Abstract
Gulf War Illness (GWI) is a chronic, multi-symptom illness that affects approximately 25% of Gulf veterans, with cognitive fatigue as one of its primary symptoms. Here, we investigated the neural networks associated with cognitive fatigue in GWI by asking 35 veterans with GWI and 25 healthy control subjects to perform a series of fatiguing tasks while in the MRI scanner. Two types of cognitive fatigue were assessed: state fatigue, which is the fatigue that developed as the tasks were completed, and trait fatigue, or one's propensity to experience fatigue when assessed over several weeks. Our results showed that the neural networks associated with state and trait fatigue differed. Irrespective of group, the network underlying trait fatigue included areas associated with memory whereas the neural network associated with state fatigue included key areas of a fronto-striatal-thalamic circuit that has been implicated in fatigue in other populations. As in other investigations of fatigue, the caudate of the basal ganglia was implicated in fatigue. Furthermore, individuals with GWI showed greater activation than the HC group in frontal and parietal areas for the less difficult task. This suggests that an inability to modulate brain activation as task demands change may underlie fatigue in GWI.
Highlights
-
•
Fatigue-related brain activation can be induced and measured in veterans with GWI.
-
•
A network of brain areas was associated with cognitive fatigue during a working memory task.
-
•
The fatigue network included the basal ganglia, prefrontal and parietal areas.
-
•
Persistent activation in frontal and parietal areas in the GWI group suggests dysregulation of the cognitive control network.
1. Introduction
Fatigue is a serious and disabling symptom of Gulf War Illness (GWI). Estimates from the Research Advisory Committee on Gulf War Illnesses indicate that 25% of all Gulf veterans (GVs) have GWI with fatigue being a primary symptom (Research Advisory Committee on Gulf War Veteran's Illnesses, 2008). Indeed, the symptoms of GWI are very similar to those of chronic fatigue syndrome (CFS) and more than half of GVs with GWI meet the criteria for CFS. Moreover, veterans with GWI report that their fatigue is worsened by the physical and cognitive demands of daily life resulting in a significant number of military personnel that are no longer able to perform their duties (Gulf War veterans statistics report, 1998). Cognitive fatigue, or fatigue that is exacerbated by mental demands, has remained understudied and is the focus of the current study.
One of the reasons that cognitive fatigue remains understudied and therefore poorly understood is that it has proven difficult to develop objective assessments of cognitive fatigue. Researchers have had to rely on subjective fatigue measures (reflecting subjects' perception of fatigue), which have often failed to correlate with objective behavioral performance (for review, see Deluca, 2005). Recent research has provided evidence that functional Magnetic Resonance Imaging (fMRI) may be used to assess cognitive fatigue by examining time-dependent changes in brain activity during sustained performance of a cognitive task (Cook et al., 2007; DeLuca et al., 2008; Kohl et al., 2009; Genova et al., 2013; Cook et al., 2017; Wylie et al., 2017a, Wylie et al., 2017b). Thus, functional neuroimaging methods may provide an objective representation of the patient's subjective experience; however, these methods have not yet been widely applied to the study of cognitive fatigue in veterans with GWI.
In the present study we applied these neuroimaging methods to the study of cognitive fatigue in GWI. Previous research has used fMRI (Rayhan et al., 2013a; Hubbard et al., 2014; Cook et al., 2017), diffusion tensor imaging (DTI, Rayhan et al., 2013b), and magnetoencephalography (MEG, Engdahl et al., 2016) to investigate the consequences of GWI on brain function. Using MEG, Engdahl et al. (Engdahl et al., 2016) found differences between veterans with GWI and healthy controls (HCs) in frontal and cerebellar regions. The results of investigations using DTI (Rayhan et al., 2013b) corroborated this finding by showing differences in the inferior fronto-occipital fasciculus, a white matter tract that connects orbital frontal and ventro-medial prefrontal areas of the brain with the insula – a circuit that has been shown to be important for the experience of pain (Baliki et al., 2012) and fatigue (Pardini et al., 2010; Wylie et al., 2017a, Wylie et al., 2017b). Functional studies have also found differences in frontal areas, including insula, superior frontal, caudate (Rayhan et al., 2013a) and parietal areas (Rayhan et al., 2013a; Cook et al., 2017). While all functional studies have used working memory tasks, the particular task used has varied. We chose the N-Back working memory task (see also Rayhan et al., 2013a) because it is a widely used task, which incorporates a straightforward manipulation of task difficulty. In healthy populations, difficult tasks result in more cognitive fatigue than easier tasks (e.g., Boksem et al., 2005, Boksem et al., 2006). We therefore asked all participants to perform four blocks of a difficult condition (the 2-back task) and a less difficult condition (the 0-back task) of the N-back working memory task. One of our hypotheses was that, because veterans with GWI report chronic fatigue, they would find both conditions of the N-back task to be fatiguing.
Another difficulty in studying cognitive fatigue arises from the methods used to measure fatigue. Often, fatigue is assessed by instruments that ask subjects to rate their fatigue over a period of time. For example, the Fatigue Severity Scale (FSS) (Krupp et al., 1989) asks participants to rate their fatigue over the previous week. This type of instrument can be thought of as assessing ‘trait’ fatigue (Kluger et al., 2013), or the extent to which participants are prone to experience fatigue, because participants are essentially required to assess their ‘average’ fatigue over a fairly long period of time (in the case of the FSS, a week). Other fatigue assessment instruments require subjects to report their instantaneous experience of fatigue, or their level of fatigue at the time of testing. One such test is the Visual Analog Scale of Fatigue (VAS—F) (Shahid et al., 2011). This instrument assesses ‘state’ fatigue (Kluger et al., 2013), or the extent to which one experiences fatigue ‘in the moment’. While it may be that trait measures of fatigue represent the integration of state fatigue over time, it is also possible that when subjects rate their trait fatigue, they are influenced by their recollections of other factors (e.g., depression, apathy). Despite this, state and trait measures of fatigue are rarely compared (indeed, they are rarely distinguished from one another), and this has contributed to the difficulty in studying fatigue.
We had several aims in the current study. Our first aim was to investigate differences in fatigue between veterans with GWI and healthy controls (HCs). To do this, we assessed both trait fatigue (using the FSS) and state fatigue, which we induced using a demanding working memory task and assessed using the VAS—F. Based on the prevalence of fatigue in GWI, we hypothesized that veterans with GWI would report more trait fatigue than HCs. We also hypothesized that veterans with GWI would report more state cognitive fatigue than the HCs, and that state cognitive fatigue would increase more quickly during performance of a cognitively fatiguing task in the GWI group than in the HC group. Our second aim was to better understand the mechanisms underlying both trait and state fatigue in GWI. To do this, we collected fMRI data while all participants performed the working memory task and correlated their brain activation with both the FSS and the VAS—F. This allowed us to see whether the brain networks involved in trait fatigue and state fatigue were the same or different, and how the networks associated with trait and state fatigue differed between the groups. Based on previous work in which we showed that increased state cognitive fatigue in HCs was associated with increased activation of the caudate (Wylie et al., 2017a), we hypothesized that the difficulty of the task would modulate state cognitive fatigue in the HC group; however, because we expected the GWI group to report more fatigue for both tasks, we expected this modulation would be less evident in the GWI group. Our third aim was to better understand how fatigue affected task performance, and how this differed between the groups (GWI vs. HC). To do this, we analyzed response time (RT) and accuracy during the performance of our fatigue-induction tasks, including our measure of state cognitive fatigue as a covariate (VAS—F).
2. Methods
2.1. Subjects
The sample consisted of 35 veterans with GWI and 25 healthy controls (HCs). Veterans in the GWI group met the criteria of a modified version of the Steele (2000) case definition of GWI (recommended by the Gulf War Research Advisory Committee [Washington D.C. meeting, February 28th to March 1st, 2011]), according to which these veterans had at least moderate fatigue, pain, and/or cognitive problems. Using methodology similar to Fukuda et al. (1998), inclusion further depended upon veterans endorsing at least 2 of these 3 symptoms, with the further stipulation that all veterans in the GWI group reported at least moderate fatigue. Healthy individuals were matched to the GWI sample for age (mean ± standard deviation = GWI: 49.3 ± 5.2 years; HC: 46.5 ± 11.1 years), education (GWI: 14.6 ± 2.8; HC: 15.0 ± 2.2 years), and gender distribution (GWI: 31 men, 4 women; HC: 21 men, 4 women). The HC sample included both veterans (n = 10) and civilians (n = 15). In order to ensure that the veterans and civilians did not differ in respect to their fatigue, we analyzed both their VAS-F scores and their FSS scores. The VAS-F scores were analyzed with a mixed between- and within- subjects ANOVA with the factors of Group (veterans vs. civilians), Task (0-back and 2-back) and Rating (rating 1–5). There was no significant effect of Group, nor was it part of any interaction. The FSS scores were compared with a t-test, and there was no significant difference between the groups.
For both the GWI and HC groups, subjects were: (1) free of a history of prior neurological insult or disease such as stroke, seizures, or brain tumor; (2) free from significant psychiatric history (such as schizophrenia or bipolar disorder) due to the potential influence of such disorders on cognitive functioning (assessed by self-report corroborated by medical records); (3) right handed due to the effect of mixed hand dominance on cerebral organization; (4) free of alcohol or drug abuse history. Subjects currently taking benzodiazepines, neuroleptics, or psycho-stimulants were excluded due to the potential effects of these medications on cognition and the hemodynamic response. For all study participants, additional exclusionary criteria associated with MRI (ferrous metal in the body) were discussed and strictly enforced.
The Institutional Review Boards of The Department of Veterans' Affairs and Kessler Foundation approved the study, and the study was performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all subjects.
2.1.1. Data collection
Behavioral data acquisition, randomization and stimulus presentation was administered using the E-Prime software (Schneider et al., 2002). The N-back paradigm was presented in the scanner in an event-related design.
Neuroimaging data collection began on a 3-Tesla Siemens Allegra scanner (15 HCs) and was completed on a 3-Tesla Siemens Skyra scanner (10 HCs and all 35 veterans with GWI). For this reason, a regressor for scanner was included in all group-level analyses, as has been done in previous research utilizing more than one scanner (Stonnington et al., 2008; Biswal et al., 2010). A T2*-weighted pulse sequence was used to collect functional images during eight blocks (four at each of two difficulty levels), resulting in 140 acquisitions per block (Allegra: echo time = 30 ms; repetition time = 2000 ms; field of view = 22 cm; flip angle = 80°; slice thickness = 4 mm, 32 slices, matrix = 64 × 64, in-plane resolution = 3.438 × 3.438 mm; Skyra: echo time = 30 ms; repetition time = 2000 ms; field of view = 22 cm; flip angle = 90°; slice thickness = 4 mm, 32 slices, matrix = 92 × 92, in-plane resolution = 2.391 × 2.391 mm). A high-resolution magnetization prepared rapid gradient echo (MPRAGE) image was also acquired (Allegra: TE = 4.38 ms; TR = 2000 ms, FOV = 220 mm; flip angle = 8°; slice thickness = 1 mm, NEX = 1, matrix = 256 × 256, in-plane resolution = 0.859 × 0.859 mm; Skyra: TE = 3.43 ms; TR = 2100 ms, FOV = 256 mm; flip angle = 9°; slice thickness = 1 mm, NEX = 1, matrix = 256 × 256, in-plane resolution = 1 × 1 mm), and was used to normalize the functional data into standard space.
2.1.2. Behavioral paradigm
All participants completed a series of practice trials before scanning, exposing them to the two difficulty levels of the N-Back task. During the fMRI scan, participants were presented with the N-Back working memory task in which task difficulty was varied by presenting the 0-back condition, which places a relatively low load on working memory, and the 2-back condition, which places a higher load on working memory. There were 4 blocks of each level of the N-back task (8 blocks total), with 65 trials per block (16 of which were targets). The 4 blocks of each task were always presented together (that is, the two tasks were not interleaved), and the order of presentation (0-back first vs. 2-back first) was counterbalanced across subjects. During the 0-back task, participants were asked to respond each time the target letter “K” was presented on the screen, while during the 2-back task, participants were asked to respond when the target letter corresponded to the letter presented two trials previously. In all cases, the letter stimuli remained on the screen for 1.5 s. and there was an inter-trial interval of 500 ms. In the analyses of the fMRI data, only correct trials were modeled.
2.1.3. Visual analog scale of fatigue
To evaluate the level of on-task ‘state’ fatigue, participants were presented with a visual analogue scale of fatigue before and after each block of the N-back task. Participants were asked: “How mentally fatigued are you?” and were asked to indicate their level of fatigue on a scale from 0 to 100, with 0 being not at all fatigued and 100 being extremely fatigued. In order to mask the purpose of the study, five additional VASs were administered as well, in randomized order. These assessed happiness, sadness, pain, tension and anger.
2.1.4. Questionnaires
Prior to the completion of the fMRI procedure, participants filled out the Fatigue Severity Scale (FSS) and Chicago Multiscale Depression Inventory (CMDI). These questionnaires provided a measure of trait fatigue and depression.
2.2. Data analysis
2.2.1. Behavioral data
The response time (RT) and accuracy data were each analyzed with linear mixed effects models using the R statistical analysis package (version 1.0.136). The between-subjects factor was Group (GWI vs. HC) and the within-subjects factors were Task (0-back vs. 2-back) and Block (block 1–4 of each task; this was included as a fixed effect to account for any order effects because the four blocks of each task were run sequentially). Only the data from blocks in which subjects performed at 70% accuracy or better were included in the analysis. This criterion resulted in the removal of 9 blocks of data from all analyses (5 from the HC group and 4 from the GWI group), which represented 2% of the full dataset. In order to model subjects' on-task, state fatigue, the VAS-F scores were included as a quantitative variable (covariate). The VAS-F score used for each block was the average of the VAS-F score reported before and after that block. Because the VAS-F scores differed between the groups, the scores were centered separately for each group prior to analysis (i.e., the group mean was subtracted from each score). The behavioral data from four HCs was lost due to equipment failure during scanning (their neuroimaging data are included in the neuroimaging analyses).
The VAS data were analyzed with a mixed, between- and within- subjects ANOVA. The between-subjects factor was Group (GWI vs. HC) and the within-subjects factors were Task (0-back vs. 2-back) and Rating (1st, 2nd, 3rd, 4th, 5th rating).
2.2.2. fMRI data
For each of the fMRI blocks, the first three images were discarded to ensure steady state magnetization. All images were preprocessed using the Broccoli software package (Eklund et al., 2014, Eklund et al., 2016) which performed the slice timing correction, motion correction and smoothing (using a 6 × 6 × 6 mm Gaussian smoothing kernel), as well as coregistering the functional data to the high resolution MPRAGE and warping all data into standard (Montreal Neurological Institute [MNI]) space using a non-linear approach (Eklund et al., 2014). Each of the four blocks of each task (0-back and 2-back) were then deconvolved separately (using 3dDeconvolve). Motion parameters and two polynomial regressors (to model signal drift) were included as regressors of no interest.
Two analyses were performed with the fMRI data (using 3dLME, a script provided in the AFNI software suite (version AFNI_16.0.00) that uses the R statistical package (version 3.3.1)): one to investigate state fatigue (using the VAS-F scores) and the other to investigate trait fatigue (using the FSS scores). Because the correlation between the VAS-F scores and the FSS scores was close to significant (r = 0.25, p = 0.056, r2 = 0.06), we performed two, separate analyses. For both analyses a linear mixed effects model was used with a between-subjects factor of Group (GWI vs. HC), within-subjects factors of Task (0-back vs. 2-back) and Block (block 1–4 of each task), and the covariate of fatigue. For the analysis of state fatigue, the covariate was the VAS-F scores; for the analysis of trait fatigue, the covariate was the FSS scores. All group-level statistical maps were thresholded using both the alpha level and cluster size correction (extent of activation). The alpha level was set at p < 0.01 and the cluster size was set at 93 contiguous voxels. The results of Monte Carlo simulations showed that this combination resulted in a corrected alpha level of p < 0.05. Because we had a prior hypothesis about the involvement of the caudate, a separate Monte Carlo simulation was conducted on just the caudate. Based on this, clusters of at least 26 voxels within the caudate were also considered significant.
3. Results
3.1. Behavioral results
For the reaction time data, there was a significant main effect of Task (F(1,373.1) = 273.34, p < 0.0001). Subjects responded with longer latencies during the 2-back task (783.6 ms) than during the 0-back task (622.0 ms) as expected. No other effects or interactions were significant.
For the accuracy data, there was a significant main effect of Task (F(1,372.0) = 345.73, p < 0.0001). Subjects responded with lower accuracy during the 2-back task (84.8%) than during the 0-back task (92.3%) as expected. The main effect of Group was also significant (F(1,53.0) = 16.49, p < 0.0002) and derived from veterans in the GWI group responding less accurately (86.0%) than veterans in the HC group (91.1%). The main effect of Fatigue was significant (F(1,409.4) = 4.58, p = 0.03) such that greater state fatigue was associated with lower accuracy (correlation coefficient = −0.14: that is, for a unit increase in VAS-F score, accuracy was estimated to decrease by 0.14%). The only significant interaction was between Task and Group (F(1,372.0) = 7.94, p = 0.005). This derived from a larger decrease in accuracy from the 0-back to the 2-back task in the GWI group (0back: 90.3%; 2-back: 81.7%) than for the HC group (0-back: 94.3%; 2-back: 87.9%).
3.2. VAS-F results
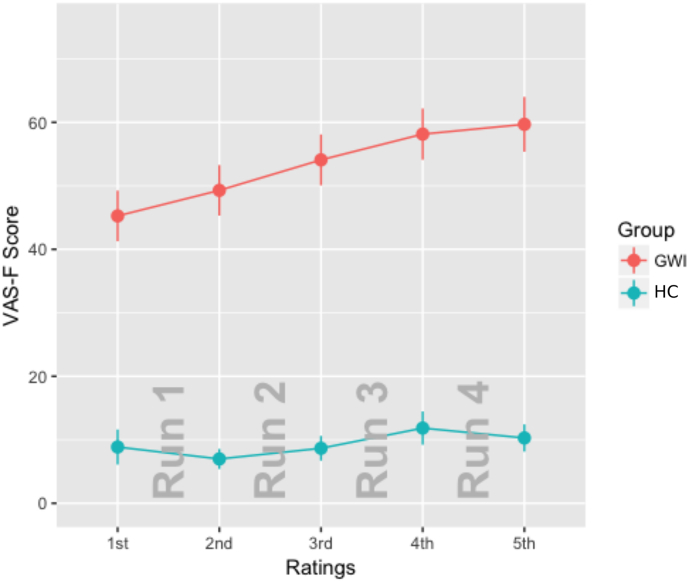
The main effect of Group was significant (F(1,58) = 59.20, p < 0.0001, η2 = 0.43). As expected, the GWI group reported significantly more fatigue than the HC group (the VAS-F scores were 53.3 and 9.4 for the GWI and HC groups respectively). The main effect of Rating was also significant (F(4,232) = 16.37, p < 0.0001, η2 = 0.02). Subjects reported progressively more fatigue over the four blocks of the two tasks (VAS-F scores were 30.1, 31.7, 35.2, 38.9, and 39.3 for the 1st, 2nd, 3rd, 4th, 5th rating, respectively). The interaction between Group and Rating was significant (F(4,232) = 5.42, p < 0.001, η2 = 0.007), such that the VAS-F scores reported by the GWI group increased at a faster rate than the scores reported by the HC group (see Fig. 1).
Fig. 1.
Mean Visual Analog Scale of Fatigue (VAS-F) scores for each group. The data from the GWI group is plotted in red and the data from the controls (HC) is plotted in blue. Error bars show the standard error of the mean. The VAS-F scores were acquired before and after each of the four runs of the task (denoted in the Figure with the words Run 1–4 in grey). The VAS-F scores of the GWI group increased across the four runs of the tasks while the VAS-F scores of the HC group remained fairly stable. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. FSS results
There was a significant difference in the FSS scores between the groups (t(57.46) = 13.09, p < 0.0001). The GWI group reported more trait fatigue (51.91) than the HC group (20.94).
3.4. BOLD state fatigue (VAS—F) results
Because we were primarily interested in the differential effects of fatigue in the two groups, we will concentrate on interactions that involve VAS-F and Group. However, for the sake of completeness, other effects are included.
There was a significant main effect of VAS-F in several areas, including the basal ganglia (putamen), postcentral areas, temporal areas (middle and superior temporal gyri) and parietal areas (superior parietal lobule). These are listed in Table 1. Because Group did not interact with VAS-F in these areas, the correlation between brain activation and VAS-F in these areas represents the response to fatigue that is unaffected by GWI. That is, in these areas there was a correlation between brain activation (across Task) and VAS-F across the whole sample. In all cases, the relationship between brain activation and VAS-F was negative, meaning that as brain activation increased, less fatigue was reported: postcentral correlation coefficient = −0.010; parietal correlation coefficient = −0.009; superior temporal correlation coefficient = −0.010; middle temporal correlation coefficient = −0.018; putamen correlation coefficient = −0.012 (see Table 2).
Table 1.
Areas of significant brain activation for the analysis of state fatigue during the N-Back task. ‘BA’ denotes Brodmann Area; ‘X’, ‘Y’, and ‘Z’ denote the location of the voxel in the cluster with the highest activation; ‘Voxels’ denotes the number of voxels in each cluster; ‘F stat.’ denotes the F-statistic associated with the voxel of highest activation.
| VAS-F (state) Fatigue and N-Back related activation | ||||||
|---|---|---|---|---|---|---|
| BA | X | Y | Z | Voxels | F stat. | |
| VAS-F Main effect | ||||||
| Postcentral Gyrus | 43 | −62 | −6 | 14 | 1709 | 16.58 |
| Middle Temporal Gyrus | 20 | 46 | −2 | −26 | 218 | 16.81 |
| Superior Temporal Gyrus | 40 | −60 | −36 | 24 | 140 | 11.65 |
| Superior Parietal Lobule | 7 | −22 | −74 | 50 | 125 | 12.32 |
| Putamen | – | 22 | −4 | 12 | 113 | 16.79 |
| Group Main effect | ||||||
| Temporal Pole/Inferior Frontal Gyrus | 22/44 | −60 | 4 | 2 | 153 | 17.17 |
| Superior Temporal Gyrus | 13 | −38 | −18 | −8 | 255 | 13.92 |
| Middle Temporal Gyrus | 39 | −52 | −72 | 24 | 1139 | 23.34 |
| Inferior Parietal Lobule | 40 | 42 | −52 | 40 | 113 | 11.48 |
| Inferior Parietal Lobule | 40 | −52 | −34 | 48 | 110 | 10.65 |
| Cuneus | 18 | −2 | −80 | 28 | 435 | 15.72 |
| Cerebellar Vermis | – | 0 | −48 | −2 | 386 | 20.66 |
| Group × VAS-F Interaction | ||||||
| Inferior Frontal Gyrus | 13 | 30 | 14 | 24 | 208 | 10.40 |
| Inferior Frontal Gyrus | 44 | −48 | 18 | 12 | 107 | 10.90 |
| Caudate Nucleus | – | −14 | 14 | 22 | 161 | 9.71 |
| Middle Temporal Gyrus | 20 | 46 | −2 | −26 | 106 | 12.18 |
| Task × VAS-F Interaction | ||||||
| Caudate Tail | – | 28 | −38 | 26 | 123 | 18.96 |
| Hippocampus | 22 | 40 | −24 | −8 | 314 | 15.69 |
| Hippocampus | – | 36 | −42 | 12 | 104 | 15.00 |
| Hippocampus | 28 | −10 | −12 | −14 | 127 | 26.50 |
| Thalamus | – | 16 | −28 | −2 | 128 | 21.14 |
| Group × Task Interaction | ||||||
| Middle Orbital Gyrus | 11 | −4 | 40 | −18 | 938 | 13.03 |
| Middle Frontal Gyrus | 10 | 40 | 58 | 10 | 131 | 10.29 |
| Middle Frontal Gyrus | 6 | −44 | 6 | 50 | 180 | 15.42 |
| Inferior Frontal Gyrus | 47 | −42 | 24 | −6 | 367 | 13.07 |
| Caudate Nucleus | – | 24 | 26 | −2 | 368 | 19.21 |
| Calcarine Gyrus | 17 | −6 | −68 | 10 | 239 | 12.10 |
| Thalamus | – | 8 | −26 | −8 | 224 | 10.43 |
| Group × Task × VAS-F Interaction | ||||||
| Caudate Tail | – | 28 | −38 | 26 | 267 | 20.76 |
| Hippocampus | 22 | 38 | −22 | −10 | 966 | 26.61 |
| Hippocampus | – | −34 | −32 | −4 | 117 | 11.62 |
Table 2.
Coefficients from the analysis of state fatigue prior to the N-Back task.
| Coefficients for VAS-F (State) Fatigue and N-Back related activation | ||||
|---|---|---|---|---|
| Coefficients (p-value) | ||||
| VAS-F Main effect | ||||
| Postcentral Gyrus | −0.0102*** | |||
| Middle Temporal Gyrus | −0.0184*** | |||
| Superior Temporal Gyrus | −0.0104** | |||
| Superior Parietal Lobule | −0.0088** | |||
| Putamen | −0.0117*** | |||
| Group Main effect | HC | GWI | ||
| Temporal Pole/Inferior Frontal Gyrus | −0.2780** | 0.2540* | ||
| Superior Temporal Gyrus | −0.2560*** | 0.1210 ns | ||
| Middle Temporal Gyrus | −0.3790*** | 0.1520 | ||
| Inferior Parietal Lobule (right) | 0.2796*** | −0.0701 ns | ||
| Inferior Parietal Lobule (left) | −0.3130* | 0.4460* | ||
| Cuneus | −0.5120*** | −0.0390 ns | ||
| Cerebellar Vermis | −0.2940*** | 0.2590* | ||
| Group × VAS-F Interaction | ||||
| Inferior Frontal Gyrus | −0.0051*** | 0.0017 ns | ||
| Inferior Frontal Gyrus | −0.0099* | 0.00001 ns | ||
| Caudate Nucleus | −0.0110** | 0.0012 ns | ||
| Middle Temporal Gyrus | −0.0193*** | 0.00001 ns | ||
| Task × VAS-F Interaction | 0-back | 2-back | ||
| Caudate Tail | −0.0131*** | 0.0067 ns | ||
| Hippocampus | −0.0193*** | 0.0052 ns | ||
| Hippocampus | −0.0147*** | 0.0043 ns | ||
| Hippocampus | −0.0098* | 0.0136*** | ||
| Thalamus | −0.0145** | 0.0098* | ||
| HC | GWI | |||
| Group × Task Interaction | 0-back | 2-back | 0-back | 2-back |
| Middle Orbital Gyrus | −0.2720* | −0.2890* | 0.1580 ns | −0.6370*** |
| Middle Frontal Gyrus | −0.1560 ns | 0.3990* | 0.2120 ns | −0.1680 ns |
| Middle Frontal Gyrus | −0.3556* | 0.4145* | 0.0372 ns | −0.0032 ns |
| Inferior Frontal Gyrus | −0.1346 | 0.2140** | 0.1356 ns | 0.0129 ns |
| Caudate Nucleus | −0.1161 | 0.1779** | 0.1109 ns | 0.0093 ns |
| Calcarine Gyrus | −0.4630*** | −0.0774 ns | 0.0172 ns | −0.1372 ns |
| Thalamus | −0.2355** | 0.1616* | 0.0979 ns | −0.0288 ns |
| Group × Task × VAS-F Interaction | ||||
| Caudate Tail | −0.0114* | 0.0088* | 0.0008 ns | −0.0013 ns |
| Hippocampus (right) | −0.0157*** | 0.0059* | 0.0009 ns | −0.0015 ns |
| Hippocampus (left) | −0.0093** | 0.0111** | 0.0009 ns | −0.0009 ns |
All significance tests relative to zero: ‘ns’ denotes not significant; ‘⋅’ p < 0.1; * p < 0.05; ** p < 0.01; *** p < 0.001.
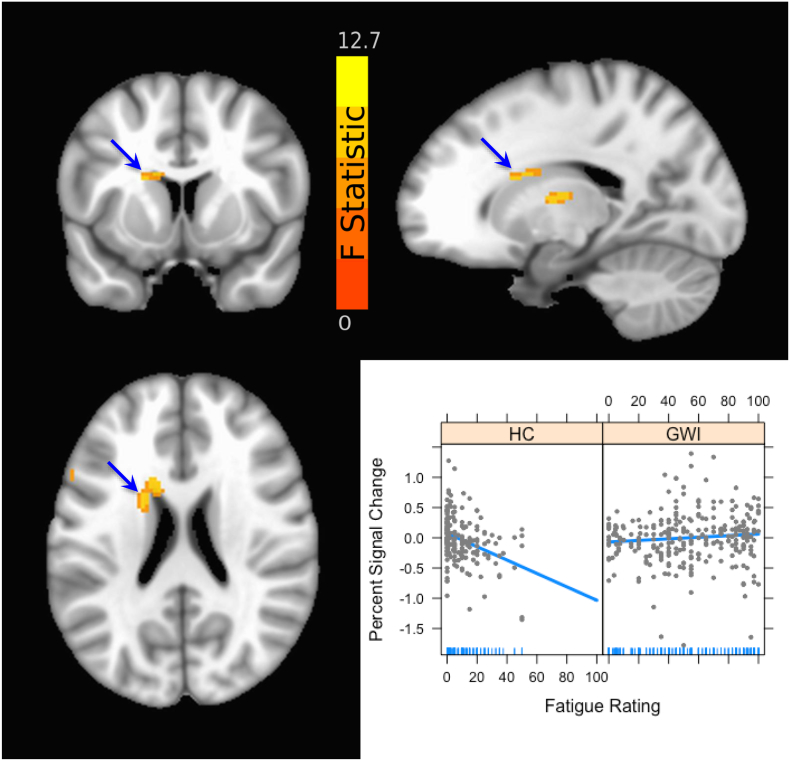
There was a significant main effect of Group in several areas, particularly in temporal and parietal cortex (see Table 1, Table 2). Of note are several areas in which the GWI group showed a significant increase in activation while the HC showed a significant decrease in activation: temporal pole/inferior frontal gyrus, inferior parietal lobule, and the cerebellum (see Table 2 and Fig. 2). Moreover, Group interacted with VAS-F in the basal ganglia (caudate nucleus, see Fig. 3), in inferior frontal areas and in the medial temporal gyrus. As the plot (inset) shows, this interaction is due to a strong negative relationship between activation in the caudate and cognitive fatigue in the HC group (correlation coefficient = −0.011, p < 0.01), and no relationship in the GWI group (correlation coefficient = 0.001, p = 0.4). Thus, for the HC group, as activation increased in the caudate head they reported less cognitive fatigue. In contrast, there was no detectable relationship between brain activation and cognitive fatigue in this area of the caudate in the GWI group.
Fig. 2.
Brain activation showing the main effect of Group (HC vs. GWI). The two top panels show the coronal (top left) and sagittal (top right) views of the brain. The bottom left panel shows the axial view. The blue arrow indicates the left inferior parietal lobe (XYZ coordinates = −52, −34, 48 in MNI space), and the data from this area is shown in the inset graph (bottom right). The colors represent the F statistic, ranging from 0 to 23.3. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Brain activation showing the Group × Fatigue interaction (that is, areas where the effect of fatigue differed between the GWI and HC groups). The two top panels show the coronal (top left) and sagittal (top right) views of the brain. The bottom left panel shows the axial view. The blue arrow indicates the caudate nucleus (XYZ coordinates = −14, 14, 22 in MNI space), and the data from this area is shown in the inset graph (bottom right). The colors represent the F statistic, ranging from 0 to 12.7. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
There was a significant Group × Task interaction in frontal (orbital extending up into anterior cingulate cortex, middle and inferior), occipital areas, and thalamus, but also in the caudate nucleus (Table 1). For the HC group, caudate activation was modulated by task, with more activation for the difficult 2-back task than for the less difficult 0-back task (0.178 and −0.116, respectively). For the GWI group, by contrast, activation in the caudate was high for the less difficult 0-back task (0.111) but lower for the more difficult 2-back task (0.009). When these differences were tested (Tukey's test), the GWI group was found to have significantly higher activation than the HC group during the 0-back task (p = 0.01), but the HC group was found to have significantly higher activation during the 2-back task (p = 0.025).
There was a Task × VAS-F interaction in the caudate tail, the hippocampus and thalamus. Furthermore these areas were also sensitive to the three-way interaction of Group × Task × VAS-F (Table 1). This three-way interaction resulted from a similar pattern across the conditions: for the HC group there was a negative relationship between fatigue and brain activation on the 0-back task, and a positive relationship on the 2-back task. For the GWI group, the slope of the relationship was close to zero for both tasks. For example, in the caudate tail, the relationship between brain activation and fatigue in the HC group was −0.0059 for the 0-back and 0.0075 for the 2-back task. For the GWI group, the relationship was 0.0008 for the 0-back task and −0.0011 for the 2-back task.
3.5. BOLD trait fatigue (FSS) results
As in the analysis of the state fatigue (VAS—F) results, we concentrated on the effect of FSS and on interactions between the FSS scores and Group. There was a main effect of FSS in several brain areas: in orbital frontal areas, in precentral areas, in superior temporal areas, and in the thalamus (Table 3). In all cases, the relationship between the FSS and brain activation was negative (higher scores on the FSS were associated with less brain activation in these areas): orbital frontal correlation coefficient: −0.041; precentral correlation coefficient: −0.032; superior temporal correlation coefficient: −0.011; thalamic correlation coefficient: −0.019 (see Table 4).
Table 3.
Areas of reliable brain activation for the analysis of trait fatigue prior to the N-Back task. ‘BA’ denotes Brodmann Area; ‘X', ‘Y', and ‘Z' denote the location of the voxel in the cluster with the highest activation; ‘Voxels' denotes the number of voxels in each cluster; ‘F stat.’ denotes the F-statistic associated with the voxel of highest activation.
| FSS (trait) Fatigue and N-Back related activation | ||||||
|---|---|---|---|---|---|---|
| BA | X | Y | Z | Voxels | F stat. | |
| FSS Main effect | ||||||
| Middle Orbital Gyrus | 10 | −32 | 58 | 0 | 174 | 13.19 |
| Precentral Gyrus | 6/9 | −54 | 2 | 38 | 909 | 23.30 |
| Superior Temporal Gyrus | 41 | −38 | −28 | 2 | 235 | 14.05 |
| Thalamus | – | 0 | −16 | 2 | 182 | 19.67 |
| Group × FSS Interaction | ||||||
| Middle Occipital Gyrus | 19 | −36 | −78 | 32 | 306 | 37.98 |
| Group × Task × FSS Interaction | ||||||
| Superior Medial Gyrus | 8 | −4 | 40 | 40 | 780 | 15.45 |
| Superior Medial Gyrus | 10 | 6 | 62 | 24 | 282 | 18.47 |
Table 4.
Coefficients from the analysis of trait fatigue prior to the N-Back task.
| Coefficients for FSS (trait) Fatigue and N-Back related activation | ||||
|---|---|---|---|---|
| Coefficients (p-value) | ||||
| FSS Main effect | ||||
| Middle Orbital Gyrus | −0.0407** | |||
| Precentral Gyrus | −0.0323*** | |||
| Superior Temporal Gyrus | −0.0113** | |||
| Thalamus | −0.0192*** | |||
| Group × FSS Interaction | HC | GWI | ||
| Middle Occipital Gyrus | −0.0350*** | 0.0192 | ||
| HC | GWI | |||
| Group × Task × FSS Interaction | 0-back | 2-back | 0-back | 2-back |
| Superior Medial Gyrus (left) | −0.0372 ns | 0.0318 ⋅ | 0.0194 ns | −0.0225 ns |
| Superior Medial Gyrus (right) | −0.0475** | 0.0258 ns | 0.0156 ns | −0.0088 ns |
All significance tests relative to zero: ‘ns’ denotes not significant; ‘⋅’ p < 0.1; * p < 0.05; ** p < 0.01; *** p < 0.001.
Trait Fatigue (FSS) interacted with Group in the orbital cortex (see Table 3). This was because the HC group showed a strong negative relationship between their FSS scores and brain activation (coefficient = −0.0350) whereas the GWI group showed a weaker positive relationship (coefficient = 0.0192; Table 4).
There was also an interaction between Group, Task and Trait Fatigue (FSS) in bilateral superior medial cortex (Table 3). In the left superior medial cortex, this resulted from the HC group showing a positive relationship between FSS and brain activation for the 2-back (coefficient = 0.0318); the relationship between FSS and brain activation was not significant for either group for the 0-back, nor was it significant for the GWI group for the 2-back task. In the right superior medial cortex, the HC group showed a negative relationship between FSS and brain activation for the 0-back task; the relationship between FSS and brain activation was not significant for either group for the 2-back, nor was it significant for the GWI group for the 0-back task (see Table 4).
4. Discussion
This study was designed to better understand the neural substrates of cognitive fatigue in veterans with GWI. Based on the emerging literature on GWI, as well as the cognitive fatigue literature in other populations, we expected that the fronto-striatal-thalamic circuit would be involved in cognitive fatigue in GWI (Chaudhuri and Behan, 2000; Dobryakova et al., 2013). Our results support this hypothesis. Activation in the caudate nucleus of the basal ganglia is associated with state fatigue in HCs, and this relationship is essentially absent in the GWI group. This pattern suggests that having a fatiguing illness such as GWI that is associated with cognitive decrements and requires the expenditure of substantial cognitive resources for even simple tasks (e.g., the 0-back task), interferes with the activation in the fronto-striatal-thalamic circuit (i.e., the reward network, see below).
The idea that individuals with GWI expended more cognitive resources to perform the tasks in this study is supported by two results. First, veterans with GWI made more errors than the HCs. This finding is consistent with previous research investigating performance of the N-back task in individuals with GWI (Rayhan et al., 2013a), and suggests that individuals with GWI found the tasks to be more difficult than the HCs. An additional argument to support the idea that individuals with GWI expended more cognitive resources to perform the tasks used here is in the group differences in the fMRI activation. For example, the GWI group showed persistently high activation in parietal areas, inferior frontal areas and cerebellar areas relative to the HCs (see Table 2, main effect of Group). These areas, which have been shown to respond abnormally in veterans with GWI in previous studies (Rayhan et al., 2013a; Cook et al., 2017), are part of the executive control network (D'Esposito and Postle, 2015) and their persistent activation in GWI suggests that this group may require more involvement of executive processes to perform these tasks than HCs (Cook et al., 2017). This persistent activation of executive control circuitry, even during the performance of the simple 0-back task, may underlie the increased fatigue experienced by the GWI group.
Finally, this interpretation is supported by the interaction between Group and Task in the caudate nucleus. The HC group showed little activation of this area for the less difficult 0-back task, and significant activation for the more difficult 2-back task, suggesting that they either required more motivation to perform the more difficult task or found it to be more rewarding than the easier task. This ability to deploy the motivation and reward circuitry as task demands increased was not seen in the GWI group. The GWI group showed significantly more activation than the HC group during the 0-back task, and significantly less activation during the 2-back task. Thus, as with the executive control circuitry, the GWI group showed an inability to modulate the motivation and reward circuitry as task demands changed.
4.1. Relating GWI to CFS
The relationships between cognitive fatigue, cognitive performance and brain responses differed as a function of disease status. For HCs fatigue was negatively associated with activity in striatal regions. This relationship was largely absent for GWI. These results are consistent with a recent study by Cook et al. (2017) that reported negative relationships between fatigue ratings and brain activity within the posterior attention system (parietal and temporal cortices) for controls, but positive relationships for patients with myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) during the performance of a fatiguing cognitive task when symptoms were exacerbated post-exercise (i.e. during the experience of post-exertional malaise). Activity in the parietal regions was also positively associated with self-reported “difficulty concentrating” for ME/CFS patients, but not controls. Although the brain regions that interact with the experience of fatigue differed somewhat between Cook et al. (2017) and the present investigation, which may be due to the different populations studied and the influence of acute exercise in Cook et al.'s study, the presence of chronic fatigue appears to be associated with inefficient cognitive processing and a dysregulated response to more difficult cognitive demand. This was evident in the present study as increased activation of frontal and parietal areas and a failure of the fronto-striatal-thalamic circuit to respond to the more difficult cognitive demands of the 2-back task. This persistent activation of areas associated with cognitive control may result in fatigue because brain activation is not down regulated for less difficult tasks.
4.2. State vs. trait fatigue
Another goal of the work presented here was to investigate whether state and trait measures of fatigue rely on the same neural networks. The results are clear. Whereas our measure of state fatigue (the VAS—F) involved the fronto-striatal-thalamic circuit, our measure of trait fatigue (the FSS) did not. Rather, the FSS was associated with brain circuits associated with memory (e.g., temporal areas) to a far greater extent than the VAS—F. While this may not seem unexpected, because trait measures require participants to recall previous experience to a greater extent than state measures, it should be remembered that participants were not performing the FSS in the scanner. Rather, the FSS from outside the scanner was associated with activation in these areas. Inasmuch as this relationship was negative (for the most part) this suggests that individuals with more activation in memory-related areas report less trait fatigue. However, more broadly, it is clear that the neural networks associated with measures of state fatigue are substantially different from those associated with trait fatigue.
4.3. Limitations
The idea that frontal and parietal areas are persistently active in the GWI group is consistent with the symptomatology of these veterans (who report consistently high levels of fatigue). Moreover, it helps to explain the large difference in self-reported fatigue between the groups (see Fig. 1). However, it does not explain the interaction seen in the VAS-F scores between Group and Rating. That is, it does not explain why veterans with GWI report more fatigue more quickly (i.e., a steeper slope in VAS-F scores over time) than the HC group. It may be that there is an interaction in the activation data from frontal and parietal areas that would help to explain the interaction between Group and Rating in the VAS-F scores, but that the interaction is subtile, and is masked by the persistently high levels of activation in the circuit in the GWI group. If this is the case, larger samples might be able to detect differences not detectable here.
5. Conclusions
The results of the present study suggest that cognitive fatigue in GWI results from chronic activation in the executive control network. This may explain why GWI patients not only experience a chronic sense of fatigue, but also why increased physical and mental work has a particular impact on fatigue, often experienced even days following exertion.
Acknowledgements
This work was funded by a grant from the Department of Veterans Affairs (5I01CX000893) to GRW.
References
- Baliki M.N., Petre B., Torbey S., Herrmann K.M., Huang L., Schnitzer T.J., Fields H.L., Apkarian A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.-N., Gohel S., Kelly C., Smith S.M., Beckmann C.F., Adelstein J.S., Buckner R.L., Colcombe S., Dogonowski A.-M., Ernst M., Fair D., Hampson M., Hoptman M.J., Hyde J.S., Kiviniemi V.J., Kötter R., Li S.-J., Lin C.-P., Lowe M.J., Mackay C., Madden D.J., Madsen K.H., Margulies D.S., Mayberg H.S., McMahon K., Monk C.S., Mostofsky S.H., Nagel B.J., Pekar J.J., Peltier S.J., Petersen S.E., Riedl V., Rombouts S. a R.B., Rypma B., Schlaggar B.L., Schmidt S., Seidler R.D., Siegle G.J., Sorg C., Teng G.-J., Veijola J., Villringer A., Walter M., Wang L., Weng X.-C., Whitfield-Gabrieli S., Williamson P., Windischberger C., Zang Y.-F., Zhang H.-Y., Castellanos F.X., Milham M.P. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksem M.A., Meijman T.F., Lorist M.M. Effects of mental fatigue on attention: an ERP study. Cogn. Brain Res. 2005;25:107–116. doi: 10.1016/j.cogbrainres.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Boksem M.A.S., Meijman T.F., Lorist M.M. Mental fatigue, motivation and action monitoring. Biol. Psychol. 2006;72:123–132. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Behan P.O. Fatigue and basal ganglia. J. Neurol. Sci. 2000;179:34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Cook D.B., O'Connor P.J., Lange G., Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. NeuroImage. 2007;36:108–122. doi: 10.1016/j.neuroimage.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Cook D.B.B., Light A.R.R., Light K.C.C., Broderick G., Shields M.R.R., Dougherty R.J.J., Meyer J.D.D., Vanriper S., Stegner A.J.J., Ellingson L.D.D., Vernon S.D.D. Neural consequences of post-exertion malaise in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Brain Behav. Immun. 2017;62:87–99. doi: 10.1016/j.bbi.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Deluca J., editor. Fatigue as a Window to the Brain. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- DeLuca J., Genova H.M., Hillary F.G., Wylie G. Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J. Neurol. Sci. 2008;270:28–39. doi: 10.1016/j.jns.2008.01.018. [DOI] [PubMed] [Google Scholar]
- D'Esposito M., Postle B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015;66:115–142. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova E., DeLuca J., Genova H.M., Wylie G.R. Neural correlates of cognitive fatigue: cortico-striatal circuitry and effort-reward imbalance. J. Int. Neuropsychol. Soc. 2013;19:849–853. doi: 10.1017/S1355617713000684. [DOI] [PubMed] [Google Scholar]
- Eklund A., Dufort P., Villani M., LaConte S. BROCCOLI: software for fast fMRI analysis on many-core CPUs and GPUs. Front. Neuroinform. 2014;8:24. doi: 10.3389/fninf.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. 2016;201602413 doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl B.E., James L.M., Miller R.D., Leuthold A.C., Lewis S.M., Carpenter A.F., Georgopoulos A.P. A Magnetoencephalographic (MEG) study of Gulf War Illness (GWI) EBioMedicine. 2016;12:127–132. doi: 10.1016/j.ebiom.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Nisenbaum R., Stewart G., Thompson W.W., Robin L., Washko R.M., Noah D.L., Barrett D.H., Randall B., Herwaldt B.L., Mawle A.C., Reeves W.C. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA. 1998;280:981–988. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- Genova H.M., Rajagopalan V., Deluca J., Das A., Binder A., Arjunan A., Chiaravalloti N., Wylie G. Examination of cognitive fatigue in multiple sclerosis using functional magnetic resonance imaging and diffusion tensor imaging. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0078811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulf War veterans statistics report . 1998. Department of Veterans Affairs Office of Policy and Planning. Washington, D.C. [Google Scholar]
- Hubbard N.A., Hutchison J.L., Motes M.A., Shokri-kojori E., Bennett I.J., Brigante R.M., Haley R.W., Rypma B. Central executive dysfunction and deferred prefrontal processing in veterans with Gulf War Illness. Clin. Psychol. Sci. 2014;2:319–327. doi: 10.1177/2167702613506580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger B.M., Krupp L.B., Enoka R.M. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80:409–416. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl A.D., Wylie G.R., Genova H.M., Hillary F.G., Deluca J. The neural correlates of cognitive fatigue in traumatic brain injury using functional MRI. Brain Inj. 2009;23:420–432. doi: 10.1080/02699050902788519. [DOI] [PubMed] [Google Scholar]
- Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Pardini M., Krueger F., Raymont V., Grafman J. Ventromedial prefrontal cortex modulates fatigue after penetrating traumatic brain injury. Neurology. 2010;74:749–754. doi: 10.1212/WNL.0b013e3181d25b6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayhan R.U., Stevens B.W., Raksit M.P., Ripple J.A., Timbol C.R., Adewuyi O., VanMeter J.W., Baraniuk J.N. Exercise challenge in Gulf War Illness reveals two subgroups with altered brain structure and function. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayhan R.U., Stevens B.W., Timbol C.R., Adewuyi O., Walitt B., VanMeter J.W., Baraniuk J.N. Increased brain white matter axial diffusivity associated with fatigue, pain and hyperalgesia in Gulf War Illness. PLoS One. 2013;8 doi: 10.1371/journal.pone.0058493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Advisory Committee on Gulf War Veteran's Illnesses . 2008. Gulf War Illness and the Health of Gulf War Veterans: Scientific Findings and Recommendations. [Google Scholar]
- Schneider W., Eschman A., Zuccolotto A. 2002. E-Prime user's Guide. Pittsburg. [Google Scholar]
- Shahid A., Wilkinson K., Marcu S., Shapiro C.M. Visual Analogue Scale to Evaluate Fatigue Severity (VAS-F) In: Marcu S., Shapiro C.M., editors. STOP, THAT and One Hundred Other Sleep Scales. Springer New York; New York, NY: 2011. pp. 399–402. [Google Scholar]
- Steele L. Prevalence and patterns of Gulf War Illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am. J. Epidemiol. 2000;152:992–1002. doi: 10.1093/aje/152.10.992. [DOI] [PubMed] [Google Scholar]
- Stonnington C.M., Tan G., Klöppel S., Chu C., Draganski B., Jack C.R., Chen K., Ashburner J., Frackowiak R.S.J., Frackowiak R.S.J. Interpreting scan data acquired from multiple scanners: a study with Alzheimer's disease. NeuroImage. 2008;39:1180–1185. doi: 10.1016/j.neuroimage.2007.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie G., Dobryakova E., DeLuca J., Chiaravalloti N., Essad K., Genova H. Cognitive fatigue in individuals with traumatic brain injury is associated with activation of the caudate. Sci. Rep. 2017;7:8973. doi: 10.1038/s41598-017-08846-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie G.R., Genova H.M., DeLuca J., Dobryakova E. The relationship between outcome prediction and cognitive fatigue: a convergence of paradigms. Cogn. Affect. Behav. Neurosci. 2017;17:838–849. doi: 10.3758/s13415-017-0515-y. [DOI] [PubMed] [Google Scholar]