Abstract
Summary
Background
Routine data from Malawi’s prevention of mother-to-child transmission (MTCT) option B+ programme suggest high uptake of antiretroviral therapy (ART) among pregnant women. Malawi’s Ministry of Health led the National Evaluation of Malawi’s PMTCT Program to obtain nationally representative data on maternal ART coverage and prevention of MTCT effectiveness. Here, we present the early transmission data for infants aged 4–12 weeks.
Methods
We used a multistage cluster design to recruit a nationally representative sample of HIV-exposed infants and their mothers in Malawi. Between October 16, 2014, and May 17, 2016, we screened for HIV in all mothers attending an under-5 vaccination or outpatient sick-child clinic with infants aged 4–26 weeks at 54 health facilities selected across ten districts and four regional sampling zones. Infants with mothers identified as HIV-infected were enrolled in the cohort. We calculated weighted MTCT rates for only the subset of infants aged 4–12 weeks at screening, thereby capturing MTCT from early pregnancy, to delivery, and early breastfeeding. We collected data on maternal and infant demographics and self-reported use of HIV services, ART, and antenatal clinics. We tested HIV-exposed infants for the virus and assessed associations of certain variables with infant HIV status.
Findings
We confirmed HIV exposure in 3542 (10.4%) of 33 980 mother (guardian)-infant pairs with infants aged 4–26 weeks. Of those, 2530 (2514 mothers and 16 guardians) had infants aged 4–12 weeks at the time of screening (2498 singlets and 32 twins). We excluded 25 infants from the analysis because no information was available about their HIV status. 91.3% (95% CI 85.6–96.9) of mothers were on ART during pregnancy. The MTCT rate was 3.7% (2.3–6.0) overall and ranged from 1–4% (0.4–4.4) in women who initiated ART before pregnancy to 19–9% (13.4–28.6) in women not on ART. In multivariable logistic regression analysis, the odds of early MTCT were higher in mothers starting ART post partum (adjusted odds ratio 16.7, 95% CI 1–6-171.5; p=0–022) and in those not on ART with an unknown HIV status during pregnancy (19.1, 8–5.43–0; p<0–0001) than in mothers on ART before pregnancy. Among HIV-exposed infants, 98–0% (95% CI 96.9–99-1) were reported by the mother to have received infant nevirapine prophylaxis, and only 45–6% (34.8–56.4) were already enrolled in an exposed infant HIV care clinic at the time of study screening.
Interpretation
These data suggest that Malawi’s decentralisation of ART services has resulted in higher ART coverage and lower early MTCT. However, the uptake of services for HIV-exposed infants remains suboptimal.
Introduction
In 2011, the Malawi Ministry of Health introduced option B+, a universal treatment strategy for the prevention of mother-to-child transmission (MTCT) of HIV.1 Under option B+, all pregnant or breastfeeding women with HIV are eligible for lifelong antiretroviral therapy (ART) regardless of clinical stage or CD4 count (health-care providers can initiate ART without any laboratory testing).1 The rationale for this strategy, which went beyond WHO recommendations at the time, included bypassing limited laboratory capacity, protecting against MTCT in future pregnancies in a setting with a high fertility rate, and ultimately improving the effectiveness of prevention strategies and long-term maternal health.2 A key priority for Malawi was to reduce MTCT to below WHO’s 5% threshold by the end of breastfeeding to meet WHO criterion for virtual elimination of MTCT of HIV.3
Although the potential benefits of a treat-all strategy were clearly stated, including the observed reduction of MTCT to less than 5% when used in clinical or highincome settings,2 such a strategy had not been previously implemented at the national level in a high-prevalence, resource-constrained setting. Thus, evidence of the effectiveness of option B+ was not available, and stakeholders raised concerns about feasibility and affordability,4 along with ethical issues related to the potential for coercion of immediate ART initiation in women who test positive for HIV at antenatal clinics.5
Option B+ was introduced in Malawi over a 6-month period between 2011 and 2012 and facilitated a greater than 700% increase in ART coverage of pregnant women with HIV in its first year.6 Several operational studies in the region have documented successes and challenges of the option B+ strategy in relation to uptake of HIV testing during pregnancy and breastfeeding;7 initiation of, and retention in, ART care;8,9 adherence to ART;9 and outcomes of multiple implementation models.10
In 2016, the Malawi Ministry of Health reported that 2.6% of HIV-exposed infants were infected at age 2 months; however, routinely collected programme data do not allow investigation of programme effectiveness at the population level because only 35% of known HIV-exposed infants have been tested for HIV by age 2 months.11 To date, there are no reliable, nationally representative data that show the effectiveness of option B+ in the prevention of MTCT. A 2014 national evaluation of the option A prevention strategy in South Africa found that MTCT in infants aged 4–8 weeks whose mothers were on ART during pregnancy was 2% compared with 10% in infants whose mothers were not on ART.12 Moreover, a 2018 national evaluation from Zimbabwe during the country’s transition from an option A to an option B+ strategy showed a cumulative MTCT rate of 3–3% at 6 weeks and 6–9% at 18 months post partum.13
The National Evaluation of Malawi’s PMTCT Program (NEMAPP) longitudinal study aimed to measure MTCT and HIV-free survival in a nationally representative cohort of HIV-exposed infants aged 4–26 weeks. The objective of this analysis is to report population-level MTCT rates under the option B+ programme at 4–12 weeks post partum to capture transmission from early pregnancy, to delivery and early breastfeeding, and to identify factors associated with transmission.
Methods
Study design and participants
We used a multistage cluster design to recruit a nationally representative sample of HIV-exposed infants aged 4–26 weeks and their mothers at 54 health facilities across ten districts and four regional sampling zones in Malawi. Follow-up lasted until the infants were aged 24 months or had stopped breastfeeding. Inclusion criteria for the mother-infant pairs were confirmed HIV exposure in infants aged 4–26 weeks at the time of screening and the mother either present at screening or confirmed dead by a legal guardian. Infants with live biological mothers who presented with a caregiver were excluded to maintain confidentiality of the mother’s HIV status. There was no lower age limit for mothers; according to Malawi law, young mothers are legally classified as adults or emancipated minors because they are sexually active.
Ethical approval for the study was provided by Malawi’s National Health Sciences Research Committee (approval number 1262) and the institutional review board of the University of Toronto (approval number 30448). All mothers or caregivers provided written informed consent for screening and then, if they tested positive for HIV, for infant enrolment in the cohort. The study was reviewed in accordance with the Centers for Disease Control and Prevention (CDC) human research protection procedures and was determined to be research, but CDC involvement did not constitute engagement in human subjects’ research (CDC approval number 2014–057).
Procedures
We screened for HIV in all mothers (or caregivers if the mother had died) with infants aged 4—26 weeks who attended an under-5 vaccination or outpatient sick-child clinic between October 16, 2014, and May 17, 2016. The under-5 clinic was selected as ideal for screening and enrolment because 97% of infants in Malawi receive their first vaccinations by age 6 weeks.14 Therefore, this entry point allowed for the broadest population-based selection of mother-infant pairs, which would not be biased by antenatal clinic attendance or HIV service use. Screening continued at each site until the required sample size of HIV-exposed infants was reached.
Trained study staff interviewed mothers and caregivers at a private location in the clinic. We abstracted data from patient-held records, antenatal clinic registers, and ART clinic records and used them to verify the mothers’ selfreported HIV status and ART uptake. After the interview, each mother received HIV pre-test counselling. Following the national protocol, HIV rapid testing in a serial algorithm was done on site for all mothers, regardless of self-reported HIV status, with Determine (Alere, Tel Aviv, Israel) as the first test and Uni-Gold (Trinity Biotech, Bray, Ireland) for confirmation; test results were returned immediately.1 We also tested for HIV antibodies in dried blood spot specimens in a reference laboratory using an EIA (Murex HIV-1.2.0; DiaSorin, Dartford, UK). In the event of subsequent discordant results between the rapid test and the EIA, we repeated the process at the next patient visit. A positive rapid test and positive EIA result in the mother indicated confirmed HIV infection. We retested all maternal samples with discordant rapid test and EIA results after the second test using the Geenius HIV 1/2 Supplemental Assay (Bio-Rad Laboratories, Hercules, CA, USA). A positive EIA result in the mother, infant, or both indicated infant HIV exposure. We did a qualitative HIV-1 DNA PCR test (COBAS AmpliPrep/COBAS TaqMan HIV-1 Qualitative Assay version 2.0; Roche Diagnostics, Indianapolis, IN, USA) on dried blood spot samples from all HIV-exposed infants to ascertain their HIV status.
Statistical analysis
We aimed to draw a representative sample of participants across Malawi to provide national and subnational estimates of MTCT of HIV. We identified four strata that were similar in terms of HIV epidemiology and service uptake: north-central rural, which consisted of north, central-west, and central-east zones; south rural, which consisted of southwest and southeast zones; and Blantyre and Lilongwe, which are the two largest urban areas in Malawi and were considered as separate and individual strata. In the first stage of sampling, we selected all districts in Blantyre and Lilongwe and then, using simple random sampling, four districts from each of the two remaining strata; thus, we included ten of the 28 districts in Malawi. In the second stage, 54 of 290 possible health facilities were sampled from within the ten selected districts (14 in north-central rural strata, nine in Lilongwe, 22 in south rural strata, and nine in Blantyre) with probability proportional to size sampling with replacement; facility size was categorised according to annual antenatal clinic attendance in 2013.15 In the third stage, we consecutively sampled all eligible mother-infant pairs. We calculated sampling weights as the inverse of the product of the three-stage selection probabilities and adjusted for variations in the length of the enrolment period between health facilities.
We based sample size calculations for NEMAPP on estimated, population-based MTCT rates for infants aged 4–12 weeks, 12 months, or 24 months using Malawi Ministry of Health data that included known distributions of ART regimens and timing of mother’s ART initiation. We calculated sample sizes required to measure MTCT in infants aged 4–12 weeks or 24 months using an online tool,16 requiring a precision of 2.5% for national estimates and 5% for strata estimates with 95% CIs. To account for the complex design of the survey in the sample size calculations, we inflated national and stratum-level sample sizes based on design effects, which we assumed to be 2.0 and 1.5, respectively. We calculated sample sizes by stratum first and then adjusted upwards proportionally to reach the required national sample size. The final national sample size required to estimate MTCT up to age 24 months was 3376 infants, including at least 1448 infants aged 4–12 weeks for estimation of early transmission.
We did a domain analysis by HIV status of exposed infants aged 4–12 weeks whose mothers were HIV positive.17 We did Rao-Scott χ2 tests to assess the strength of associations between variables and infant HIV status. We then used logistic regression analysis to assess the effect of timing of ART initiation on MTCT. We identified potential confounders using a six-step directed acyclic graph approach (figure 1).18 We included confounders that blocked backdoor paths from timing of ART initiation to MTCT as covariates in multivariable analysis.18 All statistical analyses were done with Stata release 14.2 and accounted for NEMAPP’s complex survey design (ie, stratification, weighting, and clustering).
Figure 1: Directed acyclic graph of the hypothesised causal effect of mother’s reported timing of ART initiation on early transmission to infants aged 4–12 weeks.
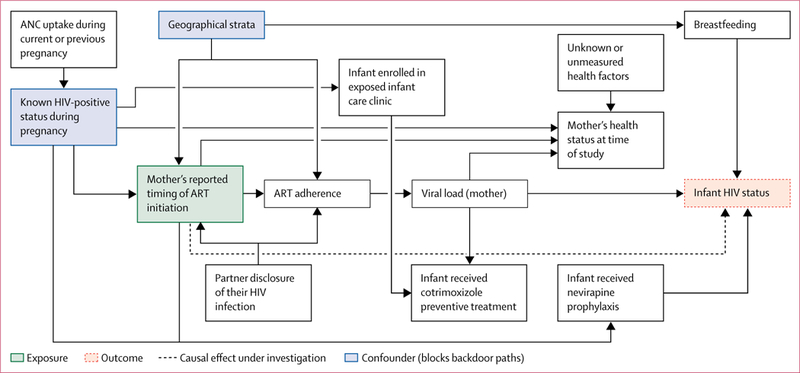
ANC=antenatal clinic. ART=antiretroviral therapy.
Role of the funding source
Funding was provided by the President’s Emergency Plan for AIDS Relief (PEPFAR), through the Centers of Disease Control and Prevention (CDC). The Malawi Ministry of Health in collaboration with CDC-Malawi, oversaw this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Oct 16, 2014, and May 17, 2016, we screened 34637 mother (or guardian)-infant pairs with infants aged 4–26 weeks for study inclusion, of whom 657 (1–9%) were excluded (figure 2). Among the 33 980 mother-infant pairs screened for HIV, including 236 guardians of infants whose mothers were confirmed dead, 30 270 were confirmed to be HIV negative, 3542 were confirmed to be exposed to HIV, and 168 had inconclusive test results. 2530 (2514 mothers and 16 guardians) of 3542 HIV- exposed mother-infant pairs had infants aged 4–12 weeks at the time of screening (2498 singlets and 32 twins). We excluded 25 infants from the analysis because no information was available about their HIV status.
Figure 2: Flow chart of mother-infant pair recruitment.
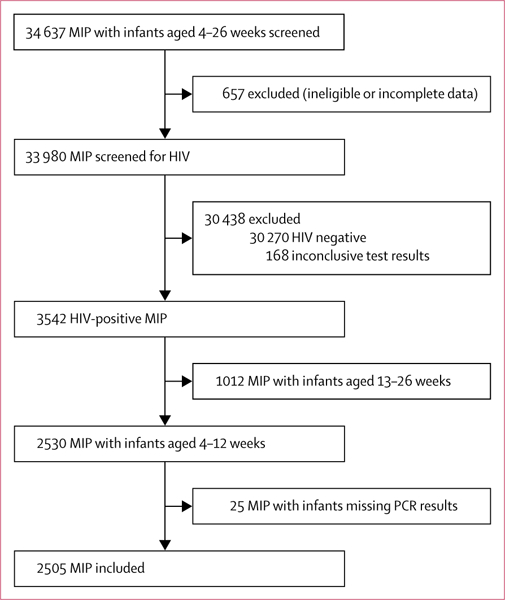
MIP=mother (or guardian)–infant pairs.
The age ofHIV-infected mothers ranged from 12 years to 53 years, with nearly a quarter being younger than 25 years (table 1). Nearly 90% of HIV-infected women reported having a cohabitating partner, and of those, over 90% reported they had disclosed their HIV status to their partner (“mother disclosed”) and their partner had shared their HIV status with them (“partner disclosed”). Almost all mothers attended antenatal clinics and reported being on ART during pregnancy: 52.3% reported starting ART before the previous pregnancy, 38.9% during the last pregnancy, 1.5% reported starting ART post partum, and 0.2% reported they had stopped ART during pregnancy. Among those on ART, 82.5% reported no missed doses in the last 30 days (table 1).
Table 1:
Characteristics of 2505 HIV-positive mothers with infants aged 4–12 weeks
| Unweighted frequency |
Weighted proportions (%)* |
|
|---|---|---|
| Regional strata | ||
| South urban | 558 | 8.0% (3.6 to 12.5) |
| South rural | 653 | 61.7% (44.4 to 78.9) |
| North urban | 663 | 10.1% (4.7to 15.5) |
| North rural | 631 | 20.2% (7.7 to 32.7) |
| Parity | ||
| One | 312 | 9.0% (6.0 to 12.0) |
| Two or more | 2193 | 91.0% (88.0 to 94.0) |
| Mother’s age | ||
| <25 years | 666 | 24.0% (20.6 to 27.4) |
| 25–34 years | 1297 | 51.5% (47.9 to 55.1) |
| ≥35 years | 542 | 24.5% (20.8 to 28.1) |
| ANC use during last pregnancy | ||
| No | 3 | 0.0% (0.0 to 0.1) |
| Yes | 2424 | 100.0% (99–9 to 100.0) |
| Missing | 78 | ·· |
| Self-reported HIV status in pregnancy | ||
| Negative | 134 | 4.3% (0.7to 7.9) |
| Positive | 2344 | 94.5% (89.3 to 99.6) |
| Not tested | 27 | 1.2% (−0.7 to 3.0) |
| Spouse or cohabitating partner | ||
| No | 184 | 10.2% (6.8 to 13.7) |
| Yes | 2264 | 89.8% (86.3 to 93.2) |
| Missing | 57 | ·· |
| Mother disclosed HIV status to partner | ||
| No | 142 | 5.1% (3–8 to 11.5) |
| Yes | 1978 | 94.9% (935 to 94.6) |
| Missing | 385 | ·· |
| Partner disclosed HIV status to mother | ||
| No | 188 | 8.0% (6.4 to 9.7) |
| Yes | 1938 | 92.0% (90.3 to 93 6) |
| Missing | 379 | |
| ART use during last pregnancy | ||
| No | 258 | 8.7% (3.1 to 14.4) |
| Yes | 2229 | 91.3% (85.6 to 96.9) |
| Missing | 18 | ·· |
| Reported timing of ART initiation | ||
| Before this pregnancy | 1148 | 52.3% (47.2 to 57.5) |
| During this pregnancy | 1081 | 38.9% (31.2 to 46.6) |
| Post partum | 33 | 1.5% (0.6 to 2.4) |
| Stopped ART | 17 | 0.2% (0.0 to 0.4) |
| Not on ART | 208 | 7.1% (2.2 to 12.0) |
| Missing | 18 | ·· |
| Reported having missed ART in the past 30 days | ||
| No | 1798 | 82·5% (79·6 to 85·4) |
| Yes | 403 | 17·5% (14·6 to 20·4) |
| Not applicable† | 225 | ·· |
| Missing | 79 | ·· |
| Reported travel time to the clinic | ||
| <1 h | 1119 | 38·6% (32·7 to 44·5) |
| 1–2 h | 911 | 37·1% (30·9 to 43·4) |
| >2 h | 408 | 24·3% (17·9 to 30·6) |
| Missing | 67 | ·· |
ANC=antenatal clinic. ART=antiretroviral therapy.
Data are % (95% CI).
Includes mothers who stopped, or were never on, ART
94.4% of HIV-exposed infants were delivered in a health facility, and 98.0% were reported by the mother to have received nevirapine prophylaxis (table 2). 45.6% were enrolled in an exposed infant HIV care clinic at the time of study screening (table 2), with the proportion varying by age group (40.2% [95% CI 28.8–52.8] of infants aged 4–7 weeks vs 55.2% [47.4–62.7] of infants aged 8–12 weeks; p=0.01). 39.6% of HIV-exposed infants had been prescribed cotrimoxazole preventive treatment (table 2), with the proportion varying by age group (29.8% [95% CI 22.3–38.6] of infants aged 4–7 weeks vs 56.8% [49.0–64.4] of infants aged 8–12 weeks; p<0–0001). Only 3–0% of mothers reported ever having fed their infants anything other than breastmilk (table 2), with 1.9% (95% CI 1.1–3.3) of infants aged 4–7 weeks having been fed something other than breastmilk compared with 4–9% (3.1–7.8) of infants aged 8–12 weeks (p=0–006). At screening,9.4% of mothers reported their HIV-exposed infant to be unwell that day, and 16.9% had presented sick at a clinic once previously (table 2).
Table 2:
Characteristics of 2505 HIV-exposed infants aged 4–12 weeks
| Unweighted frequency |
Weighted proportion (%)* |
|
|---|---|---|
| Delivered in a health facility | ||
| No | 127 | 5·6% (4·1–7·1) |
| Yes | 2320 | 94·4% (92·9–95·9) |
| Missing | 58 | ·· |
| Infant age | ||
| 4 weeks | 104 | 6·8% (2·9–10·7) |
| 5 weeks | 235 | 11·8% (9·8–13·7) |
| 6 weeks | 941 | 30·0% (25·3–34·7) |
| 7 weeks | 408 | 15·4% (12·5–18·3) |
| 8 weeks | 175 | 8·0% (6·4–9·6) |
| 9 weeks | 118 | 5·5% (4·2–6·9) |
| 10 weeks | 201 | 8·4% (6·9–9·9) |
| 11 weeks | 197 | 7·5% (6·3–8·7) |
| 12 weeks | 126 | 6·6% (4·7–8·5) |
| Received nevirapine prophylaxis (any duration) | ||
| No | 54 | 2·0% (0·9–3·1) |
| Yes | 2244 | 98·0% (96·9–99·1) |
| Missing | 207 | ·· |
| Duration of nevirapine prophylaxis | ||
| 0 weeks | 54 | 1·8% (0·8–2·7) |
| <2 weeks | 21 | 0·6% (0·1–1·1) |
| ≥2 weeks | 2430 | 97·6% (96·5–98·7) |
| Enrolled in exposed infant care clinic | ||
| No | 1334 | 54·4% (43·6–65·2) |
| Yes | 1101 | 45·6% (34·8–56·4) |
| Missing | 70 | ·· |
| Prescribed cotrimoxazole preventive treatment | ||
| No | 1408 | 60·4% (51·9–68·8) |
| Yes | 906 | 39·6% (31·2–48·1) |
| Missing | 191 | ·· |
| Health status at time of study | ||
| Well | 2221 | 90·6% (88·6–92·7) |
| Sick | 227 | 9·4% (7·3–11·4) |
| Missing | 57 | ·· |
| Received food other than breastmilk | ||
| No | 2377 | 97·0% (96·1–97·9) |
| Yes | 73 | 3·0% (2·1–3·9) |
| Missing | 55 | ·· |
| Number of times visited a clinic because of sickness | ||
| Never | 2004 | 82·5% (74·0–91·1) |
| Once | 370 | 16·9% (8·8–25·0) |
| Two or more | 14 | 0·5% (0·0–1·1) |
| Missing | 117 | ·· |
| Ever admitted to hospital | ||
| No | 2369 | 97·0% (95·7–98·3) |
| Yes | 74 | 3·0% (1·7–4·3) |
| Missing | 62 | ·· |
Data are % (95% CI).
99 of 2505 infants tested positive for HIV at the time of the study, giving a weighted early MTCT rate at age 12 weeks or less of 3–7% (table 3). Early infant transmission did not differ according to age group or regional strata but was lower in women who reported being on ART during pregnancy than in those who reported not being on ART (table 3). Transmission varied by the mother’s self-reported timing of ART initiation, with the lowest rate in women who started ART before pregnancy and the highest in those not on ART (table 3).
Table 3:
Early MTCT by mother’s self-reported ART coverage, ART timing, regional strata, and HIV-positive status
| Unweighted MTCT frequency |
Weighted proportion (%)* |
p value | |
|---|---|---|---|
| Overall transmission | 99/2505 | 3·7% (2·3–6·0) | ·· |
| Infant age group | ·· | ·· | 0·0733 |
| 4–7 weeks | 54/1688 | 2·9% (1·5–5·3) | ·· |
| 8–12 weeks | 45/817 | 5·2% (3·0–8·9) | ·· |
| MTCT option B+ coverage | ·· | ·· | <0·0001 |
| On ART | 52/2229 | 2·3% (1·3–4·0) | ·· |
| Not on ART | 47/258 | 19·6% (14·3–26·3) | ·· |
| Missing | 18 | ·· | ·· |
| Mother’s reported timing of ART initiation | ·· | ·· | <0·0001 |
| During this pregnancy | 34/1081 | 3·4% (1·5–7·6) | ·· |
| Post partum | 4/33 | 19·1% (4·6–53·5) | ·· |
| Stopped ART | 2/17 | 10·2% (2·5–33·2) | ·· |
| Not on ART | 41/208 | 19·9% (13·4–28·6) | ·· |
| Before this pregnancy | 18/1148 | 1·4% (0·4–4·4) | ·· |
| Missing | 18 | ·· | ·· |
| Regional strata | ·· | ·· | 0·651 |
| Blantyre | 21/558 | 3·3% (1·9–5·8) | ·· |
| South rural | 26/653 | 3·8% (1·8–7·9) | ·· |
| Lilongwe | 30/663 | 4·8% (3·8–6·0) | ·· |
| North-central rural | 22/631 | 3·1% (1·8–5·3) | ·· |
| Self-reported HIV status during pregnancy | ·· | ·· | <0·0001 |
| Positive | 64/2344 | 2·6% (1·6–4·0) | ·· |
| Negative | 24/134 | 20·9% (12·0–33·8) | ·· |
| Not tested | 27 | ·· | ·· |
ART=antiretroviral therapy. MTCT=mother-to-child transmission.
Data are % (95% CI).
In weighted multivariable logistic regression analysis, mothers who started ART post partum and those who had previously started and then stopped ART were 17 times and nine times more likely, respectively, to transmit HIV to their infants than were mothers who started ART before pregnancy (table 4). Mothers not on ART who were unaware of their HIV-positive status during pregnancy were 19 times more likely to transmit HIV to their infants than were mothers who started ART before pregnancy.
Table 4:
Logistic regression analysis of variables associated with results of DNA PCR HIV testing at screening of HIV-exposed infants aged 4–12 weeks
| Unweighted MTCT frequency |
Weighted proportion (%) |
Odds ratio (95% CI) |
p value | Adjusted odds ratio (95% CI) |
p value | |
|---|---|---|---|---|---|---|
| Mother’s reported timing of ART initiation | ||||||
| During this pregnancy | 34/1081 | 3·4%(1·5–7·6) | 2·5(0·5–11·9) | 0·22 | 2·5(0·5–11·9) | 0·22 |
| Post partum | 4/33 | 19·1%(4·6–53·5) | 16·5(1·6–169·5) | 0·0224 | 16·7(1·6–171·5) | 0·0223 |
| Stopped ART | 2/17 | 10·2%(2·5–33·2) | 8·0(1·3–50·3) | 0·0305 | 8·5(1·6–45·4) | 0·0171 |
| Not on ART | ||||||
| HIV-positive status known during | 6/49 | 4·1%(1·3–12·5) | 3·0(0·6–13·8) | 0·15 | 3·0(0·7–13·5) | 0·13 |
| pregnancy | ||||||
| HIV-positive status unknown during | 24/132 | 21·1%(12·3–33·9) | 18·7(8·3–42·1) | <0·0001 | 19·1(8·5–43·0) | <0·0001 |
| pregnancy | ||||||
| Before this pregnancy | 18/1148 | 1·4%(0·4–4·4) | 1·00(ref) | ·· | 1·00(ref) | ·· |
| Regional strata | ||||||
| Blantyre | 20/551 | 3·3%(1·8–5·8) | 1·2(0·5–2·7) | 0·72 | 1·1(0·4–2·5) | 0·90 |
| South rural | 21/635 | 3·5% (1·5–7·7) | 1·2(0·4–3·5) | 0·67 | 1·4(0·5–3·5) | 0·47 |
| Lilongwe | 27/653 | 4·2%(3·3–5·3) | 1·5(0·8–2·9) | 0·21 | 1·4(0·7–2·8) | 0·27 |
| North-central rural | 20/621 | 2·8%(1·6–5·1) | 1·00(ref) | ·· | 1·00(ref) | ·· |
Analyses are based on 2460 mother–infant pairs; 18 had missing data for mother’s reported timing of ART initiation and 27 had missing data for known HIV-positive status during pregnancy. Mother’s reported timing of ART initiation and known HIV-positive status during pregnancy were combined into a single variable. Not on ART was split into known and unknown HIV-positive status during pregnancy. MTCT=mother-to-child transmission. ART=antiretroviral therapy.
Discussion
This large, nationally representative study of Malawi’s option B+ programme suggests that the prevalence of early MTCT of HIV in Malawi is low and geographically homogeneous. We observed that antenatal clinic attendance and HIV testing during pregnancy were nearly universal and ART coverage in pregnant women was greater than 90%. As expected, our analysis shows that ART uptake and the timing of its initiation are the most important interventions in the cascade of care for prevention of MTCT. MTCT was strongly associated with the mother not being on ART during pregnancy and timing of ART initiation, and in women on ART before pregnancy the rate of MTCT was similar to that observed in high-income countries.2
WHO has defined minimum criteria for designating the virtual elimination of MTCT, which include meeting two impact targets for 1 year (ie, <50 new infant HIV infections per 100 000 livebirths and a <5% transmission rate among breastfeeding populations) and three HIV- related process targets in 2 years (ie, >95% antenatal clinic coverage, >95% HIV testing at antenatal clinics, and >90% ART coverage in known HIV-positive pregnant women).19 Malawi has successfully met the three process indicators for elimination of MTCT; we will report progress on the impact indicators in a subsequent manuscript.
We did not detect any significant geographical variation in early infant transmission, which was not surprising given that Malawi successfully decentralised prevention of MTCT and claims near universal access to option B+ services.20,21 Nearly all women reported attending an antenatal clinic during pregnancy, and only 1.2% of women reported not being screened or tested for HIV during the course of pregnancy. Additionally, ART coverage in those known to be infected with HIV was greater than 90%, indicating that in pregnant women, Malawi has achieved the first two 90s in the UNAIDS 90–90-90 by 2020 objectives: 90% of HIV- positive individuals know their status, and 90% of those with known status are on ART.20
Although self-reported use of nevirapine prophylaxis in infants was almost 100%, formal follow-up of HIV-exposed infants post partum remains sub-optimal: Malawi has not implemented the infant components of the continuum of prevention of MTCT services in maternity or under-5 clinics with the same rigour as it has implemented maternal prevention services in the antenatal and maternity settings. General reasons for loss to follow-up in the prevention of MTCT cascade include fear of disclosure, stigma, and insufficient social support.21,22 A qualitative study23 in Zambia has additionally linked low prevalence of early infant testing with intimate partner violence, maternal disclosure of HIV status to her partner, and ART adherence. The Malawi Ministry of Health HIV guidelines instruct health-care workers to provide infant care and early diagnostic testing at multiple entry points, including family care, under-5, and outpatient clinics.24
Further research is needed to understand the incomplete implementation of the national guidelines, including how, where, and when infant services are provided in relation to the mother’s services, and what health system changes could be implemented to more closely align infant HIV care with the mothers’ care schedule to increase uptake of early infant testing for HIV.25,26 Although one study11 in Malawi has shown increased uptake of early infant testing since before implementation of option B+, it was not enough to facilitate subsequent initiation of ART in infants. Reasons for this low uptake have not been described; however, we observed in this study that current national guidelines that require registration of HIV-exposed infants into care at maternity wards have not been comprehensively implemented, which would result in low ART coverage among infants.
This study has some limitations. First, although this is a cohort study, the results are cross-sectional and not causative because we did this analysis using only study enrolment data. This study provides an estimate of MTCT in infants aged 4–12 weeks; future publications will provide estimates at age 1 year and post-weaning, thus providing a complete measure of how close Malawi is to the virtual elimination of MTCT of HIV. Second, the health-care workers who implemented the study were unable to consistently track mothers who did not consent to study participation. Although, anecdotally, they reported almost complete acceptance, we cannot confirm that report, which could potentially result in underestimation of the national MTCT rate. Third, the study does not capture the 3% of mothers who do not take their infants to under-5 clinics, and these mother-infant pairs might have a higher risk of MTCT than those who attend these clinics, which might also lead to underestimation of the national MTCT rate. Fourth, we are unable to comment on several risk factors that might affect MTCT, such as maternal viral load, primary HIV drug resistance, socioeconomic factors, intensive adherence measures, intimate partner violence, and depression, and data collection via self-reporting might have introduced bias. However, we validated mothers’ HIV service use self-report using antenatal clinic registers and ART clinic records. Fifth, infants who died before the first visit to an under-5 vaccination or outpatient sick-child clinic were not included in this study; this omission could result in an underestimated transmission rate. Finally, we used an EIA as a confirmatory test in the laboratory testing algorithm, which is known to have a high false-positive rate (Clayton Onyango, CDC Kenya, personal communication). To confirm a positive result using dried blood spots, it would have been more appropriate to use a more specific serological assay. However, to ensure accurate confirmatory testing at the time of study implementation, all maternal samples with discordant rapid test and EIA results were retested at CDC in Atlanta using the Geenius HIV 1/2 Supplemental Assay, which can better detect maternal antibodies.
In conclusion, prevention of MTCT services are effectively decentralised and well used in Malawi, resulting in low early MTCT at the national level. As ART coverage in pregnant women reaches saturation, most of the remaining vertical transmission will be caused by incident maternal infections in the perinatal and breastfeeding period. This mode of transmission might be the most challenging to prevent because of the need for frequent retesting of a large breastfeeding population to diagnose incident infections early and initiate ART rapidly to curb the extremely high transmission risk in the early stages of maternal infection. At this stage in the HIV epidemic response, primary prevention interventions for perinatal and breastfeeding women and measures to maximise maternal ART adherence and rapid and sustained viral load suppression will be required to reach elimination targets.
Research in context.
Evidence before this study
Under Malawi’s prevention of mother-to-child transmission (MTCT) option B+ programme, all pregnant or breastfeeding women with HIV are offered lifelong antiretroviral therapy (ART) regardless of clinical stage or CD4 count. The rationale for this strategy, which went beyond WHO recommendations at the time of its implementation, was to bypass low CD4 enumeration capacity in the country, protect against MTCT in future pregnancies in a setting with a high fertility rate, and improve long-term maternal health. Although the potential benefits of option B+ were logical, evidence of its effectiveness at the national level were scarce, particularly because it was the first population-level, treat-all strategy to be implemented. We searched PubMed, MEDLINE, and Web of Science databases up to Nov 7, 2012, using the keywords “HIV”, “PMTCT”, “Africa”, “evaluation”, and “national”. No language or date restrictions were applied. We also did a general internet search using the same keywords to identify any reports not published in the peer-reviewed literature. This search did not identify any additional information relevant to the study objectives.
Added value of this study
This study is the first national evaluation of the option B+ strategy for prevention of MT CT. We investigated early MT CT under this programme, finding that the prevalence of early MTCT was low and geographically homogeneous. Furthermore, we observed that antenatal clinic attendance was nearly universal, ART coverage in pregnant women was greater than 90%, and uptake of infant nevirapine prophylaxis was almost 100%, although enrolment of infants into HIV services, including DNA PCR testing, remains suboptimal. MTCT was strongly associated with lack of ART during pregnancy and timing of ART initiation.
Implications of all the available evidence
We showed that Malawi has successfully met the three process indicators established by WHO for the virtual elimination of MTCT: greater than 95% antenatal clinic coverage and HIV testing at antenatal clinics and greater than 90% ART coverage in pregnant women with a known HIV-positive status. Furthermore, the country has met the first two 90s in the UNAIDS 90–90-90 objectives. High adherence to ART by mothers and adequate follow-up of infants throughout breastfeeding will be crucial to maximising prevention of MTCT effectiveness in this treat-all era.
Acknowledgments
This study is supported by the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Funding President’s Emergency Plan for AIDS Relief.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Beth A Tippett Barr, Division of Global HIV & Tuberculosis, Center for Global Health, Centers for Disease Control and Prevention, Lilongwe, Malawi; Center for Global Health, Centers for Disease Control and Prevention, Kisumu, Kenya.
Monique van Lettow, Dignitas International, Zomba, Malawi.
Joep J van Oosterhout, Department of Medicine, College of Medicine, University of Malawi, Blantyre, Malawi; Dignitas International, Zomba, Malawi.
Megan Landes, Department of Family and Community Medicine, University of Toronto, Toronto, ON, Canada; Dignitas International, Zomba, Malawi.
Ray W Shiraishi, Division of Global HIV & Tuberculosis, Center for Global Health.
Ermias Amene, Division of Violence Prevention, National Center for Injury Prevention and Control, ICF International, Atlanta, GA, USA; Centers for Disease Control and Prevention, Atlanta, GA, USA.
Erik Schouten, Management Sciences for Health, Lilongwe, Malawi.
Nellie Wadonda-Kabondo, Division of Global HIV & Tuberculosis, Center for Global Health, Centers for Disease Control and Prevention, Lilongwe, Malawi.
Sundeep Gupta, Division of Global HIV & Tuberculosis, Center for Global Health, Centers for Disease Control and Prevention, Lilongwe, Malawi; David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA.
Andrew F Auld, Division of Global HIV & Tuberculosis, Center for Global Health, Centers for Disease Control and Prevention, Lilongwe, Malawi.
Thokozani Kalua, Department of HIV and AIDS, Malawi Ministry of Health, Lilongwe, Malawi.
Andreas Jahn, Department of HIV and AIDS, Malawi Ministry of Health, Lilongwe, Malawi; International Training and Education Center for Health, University of Washington, Seattle, WA, USA.
References
- 1.Department of HIV and AIDS of the Ministry of Health.Clinical management of HIV in children and adults. Malawi integrated guidelines for providing HIV Services. Lilongwe: Malawi Ministry of Health, 2011. [Google Scholar]
- 2.Kalua T, Tippett Barr BA, van Oosterhout JJ, et al. Lessons learned from option B+ in the evolution toward “test and start” from Malawi, Cameroon and the United Republic of Tanzania. J Acquir Immune Defic Syndr 2017; 75: S43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive, 2011–15. Geneva: Joint United Nations Programme on HIV/AIDS, 2011. [Google Scholar]
- 4.Coutsoudis A, Goga A, Desmond C, Barron P, Black V, Coovadia H. Is option B+ the best choice? Lancet 2013; 381: 269–71. [DOI] [PubMed] [Google Scholar]
- 5.Bewley S, Welbourn A. Should we not pay attention to what women tell us when they vote with their feet? AIDS 2014; 28: 1995. [DOI] [PubMed] [Google Scholar]
- 6.Tippett Barr B, Mhango E, Tenthani L, et al. Uptake and retention in Malawi’s “option B+” PMTCT Program: lifelong ART for all HIV-infected pregnant or lactating women. Conference on Retroviruses and Opportunistic Infections; Atlanta, GA, USA; March 3–6, 2013. Oral abstract 82. [Google Scholar]
- 7.Tenthani L, Haas A, Egger M, et al. Brief report: HIV testing among pregnant women who attend antenatal care in Malawi.J Acquir Immune Defic Syndr 2015; 15: 610–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tenthani L, Haas A, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘option B+’) in Malawi. AIDS 2014;28: 589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas A, Msukwa MT, Egger M, et al. Adherence to antiretroviral therapy during and after pregnancy: cohort study on women receiving care in Malawi’s “option B+” programme.Clin Infect Dis 2016; 63: 1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Lettow M, Bedell R, Mayuni I, et al. Towards elimination of mother-to-child transmission of HIV: performance of different models of care for initiating lifelong antiretroviral therapy for pregnant women in Malawi (option B+). J Int AIDS Soc 2014; 17: 18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Government of Malawi Ministry of Health. Integrated HIV program report: January-March 2016. Lilongwe: Malawi Ministry of Health, 2016. [Google Scholar]
- 12.Goga AE, Dinh TH, Jackson DJ, et al. Population-level effectiveness of PMTCT option A on early mother-to-child (MTCT) transmission of HIV in South Africa: implications for eliminating MTCT. J Glob Health 2016; 6: 020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinh T, Mushavi A, Shiraishi R, et al. Impact of timing of antiretroviral treatment and birth weight on mother-to-child human immunodeficiency virus transmission: findings from an 18-month prospective cohort of a nationally representative sample of mother-infant pairs during the transition from option A to option B+ in Zimbabwe. Clin Infect Dis 2018; 66: 576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Statistical Office, ICF. Malawi Demographic and Health Survey 2015–16. Zomba: NSO and ICF, 2017 [Google Scholar]
- 15.Government of Malawi Ministry of Health. Integrated HIV program report: October-December 2015. Lilongwe: Malawi Ministry of Health, 2016. [Google Scholar]
- 16.Open Source Statistics for Public Health. Sample size for a proportion or descriptive study, http://www.openepi.com/SampleSize/SSPropor.htm (accessed Feb 12, 2011).
- 17.Cochran WG. Sampling techniques, 3rd edn New York, NY: Wiley, 1977. [Google Scholar]
- 18.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Global guidance on criteria and processes for validation: elimination of mother-to-child transmission of HIV and syphilis. 2014. http://apps.who.int/iris/bitstream/10665/112858/1/9789241505888_eng.pdf?ua=1&ua=1 (accessed Nov 12, 2016).
- 20.UNAIDS. 90–90-90: an ambitious treatment target to help end the AIDS epidemic. 2014. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf (accessed Nov 12, 2016).
- 21.Flax VL, Hamela G, Mofolo I, Hosseinipour MC, Hoffman IF, Maman S. Factors influencing postnatal option B+ participation and breastfeeding duration among HIV-positive women in Lilongwe district, Malawi: a qualitative study. PLoS One 2017; 12: e0175590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cataldo F, Chiwaula L, Nkhata M, et al. Exploring the experiences of women and health care workers in the context of PMTCT option B Plus in Malawi. J Acquir Immune Defic Syndr 2017; 74: 517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampanda KM, Nimz AM, Abuogi LL. Barriers to uptake of early infant HIV testing in Zambia: the role of intimate partner violence and HIV status disclosure within couples. AIDS Res Ther 2017; 14: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malawi Ministry of Health. Clinical management of HIV in children and adults. http://www.emtct-iatt.org/wp-content/uploads/2015/09/Malawi-HIV-Guidelines-2014.pdf (accessed Nov 12, 2016).
- 25.Kellerman SE, Ahmed S, Feeley-Summerl T, et al. Beyond PMTCT: keeping HIV exposed and positive children healthy and alive. AIDS 2013; 27: S225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambia J, Mandala J. A systematic review of interventions to improve prevention of mother-to-child HIV transmission service delivery and promote retention. J Int AIDS Soc 2016; 19: 20309. [DOI] [PMC free article] [PubMed] [Google Scholar]


