Abstract
Random noise can enhance the detectability of weak signals in nonlinear systems, a phenomenon known as stochastic resonance (SR). This concept is not only applicable to single threshold systems but can also be applied to dynamical systems with multiple attractor states, such as observed during the phenomenon of binocular rivalry. Binocular rivalry can be characterized by marginally stable attractor states between which the brain switches in a spontaneous, stochastic manner. Here we used a computational model to predict the effect of noise on perceptual dominance durations. Subsequently we compared the model prediction to a series of experiments where we measured binocular rivalry dynamics when noise (zero-mean Gaussian random noise) was added either to the visual stimulus (Exp. 1) or directly to the visual cortex (Exp. 2) by applying transcranial Random Noise Stimulation (tRNS 1 mA, 100–640 Hz zero -mean Gaussian random noise). We found that adding noise significantly reduced the mixed percept duration (Exp. 1 and Exp. 2). Our results are the first to demonstrate that both central and peripheral noise can influence state-switching dynamics of binocular rivalry under specific conditions (e.g. low visual contrast stimuli), in line with a SR-mechanism.
Introduction
Noise is detrimental for the transfer of information in linear systems1. However, in nonlinear systems such as the brain, noise can enhance information transfer via a stochastic resonance (SR) mechanism1,2. SR can be experimentally observed when noise is added to a non-linear system which enhances (i) the output of the system e.g. by improving the signal-to-noise ratio (SNR)3,4, (ii) the signal amplitude5 or (iii) the degree of coherence within neural networks6. In humans, the SR-effect has been observed in multiple sensory modalities when both signal and experimentally controlled noise are added to the peripheral nervous system7–10. Recently, we have demonstrated that central mechanisms of perception are also sensitive to an SR-effect3. We showed that transcranial random noise stimulation (tRNS), a type of non-invasive brain stimulation, applied over visual cortex can enhance the detection performance of weak subthreshold visual stimuli.
Theoretical considerations predict that SR not only plays a role in signal enhancement but that it can also influence the dynamics of bistable-systems11. One paradigm that allows the observation and measurement of how the brain dynamically transitions between different perceptual states is binocular rivalry. Binocular rivalry is a perceptual phenomenon that occurs when different stimuli are simultaneously presented to each eye. During binocular rivalry visual awareness switches spontaneously between the two stimuli12 so that at any given time participants perceive either one of the two images (exclusive percept) or a combination of both (mixed percept).
Models of binocular rivalry propose that it reflects a competition between neural populations coding for each image13. The neural population coding for the dominant percept inhibits neurons that code for the suppressed image. However, over time the inhibition of the dominant population becomes weaker due to adaptation or fatigue, which allows the suppressed image to become dominant13,14. This results in a deterministic anti-phase oscillation of the firing rates of the two neuronal populations15. If adaptation was the only driving factor of binocular rivalry, however, perception would change fairly regularly. In fact, the dynamics of binocular rivalry are highly nonlinear and stochastic, leading to the proposal that noise associated with the activity of the two neuronal populations causes a random distribution of dominance durations16.
Noise is thought to play a crucial role in the occurrence of the perceptual switches, and it has been suggested to represent an essential driving force of rivalry dynamics17,18. Consequently, there are many successful models of binocular rivalry which contain a noise component to describe the rivalry dynamics15,19,20. While it has been demonstrated that rivalry dynamics can be influenced by adaptation21, the effect of adding noise directly to the brain during binocular rivalry has not been empirically tested. Rivalry dynamics can conceptually be represented by a double-well energy landscape, with each well representing one of the images. Neuronal adaptation can be represented as changes in the energy-landscape: adaptation reduces the depth of the well so that the current state is less stable, thus making a switch to the competing percept more likely. Adding noise to such a system can change its dynamics in a specific way11,22. Here we investigated whether the attractor dynamics of binocular rivalry can be modulated by adding noise to either the visual stimulus or directly to the visual cortex with tRNS23,24.
Previously it has been demonstrated that 10 minutes of continuous tRNS increases cortical excitability for up to an hour after stimulation24. In the current study, however, we were interested in the direct online effects of noise on rivalry dynamics. Therefore, noise and no-noise conditions were randomly intermixed, and noise was applied non-continuously to reduce the likelihood of overall changes in neural excitability. Two experiments were performed: In Experiment 1, noise was added to low contrast or high contrast visual stimuli to test whether an SR-effect is induced when noise is added to the periphery. In Experiment 2, we added noise to the visual cortex with tRNS to test whether central mechanisms of perception are sensitive to an SR-effect. The results of these experiments suggest that rivalry dynamics can be influenced by noise when there are three stable states, namely, perception of the left-eye image, the right-eye image and a combination of both images. Since SR occurs preferentially for weak but not for strong stimuli3,7–10,25 we tested a low- and a high-contrast condition in both experiments. In order to make clear predictions as to which outcome parameters were most likely to be affected by adding noise to rivalry dynamics, we simulated different experimental conditions with a computational model19 prior to data collection.
Results
Computational modelling results
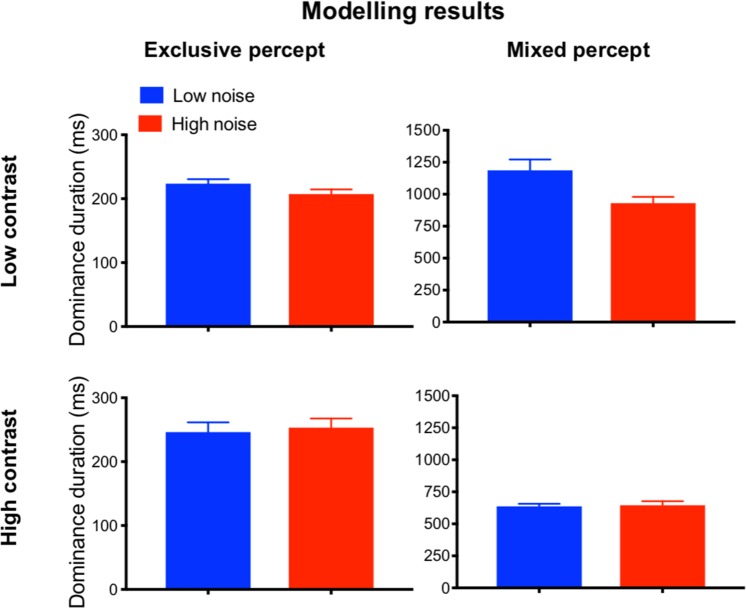
We used a conventional binocular rivalry model19 which relies on competition between neurons tuned to orthogonal orientations. Perceptual switches result from the interplay of mutual inhibition between these neurons and a noise component. We added a common noise term to the neuronal populations since tRNS is most likely to add correlated noise to many neuronal populations. When adding noise to the model, the duration of exclusive percepts decreased by 7% for the low contrast stimulus (low noise mean[s.d.]: 223.5[7.1] ms, high noise mean[s.d.]: 207.4[7.2]) ms) and increased by 3% for the high contrast stimulus (low noise mean[s.d.]: 246.3[15.5] ms, high noise mean[s.d.]: 253.3[14.4] ms, Fig. 1, left panel). The strongest effect of adding noise was observed as a substantial reduction of the mixed percept (Fig. 1, right panel) which was, however, different depending on contrast intensity: there was a stronger reduction of mixed percept durations for low contrast trials (−22% change, low noise mean[s.d.]: 1187.3[84] ms, high noise mean[s.d.]: 931[48.2]) ms) than for high contrast trials (1% change, low noise mean[s.d.]: 636.8[19.9] ms, high noise mean[s.d.]: 645.3[31.9]) ms). These modelling results are in line with previous research suggesting that mixed percept duration is affected by neuronal noise26–28. Based on these studies and our own modelling findings, the primary outcome measure for the subsequent binocular rivalry experiments was the mixed percept duration. In particular, we hypothesized that the mixed percept duration would be more strongly influenced by adding noise to low contrast stimuli than to high contrast stimuli. As a secondary outcome measure we further calculated the number of perceptual switches over the 12 minutes of modelled rivalry. The number of switches increased most with increased noise in the low-contrast condition (low noise mean[s.d.]: 310.3[11.41], high noise mean[s.d.]: 397.8[14.4]; 28% change), whereas there was no change in the number of switches in the high-contrast condition (low noise mean[s.d.]: 425.3[16.6], high noise mean[s.d.]: 423.8[12.6]; 0% change).
Figure 1.
Computational modelling results. We modelled the effect of adding noise to a rivalry model, and determined dominance durations for the exclusive and mixed percepts. The modelled results indicate that adding noise to the rivalry process reduces the duration of the mixed percept for low contrast visual stimuli by 22%. For the high contrast visual stimuli this reduction was 1%. For the exclusive percept, the dominance duration decreased with an increasing noise level for the low-contrast visual stimuli (7% reduction) and increased for the high-contrast visual stimuli (3% increase). Error bars represent standard deviations (s.d.).
Behavioural results
Experiment 1
Participants were seated in front of the monitor and viewed the images foveally through a mirror stereoscope (Fig. 2). The task for the participants was to continuously report on a keyboard whether they perceived the left or right tilted grating, or a mixture of both.
Figure 2.
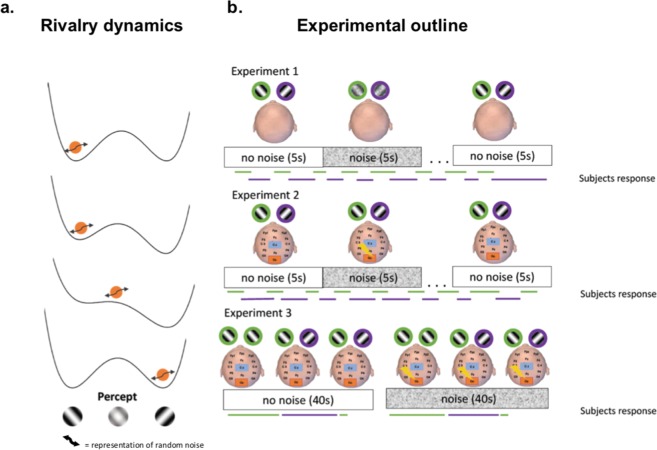
(a) Representation of rivalry dynamics. Binocular rivalry can be represented by a double-well energy landscape. The orange ball determines the current percept. Binocular rivalry is thought to be driven by adaptation and noise. Adaptation changes the landscape, meaning one of the wells becomes less shallow. Noise (arrow) causes the percept to change more quickly when the boundary between the two wells is low. (b) Experimental outline. In Experiment 1 noise (zero-mean Gaussian random noise) was applied to the visual stimulus for 5 seconds followed by 5.5–7 seconds of no stimulation. The same noise was applied to the left and right eye. The noise intensity was subthreshold for each individual participant. Experiment 2 followed the same protocol, except that the noise was applied to the visual cortex directly with tRNS (zero-mean Gaussian random noise, 100–640 Hz).
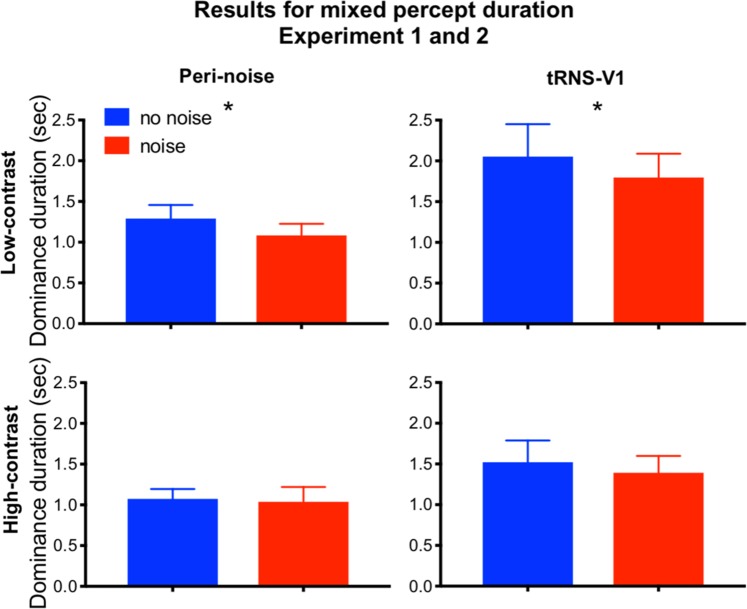
We investigated two separate cohorts (each n = 10) which performed the task either with low contrast or high contrast stimuli (see methods for further details). Adding noise to low contrast visual stimuli during binocular rivalry significantly reduced the median dominance duration of the mixed percept (low-noise mean[s.d.]: 1.29[0.53]sec, high-noise mean[s.d.]: 1.08[0.45] sec; t(9) = 2.938, p = 0.017; Cohen’s d = 0.65; −16% change in dominance duration; Fig. 3). Adding noise to high contrast visual stimuli did not significantly affect the mixed dominance duration (low-noise mean[s.d.]: 1.07[0.38] sec, high-noise mean[s.d.]: 1.04[0.58] sec; t(9) = 0.356, p = 0.730; Cohen’s d = 0.08; −4% change in dominance duration). Note that we observed a medium effect size for the low contrast stimuli, whereas there was only a small effect for high contrast stimuli suggesting that the noise effect is larger for the former.
Figure 3.
Behavioural results from Experiments 1 (left) and 2 (right). Adding noise to the visual stimulus significantly reduced the dominance duration of the mixed percept for low contrast visual stimuli by 16%, whereas the dominance duration for the high contrast visual stimuli was reduced by 4%. In Experiment 2 (right) the noise was added to the cortex with tRNS. This resulted in a reduction of 15% in median mixed percept duration. The dominance duration for the high contrast visual stimuli was reduced with 6% There was no significant effect of adding noise on the dominance duration of the exclusive percept. Error bars represent standard errors (s.e.m.).
Additionally, we tested the number of perceptual switches as a secondary outcome parameter: The number of perceptual switches was not significantly affected by peripheral noise for low contrast stimuli (low noise mean[s.d.]: 173.2[41.8], high noise mean[s.d.]: 181.7 [29.1]; t(9) = −1.284, p = 0.23; 5% change) or high contrast stimuli (low noise mean[s.d.]: 259.2[119.4], high noise mean[s.d.]: 258[113.9]; t(9) = 0.272, p = 0.79; 0% change).
Experiment 2
In Experiment 2 we added noise directly to the visual cortex with tRNS. Consistent with previous investigations, no participant reported awareness of the tRNS stimulation during de-briefing29,30. Adding noise to visual cortex with tRNS yielded a similar pattern of results to Experiment 1. Specifically, the median mixed dominance duration of the low contrast visual stimulus was significantly reduced (low-noise mean[s.d.]: 2.05[1.53] sec, high-noise mean[s.d.]: 1.79[1.14] sec; t(14) = 2.263, p = 0.04; Cohen’s d = 0.41, −13% change in dominance duration). Adding noise to a high contrast visual stimulus did not significantly affect the mixed dominance duration (low-noise mean[s.d.]: 1.51[1.03] sec, high-noise mean[s.d.]: 1.39[0.8] sec; t(14) = 0.930 p = 0.368, Cohen’s d = 0.16, −8% change in dominance duration). The number of perceptual switches was not significantly affected by peripheral noise for low contrast stimuli (low noise mean[s.d.]: 186.5[71.6], high noise mean[s.d.]: 194.2[61]; t(14) = −1.581, p = 0.136; 4% change) or high contrast stimuli (low noise mean[s.d.]: 184.7[49.9], high noise mean[s.d.]: 183.3[50.4]; t(14) = 0.428, p = 0.68; 0% change).
Discussion and Conclusion
Our results demonstrate for the first time that adding noise to a visual stimulus during binocular rivalry can significantly influence rivalry dynamics by reducing mixed percept durations. The same results were obtained when noise was added to the visual cortex directly with tRNS. This effect only occurred when the visual stimuli had a low contrast. These results are in line with the predictions made by the binocular rivalry model (see Fig. 1). Exclusive percept dominance durations in Experiments 1 and 2 were not affected by noise.
Adding noise to the visual stimuli (peripheral noise) resulted in the same behavioural results as adding noise to the brain directly with tRNS (central noise), namely, a significant reduction in mixed percept duration when the stimuli had a low contrast. No effect was observed when the stimuli were presented with a high contrast, suggesting that the stimulus contrast is a crucial parameter. Previously we have shown that noise added either peripherally or centrally can enhance performance on a contrast detection task3 for weak visual stimuli according to an SR-effect. An important difference between these two noise application methods is the location in the nervous system at which they influence visual processing for the first time. The initial influence of peripheral noise is at the receptor level. tRNS, on the other hand, influences visual processing at the cortical level. By applying tRNS during binocular rivalry we found that cortical noise can causally influence mixed percept duration, as predicted by the model.
Our secondary outcome measurement, the number of perceptual switches, changed in line with the model prediction but the effect was small and did not reach significance in the behavioural experiment. Note that we adopted an existing model which was developed and validated in a different experimental context19. Since the model was not optimized for the current experiment, its predictions were qualitatively similar to our behavioural results but not quantitatively. For example, the absolute dominance durations predicted by the model were much shorter than those observed experimentally. This discrepancy might have arisen because we did not model inter-subject variability, which has been shown to be substantial in response to transcranial electrical stimulation31. It is unclear how the intensity of “neural noise” induced by tRNS over visual cortex could be determined at the level of the individual, or how it could be parametrized in the model. More sophisticated, biologically plausible models might shed further light on these questions.
The processes underlying the occurrences of mixed percepts during binocular rivalry are unclear, but one possible mechanism involves changes in the strength of mutual inhibition between the two neuronal pools, each coding for a different percept32,33. It is thought that stronger stimuli (stimuli with a higher signal-to-noise ratio) result in an increase in mutual inhibition, which leads to a reduction in the mixed percept duration33. Adding noise could have strengthened mutual inhibition due to an increase in the SNR of the stimulus representation in the brain. Related to this finding, a recent study demonstrated that alcohol intake enhances mixed percept duration34. It has been shown in cats that alcohol reduces the signal-to-noise ratio (i.e. increases noise) in primary visual cortex35. In our study, we likely increased the SNR in visual cortex, resulting in a reduction of mixed percept duration. Another possible mechanism that effects mixed percept duration is a change in the balance between excitatory and inhibitory neural activity36. An imbalance between cortical excitation and inhibition is an important factor in autism spectrum disorder (ASD) models28. However, it is still unknown whether lower biological noise levels are also involved in ASD37. Interestingly, our finding that only mixed percepts are sensitive to a noise effect is supported by a study of binocular rivalry in ASD28. Robertson and colleagues did not find any difference between healthy controls and people with ASD on exclusive percept duration, but did find an increase in mixed percept duration in people with ASD28.
Our rivalry stimuli were relatively large (4°), which is larger than the receptive fields of individual V1 cells. Therefore, participants could have experienced ‘piecemeal rivalry’, which occurs when patches of both images are perceived together38. We did not test how much piecemeal rivalry participants experienced, but previous research suggests that it would have been less prominent in the low contrast condition than in the high contrast condition18. Previous research suggests that piecemeal rivalry is not affected by peripheral noise39.
It is known that tRNS is able to enhance cortical excitability after 4 minutes of continuous stimulation40, which results in a change in the excitation-inhibition balance. Here we alternated tRNS and non-tRNS runs in random order across participants, making it unlikely that the observed effects were driven by an overall change of excitability. Additionally, tRNS runs in Experiment 2 applied the stimulation for relatively short periods of 5 seconds, and these were followed by 5.0 to 7.7 s with no stimulation to reduce effects on overall excitability. Besides this, adding noise to the visual stimulus resulted in similar effects, and as far as we know cortical excitability cannot be modulated by peripheral noise. Therefore, our results are most likely explained by an SR-effect that modulates perceived stimulus contrast. It has been previously demonstrated that binocular rivalry is modulated by an SR-mechanism when evoked by peripheral noise41. In contrast to our study, Kim and colleagues did not add noise to the rivalry process, but changed the strength of the driving signal by changing the contrast of the two images in counter phase. The idea is that SR will occur when the driving signal matches biological noise levels. The reason we found no effect of noise on the dominance duration of exclusive percepts in our study could because the noise added might not have been optimal to introduce an SR-effect in a bistable system. The noise properties we applied were based on the findings of our previous study3 where we demonstrated that tRNS and peripheral noise are able to enhance performance on a contrast detection task. The fact that we might not have added an optimal noise level for everyone might explain why the effect sizes for the tRNS noise were relatively low.
In order to determine whether the tRNS-induced electrical noise reached the cortex, we have previously estimated that the applied field strength is too small to directly depolarize single neurons3. However, it has been proposed that the tRNS effect is amplified because thousands of neurons are modulated simultaneously42. Thereby even small depolarization may be enough to elicit action potentials (APs) in neurons that are close to their firing threshold, which results in a weak stimulus reaching the threshold for AP generation earlier.
Although it is still debated where in the brain binocular rivalry is resolved, we targeted the visual cortex. In particular, we selected an electrode montage and chose an intensity which is suitable for stimulating visual cortex as indicated by previous modelling work3. The reason we targeted this region is because neuronal activity in primary visual cortex (V1) is linked to subjective percepts during binocular rivalry43–45. Changes in higher order brain areas, including frontoparietal cortex, are thought to reflect the consequences of perceptual alternations, rather than being the cause of them46. However, there is also evidence that frontoparietal activity might play a causal role47–49. Evidence that mixed percepts may be more influenced by low-level visual processes rather than higher order processes50,51 supports the interpretation that our effect is driven by stimulation of these low-level visual cortices. The results of our study show that the effect of noise on the rivalry dynamics occurs at a cortical level. It has been shown before that peripheral noise can enhance the perceived contrast of a visual stimulus at the receptor level10,52. Therefore, finding an effect of adding noise peripherally does not necessarily mean that rivalry dynamics are sensitive to an SR effect, because any such effect could be explained at the receptor level.
In conclusion, our results are the first to demonstrate that mixed percept durations can be altered by applying noise to the visual cortex directly with tRNS, likely due to an enhancement of stimulus contrast. Our results open up new ways of manipulating noise levels within the brain, and provide a better understanding of the role noise plays in the brain when it is in a dynamical state.
Materials and Methods
The study was approved by the Kantonale Ethikkomission Zürich, Switzerland (KEK-ZH-Nr. 2014-0269) and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants before the start of the experiment.
Computational model of rivalry dynamics
We applied a computational model to predict how noise influences rivalry dynamics19. We used a conventional binocular rivalry model which relies on competition between neurons tuned to orthogonal orientations. The model relies on mutual inhibition and it also includes a noise component. The model contains two different neuronal populations and calculates the difference in firing rate between these populations. Over time inhibition on the suppressed neuronal population weakens, which eventually results in the inhibited population becoming dominant and suppressing the other population. When one population is firing fully the other population will be inhibited, resulting in the percept related to the fully firing population being dominant. When the difference in firing rate between the two populations is small then there is no winning population, resulting in a mixed percept. We introduced a criterion for mixed percepts, which was a difference in firing rate between the two neuronal populations of smaller than 0.1. In calculating dominance durations, we only included dominance times longer than 150 ms. In the model, we changed the strength of the visual stimulus and the amount of noise according to our experimental parameters. All other model parameters were identical to the original model parameters19. The simulation was run 100 times in order to derive an estimate of the variability of the model.
General procedures of Experiments 1 and 2
All experiments took place in a dark and quiet room. Visual stimuli (left and right tilted gratings) were generated using MATLAB version 2012b (MathWorks, Natick, USA) and the Psychophysics Toolbox53–55. Stimuli were controlled by a HP Elitedesk 800 G1 running Windows 7 (2009). Stimuli were presented on a Sony CPD-G420 colour monitor with a calibrated linearized output at a resolution of 1280 × 1024 pixels, with a refresh rate of 75 Hz. The two images were placed on the horizontal meridian to the left and right hand side of the screen on a uniform grey background (53 Cd/m2). The visual stimuli were orthogonal Gaussian gratings (orientation ± 45°, diameter 3 cm, contrast 20%, visual angle 4°, spatial-frequency 2.2 cycles per degree) surrounded by a white square (diameter 3.5 cm), to promote binocular fusion. Participants were seated in front of the monitor and viewed the images through a mirror stereoscope from a distance of 45 cm while resting their head on a chin rest. The images were viewed foveally (through the stereoscope).
Each participant performed 8 runs of binocular rivalry, each lasting 3 minutes. The task for the participants was to continuously report on a keyboard whether they perceived the left-eye or right-eye tilted grating or a mixture of both. In each experiment the noise was applied in 4 runs to either the screen (Exp. 1) or directly to the visual cortex with tRNS (Exp. 2). The order of the noise runs was randomized over participants. In these runs the noise was applied for 5 seconds followed by a 5.5–7 seconds’ interval of no stimulation, and in total 18 times per run. In each experiment participants received a total of 360 seconds’ visual noise (exp. 1) or tRNS (exp. 2). Participants performed one practice run without any noise before the start of the experiment.
Experiment 1: Peri-noise condition
In this experiment, we tested whether adding noise (zero-mean Gaussian random noise) to the visual stimuli influences binocular rivalry dynamics. The same noise was applied to the left and right eye. Previous research demonstrated that a noise intensity corresponding to 60% of threshold effectively induces an SR-effect3. Therefore, we used a simple up-down method to estimate each individual’s 60% correct noise-threshold before the experiment started, where 75% correct reflects threshold. We tested two cohorts of participants, one with a low contrast visual stimulus (20% contrast, n = 10, mean age = 23 years) and the other with a high contrast visual stimulus (70% contrast, n = 10, mean age = 24 years).
Experiment 2: tRNS-V1 condition
In this experiment, we tested the hypothesis that adding noise directly to the visual cortex with tRNS can influence rivalry dynamics. Noise was applied centrally with tRNS (100–640 Hz, zero-mean Gaussian random noise). Electrode placement was determined using the 10–20 system. The stimulation electrode was placed over the occipital region (Oz in the 10–20 EEG system) and the reference electrode over the vertex (Cz in the 10–20 EEG system). Electroconductive gel was applied to the contact side of the electrode (5 × 7 cm) to reduce skin impedance. Electrodes were held in place with a bandage. This setup has been demonstrated to be suitable for stimulation of the visual cortex3,56,57. More specifically, previous work has estimated the electrical field strength produced by this montage in visual cortex3. In brief, modelling the electrical field for specific frequencies between 100 and 500 Hz (50 Hz steps,) and the current flow across the brain revealed sufficiently focal stimulation of visual cortex. The induced electrical field was estimated to reach a maximum of 2 V/m with all frequencies being transmitted to the brain. Stimulation was delivered by a battery-driven electrical stimulator (DC-Stimulator Plus, neuroConn). An intensity of 1 mA was applied since it has been demonstrated that this intensity effectively induces an SR-effect in most subjects3. The maximum current density in this experiment was 28.57 μA/cm2, which is within current safety limits58. We again tested two cohorts of participants, one where the visual stimulus had a low contrast (20% contrast, n = 15, mean age = 23 years) and the other where the visual stimulus had a high contrast (70% contrast, n = 15, mean age = 24 years). We tested more participants in Experiment 2 because it was expected there would be less power to detect a reliable effect of tRNS relative to peripheral visual noise3.
Data analysis and statistics
Statistical analyses were performed using SPSS (version 20.0, IBM). The same statistical procedures were applied to both experiments. For each participant, we calculated the median dominance duration for the exclusive percepts. We also calculated the mixed percept dominance durations. Times where no button was pressed, or when the dominance duration was shorter than 150 ms, were excluded from analysis. Dominance durations terminated by the end of a block were not included in the analyses. In each experiment, we also calculated the number of perceptual switches. To test for the effect of the added noise, dominance durations and number of perceptual switches were subjected to a two-sided within-subject t-test. The α-level was set to 0.05 for all tests.
Acknowledgements
O.v.d.G was supported by the Swiss National Science Foundation (grant 165189 and 320030_149561), N.W. was supported by the Swiss National Science Foundation (grant 320030_149561), and J.B.M. was supported by the Australian Research Council (ARC) Centre of Excellence for Integrative Brain Function (ARC Centre Grant CE140100007) and by an ARC Australian Laureate Fellowship (FL110100103).
Author Contributions
All authors designed the research; O.v.d.G. performed research, analysed the data and all authors contributed to writing the paper.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McDonnell MD, Abbott D. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput Biol. 2009;5:e1000348. doi: 10.1371/journal.pcbi.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward LM, Neiman A, Moss F. Stochastic resonance in psychophysics and in animal behavior. Biological cybernetics. 2002;87:91–101. doi: 10.1007/s00422-002-0328-z. [DOI] [PubMed] [Google Scholar]
- 3.van der Groen O, Wenderoth N. Transcranial Random Noise Stimulation of Visual Cortex: Stochastic Resonance Enhances Central Mechanisms of Perception. J Neurosci. 2016;36:5289–5298. doi: 10.1523/JNEUROSCI.4519-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell DF, Wilkens LA, Moss F. Use of behavioural stochastic resonance by paddle fish for feeding. Nature. 1999;402:291. doi: 10.1038/46279. [DOI] [PubMed] [Google Scholar]
- 5.Manjarrez E, Diez-Martinez O, Mendez I, Flores A. Stochastic resonance in human electroencephalographic activity elicited by mechanical tactile stimuli. Neuroscience Letters. 2002;324:213–216. doi: 10.1016/S0304-3940(02)00212-4. [DOI] [PubMed] [Google Scholar]
- 6.Ward, L. M., MacLean, S. E. & Kirschner, A. Stochastic Resonance Modulates Neural Synchronization within and between Cortical Sources. Plos One5, ARTN e14371, 10.1371/journal.pone.0014371 (2010). [DOI] [PMC free article] [PubMed]
- 7.Collins JJ, Imhoff TT, Grigg P. Noise-enhanced tactile sensation. Nature. 1996;383:770–770. doi: 10.1038/383770a0. [DOI] [PubMed] [Google Scholar]
- 8.Collins JJ, Imhoff TT, Grigg P. Noise-mediated enhancements and decrements in human tactile sensation. Phys Rev E. 1997;56:923–926. doi: 10.1103/Physreve.56.923. [DOI] [Google Scholar]
- 9.Zeng FG, Fu QJ, Morse R. Human hearing enhanced by noise. Brain Res. 2000;869:251–255. doi: 10.1016/S0006-8993(00)02475-6. [DOI] [PubMed] [Google Scholar]
- 10.Simonotto E, et al. Visual perception of stochastic resonance. Physical Review Letters. 1997;78:1186–1189. doi: 10.1103/Physrevlett.78.1186. [DOI] [Google Scholar]
- 11.Gammaitoni L, Marchesoni F, Menichella-Saetta E, Santucci S. Stochastic resonance in bistable systems. Phys Rev Lett. 1989;62:349–352. doi: 10.1103/PhysRevLett.62.349. [DOI] [PubMed] [Google Scholar]
- 12.Levelt, W. J. M. On binocular rivalry, Gorcum, (1965).
- 13.Tong F, Meng M, Blake R. Neural bases of binocular rivalry. Trends Cogn Sci. 2006;10:502–511. doi: 10.1016/J.Tics.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Blake R. A neural theory of binocular rivalry. Psychol Rev. 1989;96:145–167. doi: 10.1037/0033-295X.96.1.145. [DOI] [PubMed] [Google Scholar]
- 15.Shpiro A, Moreno-Bote R, Rubin N, Rinzel J. Balance between noise and adaptation in competition models of perceptual bistability. Journal of Computational Neuroscience. 2009;27:37–54. doi: 10.1007/s10827-008-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lankheet MJ. Unraveling adaptation and mutual inhibition in perceptual rivalry. J Vis. 2006;6:304–310. doi: 10.1167/6.4.1. [DOI] [PubMed] [Google Scholar]
- 17.Huguet G, Rinzel J, Hupe JM. Noise and adaptation in multistable perception: noise drives when to switch, adaptation determines percept choice. J Vis. 2014;14:19. doi: 10.1167/14.3.19. [DOI] [PubMed] [Google Scholar]
- 18.Brascamp JW, van Ee R, Noest AJ, Jacobs RH, van den Berg AV. The time course of binocular rivalry reveals a fundamental role of noise. J Vis. 2006;6:1244–1256. doi: 10.1167/6.11.8. [DOI] [PubMed] [Google Scholar]
- 19.Said CP, Heeger DJ. A model of binocular rivalry and cross-orientation suppression. PLoS Comput Biol. 2013;9:e1002991. doi: 10.1371/journal.pcbi.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Bote R, Rinzel J, Rubin N. Noise-induced alternations in an attractor network model of perceptual bistability. Journal of Neurophysiology. 2007;98:1125–1139. doi: 10.1152/jn.00116.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roumani, D. & Moutoussis, K. Binocular rivalry alternations and their relation to visual adaptation. Frontiers in Human Neuroscience 6, ARTN 35, 10.3389/fnhum.2012.00035 (2012). [DOI] [PMC free article] [PubMed]
- 22.Rajasekar, S. & Sanjuan, M. A. F. Nonlinear resonances.
- 23.Onorato I, et al. Noise Enhances Action Potential Generation in Mouse Sensory Neurons via Stochastic Resonance. PLoS One. 2016;11:e0160950. doi: 10.1371/journal.pone.0160950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. 2008;28:14147–14155. doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gammaitoni L, Hänggi P, Jung P, Marchesoni F. Stochastic resonance. Reviews of modern physics. 1998;70:223. doi: 10.1103/RevModPhys.70.223. [DOI] [Google Scholar]
- 26.Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic Action in the Autistic Brain. Curr Biol. 2016;26:80–85. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Freyberg J, Robertson CE, Baron-Cohen S. Reduced perceptual exclusivity during object and grating rivalry in autism. J Vis. 2015;15:11. doi: 10.1167/15.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson CE, Kravitz DJ, Freyberg J, Baron-Cohen S, Baker CI. Slower rate of binocular rivalry in autism. J Neurosci. 2013;33:16983–16991. doi: 10.1523/JNEUROSCI.0448-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambrus GG, Paulus W, Antal A. Cutaneous perception thresholds of electrical stimulation methods: Comparison of tDCS and tRNS. Clinical Neurophysiology. 2010;121:1908–1914. doi: 10.1016/J.Clinph.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Fertonani A, Pirulli C, Miniussi C. Random noise stimulation improves neuroplasticity in perceptual learning. J Neurosci. 2011;31:15416–15423. doi: 10.1523/JNEUROSCI.2002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014;7:468–475. doi: 10.1016/j.brs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Klink PC, Brascamp JW, Blake R, van Wezel RJ. Experience-driven plasticity in binocular vision. Curr Biol. 2010;20:1464–1469. doi: 10.1016/j.cub.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollins M. The Effect of Contrast on the Completeness of Binocular-Rivalry Suppression. Perception & psychophysics. 1980;27:550–556. doi: 10.3758/Bf03198684. [DOI] [PubMed] [Google Scholar]
- 34.Cao D, Zhuang X, Kang P, Hong SW, King AC. Acute Alcohol Drinking Promotes Piecemeal Percepts during Binocular Rivalry. Front Psychol. 2016;7:489. doi: 10.3389/fpsyg.2016.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen B, Xia J, Li G, Zhou Y. The effects of acute alcohol exposure on the response properties of neurons in visual cortex area 17 of cats. Toxicol Appl Pharmacol. 2010;243:348–358. doi: 10.1016/j.taap.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 36.Said CP, Egan RD, Minshew NJ, Behrmann M, Heeger DJ. Normal binocular rivalry in autism: implications for the excitation/inhibition imbalance hypothesis. Vision Res. 2013;77:59–66. doi: 10.1016/j.visres.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis G, Plaisted-Grant K. Low endogenous neural noise in autism. Autism: the international journal of research and practice. 2015;19:351–362. doi: 10.1177/1362361314552198. [DOI] [PubMed] [Google Scholar]
- 38.O’Shea RP, Sims AJ, Govan DG. The effect of spatial frequency and field size on the spread of exclusive visibility in binocular rivalry. Vision Res. 1997;37:175–183. doi: 10.1016/S0042-6989(96)00113-7. [DOI] [PubMed] [Google Scholar]
- 39.Skerswetat J, Formankiewicz MA, Waugh SJ. More superimposition for contrast-modulated than luminance-modulated stimuli during binocular rivalry. Vision Res. 2018;142:40–51. doi: 10.1016/j.visres.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Chaieb L, Paulus W, Antal A. Evaluating aftereffects of short-duration transcranial random noise stimulation on cortical excitability. Neural plasticity. 2011;2011:105927. doi: 10.1155/2011/105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YJ, Grabowecky M, Suzuki S. Stochastic resonance in binocular rivalry. Vision Res. 2006;46:392–406. doi: 10.1016/j.visres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Francis JT, Gluckman BJ, Schiff SJ. Sensitivity of neurons to weak electric fields. J Neurosci. 2003;23:7255–7261. doi: 10.1523/JNEUROSCI.23-19-07255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SH, Blake R, Heeger DJ. Hierarchy of cortical responses underlying binocular rivalry. Nat Neurosci. 2007;10:1048–1054. doi: 10.1038/nn1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polonsky A, Blake R, Braun J, Heeger DJ. Neuronal activity in human primary visual cortex correlates with perception during binocular rivalry. Nat Neurosci. 2000;3:1153–1159. doi: 10.1038/80676. [DOI] [PubMed] [Google Scholar]
- 45.Leopold DA, Logothetis NK. Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry. Nature. 1996;379:549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- 46.Knapen T, Brascamp J, Pearson J, van Ee R, Blake R. The role of frontal and parietal brain areas in bistable perception. J Neurosci. 2011;31:10293–10301. doi: 10.1523/JNEUROSCI.1727-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanai R, Carmel D, Bahrami B, Rees G. Structural and functional fractionation of right superior parietal cortex in bistable perception. Curr Biol. 2011;21:R106–107. doi: 10.1016/j.cub.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carmel D, Walsh V, Lavie N, Rees G. Right parietal TMS shortens dominance durations in binocular rivalry. Curr Biol. 2010;20:R799–800. doi: 10.1016/j.cub.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 49.Zaretskaya N, Thielscher A, Logothetis NK, Bartels A. Disrupting parietal function prolongs dominance durations in binocular rivalry. Curr Biol. 2010;20:2106–2111. doi: 10.1016/j.cub.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 50.Blake R, Oshea RP, Mueller TJ. Spatial Zones of Binocular-Rivalry in Central and Peripheral-Vision. Visual Neurosci. 1992;8:469–478. doi: 10.1017/S0952523800004971. [DOI] [PubMed] [Google Scholar]
- 51.Antinori A, Carter OL, Smillie LD. Seeing it both ways: Openness to experience and binocular rivalry suppression. J Res Pers. 2017;68:15–22. doi: 10.1016/j.jrp.2017.03.005. [DOI] [Google Scholar]
- 52.Srebro R, Malladi P. Stochastic resonance of the visually evoked potential. Phys Rev E. 1999;59:2566–2570. doi: 10.1103/Physreve.59.2566. [DOI] [Google Scholar]
- 53.Kleiner M, Brainard D, Pelli D. What’s new in Psychtoolbox-3? Perception. 2007;36:14–14. [Google Scholar]
- 54.Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. doi: 10.1163/156856897x00357. [DOI] [PubMed] [Google Scholar]
- 55.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. doi: 10.1163/156856897x00366. [DOI] [PubMed] [Google Scholar]
- 56.Neuling T, Wagner S, Wolters CH, Zaehle T, Herrmann CS. Finite-Element Model Predicts Current Density Distribution for Clinical Applications of tDCS and tACS. Frontiers in psychiatry. 2012;3:83. doi: 10.3389/fpsyt.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Groen O, Tang MF, Wenderoth N, Mattingley JB. Stochastic resonance enhances the rate of evidence accumulation during combined brain stimulation and perceptual decision-making. PLoS Comput Biol. 2018;14:e1006301. doi: 10.1371/journal.pcbi.1006301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fertonani A, Ferrari C, Miniussi C. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clinical Neurophysiology. 2015;126:2181–2188. doi: 10.1016/j.clinph.2015.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.