Abstract
Background
Repetitive transcranial magnetic stimulation (rTMS) can modulate cortical excitability, and may be beneficial for motor recovery after stroke. However, the neuroplasticity effects of rTMS have not been thoroughly investigated in the early stage after stroke.
Objective
To comprehensively assess the effects of high- and low-frequency repetitive transcranial magnetic stimulations on motor recovery in early stroke patients, using a randomized controlled trial based on clinical, neurophysiological and functional imaging assessments.
Methods
Sixty hospitalized, first-ever ischemic stroke patients (within 2 weeks after stroke) with motor deficits were randomly allocated to receive, in addition to standard physical therapy, five consecutive sessions of either: (1) High-frequency (HF) rTMS at 10 Hz over the ipsilesional primary motor cortex (M1); (2) Low-frequency (LF) rTMS at 1 Hz over the contralesional M1; (3) sham rTMS. The primary outcome measure was a motor impairment score (Upper Extremity Fugl-Meyer) evaluated at baseline, after rTMS intervention, and at 3-month follow-up. Cortical excitability and functional magnetic resonance imaging (fMRI) data were obtained within 24 h before and after rTMS intervention. Analyses of variance were conducted to compare the recovery effects among the three rTMS groups, assessed using clinical, neurophysiological and fMRI tests.
Results
Motor improvement was significantly larger in the two rTMS groups than in the control group. The HF-rTMS group showed significantly increased cortical excitability and motor-evoked fMRI activation in ipsilesional motor areas, whereas the LF-rTMS group had significantly decreased cortical excitability and motor-evoked fMRI activation in contralesional motor areas. Activity in ipsilesional motor cortex significantly correlated with motor function, after intervention as well as at 3-month follow-up.
Conclusion
HF- and LF-rTMS can both improve motor function by modulating motor cortical activation in the early phase of stroke.
Keywords: Transcranial magnetic stimulation, Motor recovery, Stroke, Neuroplasticity, fMRI, Interhemispheric inhibition
Highlights
-
•
rTMS is an effective neurorehabilitative strategy for enhancing motor recovery in the early stage after stroke.
-
•
Effects of high- versus low-frequency rTMS on motor regions reflect different neuroplastic mechanisms supporting motor recovery.
-
•
Functional reorganization of the ipsilesional M1 after stroke plays a critical role in long-term motor recovery.
1. Introduction
Motor impairment is a leading cause of long-term disability from stroke worldwide (Hankey et al., 2002). Over 60% of stroke survivors continue to suffer from motor impairment despite undergoing intensive rehabilitative therapies (Mayo et al., 1999). Neuroplasticity-induced cortical reorganization is an important process mediating motor recovery after stroke (Buma et al., 2013). Studies in animal models (Murphy and Corbett, 2009) and in patients (Langhorne et al., 2011) suggested that the early post-stroke stage is crucial for enhancing neuroplasticity. However, evidence in favor of early utilization of plasticity-inducing interventions for stroke rehabilitation is still lacking (Ward, 2005).
Noninvasive brain stimulation, as for instance repetitive transcranial magnetic stimulation (rTMS), can promote brain plasticity by modifying cortical excitability (Adeyemo et al., 2012). Importantly, it has proven to be a promising tool for promoting motor function recovery after stroke (Adeyemo et al., 2012; Cramer and Riley, 2008; Khedr et al., 2005). However, most brain stimulation studies were conducted in chronic stroke patients (Adeyemo et al., 2012). To date, only a few studies were focused on early stroke patients (Khedr et al., 2005). Furthermore, very limited follow-up data on the long-term effects of rTMS intervention for stroke rehabilitation are available.
The interhemispheric inhibition (IHI) model (Murase et al., 2004; Nowak et al., 2009) is the theoretical model commonly adopted for guiding the use of rTMS in motor rehabilitation after stroke. According to the IHI model, neuronal excitability of each cerebral hemisphere exerts an inhibitory effect on the contralateral one, such that brain activity is typically balanced between hemispheres. In stroke patients, however, damage resulting from stroke disrupts the balance by decreasing the IHI of the affected hemisphere, and shifting the equilibrium towards the unaffected hemisphere. This leads to an over-activity of the unaffected hemisphere as compared to the affected one. Therefore, facilitating the excitability of the ipsilesional motor cortex (high frequency rTMS, >1 Hz) or suppressing the excitability of the contralesional motor cortex (low frequency rTMS, ≤1 Hz) could be beneficial for correcting the interhemispheric imbalance and facilitating post-stroke motor recovery (Ludemann-Podubecka et al., 2015; Le et al., 2014).
Unfortunately, the findings from previous rTMS experiments were generally constrained to small samples size and exploratory studies (Li et al., 2016; Chang et al., 2012), and little evidence from randomized controlled trials is available (McDonnell and Stinear, 2017). Most importantly, the differential effects of high-frequency versus low-frequency rTMS early after stroke have not been clearly documented, and their possible effects on motor cortical activation remain to be investigated.
Most studies evaluated rTMS-based therapy by using behavioral and neurophysiological measures (Chang et al., 2015; Du et al., 2016), which could not provide information on neural activity with high spatial resolution. In this regard, functional magnetic resonance imaging can be used to examine the underlying mechanisms of motor reorganization in stroke therapy (Rehme et al., 2012; Stagg et al., 2012).
To address the aforementioned limitations in the field, we conducted a randomized controlled rTMS study in a larger cohort of patients in the acute stage after stroke, which was based on the IHI theoretical model (Grefkes and Fink, 2014). Specifically, we investigated whether motor function recovery effects of high frequency rTMS on the ipsilesional hemisphere versus low frequency rTMS on the contralesional hemisphere. We hypothesized that rTMS intervention of 10 Hz and 1 Hz frequencies in the ipsilesional/contralesional hemispheres, respectively, would induce the change of neural activity of motor cortex, facilitating motor function recovery after stroke. We related our treatment results to task-evoked fMRI and neurophysiological measures, to provide novel insight into the mechanism of action of rTMS in the early post-stroke phase. In addition, 3-month patient follow-ups for the assessment of motor function were also conducted to substantiate the long-term beneficial effects of the rTMS treatment.
2. Methods
2.1. Patients
Ninety stroke patients with motor deficits were consecutively recruited from the Department of Neurology, Jinling Hospital, Jiangsu Province, China. Sixty patients met the following inclusion criteria: (1) first-ever ischemic stroke; (2) 2 weeks within symptom onset; (3) stroke lesions located within the middle cerebral artery (MCA) territory, as verified by magnetic resonance imaging (MRI); (4) unilateral upper limb motor deficit. Exclusion criteria were: (1) hemorrhagic stroke; (2) having other underlying neurological diseases; (3) severe aphasia or cognitive impairment; (4) medication of antidepressants or benzodiazepines; (5) having any contraindications to TMS and/or MRI.
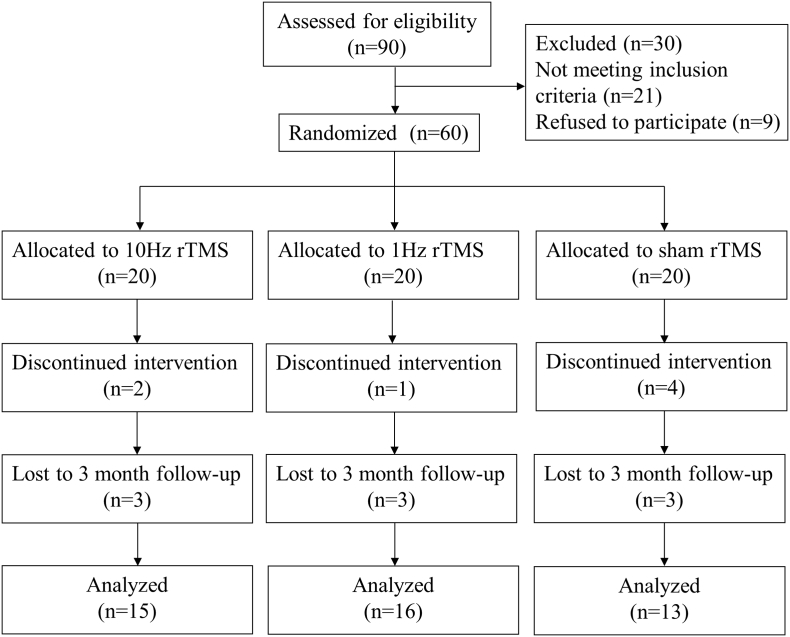
This study was performed in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and approved by the Internal Review Board of Jinling Hospital. Written informed consent was obtained from each participant. This trial was registered at the Chinese Clinical Trial Registry (ChiCTR-IOR-14005394) and was reported following the guidelines of the Consolidated Standards of Reporting Trials (CONSORT) group. The participants' flowchart is shown in Fig. 1.
Fig. 1.
Consolidated Standards of Reporting Trials flow diagram. rTMS, repetitive transcranial magnetic stimulation.
3. Study design
We used a randomized, sham-controlled, double-blinded design. Enrolled patients were randomly allocated to one of either: the high-frequency (HF, 10-Hz), the low-frequency (LF, 1-Hz) or the control (sham) rTMS group. Treatment allocations were kept in sequentially-numbered sealed opaque envelopes, which were opened only at the time of enrollment. Each patient received rTMS daily for five consecutive days and underwent clinical, cortical excitability and fMRI assessments within 24 h before and after the rTMS intervention. In addition, patients participated in follow-up motor function assessments 3 months after stroke. Both the patients and the therapist were blind to the treatment allocation. Also, a clinical assessment was conducted by an experienced neurologist blinded to patients' group allocation. rTMS procedures were performed by an investigator who was not involved in clinical assessment, patient follow up, or data analysis.
4. Intervention
The rTMS protocol was defined following the recommendations of the International Federation of Clinical Neurophysiology (Groppa et al., 2012; Rossini et al., 2015). Each patient received rTMS daily for five consecutive days. Patients in the HF-rTMS group were treated as follows: 10 Hz stimulation for 4 s per session, with a 40s interval between sessions, 30 sessions per treatment, totaling 1200 pulses at 100% resting motor threshold (rMT) over M1 of the affected hemisphere. Patients in the LF-rTMS group were treated as follows: 1 Hz stimulation for 120 s per session, with a 40s interval between sessions, 10 sessions per treatment, totaling 1200 pulses at 100% rMT on M1 of the unaffected hemisphere. The sham group received rTMS with the same parameters (noise, time and frequency) as the LF-rTMS group on M1 of the unaffected hemisphere but with the coil rotated 90° away from the scalp, so that minimal or no current flow was induced in the brain.
All patients received the same physiotherapy and medical therapies during the period spent in hospital. The standardized physiotherapy protocol (1 h daily) consisted of active and passive motor exercises of the affected extremity after each rTMS session, which was conducted by a specialized therapist. Medical therapy consisted of standard anti-platelet, statin, anti-coagulation, and anti-hypertensive drugs.
5. Clinical assessments
Motor impairment was evaluated using the upper extremity Fugl–Meyer Assessment (FMA) (Gladstone et al., 2002) and the Medical Research Council (MRC) scale (Compston, 2010). Primary outcome measure was the FMA score, a standardized motor impairment scale, which ranges from 0 (complete hemiplegia) to a maximum of 66 points (normal motor performance) for the upper extremity (Gladstone et al., 2002). MRC was used to assess muscle strength of the hemiplegic side (ranging from 0 to 5; 5 = normal power and 0 = no movement) (Compston, 2010). Stroke severity and disability was determined using the National Institutes of Health Stroke Scale (NIHSS) (Brott et al., 1989) and Modified Rankin Scale (mRS) (Wilson et al., 2002). Between-group differences were also assessed based on the intention to treat. To this end, the data used for the patients who withdraw from the study or did not participate in the follow-up was the one of the last measurement.
6. Analysis of cortical excitability
Motor cortical excitability was evaluated by single pulse TMS in the affected and unaffected hemispheres of all patients before and after rTMS therapy (Groppa et al., 2012; Rossini et al., 2015). Magnetic stimulation was performed using a Magpro 9100 stimulator (MagVenture Company, Farum, Denmark) with a figure-eight coil (outer diameter of one wing, 9 cm). We determined the optimal stimulation site (“hot spot”) where the largest motor evoked potential (MEP) could be consistently elicited over the motor representation of the contralateral abductor pollicis brevis. Once the site was identified, it was marked on the scalp to ensure consistent coil placement. Subsequent neurophysiological assessments used the same “hot spot” (usually primary motor cortex, M1).
The resting motor threshold (rMT) was defined as the minimal stimulus intensity that produced an MEP response of at least 50 μV amplitude with the target muscle at rest in at least 5 of 10 subsequent trials. MEP amplitude and latency were measured as peak to peak (μV) and the time period (ms) between stimulation onset and start of the largest MEP, respectively. The corticomotor conduction time (CMCT) was calculated by subtracting the peripheral motor conduction time (PMCT, which provides a measure of conduction time along the peripheral motor axon, using a coil centered over the C7/C8 cervical spine with the windings of the coil following the orientation of the target root) from the shortest corticomotor latency (CML, which corresponds to the fastest corticomotor conduction time) (Groppa et al., 2012). If no MEP could be obtained from the ipsilesional M1, the “hot spot” was defined as the symmetric location in the contralesional M1 measured to the ipsilesional M1. The patients for whom MEP at baseline could not be evoked for the affected hemisphere were excluded from rTMS analyses.
7. Magnetic resonance imaging
7.1. Motor tasks
A block-designed motor task, for which the thumb and the forefinger of the affected and unaffected hand had to be tapped together at a rate of 1 Hz, was used as an fMRI activation paradigm. The MRI session consists of five trains, lasting 5 min in total. Each train contained three blocks: a 20-second block of affected hand movement, a 20-second block of unaffected hand movement, and a 20-second rest block. Two motor task blocks were pseudo-randomized and separated by a rest block. Every block was repeated 5 times in total. An investigator carried out the passive movement in the scanner room and ensured the consistency of the motor tasks. All patients underwent motor task training before MRI scanning and performed the passive movements during task-evoked fMRI scanning.
7.2. MRI acquisition
MR scans were collected using a GE MR750 3.0 Tesla Scanner (General Electric, USA) installed at the Jinling hospital. A gradient echo planar imaging (EPI) sequence was used for fMRI, with the following parameters: TR = 2000 ms, TE = 30 ms, flip angle = 80°, FOV = 240 mm, matrix = 64 64, slice thickness = 3.2 mm, no gap, number of slices = 43, number of volumes = 160. The slices covered the whole brain, including the fronto-parietal cortex and the lower parts of the cerebellum. We also collected high-resolution T1-weighted structural images, using a 3D-BRAVO sequence with the following parameters: TR = 8.2 ms, TE = 3.2 ms, flip angle = 12°, FOV = 220 mm 20 mm, Matrix = 256,256, slice thickness = 1 mm. Diffusion tensor imaging (DTI) data were collected using a single shot spin echo EPI pulse sequence with following parameters: TR = 5000 ms, TE = 98.8 ms, flip angle = 90°, FOV = 220 mm, matrix = 256 × 256, slice thickness = 4 mm, number of slices = 30, gradient orientations = 25, b = 1000s/mm2.
To prepare MR image data for analysis, images from patients with right-sided lesions were flipped with respect to the midsagittal plane, so that the affected hemisphere corresponded to the left hemisphere in all patients.
7.3. Analysis of structural damage
Lesion maps were manually outlined on the T1-weighted MR image, slice by slice, using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron). A lesion was classified as cortical if it mainly involved cortical structures, subcortical if it mainly involved corona radiata or intetrnal capsule, or both if it involved cortical as well as subcortical areas. DTI data processing, and in particular the calculation of the fractional anisotropy (FA) map, was performed with the Diffusion Toolkit (http://www.trackvis.org/dtk/). The mean fractional anisotropy (FA) was calculated within the posterior limb of each internal capsule (PLIC) to quantify the structural integrity of CST. The bilateral PLIC of each patient was manually delineated by 2 radiologists. The degree of corticospinal tract (CST) damage was quantified by calculating the asymmetry of mean fractional anisotropy (FA) values: FA asymmetry index = (FAunaffected − FAaffected) / (FAunaffected + ) (Stinear et al., 2007).
7.4. Spatial normalization of MR images
A cost-function modification was used to remove the effect of stroke lesion on spatial normalization. First, the individual 3D T1-weighted image was normalized to the standard space of the Montreal Neurological Institute (MNI) by using a 12-paramter affine transformation with nonlinear adjustments with 7 × 8 × 7 basis functions. This transformation was calculated by excluding the lesion mask from the calculation of the cost function. All individual normalized 3D T1-weighted anatomical images were averaged to generate a sample-specific brain template in MNI space. Also, a mask in MNI space containing only structurally intact voxels in all patients was obtained. Second, the averaged 3D T1-weighted anatomical image was segmented using the unified segmentation function of SPM8, using the group-level mask in MNI space for the calculation of the cost-function. Third, the individual space 3D T1-weighted images were segmented using unified segmentation function, using the tissue probability maps obtained in the previous segmentation step, and excluding the individual lesion mask for the calculation of the cost-function. This segmentation step produced a deformation field, which was used for normalizing the fMRI images (Wei et al., 2016; Stebbins et al., 2008).
7.5. fMRI preprocessing
fMRI data preprocessing was performed by means of DPARSF (http://rfmri.org/DPARSF) and SPM8 (http://www.fil.ion.ucl.ac.uk). First, slice-timing and realignment was performed, in which translation or rotation parameters in any given data set did not exceed 1.5 mm or 1.5°. Second, the functional images were co-registered to the individual 3D T1-weighted images. Third, normalized functional images were obtained by applying the deformation field to the co-registered functional images, with resampling at 3 mm isotropic voxel size. Fourth, spatial smoothing (FWHM = 8 mm) was applied to the normalized functional images (Wei et al., 2016; Stebbins et al., 2008).
7.6. fMRI data analysis
fMRI data were not analyzed if: (1) the patient was unable to cooperate and complete the fMRI scanning (2) the images were of poor quality. For each remaining fMRI dataset, the experimental conditions were modeled in SPM8 using a General linear model (GLM). A boxcar function reflecting task execution was convolved with a canonical hemodynamic response function to create a task-related regression. The first-order temporal derivative of this regressor was also generated and included in the GLM, together with the head motion parameters. The beta weight maps related to the movement of the affected hand in each patient were used to perform group-level statistical analyses using SPM8.
8. Statistics
8.1. Group-level comparisons
For behavioral performance and cortical excitability data, statistical analyses were performed with SPSS for Windows version 22 (IBM Corp. Armonk, NY, USA). The mean values among the groups were compared by either a one-way ANOVA for continuous data or a chi-squared test for categorical data. The non-parametric Kruskal–Wallis test was used to compare ranked data among the three groups. To investigate the effects of the different conditions, we used a two factor ANOVA with factors “group” × “time” on motor function score (FMA) and neurophysiological measurements respectively. Bonferroni or Least Significant Difference (LSD) correction was applied for post hoc tests. The statistical threshold was set to p < 0.05.
For fMRI data, a mixed-design ANOVA test was performed using GLM flex software (http://mrtools.mgh.harvard.edu/index.php?title=Main_Page) to compare the between-subject factor “Group” (3 levels: HF, LF, sham), the within-subject factor “Time” (2 levels: baseline, post-intervention) and the “group” × “time” interaction effects on fMRI data. The statistical threshold was set to p < 0.05, with AlphaSim correction (http://www.restfmri.net). The averaged fMRI activation values of the interaction contrast between group and time were extracted for each patient.
8.2. Correlation analysis
Pearson correlations were used to test the relationship between the fMRI activation values of significant regions in “group” × “time” interaction effects and FMA scores at baseline, post intervention and at 3 months follow-up. These statistical analyses were conducted using a threshold of p < 0.05.
8.3. Sample size calculation
Sample size calculation was based on one of our previous studies (Stagg et al., 2012), in which patients improved by 5 points on the Upper Extremity Fugl-Meyer assessment after 5 days of rTMS intervention. A power analysis revealed the necessary sample size to be n = 16 per group to achieve a statistical power of at least 90% (α = 0.05). Considering the estimated loss rate 10–15%, the required sample size was no less than 53 patients.
9. Results
At baseline, there were no significant differences among the three groups in their demographic, clinical and imaging characteristics (Table 1). Seven out of 60 patients dropped out during the trial for different reasons, notably symptom exacerbation, stent implantation or incompliance. Fifty-three patients successfully completed the intervention protocol with no adverse effects, except for two transient headaches at the beginning of stimulation (both in HF-rTMS groups). Task-evoked fMRI data were collected both before and after the intervention in 42 patients. Of the 60 patients enrolled in our study, 44 patients underwent the motor function assessment during the 3-month follow-up.
Table 1.
Demographic and clinical characteristics of all patients in three groups.
| Variables | HF-rTMS (n = 20) | LF-rTMS (n = 20) | Sham (n = 20) | P value |
|---|---|---|---|---|
| Age (years) | 54 ± 12 (30–68) | 56 ± 9 (32–69) | 56 ± 11 (35–72) | 0.714 |
| Male (%) | 14 (70) | 18 (90) | 16 (80) | 0.287 |
| Time since stroke (days) | 5 ± 4 (1–14) | 6 ± 4 (2–14) | 4 ± 3 (1−13) | 0.356 |
| Hypertension (%) | 16 (80) | 11 (55) | 10 (50) | 0.112 |
| Diabetes mellitus (%) | 7 (35) | 6 (30) | 6 (30) | 0.926 |
| Dyslipidemia (%) | 10 (50) | 7 (35) | 11 (55) | 0.419 |
| Smoking (%) | 11 (55) | 12 (60) | 11 (55) | 0.934 |
| Handedness (% right) | 20 (100) | 19 (95) | 20 (100) | 0.362 |
| Lesion side (% left) | 15 (75) | 8 (40) | 10 (50) | 0.072 |
| Lesion volume (cm3) | 4.13 ± 3.11 | 8.58 ± 10.23 | 9.86 ± 17.9 | 0.295 |
| FA asymmetry | 0.02 ± 0.02 | 0.04 ± 0.04 | 0.04 ± 0.07 | 0.476 |
| Lesion location (%) | 0.819 | |||
| Cortical | 1 (5) | 0 | 1 (5) | |
| Subcortical | 16 (80) | 15 (75) | 15 (75) | |
| Both | 3 (16.7) | 4 (21.1) | 4 (25.0) | |
| Large artery stenosis or occlusion (%) | 7 (35) | 8 (40) | 8 (40) | 0.932 |
| NIHSS score | 7 (5–12) | 8 (5–10) | 7 (7–11) | 0.728 |
| FMA score | 29 ± 16 (2–59) | 30 ± 17 (4–56) | 26 ± 14 (2–53) | 0.591 |
| MRC score | 3 (2–3) | 3 (1–3) | 2 (1–3) | 0.550 |
| mRS score | 4 (3–4) | 4 (3–4) | 4 (3–4) | 0.430 |
| MEP status (+) (%) | 12 (60) | 12 (60) | 12 (60) | 1.0 |
Values presented are mean ± SD (range) or median [IQR]. HF, high frequency; LF, low frequency; FMA, Fugl–Meyer assessment of upper extremity; MRC, Medical Research Council Scale for hand muscles; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; FA, fractional anisotropy; MEP, motor-evoked potential of the affected hemisphere.
9.1. Motor performance
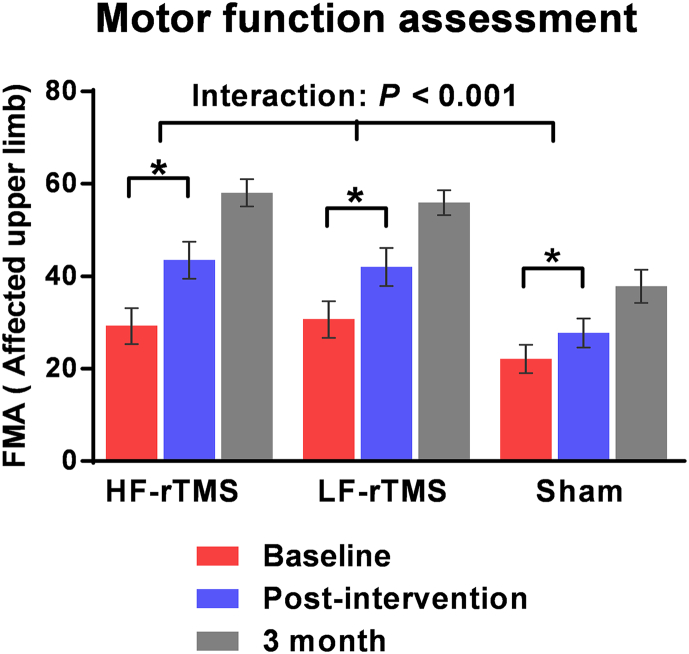
Two-factor ANOVA revealed a main effect of “time” for the FMA score (F = 248.363, df = 1, p < 0.001), indicating that all patients significantly improved in motor function post-intervention compared to baseline assessment (n = 53). A significant interaction between “group” and “time” was also found (F = 13.996, df = 2, p < 0.001), when comparing the improvement in FMA scores over time between groups (HF-rTMS vs. sham, p = 0.039; LF-rTMS vs. sham, p = 0.037; HF-rTMS vs. LF-rTMS, p = 0.997). A significant interaction between “group” and “time” (F = 2.855, df = 4, p = 0.047) was still observed when we recalculated the analyses for patients who were tested at 3 months follow-up (n = 44). Post-hoc t-tests revealed that FMA scores were significantly higher in both rTMS groups compared to the sham group (HF-rTMS vs. sham, p = 0.011; LF-rTMS vs. sham, p = 0.022; HF-rTMS vs. LF-rTMS, p = 1.0) (Fig. 2).
Fig. 2.
Motor impairment of upper extremity: Fugl–Meyer Assessment at 3 time points: baseline (red), post-intervention (blue), and 3 months follow-up (gray), for high frequency (HF) rTMS, low frequency (LF) rTMS and sham group. Values are mean ± SEM. Patients in all groups significantly improved at post-intervention compared to baseline (*p < 0.001). Both the real rTMS groups had significantly larger improvement in the FMA score than the sham group (ANOVA “group × time” interaction: p < 0.001). Finally, motor function was still significantly different between groups (ANOVA “group × time” interaction: p = 0.047) when adding patients with 3 months follow-up data (n = 44). rTMS, repetitive transcranial magnetic stimulation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The intention to treat analysis yielded comparable results (n = 60). Indeed, we observed a main effect of “time” for all groups with regard to the FMA score (F = 160.9, df = 1.3, p < 0.001). The interaction effect remained significant (F = 5.521, df = 4, p = 0.003). The post-hoc t-tests still revealed significant post-intervention improvements in the rTMS groups, but not in the sham group (HF-rTMS vs. sham, p = 0.027; LF-rTMS vs. sham, p = 0.032; HF-rTMS vs. LF-rTMS, p = 0.933).
The mRS scores were clearly different between group at post-intervention (p = 0.037) and 3 months (p = 0.002), indicating that the promising motor recovery indexed by FMA might translate into reduction of stroke disability.
9.2. Neurophysiological measures
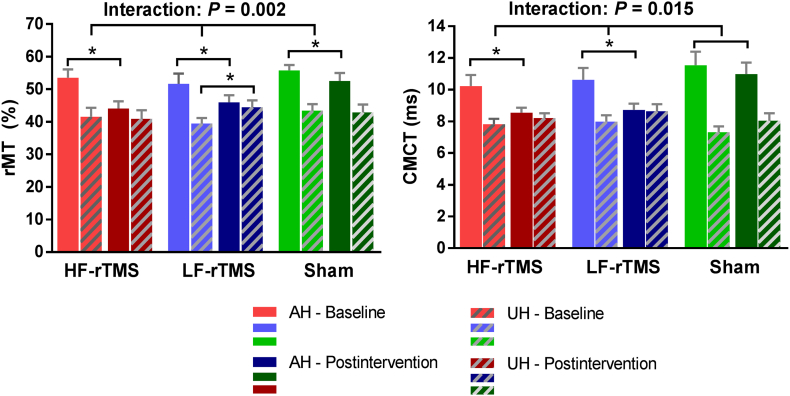
Of the 53 patients who completed the intervention, we excluded from the neurophysiological analysis 22 patients who had no evoked response in the affected hemisphere at baseline. No significant difference was evident for neurophysiological measurements at baseline among groups (All p > 0.2). However, we found significant interactions between “group” and “time” for rMT (F = 7.786, p = 0.002) and CMCT (F = 4.888, p = 0.015) of the affected hemisphere, indicating that cortical excitability of the affected hemisphere increased (decreased rMT and CMCT) in both HF-rTMS and LF-rTMS groups (all p < 0.01) between baseline and post-intervention measurements. A significant suppression of the unaffected hemisphere was observed in the LF-rTMS group (increased rMT, p = 0.013), whereas no prominent changes in the HF-rTMS and sham groups were found (Table 2, Fig. 3).
Table 2.
Changes in neurophysiological measures after rTMS in patients with MEP (+) (n = 31).
| Variables | Group | Pre | Post | Difference | P value |
|---|---|---|---|---|---|
| rMT of AH (%) | 0.002a | ||||
| HF-rTMS | 53.55 ± 8.42 | 44.09 ± 7.23 | −9.46 ± 3.11 | <0.001b | |
| LF-rTMS | 51.64 ± 10.53 | 46.0 ± 7.14 | −5.64 ± 4.11 | 0.001b | |
| Sham | 55.78 ± 4.94 | 52.56 ± 7.33 | −3.2 ± 3.42 | 0.022b | |
| rMT of UH (%) | 0.006a | ||||
| HF-rTMS | 41.55 ± 9.22 | 40.90 ± 8.78 | −0.64 ± 3.1 | 0.512b | |
| LF-rTMS | 39.45 ± 5.68 | 44.45 ± 7.02 | 5.0 ± 5.46 | 0.013b | |
| Sham | 43.44 ± 5.94 | 42.89 ± 7.29 | −0.56 ± 3.58 | 0.653b | |
| MEP latency of AH (ms) | 0.922a | ||||
| HF-rTMS | 23.42 ± 2.53 | 22.42 ± 2.23 | −1.0 ± 1.89 | 0.111b | |
| LF-rTMS | 24.08 ± 2.46 | 23.39 ± 2.22 | −0.69 ± 1.91 | 0.259b | |
| Sham | 24.11 ± 23.12 | 23.12 ± 2.7 | −0.99 ± 2.23 | 0.221b | |
| MEP latency of UH (ms) | 0.424a | ||||
| HF-rTMS | 20.72 ± 1.96 | 21.01 ± 1.87 | 0.29 ± 0.37 | 0.025b | |
| LF-rTMS | 21.45 ± 1.72 | 21.98 ± 1.5 | 0.54 ± 1.26 | 0.189b | |
| Sham | 20.26 ± 0.74 | 21.79 ± 3.87 | 1.53 ± 3.78 | 0.259b | |
| MEP amplitude of AH (μV) | 0.275a | ||||
| HF-rTMS | 0.84 ± 1.0 | 1.13 ± 1.11 | 0.29 ± 0.31 | 0.012b | |
| LF-rTMS | 0.73 ± 0.89 | 1.07 ± 1.02 | 0.34 ± 0.50 | 0.048b | |
| Sham | 0.31 ± 0.42 | 0.39 ± 0.54 | 0.08 ± 0.19 | 0.25b | |
| MEP amplitude of UH (μV) | 0.662a | ||||
| HF-rTMS | 5.54 ± 2.19 | 5.25 ± 2.26 | −0.29 ± 1.23 | 0.459b | |
| LF-rTMS | 5.59 ± 2.82 | 4.71 ± 2.49 | −0.86 ± 1.81 | 0.144b | |
| Sham | 5.71 ± 1.69 | 5.07 ± 1.6 | −0.64 ± 1.33 | 0.189b | |
| CMCT of AH (ms) | 0.015a | ||||
| HF-rTMS | 10.23 ± 2.3 | 8.77 ± 1.21 | −1.45 ± 1.44 | 0.007b | |
| LF-rTMS | 10.63 ± 2.45 | 9.18 ± 1.82 | −1.45 ± 1.2 | 0.003b | |
| Sham | 11.66 ± 2.76 | 11.54 ± 2.55 | −0.11 ± 1.07 | 0.765b | |
| CMCT of UH (ms) | 0.753a | ||||
| HF-rTMS | 7.82 ± 1.14 | 8.21 ± 1.02 | 0.39 ± 0.86 | 0.163b | |
| LF-rTMS | 7.98 ± 1.34 | 8.64 ± 1.47 | 0.65 ± 1.14 | 0.086b | |
| Sham | 7.32 ± 1.07 | 8.04 ± 1.38 | 0.72 ± 1.15 | 0.097b |
rTMS, repetitive transcranial magnetic stimulation; MEP, motor-evoked potential of the affected hemisphere; AH, affected hemisphere; UH, unaffected hemisphere; HF, high frequency; LF, low frequency; rMT, resting motor threshold; CMCT, corticomotor conduction time. Values represent (mean ± standard deviation). Figures marked in bold indicate significant results (P < 0.05).
P values determined using repeated-measure ANOVA, represent differences between groups from baseline to post-intervention.
P values determined using a paired t-test, represent changes within groups from baseline to post-intervention.
Fig. 3.
Neurophysiological changes from baseline to post-intervention assessments for high frequency (HF) rTMS, low frequency (LF) rTMS and sham group. Values are mean ± SEM. There was a significant increase in cortical excitability of the affected hemisphere as demonstrated by decreased resting motor threshold (rMT) (A) and central motor conduction time (CMCT) of affected hemisphere (B) in the rTMS groups compared with the sham group (ANOVA “group × time” interaction: p = .002 and p = 0.015, respectively). A significant excitability suppression of the unaffected hemisphere was observed in the LF-rTMS group (increased rMT; p = 0.013), whereas no such changes were found in the HF-rTMS and sham groups. AH, affected hemisphere; UH, unaffected hemisphere; rTMS, repetitive transcranial magnetic stimulation. * indicates p < 0.05.
9.3. fMRI results
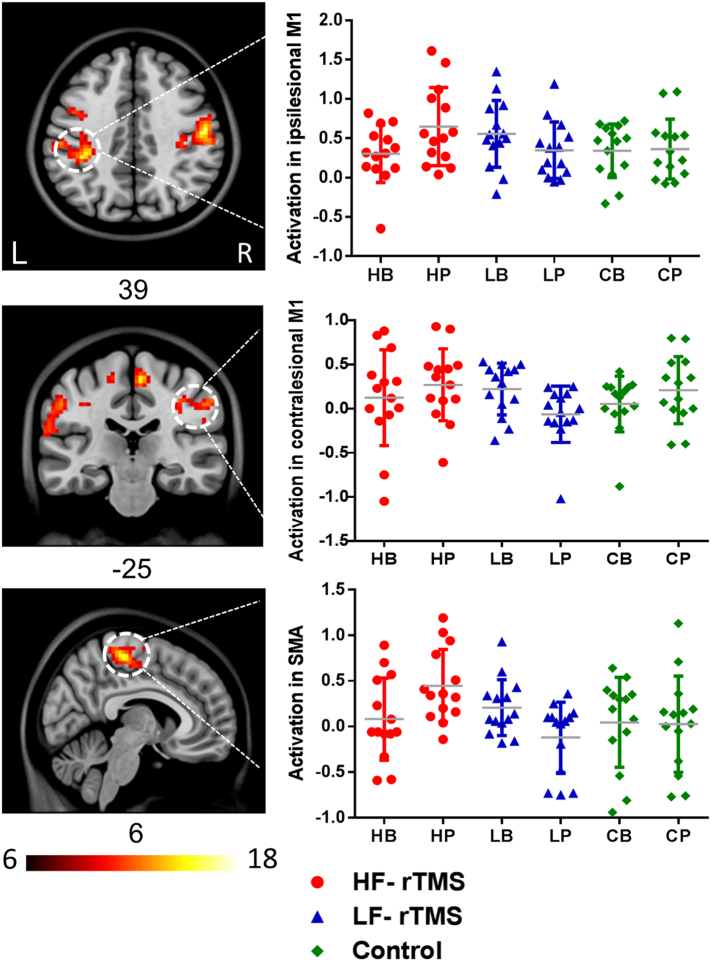
Brain activity at baseline during the movement of the affected hand, measured in the 42 patients who underwent fMRI both before and after the intervention, did not show significant differences among the three groups. All patients exhibited increased fMRI activation in the ipsilesional M1 from baseline to post-intervention (Main effect of “time”, p < 0.05). The interaction map between “group” and “time” isolated fMRI activation in bilateral M1 and SMA (p < 0.01, AlphaSim corrected). Then we further compared the changes of fMRI activation among the three groups on a region of interest level (“group” × “time” interaction) (Table 3). HF-rTMS group had a significantly increased fMRI activation in the ipsilesional M1 and SMA, compared with the LF-rTMS and sham groups (p < 0.05), and the LF-rTMS group had a significant reduction in fMRI activation in the contralesional M1, compared with the HF-rTMS and sham groups (p < 0.05) (Fig. 4).
Table 3.
rTMS-induced changes in fMRI activation within the motor cortex during movement of the affected hand.
| The fMRI ROI |
HF-rTMS |
LF-rTMS |
Sham |
P Value |
HF vs. Sham |
LF vs. Sham |
HF vs. LF |
|---|---|---|---|---|---|---|---|
| Group × Time interaction | (n = 14) | (n = 14) | (n = 14) | Adjusted P-value | Adjusted P-value | Adjusted P-value | |
| Ipsilesional M1 | |||||||
| Baseline | 0.20 ± 0.49 | 0.24 ± 0.44 | 0.11 ± 0.39 | 0.738 | |||
| Post-intervention | 0.51 ± 0.53 | 0.08 ± 0.34 | 0.10 ± 0.46 | 0.026 | 0.021 | 0.92 | 0.017 |
| Change | 0.31 ± 0.58 | −0.16 ± 0.34 | −0.01 ± 0.41 | 0.028 | 0.066 | 0.4 | 0.009 |
| Contralesional M1 | |||||||
| Baseline | 0.12 ± 0.54 | 0.22 ± 0.29 | 0.05 ± 0.32 | 0.541 | |||
| Post-intervention | 0.27 ± 0.41 | −0.06 ± 0.32 | 0.21 ± 0.38 | 0.05 | 0.667 | 0.058 | 0.022 |
| Change | 0.15 ± 0.45 | −0.29 ± 0.43 | 0.16 ± 0.48 | 0.021 | 0.954 | 0.014 | 0.017 |
| SMA | |||||||
| Baseline | 0.08 ± 0.45 | 0.21 ± 0.31 | 0.05 ± 0.49 | 0.573 | |||
| Post-intervention | 0.45 ± 0.40 | −0.12 ± 0.40 | 0.03 ± 0.53 | 0.005 | 0.016 | 0.388 | 0.002 |
| Change | 0.37 ± 0.48 | −0.33 ± 0.48 | −0.02 ± 0.74 | 0.012 | 0.087 | 0.169 | 0.003 |
Note: Values are expressed as mean ± SD (range). P-value adjustment for multiple comparisons was applied by the Bonferroni method. Bold font indicates statistically significant difference at P < 0.05.
Fig. 4.
Changes in neural activity during movements of the affected hand from baseline to post-intervention were significant in bilateral M1 and SMA for high frequency (HF) rTMS, low frequency (LF) rTMS and control group (ANOVA “group × time” interaction: p < 0.01). fMRI activation of the ipsilesional M1 and SMA significantly increased in the HF-rTMS group, compared with the LF-rTMS and control groups. There was a decrease in fMRI activation of the contralesional M1 in the LF-rTMS group, compared with the HF-rTMS and control groups. rTMS, repetitive transcranial magnetic stimulation; HB, HF-rTMS group at baseline; HP, HF-rTMS group at post-intervention; LB, LF-rTMS group at baseline; LP, LF-rTMS at post-intervention; CB, control group at baseline; CP, control group at post-intervention.
9.4. Correlation between motor performance and neural activity
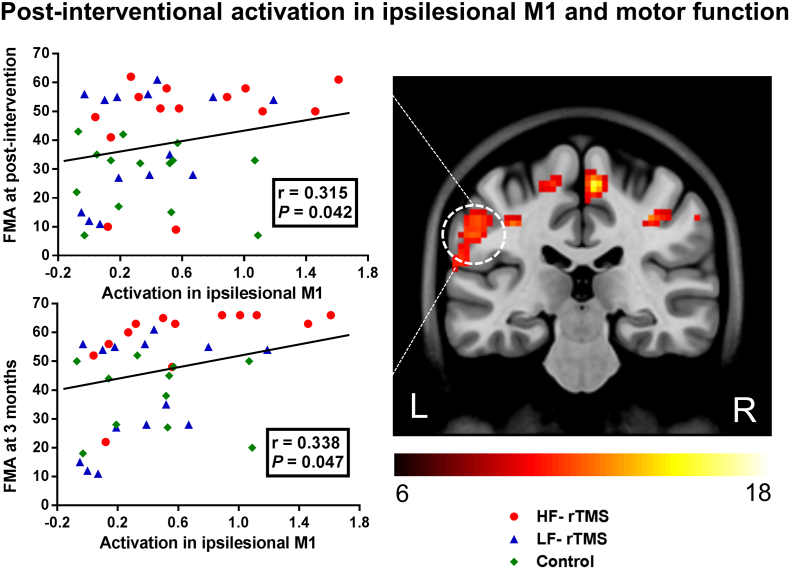
We observed a significant positive correlation between post-interventional fMRI activation in the ipsilesional M1 and motor function (r = 0.315, p = 0.042). Importantly, there was also a significant correlation between post-interventional activation in the ipsilesional M1 and motor function at 3 months (r = 0.338, p = 0.047) (Fig. 5). Thus, patients with better motor outcome had increased fMRI activation within ipsilesional M1 in the early phase of stroke. There was no significant correlation between the rTMS-induced change in neural activity and motor improvement from baseline to post-intervention.
Fig. 5.
Correlation between neural activity and motor performance. There were positive significant correlations between ipsilesional M1 activation and motor function both at post-intervention (r = 0.315, p = 0.042) and 3 month follow-up (r = 0.338, p = 0.047). Increased fMRI activation in ipsilesional M1 was observed in patients with good motor outcome. FMA, Fugl–Meyer Assessment; M1, primary motor cortex.
10. Discussion
Our study revealed significant motor improvements in stroke patients who received either HF- or LF-rTMS treatment as compared to sham stimulation. Notably, all patients underwent standard physical and medical therapy, and showed rTMS-induced changes in motor cortical activity, demonstrated by fMRI and cortical excitability assessments. Most importantly, the effects of rTMS persisted beyond the intervention up to at least 3 months.
For the first time, we conducted a randomized, sham-controlled clinical trial to examine the differential effects of HF- and LF-rTMS on motor cortical activation in a large sample of stroke patients. Our results confirmed the clinical therapeutic effect of rTMS on motor recovery in the early stage after stoke, which is consistent with previous studies conducted in chronic stroke patients (Chang et al., 2012; Ameli et al., 2009; Sung et al., 2013). In this study, we included stroke patients with cortical and subcortical infarcts involving the motor pathway. It is considered that rTMS can modulate the whole corticospinal tract excitability in response to the stimulate output (Auriat et al., 2015). Moreover, a number of studies demonstrated that rTMS modulate neuroplasticity not only locally below the magnetic coil but also in remote cortical and subcortical regions through functional connectivity of motor network (Bestmann et al., 2004; Cheng et al., 2014). Nevertheless, the differential mechanisms of rTMS in cortical and subcortical stroke should be further clarified in future study.
We provided multimodal evidence concerning motor recovery, as gathered through clinical, neurophysiological and fMRI assessments, and underpinned neuroplastic mechanisms of different rTMS protocols in acute and subacute stages after stroke. Neuroplasticity is generally beneficial, but in certain situations can also be maladaptive and hamper recovery (Langhorne et al., 2011; Johnston, 2009). The rationale for rTMS application over the motor cortex was based on the IHI theory, which provides an account of how maladaptive plasticity process that occurs after stroke may interfere with functional recovery (Marque et al., 2014). Our fMRI data, in accordance with cortical excitability measures, showed that HF-rTMS applied over ipsilesional M1 enhanced the neural activity of the ipsilesional motor areas, and that LF-rTMS applied over contralesional M1 reduced the over-activity of the contralesional motor areas. In both cases, rTMS was able to counteract maladaptive patterns of cortical activity after stroke.
Noteworthy, the effect on motor-related ipsilesional M1 activation of inhibitory rTMS over the contralesional M1 was much less pronounced as compared to the effect of facilitatory rTMS. It was previously suggested that inhibitory rTMS on the contralesional M1 could improve motor performance by rebalancing altered connectivity in the motor network (Grefkes et al., 2010). Univariate analyses of functional MRI data from our study do easily provide insights into motor network architecture. As such, future studies focused on connectivity analysis could possibly be used to test whether modulation of cortical network with inhibitory rTMS is a critical mediator for the behavioral effects observed in our study (Grefkes and Fink, 2014).
Importantly, we also found a relationship between motor-related fMRI activations and FMA scores in stroke patients. Specifically, motor-related brain activation in the ipsilesional M1 after rTMS was correlated with motor function, measured both within 24 h after intervention and after 3 months. This suggested that the restitution of normal ipsilesional M1 activity levels might play a critical role in motor recovery (Favre et al., 2014). As such, ipsilesional M1 activation could be possibly used as a physiological target for rehabilitative therapy early after stroke.
Considering the limitations of our study, it is worth mentioning that we have not assessed the immediate after-effects of rTMS on motor cortex by use of fMRI, because our fMRI acquisition was done at least 24 h after intervention. As such, our data do not reflect short-term rTMS-induced neuroplastic effects. Furthermore, although comparisons of patient assessments at baseline showed no statistical differences, the average lesion volume in the sham group was larger than in the rTMS groups, which may influence the results to a certain extent. Finally, since fMRI data were collected in stroke patients with a wide range of motor impairments, we conducted the fMRI experiment using passive movements of the affected hand to ensure task consistency. To the best of our knowledge, passive and active motor tasks have been found to induce nearly identical patterns of brain activation in healthy individuals and stroke patients (Tombari et al., 2004).
11. Conclusion
Our study demonstrates that plasticity-inducing rTMS intervention promotes motor recovery in the acute and subacute phase after stroke. Strikingly, an early modulation of motor cortical plasticity by rTMS can contribute to an improved recovery of motor function. Our findings provide robust multi-modal evidence supporting rTMS as a means to enhance motor rehabilitation, and also shed light on the neuroplastic mechanisms of rTMS on motor recovery after stroke.
Acknowledgments
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81530038, 81501193, 81220108008, 81701299, 81771424), Jiangsu Province Foundation of China (no. BK20141373) and the special scientific research fund of public welfare profession of national health and family planning commission of China (no. 201402019).
Conflicts of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.101620.
Contributor Information
Zhiqiang Zhang, Email: zhangzq2001@126.com.
Guangming Lu, Email: cjr.luguangming@vip.163.com.
Xinfeng Liu, Email: xfliu2@vip.163.com.
Appendix A. Supplementary data
Supplementary material
References
- Adeyemo B.O., Simis M., Macea D.D., Fregni F. Systematic review of parameters of stimulation, clinical trial design characteristics, and motor outcomes in non-invasive brain stimulation in stroke. Front. Psychiatry. 2012;3:88. doi: 10.3389/fpsyt.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameli M., Grefkes C., Kemper F., Riegg F.P., Rehme A.K., Karbe H. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann. Neurol. 2009;66:298–309. doi: 10.1002/ana.21725. [DOI] [PubMed] [Google Scholar]
- Auriat A.M., Neva J.L., Peters S., Ferris J.K., Boyd L.A. A review of transcranial magnetic stimulation and multimodal neuroimaging to characterize post-stroke neuroplasticity. Front. Neurol. 2015;6:226. doi: 10.3389/fneur.2015.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S., Baudewig J., Siebner H.R., Rothwell J.C., Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur. J. Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Brott T., Adams H.J., Olinger C.P., Marler J.R., Barsan W.G., Biller J. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- Buma F., Kwakkel G., Ramsey N. Understanding upper limb recovery after stroke. Restor. Neurol. Neurosci. 2013;31:707–722. doi: 10.3233/RNN-130332. [DOI] [PubMed] [Google Scholar]
- Chang W.H., Kim Y.H., Yoo W.K., Goo K.H., Park C.H., ST Kim. rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restor. Neurol. Neurosci. 2012;30:179–189. doi: 10.3233/RNN-2012-110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M.C., Kim D.Y., Park D.H. Enhancement of cortical excitability and lower limb motor function in patients with stroke by transcranial direct current stimulation. Brain Stimul. 2015;8:561–566. doi: 10.1016/j.brs.2015.01.411. [DOI] [PubMed] [Google Scholar]
- Cheng M.Y., Wang E.H., Woodson W.J., Wang S., Sun G., Lee A.G. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc. Natl. Acad. Sci. U. S. A. 2014;111(35):12913–12918. doi: 10.1073/pnas.1404109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty's Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O'Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain. 2010;133:2838–2844. doi: 10.1093/brain/awq270. [DOI] [PubMed] [Google Scholar]
- Cramer S.C., Riley J.D. Neuroplasticity and brain repair after stroke. Curr. Opin. Neurol. 2008;21:76–82. doi: 10.1097/WCO.0b013e3282f36cb6. [DOI] [PubMed] [Google Scholar]
- Du J., Tian L., Liu W., Hu J., Xu G., Ma M. Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur. J. Neurol. 2016;23:1666–1672. doi: 10.1111/ene.13105. [DOI] [PubMed] [Google Scholar]
- Favre I., Zeffiro T.A., Detante O., Krainik A., Hommel M., Jaillard A. Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: a meta-analysis. Stroke. 2014;45:1077–1083. doi: 10.1161/STROKEAHA.113.003168. [DOI] [PubMed] [Google Scholar]
- Gladstone D.J., Danells C.J., Black S.E. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil. Neural Repair. 2002;16:232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- Grefkes C., Fink G.R. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014;13:206–216. doi: 10.1016/S1474-4422(13)70264-3. [DOI] [PubMed] [Google Scholar]
- Grefkes C., Nowak D.A., Wang L.E., Dafotakis M., Eickhoff S.B., Fink G.R. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. NeuroImage. 2010;50:233–242. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L.G., Mall V. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey G.J., Jamrozik K., Broadhurst R.J., Forbes S., Anderson C.S. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989–1990. Stroke. 2002;33:1034–1040. doi: 10.1161/01.str.0000012515.66889.24. [DOI] [PubMed] [Google Scholar]
- Johnston M.V. Plasticity in the developing brain: implications for rehabilitation. Dev. Disabil. Res. Rev. 2009;15:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- Khedr E.M., Ahmed M.A., Fathy N., Rothwell J.C. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Langhorne P., Bernhardt J., Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- Le Q., Qu Y., Tao Y., Zhu S. Effects of repetitive transcranial magnetic stimulation on hand function recovery and excitability of the motor cortex after stroke: a meta-analysis. Am. J. Phys. Med. Rehabil. 2014;93:422–430. doi: 10.1097/PHM.0000000000000027. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang X.W., Zuo Z.T., Lu J., Meng C.L., Fang H.Y. Cerebral functional reorganization in ischemic stroke after repetitive transcranial magnetic stimulation: an fMRI study. CNS Neurosci. Ther. 2016;22:952–960. doi: 10.1111/cns.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludemann-Podubecka J., Bosl K., Nowak D.A. Repetitive transcranial magnetic stimulation for motor recovery of the upper limb after stroke. Prog. Brain Res. 2015;218:281–311. doi: 10.1016/bs.pbr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Marque P., Gasq D., Castel-Lacanal E., De Boissezon X., Loubinoux I. Post-stroke hemiplegia rehabilitation: evolution of the concepts. Ann. Phys. Rehabil. Med. 2014;57:520–529. doi: 10.1016/j.rehab.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Mayo N.E., Wood-Dauphinee S., Ahmed S., Gordon C., Higgins J., McEwen S. Disablement following stroke. Disabil. Rehabil. 1999;21:258–268. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- McDonnell M.N., Stinear C.M. TMS measures of motor cortex function after stroke: a meta-analysis. Brain Stimul. 2017;10:721–734. doi: 10.1016/j.brs.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Murase N., Duque J., Mazzocchio R., Cohen L.G. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Murphy T.H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Nowak D.A., Grefkes C., Ameli M., Fink G.R. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil. Neural Repair. 2009;23:641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- Rehme A.K., Eickhoff S.B., Rottschy C., Fink G.R., Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59:2771–2782. doi: 10.1016/j.neuroimage.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Bachtiar V., O'Shea J., Allman C., Bosnell R.A., Kischka U. Cortical activation changes underlying stimulation-induced behavioural gains in chronic stroke. Brain. 2012;135:276–284. doi: 10.1093/brain/awr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins G.T., Nyenhuis D.L., Wang C., Cox J.L., Freels S., Bangen K. Gray matter atrophy in patients with ischemic stroke with cognitive impairment. Stroke. 2008;39:785–793. doi: 10.1161/STROKEAHA.107.507392. [DOI] [PubMed] [Google Scholar]
- Stinear C.M., Barber P.A., Smale P.R., Coxon J.P., Fleming M.K., Byblow W.D. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Sung W.H., Wang C.P., Chou C.L., Chen Y.C., Chang Y.C., Tsai P.Y. Efficacy of coupling inhibitory and facilitatory repetitive transcranial magnetic stimulation to enhance motor recovery in hemiplegic stroke patients. Stroke. 2013;44:1375–1382. doi: 10.1161/STROKEAHA.111.000522. [DOI] [PubMed] [Google Scholar]
- Tombari D., Loubinoux I., Pariente J., Gerdelat A., Albucher J.F., Tardy J. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. NeuroImage. 2004;23:827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Ward N.S. Mechanisms underlying recovery of motor function after stroke. Postgrad. Med. J. 2005;81:510–514. doi: 10.1136/pgmj.2004.030809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Zhang Z., Xu Q., Yang F., Sun K., Lu G. More severe extratemporal damages in mesial temporal lobe epilepsy with hippocampal sclerosis than that with other lesions: a multimodality MRI study. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.T., Hareendran A., Grant M., Baird T., Schulz U.G., Muir K.W. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33:2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material