Abstract
Autism spectrum disorder (ASD) is clinically characterized by extremely slow and inflexible behavior. The neuronal mechanisms of these symptoms remain unclear though. Using fMRI, we investigate the resting state's temporal structure in the frequency domain (scale-free activity as measured with Power-Law Exponent, PLE, and Spectral Entropy, SE) and temporal variance (neural variability) in high-functioning, adult ASD comparing them with schizophrenic and neurotypical subjects. We show that ASD is characterized by high PLE in salience network, especially in dorsal anterior cingulate. This increase in PLE was 1) specific for salience network; 2) independent of other measures such as neuronal variability/SD and functional connectivity, which did not show any significant difference; 3) detected in two independent samples of ASD but not in the schizophrenia sample. Among salience network subregions, dorsal anterior cingulate cortex exhibited PLE differences between ASD and neurotypicals in both samples, showing high robustness in ROC curves values. Salience network abnormal temporal structure was confirmed by SE, which was strongly anticorrelated with PLE and thus decreased in ASD. Taken together, our findings show abnormal temporal structure (but normal temporal variance) in resting state salience network in adult high-functioning ASD. The abnormally high PLE indicates a relative predominance of slower over faster frequencies, which may underlie the slow adaptation to unexpected changes and the inflexible behavior observed in autistic individuals. The specificity of abnormal PLE in salience network suggests its potential utility as biomarker in ASD.
Keywords: ASD, Schizophrenia, Resting state fMRI, Salience network, Power-law exponent, Spectral entropy
Highlights
-
•
Lower frequencies dominate fMRI power spectrum in high functioning autism at rest.
-
•
Increased Power-Law Exponent in salience network and dorsal anterior cingulate.
-
•
Spectral measures in frequency domain reach fair diagnostics levels.
-
•
Findings are replicated in a second sample in autism but absent in schizophrenia.
-
•
Specificity is confirmed by negative findings of other measures in the same regions.
1. Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder that includes a heterogeneous set of conditions with shared clinical features, such as impairment in social communication, inflexible and slow behavior, rigid interests, stereotypies, and atypical sensory processing (Association AP, 2013). The specificity of the clinical picture is complicated by the close relationship between ASD and schizophrenia. ASD patients can display schizophrenic symptoms like hallucinations and delusions (Konstantareas and Hewitt, 2001; Cochran et al., 2013), while schizophrenic patients can show strong autistic symptoms, including autism as core-symptom (Volkmar and Cohen, 1991). Neurobiological investigation of ASD may therefore ideally include schizophrenic patients to test for the specificity of the respective neurobiological markers (Pinkham et al., 2008).
In the search for specific neurobiological markers of ASD, functional MRI (fMRI) has been proven to be a useful tool for describing the neurophysiology underlying this condition (Cherkassky et al., 2006; Dichter et al., 2009; Perez Velazquez et al., 2009; Paakki et al., 2010; Uddin et al., 2013; Maximo et al., 2014; Poulin-Lord et al., 2014; Itahashi et al., 2015; Green et al., 2016; Hull et al., 2016; O'Reilly et al., 2017; Zhou et al., 2016; Tu et al., 2016; Abbott et al., 2016; Chen et al., 2017; Neufeld et al., 2018; Lynch et al., 2017). Particularly, recent attention has been devoted to resting state fMRI (when the subject is awake but not involved in specific tasks) which measures the spontaneous neural activity or the brain's predisposition to react, i.e., change and adapt to external stimuli (Northoff et al., 2010; Raichle, 2015; He, 2013; Huang et al., 2017).
Resting state fMRI in ASD specifically focused on functional connectivity (FC) to describe the relationships between different regions, networks, and their fluctuations (Gotts et al., 2012; Bernas et al., 2018; Mastrovito et al., 2018). Findings using FC in ASD show both hyper- and/or hypo-connectivity in various networks, such as default-mode network (DMN), frontoparietal task control network (FPTC), and salience network (SN) (Hull et al., 2016; Duan et al., 2017). The situation becomes of increasing complexity given that similar FC findings have been reported in other psychiatric disorders like schizophrenia (Pankow et al., 2015; Chen et al., 2016). In order to increase the specificity of resting state measures in ASD, we focused (rather than on the relationship between different regions) on the intrinsic features of the regions themselves, such as their temporal structure and variance. The fMRI signal is based on the fluctuations in the infraslow frequencies (0.01 to 0.1 Hz) of the brain's neural activity (Raichle, 2015; Logothetis, 2008). Recent findings suggest that the fluctuations in these infraslow frequencies are not mere noise but can be characterized by a certain temporal structure with long-range temporal correlations (LRTCs) (Huang et al., 2017; Fransson et al., 2013; He et al., 2010; He, 2011; He, 2014; Hardstone et al., 2012; Palva et al., 2013). The LRTCs describe the relationship in power between different frequencies, with slower frequencies showing more power than faster ones.
LRTCs are the manifestation of the brain's activity invariance across different temporal scales (Linkenkaer-Hansen et al., 2001). This “scale-free” structure allows to describe dynamics characterized by self-similarity, long memory, and power law distributions, where the pattern observed in a specific scale resembles to the ones found in different temporal scales (Boonstra et al., 2013; Stadnitski, 2012). This fundamental property can be represented plotting frequencies and respective powers of a BOLD time series on the two axes of a graph: the obtained curve (i.e. “power spectrum”) will describe the relationship between frequency and power, being similar to an exponential in which lower frequencies show greater power than faster ones. An exponential curve acquires the shape of a line when the values in both frequency and power axes are converted into logarithms. The slope of this line can be measured by the so-called Power-Law Exponent (PLE), in which the power spectrum follows a power-law (P ∝ 1/fβ), where β indicates the PLE (He et al., 2010; Linkenkaer-Hansen et al., 2001). PLE is directly proportional to the LRTCs: higher PLE correspond to stronger LRTC in the infraslow-frequency BOLD fluctuations (Fransson et al., 2013; He, 2011; He, 2014).
1.1. Scale-free activity, PLE, and the brain
Scale-freeness is observed in a wide variety of natural systems (Mandelbrot, 1967). In the human brain, scale-free dynamics alterations have been related to several behavioral and neurological conditions such as Alzheimer's disease (Maxim et al., 2005; Akhrif et al., 2018). Concerning specific psychiatric conditions, structural MRI studies reported differences in the gray matter organization as indexed by scale-free geometry in schizophrenia and bipolar disorder (Squarcina et al., 2015; Sandu et al., 2008), as well as in ASD (Zhao et al., 2018). Only in the last years few initial studies focused on comparing scale-free activity between neurotypical subjects and psychiatric patients. If on one hand resting state fMRI analyses showed altered levels of complexity in schizophrenia (Sokunbi et al., 2014; Yang et al., 2015) and in children with ASD (Dona et al., 2017), on the other no study measured scale-free activity as indexed by PLE in neither schizophrenia nor ASD.
What has been demonstrated in neurotypical subjects is that the resting state's temporal structure, as indexed by the PLE of the infraslow frequencies fluctuations, is not uniform among the various regions and networks (He, 2011; Ciuciu et al., 2012). For instance, networks like DMN and SN show rather strong power in infraslow frequency fluctuations and thus a high PLE (Huang et al., 2017). Interestingly, scale-free activity in both SN and DMN has been associated with the psychological construct of the sense of self (Huang et al., 2016; Scalabrini et al., 2017; Qin et al., 2013). Since ASD shows both abnormal sense of self with inflexible behavior (Cheng et al., 2015; Chanel et al., 2016) as well as FC changes in DMN and SN (see above), an abnormal temporal structure, i.e., PLE, in these regions and networks could be hypothesized.
The resting state's temporal structure with its power spectrum and scale-free activity needs to be distinguished from yet another temporal feature of the brain's spontaneous activity, namely temporal variance. Temporal variance concerns the variability in the amplitude of the neural activity as measured in fMRI, i.e., neural variability (Garrett et al., 2010). Neural variability can be measured as the standard deviation (SD) of the amplitude (Garrett et al., 2013) and remains different of the PLE (Zhang et al., 2018). Most interestingly, recent findings show abnormalities in the SD in other psychiatric disorders like bipolar disorder (Martino et al., 2016; Northoff et al., 2018). However, as in the case of the resting state's temporal structure, i.e., PLE, the temporal variance of the resting state, as indexed by SD, remains to be investigated in high-functioning adults with ASD.
1.2. Primary aim
The primary aim of the present study consisted on investigating the resting state's temporal structure, i.e., PLE, in adults with high-functioning ASD. To achieve high clinical specificity, we focused on high-functioning adults with ASD selected from the largest available sample of the Autism Brain Imaging Data Exchange (ABIDE) data set (http://fcon_1000.projects.nitrc.org/indi/abide/abide_II.html). The primary end-point is thus to explore LRTC dynamics in ASD, in a first attempt to relate clinical manifestations with the phenomenological valence of the scale-free activity in fMRI (He, 2014).
1.3. Secondary aims
Since most of the FC findings in previous ASD literature were obtained in three core networks, (DMN, FPTC and SN) (Green et al., 2016; Zhou et al., 2016; Abbott et al., 2016; Neufeld et al., 2018; Mastrovito et al., 2018; Plitt et al., 2015a; 2015b), we focused PLE analyses on those networks. Given the established interaction between functional connectivity (FC) and other measures of the scale-free dynamics (Ciuciu et al., 2014), FC was included as a control analysis in order to explore its relationship with PLE.
We planned to replicate possible positive findings, performing the same analyses in another group of high-functioning ASD patients. Moreover, to allow for clinical or diagnostic specificity, we also performed the same analyses in a group of schizophrenic patients.
In addition to PLE, we calculated another frequency domain measure, namely Spectral Entropy (SE). Like PLE, SE indexes temporal structure of the fMRI signal but focuses more on the degree of order or disorder among the various frequencies in the power spectrum (Liang et al., 2015; Gomez-Pilar et al., 2015; Rosso et al., 2001). Given the nature of both PLE and SE, it is plausible that higher PLE should go along with lower SE, since strong power in the slow frequencies resulting in high PLE should decrease the disorder in the power spectrum leading to low SE (Sleigh et al., 2004). Hence, applying SE could represent a way to confirm and validate PLE findings. Controlling for the neuronal specificity of PLE and SE and differentiate temporal structure from temporal variance we also calculated SD.
2. Materials and methods
2.1. Samples
Two independent ASD samples were obtained from ABIDE dataset (http://fcon_1000.projects.nitrc.org/indi/abide/) and the schizophrenia control sample was obtained from COBRE dataset (http://fcon_1000.projects.nitrc.org/indi/retro/cobre.html). In each of the three samples ASD/schizophrenic subjects were matched for age, sex, IQ and handedness with a control group (TYP). Scanning and samples characteristics are reported in Table 1a and b. Both datasets are public: additional, open access information is available on the respective websites.
Table 1.
Characteristics of MR procedure (1a) and included samples (1b).
| Table 1a |
MR characteristics |
Functional imaging |
||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Source | Scanner/Software | Anatomical resolution | Scan duration | TR (ms) | TE (ms) | Flip angle | Resolution |
| Main ASD | ABIDE - BNI | 3.0 Tesla Ingenia | 1.11 × 1.11 × 1.20 mm | 369 s | 3000 | 25 | 80 | 3.75 × 3.75 × 4.00 mm |
| Control ASD | ABIDE - IU | 3.0 Tesla Siemens TrioTim | 0.70 × 0.70 × 0.70 mm | 352 s | 813 | 28 | 3.40 × 3.40 × 3.40 mm | |
| SCH | COBRE | 3.0 Tesla Siemens TrioTim | 1.00 × 1.00 × 1.00 mm | 300 s | 2000 | 29 | 75 | 3.80 × 3.80 × 3.50 mm |
| Table 1b |
Sample characteristics |
||||||
|---|---|---|---|---|---|---|---|
| Sample | Subj (n) | Subj (n) after motion correction | Mean Age (y) | Sex (M) | IQ | Medications | Eyes |
| Main ASD | 29 ASD 29 TYP |
25 ASD 26 TYP |
ASD: 36.54 ± 15.46 TYP: 40.11 ± 15.06 |
All | ASD: 107.11 ± 13.82 TYP: 111.85 ± 12.32 (KBIT2) |
Antidepressant: 8 ASD | Closed |
| Control ASD | 21 ASD 21 TYP |
17 ASD 18 TYP |
ASD: 25.65 ± 9.96 TYP: 24.25 ± 5.27 |
All | ASD: 116.18 ± 12.84 TYP: 116.39 ± 11.04 (WASI-II) |
Information not available | Open |
| SCH | 72 SCH 75 TYP |
41 SCH 55 TYP |
SCH: 38.02 ± 13.54 TYP: 35.53 ± 11.59 |
SCH: 32 TYP: 38 |
> 80 (inclusion criteria) | Antipsychotic: 32 SCH | Open |
Note: ASD = autism; SCH = schizophrenia; TYP = neurotypicals.
All the recordings were collected in accordance to the Declaration of Helsinki.
2.2. fMRI data preprocessing
Preprocessing steps were implemented in AFNI (http://afni.nimh.nih.gov/afni, Cox 1996).
Preprocessing steps were adopted to maximize the conformity with previous studies (Huang et al., 2016) including: (Association AP, 2013) discarding the first 4 frames of each fMRI run; (Konstantareas and Hewitt, 2001) slice timing correction; (Cochran et al., 2013) alignment of the epi and anatomical image; (Volkmar and Cohen, 1991) frame-wise regulation of the epi time series, estimating head motion parameters. Censoring methods such as DVARS and Framewise Displacement could not be applied as to preserve the continuity of the timeseries was pivotal for the frequency domain analyses. Thus, to avoid bias due to motion, after the estimated motion parameters were visually inspected, subjects with head motion larger than ±1.5 mm or rotations higher than ±1° were eliminated (Johnstone et al., 2006); (Pinkham et al., 2008) co-registration with high-resolution anatomical images; (Cherkassky et al., 2006) spatial normalization into Talairach stereotactic space and resampling of the epi resolution to 3.5 × 3.5 × 3.5 mm; (Dichter et al., 2009) spatial smoothing using a kernel of full-width at half maximum (FHMW) of 6 mm; (Perez Velazquez et al., 2009) temporal band-pass filtered (0.01 < f < 0.1 Hz) to reduce low-frequency drift and high-frequency respiratory/cardiac noise, head motion and motion derivatives regression, and mean time series from the white matter (WM) and cerebrospinal fluid (CSF) regression to control for non-neural noise (Fox et al., 2005). WM and CSF masks were eroded by one voxel (Chai et al., 2012) to minimize partial volume overlapping with gray matter.
2.3. Power-law exponent
PLE is considered a suitable measure for the scale-free dynamics of fMRI data, as it gives specific information on the relationship between slower and faster frequencies with low computational cost (He et al., 2010; He, 2011; 2014). Among the different methods for computing fMRI time series complexity, Rubin and colleagues (Rubin et al., 2013) demonstrated power spectrum based methods such as PLE to be among the most robust measures. To obtain PLE, the fMRI signal was normalized to zero mean and unit variance (z-value) (Stephens et al., 2013) and then transposed to the frequency domain using a Fast Fourier Transform with AFNI program: 3dPeriodogram (Scalabrini et al., 2017). The power spectrum was further smoothed with a Hamming Window (HW) of 7 neighboring frequency bins (HW = 7) (Huang et al., 2017; Scalabrini et al., 2017; Huang et al., 2014).
Power spectra were averaged across all the voxels for each of the three networks considered (SN, DMN and FPTC). These networks were taken from a well-established node template from previous studies (Cole et al., 2014; Power et al., 2011) containing 264 putative functional areas (10 mm diameter spheres, 30 voxels per sphere) across the whole brain. As in (Scalabrini et al., 2017), the power spectrum was fitted with a power-law function P ∝ 1/fβ using a least square estimation (in a log frequency by log power plot). The PLE of each subject's network was defined as the absolute value of the slope of the linear regression of log-power on log-frequency.
2.4. Control analyses
Positive results in the main sample were tentatively replicated in the other samples.
The present study includes the following control analyses:
-
1)
In addition to the standard Hamming Window (HM) of 7, we applied different HM () on the PLE calculations of the main sample to test whether the PLE values in SN, DMN, and FPTC could be affected by different smoothing parameters.
-
2)
SD describes the temporal variance of brain activity across time within a particular region (Garrett et al., 2010). A recent study showed how PLE and SD are correlated in the awake individual, but not when the subject is anesthetized (Zhang et al., 2018). Thus, the PLE increase in SN observed in our sample may have been related to variations of the SD. To further clarify whether the increase was specific for PLE (rather than SD), SD was also performed as specificity control. It was calculated extracting the standard deviation of the BOLD signal change across the averaged time series of all voxels in the SN (Huang et al., 2014).
-
3)
To further validate the PLE results in SN, we used the spectral entropy (SE). First, Fast Fourier Transform was applied in each voxel of interest of the fMRI signal. After computing the power spectrum density for SN and averaged it across voxels, it was normalized in all the frequency range. Thus, the resulting function can be interpreted as a probability density function (Rosso et al., 2001). Finally, SE can be defined as follows:
| (1) |
where M is the number of samples of the power spectrum in that frequency range and PSDn represents the normalized power spectral density as function of the frequency. To independently calculate SE from PLE we used customized scripts from a different software (Matlab, version 2017b) to perform this analysis. This is the first study in our knowledge to apply SE to fMRI power spectra in ASD.
-
4)
As in previous studies (He, 2011; Tagliazucchi et al., 2013), we tested the goodness-of-fit for scale invariance of the fMRI signal within SN by using a goodness-of-fit test of the BOLD fluctuations to a power-law distribution. SN time series was extracted from each participant and subjected to PLE analysis. As fractional Gaussian noise is a parsimonious model of stationary scale-free dynamics (Beran, 1994), we generated 1000 time series of fractional Gaussian noise (fGn) with the same length and standard deviation as the original time series from SN. Each of these fGn signals was subjected to the same PLE analysis as the original SN time series. The p-value is defined as the fraction of synthetic time series with standard deviations of residuals from best fit that is larger than the original standard deviations of residuals from best fit of the fMRI time series. The larger the p-value, the more plausible the fGn model is for representing the original fMRI time series, and the better the fit of the original data to a scale-free distribution. The hypothesis that the fMRI signal is scale-free is plausible if the resulting p-value is >0.1, otherwise it is ruled out (He, 2011; Tagliazucchi et al., 2013; Clauset et al., 2009).
-
5)
Connectivity (FC) between significant and replicable SN subregions and the average time series of the three original networks (SN, DMN, FPTC) was measured as a control analysis. Those values were then correlated with PLE values in the same networks and the difference between TYP and ASD of such obtained correlations was measured using a z test (Steiger, 1980).
-
6)
Some subjects were not medication free. We thus took into account medication dosage, if available (schizophrenia sample), testing a possible confounding effect in a correlation analysis with the PLE of each network. As 8 subjects in the main ASD sample were assuming an antidepressant therapy without the dosage being specified, we split the ASD sample in two subgroups (medication-free vs medication) and tested for differences in PLE with a t-test between the two groups. No information on medications was available for the second ASD sample.
-
7)
ADOS-2 scores (Pruette, 2013) were provided for ASD subjects of both samples. Correlations between PLE values and the ADOS total and sub-scores (communication, social interaction, stereotyped behaviors, creativity) were computed to assess possible relationships with the parameters measured by ADOS-2.
2.5. Statistical analyses
Statistical analyses included t-test for the measure of PLE differences between typically developing controls (TYP) and ASD, and Spearman's Rho to measure correlations/anticorrelations of PLE with SE and medications. To test for the diagnostic potential and the robustness of the PLE as a neurophysiological marker, we evaluated the area under a receiver operating characteristic (ROC) curve within SN and those SN subregions showing a significant PLE difference in both ASD samples. Areas under the curves (AUC), were then calculated for SN and the significant subregions.
3. Results
3.1. PLE and SD in ASD
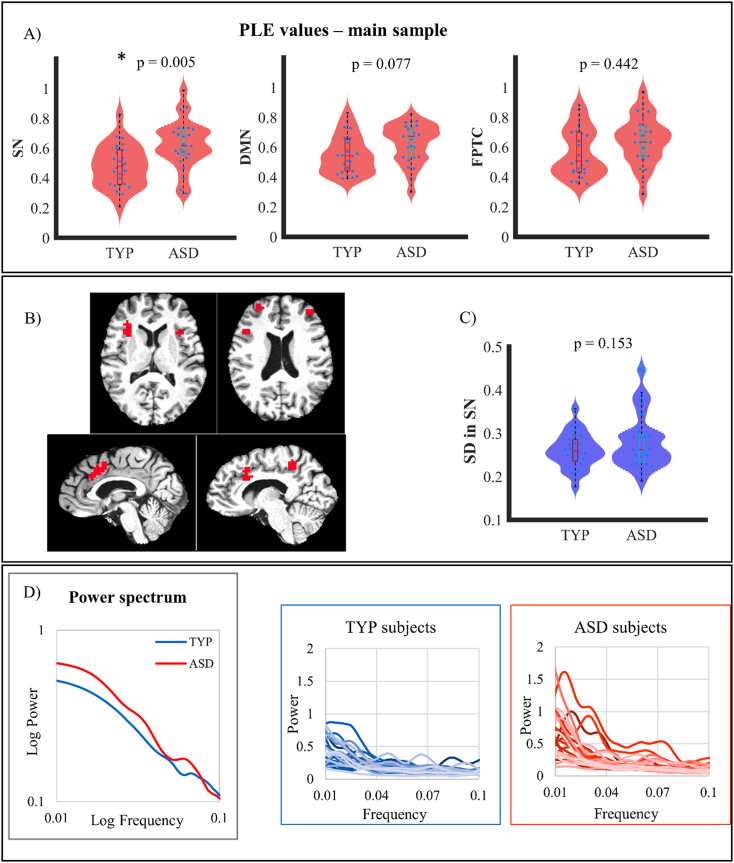
In a first step, we calculated PLE and SD for the different networks (SN, FTPC, and DMN) in ASD and TYP. This yielded significantly higher PLE in SN (but not in FTPC and DMN) (Fig. 1a and Table 2) in ASD when compared to TYP (Fig. 1b). Moreover, our findings show that, unlike PLE, SD was not increased n ASD when compared to TYP (Fig. 1c; see below for more details).
Fig. 1.
A) Violin plot showing PLE differences between neurotypicals (TYP) and autistic subjects (ASD) in Salience Network (SN), Default Mode Network (DMN) and Fronto Parietal Task Control (FPTC). p-values are Bonferroni corrected. B) Visual representation of the regions considered as part of the SN (Power et al., 2011). C) Violin plot showing neural variability (SD) differences between TYP and ASD in SN. D) On the left, SN power spectra averaged for all subjects are shown. In the two graphs on the right, power spectra are plotted for each subject.
Table 2.
PLE differencies between TYP and ASD with Hamming Window of 3, 7 and 11.
| Hamming window | Network | Mean PLE ASD | Mean PLE TYP | p-Value (Bonferroni corrected) |
|---|---|---|---|---|
| 3 | SN | 0.666 | 0.506 | 0.003⁎⁎ |
| DMN | 0.669 | 0.575 | 0.068 | |
| FPTC | 0.678 | 0.601 | 0.392 | |
| 7 | SN | 0.656 | 0.504 | 0.005⁎⁎ |
| DMN | 0.667 | 0.574 | 0.077 | |
| FPTC | 0.670 | 0.598 | 0.442 | |
| 11 | SN | 0.644 | 0.496 | 0.006⁎⁎ |
| DMN | 0.663 | 0.569 | 0.068 | |
| FPTC | 0.661 | 0.591 | 0.490 |
Note: PLE = Power-law Exponent; TYP = neurotypical subjects; ASD = autistic subjects; SN = Salience Network; DMN = Default Mode Network; FPTC = Fronto-Parietal Task Control Network
significant difference between TYP and ASD.
The representation of the power spectrum shows that ASD subjects exhibit significantly more power in the very slow frequency ranges, thus the power in the slower ranges predominates (relatively) over the power of faster frequencies (Fig. 1d). Finally, as in a previous study on healthy subjects (Huang et al., 2016), we observed marked inter-individual differences in the power spectrum in both ASD and TYP (Fig. 1d).
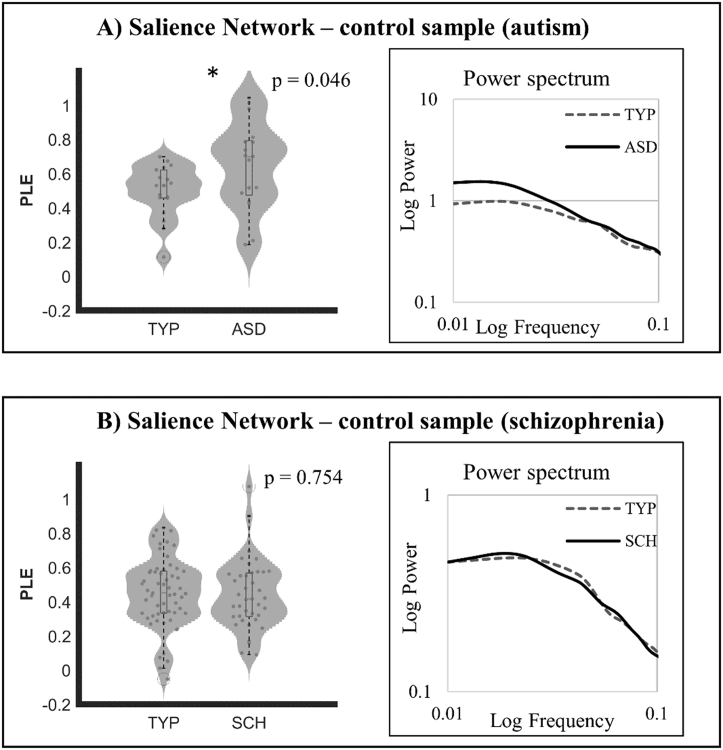
3.2. Replication of PLE in ASD and control in schizophrenia
In order to replicate the positive findings from the first ASD sample, a second ASD dataset was used. We could again observe increased PLE in SN in this second ASD group with similarly increased power in the slower frequencies (Fig. 2a). Moreover, we investigated a sample of schizophrenic patients for clinical specificity, which did not show any abnormal PLE changes in SN (Fig. 2b).
Fig. 2.
On the left, violin plots show PLE differences in SN between groups. A) neurotipical (TYP) versus autistics (ASD). B) TYP versus schizophrenics (SCH). On the left, power spectra averaged for all subjects are shown.
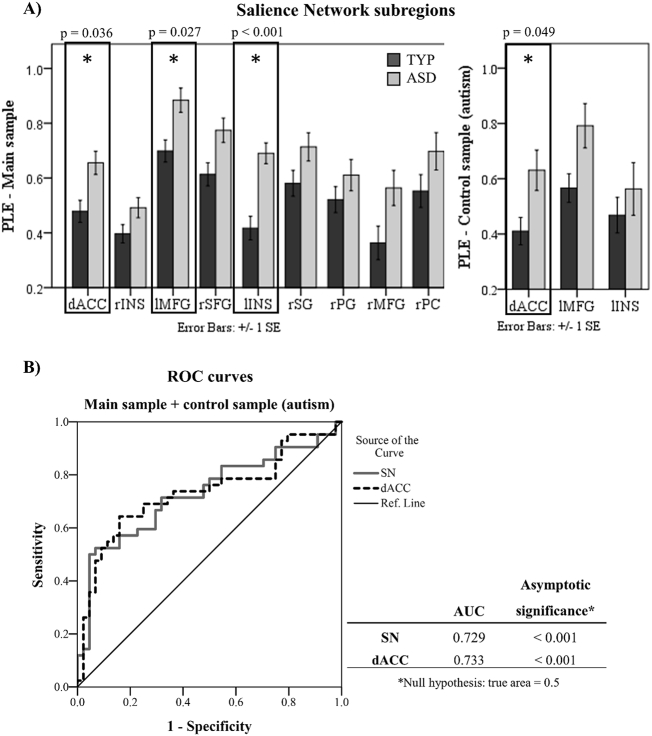
Next, we investigated PLE in SN subregions. We observed statistically significant increased PLE in the dorsal anterior cingulate cortex (dACC) in ASD in both main and replication samples (Fig. 3a). Left insula also showed increased PLE, although this result was not replicated in the control sample. We also tested diagnostic robustness of PLE to predict individual cases in SN, and dACC with ROC curves, which yielded AUCs higher than 0.7 (Fig. 3b).
Fig. 3.
A) On the left, bars show PLE differences between neurotypicals (TYP) and autistic subjects (ASD) in SN subregions for main ASD sample. On the right, positive results were tentatively replicated in the control ASD sample. Only dorsal anterior cingulate (dACC) survived Bonferroni correction in both samples. p-values which did not reach a level of significance are reported in Inline Supplementary Materials (Table 1s). B) Receiver Operating Characteristic (ROC) curves for SN and dACC show fair levels of diagnostic discrimination (AUC = Area Under the Curve).
3.3. Control analyses
-
1)
To test for the scale-free nature of our data, we calculated the fraction of the synthetic time series deviation of the residuals from best fit (Tagliazucchi et al., 2013); this turned out to be larger than the original deviation of residuals from best fit of the fMRI time series which indicates the scale-free nature of our original fMRI series in all our groups (p > 0.1 for all groups, exact values reported in Inline Supplementary Materials).
-
2)
We investigated PLE in three different HW (Cochran et al., 2013; Dichter et al., 2009; Maximo et al., 2014) in ASD which revealed similar PLE results in ASD in all three window sizes (Table 2). Only PLE in SN survived Bonferroni correction in all of the HW sizes whereas that was not the case for the PLE in DMN and FPTC, which therefore were not considered in subsequent analyses.
-
3)
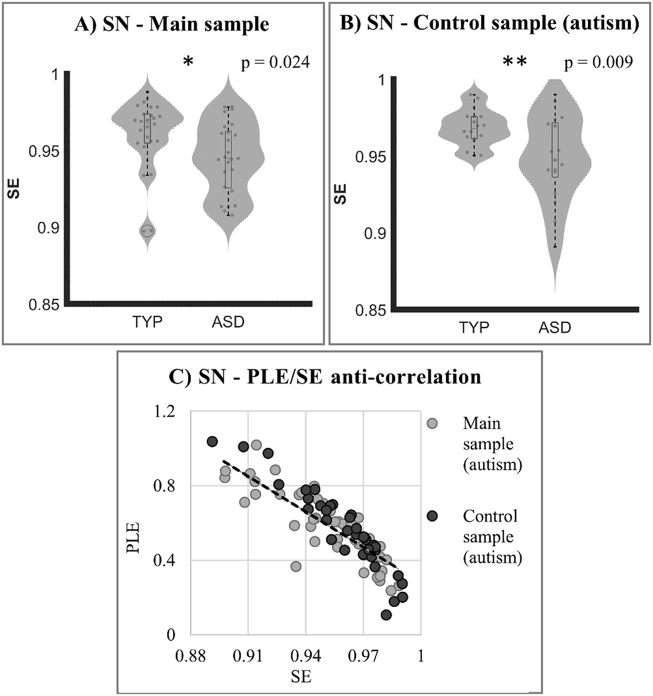
SE analysis confirmed PLE results in SN. As expected, on the basis of the increased PLE, ASD subjects (in both samples) showed significantly decreased SE (main sample: mean difference = −0.150; p = 0.024; control sample: mean difference = −0.196; p = 0.009) (Fig. 4a and b). In contrast, no significant difference in SE was found between schizophrenia and TYP (mean difference: 0.009; p > 0.1). As hypothesized, SE negatively correlated with PLE (Fig. 4c). This indicates that the increased power of the slower frequencies (relative to faster frequencies) as measured by PLE, leads to decreased disorder among the different frequencies as measured by SE.
-
4)
Neuronal variability (SD) in SN did not show any significant change in ASD when compared to TYP (mean difference = 0.21, p > 0.1). Hence, the increased PLE in SN in ASD is neuronally specific as it goes along with normal SD values in SN (Fig. 1c).
-
5)
As a control analysis, we investigated FC and its relationship with PLE. dACC was eligible as seed region as it showed marked and replicable PLE alterations in both ASD samples as shown in Fig. 3. Connectivity between dACC and the three networks which were initially considered (SN, DMN, FPTC) was measured. No significance difference between TYP and ASD was observed (for exact r and p values see Inline Supplementary Materials, Table 2s). In addition, we measured FC/PLE correlations (FC: dACC.vs.SN dACC.vs.DMN and dACC.vs.FPTC, PLE: SN, DMN, and FPTC). We found a significant correlation in ASD (main sample) between dACC.vs.DMN and PLE in SN (r = 0.553; p value = 0.036 Bonferroni corrected). However, the finding was not replicated in the control ASD sample (r = 0.040; p value = 0.875 uncorrected) confirming the controversial connectivity findings in ASD (Inline Supplementary Materials, Table 3s). As a last step, we computed group differences between TYP and ASD of FC/PLE correlations in SN, DMN, and FPTC using z-statistics. Neither the main nor the control sample showed differences surviving the Bonferroni correction. (Inline Supplementary Materials, Table 4s).
-
6)
We tested for the impact of medication on PLE in ASD. For the main ASD sample, no difference in PLE of SN was found between medicated (antidepressant) and medication-free ASD (mean difference: 0.109, p > 0.1). For the schizophrenia group no correlation between medication dosage (as measured by olanzapine equivalent; (Gardner et al., 2010)) and PLE was found in SN (Spearman's rho = −0.22; p > 0.1).
-
7)
No correlation between PLE and symptoms as measured by ADOS-2 was found (p > 0.05 for all the Pearson's rho).
Fig. 4.
A) and B) Violin plots show Spectral Entropy (SE) differences in SN between neurotypicals and autistic subjects in main and control autism samples. C) Scatter plot showing strong anti-correlation between PLE and SE values (all subjects were considered).
4. Discussion
The present study investigated BOLD spontaneous activity's temporal structure (as with PLE and SE) and temporal variance (as with SD) in high-functioning ASD adults when compared to both schizophrenic and typically developing subjects (TYP). Using resting state fMRI, our main result showed increased PLE within SN (but not in DMN and FPTC) in the main ASD group when compared to TYP. This result was replicated in a second independent ASD sample collected with a different fMRI scan and scanning parameters. Conversely, PLE was unaltered in the schizophrenia sample. The increase in PLE is determined by the fact that ASD subjects exhibit increased power in the very slow, (i.e., infraslow) frequencies in SN. The finding of increased PLE in ASD was further supported by the parallel reduction of SE in SN in the same patient sample.
Taken together, our results showed abnormal predominance of slow frequencies in SN in adults with high-functioning ASD. These findings are neuronally specific for PLE as another determinant of the power spectrum (neural variability/SD) did not show any variation compared to the TYP group. The link between PLE and ASD could not be described or explained by FC due the negativity of the FC finding and the absence of a correlation between these two measures. Moreover, increased PLE in SN is clinically specific as it was not observed in schizophrenia. Due to such neuronal and clinical specificity, these SN power spectrum abnormalities may represent a potential biomarker for high-functioning ASD in adulthood. This is supported by the fair level of discrimination that PLE values reached in the ROC curves for both SN and its subregions.
4.1. Abnormally strong slow frequency power in salience network
We observed increased PLE in ASD, specifically in SN. SN is involved in attributing salience to stimuli as well as in connecting or linking intero- and exteroceptive stimuli from body and world (Yeo et al., 2011). The strong increase in slower frequencies' power in ASD (as measured by PLE) and increased regularity and order (as measured by SE) suggests that salience attribution is dominated by slower rather than faster frequencies in ASD.
The abnormal predominance of slow frequency power in SN may predispose ASD subjects to rely on different temporal patterns when processing the salience of environmental events and integrating exteroceptive and interoceptive stimuli. This is well in accordance with clinical practice which reported that ASD patients are rather inflexible in their behavior. For instance, they show difficulties when asked to quickly answer to unexpected events or new situations (Hodgson et al., 2016) are extremely slow in adapting to variations of the daily routine (South et al., 2005), and show qualitative alterations in perception of both time flow and time structure (Vogel et al., 2018). For instance, behavioral studies such as the Wisconsin card sorting task (one of the most reliable tasks assessing cognitive flexibility) robustly showed slower switching rates and increased error ratios in ASD subjects (Van Eylen et al., 2011), thus confirming the observations during real-life conditions. Combined behavioral and fMRI experiments would be required to further validate this hypothesis. Tentatively, SE allows to formulate additional cues for hypothetical future studies: as the increased order or regularity (SE) implies the power of the signal being represented within a narrower frequency band (see lower frequencies increased power in Fig. 1D), this feature may translate into an abnormally strong salience attributed to highly selective events/objects in the environment, while others are excluded. This can indeed be observed in ASD and is a clinical hallmark in high-functioning patients (Jiujias et al., 2017).
4.2. Increased slow frequencies in anterior cingulate and behavioral inflexibility
Since SN showed significant PLE differences, we also investigated the various subregions within SN with regard to their PLE. This revealed a most consistent PLE increase in specifically dACC in ASD samples. Previous ASD literature showed that increased rigidity of the dACC functionality in task (i.e. rigid hyper- or hypo-reaction) is related not only to decreased response inhibition (Agam et al., 2010), but also to impaired social cognition (Dichter et al., 2009). As the resting state reflects the neural predisposition of the subject's behavior (He, 2013; Boly et al., 2007; Northoff, 2013), an increase in PLE (i.e. a shift towards slower frequencies) of dACC spontaneous activity may predispose to rather slow/rigid behavior resulting in decreased behavioral flexibility (Fournier et al., 2010; Anagnostou et al., 2015; Fujino et al., 2017; Uddin et al., 2015).
4.3. Interoceptive/exteroceptive binding and emotions in ASD: a possible role of the Insula?
Insula is deeply involved in the integration of intero- and exteroceptive stimuli, and PLE in that region strongly diverged from neurotypicals in the main ASD sample of our study. Even though the finding was not replicated in the second sample, an excessive relative power of the lower frequencies within the insula may further alter the subjective experience of the individual for two main reasons. First, ASD subjects not only show altered perceptual modalities, but also dramatically increased temporal binding windows when integrating cardiac (interoceptive) and auditory (exteroceptive) stimuli (Noel et al., 2018). A larger temporal binding window would fit with the slower cycles which characterize ASD’ temporal structure (see Spatiotemporal Psychopathology in section 4.5). Second, insula is involved with emotional processing by translating especially interoceptive stimuli into a more cognitive context (Menon and Uddin, 2010; Garfinkel et al., 2016). A tentative association can thus be made between slower cycles in infraslow insular frequencies and the impaired ability to adapt to the emotional context in ASD.
4.4. Methodological issues
Based on our findings, an increased PLE in dACC is related to the diagnosis of ASD in both the samples we selected. The exact nature of this bond yet remains unknown. Hopefully, future studies combining both resting state and task-evoked activity probing for behavioral flexibility (Rinehart et al., 2006) will provide empirical support to our hypotheses and/or reveal the neurobiological bases on which both fMRI features and clinical phenomena may rely.
Several methodological factors need to be considered in order to carefully interpret our data. One such issue is the specificity of the respective resting state measures. We demonstrated that ASD subjects showed abnormalities in the measures of temporal structure like PLE and SE, with the latter confirming the former. In contrast, no effects were observed in neural variability (SD) as measure of temporal variance. As PLE differences are not followed by differences in SD, we assume that there is neuronal specificity of measures indexing temporal structure (PLE, SE) as distinguished from measures indexing temporal variance (SD) that may characterize other psychiatric disorders like bipolar disorder (Martino et al., 2016; Northoff et al., 2018). Moreover, we included schizophrenia in our analyses as it is closely related to ASD from a clinical (Volkmar and Cohen, 1991) and a neurobiological (Haigh et al., 2016) point of view. We showed that the abnormalities in PLE and SE were only observed in ASD, but not in schizophrenia. PLE and SE may thus be markers that are specific for ASD as distinguished from schizophrenia.
Even though the diagnostic sensitivity/specificity reached fair levels of accuracy (similar to data from Uddin et al., 2013), we could not find any correlation of PLE/SE with clinical symptoms. The lack of PLE-symptoms correlation maybe partially due to the clinical heterogeneity of the inter-individual phenotype, which is a key aspect of ASD (Rodriguez and Thompson, 2015; Hasson et al., 2009). Neuronally, inter-individual variability may, in part, be based on the crucial role exerted by SN in balancing multiple functions in both task-positive and task-negative networks (Greicius et al., 2003; Fox et al., 2005). As also suggested by the fair level of diagnostic discrimination of ASD subjects (Fig. 3b), a basic alteration of SN temporal structure may thus be a common neural substrate influencing ASD rigidity across different clinical domains (such as motor, emotional, cognition, and social domains) rather than being related to a specific category of symptoms. Future research on brain scale-free dynamics, including extended batteries of clinical and phenomenological questionnaires, would greatly help to shed further light on the relevance of the abnormal resting state's temporal structure in ASD.
4.5. Conclusions
This is the first study reporting abnormally high scale-free activity in salience network in adults with high-functioning ASD, as distinguished from schizophrenia. Our findings showed that the spontaneous activity's temporal structure (rather than its temporal variance) in adults with high-functioning ASD is abnormally tilted towards the more powerful slow frequencies at the expense of faster frequencies in SN. Any kind of stimulus being either of internal (reflecting internal cognition) or external origin (related to perception) must be processed by and through the brain's spontaneous activity. The integration of internal and external stimuli (in which SN exerts a pivotal role) should be consequently affected by the resting state's altered temporal structure. Following the increase of PLE with stronger power in the very slow frequencies, one would expect stimuli processing in SN to be more affected by slower but powerful cycles (like a slow but powerful wave in the ocean). This may in turn produce a slow but extremely powerful and strong attribution of salience to the stimulus. Ultimately, it would be harder for the autistic patient to communicate and relate to the outside world, as she/he will have difficulties in re-focusing or switching salience attribution from one stimulus to another. Hypothetically, this points out that prominent symptoms in ASD like slow adaptation and inflexible behavior to changes in environmental contexts may have a temporal (rather than primarily cognitive) basis. This interpretation is in line with the recently introduced “Spatiotemporal Psychopathology” (Northoff, 2018a; 2018b).
Acknowledgments
Acknowledgements
This work was supported by: Canadian Institute of Health Research, Michael Smith Foundation, EJLB - Canadian Institute of Health Research, Canada Research Chair to G. Northoff and by the National Natural Science Foundation of China (No. 31271195). This work was supported by the grant from the Ministry of Science and Technology of China, National Key R&D Program of China (2016YFC1306700).
Our thanks to Dr. Cheryl Aine and Dr. Margaret King for their valuable contribution on providing additional key data from the COBRE project, and to the AFNI team for their support throughout the analysis process.
Disclosures
The authors declare that they have no competing financial interests in relation to the work described.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.101634.
Appendix A. Supplementary data
Supplementary material
References
- Abbott A.E., Nair A., Keown C.L., Datko M., Jahedi A., Fishman I. Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. Cereb. Cortex. 2016;26:4034–4045. doi: 10.1093/cercor/bhv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agam Y., Joseph R.M., Barton J.J., Manoach D.S. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage. 2010;52:336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhrif A., Romanos M., Domschke K., Schmitt-Boehrer A., Neufang S. Fractal analysis of BOLD time series in a network associated with waiting impulsivity. Front. Physiol. 2018;9:1378. doi: 10.3389/fphys.2018.01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou E., Jones N., Huerta M., Halladay A.K., Wang P., Scahill L. Measuring social communication behaviors as a treatment endpoint in individuals with autism spectrum disorder. Autism. 2015;19:622–636. doi: 10.1177/1362361314542955. [DOI] [PubMed] [Google Scholar]
- Association AP . 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) (American Psychiatric Pub) [Google Scholar]
- Beran J. 1994. Statistics for Long-Memory Processes. (Chapman & Hall/CRC) [Google Scholar]
- Bernas A., Barendse E.M., Aldenkamp A.P., Backes W.H., Hofman P.A.M., Hendriks M.P.H. Brain resting-state networks in adolescents with high-functioning autism: Analysis of spatial connectivity and temporal neurodynamics. Brain Behav. 2018;8 doi: 10.1002/brb3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M., Balteau E., Schnakers C., Degueldre C., Moonen G., Luxen A. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra T.W., He B.J., Daffertshofer A. Scale-free dynamics and critical phenomena in cortical activity. Front. Physiol. 2013;4:79. doi: 10.3389/fphys.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Castañón A.N., Öngür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanel G., Pichon S., Conty L., Berthoz S., Chevallier C., Grezes J. Classification of autistic individuals and controls using cross-task characterization of fMRI activity. Neuroimage Clin. 2016;10:78–88. doi: 10.1016/j.nicl.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Chen X., He X., Wang L., Wang K., Qiu B. Aberrant structural and functional connectivity in the salience network and central executive network circuit in schizophrenia. Neurosci. Lett. 2016;627:178–184. doi: 10.1016/j.neulet.2016.05.035. [DOI] [PubMed] [Google Scholar]
- Chen H., Uddin L.Q., Duan X., Zheng J., Long Z., Zhang Y. Shared atypical default mode and salience network functional connectivity between autism and schizophrenia. Autism Res. 2017;10:1776–1786. doi: 10.1002/aur.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Rolls E.T., Gu H., Zhang J., Feng J. Autism: reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain. 2015;138:1382–1393. doi: 10.1093/brain/awv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky V.L., Kana R.K., Keller T.A., Just M.A. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Ciuciu P., Varoquaux G., Abry P., Sadaghiani S., Kleinschmidt A. Scale-free and multifractal time dynamics of fMRI signals during rest and task. Front. Physiol. 2012;3:186. doi: 10.3389/fphys.2012.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuciu P., Abry P., He B.J. Interplay between functional connectivity and scale-free dynamics in intrinsic fMRI networks. NeuroImage. 2014;95:248–263. doi: 10.1016/j.neuroimage.2014.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauset A., Shalizi C.R., Newman M.E.J. Power-Law distributions in empirical data. SIAM Rev. 2009;51:661–703. [Google Scholar]
- Cochran D.M., Dvir Y., Frazier J.A. "Autism-plus" spectrum disorders: intersection with psychosis and the schizophrenia spectrum. Child Adolesc. Psychiatr. Clin. N. Am. 2013;22:609–627. doi: 10.1016/j.chc.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Cole M.W., Repovs G., Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist. 2014;20:652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G.S., Felder J.N., Bodfish J.W. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Soc. Cogn. Affect. Neurosci. 2009;4:215–226. doi: 10.1093/scan/nsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dona O., Hall G.B., Noseworthy M.D. Temporal fractal analysis of the rs-BOLD signal identifies brain abnormalities in autism spectrum disorder. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0190081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Chen H., He C., Long Z., Guo X., Zhou Y. Resting-state functional under-connectivity within and between large-scale cortical networks across three low-frequency bands in adolescents with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;79:434–441. doi: 10.1016/j.pnpbp.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Fournier K.A., Hass C.J., Naik S.K., Lodha N., Cauraugh J.H. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J. Autism Dev. Disord. 2010;40:1227–1240. doi: 10.1007/s10803-010-0981-3. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Metsaranta M., Blennow M., Aden U., Lagercrantz H., Vanhatalo S. Early development of spatial patterns of power-law frequency scaling in FMRI resting-state and EEG data in the newborn brain. Cereb. Cortex. 2013;23:638–646. doi: 10.1093/cercor/bhs047. [DOI] [PubMed] [Google Scholar]
- Fujino J., Tei S., Jankowski K.F., Kawada R., Murai T., Takahashi H. Role of spontaneous brain activity in explicit and implicit aspects of cognitive flexibility under socially conflicting situations: a resting-state fMRI study using fractional amplitude of low-frequency fluctuations. Neuroscience. 2017;367:60–71. doi: 10.1016/j.neuroscience.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Gardner D.M., Murphy A.L., O'Donnell H., Centorrino F., Baldessarini R.J. International consensus study of antipsychotic dosing. Am. J. Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Garfinkel S.N., Tiley C., O'Keeffe S., Harrison N.A., Seth A.K., Critchley H.D. Discrepancies between dimensions of interoception in autism: implications for emotion and anxiety. Biol. Psychol. 2016;114:117–126. doi: 10.1016/j.biopsycho.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Garrett D.D., Kovacevic N., McIntosh A.R., Grady C.L. Blood oxygen level-dependent signal variability is more than just noise. J. Neurosci. 2010;30:4914–4921. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D.D., Samanez-Larkin G.R., MacDonald S.W., Lindenberger U., McIntosh A.R., Grady C.L. Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neurosci. Biobehav. Rev. 2013;37:610–624. doi: 10.1016/j.neubiorev.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pilar J., Poza J., Bachiller A., Gómez C., Molina V., Hornero R. Neural network reorganization analysis during an auditory oddball task in schizophrenia using wavelet entropy. Entropy. 2015;17:5241–5256. [Google Scholar]
- Gotts S.J., Simmons W.K., Milbury L.A., Wallace G.L., Cox R.W., Martin A. Fractionation of social brain circuits in autism spectrum disorders. Brain. 2012;135:2711–2725. doi: 10.1093/brain/aws160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S.A., Hernandez L., Bookheimer S.Y., Dapretto M. Salience network connectivity in autism is related to brain and behavioral markers of sensory overresponsivity. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(618–626) doi: 10.1016/j.jaac.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh S.M., Gupta A., Barb S.M., Glass S.A.F., Minshew N.J., Dinstein I. Differential sensory fMRI signatures in autism and schizophrenia: analysis of amplitude and trial-to-trial variability. Schizophr. Res. 2016;175:12–19. doi: 10.1016/j.schres.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardstone R., Poil S.S., Schiavone G., Jansen R., Nikulin V.V., Mansvelder H.D. Detrended fluctuation analysis: a scale-free view on neuronal oscillations. Front. Physiol. 2012;3:450. doi: 10.3389/fphys.2012.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Avidan G., Gelbard H., Vallines I., Harel M., Minshew N. Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Res. 2009;2:220–231. doi: 10.1002/aur.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Scale-free properties of the functional magnetic resonance imaging signal during rest and task. J. Neurosci. 2011;31:13786–13795. doi: 10.1523/JNEUROSCI.2111-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Spontaneous and task-evoked brain activity negatively interact. J. Neurosci. 2013;33:4672–4682. doi: 10.1523/JNEUROSCI.2922-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Scale-free brain activity: past, present, and future. Trends Cogn. Sci. 2014;18:480–487. doi: 10.1016/j.tics.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J., Zempel J.M., Snyder A.Z., Raichle M.E. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66:353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson A.R., Freeston M.H., Honey E., Rodgers J. 2016. Facing the Unknown: Intolerance of Uncertainty in Children with Autism Spectrum Disorder. (J Appl Res Intellect Disabil) [DOI] [PubMed] [Google Scholar]
- Huang Z., Wang Z., Zhang J., Dai R., Wu J., Li Y. Altered temporal variance and neural synchronization of spontaneous brain activity in anesthesia. Hum. Brain Mapp. 2014;35:5368–5378. doi: 10.1002/hbm.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Obara N., Davis HHt, Pokorny J., Northoff G. The temporal structure of resting-state brain activity in the medial prefrontal cortex predicts self-consciousness. Neuropsychologia. 2016;82:161–170. doi: 10.1016/j.neuropsychologia.2016.01.025. [DOI] [PubMed] [Google Scholar]
- Huang X., Xu K., Chu C., Jiang T., Yu S. Weak higher-order interactions in macroscopic functional networks of the resting brain. J. Neurosci. 2017;37:10481–10497. doi: 10.1523/JNEUROSCI.0451-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull J.V., Dokovna L.B., Jacokes Z.J., Torgerson C.M., Irimia A., Van Horn J.D. Resting-state functional connectivity in autism spectrum disorders: a review. Front Psychiatr. 2016;7:205. doi: 10.3389/fpsyt.2016.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahashi T., Yamada T., Watanabe H., Nakamura M., Ohta H., Kanai C. Alterations of local spontaneous brain activity and connectivity in adults with high-functioning autism spectrum disorder. Mol. Autism. 2015;6:30. doi: 10.1186/s13229-015-0026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiujias M., Kelley E., Hall L. Restricted, repetitive behaviors in autism spectrum disorder and obsessive-compulsive disorder: a comparative review. Child Psychiatry Hum. Dev. 2017;48:944–959. doi: 10.1007/s10578-017-0717-0. [DOI] [PubMed] [Google Scholar]
- Johnstone T., Ores Walsh K.S., Greischar L.L., Alexander A.L., Fox A.S., Davidson R.J., Oakes T.R. Motion correction and the use of motion covariates in multiple-subject fMRI analysis. Hum. Brain Map. 2006;27:779–788. doi: 10.1002/hbm.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantareas M.M., Hewitt T. Autistic disorder and schizophrenia: diagnostic overlaps. J. Autism Dev. Disord. 2001;31:19–28. doi: 10.1023/a:1005605528309. [DOI] [PubMed] [Google Scholar]
- Liang Z., Wang Y., Sun X., Li D., Voss L.J., Sleigh J.W. EEG entropy measures in anesthesia. Front. Comput. Neurosci. 2015;9:16. doi: 10.3389/fncom.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K., Nikouline V.V., Palva J.M., Ilmoniemi R.J. Long-range temporal correlations and scaling behavior in human brain oscillations. J. Neurosci. 2001;21:1370–1377. doi: 10.1523/JNEUROSCI.21-04-01370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N.K. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Lynch C.J., Breeden A.L., You X., Ludlum R., Gaillard W.D., Kenworthy L. Executive dysfunction in autism spectrum disorder is associated with a failure to modulate frontoparietal-insular hub architecture. Biol. Psychiatr. Cogn. Neurosci. Neuroimag. 2017;2:537–545. doi: 10.1016/j.bpsc.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbrot B. How long is the coast of britain? Statistical self-similarity and fractional dimension. Science. 1967;156:636–638. doi: 10.1126/science.156.3775.636. [DOI] [PubMed] [Google Scholar]
- Martino M., Magioncalda P., Huang Z., Conio B., Piaggio N., Duncan N.W. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc. Natl. Acad. Sci. U. S. A. 2016;113:4824–4829. doi: 10.1073/pnas.1517558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrovito D., Hanson C., Hanson S.J. Differences in atypical resting-state effective connectivity distinguish autism from schizophrenia. Neuroimage Clin. 2018;18:367–376. doi: 10.1016/j.nicl.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxim V., Şendur L., Fadili J., Suckling J., Gould R., Howard R. Fractional Gaussian noise, functional MRI and Alzheimer's disease. NeuroImage. 2005;25:141–158. doi: 10.1016/j.neuroimage.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Maximo J.O., Cadena E.J., Kana R.K. The implications of brain connectivity in the neuropsychology of autism. Neuropsychol. Rev. 2014;24:16–31. doi: 10.1007/s11065-014-9250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld J., Kuja-Halkola R., Mevel K., Cauvet E., Fransson P., Bolte S. Alterations in resting state connectivity along the autism trait continuum: a twin study. Mol. Psychiatry. 2018;23:1659–1665. doi: 10.1038/mp.2017.160. [DOI] [PubMed] [Google Scholar]
- Noel J.P., Lytle M., Cascio C., Wallace M.T. Disrupted integration of exteroceptive and interoceptive signaling in autism spectrum disorder. Autism Res. 2018;11:194–205. doi: 10.1002/aur.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. Brain and self - a neurophilosophical account. Child Adolesc. Psychiatry Ment. Health. 2013;7:28. doi: 10.1186/1753-2000-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. The brain's spontaneous activity and its psychopathological symptoms - "Spatiotemporal binding and integration". Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;80:81–90. doi: 10.1016/j.pnpbp.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Northoff G. Springer; Neuropsychodynamic Psychiatry: 2018. Why Do We Need Psychopathology? From the Brain's Spontaneous Activity to “Spatiotemporal Psychopathology”; pp. 9–18. [Google Scholar]
- Northoff G., Duncan N.W., Hayes D.J. The brain and its resting state activity--experimental and methodological implications. Prog. Neurobiol. 2010;92:593–600. doi: 10.1016/j.pneurobio.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Northoff G., Magioncalda P., Martino M., Lee H.C., Tseng Y.C., Lane T. Too fast or too slow? Time and neuronal variability in bipolar disorder-A combined theoretical and empirical investigation. Schizophr. Bull. 2018;44:54–64. doi: 10.1093/schbul/sbx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly C., Lewis J.D., Elsabbagh M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paakki J.J., Rahko J., Long X., Moilanen I., Tervonen O., Nikkinen J. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010;1321:169–179. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- Palva J.M., Zhigalov A., Hirvonen J., Korhonen O., Linkenkaer-Hansen K., Palva S. Neuronal long-range temporal correlations and avalanche dynamics are correlated with behavioral scaling laws. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3585–3590. doi: 10.1073/pnas.1216855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow A., Deserno L., Walter M., Fydrich T., Bermpohl F., Schlagenhauf F. Reduced default mode network connectivity in schizophrenia patients. Schizophr. Res. 2015;165:90–93. doi: 10.1016/j.schres.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Perez Velazquez J.L., Barcelo F., Hung Y., Leshchenko Y., Nenadovic V., Belkas J. Decreased brain coordinated activity in autism spectrum disorders during executive tasks: reduced long-range synchronization in the fronto-parietal networks. Int. J. Psychophysiol. 2009;73:341–349. doi: 10.1016/j.ijpsycho.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Pinkham A.E., Hopfinger J.B., Pelphrey K.A., Piven J., Penn D.L. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr. Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitt M., Barnes K.A., Martin A. Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. Neuroimage Clin. 2015;7:359–366. doi: 10.1016/j.nicl.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitt M., Barnes K.A., Wallace G.L., Kenworthy L., Martin A. Resting-state functional connectivity predicts longitudinal change in autistic traits and adaptive functioning in autism. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6699–E6706. doi: 10.1073/pnas.1510098112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin-Lord M.P., Barbeau E.B., Soulieres I., Monchi O., Doyon J., Benali H. Increased topographical variability of task-related activation in perceptive and motor associative regions in adult autistics. Neuroimage Clin. 2014;4:444–453. doi: 10.1016/j.nicl.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruette J.R. 2013. Autism diagnostic observation schedule-2 (ADOS-2) (Google Scholar) [Google Scholar]
- Qin P., Duncan N., Northoff G. Why and how is the self-related to the brain midline regions? Front. Hum. Neurosci. 2013;7:909. doi: 10.3389/fnhum.2013.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The restless brain: how intrinsic activity organizes brain function. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015;370 doi: 10.1098/rstb.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart N.J., Bradshaw J.L., Moss S.A., Brereton A.V., Tonge B.J. Pseudo-random number generation in children with high-functioning autism and Asperger's disorder: further evidence for a dissociation in executive functioning? Autism. 2006;10:70–85. doi: 10.1177/1362361306062011. [DOI] [PubMed] [Google Scholar]
- Rodriguez N.M., Thompson R.H. Behavioral variability and autism spectrum disorder. J. Appl. Behav. Anal. 2015;48:167–187. doi: 10.1002/jaba.164. [DOI] [PubMed] [Google Scholar]
- Rosso O.A., Blanco S., Yordanova J., Kolev V., Figliola A., Schurmann M. Wavelet entropy: a new tool for analysis of short duration brain electrical signals. J. Neurosci. Methods. 2001;105:65–75. doi: 10.1016/s0165-0270(00)00356-3. [DOI] [PubMed] [Google Scholar]
- Rubin D., Fekete T., Mujica-Parodi L.R. Optimizing complexity measures for FMRI data: algorithm, artifact, and sensitivity. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0063448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandu A.L., Rasmussen I.A., Jr., Lundervold A., Kreuder F., Neckelmann G., Hugdahl K. Fractal dimension analysis of MR images reveals grey matter structure irregularities in schizophrenia. Comput. Med. Imaging Graph. 2008;32:150–158. doi: 10.1016/j.compmedimag.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Scalabrini A., Huang Z., Mucci C., Perrucci M.G., Ferretti A., Fossati A. How spontaneous brain activity and narcissistic features shape social interaction. Sci. Rep. 2017;7:9986. doi: 10.1038/s41598-017-10389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh J.W., Steyn-Ross D.A., Steyn-Ross M.L., Grant C., Ludbrook G. Cortical entropy changes with general anaesthesia: theory and experiment. Physiol. Meas. 2004;25:921–934. doi: 10.1088/0967-3334/25/4/011. [DOI] [PubMed] [Google Scholar]
- Sokunbi M.O., Gradin V.B., Waiter G.D., Cameron G.G., Ahearn T.S., Murray A.D. Nonlinear complexity analysis of brain FMRI signals in schizophrenia. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0095146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M., Ozonoff S., McMahon W.M. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. J. Autism Dev. Disord. 2005;35:145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Squarcina L., De Luca A., Bellani M., Brambilla P., Turkheimer F.E., Bertoldo A. Fractal analysis of MRI data for the characterization of patients with schizophrenia and bipolar disorder. Phys. Med. Biol. 2015;60:1697–1716. doi: 10.1088/0031-9155/60/4/1697. [DOI] [PubMed] [Google Scholar]
- Stadnitski T. Measuring fractality. Front. Physiol. 2012;3:127. doi: 10.3389/fphys.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger J.H. Tests for comparing elements of a correlation matrix. Psychol. Bull. 1980;87:245. [Google Scholar]
- Stephens G.J., Honey C.J., Hasson U. A place for time: the spatiotemporal structure of neural dynamics during natural audition. J. Neurophysiol. 2013;110:2019–2026. doi: 10.1152/jn.00268.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E., von Wegner F., Morzelewski A., Brodbeck V., Jahnke K., Laufs H. Breakdown of long-range temporal dependence in default mode and attention networks during deep sleep. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15419–15424. doi: 10.1073/pnas.1312848110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P.C., Hsu J.W., Lan C.C., Liu C.C., Su T.P., Chen Y.S. Structural and functional correlates of a quantitative autistic trait measured using the social responsive scale in neurotypical male adolescents. Autism Res. 2016;9:570–578. doi: 10.1002/aur.1535. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Lynch C.J., Khouzam A., Phillips J., Feinstein C. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatr. 2013;70:869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Lynch C.J., Cheng K.M., Odriozola P., Barth M.E. Brain state differentiation and behavioral inflexibility in autism. Cereb. Cortex. 2015;25:4740–4747. doi: 10.1093/cercor/bhu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eylen L., Boets B., Steyaert J., Evers K., Wagemans J., Noens I. Cognitive flexibility in autism spectrum disorder: explaining the inconsistencies? Res. Autism Spectr. Disord. 2011;5:1390–1401. [Google Scholar]
- Vogel D., Falter-Wagner C.M., Schoofs T., Kramer K., Kupke C., Vogeley K. Interrupted time experience in autism spectrum disorder: empirical evidence from content analysis. J. Autism Dev. Disord. 2018:1–12. doi: 10.1007/s10803-018-3771-y. [DOI] [PubMed] [Google Scholar]
- Volkmar F.R., Cohen D.J. Comorbid association of autism and schizophrenia. Am. J. Psychiatry. 1991;148:1705–1707. doi: 10.1176/ajp.148.12.1705. [DOI] [PubMed] [Google Scholar]
- Yang A.C., Hong C.J., Liou Y.J., Huang K.L., Huang C.C., Liu M.E. Decreased resting-state brain activity complexity in schizophrenia characterized by both increased regularity and randomness. Hum. Brain Mapp. 2015;36:2174–2186. doi: 10.1002/hbm.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Huang Z., Chen Y., Zhang J., Ghinda D., Nikolova Y. Breakdown in the temporal and spatial organization of spontaneous brain activity during general anesthesia. Hum. Brain Mapp. 2018;39:2035–2046. doi: 10.1002/hbm.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Walsh K., Long J., Gui W., Denisova K. Reduced structural complexity of the right cerebellar cortex in male children with autism spectrum disorder. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0196964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Shi L., Cui X., Wang S., Luo X. Functional connectivity of the caudal anterior cingulate cortex is decreased in autism. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0151879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material