032. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl. 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Xu M, Liang H, Xu L. Comparison of CT and MRI in diagnosis of cerebrospinal leak induced by multiple fractures of skull base. Radiol Oncol. 2011;45:91–96. doi: 10.2478/v10019-011-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Union Against Cancer . 7th ed. Wiley-Liss; NY: 2009. TNM classification of malignant tumours; pp. 138–146. [Google Scholar]
- 15.Kwee TC, Takahara T, Ochiai R, Koh DM, Ohno Y, Nakanishi K, Niwa T, Chenevert TL, Luijten PR, Alavi A. Complementary roles of whole-body diffusion-weighted MRI and 18F-FDG PET: the state of the art and potential applications. J Nucl Med. 2015;51:1549–1558. doi: 10.2967/jnumed.109.073908. [DOI] [PubMed] [Google Scholar]
- 16.Ciliberto M, Maggi F, Treglia G, Padovano F, Calandriello L, Giordano A, Bonomo L. Comparison between whole-body MRI and Fluorine-18-Fluorodeoxyglucose PET or PET/CT in oncology: a systematic review. Radiol Oncol. 2013;47:206–218. doi: 10.2478/raon-2013-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SH, Moon WK, Cho N, Chang JM, Im SA, Park IA, Kang KW, Han W, Noh DY. Comparison of diffusion-weighted MR imaging and FDG PET/CT to predict pathological complete response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol. 2012;22:18–25. doi: 10.1007/s00330-011-2236-x. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L, Tunariu N, Collins DJ, Blackledge MD, Riddell AM, Leach MO, Popat S, Koh DM. Response evaluation in mesothelioma: Beyond RECIST. Lung Cancer. 2015;90:433–441. doi: 10.1016/j.lungcan.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Song I, Kim SH, Lee SJ, Choi JY, Kim MJ, Rhim H. Value of diffusion-weighted imaging in the detection of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer: comparison with T2 weighted and PET/CT imaging. Br J Radiol. 2012;85:577–586. doi: 10.1259/bjr/68424021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohno Y, Koyama H, Yoshikawa T, Matsumoto K, Aoyama N, Onishi Y, Sugimura K. Diffusion-weighted MRI versus 18F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol. 2012;198:75–82. doi: 10.2214/AJR.11.6525. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Kellokumpu-Lehtinen PL, Pertovaara H, Korkola P, Soimakallio S, Eskola H, Dastidar P. Diffusion-weighted MRI in early chemotherapy response evaluation of patients with diffuse large B-cell lymphoma—a pilot study: comparison with 2-deoxy-2-fluoro-D-glucose-positron emission tomography/computed tomography. NMR Biomed. 2011;24:1181–1190. doi: 10.1002/nbm.1689. [DOI] [PubMed] [Google Scholar]
- 22.Yabuuchi H, Hatakenaka M, Takayama K, Matsuo Y, Sunami S, Kamitani T, Jinnouchi M, Sakai S, Nakanishi Y, Honda H. Non-small cell lung cancer: detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology. 2011;261:598–604. doi: 10.1148/radiol.11101503. [DOI] [PubMed] [Google Scholar]
- 23.Nunes TF, Szejnfeld D, Szejnfeld J, Kater CE, Faintuch S, Castro CH, Goldman SM. Assessment of early treatment response with DWI after CT-guided radiofrequency ablation of functioning adrenal adenomas. AJR Am J Roentgenol. 2016;207:804–810. doi: 10.2214/AJR.16.16207. [DOI] [PubMed] [Google Scholar]
- 24.Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, Husband JE. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188:1001–1008. doi: 10.2214/AJR.06.0601. [DOI] [PubMed] [Google Scholar]
- 25.Heijmen L, Verstappen MC, Ter Voert EE, Punt CJ, Oyen WJ, de Geus-Oei LF, Hermans JJ, Heerschap A, van Laarhoven HW. Tumour response prediction by diffusion-weighted MR imaging: ready for clinical use? Crit Rev Oncol Hematol. 2012;83:194–207. doi: 10.1016/j.critrevonc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Darge K, Jaramillo D, Siegel MJ. Whole-body MRI in children: current status and future applications. Eur J Radiol. 2008;68:289–298. doi: 10.1016/j.ejrad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Kwee TC, Takahara T, Ochiai R, Koh DM, Ohno Y, Nakanishi K, Niwa T, Chenevert TL, Luijten PR, Alavi A. Complementary roles of whole-body diffusion-weighted MRI and 18F-FDG PET. The state of the art and potential application. J Nucl Med. 2010;51:1549–1558. doi: 10.2967/jnumed.109.073908. [DOI] [PubMed] [Google Scholar]
- 28.Desprechins B, Stadnik T, Koerts G, Shabana W, Breucq C, Osteaux M. Use of diffusion-weighted MR imaging in differential diagnosis between intracerebral necrotic tumors and cerebral abscesses. Am J Neuroradiol. 1999;20:1252–1257. [PMC free article] [PubMed] [Google Scholar]
- 29.Ebisu T, Tanaka C, Umeda M, Kitamura M, Naruse S, Higuchi T, Ueda S, Sato H. Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging. 1996;14:1113–1116. doi: 10.1016/s0730-725x(96)00237-8. [DOI] [PubMed] [Google Scholar]
- 30.Nasu K, Kuroki Y, Minami M. Diffusion-weighted imaging findings of mucinous carcinoma arising in the ano-rectal region. Comparison of apparent diffusion coefficient with that of tubular adenocarcinoma. Jpn J Radiol. 2012;30:120–127. doi: 10.1007/s11604-011-0023-x. [DOI] [PubMed] [Google Scholar]