Abstract
Neuroadaptations caused by chronic methamphetamine (MA) use are likely major contributors to high relapse rate following treatment. Thus, focusing intervention efforts at pre-empting addiction in vulnerable populations, thereby preventing MA-use-induced neurological changes that make recovery so challenging, may prove more effective than targeting chronic users. One approach is studying casual/recreational users, not diagnosed with substance use disorder. This group may be at high risk for addiction due to their experience with MA. On the other hand, they may be resilient against addiction since they were able to maintain casual use over the years and not become addicted. Understanding their neuro-cognitive characteristics during decision-making and risk-taking would help solve this dilemma and, may help identify intervention strategies. Unfortunately, research on neuro-cognitive characteristics of casual MA users is currently lacking. In this work we begin to address this deficit.
This study was part of a larger investigation of neural correlates of risky sexual decision-making in men who have sex with men. While undergoing functional magnetic resonance imaging, 31 casual MA users and 66 non-users performed the CUPS task, in which they decided to accept or refuse a series of mixed gambles. Convergent results from whole brain, region of interest and psychophysiological interaction (PPI) analyses are presented.
Whole brain analysis identified an amygdala-striatal cluster with weaker activation in casual MA users compared to non-users during decision-making. Activity in that cluster inversely correlated with decisions to gamble: lower activation corresponded to higher risk taking. Using this cluster as a seed in PPI analyses, we identified a wide range of neural network differences between casual MA users and non-users. Parametric whole brain analyses identified clusters in the ventral striatum, posterior insula and precuneus where activations modulated by risk and reward were significantly weaker in casual MA users than in non-users. The striatal cluster identified in these analyses overlapped with the amygdala-striatal cluster.
This work identified neural differences in casual MA users' reward processing and outcome learning systems which may underlie their increased real-world risk-taking. It suggests that while making decisions casual MA users focus primarily on potential gain unlike non-users who also take the riskiness of the choice into consideration.
Keywords: Methamphetamine, Risk taking, Decision, fMRI, Amygdala, Striatum, Insula
Highlights
-
•
Neurocognitive processes of casual meth users, not yet addicted but at high risk.
-
•
Convergent results from whole brain, psychophysiological interaction and ROI.
-
•
Isolated key deficits in reward processing and outcome learning.
-
•
Identified deficits may contribute to increased lab and real-world risk-taking.
1. Introduction
Amphetamine-type stimulants, including methamphetamine (MA), have become the second (after cannabis) most widely used class of illicit drugs (United Nations Office on Drugs and Crime, 2017), affecting 37 million people worldwide. Disorders related to use of amphetamines are second only to those related to opioids. Moreover, according to the available data, methamphetamine represents the highest global health threat among amphetamines (UN Office on Drugs and Crime, 2017). A growing body of research has identified cognitive deficits characteristic of MA abuse/dependence (London et al., 2015; Paulus et al., 2002; Scott et al., 2007; Stewart et al., 2014; Tolliver et al., 2012). However, the question whether these differences are pre-existing or caused by methamphetamine use has not yet been answered. Dean and colleagues attempted to address this question (Dean et al., 2013) by reviewing evidence from animal studies, cross-sectional human studies, a twin study, studies of changes in cognition with abstinence, studies of changes in brain structure and function with abstinence, and studies examining how severity of MA abuse relates to levels of cognitive decline. Although the authors reported mixed findings, they found some evidence of a causal relationship between MA abuse and cognitive decline. The reported cognitive deficits in the areas of executive functioning (Scott et al., 2007), response inhibition (Monterosso et al., 2005) and delay discounting (Monterosso et al., 2007) may be the key contributors to a relapse rate as high as 77% within 5 years from treatment (Brecht and Herbeck, 2014). Another key change that is likely to contribute to a high relapse rate is the adaptation of the reward processing system: in order to handle the high surge of dopamine that methamphetamine use evokes, the reward system down-regulates its dopamine receptors and becomes less sensitive to rewards (Volkow et al., 2004; Volkow et al., 2010). This in turn reduces the salience of non-drug related rewards, so that they become less rewarding in comparison to drug-reward.
Focusing intervention efforts at pre-empting addiction in vulnerable populations seems a more promising strategy, since it would prevent MA-use-induced neurological changes that make recovery so challenging. One approach is studying casual/recreational users, not diagnosed with substance use disorder (SUD). This group may be at high risk for addiction due to their experience with MA. On the other hand, they may be resilient against addiction since they were able to maintain casual use over the years and not become addicted. Understanding their neuro-cognitive characteristics during decision-making and risk-taking would help solve this dilemma and, may help identify intervention strategies. Unfortunately, research on cognitive and neural characteristics of casual MA users is currently lacking, with the vast majority of existing research focusing on addicted individuals. In this paper we are starting to bridge this gap by specifically identifying neurocognitive differences characteristic of casual MA users compared to non-users. This knowledge may also help elucidate the extent to which observed neurocognitive changes in chronic MA users are pre-existing versus meth-use-induced.
Another challenge when identifying neural patterns specific to methamphetamine users is the co-occurrence of other risky behaviors characteristic of this population; specifically, consistently strong associations exist among binge drinking, methamphetamine use and risky sexual behavior (Vosburgh et al., 2012). In this study we employed two strategies in order to disentangle unique behavioral and neural processes related to MA use. First, binge drinkers were excluded from the study. Second, all participants reported involvement in risky sexual behavior; thus, when contrasting differences between MA users and non-user we could isolate unique characteristics of casual MA use.
Understanding neural characteristics of casual MA users, particularly during risky decision-making, may help identify addiction-vulnerable phenotypes that could influence treatment response and thus represent novel therapeutic targets (London et al., 2015). The goal of this paper is to isolate aspects of the decision process that cognitively and neurally distinguish casual MA users from non-users, in order to inform future treatment development.
We conceptualize the decision-making process as consisting of two phases: the decision phase, where the evaluation and action selection are performed, and the feedback phase for the outcome processing. Although neuro-cognitive processes sub-serving each phase are temporally and functionally distinct, they are supported by the same distributed network, but with its components engaged differently in each phase: amygdala, insula, ventral striatum (VS), dorsal striatum, prefrontal cortex (PFC), anterior cingulate cortex ACC, pre-supplementary motor area, superior/intraparietal lobule and superior temporal gyrus (STG) (Bechara and Damasio, 2005; Ernst and Paulus, 2005). We considered it important to be able to identify the differences in neural processing found between the groups, specific to each phase of the decision-making process. To address this, we used a specially designed decision-making task, the CUPS task (Xue et al., 2010) that allowed us to separately examine decision and feedback phases. The CUPS task was similarly used to examine neurocognitive characteristics specific to individuals with internet gambling disorder (IGD) during the decision process (Liu et al., 2017). In this work the researchers found weaker risk-modulated activation in dorsolateral PFC (dlPFC) and inferior parietal lobule (IPL) in the IGD group during the decision phase and greater outcome-related activation in the ventromedial PFC (vmPFC), and VS in the IGD participants during outcome processing.
We hypothesized that risk taking in a laboratory gambling task will be reflective of real-life sexual risk taking; thus, the differences in neuro-cognitive processing of decisions during the task would illuminate the neural characteristics of casual MA users that are linked to their real-life risk-taking behavior.
In the current functional magnetic resonance imaging (fMRI) study, we performed a five-step analysis to identify the neuro-cognitive group differences during risky decision making and examine how they relate to risky behavior both during the task and in real life. In step one we used whole brain analysis to identify areas with differential activation in casual MA users, compared to non-users, during decisions and feedback processing. The purpose of step two was to examine whether the identified differences in activation are related to risk-taking in the task, thus we performed an independent ROI analysis, and correlated the average activation in the cluster identified in step one with risk-taking in the task. The goal of step three was to flush out the larger network differences between the groups, to see if there are differences in functional connectivity of the area we identified in step one with the other neural components key to decision-making. Thus, we utilized a generalized form of psycho-physiological interaction analysis (McLaren et al., 2012), using the ROIs resulting from the whole brain analysis during decisions (step one) as a seed. In step four we wanted to identify areas where activation was modulated by risk or reward differently between the groups. In similar work comparing methamphetamine-dependent individuals with control participants (Kohno et al., 2014) researchers found differences between the groups in parametric modulation of the activation by risk during risky decisions: in methamphetamine-dependent individuals it was more strongly modulated in VS and more weakly modulated in dlPFC. We wanted to see if casual meth-users demonstrate a similar neuro-behavioral profile as methamphetamine-dependent individuals during risky decisions. To test this we performed two whole brain analyses with parametrically modulated regressors to identify areas where activation modulated by risk or reward differs between the groups. Finally, in step five, we computed a meth-use index and used it as a regressor in a group level whole brain analysis to identify neural areas moderated by intensity of meth use.
2. Methods and materials
2.1. Participants
Ninety seven sexually risky men (31 White, 30 Black and 36 Hispanic/Latino; age range 18.2–30 years, M = 25.2 years) who have sex with men (31 casual MA users and 66 non-users), participants in a larger investigation of neural correlates of risky sexual decision-making in men who have sex with men, were included in this study. SUD diagnoses for any substance other than nicotine, binge drinking (>2 drinks on a weekday or >5 on a weekend), and regular use of any illicit substance except for marijuana were exclusionary (see Table 1 for substance use characteristics). Data were collected between 2012 and 2014, prior to widespread use of pre-exposure prophylaxis (PrEP) drugs to inhibit HIV infection, thus unprotected sex carried a serious risk of HIV infection. Since methamphetamine use is prevalent in this population (Shoptaw and Reback, 2006), one goal of the larger project was to identify possible neural correlates associated with methamphetamine use; thus we targeted 33% of the recruited subjects to be MA users. We recruited 177 participants using internet advertisements, 155 of whom participated in this task. Three participants were removed because no valid structural data was collected from them. Along with other measures, participants reported the number of risky sexual occurrences (defined as an instance of condomless anal sex) in the past 90 days, (range = 0 to 89 instances); this value was used as their real-world sexual risk-taking index. Participants were recruited into three groups based on this real-world sexual risk-taking index and methamphetamine use: safe (# risky sex occurrences = 0; N = 50), sexually risky (# risk sex occurrences >0) non-users (N = 66) and sexually risky MA users (N = 37). Since the purpose of the current study was to isolate the neural mechanisms associated with MA use from those associated with risky sexual behavior we only included the participants from the two risky groups. Participants were considered MA users if they self-reported MA use at any point in their life, even if currently abstinent. One participant originally assigned to the MA group was excluded due to a contradiction in his MA-use history that made it unclear whether he was a user or not. Due to our focus on casual MA use, we evaluated MA group participants in accordance with DSM 5 diagnostic criteria of substance use disorder (Hasin et al., 2013) that included, in addition to frequency and length of use, cravings, withdrawal symptoms, impact on life and overdose history, in order to identify the participants who may currently suffer from addiction or have suffered in the past and were not included as casual MA users. Based on these criteria, we excluded 5 participants, leaving 31 participants in the casual MA group. Informed consent was obtained from each participant before the experiment. The protocol of the study was approved by the University Institutional Review Board.
Table 1.
Research participants substance use characteristics.
| % of participants used in last 90 days | Casual Meth-users | Non-users |
|---|---|---|
| Nicotine | 54.83 | 36.92 |
| Marijuanaa | 87.10 | 62.12 |
| All Other Drugsa | 67.74 | 42.42 |
| Alcohol use, drinks per day* | 1.42 | 1.06 |
- Significant difference between the groups, p < .05
3. fMRI CUPS task
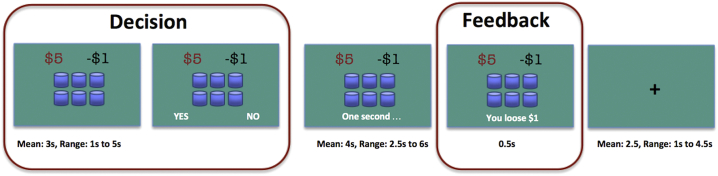
While undergoing fMRI, participants performed the modified CUPS task (Levin et al., 2007; Xue et al., 2010), in which they had to decide whether to accept or refuse a series of mixed gambles. In each trial participants were presented with a set of cups (3 to 11). They were informed that one of the cups contained a gain (amount ranging between $3 and $8) and the rest of the cups contained a loss of $1, and they were asked to accept or reject the gamble (Fig. 1). When the gamble was accepted, the participant was informed of the gain or loss after a short waiting period. When the gamble was rejected, the participant did not win or lose any money. When no selection was made during the response window, the participant lost $1.
Fig. 1.
CUPS task.
The probability and magnitude of the potential gain were independently manipulated so that it created three expected value (EV) based categories: risk equivalent (RE, EV = 0, e.g., $6 gain in one cup and $1 loss in 6 cups), risk advantageous (RA, EV > 0, e.g., $8 gain in one cup and $1 loss in 6 cups) and risk disadvantageous (RD, EV < 0, e.g., $6 gain in one cup and $1 loss in 8 cups). Half of the gambles were RE, one third of the other half of the gambles were RD, and the rest were RA.
First the gamble was presented for the participant to contemplate, but no action was expected or accepted. After a variable delay (mean 3 s, ranging from 1.5 to 5 s, drawn from an exponential distribution), the response options appeared and participants were required to respond within 3 s, otherwise they would lose $1. The position of the response cue varied from trial to trial to prevent preplanned motor response at the decision stage. After the response and a variable delay (2.5 to 6 s, mean 4 s), the participant was informed of the gamble resolution (presented for 0.5 s). During the variable inter-trial interval (mean 2.5 s, ranged from 1 to 4.5 s), the fixation cross was presented.
The accumulated win/loss total was displayed once at the end of the task. Participants received their winnings at the end of the experiment; however, participants who ended up with a net loss did not actually lose money. The task consisted of 72 trials and lasted 12 min.
The intervals between the trials, the delays between gamble presentation and decision acceptance, and gamble resolution delays were randomly jittered and the sequence was optimized for design efficiency (Christakou et al., 2009; Rogers et al., 2004) using an in-house program.
3.1. Functional imaging procedure
Participants lay supine in the scanner, and viewed visual stimuli back-projected onto a screen through a mirror attached to the head coil. Foam pads were utilized to minimize head motion. Stimulus presentation and timing of all stimuli and response events were controlled by Matlab (The Mathworks, Inc.) programs based on Psychtoolbox (http://www.psychtoolbox.org) extensions on a MacBook Pro. Participants' responses were collected online using an MRI-compatible button box. Participants completed the CUPS task as part of a larger battery of structural and functional MRI data collection for approximately 1.2 h of scanning (task order during the session stayed constant for all the participants) in a 3 T Siemens MAGNETOM Tim Trio scanner. A T1-weighted anatomical image, a set of diffusion-weighted images, and several task-related functional image sequences were collected. Task-related fMRI data were acquired using T2*-weighted (TR = 2000 ms, TE = 25 ms, 64 × 64 matrix size with a resolution of 3 mm2, using 41 3.0-mm axial slices) imaging.
3.2. fMRI data preprocessing and statistical analysis
Image preprocessing and statistical analysis were carried out using FEAT (FMRI Expert Analysis Tool) version 6.00, part of the FSL package (FMRIB software library, version 4.1.8, www.fmrib.ox.ac.uk/fsl). The data were temporally filtered using a non-linear high pass filter with a 100 s cut-off, and spatially smoothed using a 5 mm full-width-half-maximum (FWHM) Gaussian kernel. A two-step registration procedure was used whereby images were first registered to the MPRAGE structural image, and then into the standard Montreal Neurological Institute MNI-152 T1 template brain, using affine transformations with FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001). Registration from MPRAGE structural images to standard space was further refined using FNIRT nonlinear registration (Andersson et al., 2007). Statistical analyses were performed in the native image space, with the statistical maps normalized to the standard space prior to higher-level analysis. Melodic ICA was used to de-noise the preprocessed functional data (Beckmann and Smith, 2004). The FIX software package was used to automatically identify noise components (Griffanti et al., 2014; Salimi-Khorshidi et al., 2014).
Data were modeled at the first level using a general linear model within FSL's FILM module. In the whole brain analysis, four predictors of interest (2 trial types based on the decision to take risk (risky = gamble accepted, non-risky = gamble rejected) X 2 phases: (decision = time from the gamble display to the button press to accept or reject (2–8 s), feedback = time when gamble resolution was displayed (0.5 s)) were modeled separately. We modeled the main effect of each type of decision for each decision phase by contrasting against baseline (inter-trial break periods when fixation cross was displayed) as well as contrasts between risky and non-risky trials for each phase. At the group level we examined whether any lower-level effects differed between casual MA users and non-users.
3.3. Generalized psychophysiological interaction (PPI) analysis
Psychophysiological interaction analysis (PPI) can provide information about functional integration of the brain under certain contexts, such as task condition (Friston et al., 1997). Here, we used a generalized PPI approach [(McLaren et al., 2012)] to identify regions whose connectivity differs between the groups on the basis of task conditions. Like other functional connectivity approaches, generalized PPI computes connectivity between a seed region and other voxels in the brain, where seed, as in the original PPI, is usually a region significantly activated in the task in the same data set (Friston et al., 1997; McLaren et al., 2012). However, unlike the original method, generalized PPI can use all available data in the estimation of connectivity. By modeling the entire experimental space, this approach provides a better model fit and achieves greater sensitivity and specificity than the original method of PPI (McLaren et al., 2012). Thus, compared to the traditional PPI method, the generalized PPI (further referred to as PPI) can help reduce the probabilities of both false positives and false negatives.
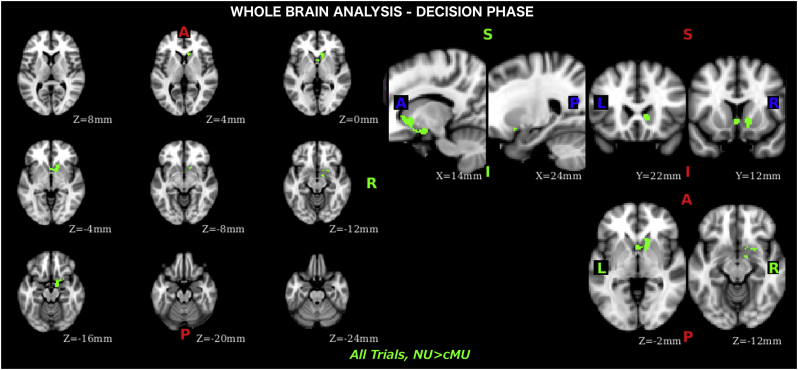
An amygdala-striatal cluster identified during the whole brain analysis, where activation during the decisions was attenuated in casual MA users compared to non-users (Fig. 2), was used as the seed region in the PPI analyses. In these analyses, we first transformed the PPI seed region of interest (ROI) into the native space for each participant. The time course of the seed ROI was then extracted. Next, a PPI model was set up to explore effective connectivity between the seed ROI and other voxels in the brain. Nine covariates were included in this model, including the same four predictors used in the whole brain analysis (2 trial types based on the decision to take risk: risk/non-risk X 2 phases: decision and feedback) as the psychological factors, time course of the seed region as the physiological factor, four interaction covariates between the psychological and physiological factors. In addition to the main effect for each phase, two contrasts were set up in the first level model to examine PPI difference between risky and non-risky trials during decision and feedback processing.
Fig. 2.
Whole brain analysis, results of the decision phase where non-users' activation is higher than casual meth-users'.
3.4. Whole brain analyses with risk and reward as parametric modulators
In order to identify brain areas differentially modulated by risk or potential reward between the groups, we performed two whole brain analyses with parametric modulators. Four parametric regressors1 (2 trial types based on the decision to take risk X 2 phases, decision & feedback) were used in each analysis; the risk parameter in analysis 1 was represented by the number of cups, the gain parameter in analysis 2 was represented by the potential win amount. As in the first whole brain analysis discussed above, we modeled the main effect of each trial type at each phase by contrasting against baseline as well as computing the contrasts between risky and non-risky trials for each phase. At the group level we examined whether any lower-level effects differed between casual MA users and non-users.
3.5. Meth-use index analysis
We used the following algorithm to compute the meth-use index, a continuous variable representing individual differences in the severity of use across participants:
if never used set index to 0, if ever used start with 1, if use initiated before age of 18 add 1, if current user (used in the last 3 months) add 2, if used > 10 times in the last 3 month add 2. This resulted in a range of use from 0 to 6. We used demeaned meth-use index as a covariate in the group level analysis.
All group analyses (whole brain, PPI and meth-index), were performed using random-effects FLAME (FMRIB's Local Analysis of Mixed Effects) stage 1 simple mixed effect model (Beckmann et al., 2003; Woolrich, 2008; Woolrich et al., 2004). Group images were then thresholded using cluster detection statistics with a height threshold of z > 2.3 and a cluster probability of p < .05, corrected for whole-brain multiple comparisons using Gaussian Random Field Theory.
4. Results
4.1. Behavioral results
There were no significant differences in the risk taken, response time, number of misses, amount or number of wins or losses between the groups. However, casual MA users took marginally more risk in the task (M = 0.54) then non-users (M = 0.46), Mdiff = 0.08, 95% CI = [−0.157, 0.004], p = .06 and lost (marginally) more money (M = 27.03) then non-users(M = 23.00), Mdiff = 4.03, 95% CI = [−8.66, 0.6.], p = .086. Real-life sexual risk taking, significantly correlated with risk taking in the laboratory CUPS task (measured as ratio of risky choices to all trials) across all subjects, r(95) = 0.27, p = .007. This relationship was driven by participants' behavior on risk-equivalent trials (EV = 0), r(95) = 0.29, p = .004.
4.2. fMRI results – Whole brain analysis
During the decision phase, averaging across all trials, we found weaker BOLD response in casual MA users (compared to non-users) on the right side in the amygdala, nucleus accumbens (NAcc), and surrounding ventral regions of the putamen and caudate (or ventral striatum) (Fig. 2, Table 2). No differences were observed when contrasting risky and non-risky trials or examining each type of trial (risky/non-risky) separately; no differences were found during the feedback phase.
Table 2.
Whole brain analysis results during evaluation phase where activation for non-users was higher than for meth-users. The value in the “max” column is the maximum z-stat in each local maxima. X, Y, and Z are x, y, and z coordinates in MNI-152 space in mm. Cluster size is 260 voxels.
| Z-MAX | Z-MAX X | Z-MAX Y | Z-MAX Z | Notes |
|---|---|---|---|---|
| 3.91 | 12 | −2 | −16 | R Amygdala |
| 3.46 | 10 | 22 | 2 | R Caudate |
| 3.32 | 14 | 14 | −4 | R Caudate/Putamen |
| 2.98 | 12 | 8 | −14 | R Putamen |
| 2.83 | 18 | 8 | −16 | R Ventromedial Prefrontal Cortex (vmPFC) |
4.3. ROI analysis and decision to gamble
To examine whether the activation in the amygdala-striatal ROI differentiating casual MA users and non-users during decisions was related to the choice to gamble or not, we conducted an independent ROI analysis by extracting the percent signal change for each subject (N = 97). The amygdala-striatal ROI was defined as group difference (non-users > MA users) during the gamble evaluation (time from the moment the gamble is displayed till the user presses the button with his selection) for all trials (both risky and non-risky trials included, so risk taking is not part of the ROI selection). It was correlated with the average risk taken in the task (average risk calculated as the ratio of accepted gambles to number of trials). We found a significant negative effect (r = −0.31, p = .002), so that the stronger the activation in the amygdala-striatal ROI was associated with less within-task risk.
5. fMRI results – Generalized PPI analysis
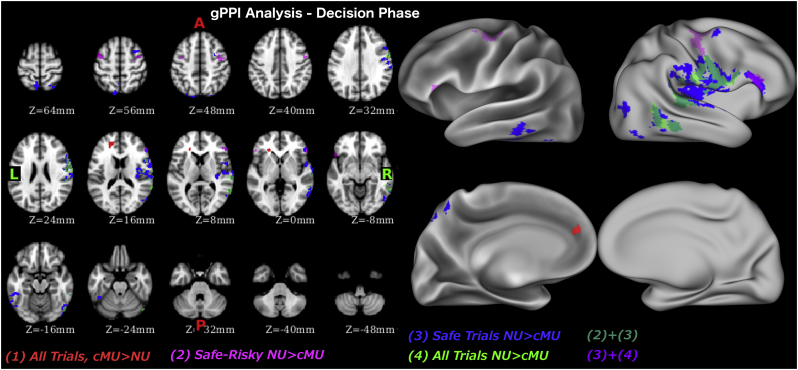
During the decision phase, averaging across all trials, casual MA users had stronger connectivity between the amygdala-striatal seed region and the vmPFC than non-users. Non-users on the other hand, had stronger connectivity between the amygdala-striatal seed region and the posterior insular cortex (PIC), STG and middle temporal gyrus (MTG) (Fig. 3, Table 3) than did casual MA users. When isolating the decisions on which participants subsequently decided to take risk (risky trials) from those they didn't (non-risky trials), we found no difference in connectivity with the amygdala-striatal seed region during the trials when risk was taken. However, during the non-risky trials non-users had stronger connectivity of seed ROI with PIC, MTG, medial frontal gyrus (MFG), and inferior temporal gyrus (ITG). Similarity of these results for non-risky trials with the ones for all trials, suggests that all trial's results are likely driven by the non-risky trials. When contrasting non-risky and risky trials, non-users had stronger connectivity between the seed ROI and clusters in bilateral inferior frontal gyrus (IFG) and MFG.
Fig. 3.
Psycho-physiological interaction analysis results during decision phase.
Table 3.
Psycho-physiological interaction analysis results during evaluation phase. Each row refers to activity related to one cluster. The value in the “max” column is the maximum z-stat in each cluster. X, Y, and Z are x, y, and z coordinates in MNI-152 space in mm. The number of voxels in the cluster is recorded in the “voxels” column.
| Z-MAX | Voxels | Z-MAX X | Z-MAX Y | Z-MAX Z | Notes |
|---|---|---|---|---|---|
| All trials | Meth > NonMeth | ||||
| 3.27 | 132 | −18 | 46 | 16 | L Ventromedial Prefrontal Cortex (vmPFC) |
| All trials | NonMeth > Meth | ||||
| 3.96 | 700 | 66 | −26 | 8 | R Superior Temporal Gyrus/Posterior Insular Cortex (PIC) |
| 3.68 | 298 | 64 | −48 | 12 | R Middle Temporal Gyrus (MTG) |
| 3.80 | 104 | 52 | −72 | −16 | R Lateral Occipital Cortex |
| Safe trials | NonMeth > Meth | ||||
| 4.37 | 1713 | 36 | −24 | 10 | R PIC |
| 3.49 | 318 | 58 | −36 | −12 | R MTG |
| 4.81 | 253 | 52 | −68 | −16 | R Lateral Occipital Cortex |
| 3.85 | 179 | 56 | 32 | 16 | R Inferior Frontal Gyrus (IFG) |
| 4.07 | 126 | −46 | −38 | −18 | L Inferior Temporal Gyrus |
| 3.48 | 108 | −54 | −64 | −20 | L Lateral Occipital Cortex |
| 3.20 | 91 | 30 | 8 | 54 | R Medial Frontal Gyrus |
| 3.71 | 87 | 30 | −66 | 62 | R Lateral Occipital Cortex |
| Safe-Risky | NonMeth > Meth | ||||
|---|---|---|---|---|---|
| 3.78 | 190 | 44 | −8 | 48 | R Precentral Gyrus/Medial Frontal Gyrus |
| 3.25 | 151 | 56 | 32 | 18 | R IFG |
| 3.16 | 112 | −44 | 4 | 54 | L Precentral Gyrus/Medial Frontal Gyrus |
| 3.42 | 74 | −50 | 20 | −10 | L IFG |
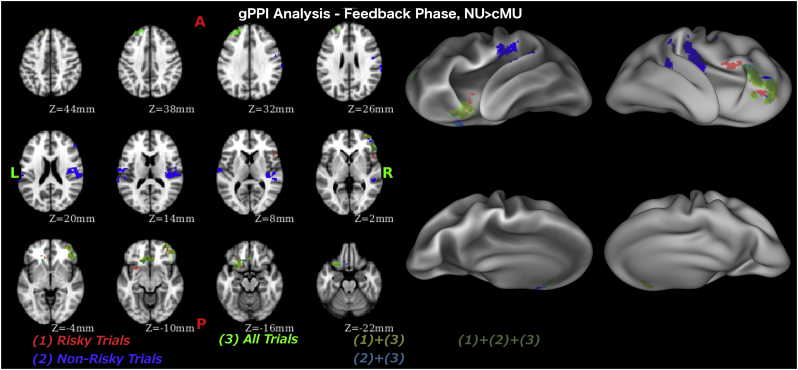
During the feedback phase, stronger connectivity between the amygdala-striatal seed region and the vmPFC, including the medial orbitofrontal area, dlPFC and ventrolateral PFC (vlPFC), and IFG was evident in non-users (Fig. 4, Table 4) compared to casual MA users. Examining risky and non-risky trials separately we found stronger connectivity between the amygdala-striatal seed region and the PIC, vmPFC, and IFG during non-risky trials and stronger connectivity between the amygdala-striatal seed region and the vmPFC, vlPFC and IFG during risky trials in non-users compared to casual MA users.
Fig. 4.
Psycho-physiological interaction analysis results during feedback phase.
Table 4.
Psycho-physiological interaction analysis results during feedback phase. Each row refers to activity related to one cluster. The value in the “max” column is the maximum z-stat in each cluster. X, Y, and Z are x, y, and z coordinates in MNI-152 space in mm. The number of voxels in the cluster is recorded in the “voxels” column.
| Z-MAX | Voxels | Z-MAX X | Z-MAX Y | Z-MAX Z | Notes |
|---|---|---|---|---|---|
| All trials | NonMeth > Meth | ||||
| 4.21 | 714 | 52 | 32 | 2 | R Inferior Frontal Gyrus (IFG)/Ventrolateral Prefrontal Cortex (vlPFC) |
| 4.51 | 509 | −4 | 30 | −12 | L Ventromedial PFC |
| 3.35 | 320 | −30 | 48 | 32 | L Dorsolateral PFC |
| Safe trials | NonMeth > Meth | ||||
| 4.36 | 733 | 56 | −20 | 20 | R Posterior Insular Cortex |
| 3.90 | 292 | 52 | 32 | 4 | R IFG |
| 3.16 | 211 | −64 | −12 | 16 | L Postcentral Gyrus |
| 4.01 | 161 | −10 | 24 | −20 | L vmPFC |
| Risky trials | NonMeth > Meth | ||||
| 3.91 | 512 | 40 | 38 | −4 | R vlPFC, IFG |
| 4.15 | 404 | −4 | 30 | −12 | L vmPFC |
5.1. Post-hoc behavioral analysis
Stronger connectivity of the amygdala-striatal ROI in casual MA users with the vmPFC, an area responsible for value calculation (O'Doherty, 2004), and in non-users with the insula, a region implicated in risk processing (Droutman et al., 2015), during decision, suggested the possibility that casual MA users were more focused on the potential gains and non-users were more attuned to possible risks, represented in the task by the number of cups. Therefore, we conducted additional analyses of the behavioral data to explore this idea further. We ran logistic regression with repeated measures using the glmer function from the lme4 package in R, fitting a general linear model using the maximum likelihood method. We regressed the decision to accept the gamble (risk) on group (casual MA users vs. non-users), number of cups, potential gain, trial type and all interactions. To ensure that adding the interaction terms significantly improves the model, we also fitted a model without interaction terms and compared the two models using a log likelihood ratio test (using the ANOVA function in R). Akaike Information Criterion (AIC; Akaike, 1974) values as well as Bayesian Information Criterion (BIC; Schwarz, 1978) were used along with chi square to identify the model with a better fit. This revealed the superiority of the model with interaction terms, since it had lower AIC/BIC and p (chi square) < 0.001 (Table 5). We were particularly interested in the interactions between group and the potential gain and between group and the number of cups. The interaction of group and number of cups turned out to be significant (p < .001). We proceeded with stratifying by group and found that while for non-users both potential gain and number of cups were significant predictors of risk, for casual MA users only potential gain but not the number of cups was a predictor (Table 6).
Table 5.
Parameters for model fit evaluation for post hoc logistical regression analysis.
| Model | AIC | BIC |
|---|---|---|
| Model1 - without interaction terms | 8344.3 | 8385.4 |
| Model2 - with interaction terms | 8238.5 | 8355.0 |
Model1 regresses decision to accept the gamble (risk) on group (cMU vs. NU), number of cups, potential gain and trial type. Model2 regresses decision to accept the gamble (risk) on group (cMU vs. NU), number of cups, potential gain, trial type and all interactions.
Table 6.
Results of logistical regression of decision to accept the gamble (risk) on potential gain, number of cups and EV category stratified by group.
| GROUP |
METH |
NON-USERS |
|---|---|---|
| Predictors | Estimate [95%CI]/z/p | Estimate [95%CI]/z/p |
| PayOff | 0.47 [0.10, 0.85]/2.37/0.02 | 0.30 [0.02, 0.56]/2.14/0.03 |
| NumCups | 0.06[−0.24, 0.38]/0.38/ns | −0.60 [−0.82, −0.39]/-5.37/<0.001 |
| Trial Type | 2.40 [0.99, 4.01]/2.96/0.003 | 2.05 [0.98, 3.15]/3.69/<0.001 |
5.2. Whole brain analyses with risk and reward as parametric modulators
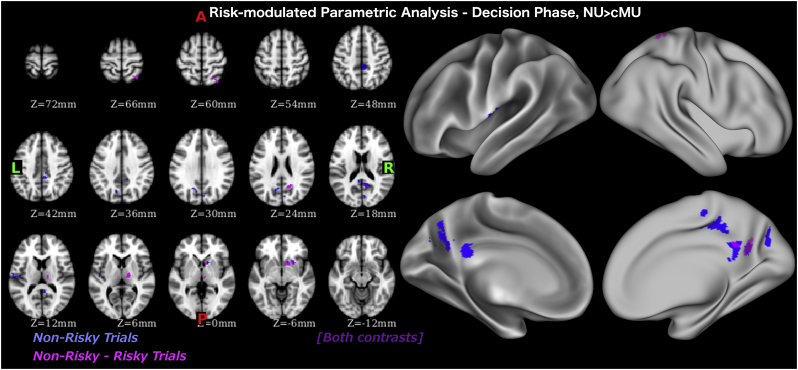
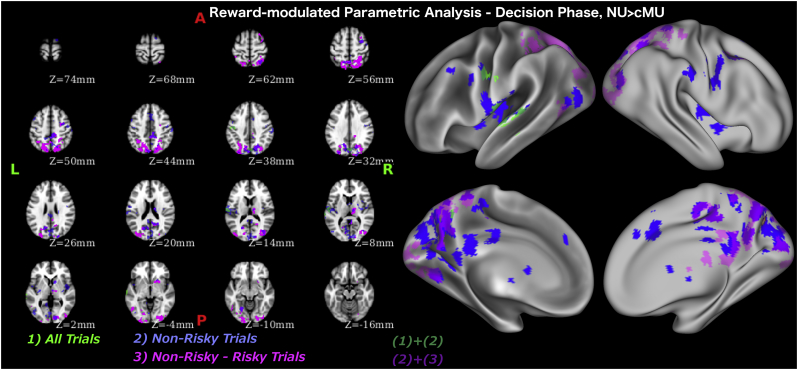
To examine the full spectrum of neural differences in processing risk and potential reward between the groups, we performed two parametrically modulated fMRI analyses, one identifying activation modulated by risk (number of cups) and one identifying activation modulated by potential reward. It revealed that during the decision phase, on non-risky trials, non-users had higher risk-modulated and gain-modulated activation in the ventral striatum (a cluster within the amygdala-striatal ROI differentiating non-users and casual MA users during the decision phase), posterior cingulate cortex (PCC), PIC, precuneus and cuneal cortex (Table 7, Table 8, Fig. 5, Fig. 6). Similar differences were evident when contrasting non-risky and risky trials.
Table 7.
Whole brain analysis with risk (number of cups) as parametric modulator during evaluation phase. Each row refers to activity related to one cluster. The value in the “max” column is the maximum z-stat in each cluster. X, Y, and Z are x, y, and z coordinates in MNI-152 space in mm. The number of voxels in the cluster is recorded in the “voxels” column.
| Z-MAX | Voxels | Z-MAX X | Z-MAX Y | Z-MAX Z | Notes |
|---|---|---|---|---|---|
| Safe trials | NonMeth > Meth | ||||
| 3.72 | 157 | 6 | −38 | 46 | R Posterior Cingulate Cortex (PCC) |
| 3.37 | 140 | 10 | −54 | 18 | R Precuneus |
| 2.96 | 81 | −16 | −62 | 30 | L Precuneus |
| 2.94 | 68 | 4 | −78 | 32 | R Cuneal Cortex |
| 2.87 | 67 | 14 | 12 | −4 | R Caudate/Putamena |
| 3.25 | 64 | −46 | −16 | 10 | L Posterior Insular Cortex |
| Safe-Risky | NonMeth > Meth | ||||
| 3.70 | 98 | 14 | 12 | −4 | R Caudate/Putamena |
| 3.59 | 86 | 10 | −56 | 18 | R Precuneus |
| 3.48 | 74 | 14 | −14 | 6 | R Thalamus |
| 3.12 | 70 | 34 | −58 | 68 | R Superior Parietal Lobe |
This cluster is within amygdala-striatal ROI identified by whole brain analysis as differentiating cMU and NU during decision phase.
Table 8.
Whole brain analysis with gain (potential win amount) as parametric modulator during evaluation phase. Each row refers to activity related to one cluster. The value in the “max” column is the maximum z-stat in each cluster. X, Y, and Z are x, y, and z coordinates in MNI-152 space in mm. The number of voxels in the cluster is recorded in the “voxels” column.
| Z-MAX | Voxels | Z-MAX X | Z-MAX Y | Z-MAX Z | Notes |
|---|---|---|---|---|---|
| All trials | NonMeth > Meth | ||||
| 3.6 | 264 | −58 | −18 | 14 | L Posterior Insular Cortex (PIC) |
| 3.3 | 124 | 4 | −78 | 36 | R Cuneal Cortex |
| 3.28 | 80 | −44 | −14 | 48 | L Precentral Gyrus |
| Safe trials | NonMeth > Meth | ||||
| 3.72 | 2521 | 12 | −88 | 4 | R Intracalcarine Cortex |
| 3.93 | 647 | −58 | −18 | 14 | L PIC |
| 4.49 | 403 | 6 | −40 | 46 | R Precuneus/Posterior Cingulate Cortex |
| 3.25 | 210 | 0 | 18 | 44 | Paracingulate Gyrus/Anterior Cingulate Cortex |
| 3.34 | 185 | 44 | 0 | 2 | R PIC |
| 3.75 | 184 | 48 | −10 | 52 | R Precentral Gyrus |
| 3.78 | 132 | 34 | −12 | 6 | R PIC |
| 3.11 | 128 | 6 | −12 | 2 | R Thalamus |
| 3.56 | 128 | 64 | −18 | 42 | R Supramarginal Gyrus |
| 3.71 | 125 | 34 | −4 | 54 | R Middle Frontal Gyrus (MFG) |
| 3.79 | 103 | 36 | −74 | 12 | R Lateral Occipital |
| 3.27 | 98 | −46 | −76 | 2 | L Lateral Occipital |
| 3.74 | 95 | 12 | 10 | 4 | R Caudate* |
| 3.83 | 89 | −22 | −64 | −10 | L Lingual Gyrus |
| 2.86 | 86 | −50 | 6 | 44 | L MFG |
| 3.43 | 86 | 30 | −58 | 60 | R Lateral Occipital |
| 3.91 | 75 | 18 | 2 | 74 | R Superior Frontal Gyrus |
| 2.96 | 72 | −42 | −4 | 4 | L PIC |
| 3.16 | 70 | −18 | −88 | 40 | L Lateral Occipital |
| 3.54 | 67 | 0 | −32 | 26 | Posterior Cingulate Cortex |
| 3.01 | 65 | −44 | −14 | 38 | L Precentral Gyrus |
| Safe - Risky | NonMeth > Meth | ||||
| 4.43 | 4109 | −12 | −78 | 50 | L Lateral Occipital |
| 3.81 | 840 | 20 | −90 | −6 | R Occipital Pole/Fusiform Gyrus |
| 4.21 | 323 | 4 | −40 | 46 | R Precuneus/Posterior Cingulate Cortex |
| 3.51 | 301 | −28 | −98 | −4 | L Occipital Pole |
| 3.57 | 283 | 16 | −58 | 24 | R Precuneus |
| 4.04 | 253 | 14 | −14 | 8 | R Thalamus |
| 3.62 | 230 | 36 | −2 | 54 | R MFG |
| 3.85 | 172 | −22 | −66 | −10 | L Occipital Fusiform Gyrus |
| 3.11 | 119 | 24 | 12 | −4 | R Putamena |
| 3.34 | 105 | −48 | −62 | −6 | L Lateral Occipital |
This cluster is within amygdala-striatal ROI identified by whole brain analysis as differentiating cMU and NU during decision phase.
Fig. 5.
Whole brain analysis with risk modulated regressors during decision phase.
Fig. 6.
Whole brain analysis with reward modulated regressors during decision phase.
5.3. Meth-use Index effect
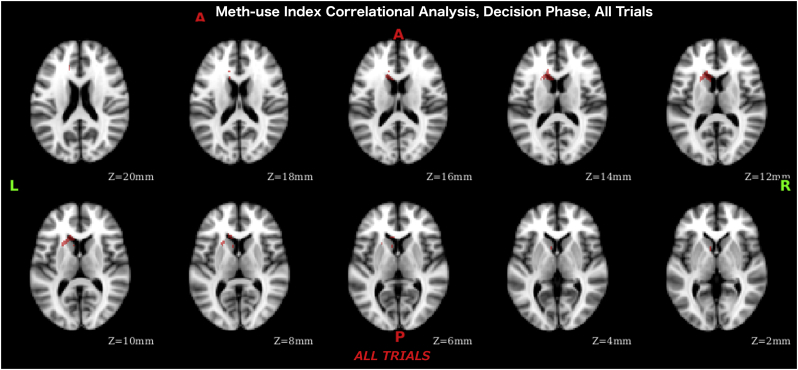
During the decision phase, averaging across all trials, we found significant negative correlation of the meth-use index (more intense use corresponding to weaker activation) with activation levels in a cluster in the ventral striatum (Table 9, Fig. 7).
Table 9.
Meth-use index whole brain analysis results during decision phase where more intense use corresponds to weaker activation. The value in the “max” column is the maximum z-stat in each local maxima. X, Y, and Z are x, y, and z coordinates in MNI-152 space in mm. Cluster size is 160 voxels.
| Z-MAX | Z-MAX X | Z-MAX Y | Z-MAX Z | Notes |
|---|---|---|---|---|
| 3.35 | −22 | 16 | 10 | L Putamen |
| 3.05 | −14 | 20 | 12 | L Caudate |
| 2.96 | −10 | 18 | 14 | L Caudate |
| 2.75 | −10 | 24 | 8 | L Caudate |
| 2.62 | −8 | 10 | 4 | L Caudate |
| 2.49 | −14 | 32 | 20 | L Paracingulate Gyrus/Anterior Cingulate Gyrus |
Fig. 7.
Meth-use index correlational analysis results (across all subjects) during decision phase.
6. Discussion
This study examined neural-network differences between casual MA users and non-users in the decision-making process in order to identify characteristics of casual MA users that may be responsible for real-life risk. The observed significant correlation of the amount of risk participants took during the experimental task and real-life sexual risk (measured by amount of condomless anal sex over the previous 90 days) suggests that our findings may generalize to real-life risk-taking behavior. An examination of the behavioral and neuroimaging findings suggests that casual MA users may have a lower risk sensitivity compared to non-users. Specifically, both groups considered the magnitude of the potential win but only non-users consider the chances of winning while making their decisions.
Our research suggested that an amygdala-striatal cluster could be the key to the neural differences underlying risk related decisions in casual MA users. This cluster emerged as a group differentiator during the decisions in a whole brain analysis with weaker activation in casual meth-users. In light of previous findings suggesting this area's role in reward evaluation (O'Doherty, 2004; Elliott et al., 2003; Baxter and Murray, 2002), reward prediction and error computation (Hare et al., 2008; Knutson et al., 2001; Pagnoni et al., 2002), the current findings may suggest that casual MA users' behavior during the task arises from a deregulated reward processing system.
Weaker striatal activation is consistent with the neuroadaptation characteristic of chronic MA users (Volkow et al., 2004, Volkow et al., 2010). Since MA users in our study are casual users there are two possible explanations of this finding: (1) adaptation of the reward system happens rapidly enough with methamphetamine use so that it manifests itself even in casual users, and (2) weaker neural response to reward may be a pre-existing characteristic of methamphetamine users. Correlational whole brain analysis of meth use intensity also revealed a cluster in the ventral striatum where higher intensity of use corresponded to weaker activation during decisions, thus providing support for the first explanation. This explanation also aligns well with prior research that found a disruption of value-related signals after a single MA challenge (Bernacer et al., 2013).
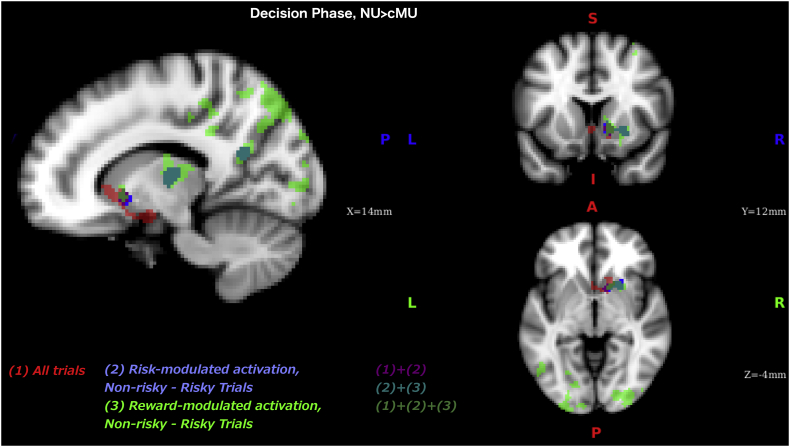
Our parametrically modulated analyses also supports the deregulation of reward-system hypothesis: it identified a striatal cluster (overlapping with the amygdala-striatal cluster found in the whole brain analysis) where risk- and reward-modulated activation was stronger in non-users compared to casual MA users during decisions that resulted in safe choices (as contrasted with risky choices or baseline, Fig. 8). Interestingly these results differ from the prior work that examined differences in risk-modulated activation between methamphetamine-dependent individuals and controls (Kohno et al., 2014), that reported no difference in risk-modulation between the groups during safe events (cash outs in Balloon Analog Reward Task, BART). On the other hand, they found higher risk-modulated activation in VS and weaker risk-modulated activation in dlPFC in methamphetamine-dependent group on risky trials (active balloon pumps in BART), where we found no group difference on risky trials. Although it is difficult to draw conclusions when comparing the results from two such differently designed studies, these findings point to possible differences between casual meth-users and methamphetamine-dependent individuals that can be tested in future studies.
Fig. 8.
Combined results of 3 whole brain analyses during decision phase where activation in non-users was greater that in casual meth-users, from Fig. 2, Fig. 5, Fig. 6) identifying the overlapping areas: (1) - All trials contrasted with baseline, (2) – risk-modulated activation in safe trials contrasted with risky trials, (3) – reward-modulated activation in safe trials contrasted with risky trials.
Novel to the current investigation, the activation in the amygdala-striatal cluster during decisions significantly predicted how much risk participants took in the task, such that higher activation corresponded to less risk taking. This finding is somewhat paradoxical, since it shows that lesser activation in the reward area led to higher gambling. One possible explanation may be that people with lower phasic levels of dopamine (Grace, 1991), resulting in lower activation in striatal areas, are more reward-seeking in order to elicit homeostatic compensation and increase overall dopaminergic activity, which results in a generally higher level of risk taking.
To further examine this possible reward system deficit we performed a generalized PPI analysis using this cluster as the seed region. This analysis uncovered further neural-network differences between the groups. Specifically, during the decisions the connectivity of the amygdala-striatal cluster was stronger in casual MA users with the vmPFC and in non-users with the insular cortex (IC), IFG and MFG. We theorize that these connectivity differences may reflect different cognitive processes underlying decision-making. Since the vmPFC has been implicated in value calculation (O'Doherty, 2004), the greater vmPFC-amygdala-striatal connectivity in casual MA users during decision-making could reflect a focus on the value of choices; this increased connectivity may also be a compensatory measure that balances the weaker amygdala-striatal activation in casual MA users.
In contrast, the IC has been implicated in risk assessment and risk prediction error calculation (Droutman et al., 2015), the IFG in risk related inhibition of the suboptimal choices (Stewart et al., 2013) and MFG in response inhibition (Batterink et al., 2010), suggesting that the increased connectivity among these regions in non-users may reflect a greater focus on the risk of a choice, as represented by the likelihood of potential outcomes, and inhibition of reward seeking response. Converging evidence for this hypothesis comes from the parametrically modulated analysis, which revealed risk-modulated activation in insula during safe trials was stronger in non-users than casual MA users. The behavioral analysis supports this as well, since the potential gain amount equally strongly predicted the gambling decisions for casual MA users and non-users whereas the risk represented by the number of cups was only a significant predictor for non-users.
The somatic marker hypothesis [SMH, (Bechara and Damasio, 2005)] considers the vmPFC a repository of the linkage between factual knowledge and bioregulatory states and a substrate for learning the association between a complex situation and an emotional state during our experiences (Bechara et al., 2000). When similar situations are encountered, these somatic markers are invoked and the emotional responses are re-enacted, providing for optimal decision-making. SMH is operative during the feedback phase of decision-making, since the function of this phase is to learn the actual values of options for the goal of adaptive behavior (Ernst and Paulus, 2005). Weaker connectivity of the amygdala-striatal cluster with the key neural components necessary for successful somatic marker association (Bechara, 2005; Verdejo-Garcia and Bechara, 2009), the vmPFC, working memory processing (dlPFC), and somato-emotional processing (IC), in casual MA users during feedback processing may suggest deregulation of the critical learning mechanism that impacts sub-optimal choice during following decisions.
7. Concluding remarks
The current paper employed a network-based approach to isolate several key differences that may contribute to drug use in casual MA users. First, a dysregulation of the reward processing system was evident during the decision phase. We identified an amygdala-striatal cluster with attenuated activation in casual MA users compared to non-users during decisions in general, and, the striatal portion of this cluster had attenuated risk and reward modulated activation. Moreover, activation in this cluster was predictive of risk taking so that higher activation related to lesser risk taking. Our analysis suggests that when making decisions, non-users took into consideration both the potential gain and riskiness of the gambles, whereas casual MA users focused primarily on potential gain. On a neural level these behavioral findings are supported by stronger risk modulated activation in IC in non-users and higher functional connectivity of the amygdala-striatal cluster with the IC, MFG and IFG in non-users and the vmPFC in casual MA users. Second, during the feedback phase, weaker connectivity of the amygdala-striatal region with the vmPFC, dlPFC and PIC in casual MA users could indicate a suboptimal feedback processing and outcome learning mechanisms. Finally, the correlation of risk taking in the task with real-life self-reported sexual risk taking suggests that these uncovered differences in reward processing and outcome learning may also underlie real-life risky behavior in casual MA users.
Acknowledgments
Acknowledgements
Funding: This work was supported by the National Institutes of Drug Abuse [R01DA031626] and National Institute of General Medical Science [R01GM109996].
Compliance with ethical standards
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Informed consent was obtained for all experimentation with human subjects. The privacy rights of human subjects were observed.
Footnotes
A three-column file format was used that contained onset time for each event, response time and demeaned parameter value (number or cups or gain amount for the trial).
References
- Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974;19(6):716–723. [Google Scholar]
- Andersson J.L., Jenkinson M., Smith S. FMRIB Analysis Group of the University of Oxford; 2007. Non-linear Registration, Aka Spatial Normalisation FMRIB Technical Report TR07JA2. [Google Scholar]
- Batterink L., Yokum S., Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. NeuroImage. 2010;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M.G., Murray E.A. The amygdala and reward. Nat. Rev. Neurosci. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat. Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R. The somatic marker hypothesis: a neural theory of economic decision. Games Econ. Behav. 2005;52(2):336–372. [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann C., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bernacer J., Corlett P.R., Ramachandra P., McFarlane B., Turner D.C., Clark L.…Murray G.K. Methamphetamine-induced disruption of frontostriatal reward learning signals: relation to psychotic symptoms. Am. J. Psychiatr. 2013;170(11):1326–1334. doi: 10.1176/appi.ajp.2013.12070978. [DOI] [PubMed] [Google Scholar]
- Brecht M.-L., Herbeck D. Time to relapse following treatment for methamphetamine use: a long-term perspective on patterns and predictors. Drug Alcohol Depend. 2014;139:18–25. doi: 10.1016/j.drugalcdep.2014.02.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A., Brammer M., Giampietro V., Rubia K. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. J. Neurosci. 2009;29(35):11020–11028. doi: 10.1523/JNEUROSCI.1279-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A.C., Groman S.M., Morales A.M., London E.D. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology. 2013;38(2) doi: 10.1038/npp.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droutman V., Bechara A., Read S.J. Roles of the different sub-regions of the insular cortex in various phases of the decision-making process. Front. Behav. Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R., Newman J.L., Longe O.A., Deakin J.F.W. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J. Neurosci. 2003;23(1):303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Paulus M.P. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol. Psychiatry. 2005;58(8):597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Grace A.A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Griffanti L., Salimi-Khorshidi G., Beckmann C.F., Auerbach E.J., Douaud G., Sexton C.E.…Smith S.M. ICA-based artefact and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage. 2014;95:232–247. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., O'Doherty J., Camerer C.F., Schultz W., Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J. Neurosci. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D.S., O'Brien C.P., Auriacombe M., Borges G., Bucholz K., Budney A.…Grant B.F. DSM-5 criteria for substance use disorders: recommendations and rationale. Am. J. Psychiatr. 2013;170(8):834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M., Morales A.M., Ghahremani D.G., Hellemann G., London E.D. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry. 2014;71(7):812. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin I.P., Weller J.A., Pederson A.A., Harshman L.A. Age-related differences in adaptive decision making: sensitivity to expected value in risky choice. Judgm. Decis. Mak. 2007;2(4):9. [Google Scholar]
- Liu L., Xue G., Potenza M.N., Zhang J.-T., Yao Y.-W., Xia C.-C.…Fang X.-Y. Dissociable neural processes during risky decision-making in individuals with Internet-gaming disorder. NeuroImage. 2017;14:741–749. doi: 10.1016/j.nicl.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E., Kohno M., Morales A., Ballard M. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging., chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2015;1628(Pt A):174–185. doi: 10.1016/j.brainres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso J.R., Aron A.R., Cordova X., Xu J., London E.D. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Monterosso J.R., Ainslie G., Xu J., Cordova X., Domier C.P., London E.D. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum. Brain Mapp. 2007;28(5):383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J.P. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Pagnoni G., Zink C.F., Montague P.R., Berns G.S. Activity in human ventral striatum locked to errors of reward prediction. Nat. Neurosci. 2002;5(2):97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Hozack N.E., Zauscher B.E., Frank L., Brown G.G., Braff D.L., Schuckit M.A. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Nature. 2002;26(1):53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Rogers R.D., Ramnani N., MacKay C., Wilson J.L., Jezzard P., Carter C.S., Smith S.M. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol. Psychiatry. 2004;55(6):594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functional mri data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann. Stat. 1978;6(2):461–464. [Google Scholar]
- Scott J.C., Woods S.P., Matt G.E., Meyer R.A., Heaton R.K., Atkinson J.H., Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol. Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shoptaw S., Reback C.J. Associations between methamphetamine use and HIV among men who have sex with men: a model for guiding public policy. J. Urban Health. 2006;83(6):1151–1157. doi: 10.1007/s11524-006-9119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.L., Flagan T.M., May A.C., Reske M., Simmons A.N., Paulus M.P. Young adults at risk for stimulant dependence show reward dysfunction during reinforcement-based decision making. Biol. Psychiatry. 2013;73(3):235–241. doi: 10.1016/j.biopsych.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.L., Connolly C.G., May A.C., Tapert S.F., Wittmann M., Paulus M.P. Striatum and insula dysfunction during reinforcement learning differentiates abstinent and relapsed methamphetamine-dependent individuals: striatum dysfunction associated with relapse. Addiction. 2014;109(3):460–471. doi: 10.1111/add.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver B.K., Price K.L., Baker N.L., Larowe S.D., Simpson A.N., McRae-Clark A.L.…Brady K.T. Impaired cognitive performance in subjects with methamphetamine dependence during exposure to neutral versus methamphetamine-related cues. Am. J. Drug Alcohol Abuse. 2012;38(3):251–259. doi: 10.3109/00952990.2011.644000. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime . United Nations; 2017. World Drug Report 2017. [Google Scholar]
- Verdejo-Garcia A., Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56(Suppl. 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S., Wang G.-J. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Fowler J.S., Tomasi D., Telang F., Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. BioEssays. 2010;32(9):748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosburgh H.W., Mansergh G., Sullivan P.S., Purcell D.W. A review of the literature on event-level substance use and sexual risk behavior among men who have sex with men. AIDS Behav. 2012;16(6):1394–1410. doi: 10.1007/s10461-011-0131-8. [DOI] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E.J., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Xue G., Lu Z., Levin I.P., Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. NeuroImage. 2010;50(2):709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]