Abstract
Activation of TRPM8 channel through oxidative stress may induce Ca2+ and pro-apoptotic signals in prostate cancer and kidney cells. The aim of this study was to evaluate activation of TRPM8 can increase apoptosis and oxidative stress in the prostate cancer (Du145M8), TRPM8 knock out (Du 145M8KO), transfected (HEK293TM8) and non-transfected human kidney (HEK293) cells. Intracellular Ca2+ responses to TRPM8 activation were increased in the Du145M8 and HEK293TM8 cells from coming cumene hydrogen peroxide (CHPx), menthol, ADP-Ribose (ADPR), but not in the HEK293 and Du 145M8KO cells. The intracellular Ca2+ responses to both ADPR and CHPx were totally inhibited by the thiol cycle antioxidant glutathione, and TRPM8 blockers (N-(p-amylcinnamoyl)anthranilic acid and capsazepine). Apoptosis, Annexin V, mitochondrial membrane depolarization, intracellular ROS, caspase 3 and 9 values were increased through TRPM8 activation in the Du 145M8 but not in the Du 145M8KO and non-transfected HEK293 cells by CHPx and hydrogen peroxide. In conclusion, apoptotic and oxidant effects on the cells were increased activation of TRPM8 by oxidative stress and ADPR. Activation of TRPM8 through oxidative stress and ADPR in the cells could be used as an effective strategy in the treatment of prostate cancer cells.
Introduction
Oxidative stress occurs during the physiological functions such as phagocyte activity and mitochondrial function. The oxidative stress is controlled by the antioxidants such as glutathione (GSH) and glutathione peroxidase (GSH-Px). GSH as a member of thiol cycle antioxidants endogenously synthesized all mammalian cells and it has several physiological functions such as antioxidant defense, inhibition of prostate cancer and transport of cysteine1,2. GSH and N acetyl cysteine (NAC) treatments as a member of thiol redox system, induced transient receptor (TRP) melastatin 2 (TRPM2) and 8 (TRPM8) channel inhibitor roles3–6. ADP-Ribose (ADPR) is synthesized in the nucleus beta nicotinamide adenine dinucleotide by activation CD38 enzyme through hydrogen peroxide (H2O2) production7,8. The H2O2 has been using for investigation of oxidative stress dependent TRP channel activations such as TRPM2 and TRPV17–9. The TRPM8 channel is activated by cold and menthol10,11. However, there is no report ADPR and H2O2 dependent activation of TRPM8 in the prostate cancer and human embryonic kidney cells 293 (HEK293) cells.
Intracellular free calcium ion ([Ca2+]i) concentration is a major intracellular second messenger factor that regulates many physiological and pathophysiological functions including cell migration12,13. Apoptosis, proliferation, differentiation and migration in cells are controlled by the Ca2+ signaling pathways. Prostate cancers are a most common diagnosis in men. It is also well known that an increase of [Ca2+]i concentration involved in prostate cancer carcinogenesis and in metastasis development14. The Ca2+ passes the cell membranes through different cation channels including TRP channels. As a member of the TRP superfamily, TRPM8 channel, changes in its expression level is involved in the etiology of prostate cancers and it seems to be one of the most promising potential drug target channels in the treatment of prostate cancers15. Androgen-dependent expression of TRPM8 increases in both benign prostate hyperplasia and in prostate carcinoma cells15,16. Involvement of transmembrane domains-isoforms of TRPM8 in the mitochondria of keratinocyte cells for the regulating [Ca2+]i concentration was recently reported17. In addition, an increase of [Ca2+]i concentration through menthol activation of TRPM8 channels in the prostate cancer cells induced increase the rate of mitochondrial oxidative stress, resulting apoptosis of the cancer cells18. Hence, activation of TRPM8 through oxidative stress may induce pro-apoptotic signals in prostate cancer cells, but it remains unclear.
To our knowledge, there is no report on the oxidative stress and ADPR dependent activation of TRPM8 channels in TRPM8 positive androgen insensitive prostate cancer (Du 145M8) and overexpressing human TRPM2 channel HEK293 (HEK293TM8) cells. Therefore, we propose that investigation of the involvement of oxidative stress in the TRPM8 activation might represent two of the mechanisms controlling up-regulation of mitochondrial oxidative stress, apoptosis and [Ca2+]i concentration in the Du 145M8 and HEK293TM8 cells.
Results
Oxidative stress activates TRPM8 in the Du 145M8 cells
As the first step in the current study whether activation of TRPM8 channel is related to oxidative stress (cumene hyroperoxide, CHPx) activator and menthol, the influences of the channel on Ca2+ fluorescence intensity in the Du 145 cells were investigated by using the activators and inhibitors (thiol cycle antioxidant GSH and TRPM8 channel blocker [N-(p-amylcinnamoyl)anthranilic acid (ACA)]. The confocal microscope images (Fig. 1a) and columns (Fig. 1b) of Ca2+ fluorescence intensity in Du 145M8 are presented in Fig. 1. The Ca2+ fluorescence intensity was increased in the cells by CHPx stimulations. On the other word, the Ca2+ fluorescence intensity was significantly (p ≤ 0.001) higher in the control + CHPx groups as compared to control. However, the Ca2+ fluorescence intensity was markedly (p ≤ 0.001) decreased in the control + CHPx + ACA group as compared to the CHPx group by the ACA treatment. This increase in Ca2+ fluorescence intensity was totally prevented by pretreatment with GSH and the Ca2+ fluorescence intensity was markedly (p ≤ 0.001) lower in the GSH, GSH + CHPx and GSH + CHPx + ACA than in the control + CHPx and control + CHPx + ACA groups.
Figure 1.
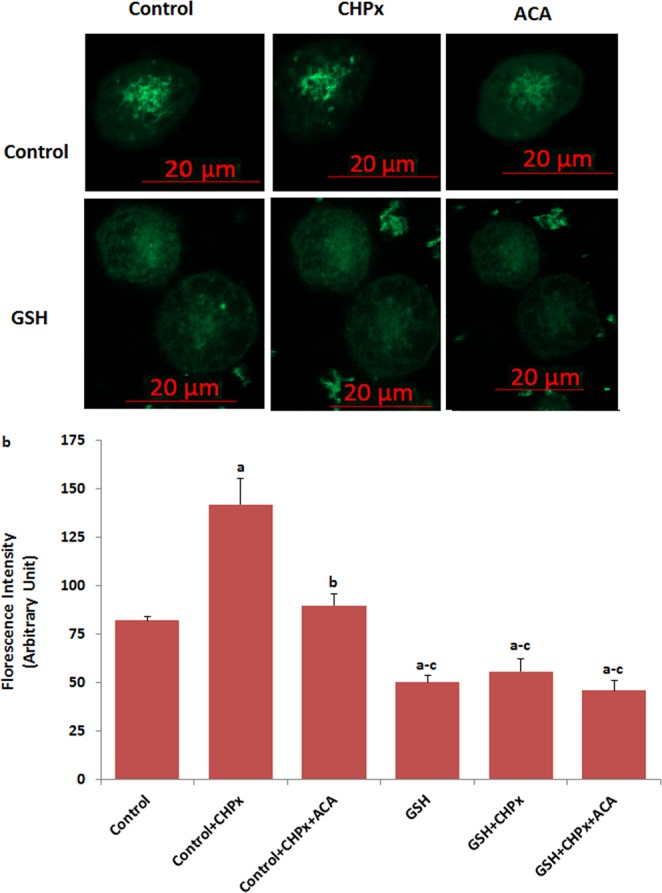
Activation of TRPM8 in the Du 145M8 cells by oxidative stress. (mean ± SD). The cells were stained with Fluo-3 calcium dye and mean ± SD of fluorescence in 15 mm2 of cell as arbitrary unit are presented; n = 10–20 independent experiments. In GSH experiments, the cells were pretreated with GSH (10 mM for 2 hours). The cells were extracellularly stimulated by cumene hyroperoxide (CHPx and 1 mM for 5 min) but they were extracellularly inhibited by ACA (25 μM for 10 min). The samples were analyzed by the laser confocal microscopy fitted with a 40× oil objective. The scale bar was 20 µm. Representative images and fluorescence intensities of the CHPx, ACA and GSH effect on the TRPM8 activation in the laser confocal microscope analyses are shown in (a,b) respectively. (ap ≤ 0.001 versus control. bp ≤ 0.001 versus control + CHPx group. cp ≤ 0.001 versus control + CHPx + ACA group).
Oxidative stress has no TRPM8 activation in the absence of TRPM8 and extracellular Ca2+ in the Du 145M8 and Du 145M8KO cells
After observation of oxidative stress dependent activation of TRPM8 in the cells, we tested the effects of absence or presence of extracellular Ca2+ (+Ca2+, Ca2+-containing extracellular buffer; −Ca2+, Ca2+-free buffer) or deletion of TRPM8 (Du 145M8KO) in the Ca2+ fluorescence intensity of Du 145M8 cells. Du 145M8KO cells, which do not express TRPM8 channels18, showed no detectable TRPM8 response-induced Ca2+ fluorescence intensity (Fig. 2a,b). Addition of CHPx and menthol in the presence of Ca2+ led to a significant increase in the Ca2+ fluorescence intensity in the Du 145M8KO cells, which was decreased by the addition of ACA, the TRPM2 channel specific inhibitor (Fig. 2a,b). In contrast, CHPx and menthol treatments induced no increase in the Ca2+ fluorescence intensity level in the absence of Ca2+ (Fig. 2a,b). Furthermore, the Ca2+ fluorescence intensity increases were not observed in the absence of TRPM8 in the Du 145M8KO cells. These results exclude the Ca2+ release from intracellular organelles such as the endoplasmic reticulum and mitochondria and more importantly, for the first time, demonstrate the existence of a specific mechanism for Ca2+ influx involving TRPM2 channels.
Figure 2.
There is no activation of TRPM8 in the Du 145M8 and Du 145M8KO cells without extracellular Ca2+ by oxidative stress (CHPx) and menthol. (mean ± SD). (+Ca2+, Ca2+-containing extracellular buffer; −Ca2+, Ca2+-free buffer). The cells were stained with Fluo-3 calcium dye and mean ± SD of fluorescence in 15 mm2 of the cells as arbitrary unit are presented; n = 10–20 independent experiments. The Du 145M8 and Du 145M8KO cells with +Ca2+and −Ca2+ buffers in the TRPM8 experiments were stimulated by CHPx (1 mM for 10 min) but they were inhibited by ACA (25 μM for 10 min). The samples were analyzed by the laser confocal microscopy fitted with a 40× oil objective. The scale bar was 20 µm. Representative images and fluorescence intensities of the CHPx, ACA and menthol effects on the TRPM8 activation in the laser confocal microscope analyses are shown in Fig. 1a,b, respectively. (ap ≤ 0.001 versus control. bp ≤ 0.001 versus control + CHPx group. cp ≤ 0.001 versus control + CHPx + ACA group. cp ≤ 0.001 versus Du 145M8 (without Ca2+ groups).
TRPM2 blocker (ACA) inhibits the ADPR -induced TRPM8 currents in the Du 145M8KO cells
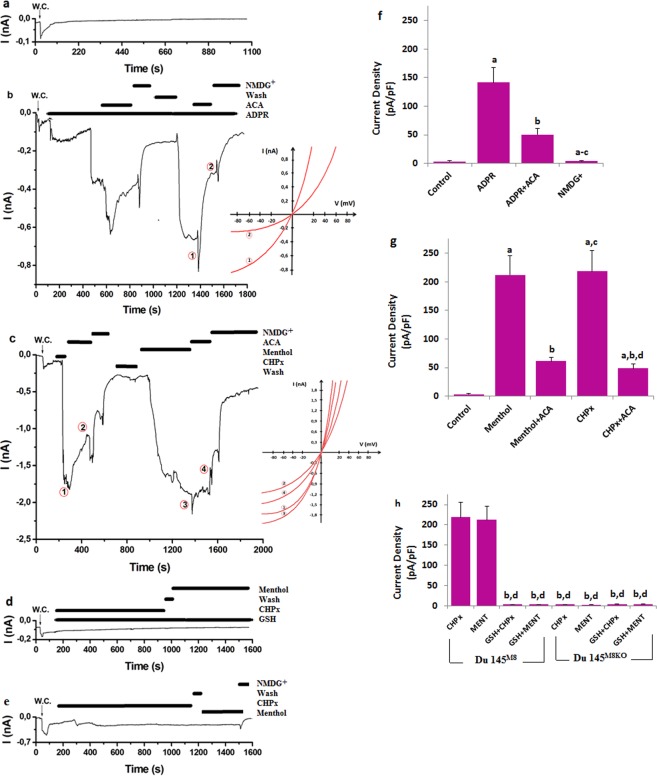
ADPR is synthesized in the nucleus beta nicotinamide adenine dinucleotide by activation CD38 enzyme through extracellular H2O2 production19,20. As a member of TRP superfamily, TRPM2 channel is activated by ADPR19,20 but there is no report on the ADPR-induced TRPM8 in cells. Therefore, we firstly tested involvement of ADPR on the TRPM8 activation in the Du 145 cells. TRPM8 channel in the patch-clamp experiments was gated in the Du 145M8 cells by ADPR (1 mM in the patch-pipette), although they were reversibly blocked by ACA and NMDG+ (replacement of Na+) (Fig. 3b). There were no currents in the absence of the TRPM8 agonists (ADPR, CHPx and menthol) and antagonists (and ACA) (Fig. 3a). Treatment of wild type (Du 145M8) cells with the 25 μM ACA as a TRPM2 channel inhibitor, strongly suppressed ADPR-induced current densities (Fig. 3b,c). On the other word, the current densities in the cells were significantly higher in the control + ADPR group compared with the control group (p ≤ 0.001); however, the current density of TRPM8 was significantly (p ≤ 0.001) lower in the control + ADPR + ACA group than in the control + ADPR group (Fig. 3b,f).
Figure 3.
Effect of oxidative stress (CHPx) and ADPR on the TRPM8 current densities (pA/pF) in the Du 145M8 and Du 145M8KO cells. (mean ± SD and n = 3). The TRPM8 currents in the Du 145M8 and Du 145M8KO cells were induced either by intracellular ADPR (1 mM in patch-pipette) or extracellular CHPx (10 mM) and 0.1 mM menthol, but they were blocked by extracellular ACA (25 μM) in the patch-chamber. Intracellular GSH (2 mM) was given to the cells in the patch pipette. W.C.: Whole cell. (a) Control: Original recordings from control neuron. (b) ADPR group. (c) Menthol and CHPx group. (d) GSH group. (e) the Du 145M8KO cells group. The (f–h) were currents densities of (b–d) +e patch clamp records, respectively. (ap ≤ 0.001 versus control. bp ≤ 0.001 versus ADPR and menthol groups. cp ≤ 0.001 versus ADPR + ACA and menthol + ACA groups. dp ≤ 0.001 versus CHPx group).
The H2O2 has been using for investigation of oxidative stress dependent TRP channel activation such as TRPM2 and TRPV17,9. To further investigate the relative contribution of oxidative stress in the TRPM8 activation, the effect of CHPx was studied in the TRPM8 present (Du 145M8) and knockout (Du 145M8KO) prostate cancer cells (Fig. 3c–e). In addition, we used specific agonist of TRPM8 (menthol) as positive control records. The current densities in the neurons were increased in CHPx and menthol groups (Fig. 3g), and they were decreased in the CHPx + ACA and menthol + ACA groups by the ACA treatments (p ≤ 0.001) (Fig. 3g). Hence, these effects of CHPx and menthol were partially abolished by ACA.
In patch clamp experiment, we also tested the role of antioxidant GSH and deletion of TRPM8 on the TRPM8 activation in the Du 145M8 cells. The menthol and CHPx-induced currents were completely blocked in the presence of intracellular GSH (2 mM in the patch pipette) (Fig. 3d) and deletion of TRPM8 (Fig. 3e). The current densities were markedly (p ≤ 0.001) lower in the Du 145M8 + GSH, Du 145 GSH + menthol, Du 145M8 + GSH + CHPx and Du 145M8KO groups than in the Du 145M8KO + menthol and Du 145M8KO + CHPx groups.
These results clearly indicated that oxidative stress induced excessive Ca2+ influx through the TRPM8 channel. However, the oxidative stress-induced TRPM8 currents through ROS production modulation were decreased by treatment with the antioxidant (GSH).
Hydrogen peroxide induces TRPM8-dependent increase of Ca2+ fluorescence intensity in the HEK293 cells overexpressing human TRPM8 channel (HEK293TM8) cells
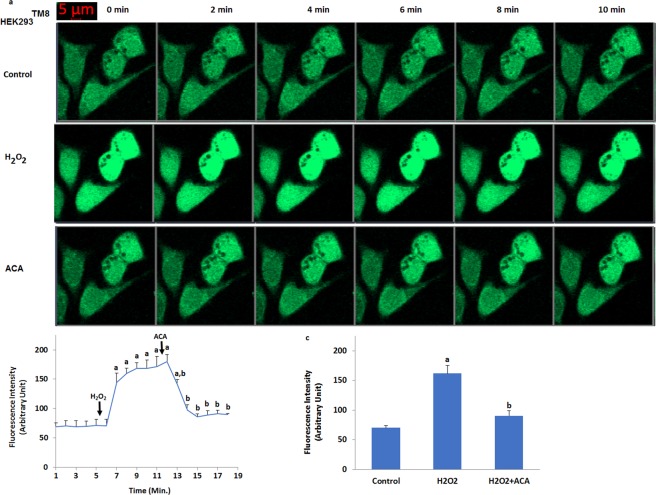
After observation of oxidative stress dependent activation of TRPM8 in the cells, we tested effects of oxidative stress (H2O2) on the fluorescence intensity (Figs 4 and 5) through TRPM8 activation in the HEK293TM8 cells. It is well known that TRPM2 channel is activated within 2–5 minutes in different cell lines by oxidative stress and ADPR19,20. Similarly, we observed activation of TRPM8 in HEK293TM8 cell within 2–5 minutes by ADPR and H2O2 (Fig. 4b). There was no increase in the fluorescence intensity of Ca2+ in the control HEK293TM8 cells within 6 minutes (Fig. 4b). The intensity was markedly (p ≤ 0.001) increased in the cell by H2O2 (Fig. 4b,c), although it was decreased TRPM2 channel blocker (ACA). However, HEK293 cells, which do not express TRPM8 channels, showed no detectable TRPM8 response-induced Ca2+ fluorescence intensity through activation of TRPM8 by the H2O2 stimulation (Fig. 5a,b) and the fluorescence intensity levels did not change in the control, H2O2 and ACA groups, statistically. It is well known that several TRP channels such as TRPM2 and TRPM7 can be activated by oxidative stress12. These results in the TRPM8 expressing the HEK293TM8 cells exclude involvement of oxidative stress dependent activated other TRP channels and more importantly, for the first time, demonstrate the existence of a specific mechanism for oxidative stress-induced Ca2+ influx involving TRPM8 channels.
Figure 4.
Activation of TRPM8 in the non-transfected (HEK293) and transfected (HEK293TM8) human HEK293 cells by hydrogen peroxide (H2O2). (mean ± SD). The cells were stained with Fluo-3 calcium dye and mean ± SD of fluorescence in 15 mm2 of the cells as arbitrary unit are presented; n = 10–20 independent experiments. The HEK293TM8 cells were stimulated by H2O2 (1 mM for 10 min) but they were inhibited by ACA (25 μM for 10 min). The samples were analyzed by the laser confocal microscopy fitted with a 40× oil objective. The scale bar was 5 µm. Representative images (a), line (b) and column (c) of fluorescence intensities of the H2O2 and ACA on the TRPM8 activation in the laser confocal microscope analyses are shown in Figs a–c, respectively. (ap ≤ 0.001 versus control. bp ≤ 0.001 versus H2O2 group).
Figure 5.
No activation of TRPM8 in the HEK293 without overexpressing human TRPM2 channel by hydrogen peroxide (H2O2). (mean ± SD). The cells were stained with Fluo-3 calcium dye and mean ± SD of fluorescence in 15 mm2 of the cells as arbitrary unit are presented; n = 10–20 independent experiments. The HEK293 cells were stimulated by H2O2 (1 mM for 10 min) but they were inhibited by ACA (25 μM for 10 min). The samples were analyzed by the laser confocal microscopy fitted with a 40× oil objective. The scale bar was 5 µm. Representative images, line and column of fluorescence intensities of the H2O2 and ACA on the TRPM8 activation in the laser confocal microscope analyses are shown in Figs a–c, respectively.
ADPR and hydrogen peroxide induce TRPM8-dependent increase of [Ca2+]i concentration in the HEK293 cells overexpressing human TRPM8 channel (HEK293TM8) cells: Single cell patch clamp records
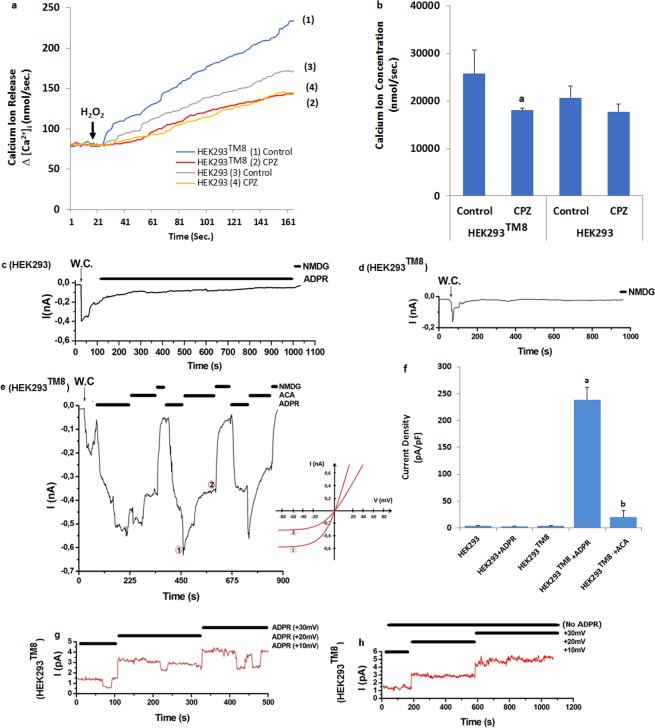
After observation of the oxidative stress dependent increase of TRPM8 in the cells, we tested the effects of ADPR and oxidative stress (H2O2) on the Ca2+ fluorescence intensity in the overexpressing human TRPM8 channel (HEK293TM8) cells, we wanted further confirms the results of measurements of [Ca2+]i concentration via Fura-2 analyses and current density via patch-clamp analyses. Again, the HEK293 cells, which do not express TRPM8 channels, showed no detectable TRPM8 response-induced [Ca2+]i concentration (Fig. 6a,b) current density (Fig. 6c,f) through activation of TRPM8 by the H2O2 and ADPR stimulations. Induction of TRPM2 expression using a transfection system, however resulted in decrease ADPR and oxidative stress-sensitive [Ca2+]i concentration (Fig. a,b) and current density (Fig. 6e,f) through ACA treatment. In addition, we observed ADPR dependent activation in the single channel (inside out) patch clamp records (Fig. 6g). However there was no the single channel currents in the absence of ADPR (Fig. 6h). The single channel results exclude the involvement of second messengers for the activation of TRPM8 via oxidative stress and ADPR. On the other word, it is more importantly, for the first time, demonstrate the existence of a specific mechanism as a TRPM2 channel for Ca2+ involving TRPM8 channels.
Figure 6.
Effects of ADPR and oxidative stress (H2O2) on the TRPM8 current densities (pA/pF) in the HEK293 and HEK293TM8 cells. (mean ± SD and n = 6). Fura-2-loaded the HEK293 and HEK293TM8 cells were stimulated with H2O2 (1 mM) in the presence of normal extracellular calcium (1.2 mM) for 160 seconds. The results were expressed as lines (a) and columns (b). In patch-clamp experiments, the TRPM8 currents in the HEK293 and HEK293TM8 cells were induced by intracellular ADPR (1 mM in patch-pipette), but they were blocked by extracellular ACA (25 μM) in the patch-chamber. W.C.: Whole cell. (c) Original recordings from HEK293 with ADPR stimulation. (d) Original recordings from HEK293TM8 without ADPR stimulation. (e) Original recordings from HEK293TM8 with ADPR stimulation and ACA inhibition. The f was current densities of e patch clamp records. (g) ADPR. Single cell records from HEK293TM8 cells. (g) No ADPR. Control single cell records from HEK293TM8 cells. (ap ≤ 0.001 versus HEK293TM8 group. bp ≤ 0.001 versus HEK293TM8 + ADPR group).
Involvement of TRPM8 in oxidative stress-induced Du 145M8 cell apoptosis and ROS generation
The excessive Ca2+ entry is an important source of ROS that induce cell death and ROS is known to activate several TRP channels. Next, we examined whether TRPM8 were attenuated in ROS-induced apoptosis, cell viability and caspase activation by determining the effects of ACA as a TRPM8 inhibitor, on oxidative stress-induced prostate cancer cell apoptosis and generation of ROS. The results of MTT (Fig. 7a), apoptosis (Fig. 7b), caspase 3 (Fig. 7c), caspase 9 (Fig. 7d), intracellular ROS production (Fig. 7e) and mitochondrial membrane depolarization (JC1) (Fig. 7f) in the four groups of Du 145M8 and Du 145M8KO cells are shown in Fig. 7. Compared with control, CHPx treatment in the Du 145M8KO cells increased the levels of apoptosis, ROS, JC1, caspase 3 and 9 (p ≤ 0.001), although MTT levels in the cells was decreased by the CHPx treatment (Fig. 7a) (p ≤ 0.001). However, there were no differences in the values in the four groups of Du 145M8 and Du 145M8KO cells. More importantly, we found ACA reduced the levels of apoptotic cells through the decrease of the ROS, JC1, caspase 3 and 9 values and increase of the MTT levels in the cells (p ≤ 0.001). However, Du 145M8KO cells, which do not express TRPM8 channels, showed no detectable TRPM8 response-induced apoptosis, ROS, JC1, caspase 3 and 9 through activation of TRPM8 by the CHPx stimulation (p ≥ 0.05). Our data suggested that the involvement of TRPM8 channels on the oxidative stress-induced apoptosis in the cancer cells, because oxidative stress-induced apoptosis, which could be inhibited by TRPM8 blocker (ACA) treatment.
Figure 7.
Effect of CHPx (1 mM) and ACA on the cell viability (MTT) (a), apoptosis (b), caspase 3 (c), caspase 9 (d), intracellular ROS production (e) and mitochondrial membrane depolarization (JC1) (f) levels in the Du 145 wild type (Du145M8) and Du 145-knockout (Du145M8KO) cells. (mean ± SD and n = 3). The TRPM8 currents in the Du 145M8 cells were induced by with CHPx (1 mM for 10 min) but they were blocked by extracellular ACA (25 μM for 10 min). Then, cells in the four groups were further stimulated by CHPx (1 mM). (ap ≤ 0.001 versus control group. bp ≤ 0.001 versus CHPx group).
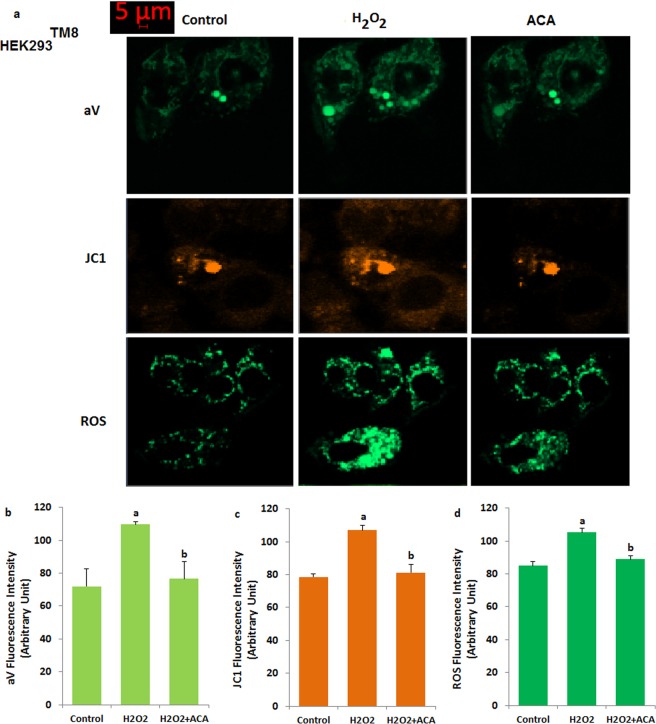
Involvement of TRPM8 in fluorescence intensity of Annexin V (aV), mitochondrial membrane depolarization (JC1) and intracellular ROS production levels in the non-transfected (HEK293) and transfected human HEK293 (HEK293TM8) cells
We further studied certain mitochondrial oxidative stress-related apoptosis (aV) induced by the H2O2. The fluorescence intensity of aV (a and b), JC1 (a and c) and ROS (a and d) results are shown in Fig. 8. The aV, JC1 and ROS levels were increased by the H2O2 incubation. On the other word, the aV, JC1 and ROS levels were markedly (p ≤ 0.001) higher in the H2O2 group as compared to control. In addition, the increased aV, ROS and JC1 levels were markedly (p ≤ 0.001) decreased in the ACA and ACA + H2O2 groups by the ACA treatment. However, there were no differences on the aV, JC1 and ROS values in the control, H2O2 and H2O2 + ACA groups of non-transfected HEK293 cells (Data are not shown).
Figure 8.
Effect of H2O2 and ACA on apoptosis (Annexin V, aV) (a,d), mitochondrial membrane depolarization (JC1) (a,c) and intracellular ROS production (a,d) fluorescence intensity levels in the transfected HEK293 (HEK293TM8) cells. (mean ± SD and n = 10–20). The cells were stimulated with H2O2 (1 mM for 10 min), but they were blocked by extracellular ACA (25 μM for 10 min). Then, the cells in the four groups were further stimulated by H2O2 (1 mM). (ap ≤ 0.001 versus control group. bp ≤ 0.001 versus H2O2 group).
Discussion
In the current study, we found that oxidative stress and ADPR treatments could induce the TRPM8 activations resulting in the overload Ca2+ entry, apoptosis, and mitochondrial oxidative stress. More importantly, we found that GSH could protect the Du 145M8 prostate cancer cells from oxidative stress-induced apoptosis via maintaining the intracellular Ca2+ homeostasis as well as down-regulating mitochondrial oxidative stress pathway. The major findings of this study are that TRPM8 channel is separately activated in the prostate cancer cells by ADPR and oxidative stress and its sensitivity enhance to ROS.
There is debating evidence obtained from the prostate cancer and human kidney cells, that TRPM8 channel activation is associated with production of oxidative stress17,18,21. Indeed, H2O2 stimulation induced functional changes on the TRPM8 in the urothelium cell of elderly subject and human lung epithelial cells, although the changes were reduced by NAC treatments20. However, conflicting report is also presented on the subject and the TRPM8 channel was not activated in urothelium bladder cells by 1 mM H2O221. In general, induction of oxidative stress as a mechanism that may contribute to the antitumor induction effect has been gaining acceptance15. Most of chemotherapeutic agents induce excessive ROS production for killing the cancer cells14. It is well known that an increase in [Ca2+]i concentrations through activation of TRP channels such as TRPM2 and TRPV1 induces an increase of intracellular mitochondrial ROS production22,23. However, GSH as a member of thiol cycle antioxidants has been shown to inhibit CHPx-evoked increased in cell viability and decreases in intracellular levels of ROS and apoptosis13,14,23. GSH has been also reported to prevent completely ADPR and CHPx-evoked TRPM2 and TRPV1 channel activations13,14,23. Thus, the pro-apoptotic effects of oxidative stress in the cancer cells, including prostate cancer cells seem to be dependent on one single mechanism, e.g., the ability of TRPM8 activation to generate oxidative stress. We have recently identified the primary role of menthol dependent, but not oxidative stress TRPM8 activation in the Du 145 cells18. GSH and NAC treatments as two members of thiol redox system, induced TRPM2 and TRPM8 channel inhibitor roles through inhibition of oxidative stress in different cell lines3–6 . Of interest for the present discussion is the finding that ADPR and CHPx-evoked TRPM8 currents were completely abated by intracellular GSH treatment. These findings imply that oxidative stress directly gates TRPM8, but rather probably exerts this action indirectly via the generation ADPR in DNA damage of nucleus by oxidative stress byproducts that eventually target the channel in the prostate cancer cells, through the direct formation of intracellular ROS4.
In the current study, we observed increased levels of apoptosis, caspase 3, caspase 9, mitochondrial membrane depolarization and ROS values through activation of TRPM8 channel in the Du 145M8 cells, but not Du Du 145M8KO cells by CHPx and ADPR, although the values were decreased in the cells by the GSH treatment. During the treatment of tumor cells including prostate cancer cells, increase of mitochondrial oxidative stress through activation of TRPM8 channels and mitochondrial dysfunction has been suggested to account in cancer cells the induction of apoptosis24,25. Mitochondrial oxidative stress and apoptosis in human epithelial prostate cancer cells were induced by suppression of TRPM8 isoforms17, through alterations in mitochondrial membrane depolarization and ATP production26, which leads to oxidative phosphorylation through the electron transport chain and hence the formation of JC127. Thus, induction of apoptosis through overload Ca2+ entry by oxidative stress probably lead to the increase of this toxic protein aggregates inhibiting cancer cell survival. It has been reported that Ca2+ entered from the cytosol during mitochondrial stress accumulates in the mitochondria and mediates the excessive apoptosis through activation of caspase 3 and 918. ROS generation activates both survival and death signaling, depending upon the intensity of the production process. In turn, TRPM8 activation is increased by the increase of mitochondrial ROS production and then the prostate cancer cells are killed by the TRPM8 channel-induced overproduction of intracellular ROS, apoptosis and Ca2+ entry.
As a sulfur containing substance, GSH is containing sulfur groups and it is a member of thiol cycles2,28. Oxidation of thiol redox system and cysteine groups in cancer cells have the main role in the activation of thiol group containing TRP channels such as TRPA1, TRPM8 and TRPV1. Intracellular cysteine suppression reduced tumor growth in prostate cancer cells1,29. In the current study, the GSH treatment inhibited the oxidative stress and ADPR-induced TRPM8 activation through supporting the thiol cycle antioxidants such as GSH and GSH-Px in the cell line. Similarly, the protective role of GSH treatment on the oxaliplatin-induced TRPA1 activation in mouse dorsal root ganglion (DRG) neurons was reported by Materazzi et al.29. In addition, it was recently reported that redox-sensitive TRPV1, TRPC1, TRPM2, and TRPM7 channels are inhibited in human hepatoma cell line30 and rat DRG neurons5,24 by GSH and N acetyl cysteine.
In conclusion, our data clearly show that oxidative stress and ADPR stimulus increased TRPM8-mediated responses, including an increase of intracellular Ca2+ and mitochondrial ROS sensitive-apoptosis in the Du 145M8 and HEK293TM8 cells. However, these responses were attenuated by the treatment with the ROS scavenger GSH and TRPM8 blockers (ACA and CPZ). All together, these data support the hypothesis that oxidative stress is able to induce functional changes in the prostate cancer cell TRPM8 channel signaling and suggest that the killing the prostate cancer cells is susceptible to oxidative stress, with possible implications for treatment of prostate cancer.
Methods
Cell lines
Human prostate (Du 145M8) cancer cells were purchased from ATCC (Manassas, VA, USA), although HEK293 cells were obtained from the Şap Institute of Agriculture and Animal Ministry of Turkey (Ankara, Turkey). The cells were cultured in a medium consisting of 90% Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Istanbul, Turkey), 10% fetal bovine serum (FBS, Gibco, Istanbul, Turkey), and 100 μg/ml streptomycin + penicillin (100 U/ml) combination (Biochrom, Berlin, Germany) and the appropriate supplements, including 100 μg/ml sodium pyruvate (Sigma-Aldrich, Istanbul, Turkey) as suggested by the supplier in a humidified atmosphere in 5% CO2 at 37 °C. The cells were tested within 24 hours after plating onto the coverslips. The cells were tested within 24 hours after plating onto the coverslips. Then the cells were counted by using an automatic cell counter (Casy Modell TT, Roche, Germany). In plate reader and patch-clamp analyses, the cells were seeded in 6 flasks at a density of 1 × 106 cells per flask (filter cap, sterile, 260 ml, 80 cm²) (Thermo Fischer Sci. Inc., Istanbul Turkey). In confocal microscope analyses, the cells were seeded in 35 mm glass bottom dishes (Mattek Corporation Inc., Ashland, MA, USA).
Transfection of HEK293
Transient transfections of HEK293 cells with the 2 μg cDNAs of human TRPM8 (hTRPM8 and a gift from Dr. Simon Hebeisen, B’SYS GmbH, Witterswil Switzerland) were performed according to the manufacturer’s instructions (B’SYS GmbH). For control experiments, 2 μg of wild type TRPM8 empty vector hTRPM8 (C-terminal FLAG tag) plasmid (OriGene Technologies, Istanbul, Turkey) was used for 24 hours using Lipofectamine 2000 (Invitrogen; Istanbul, Turkey. The transfected HEK293 cells (HEK293TM8) seeded on glass coverslips at a suitable dilution and were maintained for 24 h in an incubator at 37 °C and 5% CO2. Then, patch-clamp, Fura-2 and laser confocal microscope experiments were carried out with cells visibly positive for EGFP.
Generation of the TRPM8 Knock out Du 145 (Du 145M8KO) cell line
Wild type Du 145M8 cells were transduced with lentivirus produced as described in a previous study18.
Testing the TRPM8 in the Du 145M8 and Du 145M8KO cell lines
Before starting the experiments we tested presence of TRPM8 in the Du 145M8 but not in Du 145M8KO. Menthol results of TRPM8 were indicated in the current study. Cold exposure to Du 145M8 and Du 145M8KO cells in patch-clamp experiments were performed by slice mini bath chamber with controller type as described in a recent study11. The TRPM8 is also activated in the Du 145M8 but not in Du 145M8KO by cold18.
Determination of intracellular free calcium ion ([Ca2+]i) concentration in the non-transfected (HEK293) and transfected human HEK293 (HEK293TM8) cells, and calcium imaging in Du 145M8 and Du 145M8KO cells
The [Ca2+]i concentrations in the HEK293TM8 and HEK293 cells were monitored using Fura-2-AM as described in a previous study23. HEK293TM8 and HEK293 grown in 96 well plates, were incubated with Fura-2-AM (4 µM) in phosphate buffer for 45 min at 37 °C in the dark. The groups were exposed to the stimulations in a water-jacketed cuvette (37 °C) with continuous magnetic stirring. Fluorescence was detected by using a Carry Eclipse Spectrofluorometer (Varian Inc, Sydney, Australia). The fluorescence at 505 nm was measured at 1 second intervals after excitation at 340 nm and 380 nm, respectively. Calculation of the [Ca2+]i concentrations was described in the previous study22, assuming a Kd of 155 nM. The [Ca2+]i concentrations in the cells were recorded by using the integral of the rise in [Ca2+]i for 160 seconds after the addition of H2O2 (1 mM) and capsazepine (CPZ and 0.1 mM) as TRPM8 blocker31. The [Ca2+]i concentration is expressed as nanomolar (nM) taking a sample every second as previously described23.
For imaging Du 145M8 and Du 145M8KO cells, the cells were analyzed by using Ca2+ indicator florescent dye (Fluo-3, Calbiochem, Darmstadt, Germany) in the dark. The Fluo-3 is a single wavelength excitation and emission dye that excited by a 488 nm argon laser from the confocal microscope32. The cells were treated with TRPM8 antagonist (ACA and 25 μM) to inhibit Ca2+ entry before stimulation of TRPM8 (CHPx and 1 mM). Fluorescence emission of the cells was inspected with a plan Apo 40×/0.2 immersion objective on a confocal microscope (LSM 800, Zeiss, Ankara, Turkey) at 515 nm. Intracellular fluorescence intensities of 10 cells were analyzed in the confocal microscope before CHPx stimulations by ZEN program. Ca2+concentration (1.2 mM) and content of the extracellular buffer were described in a previous study11. Results of a recent study expressed the importance of TRPM8 on the Ca2+ release from intracellular organelles in the prostate cancer cells33. For the clarifying importance of Ca2+ release from the intracellular organelles through TRPM8 activation we used calcium-free extracellular buffer. In the experiments where calcium-free medium was required, Ca2+ was omitted and 2 mM of the chelator EGTA was added.
Manufacturers and preparations of the ADPR, CPZ, menthol, and ACA were described in the previous studies11,18,23. The CHPx were dissolved in the extracellular buffer with and without Ca2+ (1.2 mM).
Electrophysiology
Whole-cell voltage clamp recording was taken from the Du 145M8, Du 145M8KO HEK293TM8 and HEK293 cells (EPC10 patch-clamp set, HEKA, Lamprecht, Germany). We used standard extracellular bath and pipette solutions as described in previous studies11,18,23. Holding potential of the patch-clamp analyses in the cell was −60 mV. The current-voltage (I–V) relationships were obtained from voltage ramps from −150 to +150 mV applied over 200 milliseconds. All experiments were performed at room temperature (22 ± 2 °C).
We also performed single cell record experiments in the HEK293TM8 cell as described in a previous study19.
In the whole cell and single cell experiments, TRPM8 was intracellularly gated by ADPR (1 mM), and the channels were extracellularly blocked by ACA (25 μM). In recent studies, we observed inhibitory role of intracellular GSH (2 mM) on the oxidative stress dependent activations of TRPM2 and TRPV1 channels11,14,23. Hence, the TRPM8 channels in some path-clamp experiments were treated with the intracellular GSH. The maximal current amplitudes (pA) in the Du 145 and HEK293 cells were divided by the cell capacitance (pF), a measure of the cell surface. Values of current density were expressed as pA/pF in the patch-clamp experiments.
Assay of cell viability
Cells were plated in 48-well plates, incubated after treatment with CHPx (1 mM) and ACA (25 μM). Number of viable cell was determined using the 3-(4,5-dimethylthiazol-2yl)-2,5- diphenyl tetrazolium bromide colorimetric (MTT) colorimetric assay as described previously34. Absorbance in the spectrophotometer (UV-1800) was read at 570 nm. A total of 3 experiments (n = 3) was performed for the cell viability assay. The data are presented as fold-increase over the pretreatment level.
Assay of apoptosis, caspase 3 and 9 activities
For the apoptosis spectrophotometric analysis apoptosis, we used a commercial kit and the analyses were performed according to the instructions provided by Biocolor Ltd. (Northern Ireland) and elsewhere34.
The determinations of caspase 3 and 9 activities were based on a method previously reported35,36 with minor modifications. Caspase 3 (N-acetyl-Asp-Glu-Val-Asp-7-amido-4-methylcoumarin) and 9 (N-acetyl-Leu-Glu- His-Asp-7-amino-4-methylcoumarin) substrates were purchased from Bachem (Bubendorf, Switzerland) and cleavages of the substrates were measured with a microplate reader (Infinite pro200; Tecan Austria GmbH, Groedig, Austria) with excitation wavelength of 360 nm and emission at 460 nm. The data were calculated as fluorescence units/mg protein and presented as fold-increase over the pretreatment level (experimental/control). A total of 3 experiments were performed for the caspase and apoptosis assays.
Detection of intracellular reactive oxygen species (ROS) level
Dihydrorhodamine- 123 (DHR 123) as a non-fluorescent and non-charged dye can easily diffuse across membranes34,35. The Du 145M8 and Du 145M8KO cells were washed 1xPBS and they were incubated in DHR123 (1 μl/ml) (Santa Cruz Biotechnology, Inc. Texas USA) at 37 °C in the dark for 30 min. The fluorescence intensity of the oxidized product (Rh123) was measured in the microplate reader (Infinite Pro200). Excitation and emission wavelengths were 488 and 543 nm, respectively32. The data are presented as fold-increase over the pretreatment level.
In imaging the ROS production in HEK293TM8 and HEK293 cells, the intracellular oxidative stress was monitored by DHR123 (514 nm excitation, 570 emission)36. After exposed to indicated treatments, they were incubated in culture medium containing 1 μM DHR123 for 30 min at 37 °C in the dark. Cells were washed and maintained with the phosphate buffer before images were captured using a ZEN Program Imaging System. Fluorescence intensity in 15 μm2 of each cell as arbitrary unit was measured by using ZEN program and analyzed using Image J/Imaris software. The results of JC1 and DHR123 were expressed as the mean fluorescence intensity as arbitrary unit /cell.
Measurement of mitochondrial membrane potential (ΔΨm)
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC1, Molecular Probes, Eugene, OR, USA) floresecen dye has been using for measurement of ΔΨm level36. Hence, we used the dye in the current study for measurement of ΔΨm level. The green (excitation; 485 nm and emission; 535 nm) and red (excitation; 540 nm and emission; 590 nm) JC1 signals were measured in the cell line as described in a previous study34. Fluorescence changes were analyzed using the microplate reader (Infinite Pro200). The data are presented as the fold-increase over the pretreatment level.
In imaging of mitochondrial membrane depolarization, the HEK293TM8 cells were re-suspended in 0.2 ml of phosphate buffer with calcium and then incubated with JC1 (5 μl) dye solutions for 30 min at 37 °C in the dark. The samples were then analyzed by the laser confocal microscopy. JC1 (505 nm excitation, 535 emission) was excited with a diode laser at 488 nm, an Argon laser at 488 nm36. Fluorescence intensity in 15 μm2 of each cell as arbitrary unit was measured by using ZEN program and analyzed using Image J/Imaris software. The results of JC1 were expressed as the mean fluorescence intensity as arbitrary unit/cell.
Annexin V-FITC assay by laser confocal microscope
The protective effects of DTX-induced apoptosis were determined by the laser confocal microscope (LSM-800) using the Annexin V (FITC) dye as described in the manufacturer’s guidelines (Santa Cruz). Briefly, the Annexin V apoptosis detection Kit utilizes FITC-conjugated Annexin V protein for detection of cells undergoing apoptosis. Annexin V FITC binds to the membranes of apoptotic cells, displaying a green characteristic staining pattern which was viewed by the laser confocal microscope (LSM-800).
At the end of the H2O2 treatment, the HEK293TM8 cells were washed twice with phosphate-buffered saline. The cells were re-suspended in 0.2 ml of extracellular buffer and then loaded with Annexin V-FITC (1 μl) for 15 min at room temperature in dark. The samples were then analyzed by the laser confocal microscopy fitted with a 40× oil objective. The fluorescence intensity of each cell as arbitrary unit was measured by using ZEN program and analyzed using Image J/Imaris software. The results of Annexin V-FITC were expressed as the mean fluorescence intensity as arbitrary unit /cell.
Statistical analyses
All data were represented as means ± standard deviation (SD). Statistical analysis was performed with SPSS Version 18.0 statistic software package (Chicago, Illinois, USA). P value as ≤0.05 was considered to indicate a statistically significant. Presence of significance was once detected by LSD test. Then, comparisons between groups for finding levels of p values were performed with analysis of non-parametric Mann Whitney U test.
Acknowledgements
The authors wish to thank and the technicians Fatih Şahin and Hulusi Gül (BSN Health Analyses ARGE Ltd., Teknokent, Isparta, Turkey) for helping patch-clamp analyses. In addition, the authors wish to thank Dr. Simon Hebeisen (B’SYS GmbH, Witterswil Switzerland) for gifting cDNAs of hTRPM8. The study was supported by BSN Health, Analysis and Innovation Ltd. Inc. Teknokent, Isparta, Turkey (Project No: 2018-05). There is no financial and scientific disclosure for the current study.
Author Contributions
M.N. formulated the present hypothesis and was responsible for writing the report. E.B. supervised the study. M.N. analyzed the data. L.P. obtained the Du 145M8KO cells.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cramer SL, et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat Med. 2017;23:120–127. doi: 10.1038/nm.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazıroğlu M. Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem Res. 2009;34:2181–2191. doi: 10.1007/s11064-009-0015-8. [DOI] [PubMed] [Google Scholar]
- 3.Dragoni I, Guida E, McIntyre P. The cold and menthol receptor TRPM8 contains a functionally important double cysteine motif. J Biol Chem. 2006;281(49):37353–37360. doi: 10.1074/jbc.M607227200. [DOI] [PubMed] [Google Scholar]
- 4.Lin AH, et al. Inflammatory effects of menthol vs. non-menthol cigarette smoke extract on human lung epithelial cells: A double-hit on TRPM8 by reactive oxygen species and menthol. Front Physiol. 2017;8:263. doi: 10.3389/fphys.2017.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Özgül C, Nazıroğlu M. TRPM2 channel protective properties of N-acetylcysteine on cytosolic glutathione depletion dependent oxidative stress and Ca2+ influx in rat dorsal root ganglion. Physiol Behav. 2012;106:122–128. doi: 10.1016/j.physbeh.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Gibbs GM, et al. Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proc Natl Acad Sci USA. 2011;108(17):7034–7039. doi: 10.1073/pnas.1015935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nazıroğlu M. New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res. 2007;36(11):1990–2001. doi: 10.1007/s11064-007-9386-x. [DOI] [PubMed] [Google Scholar]
- 8.Togashi K, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25(9):1804–15. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keeble JE, et al. Hydrogen peroxide is a novel mediator of inflammatory hyperalgesia, acting via transient receptor potential vanilloid 1-dependent and independent mechanisms. Pain. 2009;141(1–2):135–42. doi: 10.1016/j.pain.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Andersson DA, Chase HW, Bevan S. TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH. J Neurosci. 2004;24(23):5364–5369. doi: 10.1523/JNEUROSCI.0890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nazıroğlu M, Özgül C. Effects of antagonists and heat on TRPM8 channel currents in dorsal root ganglion neuron activated by nociceptive cold stress and menthol. Neurochem Res. 2012;37:314–320. doi: 10.1007/s11064-011-0614-z. [DOI] [PubMed] [Google Scholar]
- 12.Kumar VS, Gopalakrishnan A, Nazıroğlu M, Rajanikant GK. Calcium ion-the key player in cerebral ischemia. Curr Med Chem. 2014;21:2065–2075. doi: 10.2174/0929867321666131228204246. [DOI] [PubMed] [Google Scholar]
- 13.Pecze L, Blum W, Henzi T, Schwaller B. Endogenous TRPV1 stimulation leads to the activation of the inositol phospholipid pathway necessary for sustained Ca(2+) oscillations. Biochim Biophys Acta. 2016;1863:2905–2915. doi: 10.1016/j.bbamcr.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Peng M, et al. Overexpression of short TRPM8 variant α promotes cell migration and invasion, and decreases starvation-induced apoptosis in prostate cancer LNCaP cells. Oncol Lett. 2015;10:1378–1384. doi: 10.3892/ol.2015.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grolez GP, Gkika D. TRPM8 puts the chill on prostate cancer. Pharmaceuticals (Basel). 2016;9:E44. doi: 10.3390/ph9030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valero ML, Mello de Queiroz F, Stühmer W, Viana F, Pardo LA. TRPM8 ion channels differentially modulate proliferation and cell cycle distribution of normal and cancer prostate cells. PLoS One. 2012;7:e51825. doi: 10.1371/journal.pone.0051825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bidaux G, et al. Targeting of short TRPM8 isoforms induces 4TM-TRPM8-dependent apoptosis in prostate cancer cells. Oncotarget. 2016;7(20):29063–29080. doi: 10.18632/oncotarget.8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nazıroğlu M, et al. Menthol evokes Ca(2+) signals and induces oxidative stress independently of the presence of TRPM8 (menthol) receptor in cancer cells. Redox Biol. 2018;14:439–449. doi: 10.1016/j.redox.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazıroğlu M, Lückhoff A. A calcium influx pathway regulated separately by oxidative stress and ADP-Ribose in TRPM2 channels: single channel events. Neurochem Res. 2008;33(7):1256–1262. doi: 10.1007/s11064-007-9577-5. [DOI] [PubMed] [Google Scholar]
- 20.Nocchi L, Daly DM, Chapple C, Grundy D. Induction of oxidative stress causes functional alterations in mouse urothelium via a TRPM8-mediated mechanism: implications for aging. Aging Cell. 2014;13:540–550. doi: 10.1111/acel.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholas S, Yuan SY, Brookes SJ, Spencer NJ, Zagorodnyuk VP. Hydrogen peroxide preferentially activates capsaicin-sensitive high threshold afferents via TRPA1 channels in the guinea pig bladder. Br J Pharmacol. 2017;174:126–138. doi: 10.1111/bph.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Övey IS, Nazıroğlu M. Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: involvement of TRPM2 and TRPV1 channels. Neuroscience. 2015;284:225–133. doi: 10.1016/j.neuroscience.2014.09.078. [DOI] [PubMed] [Google Scholar]
- 23.Nazıroğlu M, Ciğ B, Ozgül C. Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: role of TRPV1 channels. Neuroscience. 2013;242:151–160. doi: 10.1016/j.neuroscience.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Tang Y, et al. Natural compound Alternol induces oxidative stress-dependent apoptotic cell death preferentially in prostate cancer cells. Mol Cancer Ther. 2014;13:1526–1536. doi: 10.1158/1535-7163.MCT-13-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camello-Almaraz C, Salido GM, Pariente JA, Camello PJ. Role of mitochondria in Ca(2+) oscillations and shape of Ca(2+) signals in pancreatic acinar cells. Biochem Pharmacol. 2002;63(2):283–292. doi: 10.1016/S0006-2952(01)00830-9. [DOI] [PubMed] [Google Scholar]
- 26.Keil VC, Funke F, Zeug A, Schild D, Müller M. Ratiometric high-resolution imaging of JC-1 fluorescence reveals the subcellular heterogeneity of astrocytic mitochondria. Pflugers Arch. 2011;462:693–708. doi: 10.1007/s00424-011-1012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2012;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 28.Kachadourian R, Day BJ. Flavonoid-induced glutathione depletion: potential implications for cancer treatment. Free Radic Biol Med. 2006;41:65–76. doi: 10.1016/j.freeradbiomed.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Materazzi S, et al. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. 2012;463:561–569. doi: 10.1007/s00424-011-1071-x. [DOI] [PubMed] [Google Scholar]
- 30.Badr, H., Kozai, D., Sakaguchi, R., Numata, T. & Mori, Y. Different contribution of redox-sensitive transient receptor potential channels to acetaminophen-induced death of human hepatoma cell line. Front Pharmacol.7, 19 (2016). [DOI] [PMC free article] [PubMed]
- 31.Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141(4):737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orabi, A. I., Nathanson, M. H. & Husain, S. Z. Measuring Ca2+ dynamics in pancreatic acini using confocal microscopy. Pancreapedia: Exocrine Pancreas Knowledge Base, 10.3998/panc.2011.30 (2011).
- 33.Bidaux G, et al. 4TM-TRPM8 channels are new gatekeepers of the ER-mitochondria Ca(2+) transfer. Biochim Biophys Acta. 2018;1865(7):981–994. doi: 10.1016/j.bbamcr.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Yüksel, E., Nazıroğlu, M., Şahin, M. & Çiğ, B. Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: Protective role of selenium. Sci Rep.7(1), 17543 (2017). [DOI] [PMC free article] [PubMed]
- 35.Espino J, et al. Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: Evidence for the involvement of mitochondria and Bax activation. J Membr Biol. 2010;233:105–118. doi: 10.1007/s00232-010-9230-0. [DOI] [PubMed] [Google Scholar]
- 36.Joshi DC, Bakowska JC. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J Vis Exp. 2011;51:2704. doi: 10.3791/2704. [DOI] [PMC free article] [PubMed] [Google Scholar]