Abstract
Integrins are cell adhesion molecules that are composed of an alpha (α) subunit and a beta (β) subunit with affinity for different extracellular membrane components. The integrin family includes 24 known members that actively regulate cellular growth, differentiation, and apoptosis. Each integrin heterodimer has a particular function in defined contexts as well as some partially overlapping features with other members in the family. As many reviews have covered the general integrin family in molecular and cellular studies in life science, this review will focus on the specific regulation, function, and signaling of integrin α2 subunit (CD49b, VLA-2; encoded by the gene ITGA2) in partnership with β1 (CD29) subunit in normal and cancer cells. Its roles in cell adhesion, cell motility, angiogenesis, stemness, and immune/blood cell regulations are discussed. The pivotal role of integrin α2 in many diseases such as cancer suggests its potential to be used as a novel therapeutic target.
Keywords: Integrin α2, CD49b, Molecular mechanisms, Regulation, Signaling
Introduction
Cell adhesion is not only essential for all multicellular organisms to interact and coordinate within cell populations but also critical for unicellular organisms to exchange signals with their microenvironment. Adhesion receptors include integrins, the immunoglobulin superfamily, cadherins, cellular adhesion molecules (CAMs), and homing receptors.1 Among all the cell adhesion receptors, integrins are important for “maintaining the integrity of the cytoskeletal-ECM linkage,” as established in the 1970's and 1980's by the seminal data of Erkki Ruoslahti and Richard O. Hynes, the founding fathers of the integrin field.2 In vertebrates, there are 24 heterodimeric combinations of integrins composed of one of 18 alpha subunits and one of 8 beta subunits (Table 1). Each of these heterodimers binds to extracellular matrix (ECM) molecules, such as collagen, fibronectin, and laminin.3 The most common beta subunit in integrin heterodimers is β1, and it can partner with one of 11 alpha subunits (α1- α11) to play important roles in multiple types of cancer due to its contributions to cell migration and stemness.3
Table 1.
The 24 integrins known to vertebrates, classified by receptor type.
| Collagen receptors | RGD receptors | Laminin receptors | Leukocyte-specific receptors |
|---|---|---|---|
| α1β1, α2β1, α10β1, α11β1 | α5β1, α8β1, αIIβ3, αvβ1, αvβ3, αvβ5, αvβ6, αvβ8 | α3β1, α6β1, α7β1, α6β4 | α9β1, α4β1, α4β7, αEβ7, αLβ2, αMβ2, αXβ2, αDβ2 |
Both subunits of integrins are transmembrane proteins with one ectodomain, one membrane spanning region, and a cytoplasmic tail. Upon activation, they transduce biochemical signals into the cell, through downstream effector proteins, and they can also transmit inside-out signaling information. The difference among various integrin heterodimers consists of ligand specificity and their abundance in particular cell types and tissues.
Dysregulated expression of integrins alters the rates of migration of cells, which is particularly relevant to cancer development and progression. This review will summarize the current knowledge of and exploratory studies on integrin α2 (CD49b). It binds only to the β1 subunit and has a widespread distribution in fibroblasts, endothelial cells, blood cells (such as platelets), and epithelial cells.4 The role of integrin α2 in diseases such as cancer is still not well defined.
Molecular mechanisms regulating the expression of integrin α2
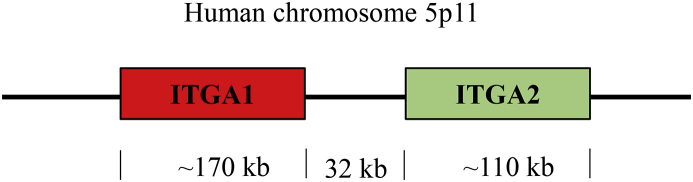
Integrin α2 (1181 amino acids) is encoded by the gene ITGA2, which is located in chromosome 5 and contains 30 exons (Fig. 1). The locus of ITGA2 is 32 kilobases downstream of ITGA1, which encodes integrin α1, another collagen- and laminin-binding integrin.5 The loci of these two integrin genes are the closest integrin-encoding genes within the human genome; however, their expression is cell-specific and they do not necessarily co-express. For example, ITGA1 is selectively suppressed in megakaryocytic lineages, while ITGA2 is not.5 Expression of ITGA2 can vary by several fold in healthy individuals, and the discrepancy in expression of ITGA2 can be partially explained by the presence of single nucleotide polymorphisms (SNPs), which can alter the transcription rate by altering the affinity for its transcription factors.6
Figure 1.
Loci of genes encoding integrin α1 (ITGA1) and α2 (ITGA2) in the human chromosome. ITGA1 is around 170 kb in length while ITGA2 is 110 kb in length. Adapted from Kulich, et al 2002.7
Single nucleotide polymorphisms
SNPs can alter human susceptibility to certain diseases. ITGA2 has been found to have SNPs that are relevant to multiple types of cancer as well as blood disorders (Table 2). Studies in human platelets revealed differences in platelet adhesion between samples of 27 normal subjects by measuring the time required for type I collagen-induced platelet aggregation in platelet-rich plasma.7 These studies observed differences in α2β1 collagen receptor activity and suggest polymorphisms or variable regulation by another gene product. Research directed by Yann Cheli et al suggests that certain SNPs can enhance the binding of transcription co–activator complexes to increase transcription of ITGA2. In their research they observed this behavior in a CA repeat polymorphism in the 5′-regulatory region of ITGA2, which increased the binding of PARP-1 and Ku80/70 and in turn increased levels of integrin α2β1 in platelets.8
Table 2.
ITGA2 gene polymorphisms associated with multiple types of cancer.
| Type of Cancer | ITGA2 status/role |
|---|---|
| Breast Cancer | ITGA2 1648G > A (95% of the breast cancer patients expressed the 1648AA genotype).9 |
| Colorectal cancer | ITGA2 807C > T polymorphism was associated with reduced colorectal cancer risk but no effects on overall survival or relapse-free survival.10, 11 |
| Gastric Cancer | ITGA2 807C > T polymorphism may be associated with an increased risk of gastric cancer, differentiation and invasion of gastric cancer.12 |
| Lymph node positive breast cancer | No correlation of polymorphism with disease-free survival or relapse-free survival.13 |
| Melanoma | Very conserved polymorphisms have small effects in melanoma.84 |
| Colon cancer | Up-regulated.85 |
Evidence suggests that ITGA2 SNPs also play important roles in the progression of cancer. According to a study from Austria comparing 500 breast cancer patients to 500 healthy females, there is a higher incidence of breast cancer in patients who expressed one of the two functional polymorphisms of ITGA2 (1648G > A).9 Also, a case–control study performed by the same group found that the 807C > T polymorphism was associated with reduced colorectal cancer risk in a cohort of 433 patients.10 However, a follow-up, 41-month-long study of these patients showed no effects of these SNPs on relapse-free survival or overall survival.11 Another case–control study showed the expression of ITGA2's 807C > T polymorphism may be associated with an increased risk, poor differentiation, and elevated invasion of gastric cancer,12 based on 432 blood samples from sporadic breast cancer patients with no synchronous metastasis, 216 of which were from lymph node-positive patients. No correlation was found between the 807C > T and 1648G > A polymorphisms and disease-free or overall survival of lymph node-positive breast cancer.13
Transcriptional regulation of ITGA2
The transcription of ITGA2 is largely driven by SP1, a ubiquitous transcription factor, which binds to 2 tandem recognition sequences in the proximal promoter of ITGA2.5 Other binding regions found in the ITGA2 promoter are the elements for transcription factors AP1 and AP2, 3 GATA boxes for the GATA family of transcription factors, and 4 half-sites for the estrogen receptor.14 Additional transcription factors have been found to regulate the expression of ITGA2 in different types of cancer, such as FOXL215 and β-catenin.16 A recent finding by the Daiming Fan group suggests that FOXL2 sustains the expression levels of ITGA2 in chemoresistant gastric cells.15 However, it is unknown if there is a forkhead recognition site for FOXL2 in the ITGA2 promoter. Β-catenin also serves as a transcription activator of ITGA2 in certain brain (U87MG), colon (DLD1) and lung (H1299) cancer cell lines, as confirmed by chromatin immunoprecipitation.16
Integrin switching
Recent studies have discovered that integrins can switch from one to another in response to cell signaling. The mechanism is not well understood yet, but it has been observed in different types of cancer and it is believed to be a mechanism required to provide metastatic competence.17 For example, Parvani's research found a compensatory increase in β3 expression when β1 is inhibited.18 Truong observed the same switch; however, they concluded it occurred through the TGFb-miR-200-Zeb network in triple negative breast cancer.19 Another example of integrin switching can be observed in the human colon adenocarcinoma cell line, HT-29. In this case, a switch of α2β1 to α3β1 occurs during early stages of differentiation as a response to RhoA activity.20 Further studies are expected to better elucidate the potential and mechanisms of integrin switching in cancer.
Post-translational regulation
Integrins, like any other cell surface receptors, are subject to many post-translational modifications. Particularly, tumor cells usually exhibit an altered state such as aberrant glycosylation. In the case of integrin α5β1, aberrant glycosylation on the α5 subunit can alter EGFR signaling.21 Sialylation in integrin α2 is associated with invasiveness and metastatic potential in cancer cells when it occurs at a higher or lower rate than normal.22, 23 Cleavage of sialic acids from modified surface molecules has been found to increase the adhesion of the triple negative breast cancer cell line MDA-MB-231 to the ECM, although no change in invasion or migration was observed.23 Sialylation of β1 integrin in colon cells blocks binding to gal-3 and protects them from apoptosis induced by gal-3.24 Furthermore, changes in glycosylation and sialylation patterns of α2β1 can regulate pancreatic cancer cell adhesion and invasion.25 Hence, different patterns in post-translational modifications in α2β1 could potentially explain its controversial role in tumor progression.
Structure and ligand binding
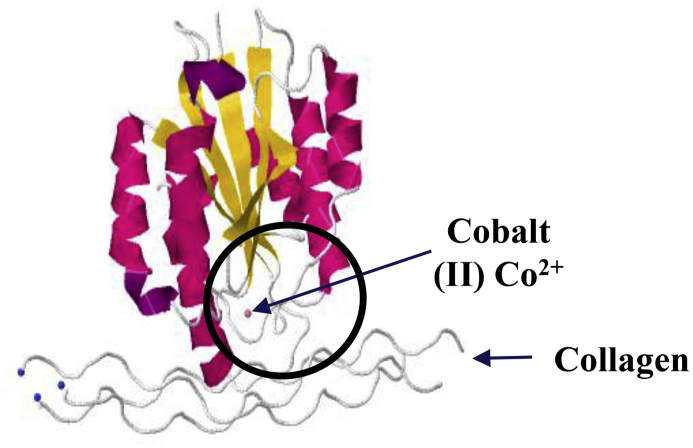
Integrin α2 forms heterodimers exclusively with β1, generating the α2β1 receptor. Meanwhile, β1 can form a heterodimer with one of at least 12 different α subunits. Alpha integrins possess a Von Willebrand factor type A domain (or I domain) in the extracellular head of the integrin that is responsible for ligand binding.26 Beta integrins possess a similar domain (βI domain) that can mediate ligand recognition when the α subunit does not contain the I domain.3 Therefore, integrin α2β1 ligand recognition occurs mainly at the alpha subunit. The α2 subunit also contains a metal ion-dependent adhesion site called the MIDAS motif, which is also required for ligand binding27, 28 (Fig. 2). Binding of integrin α2 to its ligand is cation-dependent, using divalent cations such as manganese and magnesium. However, this binding is abrogated by calcium.29 So far, only 4 integrins, α1β1, α2β1, α10β1 and α11β1, are known to be I domain-containing integrins that bind to collagen. The β1 subunit also contains two metal ion-dependent adhesion sites called the MIDAS and ADMIDAS. The ADMIDAS region contains an inhibitory calcium ion, but when bound to manganese, it leads to a conformational change that represents the active form of the integrin.3 While the alpha subunits determine the integrin ligand specificity, the beta subunits are connected to the cytoskeleton and affect multiple signaling pathways.3
Figure 2.
Protein model of integrin α2 domain I binding to collagen. The crystal that was used for determination of the structure grew in Co2+ ion, which supports collagen adhesion in vitro. The domain I is circled.
It was previously believed that integrins would bind to one specific ligand in the ECM; however, more recent findings have shown that in general integrins can bind to multiple ligands (1). The most frequently studied ligand of integrin α2β1 is collagen, to which it can bind with high affinity by recognizing the proline-hydroxylated sequence G-F-O-G-E-R in collagen.26 Other extracellular membrane components such as laminin can also be a ligand of integrin α2β126,30. Interestingly, α2β1 can also serve as a receptor for viruses such as human rotavirus A and human echoviruses 1 and 8.31, 32 Integrins are usually present at a high concentration on the cell surface but usually bind their ligand with low affinity. This system allows the cells to dynamically move around instead of being irreversibly glued to the matrix.33
Physiological functions of integrin α2
Signaling cascades
Integrins provide a connection between the ECM and the intracellular actin cytoskeleton, which allows integrins to regulate cytoskeletal organization and cellular motility. Commonly, integrins are found in an inactive (bent) state until they become activated by an extrinsic or intrinsic ligand. Integrin activation by ECM components (extrinsic ligands) induces a signaling cascade through focal adhesion complexes leading to changes in proliferation, survival, etc.33 However, activation of integrins does not exclusively rely on extracellular binding to ECM components. Evidence provided by Calderwood and others suggests that intracellular activation of integrins can occur through the binding of talin to integrin beta tails.34 In fact, it is believed that inside-out signaling, such as talin activation, increases the affinity of the integrin for ECM ligands.35 Several proteins can inhibit integrin activation by competing with talin for binding to the β-integrin tail.36
To describe the signaling of the integrins can be challenging, considering we would have to describe a network of 156 components as elucidated by Geiger and colleagues.37 However, it is important to mention the key players of integrin signaling, such as focal adhesion kinases (FAKs) and Src family kinases. FAK is recruited in focal adhesions as a response to integrin clustering. Consequently, FAK conducts autophosphorylation and generates docking sites for Src kinases and other SH2 domain-containing proteins.33, 36 Non-canonical signaling pathways have also been found for certain heterodimers such as integrin α5β3, which recruits Kras and Src to drive cellular reprogramming.
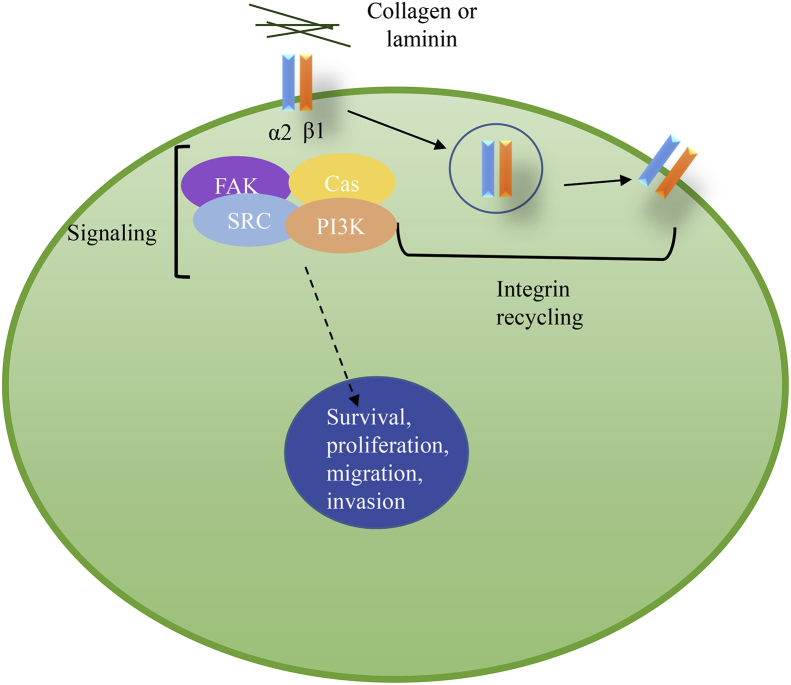
Integrin trafficking has become an important area of study. Integrins are endocytosed through clathrin- or caveolin-mediated routes and undergo endosomal sorting that determines degradation or recycling of the receptor. Research has shown that integrins are constantly recycled back to the membrane to provide a constant renewal of the pool38 (Fig. 3). However, a specific and detailed understanding of the signaling and cycling of integrin α2β1 has not yet been attained.
Figure 3.
Canonical pathway for α2β1 signaling. Upon collagen or laminin binding to integrin α2β1, a series of signaling events occur to promote survival, proliferation, migration, and invasion. Integrins are often recycled to provide a refreshed pool of integrins to the surface membrane.
Proteoglycan ligand-mediated angiogenesis
Proteoglycans, a major class of molecules found in the ECM, can modulate the function of integrins. Modulation of α2β1 by the proteoglycans decorin and perlecan has been found to have important roles associated with angiogenesis, which is a process of new blood vessel formation that benefits tumor growth.39 Decorin, a small leucine-rich proteoglycan, is expressed only in sprouting endothelial cells, but its role in angiogenesis is controversial, with evidence for both enhancement and inhibition of tube formation.40 In a study led by Fiedler, decorin enhanced α2β1 integrin-dependent endothelial cell adhesion and migration on fibrillary collagen I.40 It was concluded that decorin allosterically modulates the integrin α2β1-collagen I interaction.40 Additionally perlecan, a heparin sulfate proteoglycan, can bind to α2β1 via its C-terminal fragment, called endorepellin.39 The terminal globular domain of endorepellin, LG3, interacts directly with the α2 I domain.39 Endorepellin potently inhibits angiogenesis by exerting a dual receptor antagonism by simultaneously engaging VEGFR2 and α2β1 integrin.39 As a result, endorepellin causes disassembly of the actin cytoskeleton and focal adhesions. This type of modulation is important to control the tumor angiogenesis that can be promoted by α2β141.
Cell migration
Integrins mediate cell migration, and they have been found to play a role in tissue regeneration and repair. However, integrins can also be harmful by enhancing cancer cell migration and other disorders. Optimum speed of cellular migration occurs at intermediate levels of expression of α2β1 or intermediate concentrations of ligand,42 therefore an alteration of these concentrations can alter cell migration speed. Cell migration mediated by integrin α2β1 has been shown to play a prominent a role in multiple types of cancer, such as melanoma.30 Melanoma cell lines that showed weak attachment and low cell migration were compared to melanoma cell lines that had a high cell migration and attachment capacity.30 The cell lines with the enhanced migration were blocked with monoclonal antibodies for β1 and α2 and showed an inhibition of migration. In contrast, monoclonal antibodies blocking α3 and α6 did not inhibit the migration phenotype.30 Yoshinaga et al confirmed in their studies that the α2 subunit has a significant contribution to the motility of metastatic melanoma cell lines.43
Stem cells and differentiation
Stem cells are multipotent cells that are capable of self-renewal, proliferation, and differentiation. Studies have found that, within a tumor, a subset of cancer cells is capable of self-renewal and limited differentiation, termed cancer stem cells (CSCs). Each type of stem cell, normal or cancer, displays particular markers that help identify them in a tissue-dependent manner. CSCs in a tumor are considered responsible for therapy resistance, tumor recurrence, and metastasis.44 Therefore, it is of clinical relevance to understand the stem cell properties (stemness) and differentiation of normal stem cells and CSCs in multiple tissue types, thereby identifying possible targets for disease treatments.
Integrin α2 plays a role in cell differentiation. It is one of the surface markers of luminal progenitor cells of human and mouse mammary glands,45, 46 while luminal differentiated cells do not express this integrin.47 Integrin α2 is considered a mesenchymal stem cell marker of cells isolated from the bone marrow48 and has been found to be highly expressed in umbilical cord blood-derived mesenchymal stem cells that were identified by flow cytometry.49
Uterine leiomyoma, the most common benign tumor in reproductive-age women, is characterized by highly elevated expression of integrin α2. This particular population of cells also highly co-expresses KLF4, NANOG, SOX2, and OCT4, which are known markers of self-renewal. Furthermore, the in vitro colony formation capacity of integrin α2-positive cells, as well as the in vivo tumor-regeneration ability, confirmed it as a marker for uterine leiomyoma progenitor cells.50 In addition, integrin α2β1 is a marker of normal human prostate stem cells, and it is also enriched, along with CD44, in prostate CSCs.51
Although integrin α2 has been identified as a marker of stemness, it is not a universal marker of cancer stemness across all types of cancer. Instead, integrin β1 has been recognized widely as a stemness marker and a CSC marker across multiple types of cancer and in dimerization with multiple alpha subunits.52, 53, 54, 55
Blood and immune system
Integrin α2β1 mediates adhesion of platelets to multiple collagen types, which induces platelet aggregation.7 Furthermore, integrin α2β1 is present in many other immune cells such as mast cells, neutrophils, and a subset of T cells.56 Data suggests integrin α2β1 is required for innate immunity and regulation of autoimmune disorders. For example, integrin α2β1 is required for mast cell activation after infection with Listeria, which in turns activates cytokine secretion.57 Integrin α2 is also one of the markers used to identify CD4+ type 1 T regulatory (Tr1) cells.58 Identifying these Tr1 cells has been a challenge due to their lack of surface markers, but it is essential to identify them, since they show strong immunosuppressive activity.58 Collagen binding to integrin α2β1 promotes survival of malignant T cells in acute lymphoblastic leukemia59 and has been found to promote neutrophil recruitment and mediate inflammation of diseases such as colitis.60
Knockout mouse models of integrin α2
Integrin α2-deficient mice develop normally and are fertile, but display partially defective platelet interaction with collagen61 and diminished branching complexity of the mammary glands.62 Although α2β1 integrin-null mice show no alteration in either vasculogenesis or angiogenesis, adult mice show an enhanced angiogenic response during wound healing and tumor xenograft development.56, 62 Similarly to integrin α2 knockout models, integrin αIIb knockout models are also viable and fertile and show defective platelet aggregation, demonstrating a similar importance in platelets.63 In contrast, mutant mice that cannot make β1 integrins die at implantation, demonstrating a more essential role of β1 and a more challenging therapeutic target compared to α units, the loss of which might be compensated by other factors.33
Role of integrin α2 in diseases
Intrinsic role of integrin α2 in cancer cells
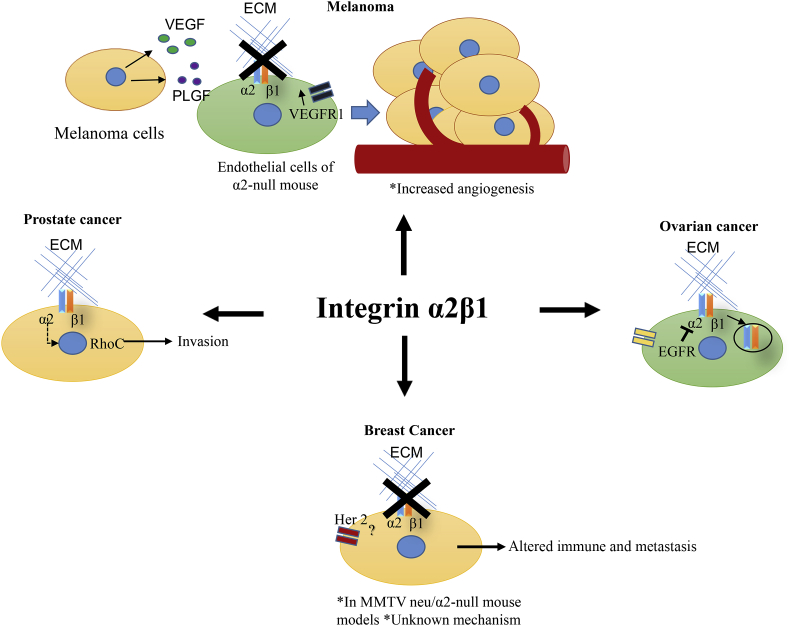
Integrin α2β1 was identified as a prostate basal marker after evaluation of multiple prostate cancer cell lines,64 and other groups have identified it as a prostate cancer stem cell marker along with integrin α6.51, 65 Integrin α2β1 has been found to promote the growth of prostate cancer cell line LnCaP within the bone66 and to promote its invasion through RhoC GTPase67 (Fig. 4). Skeletal metastasis is frequently the cause of death of prostate cancer patients, and hence reducing the expression of integrin α2 in this particular type of cancer is potentially an ideal therapy.68
Figure 4.
Roles of integrin α2β1 in multiple types of cancer. Top panel: Melanoma cells that produce high levels of VEGF and PLGF can stimulate endothelial cells of α2-null mice, which have increased VEGFR1 to stimulate angiogenesis.41 Right panel: Ovarian cancer cells that express high levels of EGFR can block integrin α2β1 signaling by increasing its internalization.86 Left panel: In prostate cancer, α2β1 promotes invasion by RhoC GTPase expression.66 Bottom panel: Absence of α2β1 in the stroma of host mice promotes spontaneous metastasis of transgenic c-neu oncogene-initiated breast cancer.72
Integrin α2β1 has also been implicated in metastasis promotion in multiple other types of cancer. For instance, in mouse models of melanoma, colon, and breast cancer, blockage of integrin α2 diminished metastasis to the liver.69 In hepatocellular carcinoma, integrin α2 has been found to activate YAP oncogenic signaling, and clinical data correlates it with a worse prognostic outcome.70 However, the mechanisms are yet to be elucidated.
Stromal integrin α2 in angiogenesis
When mice lacking integrin α2β1 are challenged with melanoma cells (B16F10), it results in increased tumor angiogenesis. The endothelial cells in the α2-null mouse model show up-regulated expression of VEGFR1. The angiogenesis effect occurs as a result of the high expression of growth factors VEFG and PLGF41 (Fig. 4). According to this study, the regulatory function of integrin α2 in cancer is partially carried out in the microenvironment, in stromal and endothelial cells.
Complex role of integrin α2 in breast cancer
Integrin α2β1 has been shown to play a role in breast cancer, although it seems to be complex. In a study in which 124 tumor samples and 33 matched adjacent tissue samples from breast cancer patients were stained for multiple stemness markers, it was found that integrin α2 was strongly stained in the tumors, associated vessels, and ducts, but weakly stained in the background or normal epithelia. A significant association was found between loss of expression of the α2 subunit (quantified by qPCR) and metastatic disease, such as increased tumor nodal status.71 In vivo studies crossing the α2 integrin–null mice with transgenic mice carrying the MMTV-c-neu oncogene demonstrated that integrin α2 works as a metastasis suppressor.72 However, it remains unclear if the phenotype is caused by the deficiency of α2β1 integrin in tumor cells, endothelial cells, or both. Of note, β1 has been shown to be a pro-metastasis integrin in breast cancer.19 Research lead by Haidari discovered that the β1 of integrin α2β1 phosphorylates vascular endothelial cadherin in endothelial cells upon induction by invasive breast cancer cells.73 Tyrosine phosphorylation of vascular endothelial cadherin has been implicated in the disruption of endothelial cell adherens junctions and in the diapedesis of metastatic cancer cells. These studies suggest that invasive breast cancer cells activate integrin α2β1 to promote transendothelial migration.73
Potential therapeutics targeting integrin α2
Several experimental inhibitors of integrin α2 have a sulfonyl group. The sulfonyl group is essential for α2β1binding. Replacing the sulfonyl group of an experimental inhibitor of α2β1 with a carbonyl group reduced the potency 600-fold.74 E7820 is a novel sulfonamide derivative that inhibits integrin α2 and can be taken orally.75 E7820 has undergone several clinical trials, including a phase II study in combination with cetuximab (an EGFR inhibitor) in subjects with metastatic and refractory colorectal cancer.76 So far, a single prolonged partial response has been observed in a KRAS-mutant patient; however, this study did not meet its primary endpoint of objective response rate76 and further results have not been published. E7820 was also used in combination with erlotinib in preclinical studies to overcome therapy resistance in non-small cell lung cancer. The study was performed with cell lines that are resistant to erlotinib and showed a synergistic antitumor effect in xenograft models77 by decreasing microvessel density and enhancing apoptosis of tumor-associated endothelial cells.
Bufalin, a component of traditional Chinese medicine, has been found to induce apoptosis in several types of cancers.78, 79 Interestingly, it has been found to suppress cervical cancer tumorigenesis and to enhance paclitaxel therapy by suppressing α2/FAK/AKT1/GSK3β signaling.80 Furthermore, a component in snake venom called aggretin can bind to integrin α2β1.81 A synthetic aggretin polypeptide successfully reduced endothelial angiogenesis by blocking integrin α2β1.82 Other toxins derived from snake venom and successfully extracted by affinity chromatography have also been shown to inhibit integrin α2β1.83 Another modality by which to potentially target integrin α2 would be through neutralizing antibodies, demonstrated to be effective by in vivo studies of inflammatory bowel disease.60 In summary, there are multiple routes by which to develop viable therapies to suppress integrin α2 in cancer.
Conclusion
Integrin discoveries have occurred over a long time, and the field remains in constant growth. As the roles of integrins in diseases are revealed, we are challenged to attain a better understanding of the mechanisms and signaling particular to each heterodimer, as well as each subunit of various heterodimers. For example, β1 has been identified as a breast cancer stem cell marker; however, it is not known if this mechanism is mediated by a particular α subunit, as β1 binds to many α subunits. Therapeutic treatments could be made more specific by targeting individual α subunits depending on the specific context. Meanwhile, the potential overlapping or compensating roles of α subunits need to be clarified and considered. In this review, we have summarized the current understanding and complex role of integrin α2 in multiple cell types and disease settings. The role of integrin α2 varies according to cell context, cancer type, and other aspects such as SNPs and post-translational sialylation. Specific targeting of integrin α2 in defined cell types and diseases seems a desirable approach in order to improve clinical outcomes. Further understanding of these mechanisms could unravel new therapeutic opportunities.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding and acknowledgment
We thank the Liu laboratory members, especially Andrew D. Hoffman, Erika Ramos, and Nurmaa K. Dashzeveg, for editing the manuscript. This manuscript is partially supported by the funds from the National Institutes of Health/National Cancer Institute (NIH/NCI) R00CA160638 (H.L.) and its Supplement for Diversity (V.A.), American Cancer Society 127951-RSG-15-025-01-CSM (H.L.), Susan G. Komen Foundation CCR15332826 (H.L.), and Department of Defense W81XWH-16-1-0021 (H.L.).
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2018.12.003.
Contributor Information
Valery Adorno-Cruz, Email: valery.adorno-cruz@northwestern.edu.
Huiping Liu, Email: huiping.liu@northwestern.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Albelda S.M., Buck C.A. Integrins and other cell adhesion molecules. FASEB J. 1990;4(11):2868–2880. [PubMed] [Google Scholar]
- 2.Hynes R.O. The emergence of integrins: a personal and historical perspective. Matrix Biol J Int Soc Matrix Biol. 2004;23(6):333–340. doi: 10.1016/j.matbio.2004.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barczyk M., Carracedo S., Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zutter M.M., Santoro S.A. Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am J Pathol. 1990;137(1):113–120. [PMC free article] [PubMed] [Google Scholar]
- 5.Cheli Y., Kanaji S., Jacquelin B., Chang M., Nugent D.J., Kunicki T.J. Transcriptional and epigenetic regulation of the integrin collagen receptor locus ITGA1-PELO-ITGA2. Biochim Biophys Acta. 2007;1769(9-10):546–558. doi: 10.1016/j.bbaexp.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Paola J., Jugessur A., Goldman T. Platelet glycoprotein I(b)alpha and integrin alpha2 beta1 polymorphisms: gene frequencies and linkage disequilibrium in a population diversity panel. J Thromb Haemostasis JTH. 2005;3(7):1511–1521. doi: 10.1111/j.1538-7836.2005.01273.x. [DOI] [PubMed] [Google Scholar]
- 7.Kunicki T.J., Orchekowski R., Annis D., Honda Y. Variability of integrin alpha 2 beta 1 activity on human platelets. Blood. 1993;82(9):2693–2703. [PubMed] [Google Scholar]
- 8.Cheli Y., Williams S.A., Ballotti R., Nugent D.J., Kunicki T.J. Enhanced binding of poly(ADP-ribose)polymerase-1 and Ku80/70 to the ITGA2 promoter via an extended cytosine-adenosine repeat. PLoS One. 2010;5(1) doi: 10.1371/journal.pone.0008743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langsenlehner U., Renner W., Yazdani-Biuki B. Integrin alpha-2 and beta-3 gene polymorphisms and breast cancer risk. Breast Cancer Res Treat. 2006;97(1):67–72. doi: 10.1007/s10549-005-9089-4. [DOI] [PubMed] [Google Scholar]
- 10.Gerger A., Hofmann G., Langsenlehner U. Integrin alpha-2 and beta-3 gene polymorphisms and colorectal cancer risk. Int J Colorectal Dis. 2009;24(2):159–163. doi: 10.1007/s00384-008-0587-9. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann G., Langsenlehner U., Langsenlehner T. Single nucleotide polymorphisms of integrin alpha-2 and beta-3 genes are not associated with relapse-free and overall survival in colorectal cancer patients. Anticancer Res. 2011;31(4):1373–1377. [PubMed] [Google Scholar]
- 12.Chen J., Liu N.N., Li J.Q. Association between ITGA2 C807T polymorphism and gastric cancer risk. World J Gastroenterol. 2011;17(23):2860–2866. doi: 10.3748/wjg.v17.i23.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knechtel G., Hofmann G., Gerger A. Analysis of common germline polymorphisms as prognostic factors in patients with lymph node-positive breast cancer. J Cancer Res Clin Oncol. 2010;136(12):1813–1819. doi: 10.1007/s00432-010-0839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zutter M.M., Painter A.A., Staatz W.D., Tsung Y.L. Regulation of alpha 2 integrin gene expression in cells with megakaryocytic features: a common theme of three necessary elements. Blood. 1995;86(8):3006–3014. [PubMed] [Google Scholar]
- 15.Dong J., Wang R., Ren G. HMGA2-FOXL2 Axis regulates metastases and epithelial-to-mesenchymal transition of chemo-resistant gastric cancer. Clin Cancer Res. 2017;23(13):3461–3473. doi: 10.1158/1078-0432.CCR-16-2180. [DOI] [PubMed] [Google Scholar]
- 16.Su Y.J., Lin W.H., Chang Y.W. Polarized cell migration induces cancer type-specific CD133/integrin/Src/Akt/GSK3beta/beta-catenin signaling required for maintenance of cancer stem cell properties. Oncotarget. 2015;6(35):38029–38045. doi: 10.18632/oncotarget.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madamanchi A., Zijlstra A., Zutter M.M. Flipping the switch: integrin switching provides metastatic competence. Sci Signal. 2014;7(318):pe9. doi: 10.1126/scisignal.2005236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvani J.G., Galliher-Beckley A.J., Schiemann B.J., Schiemann W.P. Targeted inactivation of beta1 integrin induces beta3 integrin switching, which drives breast cancer metastasis by TGF-beta. Mol Biol Cell. 2013;24(21):3449–3459. doi: 10.1091/mbc.E12-10-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong H.H., Xiong J., Ghotra V.P. beta1 integrin inhibition elicits a prometastatic switch through the TGFbeta-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci Signal. 2014;7(312):ra15. doi: 10.1126/scisignal.2004751. [DOI] [PubMed] [Google Scholar]
- 20.Gout S.P., Jacquier-Sarlin M.R., Rouard-Talbot L., Rousselle P., Block M.R. RhoA-dependent switch between alpha2beta1 and alpha3beta1 integrins is induced by laminin-5 during early stage of HT-29 cell differentiation. Mol Biol Cell. 2001;12(10):3268–3281. doi: 10.1091/mbc.12.10.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi M., Kizuka Y., Ohtsubo K., Gu J., Taniguchi N. Disease-associated glycans on cell surface proteins. Mol Aspect Med. 2016;51:56–70. doi: 10.1016/j.mam.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Vajaria B.N., Patel K.R., Begum R., Patel P.S. Sialylation: an avenue to target cancer cells. Pathol Oncol Res POR. 2016;22(3):443–447. doi: 10.1007/s12253-015-0033-6. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y., Wu L., Shen S., Wu S., Burdick M.M. Effect of alpha 2,6 sialylation on integrin-mediated adhesion of breast cancer cells to fibronectin and collagen IV. Life Sci. 2016;149:138–145. doi: 10.1016/j.lfs.2016.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuo Y., Chammas R., Bellis S.L. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J Biol Chem. 2008;283(32):22177–22185. doi: 10.1074/jbc.M8000015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassaganas S., Carvalho S., Dias A.M. Pancreatic cancer cell glycosylation regulates cell adhesion and invasion through the modulation of alpha2beta1 integrin and E-cadherin function. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0098595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emsley J., Knight C.G., Farndale R.W., Barnes M.J., Liddington R.C. Structural basis of collagen recognition by integrin alpha2beta1. Cell. 2000;101(1):47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 27.Michishita M., Videm V., Arnaout M.A. A novel divalent cation-binding site in the A domain of the beta 2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993;72(6):857–867. doi: 10.1016/0092-8674(93)90575-b. [DOI] [PubMed] [Google Scholar]
- 28.Kamata T., Puzon W., Takada Y. Identification of putative ligand binding sites within I domain of integrin alpha 2 beta 1 (VLA-2, CD49b/CD29) J Biol Chem. 1994;269(13):9659–9663. [PubMed] [Google Scholar]
- 29.Tuckwell D., Calderwood D.A., Green L.J., Humphries M.J. Integrin alpha 2 I-domain is a binding site for collagens. J Cell Sci. 1995;108(Pt 4):1629–1637. doi: 10.1242/jcs.108.4.1629. [DOI] [PubMed] [Google Scholar]
- 30.Etoh T., Thomas L., Pastel-Levy C., Colvin R.B., Mihm M.C., Jr., Byers H.R. Role of integrin alpha 2 beta 1 (VLA-2) in the migration of human melanoma cells on laminin and type IV collagen. J Invest Dermatol. 1993;100(5):640–647. doi: 10.1111/1523-1747.ep12472299. [DOI] [PubMed] [Google Scholar]
- 31.Bergelson J.M., St John N., Kawaguchi S. Infection by echoviruses 1 and 8 depends on the alpha 2 subunit of human VLA-2. J Virol. 1993;67(11):6847–6852. doi: 10.1128/jvi.67.11.6847-6852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham K.L., Halasz P., Tan Y. Integrin-using rotaviruses bind alpha2beta1 integrin alpha2 I domain via VP4 DGE sequence and recognize alphaXbeta2 and alphaVbeta3 by using VP7 during cell entry. J Virol. 2003;77(18):9969–9978. doi: 10.1128/JVI.77.18.9969-9978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruce Alberts A.J., Lewis Julian, Raff Martin, Roberts Keith, Walter Peter. 4th ed. Garland Science; New York: 2002. Integrins. Molecular Biology of the Cell. [Google Scholar]
- 34.Calderwood D.A. Talin controls integrin activation. Biochem Soc Trans. 2004;32(Pt3):434–437. doi: 10.1042/BST0320434. [DOI] [PubMed] [Google Scholar]
- 35.Calderwood D.A. Integrin activation. J Cell Sci. 2004;117(Pt 5):657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- 36.Harburger D.S., Calderwood D.A. Integrin signalling at a glance. J Cell Sci. 2009;122(Pt 2):159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaidel-Bar R., Itzkovitz S., Ma'ayan A., Iyengar R., Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9(8):858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Franceschi N., Hamidi H., Alanko J., Sahgal P., Ivaska J. Integrin traffic – the update. J Cell Sci. 2015;128(5):839–852. doi: 10.1242/jcs.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douglass S., Goyal A., Iozzo R.V. The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connect Tissue Res. 2015;56(5):381–391. doi: 10.3109/03008207.2015.1045297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiedler L.R., Schonherr E., Waddington R. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alpha2beta1 integrin activity. J Biol Chem. 2008;283(25):17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z., Ramirez N.E., Yankeelov T.E. alpha2beta1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood. 2008;111(4):1980–1988. doi: 10.1182/blood-2007-06-094680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huttenlocher A., Horwitz A.R. Integrins in cell migration. Cold Spring Harbor Perspect Biol. 2011;3(9):a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshinaga I.G., Vink J., Dekker S.K., Mihm M.C., Jr., Byers H.R. Role of alpha 3 beta 1 and alpha 2 beta 1 integrins in melanoma cell migration. Melanoma Res. 1993;3(6):435–441. doi: 10.1097/00008390-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Adorno-Cruz V., Kibria G., Liu X. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015;75(6):924–929. doi: 10.1158/0008-5472.CAN-14-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shehata M., Teschendorff A., Sharp G. Phenotypic and functional characterisation of the luminal cell hierarchy of the mammary gland. Breast Cancer Res BCR. 2012;14(5):R134. doi: 10.1186/bcr3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giraddi R.R., Shehata M., Gallardo M., Blasco M.A., Simons B.D., Stingl J. Stem and progenitor cell division kinetics during postnatal mouse mammary gland development. Nat Commun. 2015;6:8487. doi: 10.1038/ncomms9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Visvader J.E. Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 2009;23(22):2563–2577. doi: 10.1101/gad.1849509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seeberger K.L., Dufour J.M., Shapiro A.M., Lakey J.R., Rajotte R.V., Korbutt G.S. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest J Tech Methods Pathol. 2006;86(2):141–153. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 49.Lee O.K., Kuo T.K., Chen W.M., Lee K.D., Hsieh S.L., Chen T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 50.Yin P., Ono M., Moravek M.B. Human uterine leiomyoma stem/progenitor cells expressing CD34 and CD49b initiate tumors in vivo. J Clin Endocrinol Metabol. 2015;100(4):E601–E606. doi: 10.1210/jc.2014-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patrawala L., Calhoun-Davis T., Schneider-Broussard R., Tang D.G. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67(14):6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 52.Shackleton M., Vaillant F., Simpson K.J. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 53.Han J., Fujisawa T., Husain S.R., Puri R.K. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer. 2014;14:173. doi: 10.1186/1471-2407-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pruszak J., Ludwig W., Blak A., Alavian K., Isacson O. CD15, CD24, and CD29 define a surface biomarker code for neural lineage differentiation of stem cells. Stem Cell. 2009;27(12):2928–2940. doi: 10.1002/stem.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medema J.P. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15(4):338–344. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 56.Zweers M.C., Davidson J.M., Pozzi A. Integrin alpha2beta1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol. 2007;127(2):467–478. doi: 10.1038/sj.jid.5700546. [DOI] [PubMed] [Google Scholar]
- 57.McCall-Culbreath K.D., Li Z., Zutter M.M. Crosstalk between the alpha2beta1 integrin and c-met/HGF-R regulates innate immunity. Blood. 2008;111(7):3562–3570. doi: 10.1182/blood-2007-08-107664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gagliani N., Magnani C.F., Huber S. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19(6):739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 59.Naci D., Aoudjit F. Alpha2beta1 integrin promotes T cell survival and migration through the concomitant activation of ERK/Mcl-1 and p38 MAPK pathways. Cell Signal. 2014;26(9):2008–2015. doi: 10.1016/j.cellsig.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Lundberg S., Lindholm J., Lindbom L., Hellstrom P.M., Werr J. Integrin alpha2beta1 regulates neutrophil recruitment and inflammatory activity in experimental colitis in mice. Inflamm Bowel Dis. 2006;12(3):172–177. doi: 10.1097/01.MIB.0000217765.96604.83. [DOI] [PubMed] [Google Scholar]
- 61.Holtkotter O., Nieswandt B., Smyth N. Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem. 2002;277(13):10789–10794. doi: 10.1074/jbc.M112307200. [DOI] [PubMed] [Google Scholar]
- 62.Chen J., Diacovo T.G., Grenache D.G., Santoro S.A., Zutter M.M. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161(1):337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouvard D., Brakebusch C., Gustafsson E. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89(3):211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- 64.Liu A.Y. Differential expression of cell surface molecules in prostate cancer cells. Cancer Res. 2000;60(13):3429–3434. [PubMed] [Google Scholar]
- 65.Hoogland A.M., Verhoef E.I., Roobol M.J. Validation of stem cell markers in clinical prostate cancer: alpha6-integrin is predictive for non-aggressive disease. Prostate. 2014;74(5):488–496. doi: 10.1002/pros.22768. [DOI] [PubMed] [Google Scholar]
- 66.Hall C.L., Dai J., van Golen K.L., Keller E.T., Long M.W. Type I collagen receptor (alpha 2 beta 1) signaling promotes the growth of human prostate cancer cells within the bone. Cancer Res. 2006;66(17):8648–8654. doi: 10.1158/0008-5472.CAN-06-1544. [DOI] [PubMed] [Google Scholar]
- 67.Hall C.L., Dubyk C.W., Riesenberger T.A., Shein D., Keller E.T., van Golen K.L. Type I collagen receptor (alpha2beta1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008;10(8):797–803. doi: 10.1593/neo.08380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sottnik J.L., Daignault-Newton S., Zhang X. Integrin alpha2beta 1 (alpha2beta1) promotes prostate cancer skeletal metastasis. Clin Exp Metastasis. 2013;30(5):569–578. doi: 10.1007/s10585-012-9561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshimura K., Meckel K.F., Laird L.S. Integrin alpha2 mediates selective metastasis to the liver. Cancer Res. 2009;69(18):7320–7328. doi: 10.1158/0008-5472.CAN-09-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong K.F., Liu A.M., Hong W., Xu Z., Luk J.M. Integrin alpha2beta1 inhibits MST1 kinase phosphorylation and activates Yes-associated protein oncogenic signaling in hepatocellular carcinoma. Oncotarget. 2016;7(47):77683–77695. doi: 10.18632/oncotarget.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin T.A., Jiang W.G. Evaluation of the expression of stem cell markers in human breast cancer reveals a correlation with clinical progression and metastatic disease in ductal carcinoma. Oncol Rep. 2014;31(1):262–272. doi: 10.3892/or.2013.2813. [DOI] [PubMed] [Google Scholar]
- 72.Ramirez N.E., Zhang Z., Madamanchi A. The alpha(2)beta(1) integrin is a metastasis suppressor in mouse models and human cancer. J Clin Invest. 2011;121(1):226–237. doi: 10.1172/JCI42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haidari M., Zhang W., Caivano A. Integrin alpha2beta1 mediates tyrosine phosphorylation of vascular endothelial cadherin induced by invasive breast cancer cells. J Biol Chem. 2012;287(39):32981–32992. doi: 10.1074/jbc.M112.395905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi S., Vilaire G., Marcinkiewicz C., Winkler J.D., Bennett J.S., DeGrado W.F. Small molecule inhibitors of integrin alpha2beta1. J Med Chem. 2007;50(22):5457–5462. doi: 10.1021/jm070252b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Funahashi Y., Sugi N.H., Semba T. Sulfonamide derivative, E7820, is a unique angiogenesis inhibitor suppressing an expression of integrin alpha2 subunit on endothelium. Cancer Res. 2002;62(21):6116–6123. [PubMed] [Google Scholar]
- 76.Sawyer M.B., Iqbal S., Lenz H. Phase II study of E7820 in combination with cetuximab in subjects (pts) with metastatic and refractory colorectal cancer (CRC) J Clin Oncol. May 2010;28(15_suppl) ASCO Annual Meeting Abstracts; 3537–3537. [Google Scholar]
- 77.Ito K., Semba T., Uenaka T., Wakabayashi T., Asada M., Funahashi Y. Enhanced anti-angiogenic effect of E7820 in combination with erlotinib in epidermal growth factor receptor-tyrosine kinase inhibitor-resistant non-small-cell lung cancer xenograft models. Cancer Sci. 2014;105(8):1023–1031. doi: 10.1111/cas.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu Z., Feng H., Sun X. Bufalin suppresses hepatocarcinogenesis by targeting β-catenin/TCF signaling via cell cycle-related kinase. Sci Rep. 2018;8(1):3891. doi: 10.1038/s41598-018-22113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujii E., Inada Y., Kakoki M. Bufalin induces DNA damage response under hypoxic condition in myeloma cells. Oncol Lett. 2018;15(5):6443–6449. doi: 10.3892/ol.2018.8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu F., Tong D., Li H. Bufalin enhances antitumor effect of paclitaxel on cervical tumorigenesis via inhibiting the integrin alpha2/beta5/FAK signaling pathway. Oncotarget. 2016;7(8):8896–8907. doi: 10.18632/oncotarget.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang C.H., Chung C.H., Hsu C.C., Peng H.C., Huang T.F. Inhibitory effects of polypeptides derived from a snake venom C-type lectin, aggretin, on tumor cell-induced platelet aggregation. J Thromb Haemostasis JTH. 2014;12(4):540–549. doi: 10.1111/jth.12519. [DOI] [PubMed] [Google Scholar]
- 82.Chung C.H., Chang C.H., Hsu C.C., Lin K.T., Peng H.C., Huang T.F. Aggretin venom polypeptide as a novel anti-angiogenesis agent by targeting integrin alpha2beta1. Sci Rep. 2017;7:43612. doi: 10.1038/srep43612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arlinghaus F.T., Momic T., Ammar N.A. Identification of alpha2beta1 integrin inhibitor VP-i with anti-platelet properties in the venom of Vipera palaestinae. Toxicon Off J Int Soc Toxinol. 2013;64:96–105. doi: 10.1016/j.toxicon.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Lenci R.E., Rachakonda P.S., Kubarenko A.V. Integrin genes and susceptibility to human melanoma. Mutagenesis. 2012;27(3):367–373. doi: 10.1093/mutage/ger090. [DOI] [PubMed] [Google Scholar]
- 85.Bertucci F., Salas S., Eysteries S. Gene expression profiling of colon cancer by DNA microarrays and correlation with histoclinical parameters. Oncogene. 2004;23(7):1377–1391. doi: 10.1038/sj.onc.1207262. [DOI] [PubMed] [Google Scholar]
- 86.Ning Y., Buranda T., Hudson L.G. Activated epidermal growth factor receptor induces integrin alpha2 internalization via caveolae/raft-dependent endocytic pathway. J Biol Chem. 2007;282(9):6380–6387. doi: 10.1074/jbc.M610915200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.