Abstract
Background
Diffusion imaging abnormalities have been associated with schizophrenia (SZ) and bipolar disorder (BD), indicating impaired structural connectivity. Newer methods permit the automated reconstruction of major white matter tracts from diffusion-weighted MR images in each individual's native space. Using high-definition diffusion data from SZ and BP subjects, we investigated brain white matter integrity using both an automated tract-based and voxel-based methods.
Methods
Using a protocol matched to the NIH (Young-Adult) Human Connectome Project (and collected on the same customized ‘Connectom’ scanner), diffusion scans were acquired from 87 total participants (aged 18–30), grouped as SZ (n = 24), BD (n = 33) and healthy controls (n = 30). Fractional anisotropy (FA) of eighteen white matter tracks were analyzed using the TRACULA software. Voxel-wise statistical analyses of diffusion data was carried out using the tract-based spatial statistics (TBSS) software. TRACULA group effects and clinical correlations were investigated using analyses of variance and multiple regression.
Results
TRACULA analysis identified a trend towards lower tract FA in SZ patients, most significantly in the left anterior thalamic radiation (ATR; p = .04). TBSS results showed significantly lower FA voxels bilaterally within the cerebellum and unilaterally within the left ATR, posterior thalamic radiation, corticospinal tract, and superior longitudinal fasciculus in SZ patients compared to controls (FDR corrected p < .05). FA in BD patients did not significantly differ from controls using either TRACULA or TBSS. Multiple regression showed FA of the ATR as predicting chronic mania (p = .0005) and the cingulum-angular bundle as predicting recent mania (p = .02) in patients. TBSS showed chronic mania correlating with FA voxels within the left ATR and corpus callosum.
Conclusions
White matter abnormality in SZ varies in severity across different white matter tract regions. Our results indicate that voxel-based analysis of diffusion data is more sensitive than tract-based analysis in identifying such abnormalities. Absence of white matter abnormality in BD may be related to medication effects and age.
Keywords: Schizophrenia, Bipolar Disorder, DTI, TRACULA, TBSS
Highlights
-
•
Our study investigated white matter integrity in 87 young schizophrenia, bipolar disorder and control subjects with a tract-based (TRACULA) and a voxel-based (TBSS) approach, using high-definition diffusion imaging data obtained from the Human Connectome Project ‘Connectom’ scanner.
-
•
TRACULA evaluated fractional anisotropy (FA) from 18 white matter tracts. TBSS evaluated regional white matter FA.
-
•
TRACULA identified a trend towards lower tract FA in schizophrenia subjects across multiple tracts. TBSS results showed mainly unilaterally decreased FA voxels in schizophrenia subjects. FA in bipolar patients did not significantly differ from controls with either method.
-
•
With TRACULA, multiple regression showed that anterior thalamic radiation FA predicted chronic affectivity and cingulum-angular bundle FA predicted recent mania in patients. With TBSS, chronic mania correlated with FA voxels within the left anterior thalamic radiation and corpus callosum.
1. Introduction
Schizophrenia (SZ) and bipolar disorder (BD) are relatively prevalent psychiatric disorders, that cause significant disability (Eaton et al., 2008). The hallmark symptoms of schizophrenia involve psychosis (i.e. delusions or hallucinations), disorganization and negative symptoms; while bipolar disorder classically consists of varying mood episodes, usually involving mania/hypomania and depression. BD shares symptomatic overlap with SZ (Coryell et al., 2001), is prevalent at increased rates in families with SZ (Kendler et al., 1993), and shares chromosomal linkage with SZ (Goes et al., 2007; Park et al., 2004; Potash et al., 2003). Additionally, at least 50% of those with BP have experienced psychosis in their lifetime (Coryell et al., 2001). While the etiology of both SZ and BP remains elusive, there is accumulating evidence that abnormalities in brain connectivity have a major role in the neurobiology of these disorders.
Diffusion imaging is a powerful non-invasive tool for examining structural brain connectivity based on patterns of water diffusion in neural tissue. With this imaging modality, the diffusion tensor model is most commonly employed, and yields the frequently used fractional anisotropy (FA) measure, which is a measure of the asymmetry of water diffusion, and indirectly indexes “neuronal integrity”, putatively reflecting both myelination and organization of the white matter tracts. Diffusion imaging studies in SZ have shown decreased FA in long-range association tracts, including the superior longitudinal fasciculus, cingulum bundle, uncinate fasciculus, inferior longitudinal fasciculus and arcuate fasciculus; projection tracts, such as the anterior thalamic radiation; and the corpus callosum (Arnedo et al., 2015; Karlsgodt, 2016; Kyriakopoulos et al., 2008; Mamah et al., 2010; Peters and Karlsgodt, 2015; Vitolo et al., 2017; Wheeler and Voineskos, 2014). However, there has been a lot of variability in the regions affected across studies, with some reporting no significant abnormalities (Mulert et al., 2012; Peters et al., 2010; Wheeler and Voineskos, 2014). Diffusion imaging studies in BD have also shown variable results, with studies reporting decreased FA in a range of white matter tracts (Mahon et al., 2009; Nortje et al., 2013; Sexton et al., 2009), including the corpus callosum (Yurgelun-Todd et al., 2007), and in frontal and temporal regions (Adler et al., 2004; Beyer et al., 2005; Bruno et al., 2008). Differential results across studies is believed to be influenced by a variety of factors, including disease state, developmental stage, and medications (Kochunov and Hong, 2014; Peters and Karlsgodt, 2015).
Several methods are available for processing diffusion images, including region-of-interest (ROI), voxel-based and tract-based spatial statistics (TBSS) approaches (Soares et al., 2013). ROI-based analysis requires a priori delineation of white matter regions, which can be challenging for smaller regions (Mukherjee et al., 2008), while voxel-based methods can be complicated by inaccuracies with registration algorithms using tensor datasets (Mukherjee et al., 2008) and have been shown to be very sensitive to differences in processing parameters (Jones et al., 2005). Recently, TRACULA (TRActs Constrained by UnderLying Anatomy), an automated probabilistic tractography toolbox within Freesurfer (Yendiki et al., 2011), was used to segment 18 major white matter tracts in BP and showed decreased FA in multiple tracts, including the superior longitudinal fasciculus, cingulum, and corticospinal tracts (Ji et al., 2017; Sprooten et al., 2016). To our knowledge, TRACULA has not been previously used to investigate white matter tract abnormality in SZ. Studying SZ and BD subjects together using identical imaging protocols, will facilitate the understanding of the relationship and differences of brain connectivity of these disorders. A potential disadvantage of a tract-based approach, such as TRACULA, is that it could dilute the identification of FA abnormalities if these are not present universally across given tracts. Thus, we hypothesize that investigations of white matter integrity would be most informative if tract-based methods are paired with voxel-based approaches, capitalizing on the advantages of each method.
Our current study uses TRACULA and TBSS to investigate the brains of SZ and BP participants, obtained using an optimized diffusion protocol matched to the Young-Adult Human Connectome Project (HCP-YA) (Sotiropoulos et al., 2013) and collected on the same customized ‘Connectom’ scanner as the HCP-YA at Washington University. This scanner was highly modified to improve diffusion imaging, by including a Siemens SC72 gradient coil and stronger gradient power supply with maximum gradient amplitude of 100 mT/m (Sotiropoulos et al., 2013). High gradient amplitudes are beneficial for diffusion MRI (dMRI) and increase the signal-to-noise ratio (SNR) over conventional MRI systems (Sotiropoulos et al., 2013). We hypothesize that our optimized dMRI protocol will be sensitive to small group differences in white matter integrity. Both groups are hypothesized to show a pattern of decreased FA in multiple tracts, with greater impairment in the schizophrenia group. TBSS is expected to identify the regions with the greatest involvement within these abnormal tracts.
2. Materials and methods
2.1. Subjects
Participants gave written informed consent prior to participation, and all study protocols were approved by the Institutional Review Board at the Washington University School of Medicine in St. Louis, MO. Imaging data was acquired from 18 to 30-year-old individuals, who were divided into three subject groups: 30 healthy control (CN), 24 schizophrenia (SZ) and 33 bipolar disorder (BD). Table 1 shows demographic information. Participant groups were diagnosed on the basis of a consensus between a research psychiatrist and a trained research assistant who used the Structured Clinical Interview for DSM-IV Axis I Disorder (First et al., 1996). CON subjects were required to have no lifetime history of psychotic or mood disorders. Bipolar (BD) participant patients were required to meet DSM-IV criteria for Bipolar I Disorder and were classified as psychotic BD if the participant had a psychotic event over the course of their lifetime, as assessed via the Structured Clinical Interview for the DSM (SCID). Participants were excluded if they: (a) met DSM-IV criteria for substance dependence or severe/moderate abuse during the prior 3 months; (b) had a clinically unstable or severe general medical disorder; or (c) had a history of head injury with documented neurological sequelae or loss of consciousness. Additionally, to minimize clinical heterogeneity within the BD group, only participants with a history of euphoric mania (versus mania characterized by primarily irritable mood) were included in the study.
Table 1.
Baseline demographic and clinical characteristics across participant groups.
| Characteristic | CN |
SZ |
BP |
F/χ2 | p |
|---|---|---|---|---|---|
| (n = 30) | (n = 24) | (n = 33) | |||
| Age | 24.5 (3.0) | 24.6 (3.6) | 26.5 (3.0) | 3.9 | 0.02 |
| Gender (%) | 7.2 | 0.03 | |||
| Female | 15 (50.0) | 5 (20.8) | 18 (45.5) | ||
| Male | 15 (50.0) | 19 (79.2) | 15 (54.6) | ||
| Ethnicity (%) | 10.0 | 0.02 | |||
| Asian | 4 (13.3) | 1 (4.2) | 3 (9.1) | ||
| Black | 7 (23.3) | 16 (66.7) | 3 (9.1) | ||
| Hispanic | 3 (10.0) | 0 | 3 (9.1) | ||
| White | 15 (50.0) | 5 (16.7) | 22 (66.7) | ||
| Other | 1 (3.3) | 1 (4.2) | 1 (3.0) | ||
| Handedness (%) | 8.1 | 0.23 | |||
| Left | 3 (10.0) | 2 (9.5) | 3 (9.1) | ||
| Right | 27 (90.0) | 19 (90.5) | 30 (90.9) | ||
| Psychotropic medication Hx (%) | |||||
| Typical neuroleptic | 0 | 8 (33.3) | 2 (6.1) | ||
| Atypical neuroleptic | 0 | 14 (58.3) | 12 (36.4) | ||
| Lithium | 0 | 0 | 6 (18.2) | ||
| Other Mood Stabilizerb | 0 | 4 (16.7) | 12 (36.4) | ||
| SSRI/SNRI | 1 (3.3) | 6 (25.0) | 9 (27.3) | ||
| Other Antidepressanta | 0 | 2 (8.3) | 5 (15.2) | ||
| Stimulant | 1 (3.3) | 0 | 0 | ||
| Benzodiazepines | 0 | 3 (12.5) | 6 (18.2) | ||
| Anticholinergic | 0 | 4 (16.7) | 0 | ||
| None | 29 (96.7) | 4 (16.7) | 3 (9.1) | ||
| Duration of Illness (months) | N/A | 72.5 (42.4) | 119.2 (69.9) | N/A | |
| SAPSc | |||||
| Positive symptoms | 0.07 (0.4) | 3.42 (2.8) | 1.03 (1.8) | 23.4 | <0.0001 |
| Disorganization symptoms | 0.07 (0.4) | 1.04 (1.5) | 1.03 (1.7) | 5.2 | 0.007 |
| SANSd | |||||
| Negative symptoms | 0.7 (1.4) | 6.25 (3.0) | 2.88 (3.3) | 28.4 | <0.0001 |
| WERCAPe | |||||
| Mania | 4.60 (5.7) | 15.63 (9.3) | 26.21 (5.9) | 76.1 | <0.0001 |
| Psychosis | 0.77 (3.2) | 30.25 (11.2) | 10.42 (9.3) | 83.9 | <0.0001 |
| YMRS | 0.5 (1.1) | 1.9 (2.7) | 3.5 (4.3) | 7.2 | 0.001 |
| Stress | 19.6 (33.5) | 32.6 (22.7) | 40.5 (33.4) | 3.6 | 0.03 |
Values are given as means (SD) or number per group (%). Results derived from results of two-way ANOVA or Chi-Square analyses.
Refers to antidepressants other than selective serotonin reuptake inhibitors (SSRI).
Other mood stabilizers included Tegretol/Carbamazepine, Depakote/Divalproex, Trileptal/Oxcarbazepine, Topomax/Topiramate, and Lamictal/Lamotrigine.
Maximum possible score on the Structured Assessment of Positive Symptoms (SAPS) is 16.
Maximum possible score on the Structured Assessment of Negative Symptoms (SANS) is 20.
WERCAP = Washington Early Recognition Center Affectivity and Psychosis Screen. Maximum possible score on the Mania section is 49. Maximum possible score on the Psychosis section is 64.
Recent symptoms (i.e. in the last two weeks) were assessed using the Scale for the Assessment of Negative Symptoms (SANS), the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen et al., 1995), and the Young Mania Rating Scale (YMRS) (Young et al., 1978). Chronic symptoms (i.e. last several years) were assessed using the Washington Early Recognition Center Affectivity and Psychosis (WERCAP) Screen, both mania (mWERCAP) and psychosis (pWERCAP) components (Hsieh et al., 2016; Mamah et al., 2014, Mamah et al., 2016b). Psychosocial stress severity was assessed using the WERC Stress Screen (Hsieh et al., 2016; Mamah et al., 2014).
2.2. MRI scan acquisition parameters
Scans were run using a 32-channel head coil on a customized Siemens 3T “Connectom” MRI scanner, which was previously used for collecting the Human Connectome Project – Young Adult (HCP-YA) data and housed at Washington University in St. Louis (Van Essen et al., 2012). The scanning protocol used identical parameters for individual scans as that of the HCP-YA (Glasser et al., 2013; Smith et al., 2013; Van Essen et al., 2012). However, the overall structure of the HCP-YA protocol was consolidated to 3 total imaging sessions (rather than 4) by only acquiring a single T1-weighted (T1w) and T2-weighted (T2w) scan (rather than two of each). The 3 sessions were typically collected over a period of two days. Briefly, T1w MPRAGE and T2w SPACE images were acquired at 0.7 mm isotropic resolution. Oblique axial acquisitions alternate between right-to-left and left-to-right phase encoding directions in consecutive runs. Image reconstruction used SENSE1 multi-channel (Sotiropoulos et al., 2013). A full dMRI session included 6 runs (each approximately 9 min and 50 s), representing 3 different gradient tables, with each table acquired once with right-to-left and left-to-right phase encoding polarities, respectively. Each gradient table includes approximately 90 diffusion weighting directions plus 6 b = 0 acquisitions interspersed throughout each run. Diffusion weighting consisted of 3 shells of b = 1000, 2000, and 3000 s/mm2 interspersed with an approximately equal number of acquisitions on each shell within each run. The diffusion directions were obtained using a toolbox available from INRIA that returns uniformly distributed directions in multiple q-space shells. The directions were optimized so that every subset of the first M directions was also isotropic.
2.3. Image preprocessing
Diffusion scans were concatenated and run through FSL EDDY (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/EDDY), which reduces distortions caused by eddy currents and subject movement by registering the diffusion weighted images to the b0 image. The b0 image was registered to the T1-weighted image with an affine registration. After the distortion correction, the scans were divided to their respective polarities and averaged together. Each individual's T1-weighted image was registered to the 1 mm-resolution MNI-152 atlas using affine registration (Jenkinson et al., 2002) in order to compare individual subjects (Collins et al., 2004). FSL's brain extraction tool was used to remove the skull and other non-brain tissue. FSL's “dtifit” was applied to perform least-squares tensor estimations, specifically eigenvectors, eigenvalues and FA. “Bedpostx GPU” (Hernandez et al., 2013) was used to apply the ball & stick model to estimate diffusion distributions.
2.4. TRACULA
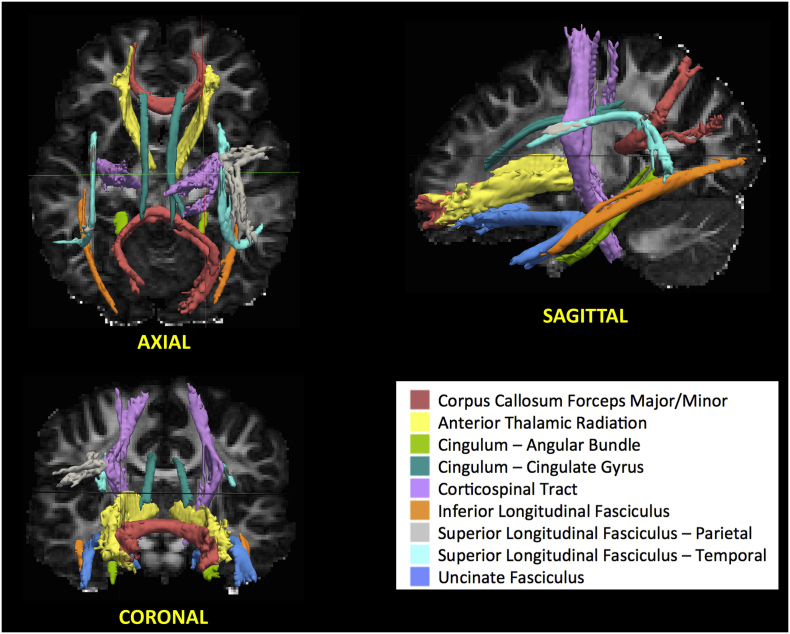
TRACULA (TRActs Contstrained by UnderLying Anatomy) is a powerful method (Yendiki et al., 2011) used to analyze global probabilistic tractography. This method uses a Bayesian framework for global tractography suggested by (Jbabdi et al., 2007) which determines the connection that best fits two selected endpoints using diffusion data. In addition, TRACULA also incorporates prior anatomical knowledge based on manually verified trajectories of tracts in the training set tested by (Yendiki et al., 2011). The anatomy of the tracts in the training subject set have been verified to not significantly deviate from those found in Yendiki's clinical group (Yendiki et al., 2011). For every subject, this method reconstructs probabilistic distributions of 18 major white matter tracts in each subject. A sample participant's estimation and identification of white matter tracts is shown in Fig. 1. More specifically, TRACULA uses the endpoints established in the training set's tracts, expands the endpoints, and transforms them into each subject's native space. Then, TRACULA establishes probabilistic streamlines accounting for the anatomical Freesurfer segmentations and uses control points to dictate the curvature of the tract. This method does not presume exact tract spatial location or shape, so the trajectory of the tract is only restricted with respect to the surrounding anatomical structures. This allows for individual variations across subjects while still establishing the same tracts for comparison. TRACULA trac-all package automates the segmentation steps and includes the training subject data that the manually inspected tract specifications are based on (refer to https://surfer.nmr.mgh.harvard.edu/fswiki/Tracula for more details).
Fig. 1.
Depiction of white matter tracts identified by TRACULA in sample control participant. This method determines the connection that best fits between two selected endpoints unique to each (18) white matter tract of interest using diffusion data. Bilateral tracts are given a single color in this figure, but were treated as independent for all analyses.
We analyzed the average Fractional Anisotropy (FA) for all tracts. FA is a commonly used DTI metric that establishes the directional asymmetry of water diffusion at each voxel and total diffusion at each voxel respectively (Adler et al., 2006; Cao et al., 2014; Nortje et al., 2013; Sprooten et al., 2016). The mean FA for each subject's tracts was calculated by TRACULA based on the probabilistic fibers that were at least 20% of the maximum probability fiber for each pathway (Yendiki et al., 2011).
2.5. TBSS
Tract-based spatial statistics (TBSS) was used to perform voxel-wise analyses of all white matter tracts, as previously described (Arnedo et al., 2015; Smith et al., 2006). FA images were calculated and projected onto the mean FA skeleton, which represents the center of white matter tracts, and thresholded at FA = 0.2.
2.6. Statistical analysis
All statistical analyses were done using SAS 9.4 (SAS Institute Inc., Cary, NC). For TRACULA studies, analysis of variance, covaried for age and sex, was used to test for diagnostic effects for each tract as well as all pairwise comparisons between diagnostic groups. Tests were Bonferroni corrected for multiple comparisons. Relationships between mean FA for each tract and clinical measures (i.e. SAPS, SANS, YMRS, mWERCAP, pWERCAP, and WERC Stress Screen) were investigated using a forward stepwise multiple linear regression analysis. We performed separate regressions to separately examine the relationship between mean FA and each behavioral variable and report the partial correlation for each significant predictor.
For voxel-wise DTI parameter analyses (i.e. TBSS), groups were compared using general linear models, covarying age and gender. Multiple comparison corrections were applied using a permutation-based statistical approach within FSL's Randomise (Smith et al., 2006). Randomise is FSL's tool for nonparametric permutation inference on neuroimaging data, and produces a test statistic image and sets of P-value images (stored as 1-P for more convenient visualization, as bigger is then “better”). Brain and behavioral measures which had significant group effects or were abnormal compared to clinical norms were selected for further correlational analysis within the SZ and BD groups, controlling for age and gender. Significance was set at p < .05.
3. Results
3.1. TRACULA group analysis
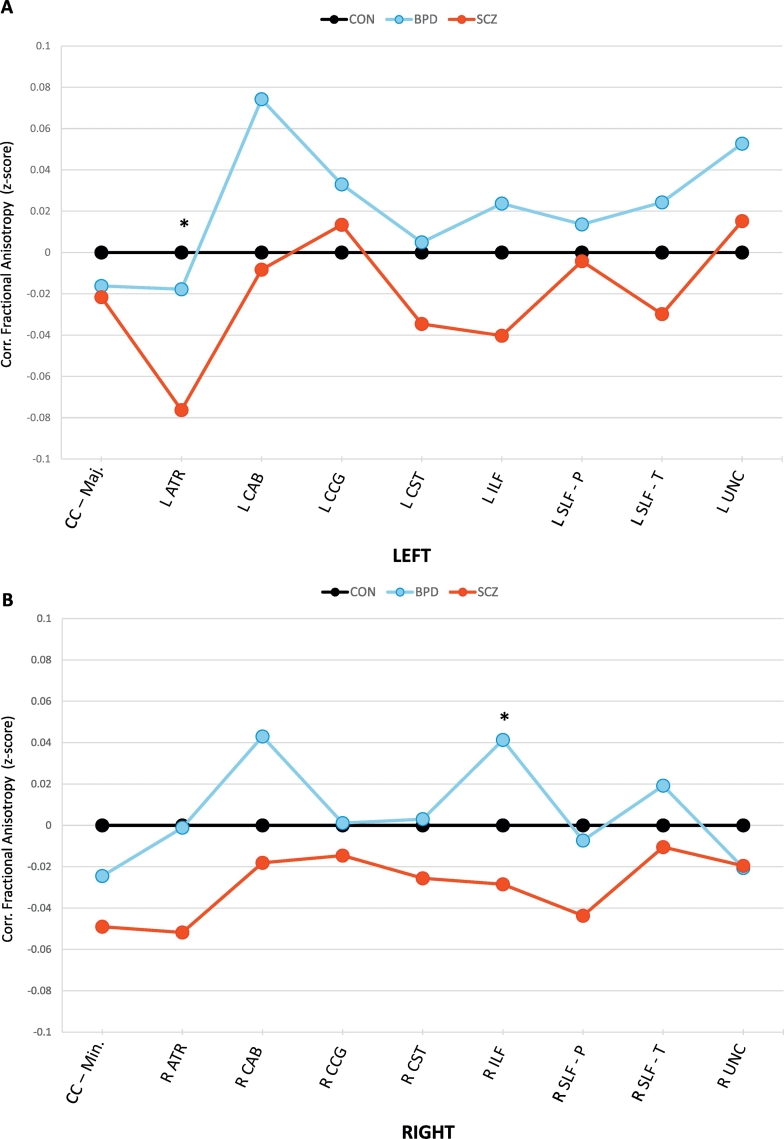
Mean tract FA in each group, and post-hoc univariate tests for each tract are presented in Table 2. The most notable group differences were seen for the left anterior thalamic radiation (p = .039) and the right inferior longitudinal fasciculus (p = .03), with the lowest FA in the SZ group. These differences however did not meet statistical significance after Bonferroni correction.
Table 2.
FA of white matter tracts in control, bipolar disorder and schizophrenia subjects.
| Tract | CON |
BPD |
SCZ |
F | p |
|---|---|---|---|---|---|
| (n = 30) | (n = 24) | (n = 33) | |||
| Corpus callosum | |||||
| F. Major | 0.60 (0.07) | 0.59 (0.07) | 0.59 (0.08) | 0.23 | 0.79 |
| F. Minor | 0.54 (0.06) | 0.52 (0.08) | 0.51 (0.10) | 0.70 | 0.50 |
| Anterior thalamic radiation | |||||
| Left | 0.42 (0.04) | 0.41 (0.03) | 0.39 (0.06) | 3.38 | 0.039* |
| Right | 0.41 (0.04) | 0.41 (0.04) | 0.39 (0.06) | 1.99 | 0.14 |
| Cingular-angular bundle | |||||
| Left | 0.37 (0.07) | 0.40 (0.07) | 0.37 (0.08) | 1.70 | 0.19 |
| Right | 0.39 (0.08) | 0.41 (0.07) | 0.38 (0.08) | 0.78 | 0.46 |
| Cingulum cingulate gyrus | |||||
| Left | 0.59 (0.10) | 0.61 (0.08) | 0.60 (0.10) | 0.37 | 0.69 |
| Right | 0.54 (0.07) | 0.54 (0.06) | 0.53 (0.08) | 0.12 | 0.88 |
| Cortico-spinal tract | |||||
| Left | 0.54 (0.04) | 0.54 (0.04) | 0.52 (0.06) | 1.61 | 0.21 |
| Right | 0.52 (0.04) | 0.53 (0.05) | 0.51 (0.05) | 0.87 | |
| Inferior longitudinal fasciculus | |||||
| Left | 0.48 (0.04) | 0.49 (0.05) | 0.46 (0.06) | 2.58 | 0.08 |
| Right | 0.48 (0.04) | 0.50 (0.05) | 0.46 (0.06) | 3.64 | 0.03* |
| Sup. longitudinal fasc. - parietal | |||||
| Left | 0.47 (0.05) | 0.48 (0.06) | 0.47 (0.06) | 0.17 | 0.84 |
| Right | 0.47 (0.05) | 0.47 (0.04) | 0.45 (0.06) | 1.34 | 0.27 |
| Sup. longitudinal fasc. – temp. | |||||
| Left | 0.49 (0.05) | 0.51 (0.04) | 0.48 (0.06) | 1.83 | 0.17 |
| Right | 0.47 (0.04) | 0.47 (0.05) | 0.46 (0.06) | 0.61 | 0.54 |
| Uncinate fasciculus | |||||
| Left | 0.37 (0.07) | 0.39 (0.07) | 0.37 (0.08) | 0.62 | 0.54 |
| Right | 0.38 (0.05) | 0.38 (0.07) | 0.38 (0.07) | 0.13 | 0.88 |
Bolded and asterisked values are statistically significant.
Fig. 2 depicts the z-scored (corrected) mean FA in all tracts across groups and shows a trend for SZ subjects to have lower FA compared to CON and BP participants. BP participants showed a trend towards higher tract FA compared to controls. An ANOVA comparing the 18 average tract z-scores across the three groups showed a significant effect (F = 4.35; p = .018). A post-hoc analysis showed significant average z-score differences between SZ and BP (p = .01), and trend level differences between SZ vs. CN (p = .08). CN vs. BP differences were not significant (p = .3).
Fig. 2.
Z-Scores of White Matter Tract Fractional Anisotropy obtained from TRACULA. Graphs depict corrected mean FA z-scores of each group's white matter tracts, obtained using TRACULA. Z-scores were corrected for gender and age, and subtracted from mean values in the control group. Figures (A) and (B) depict left and right hemispheric structures respectively, with the exception of the corpus callosum (CC) which have major and minor divisions. Plotted values represent means per group. Control = black. Schizophrenia = red. Bipolar disorder = blue. * p < .05.
3.2. TBSS analysis
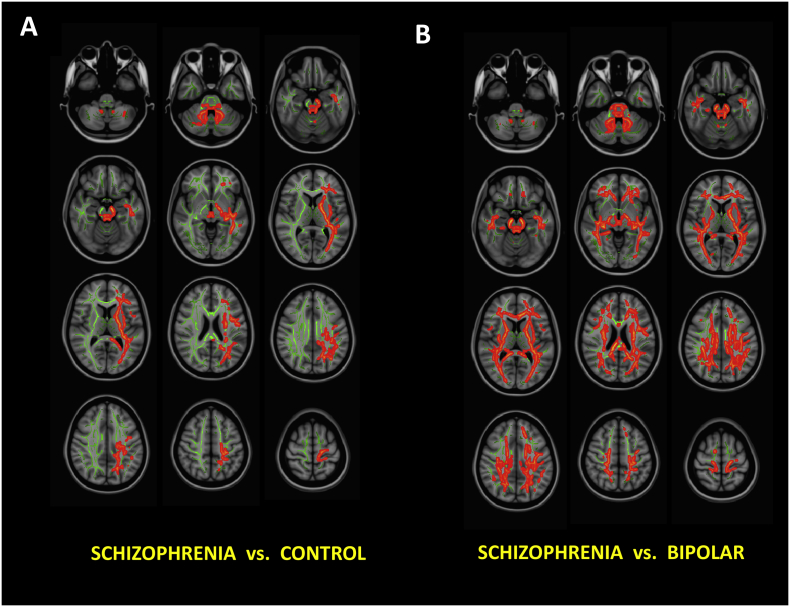
As shown in Fig. 3A, compared to control subjects, significantly decreased FA was observed in SZ patients largely in the left cerebral hemisphere, unilaterally distributed over the anterior thalamic radiation, posterior thalamic radiation, corticospinal tract and superior longitudinal fasciculi (FDR correction p < .05). Bilaterally decreased FA in the cerebellum was also observed in SZ patients compared to controls.
Fig. 3.
Voxel-based White Matter Fractional Anisotropy Group Comparisons Using TBSS. (A) Schizophrenia vs. Controls. (B) Schizophrenia vs. Bipolar Disorder. Red/orange regions are statistically significant FA differences after FDR correction. Bipolar Disorder vs. Control comparisons are not shown, as no region survived FDR correction.
Compared to controls, FA in bipolar disorder patients were not statistically different. Group FA differences between BP and SZ patients were bilateral and widespread, with decreased FA in SZ patients (Fig. 3B).
3.3. Clinical relationships: TRACULA
In order to assess the relationship between clinical variables and FA of TRACULA tracts, across BP and SZ participants, we performed separate stepwise linear regressions for chronic mania (mWERCAP), chronic psychosis (pWERCAP), stress (WERC Stress Screen), recent manic symptoms (YMRS), recent positive symptoms (SAPS), recent disorganization (SAPS), and negative symptoms (SANS), respectively. Among all white matter tracts, the regression for chronic mania identified only the anterior thalamic radiation (ATR) FA as predicting chronic mania severity, accounting for 19.9% of the variance (Β = 38.5; F = 13.7; p = .0005). A separate multiple regression showed only the cingulum-angular bundle (CAB) FA significantly predicted recent mania, accounting for 9.3% of the variance (B = 6.7; F = 5.66; p = .02). No tract predicted any other behavioral symptom across BP and SZ participants.
Multiple regression analyses were also conducted separately in BP participants (mWERCAP, YMRS) and SZ participants (pWERCAP, SAPS, SANS). In BP, the superior longitudinal fasciculus-temporal (SLF-T) FA predicted chronic mania with 12% of the variance (B = −23.9; F = 4.2; p = .049), and the CAB FA predicted recent mania with 12.9% of the variance (B = 9.7; F = 4.6; p = .04). In SZ, only the CAB FA predicted chronic psychosis with 23.5% of the variance (B = 29.5; F = 6.8; p = .016).
3.4. Clinical relationships: TBSS
We calculated the relationship between each behavioral measure with FA values across both BP and SZ participants.
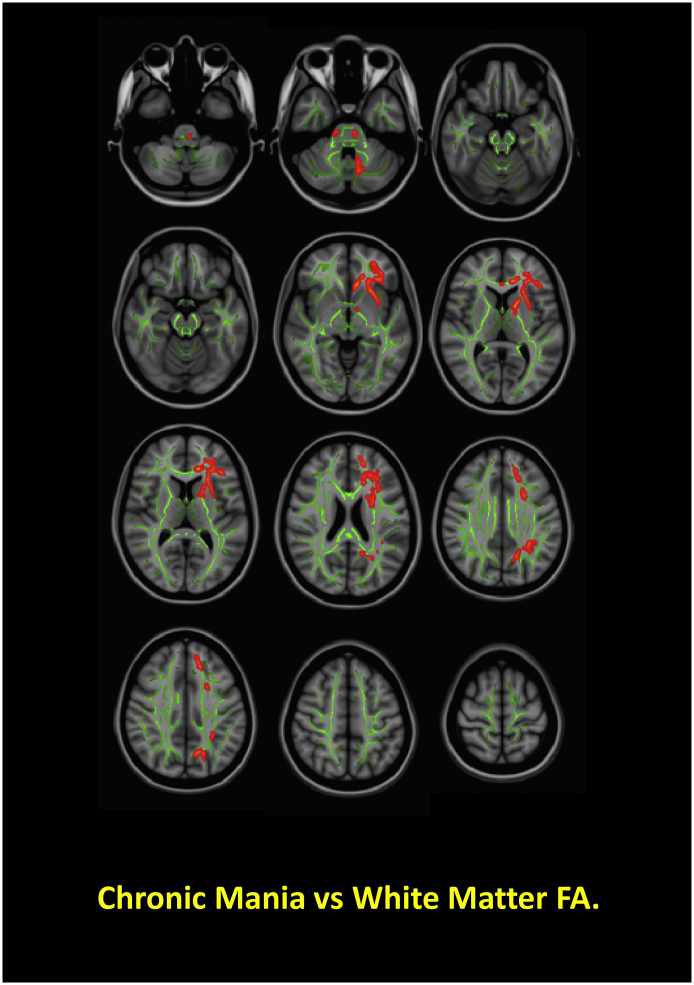
Among all behavioral measures, only chronic mania (mWERCAP) correlated with FA, specifically within the left anterior corpus callosum and left anterior thalamic radiation (Fig. 4). Increased FA was related to increased chronic mania severity.
Fig. 4.
Significant Clinical Relationships with White Matter Fractional Anisotropy in Patients. Figure depicts significant correlations of chronic mania, based on scores from the WERCAP Screen, and FA across bipolar disorder and schizophrenia patients. Red/orange regions show statistically significant FA relationships with chronic mania.
3.5. Medication relationships
Considering the highly heterogenous medications (and medication combinations) used across our patient populations, completely controlling for medications was not possible. We however investigated the effects of more commonly used medications that have been associated with structural brain changes in select patient groups: 1) typical antipsychotics vs. atypical antipsychotics vs. neither in SZ; 2) lithium vs. no lithium in BP; 3) atypical vs. no atypical in BP; and 4) SSRI/SNRI in BP.
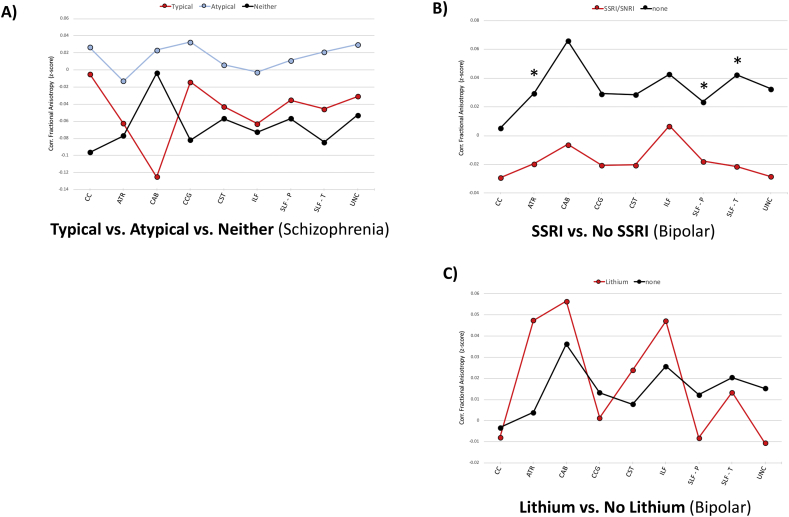
Fig. 5 shows results of individual comparisons and mean tract FA values across groups. Notably, the SSRI/SNRI group trended towards lower mean FA than the non-SSRI/SNRI group, with significant findings observed in the ATR (F = 6.8; p = .014); SLF-P (F = 4.3; p = .046); SLF-T (F = 4.7; p = .039). Group differences did not remain after correcting for multiple comparisons.
Fig. 5.
Medication Effects on TRACULA-Derived White Matter Tracts. Figures depict mean group FA z-scores, corrected for gender and age. Left and right tracks have been combined. (A) schizophrenia patients on either typical antipsychotics, atypical antipsychotics or neither; (B) bipolar disorder patients either on selective serotonin reuptake inhibitor (SSRI)/serotonin norepinephrine reuptake inhibitor (SNRI) or not; (C); bipolar disorder patients either on lithium or not. * p < .05.
4. Discussion
We investigated white matter integrity in the brain of patients with schizophrenia and bipolar disorder, scanned with an optimized diffusion imaging protocol identical to that used in the Human Connectome Project (Sotiropoulos et al., 2013) and processed using both an automated tract-based approach and a voxel-based approach. With both approaches, we found some significant reduction in white matter tract FA in schizophrenia compared to controls, and a trend towards increased FA in bipolar disorder without meeting statistical significance. The voxel-based approach (TBSS) was better at identifying significant group differences than the tract-based approach (TRACULA) across our participants, likely because the latter dilutes significant group effects if white matter abnormalities are not present across an entire tract. TRACULA did not identify significant group differences (after correcting for multiple comparisons), but showed a trend towards lower FA across several tracts in schizophrenia patients compared to the other participants, most notably in the left anterior thalamic radiation (ATR). TBSS however identified several white matter regions with significantly decreased FA in schizophrenia patients. With the exception of cerebellar white matter, these group differences involved primarily white matter of the left hemisphere. Affected regions included the left ATR, as well as the left posterior thalamic radiation, corticospinal tract and superior longitudinal fasciculus. White matter abnormality is usually found in schizophrenia patients, although with some variability in the affected tracts across studies (Arnedo et al., 2015; Karlsgodt, 2016; Kyriakopoulos et al., 2008; Mamah et al., 2010; Mulert et al., 2012; Peters et al., 2010; Peters and Karlsgodt, 2015; Vitolo et al., 2017; Wheeler and Voineskos, 2014). A recent meta-analysis of voxel-based morphometry and diffusion imaging studies indicated a widespread white matter disruption in schizophrenia, largely involving the long projection fibers, callosal and commissural fibers, part of motor descending fibers, and fronto-temporal-limbic pathways (Vitolo et al., 2017). Meta-analyses of diffusion imaging studies have also reported decreased FA in white coordinates mainly in the deep white matter of the left frontal and temporal brain regions (Ellison-Wright and Bullmore, 2009). Thus, our findings of widespread white matter involvement, predominantly on the left, is consistent with some prior studies.
We did not find lower FA for any tract in bipolar disorder patients, as observed in prior TRACULA studies (Ji et al., 2017; Sprooten et al., 2016). Rather, the FA of several tracts in the bipolar disorder group trended towards higher values compared to controls, most notably in the cingulum-angular bundle. TBSS analysis also did not show any significant abnormalities in bipolar participant, in contrast to that reported by most other groups (Nortje et al., 2013). Voxel-based studies generally report decreased FA in bipolar individuals in several broad tract categories: fronto-limbic (e.g. the cingulum bundle), interhemispheric (e.g. corpus collosum), and long-distance connections between association cortices (e.g. the superior longitudinal fasciculus) (Brambilla et al., 2001; Heng et al., 2010; Nortje et al., 2013). However, a few studies have reported increased white matter FA in bipolar disorder, suggesting increased directional coherence of certain white matter tracts in some patients (Mahon et al., 2009; Versace et al., 2008; Wessa et al., 2009). Some studies reporting normal white matter findings in bipolar disorder have involved younger or unmedicated subjects (Teixeira et al., 2014), and young adult patients often lack the cortical thinning seen in older cohorts (Mamah et al., 2016a), suggesting structural abnormalities progress significantly in some bipolar patients. In addition to the potential role of age, medications likely also influence structural connectivity in bipolar disorder patients. For example, acute antipsychotic use has been associated with increased frontal lobe intracortical myelin volume, while chronic use has been reported as having the opposite effect (Bartzokis et al., 2012; Ho et al., 2011). Decreased cingulate and frontal white matter FA in medication naïve bipolar patients after six weeks of risperidone treatment has also been reported (Wang et al., 2013). In our limited analysis of medication effects, bipolar disorder patients on SSRI-type antidepressants had lower tract FA than those not taking these antidepressants. This may indicate an effect of SSRI on reducing white matter integrity in some tracts. Antidepressant treatment has been associated with improved structural connectivity (Fan et al., 2012; Lai et al., 2013; Lugo-Candelas et al., 2018; Zeng et al., 2012), however decreased white matter structure has also been reported with treatment (Steffens et al., 2008; Zeng et al., 2012). Our results suggest that differential antidepressant use may contribute to variability of findings across studies of white matter in bipolar disorder. In the future, longitudinal diffusion imaging studies of bipolar disorder will be needed to clarify the developmental trajectory of white matter tracts in this disorder and specific medication influences.
A multiple regression identified the cingulum-angular bundle (CAB) FA as predicting recent mania severity in bipolar patients alone, as well as across both patient groups. Interestingly, the CAB FA also predicted chronic psychotic symptoms in patients. In both cases, increased FA in the CAB correlated with increased symptoms. The CAB represents the posterior part of the cingulum, which extends between the posterior cingulate gyrus and the parahippocampus and uncus of the temporal lobe (Jones et al., 2013). Fibers within this tract have been associated with cognitive functions, conveying memory information from the hippocampus and integrating it with other parts of the brain (Ezzati et al., 2016). Decreased structural connectivity of the cingulum, primarily in its anterior part, has been associated with bipolar disorder (Ji et al., 2017; Wang et al., 2008), and with mania (Martino et al., 2016). Our study is the first to our knowledge reporting a positive correlation between cingulum integrity and manic severity, and psychosis in schizophrenia. While speculative, this may reflect enhanced fiber coherence related to prolonged hippocampal and/or amygdala hyperactivity, which has been associated with bipolar disorder (Heckers and Konradi, 2015; Tregellas et al., 2014). Decreased FA in the temporal tract of the superior longitudinal fasciculus (SLF-T) also predicted chronic affective symptoms in bipolar patients suggesting that impaired integrity of this tract may be related to the neurobiology of the illness. The SLF is a major association tract in the brain that connects frontal, occipital, parietal and temporal lobes of the brain, and was the only white matter tract shown to have decreased FA in both existing TRACULA studies of bipolar disorder (Ji et al., 2017; Sprooten et al., 2016). Specifically, the impaired SLF-T integrity was also related to psychotic symptoms in one of these studies (Ji et al., 2017), suggesting this tract may be a marker of illness severity and not of a specific symptom.
The implications of FA abnormalities in disease has been well described. FA measures the asymmetry in the direction of diffusion of water. To the extent that white matter fiber tracts are structurally intact and healthy, the diffusion of water will be dominated by a coherent direction down the path of the fiber tract (Beaulieu, 2002). Axonal degradation, demyelination, and neurodegenerative effects however would be expected to produce a disruption in the coherence of water diffusion and consequently a decrease in the FA. Such findings appear to be largely related to underlying neurobiology of schizophrenia, considering low FA is often seen in medication naïve patients (Cheung et al., 2008, Cheung et al., 2011; Filippi et al., 2014; Gasparotti et al., 2009; Guo et al., 2012; Perez-Iglesias et al., 2010; Sun et al., 2015) and unaffected siblings or high-risk individuals (Epstein et al., 2014; Peters et al., 2009; von Hohenberg et al., 2014; Zhou et al., 2017). Nevertheless, the effects of confounding factors, including medications and substance use which are prevalent in these patients, may influence the pattern of white matter architecture. Differences in scan acquisition, motion correction, and analysis methods could also contribute to variability across studies. Beyond technical issues and external factors, the biological heterogeneity of schizophrenia likely manifest in differential patterns of brain connectivity, sometimes with differential symptomatology (Arnedo et al., 2015; Sun et al., 2015). For example, by unsupervised biclustering of TBSS-derived white matter data, Arnedo et al. (2015) identified four general patterns of low FA among schizophrenia subjects, predominantly involving the genu of the corpus callosum; fornix and external capsule; splenium of the corpus callosum; and anterior limb of the internal capsule respectively. Along these lines, Sun et al. (2015) applied hierarchical clustering of 36 features from fiber tracts in schizophrenia patients and found two patterns of abnormalities: one showing widespread white matter abnormalities and another with primarily superior longitudinal fasciculi abnormalities. Thus, it is likely that any given cohort of schizophrenia patients comprises of a highly heterogenous combination of participants, making broad conclusions about specific schizophrenia abnormalities impractical.
There are some limitations in our ability to interpret and extend upon the current findings. Due to the cross-sectional nature of the current study, we cannot address questions of causality in the relationship of observed white matter fractional anisotropy abnormalities. Therefore, this study is unable to address the time course of FA changes with bipolar disorder and cannot specifically rule out the effects of various forms of treatment over the course of the disease. As alluded to above, we also cannot account for all of the potential medication effects on white matter integrity in the current sample, as our study was not well powered to investigate medication effects. For example, many of our patients were taking various mood stabilizers, which could potentially have influenced structural connectivity. Specifically, the neuroprotective effects of mood stabilizing pharmaceuticals may counteract impaired connectivity results differentially throughout the brain in various samples. Lithium, for example, has been associated with the regulation of cell death as well as up-regulation of proteins responsible for axonal myelination, a vital property of cell growth that would directly affect measures of white matter integrity (Chen et al., 1999; Manji et al., 2000; Nonaka et al., 1998). Antipsychotic medications, used in the treatment of schizophrenia and often bipolar disorder, have been associated with decreased white matter FA (Szeszko et al., 2014; Wang et al., 2013) and volume (Girgis et al., 2006; Ho et al., 2011), possibly from alterations in glial cell composition or number (Szeszko et al., 2014). Indirectly, white matter connectivity could also be affected indirectly, though medication effects on cortical gray matter size (Lieberman et al., 2005). Finally, step-wise linear regression may perform poorly with the number of predictors included in cases of collinearity and could give biased coefficients that need shrinkage (Tibshirani, 1996).
In summary, we investigated white matter integrity in schizophrenia and bipolar disorder using two complementary approaches. Both approaches showed reduced white matter FA in schizophrenia, with a trend towards increased FA in bipolar disorder, compared to controls. Specifically, we found decreased FA in multiple, primarily left sided, white matter regions in schizophrenia using TBSS, a voxel-based approach. A tract-based methodology, TRACULA, showed a trend towards lower FA in multiple tracts in schizophrenia, and a trend towards higher FA in some tracts in bipolar disorder. FA in the cingulum-angular bundle predicted chronic mania, and in the superior longitudinal fasciculus predicted recent mania. These studies build on existing studies of psychiatric disorders and show decreased white matter integrity in schizophrenia. Medications, drug use, age and biological heterogeneity are likely confounds, and may have contributed to absence of findings in bipolar patients. Future studies using larger groups are needed, and longitudinal investigations of effects of external factors on structural brain connectivity.
Funding
This work was funded by NIMH grant R01 MH104414. Additionally, Dr. Mamah has received funding from Taylor Foundation Institute, Dept. Psychiatry, Washington University and the Center for Brain Research on Mood Disorders, Dept. Psychiatry, Washington University. Research reported in this publication was also supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University.
References
- Adler C.M., Holland S.K., Schmithorst V., Wilke M., Weiss K.L., Pan H., Strakowski S.M. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;6:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- Adler C.M., Adams J., DelBello M.P., Holland S.K., Schmithorst V., Levine A., Jarvis K., Strakowski S.M. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am. J. Psychiatry. 2006;163:322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Arndt S., Alliger R., Miller D., Flaum M. Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch. Gen. Psychiatry. 1995;52:341–351. doi: 10.1001/archpsyc.1995.03950170015003. [DOI] [PubMed] [Google Scholar]
- Arnedo J., Mamah D., Baranger D.A., Harms M.P., Barch D.M., Svrakic D.M., de Erausquin G.A., Cloninger C.R., Zwir I. Decomposition of brain diffusion imaging data uncovers latent schizophrenias with distinct patterns of white matter anisotropy. NeuroImage. 2015;120:43–54. doi: 10.1016/j.neuroimage.2015.06.083. [DOI] [PubMed] [Google Scholar]
- Bartzokis G., Lu P.H., Raven E.P., Amar C.P., Detore N.R., Couvrette A.J., Mintz J., Ventura J., Casaus L.R., Luo J.S., Subotnik K.L., Nuechterlein K.H. Impact on intracortical myelination trajectory of long acting injection versus oral risperidone in first-episode schizophrenia. Schizophr. Res. 2012;140:122–128. doi: 10.1016/j.schres.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beyer J.L., Taylor W.D., MacFall J.R., Kuchibhatla M., Payne M.E., Provenzale J.M., Cassidy F., Krishnan K.R. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30:2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- Brambilla P., Harenski K., Nicoletti M., Mallinger A.G., Frank E., Kupfer D.J., Keshavan M.S., Soares J.C. Differential effects of age on brain gray matter in bipolar patients and healthy individuals. Neuropsychobiology. 2001;43:242–247. doi: 10.1159/000054897. [DOI] [PubMed] [Google Scholar]
- Bruno S., Cercignani M., Ron M.A. White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar Disord. 2008;10:460–468. doi: 10.1111/j.1399-5618.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Cao H., Plichta M.M., Schafer A., Haddad L., Grimm O., Schneider M., Esslinger C., Kirsch P., Meyer-Lindenberg A., Tost H. Test-retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. NeuroImage. 2014;84:888–900. doi: 10.1016/j.neuroimage.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Chen B., Wang J.F., Hill B.C., Young L.T. Lithium and valproate differentially regulate brain regional expression of phosphorylated CREB and c-Fos. Brain Res. Mol. Brain Res. 1999;70:45–53. doi: 10.1016/s0169-328x(99)00125-4. [DOI] [PubMed] [Google Scholar]
- Cheung V., Cheung C., McAlonan G.M., Deng Y., Wong J.G., Yip L., Tai K.S., Khong P.L., Sham P., Chua S.E. A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol. Med. 2008;38:877–885. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- Cheung V., Chiu C.P., Law C.W., Cheung C., Hui C.L., Chan K.K., Sham P.C., Deng M.Y., Tai K.S., Khong P.L., McAlonan G.M., Chua S.E., Chen E. Positive symptoms and white matter microstructure in never-medicated first episode schizophrenia. Psychol. Med. 2011;41:1709–1719. doi: 10.1017/S003329171000156X. [DOI] [PubMed] [Google Scholar]
- Collins D.L., Holmes C.J., Peters T.M., Evans A.C. Automatic 3-D model-based neuroanatomical segmentation. Hum. Brain Mapp. 2004;3:190–208. [Google Scholar]
- Coryell W., Leon A.C., Turvey C., Akiskal H.S., Mueller T., Endicott J. The significance of psychotic features in manic episodes: a report from the NIMH collaborative study. J. Affect. Disord. 2001;67:79–88. doi: 10.1016/s0165-0327(99)00024-5. [DOI] [PubMed] [Google Scholar]
- Eaton W.W., Martins S.S., Nestadt G., Bienvenu O.J., Clarke D., Alexandre P. The burden of mental disorders. Epidemiol. Rev. 2008;30:1–14. doi: 10.1093/epirev/mxn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Epstein K.A., Cullen K.R., Mueller B.A., Robinson P., Lee S., Kumra S. White matter abnormalities and cognitive impairment in early-onset schizophrenia-spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(362–372):e361–e362. doi: 10.1016/j.jaac.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A., Katz M.J., Lipton M.L., Zimmerman M.E., Lipton R.B. Hippocampal volume and cingulum bundle fractional anisotropy are independently associated with verbal memory in older adults. Brain Imaging Behav. 2016;10:652–659. doi: 10.1007/s11682-015-9452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q., Yan X., Wang J., Chen Y., Wang X., Li C., Tan L., You C., Zhang T., Zuo S., Xu D., Chen K., Finlayson-Burden J.M., Xiao Z. Abnormalities of white matter microstructure in unmedicated obsessive-compulsive disorder and changes after medication. PLoS One. 2012;7:e35889. doi: 10.1371/journal.pone.0035889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M., Canu E., Gasparotti R., Agosta F., Valsecchi P., Lodoli G., Galluzzo A., Comi G., Sacchetti E. Patterns of brain structural changes in first-contact, antipsychotic drug-naive patients with schizophrenia. AJNR Am. J. Neuroradiol. 2014;35:30–37. doi: 10.3174/ajnr.A3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams Janet B.W. American Psychiatric Publishing; Washington, DC: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) [Google Scholar]
- Gasparotti R., Valsecchi P., Carletti F., Galluzzo A., Liserre R., Cesana B., Sacchetti E. Reduced fractional anisotropy of corpus callosum in first-contact, antipsychotic drug-naive patients with schizophrenia. Schizophr. Res. 2009;108:41–48. doi: 10.1016/j.schres.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Girgis R.R., Diwadkar V.A., Nutche J.J., Sweeney J.A., Keshavan M.S., Hardan A.Y. Risperidone in first-episode psychosis: a longitudinal, exploratory voxel-based morphometric study. Schizophr. Res. 2006;82:89–94. doi: 10.1016/j.schres.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M., Consortium W.U.-M.H. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goes F.S., Zandi P.P., Miao K., McMahon F.J., Steele J., Willour V.L., Mackinnon D.F., Mondimore F.M., Schweizer B., Nurnberger J.I., Jr., Rice J.P., Scheftner W., Coryell W., Berrettini W.H., Kelsoe J.R., Byerley W., Murphy D.L., Gershon E.S., Bipolar Disorder Phenome Group, Depaulo J.R., Jr., McInnis M.G., Potash J.B. Mood-incongruent psychotic features in bipolar disorder: familial aggregation and suggestive linkage to 2p11-q14 and 13q21–33. Am. J. Psychiatry. 2007;164:236–247. doi: 10.1176/ajp.2007.164.2.236. [DOI] [PubMed] [Google Scholar]
- Guo W., Liu F., Liu Z., Gao K., Xiao C., Chen H., Zhao J. Right lateralized white matter abnormalities in first-episode, drug-naive paranoid schizophrenia. Neurosci. Lett. 2012;531:5–9. doi: 10.1016/j.neulet.2012.09.033. [DOI] [PubMed] [Google Scholar]
- Heckers S., Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr. Res. 2015;167:4–11. doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng S., Song A.W., Sim K. White matter abnormalities in bipolar disorder: insights from diffusion tensor imaging studies. J. Neural Transm. (Vienna) 2010;117:639–654. doi: 10.1007/s00702-010-0368-9. [DOI] [PubMed] [Google Scholar]
- Hernandez M., Guerrero G.D., Cecilia J.M., Garcia J.M., Inuggi A., Jbabdi S., Behrens T.E., Sotiropoulos S.N. Accelerating fibre orientation estimation from diffusion weighted magnetic resonance imaging using GPUs. PLoS One. 2013;8:e61892. doi: 10.1371/journal.pone.0061892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho B.C., Andreasen N.C., Ziebell S., Pierson R., Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C.J., Godwin D., Mamah D. Utility of Washington early recognition center self-report screening questionnaires in the assessment of patients with schizophrenia and bipolar disorder. Front. Psychiatry. 2016;7:149. doi: 10.3389/fpsyt.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S., Woolrich M.W., Andersson J.L., Behrens T.E. A Bayesian framework for global tractography. NeuroImage. 2007;37:116–129. doi: 10.1016/j.neuroimage.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Ji A., Godwin D., Rutlin J., Kandala S., Shimony J.S., Mamah D. Tract-based analysis of white matter integrity in psychotic and nonpsychotic bipolar disorder. J. Affect. Disord. 2017;209:124–134. doi: 10.1016/j.jad.2016.11.038. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Symms M.R., Cercignani M., Howard R.J. The effect of filter size on VBM analyses of DT-MRI data. NeuroImage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Christiansen K.F., Chapman R.J., Aggleton J.P. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia. 2013;51:67–78. doi: 10.1016/j.neuropsychologia.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt K.H. Diffusion imaging of white matter in schizophrenia: progress and future directions. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1:209–217. doi: 10.1016/j.bpsc.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., McGuire M., Gruenberg A.M., Spellman M., O'Hare A., Walsh D. The Roscommon Family Study. II. The risk of nonschizophrenic nonaffective psychoses in relatives. Arch. Gen. Psychiatry. 1993;50:645–652. doi: 10.1001/archpsyc.1993.01820200059006. [DOI] [PubMed] [Google Scholar]
- Kochunov P., Hong L.E. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr. Bull. 2014;40:721–728. doi: 10.1093/schbul/sbu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos M., Vyas N.S., Barker G.J., Chitnis X.A., Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol. Psychiatry. 2008;63:519–523. doi: 10.1016/j.biopsych.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Lai C.H., Wu Y.T., Yu P.L., Yuan W. Improvements in white matter micro-structural integrity of right uncinate fasciculus and left fronto-occipital fasciculus of remitted first-episode medication-naive panic disorder patients. J. Affect. Disord. 2013;150:330–336. doi: 10.1016/j.jad.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Lieberman J.A., Tollefson G.D., Charles C., Zipursky R., Sharma T., Kahn R.S., Keefe R.S., Green A.I., Gur R.E., McEvoy J., Perkins D., Hamer R.M., Gu H., Tohen M., Group, H.S Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch. Gen. Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Lugo-Candelas C., Cha J., Hong S., Bastidas V., Weissman M., Fifer W.P., Myers M., Talati A., Bansal R., Peterson B.S., Monk C., Gingrich J.A., Posner J. Associations between brain structure and connectivity in infants and exposure to selective serotonin reuptake inhibitors during pregnancy. JAMA Pediatr. 2018;172:525–533. doi: 10.1001/jamapediatrics.2017.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon K., Wu J., Malhotra A.K., Burdick K.E., DeRosse P., Ardekani B.A., Szeszko P.R. A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology. 2009;34:1590–1600. doi: 10.1038/npp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D., Conturo T.E., Harms M.P., Akbudak E., Wang L., McMichael A.R., Gado M.H., Barch D.M., Csernansky J.G. Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry Res. 2010;183:144–150. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D., Owoso A., Sheffield J.M., Bayer C. The WERCAP screen and the WERC stress screen: psychometrics of self-rated instruments for assessing bipolar and psychotic disorder risk and perceived stress burden. Compr. Psychiatry. 2014;55:1757–1771. doi: 10.1016/j.comppsych.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Mamah D., Alpert K.I., Barch D.M., Csernansky J.G., Wang L. Subcortical neuromorphometry in schizophrenia spectrum and bipolar disorders. Neuroimage Clin. 2016;11:276–286. doi: 10.1016/j.nicl.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D., Musau A., Mutiso V.N., Owoso A., Abdallah A.B., Cottler L.B., Striley C.W., Walker E.F., Ndetei D.M. Characterizing psychosis risk traits in Africa: a longitudinal study of Kenyan adolescents. Schizophr. Res. 2016;176:340–348. doi: 10.1016/j.schres.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Manji H.K., Moore G.J., Chen G. Lithium up-regulates the cytoprotective protein Bcl-2 in the CNS in vivo: a role for neurotrophic and neuroprotective effects in manic depressive illness. J. Clin. Psychiatry. 2000;61(Suppl. 9):82–96. [PubMed] [Google Scholar]
- Martino M., Magioncalda P., Saiote C., Conio B., Escelsior A., Rocchi G., Piaggio N., Marozzi V., Huang Z., Ferri F., Amore M., Inglese M., Northoff G. Abnormal functional-structural cingulum connectivity in mania: combined functional magnetic resonance imaging-diffusion tensor imaging investigation in different phases of bipolar disorder. Acta Psychiatr. Scand. 2016;134:339–349. doi: 10.1111/acps.12596. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Chung S.W., Berman J.I., Hess C.P., Henry R.G. Diffusion tensor MR imaging and fiber tractography: technical considerations. AJNR Am. J. Neuroradiol. 2008;29:843–852. doi: 10.3174/ajnr.A1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulert C., Kirsch V., Whitford T.J., Alvarado J., Pelavin P., McCarley R.W., Kubicki M., Salisbury D.F., Shenton M.E. Hearing voices: a role of interhemispheric auditory connectivity? World J. Biol. Psychiatry. 2012;13:153–158. doi: 10.3109/15622975.2011.570789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S., Katsube N., Chuang D.M. Lithium protects rat cerebellar granule cells against apoptosis induced by anticonvulsants, phenytoin and carbamazepine. J. Pharmacol. Exp. Ther. 1998;286:539–547. [PubMed] [Google Scholar]
- Nortje G., Stein D.J., Radua J., Mataix-Cols D., Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J. Affect. Disord. 2013;150:192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- Park N., Juo S.H., Cheng R., Liu J., Loth J.E., Lilliston B., Nee J., Grunn A., Kanyas K., Lerer B., Endicott J., Gilliam T.C., Baron M. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol. Psychiatry. 2004;9:1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R., Tordesillas-Gutierrez D., Barker G.J., McGuire P.K., Roiz-Santianez R., Mata I., de Lucas E.M., Quintana F., Vazquez-Barquero J.L., Crespo-Facorro B. White matter defects in first episode psychosis patients: a voxelwise analysis of diffusion tensor imaging. NeuroImage. 2010;49:199–204. doi: 10.1016/j.neuroimage.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Peters B.D., Karlsgodt K.H. White matter development in the early stages of psychosis. Schizophr. Res. 2015;161:61–69. doi: 10.1016/j.schres.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B.D., Schmitz N., Dingemans P.M., van Amelsvoort T.A., Linszen D.H., de Haan L., Majoie C.B., den Heeten G.J. Preliminary evidence for reduced frontal white matter integrity in subjects at ultra-high-risk for psychosis. Schizophr. Res. 2009;111:192–193. doi: 10.1016/j.schres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Peters B.D., Blaas J., de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned? J. Psychiatr. Res. 2010;44:993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Potash J.B., Zandi P.P., Willour V.L., Lan T.H., Huo Y., Avramopoulos D., Shugart Y.Y., MacKinnon D.F., Simpson S.G., McMahon F.J., DePaulo J.R., Jr., McInnis M.G. Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic bipolar disorder. Am. J. Psychiatry. 2003;160:680–686. doi: 10.1176/appi.ajp.160.4.680. [DOI] [PubMed] [Google Scholar]
- Sexton C.E., Mackay C.E., Ebmeier K.P. A systematic review of diffusion tensor imaging studies in affective disorders. Biol. Psychiatry. 2009;66:814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Beckmann C.F., Andersson J., Auerbach E.J., Bijsterbosch J., Douaud G., Duff E., Feinberg D.A., Griffanti L., Harms M.P., Kelly M., Laumann T., Miller K.L., Moeller S., Petersen S., Power J., Salimi-Khorshidi G., Snyder A.Z., Vu A.T., Woolrich M.W., Xu J., Yacoub E., Ugurbil K., Van Essen D.C., Glasser M.F., Consortium W.U.-M.H. Resting-state fMRI in the Human Connectome Project. NeuroImage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J.M., Marques P., Alves V., Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front. Neurosci. 2013;7:31. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiropoulos S.N., Jbabdi S., Xu J., Andersson J.L., Moeller S., Auerbach E.J., Glasser M.F., Hernandez M., Sapiro G., Jenkinson M., Feinberg D.A., Yacoub E., Lenglet C., Van Essen D.C., Ugurbil K., Behrens T.E., Consortium W.U.-M.H. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. NeuroImage. 2013;80:125–143. doi: 10.1016/j.neuroimage.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten E., Barrett J., McKay D.R., Knowles E.E., Mathias S.R., Winkler A.M., Brumbaugh M.S., Landau S., Cyr L., Kochunov P., Glahn D.C. A comprehensive tractography study of patients with bipolar disorder and their unaffected siblings. Hum. Brain Mapp. 2016;37:3474–3485. doi: 10.1002/hbm.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens D.C., Chung H., Krishnan K.R., Longstreth W.T., Jr., Carlson M., Burke G.L. Antidepressant treatment and worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2008;39:857–862. doi: 10.1161/STROKEAHA.107.498097. [DOI] [PubMed] [Google Scholar]
- Sun H., Lui S., Yao L., Deng W., Xiao Y., Zhang W., Huang X., Hu J., Bi F., Li T., Sweeney J.A., Gong Q. Two patterns of white matter abnormalities in medication-naive patients with first-episode schizophrenia revealed by diffusion tensor imaging and cluster analysis. JAMA Psychiatry. 2015;72:678–686. doi: 10.1001/jamapsychiatry.2015.0505. [DOI] [PubMed] [Google Scholar]
- Szeszko P.R., Robinson D.G., Ikuta T., Peters B.D., Gallego J.A., Kane J., Malhotra A.K. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology. 2014;39:1324–1331. doi: 10.1038/npp.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira A.M., Kleinman A., Zanetti M., Jackowski M., Duran F., Pereira F., Lafer B., Busatto G.F., Caetano S.C. Preserved white matter in unmedicated pediatric bipolar disorder. Neurosci. Lett. 2014;579:41–45. doi: 10.1016/j.neulet.2014.06.061. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. Regression shrinkage and selection via the Lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996;58:267–288. [Google Scholar]
- Tregellas J.R., Smucny J., Harris J.G., Olincy A., Maharajh K., Kronberg E., Eichman L.C., Lyons E., Freedman R. Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am. J. Psychiatry. 2014;171:549–556. doi: 10.1176/appi.ajp.2013.13070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C., Ugurbil K., Auerbach E., Barch D., Behrens T.E., Bucholz R., Chang A., Chen L., Corbetta M., Curtiss S.W., Della Penna S., Feinberg D., Glasser M.F., Harel N., Heath A.C., Larson-Prior L., Marcus D., Michalareas G., Moeller S., Oostenveld R., Petersen S.E., Prior F., Schlaggar B.L., Smith S.M., Snyder A.Z., Xu J., Yacoub E., Consortium W.U.-M.H. The Human Connectome Project: a data acquisition perspective. NeuroImage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A., Almeida J.R., Hassel S., Walsh N.D., Novelli M., Klein C.R., Kupfer D.J., Phillips M.L. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch. Gen. Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo E., Tatu M.K., Pignolo C., Cauda F., Costa T., Ando A., Zennaro A. White matter and schizophrenia: a meta-analysis of voxel-based morphometry and diffusion tensor imaging studies. Psychiatry Res. Neuroimaging. 2017;270:8–21. doi: 10.1016/j.pscychresns.2017.09.014. [DOI] [PubMed] [Google Scholar]
- von Hohenberg C.C., Pasternak O., Kubicki M., Ballinger T., Vu M.A., Swisher T., Green K., Giwerc M., Dahlben B., Goldstein J.M., Woo T.U., Petryshen T.L., Mesholam-Gately R.I., Woodberry K.A., Thermenos H.W., Mulert C., McCarley R.W., Seidman L.J., Shenton M.E. White matter microstructure in individuals at clinical high risk of psychosis: a whole-brain diffusion tensor imaging study. Schizophr. Bull. 2014;40:895–903. doi: 10.1093/schbul/sbt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Jackowski M., Kalmar J.H., Chepenik L.G., Tie K., Qiu M., Gong G., Pittman B.P., Jones M.M., Shah M.P., Spencer L., Papademetris X., Constable R.T., Blumberg H.P. Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. Br. J. Psychiatry. 2008;193:126–129. doi: 10.1192/bjp.bp.107.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Cheung C., Deng W., Li M., Huang C., Ma X., Wang Y., Jiang L., Sham P.C., Collier D.A., Gong Q., Chua S.E., McAlonan G.M., Li T. White-matter microstructure in previously drug-naive patients with schizophrenia after 6 weeks of treatment. Psychol. Med. 2013;43:2301–2309. doi: 10.1017/S0033291713000238. [DOI] [PubMed] [Google Scholar]
- Wessa M., Houenou J., Leboyer M., Chanraud S., Poupon C., Martinot J.L., Paillere-Martinot M.L. Microstructural white matter changes in euthymic bipolar patients: a whole-brain diffusion tensor imaging study. Bipolar Disord. 2009;11:504–514. doi: 10.1111/j.1399-5618.2009.00718.x. [DOI] [PubMed] [Google Scholar]
- Wheeler A.L., Voineskos A.N. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front. Hum. Neurosci. 2014;8:653. doi: 10.3389/fnhum.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A., Panneck P., Srinivasan P., Stevens A., Zollei L., Augustinack J., Wang R., Salat D., Ehrlich S., Behrens T., Jbabdi S., Gollub R., Fischl B. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinform. 2011;5:23. doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D.A., Silveri M.M., Gruber S.A., Rohan M.L., Pimentel P.J. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007;9:504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- Zeng L.L., Liu L., Liu Y., Shen H., Li Y., Hu D. Antidepressant treatment normalizes white matter volume in patients with major depression. PLoS One. 2012;7:e44248. doi: 10.1371/journal.pone.0044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liu J., Driesen N., Womer F., Chen K., Wang Y., Jiang X., Zhou Q., Bai C., Wang D., Tang Y., Wang F. White matter integrity in genetic high-risk individuals and first-episode schizophrenia patients: similarities and disassociations. Biomed. Res. Int. 2017;2017:3107845. doi: 10.1155/2017/3107845. [DOI] [PMC free article] [PubMed] [Google Scholar]