Abstract
The systematics of Oriental freshwater mussels (Bivalvia: Unionidae) is poorly known. Here, we present an integrative revision of the genus Trapezoideus Simpson, 1900 to further understanding of freshwater mussel diversity in the region. We demonstrate that Trapezoideus as currently circumscribed is non-monophyletic, with its former species belonging to six other genera, one of which is new to science and described here. We recognize Trapezoideus as a monotypic genus, comprised of the type species, T. foliaceus. Trapezoideus comptus, T. misellus, T. pallegoixi, and T. peninsularis are transferred to the genus Contradens, T. subclathratus is moved to Indonaia, and T. theca is transferred to Lamellidens. Trapezoideus prashadi is found to be a junior synonym of Arcidopsis footei. Trapezoideus dallianus, T. nesemanni, T. panhai, T. peguensis, and two species new to science are placed in Yaukthwa gen. nov. This genus appears to be endemic of the Western Indochina Subregion. The two new species, Yaukthwa paiensis sp. nov. and Y. inlenensis sp. nov., are both endemic to the Salween River basin. Our results highlight that Southeast Asia is a species-rich freshwater mussel diversity hotspot with numerous local endemic species, which are in need of special conservation efforts.
Introduction
Freshwater mussels (Unionoida) are a diverse and globally distributed clade1,2. There are two major freshwater mussel biodiversity hotspots, i.e. the Southeastern USA and East, South and Southeast Asia3,4. In comparison to the Southeastern USA, Asian freshwater mussel diversity is very poorly understood3. Several recent phylogenetic studies have substantially revised the taxonomy, morphological evolution, and historical biogeography of Asian freshwater mussels4–13 but many taxa remain poorly characterized. The genus Trapezoideus Simpson, 1900 has been included in several recent phylogenetic studies5,8,12–14 but taxon sampling within the genus remains largely incomplete, and no published study has yet to include the type species Unio foliaceus Gould, 184314.
Konopleva et al.14 recently demonstrated that Trapezoideus was non-monophyletic with some of its representatives belonging to the subfamily Parreysiinae and other belonging to the Rectidentinae. Bolotov et al.8 revised the taxonomy of these two clades, describing the new genus Trapezidens (Parreysiinae: Lamellidentini) for the Unio exolescens group and suggested that Trapezoideus s. str. consisted of six species and was endemic to the rivers of western Indochina. However, that circumscription of Trapezoideus s. str. was based on morphological studies of the type species and an incomplete sample of other species previously attributed to Trapezoideus.
Recent collections of putative Unio foliaceus and several other species previously assigned to Trapezoideus (Trapezoideus comptus, T. misellus, T. pallegoixi, and T. subclathratus), as well as several morphologically similar specimens collected from the poorly characterized Salween River provide the basis for a more robust revision of the genus Trapezoideus and the tribe Contradentini more generally.
Results
Polyphyly of the genus Trapezoideus
Our multi-locus phylogeny based on the mitochondrial cytochrome c oxidase subunit I (COI), small ribosomal RNA (16 S rRNA), and the nuclear large ribosomal RNA (28 S rRNA) gene fragments clearly indicates that the genus Trapezoideus sensu Bolotov et al. 2017 in its current understanding is a polyphyletic entity (Fig. 1; Supplementary Table 1). Trapezoideus foliaceus, the type species of this genus, represents a separate phylogenetic lineage within the tribe Contradentini. Five species from western Indochina, i.e. Trapezoideus panhai, T. nesemanni, T. cf. dallianus [=T. subclathratus sensu Bolotov et al.8], and two undescribed species, form a well-supported and distinct genus-level clade, Yaukthwa gen. nov. (BPP/BS = 100/97), the range of which covers the Ayeyarwady, Sittaung and Salween river drainages (Fig. 2). Three other species from the Mekong Basin, i.e. Trapezoideus misellus, T. comptus, and T pallegoixi, cluster together with the members of the genus Contradens. Finally, the sequenced topotype specimens of Trapezoideus subclathratus belong to the Parreysiinae and this species was found to be a member of the genus Indonaia.
Figure 1.
Fifty-percent majority rule consensus phylogenetic tree recovered from Bayesian inference analysis of the complete data set of mitochondrial and nuclear sequences of the Unionidae species (five partitions: three codons of COI + 16 S rRNA + 28 S rRNA). Margaritifera dahurica and Gibbosula laosensis were used as an outgroup (not shown). Scale bar indicates the branch lengths. Black numbers near nodes are Bayesian posterior probabilities/ML bootstrap support values. The taxa previously assigned to Trapezoideus are colored red to highlight their non-monophyly. The two species new to science are colored blue.
Figure 2.
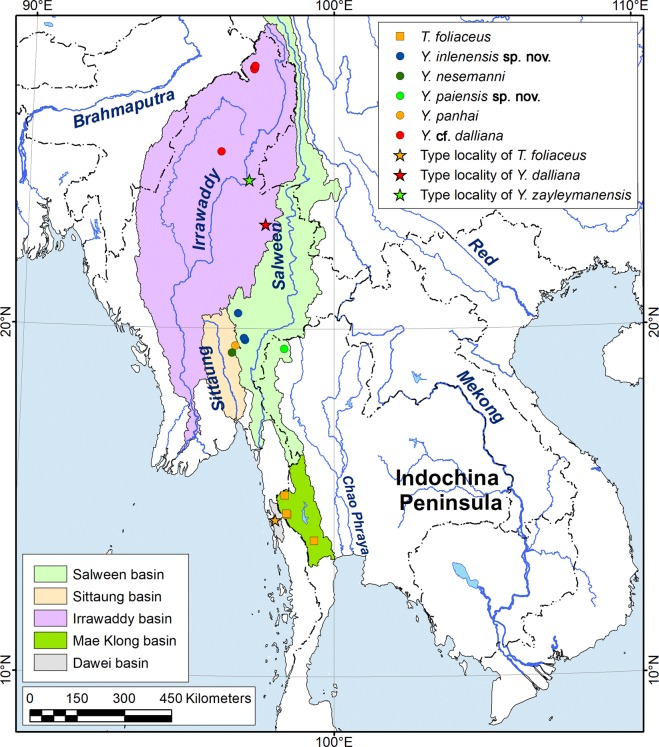
Distribution ranges of Trapezoideus foliaceus and species in the genus Yaukthwa gen. nov. The corresponding river basins are highlighted in color. The map was developed using ESRI ArcGIS 10 software (www.esri.com/arcgis). The topographic base of the map was compiled with Natural Earth Free Vector and Raster Map Data (www.naturalearthdata.com), GSHHG version 2.3.7 (http://www.soest.hawaii.edu/pwessel/gshhg)47, and the HydroSHEDS database (http://www.hydrosheds.org)48,49. (Map: Mikhail Yu. Gofarov).
Additionally, we revised the taxonomic placement of Trapezoideus peninsularis, T. theca and T. prashadi by means of a morphological approach, because the molecular sequence data for these nominal taxa is still lacking. We suggest that Trapezoideus peninsularis is a member of the genus Contradens, T. theca belongs to Lamellidens, and Trapezoideus prashadi is a junior synonym of Arcidopsis footei (see Taxonomic Account for details). Biogeographic data also supports these conclusions (see Taxonomic Account and Discussion).
Morphological analyses of Trapezoideus foliaceus and Yaukthwa gen. nov
The primary diagnostic features of shell structure of the studied Trapezoideus foliaceus specimens from the Mae Klong River basin such as thin and trapezoidal shell, shallow anterior muscle attachment scars, and slender pseudocardinal teeth correspond well to the lectotype of this nominal taxon thought to be collected from the Dawei (Tavoy) River (Figs 2, 3, 4a,b). The other Trapezoideus representatives from western Indochina studied by us have more elongated shell (although small specimens are rather similar in shell shape), more developed hinge, well-marked anterior muscle scars even for young individuals, and more elevated umbo compared with those of T. foliaceus (Figs 4 and 5). These specimens have well distinguishable morphological features and belong to the Yaukthwa gen. nov.
Figure 3.
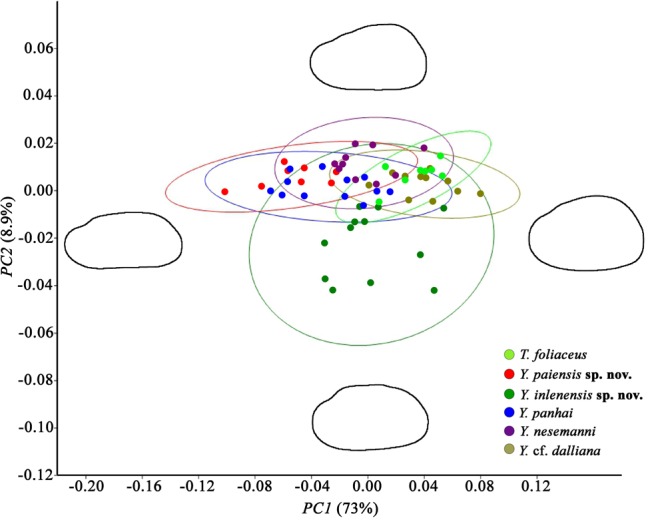
Scatter plot from principal component analysis (PCA) based on Fourier coefficients of the shell shape of Trapezoideus foliaceus and five Yaukthwa species. The color lines show 95% confidence ellipses. Colors correspond to biological species (see legend). The PC1 axis describes 73% and the PC2 axis describes 9% of a total variation. Synthetic outlines of “extreme” shell morphotypes are shown in four sides of the scatter plot.
Figure 4.
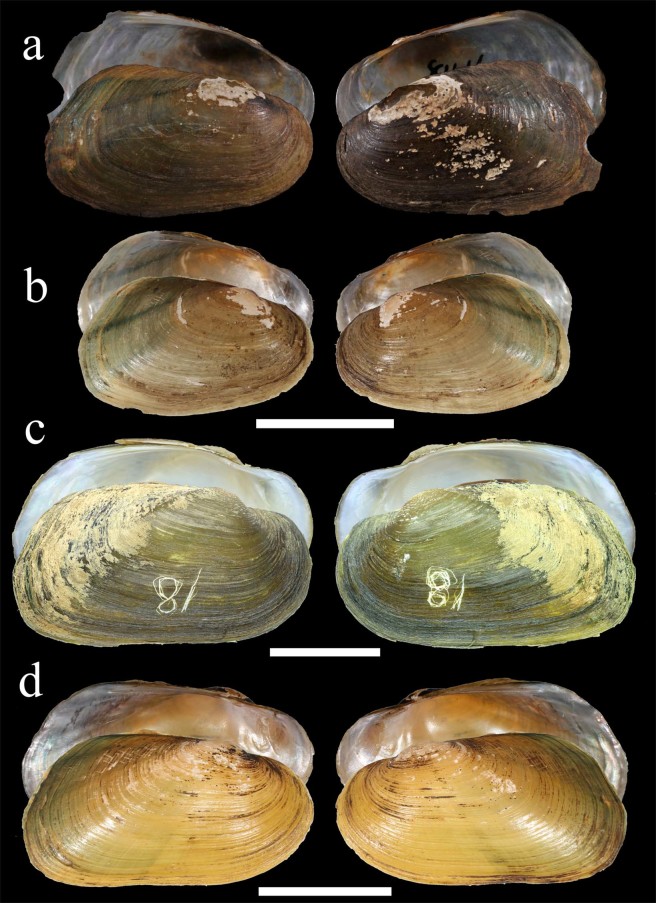
Shells of Trapezoideus and Yaukthwa species. (a) Lectotype of Trapezoideus foliaceus, Tavoy, British Burma (NMNH 84161). (b) T. foliaceus, Mae Klong River, western Thailand (UF 507865). (c) Y. inlenensis sp. nov., Salween River Basin, tributary of Nam Pilu River, Mway Stream, Myanmar (holotype RMBH biv139_18). (d) Y. paiensis sp. nov., Salween River Basin, Khong River, northwestern Thailand (holotype UF 505164). Scale bars = 2 cm. (Photos: NMNH, with permission of Dr. Ellen Strong [a], John M. Pfeiffer [b, d], and Ekaterina S. Konopleva [c]).
Figure 5.
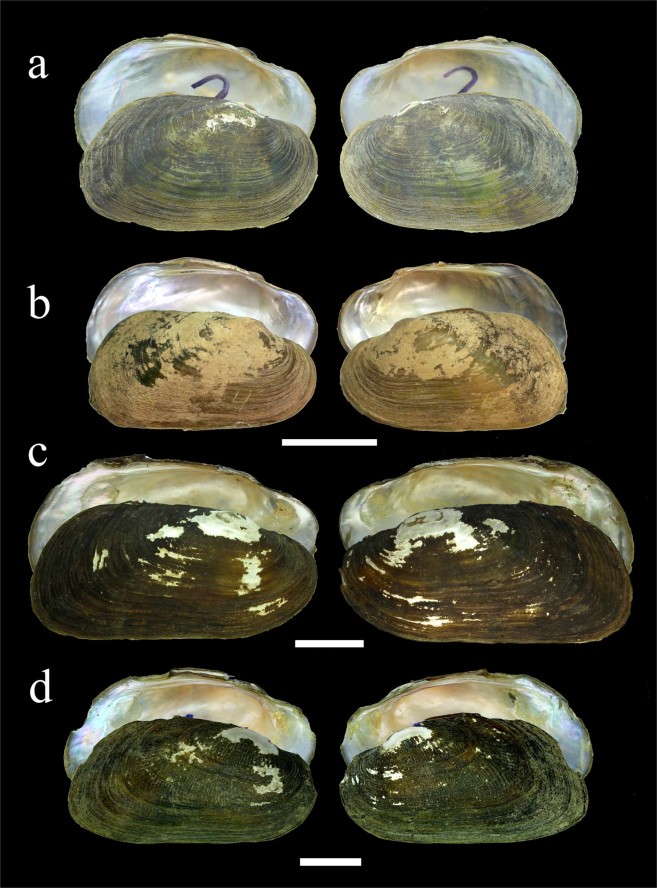
Shell morphology of Yaukthwa species and Indonaia subclathrata comb. nov. (a) Yaukthwa cf. dalliana, Nanuinhka Chaung River, Ayeyarwady Basin, Myanmar (RMBH biv111_2). (b) Y. panhai comb. nov., Kyan Hone River, Sittaung Basin, Myanmar (holotype NCSM 103033 [transferred from RMBH biv138_48]). (c) Y. nesemanni gen. & comb. nov., Thauk Ye Kupt River, Sittaung Basin, Myanmar (holotype NCSM 103036 [transferred from RMBH biv255_28]). (d) Indonaia subclathrata comb. nov., Chindwin River near Kalewa, Ayeyarwady Basin, Myanmar (topotype RMBH biv347_1). Scale bars = 2 cm [a-b, d] and 3 cm [c]. (Photos: Ekaterina S. Konopleva).
Species of the Yaukthwa and Trapezoideus were also analyzed with respect to their shell contours (Fig. 3). For morphometric analysis, two significant principal components (PC1 and PC2) of the shell shape were obtained using a principal component analysis (PCA) approach based on 20 normalized Elliptic Fourier Descriptors (EFDs). PC1 axis describes 73% of the total variation of sagittal shell shape with much higher shell variability, while PC2 axis describes only 9%. The first component shows variation in the shell height, dorsal edge elevation and curve of ventral margin. The second component reflects the position and elevation of umbo and the shape of posterior end. Four synthetic outlines of the ‘extreme’ shell forms are shown in Fig. 3. According to the PCA results, 95% confident ellipses of all the studied species mainly overlapped, with exception of Yaukthwa inlenensis sp. nov., the specimens of which form a largely separate cloud.
Taxonomic Account
Family Unionidae Rafinesque, 1820
Subfamily Rectidentinae Modell, 1942
Tribe Contradentini Modell, 1942
Type genus: Contradens Haas, 1911 (by original designation)
Genus Trapezoideus Simpson 1900
Type species: Unio foliaceus Gould, 1843 (by original designation)
Comments: This genus was thought to comprise several species inhabiting numerous freshwater drainages from India to East Asia4,15,16, but we consider it to be a monotypic genus, with a rather local distribution range in western Thailand.
Trapezoideus foliaceus (Gould, 1843)
Unio foliaceus Gould (1843): 14117.
Trapezoideus foliaceus Simpson (1900): 85818.
Trapezoideus foliaceus Konopleva et al. (2017): 21414.
Figure 4a,b
Type: Lectotype NMNH 84161.
Type locality: Tavoy, British Burma [Dawei River, Myanmar (approx. 14.50139° N, 98.15583° E)]17.
Material examined: UF 507697: Thailand, Mae Klong River basin, Pracham Mai River, 14.65983° N, 98.53422° E, 28.i.2015, 26 specimens (sequenced individuals = 2012–0443, 2012-0445), Pfeiffer & Page leg. UF 507702: Thailand, Mae Klong River basin, Song Karia River, 15.22318° N, 98.44648° E, 29.i.2015, 29 specimens (sequenced individuals = 2012-0457, 2012-0457), Pfeiffer & Page leg. UF 507865: Thailand, Mae Klong River basin, tributary of Pracham Mai River, 14.69334° N, 98.50639° E, 06.i.2017, 1 specimen (sequenced individual = ICH-02059), Pfeiffer & Page leg. UF507879: Thailand, Mae Klong River basin, Pachee River, 13.918134° N, 99.38227° E, 13.i.2017, 2 specimens (sequenced individuals = ICH-02104, ICH-02105), Pfeiffer & Page leg.
Redescription: Shell shape trapezoidal, inequilateral, not inflated, thin and small. Maximum shell length to 48.7 mm, height to 31.6 mm, width to 16.6 mm (N = 80). Posterior end broader than anterior one, somewhat oblique; anterior margin rounded. Umbo slightly elevated, corrugated; sculpture double-looped. Periostracum smooth, yellow-brown with green ribs on the posterior margin; nacre yellow-whitish. Pseudocardinal teeth slender and lamellar, two on the right valve and one on the left valve. Lateral teeth thin, elongated, slightly curved, one on the right valve and two on the left valve. Umbo cavity not deep. Muscle attachment scars shallow or reduced, oval-shaped.
Distribution: Only the type series is reported from “Tavoy” and there is some question as to the accuracy of the reported type locality (see discussion). All other records are from the Mae Klong Basin, western Thailand (Fig. 2).
Habitat: Common in small to medium sized streams and rivers with moderate current. Often found in coarse rocky substrate.
Genus Contradens Haas, 1911
Type species: Unio contradens Lea, 1838 (by original designation)
Comments: This genus is distributed in the Mekong Basin, Chao Phraya River, rivers of the Malacca Peninsula and Greater Sunda Islands8,10. Records from other areas most likely represent misidentified specimens (Supplementary Table 2).
Contradens peninsularis (Simpson, 1900) comb. nov.
Trapezoideus peninsularis Simpson (1900): 85918.
Type: Not traced.
Type locality: Sumatra18.
Distribution: Sumatra, Indonesia.
Comments: Molecular sequence data for this poorly known species is still lacking. We transfer it to the genus Contradens on the basis of available biogeographic information7–9,13 and morphological data, although this taxonomic hypothesis requires further research.
Contradens comptus (Deshayes & Jullien, 1874) comb. nov.
Unio comptus Deshayes & Jullien (1874): 126, pl. 6, Figs 3–4 19.
Diplodon ludovicianum Rochebrune (1881): 4320.
Trapezoideus misellus Haas (1969): 7621.
Trapezoideus exolescens comptus Brandt (1974): 30015.
Harmandia munensis Brandt (1974): 28415.
Trapezoideus comptus Pfeiffer et al. (2018): 313.
Type: Syntype MNHN IM-2000-1661.
Type locality: Peam Chelang, Cambodge [Mekong River, Peam Chilang village, Tboung Khmum District, Kampong Cham Province, Cambodia (approx. 12.0937° N, 105.5331° E)]19.
Distribution: Mekong Basin in Laos, Thailand, and Cambodia.
Comments: Our molecular phylogeny (Fig. 1) indicates that the specimens morphologically identified as T. comptus belong to the genus Contradens and should be transferred from Trapezoideus to Contradens.
Contradens pallegoixi (Sowerby, 1867) comb. nov.
Anodon pallegoixi Sowerby (1867): pl. 8, sp. 18, fig. 1722.
Trapezoideus pallegoixi Simpson (1900): 85918.
Type: Holotype BMNH 1965193.
Type locality: Siam22.
Distribution: Mun River drainage (Mekong Basin) in Thailand.
Comments: This species is transferred to Contradens based on the multi-locus molecular data (Fig. 1).
Contradens misellus (Morelet, 1865) comb. nov.
Unio misellus Morelet (1865): 2123.
Trapezoideus misellus Simpson (1900): 85918.
Type: Holotype BMNH 93-2-4-1593.
Type locality: Siam23.
Distribution: Chao Phraya Basin in Thailand.
Comments: Our molecular phylogeny (Fig. 1) recovered this species as a Contradens member.
Genus Yaukthwa gen. nov.
Type species: Yaukthwa nesemanni (Konopleva, Vikhrev & Bolotov, 2017) gen. & comb. nov.
Etymology: The name of this genus means “freshwater bivalve” (yaukthwa) in Burmese language.
Diagnosis: This genus represents a distinct phylogenetic clade, but is morphologically similar to the Contradens and Trapezoideus. However, adult representatives of Yaukthwa gen. nov. can be distinguished by wider and more rounded anterior end, straighter dorsal margin without developed wing, and shallow posterior muscle scar.
Description: Shell middle-sized, from obovate for juvenile specimens to trapezoidal for adults, inequilateral, rather compressed, of various thicknesses. Right valve with one lateral tooth and two linear pseudocardinal teeth. For some specimens teeth may be reduced, usually to one weak tubercle-like lateral tooth and one pseudocardinal tooth in each valve. Left valve with two somewhat curved lateral teeth and one pseudocardinal tooth. Anterior muscle scar well developed, oval-shaped. Posterior muscle scar shallow. Ectobranchous brooding in outer demibranches. Inner demibranch attached to visceral mass by its anterior end.
Distribution: Western Indochina (Ayeyarwady, Bago, Sittaung and Salween basins in Myanmar and Salween Basin in western Thailand).
Habitat: Rapidly flowing mountain streams and rivers with sandy, gravel, and clay substrate, mostly within upland areas (Supplementary Table 3 and Fig. 6). However, Yaukthwa inlenensis sp. nov. can be found in rivers and streams with moderate current and clay substrate.
Figure 6.
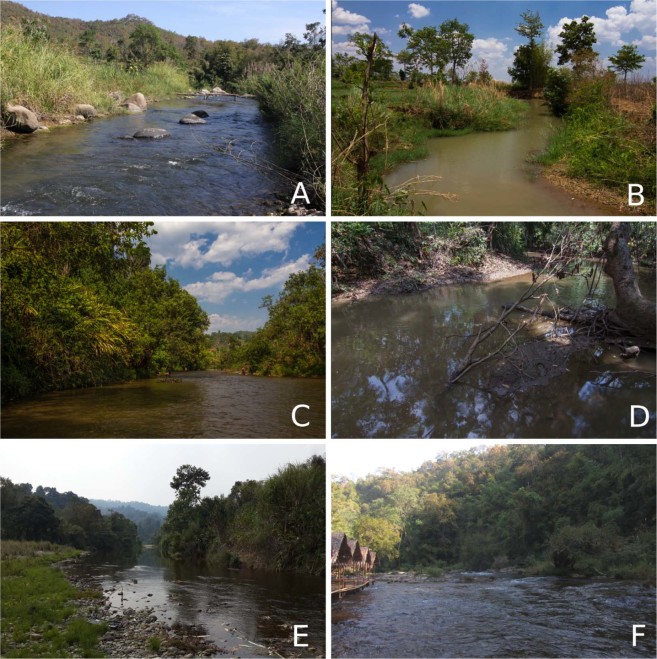
Type localities and habitats of the Yaukthwa species and habitat of Trapezoideus foliaceus. (A) Type locality of Y. paiensis sp. nov.: Khong River, Pai River basin, Salween Basin, northwestern Thailand. (B) Type locality of Y. inlenensis sp. nov.: Mway Stream, Salween Basin, Myanmar. (C) Habitat of Y. nesemanni gen. & comb. nov.: Thauk Ye Kupt River, Sittaung Basin, Myanmar. (D) Habitat of Y. panhai comb. nov.: Kyan Hone River, Sittaung Basin, Myanmar. (E) Habitat of Y. cf. dalliana comb. nov.: Nam Shu River, Malikha Basin, Ayeyarwady Drainage, Myanmar. (F) Habitat of T. foliaceus, Mae Klong Basin, western Thailand. (Photos: Zachary S. Randall [a, f] and Ilya V. Vikhrev [b-e]).
Comments: Here, we transfer five Trapezoideus taxa to the new genus and describe two additional species new to science.
Yaukthwa nesemanni (Konopleva, Vikhrev & Bolotov, 2017) gen. & comb. nov.
Trapezoideus nesemanni Konopleva, Vikhrev & Bolotov (2017): 13, Fig. 5c8.
Type: Holotype NCSM 103033 [transferred from RMBH biv255_28].
Type locality: Thauk Ye Kupt River, Sittaung Basin, Myanmar (19.3075° N, 96.7219° E)8.
Distribution: Known only from the type locality (Fig. 2).
Yaukthwa panhai (Konopleva, Bolotov & Kondakov, 2017) comb. nov.
Trapezoideus panhai Konopleva, Bolotov & Kondakov (2017): 13, Fig. 5d8.
Type: Holotype NCSM 103036 [transferred from RMBH biv138_48].
Type locality: Kyan Hone River, Sittaung Basin, Myanmar (19.5059° N, 96.8280° E)8.
Distribution: Known only from the type locality (Fig. 2).
Yaukthwa dalliana (Frierson, 1913) comb. nov.
Parreysia dalliana Frierson (1913): 14224.
Trapezoideus dallianus Haas (1919): 263, pl. 32, Fig. 4 25,26.
Trapezoideus dallianus Srinivasa Rao (1928): 46427.
?Trapezoideus subclathratus sensu Bolotov et al. (2017): 108.
Type: Lectotype SMF 13699b (by present designation). Frierson24 stated that two specimens illustrated in Haas’s work [pl. 32, Figs 3 and 4]25 are representatives of his new species. Later, Haas26 revised the type series, and concluded that only the specimen in Fig. 4 should be the representative of Trapezoideus dallianus, and that the specimen on Fig. 3 belongs to Trapezoideus foliaceus. We agree that the latter specimen is conchologically different, and, at first glance, it may be a Lamellidentini member (e.g. Trapezidens sp.). It was collected from Pegu [Bago River, Myanmar]26. Haas26 also listed another specimen from the same lot (probably, specimen no. SMF 13699a) as an additional representative of Trapezoideus dallianus. However, this specimen was not pictured by Haas25, and was unknown to Frierson. Therefore, it could not be considered a part of the type series.
Type locality: Lashio-Fluss bei Lashio, nördliche Shan-Staaten [Lashio River near Lashio, Ayeyarwady Basin, northern Shan State, Myanmar (approx. 22.9946° N, 97.7650° E)]26.
Distribution: Known from the type locality (Fig. 2). Srinivasa Rao27 recorded two subfossil shells from the Namtu River at Hsenwi, 45 km NE of the type locality. Morphologically similar specimens were collected from the headwater of the Ayeyarwady River (Malikha Basin).
Comments: Our samples of Trapezoideus subclathratus sensu Bolotov et al.8 are morphologically similar to Y. dalliana, but they were collected from the Malikha River basin, far from the type locality of Frierson’s species (Fig. 2). However, the Malikha River belongs to the same drainage, the Ayeyarwady River. Here, we preliminary consider our samples as belonging to Y. cf. dalliana, but this hypothesis should be checked in a future based on molecular sequences of the topotypes from Lashio.
Yaukthwa zayleymanensis (Preston, 1912) comb. nov.
Trapezoideus foliaceus var. zayleymanensis Preston (1912): 30728.
Type: Paratype SMF 3615.
Type locality: Bhamo [Bhamo, Ayeyarwady River (approx. 24.2669° N, 97.2210° E)]28.
Distribution: Known only from the type locality (Fig. 2) and from Zayleyman, Upper Burma28,29. We were unable to find an exact geographic position of Zayleyman, but the Upper Burma Region of British Empire included areas of the modern Shan and Kachin States of Myanmar. We suggest that Zayleyman was located somewhere on the Ayeyarwady River north of Mandalay.
Comments: Molecular data for this nominal taxon is still lacking. It is externally similar to Yaukthwa nesemanni comb. nov. from the Sittaung River by the elongated shell shape.
Yaukthwa peguensis (Anthony, 1865) comb. nov.
Unio peguensis Anthony (1865): 35130.
Type: Holotype MCZ 161875.
Type locality: Pegu, British Burmah [Bago River, Myanmar]30.
Distribution: Known only from the type locality (Fig. 2).
Comments: Molecular data for this nominal taxon is still lacking.
Yaukthwa paiensis sp. nov.
Table 1.
Shell measurements and reference DNA sequences for the type series of new Yaukthwa species from western Indochina.
| Species | Status of Specimen | Specimen Voucher* | Shell Length, mm | Shell Height, mm | Shell Width, mm | NCBI’s GenBank acc. nos. | ||
|---|---|---|---|---|---|---|---|---|
| COI | 16 S rRNA | 28 S rRNA | ||||||
| Y. inlenensis sp. nov. | Holotype | RMBH biv_139_18 | 55.3 | 30.8 | 18.0 | KX865924 | KX865678 | KX865795 |
| Paratype | RMBH biv_114_1 | 31.2 | 18.0 | 12.3 | KX865915 | KX865672 | KX865786 | |
| Paratype | RMBH biv_114_3 | 36.4 | 21.5 | 15.4 | KX865916 | KX865673 | KX865787 | |
| Paratype | RMBH biv_143_2 | 42.9 | 26.4 | 17.3 | KX865917 | KX865674 | KX865788 | |
| Paratype | RMBH biv_114_2 | 35.0 | 20.3 | 14.5 | KX865918 | KX865675 | KX865789 | |
| Paratype | RMBH biv_115_1 | 37.6 | 22.7 | 12.1 | KX865919 | n/a | KX865790 | |
| Paratype | RMBH biv_115_3 | 33.4 | 20.2 | 14.2 | KX865920 | n/a | KX865791 | |
| Paratype | RMBH biv_115_2 | 38.1 | 21.2 | 13.5 | KX865921 | n/a | KX865792 | |
| Paratype | RMBH biv_139_7 | 40.7 | 23.0 | 13.8 | KX865922 | KX865676 | KX865793 | |
| Paratype | RMBH biv_139_15 | 50.2 | 29.3 | 17.2 | KX865923 | KX865677 | KX865794 | |
| Paratype | RMBH biv_140_22 | 46.0 | 26.2 | 19.1 | KX865925 | KX865679 | KX865796 | |
| Paratype | RMBH biv_140_24 | 43.7 | 24.4 | 18.9 | KX865926 | KX865680 | KX865797 | |
| Paratype | RMBH biv_140_25 | 46.5 | 25.6 | 19.1 | KX865927 | KX865681 | KX865798 | |
| Y. paiensis sp. nov. | Holotype | UF 505164 (ICH_00638) | 36.0 | 18.9 | 11.9 | MH345970 | MH346011 | MH345991 |
| Paratype | UF 507709 (ICH_00639) | 27.1 | 14.4 | 7.7 | MH345971 | MH346012 | MH345992 | |
| Paratype | UF 507709 (ICH_00640) | 27.6 | 14.1 | 7.4 | MH345972 | n/a | n/a | |
| Paratype | UF 507709 (ICH_00637) | 42.7 | 21.5 | 11.7 | n/a | n/a | n/a | |
| Paratype | UF 507709 | 26.0 | 13.4 | 7.4 | n/a | n/a | n/a | |
| Paratype | UF 507709 | 24.2 | 13.0 | 7.3 | n/a | n/a | n/a | |
| Paratype | UF 507709 | 23.1 | 12.1 | 7.1 | n/a | n/a | n/a | |
| Paratype | UF 507709 | 21.2 | 11.3 | 6.5 | n/a | n/a | n/a | |
*RMBH – Russian Museum of Biodiversity Hotspots, Federal Center for Integrated Arctic Research, Russian Academy of Sciences, Arkhangelsk, Russia; UF – Florida Museum of Natural History, Gainesville, USA. n/a – not available.
Table 2.
Molecular diagnoses of new Yaukthwa species from western Indochina.
| Species | Mean COI p-distance from the nearest neighbor of new species, % | Nearest neighbor of new species | Fixed unique nucleotide differences based on the sequence alignment of congeners | ||
|---|---|---|---|---|---|
| COI | 16 S rRNA | 28 S rRNA | |||
| Y. inlenensis sp. nov. | 3.3 | Y. paiensis sp. nov. | 77 G, 341 C, 344 C, 482 G | 234 C, 249 A, 267 C, 464 G | None |
| Y. paiensis sp. nov. | 3.3 | Y. inlenensis sp. nov. | 26 A, 296 A, 302 G, 317 A, 429 C, 461 T, 530 C, 579 C, 629 T | 78 A, 239 T, 249 T, 254 C, 255 G, 319 C, 337 C, 448 T | 121 C, 485 G, 761 C |
Type material: Holotype UF 505164: Thailand, Salween River Drainage, Pai District, Mae Hong Son Province, Khong River, tributary of Pai River, off Rt. 1095, 19.4246° N, 98.4013° E, 10.i.2016, J. Pfeiffer & L. Page. Paratypes UF 507709: Thailand, Salween River Drainage, same locality and date as holotype, 7 specimens, J. Pfeiffer & L. Page.
Etymology: The species name is derived from the Pai River, the watershed from which the type specimen had been collected.
Diagnosis: Yaukthwa paiensis resembles its sister species, Y. inlenensis, but is distinguished by its more elongate shell outline, more parallel dorsal and ventral margins, less distinct umbo, and fixed nucleotide substitutions (Table 2).
Description: Shell outline subtrapezoidal, dorsal and ventral margins straight, dorsal margin elevated posteriorly, creating slight wing. Maximum length to 42.7 mm, height to 21.5 mm, width to 11.9 mm (Table 1). Posterior ridge broadly rounded, posterior slope gradual, often with very fine corrugations. Periostracum very smooth, yellow to brownish-yellow, often with green rays posteriorly. Nacre bluish-white, strongly iridescent posteriorly, often with orange tint near the umbo. Umbo only slightly elevated above hinge line. Pseudocardinal teeth strong, thin, elongate, one in each valve. Lateral teeth strong, thin, slightly curved, two in left valve, one in right. Umbo pocket, very shallow. Adductor muscle scars shallow, contiguous with pedal retractor scars.
Distribution: Known only from the type locality in northwestern Thailand (Fig. 2).
Habitat: The species inhabits slower flowing portions of the mountain stream, typically near sheltered and sandy banks. This species (and freshwater mussels in general) appears to be uncommon or patchily distributed in the Pai River system. No other freshwater mussel specimens (dead or alive) were found at any of our recent sampling sites in the Pai River watershed (N = 3) and there is only one other known freshwater mussel record from the Pai system (SMF 220825: 3 subfossil valves of Gibbosula laosensis)15.
Yaukthwa inlenensis sp. nov.
Trapezoideus sp. ‘Salween’ sensu Bolotov et al. (2017): 108
Figures 2 & 4c, Tables 1 and 2
Type material: Holotype RMBH biv139_18: Myanmar, Salween Basin, Mway Stream, a tributary of Nam Pilu River, 19.7266° N, 97.0992° E, 1.iv.2014, Vikhrev, Bolotov & locals leg. Paratypes: the type locality, 3 specimens (RMBH biv139_7, biv139_15, biv143_2), Myanmar, Salween Basin, Inle Lake channel, 20.4420° N, 96.9036° E, 1.iv.2014, 6 specimens (RMBH biv114_1, biv114_2, biv114_3, biv115_1, biv115_3, biv115_2), Myanmar, Salween Basin, Nam Pilu River, 3 specimens (RMBH biv140_22, biv140_24, biv140_25), 19.6746° N, 97.1352° E, 19.iv.2015, Bolotov & locals leg.
Etymology: The species name is derived from the Inle Lake, because it is widespread in tributaries and outlet of this water body.
Diagnosis: This species is very similar to Yaukthwa paiensis, but differs from it by more developed umbo and fixed nucleotide substitutions (Table 2).
Description: Shell shape variable, from elliptic to obovate, mainly with broader posterior side. Many specimens from the Nam Pilu River have constricted posterior end and more rounded ventral margin. All shells rather thick and inequilateral. Maximum shell length to 55.3 mm, height to 30.8 mm, width to 19.1 mm (Table 1). Umbo elevated with w-shaped sculpture, usually corrugated. Periostracum from olive-brown to dark-brown; the nacre whitish. Well-marked wrinkles grooves along the dorsal and posterior margin. Pseudo-cardinal teeth linear and strong, two on the right valve and one on the left valve. Lateral teeth thin, long and slightly curved, one on the right valve and two on the left valve. Umbo cavity rather deep. Anterior adductor scar oval-form and marked. Posterior adductor scar shallow or absent.
Distribution: Tributaries and the outlet of Inle Lake, Myanmar (Fig. 2).
Habitat: Moderately and slow flowing streams with clay and gravel substrate.
Subfamily Parreysiinae Henderson, 1935
Type genus: Parreysia Conrad, 1853
Tribe Lamellidentini Modell, 1942
Type genus: Lamellidens Simpson, 1900
Genus Lamellidens Simpson, 1900
Type species: Unio marginalis Lamarck, 1819 (by original designation)
Lamellidens theca (Benson, 1862) comb. nov.
Unio theca Benson (1862): 18631.
Trapezoideus theca Simpson (1900): 85918.
Type: Not traced.
Type locality: Fluvio Cane, prope Banda, Bundelkhund [Ken River, near Banda, Uttar Pradesh, central India (approx. 25.4836° N, 80.3128° E)]31.
Distribution: Known only from the type locality.
Comments: Benson (p. 187)31 noted: “This shell, of which I found a single specimen, belongs to the Corrianus type of Unio marginalis, and is remarkable for its elongate-ovate non-rhomboidal form”. Benson’s protologue31 clearly indicates that this species is a member of the genus Lamellidens. The validity of this species is in doubt and deserves further research, because it is known from a single type specimen.
Tribe Indochinellini Bolotov, Pfeiffer, Vikhrev & Konopleva, 2018
Type genus: Indochinella Bolotov, Pfeiffer, Vikhrev & Konopleva, 2018 (by original designation)
Genus Indonaia Prashad, 1918
Type species: Unio caeruleus Lea, 1831 (by original designation)
Indonaia subclathrata (Martens, 1899) comb. nov.
Unio misellus var. subclathratus Martens (1899): 4432.
Trapezoideus misellus var. subclathratus Subba Rao (1989): 19516.
Figure 5d
Type: Not traced.
Type locality: Im Chindwinfluss bei Kalewa und bei Matu < … > ; einige Stücke auch im Irawaddi selbst bei Yenangyoung [Chindwin River near Kalewa and Matu (approx. 23.1991° N, 94.3071° E), several specimens also from Ayeyarwady as far as Yenangyaung (approx. 20.4347° N, 94.8720° E)]32.
Distribution: Manipur and Chindwin rivers, and the middle reaches of the Ayeyarwady River, Myanmar.
Comments: This taxon has been considered a synonym of Trapezoideus exolescens15,16, but the latter species was found to be a member of the Lamellidentini8,14. Molecular analyses of the newly collected topotypes of T. subclathratus from Kalewa unexpectedly reveal that this species belongs to the genus Indonaia, representing another example of incorrect placement of the Parreysiinae taxa within the Rectidentinae based on an external resemblance of the shell8,14. Actually, I. subclathrata is externally quite similar to the Yaukthwa species (Fig. 5d).
Unionidae incertae sedis
Genus Arcidopsis Simpson, 1900
Type species: Unio footei Theobald, 1876 (by original designation)
Arcidopsis footei (Theobald, 1876)
Unio footei Theobald (1876): 187, pl. 14, figs. 9-9a33.
Trapezoideus prashadi Haas (1922) syn. nov.: 10134.
Type: Holotype BMNH 88-12-4-1651 (A. footei); holotype SMF 3614 (T. prashadi).
Type locality: Kistna flumine prope ‘Gutparba falls’ [Gokak Falls, Ghataprabna River, Krishna Basin, southwestern India (approx. 16.1921° N, 74.7776° E)]33.
Distribution: Krishna River drainage in Western Ghats, India.
Comments: The type locality of T. prashadi is Mysore, Südostindien [Krishna Basin, Mysuru, Karnataka, southwestern India (approx. 12.4003° N, 76.6929° E)]34. Haas25 and Prashad35 listed the type specimen of T. prashadi as A. footei. Haas34 described this specimen as a new Trapezoideus species, but used only a brief description and figures of A. footei33 to delineate these taxa. Unfortunately, the figures in Theobald’s protologue [pl. 14, figs. 9-9a]33 are hardly resemble the shells of A. footei. Later, Haas (1969) noted that A. footei could actually be a member of the Trapezoideus and may be conspecific to T. prashadi. From a conchological point of view, these taxa are identical, and the Arcidopsis appears to be a valid genus, which is not related to Trapezoideus. We therefore considered T. prashadi as a junior subjective synonym of A. footei.
Discussion
In this study, Trapezoideus was not recovered as monophyletic, and its putative species were distributed across the tribe Contradentini (five distinct lineages in three genera) and one of its former species (T. subclathratus) is placed within the distantly related tribe Indochinellini (Fig. 1). On the basis of morphological and biogeographic patterns we remove three other species from the genus Trapezoideus, i.e. T. peninsularis, T. theca, and T. prashadi. We briefly discuss each of these hypotheses in terms of their systematic and biogeographic relevance (see Taxonomic Account).
We collected putative specimens of the type species of Trapezoideus, T. foliaceus, from several headwater sites of the Mae Klong Basin in western Thailand, which is directly adjacent to the Dawei (Tavoy) Drainage, the drainage from which Unio foliaceus is presumed to be described from (Fig. 2). However, in the description of Unio foliaceus Gould17 never explicitly mentions the type locality of the species. The specimens are presumed to be from Tavoy on the basis that Rev. F. Mason, a missionary in the region, sent the specimens to Gould14. It is therefore possible that the specimens did not in fact originate from that river. The sequenced Mae Klong specimens are morphologically very similar to the lectotype of Unio foliaceus (Fig. 4a,b) and we consider them representatives of this nominal taxon. The inclusion of specimens of the type species of the genus Trapezoideus provided the material necessary to more completely revise the tribe Contradentini. Our Trapezoideus foliaceus specimens are recovered as the sister lineage to the genus Contradens (Fig. 1). The topology recovered here is completely consistent with the morphology-based hypothesis of Konopleva et al.14 who suggested that Trapezoideus foliaceus belongs to the Rectidentinae while T. exolescens belongs to the Parreyssiinae.
The taxa previously attributed to Trapezoideus from the Western Indochina Subregion belong to another genus, Yaukthwa gen. nov. This new genus comprises at least seven species, inhabiting the Salween, Sittaung, Bago and Ayeyarwady River drainages (Fig. 2). This new genus is morphologically similar to Trapezoideus, and their species are indistinguishable from each other by a morphometric shell shape analysis, with only the exception of Yaukthwa inlenensis sp. nov., showing a high variation in the shell shape, likely because of wider range of habitats. Yaukthwa gen. nov. is phylogenetically distant from the other Contradentini clades (Trapezoideus, Physunio, and Contradens) that are distributed east of the Salween – Mekong drainage divide, supporting a previously established biogeographic division of Southeast Asia9. In general, five freshwater mussel genera seem to be endemic to western Indochina: Yaukthwa gen. nov., Indochinella, Pseudodon, Leoparreysia, and Trapezidens.
All the Yaukthwa species and Trapezoideus foliaceus were recorded from lotic freshwater systems, i.e. rapidly flowing rivers and streams, mostly within upland areas (Supplementary Table 3). With respect to this evidence, dam construction, water pollution, and forest cutting appear to be the primary treats for their populations, as was shown for Laos, Borneo and Malaysia10,11,36. Taking into account local distribution ranges of the Yaukthwa and Trapezoideus species, they should be a focus of special conservation efforts from the governments, local authorities and local communities of Myanmar and Thailand, as well as international organizations. In conclusion, our results confirm a high conservation significance of the Oriental freshwater mussel fauna, because it includes numerous local endemic taxa. An integrative taxonomic approach becomes an essential tool for revisions of freshwater mussels in Southeast Asia.
Methods
Taxon sampling and molecular analysis
The samples of the Contradentini taxa were collected from Myanmar, Thailand, Laos, Cambodia, and Malaysia (Supplementary Table 1). Total genomic DNA extraction was carried out using NucleoSpin® Tissue XS Kit (Macherey-Nagel GmbH & Co. KG, Germany), following the manufacturer’s protocol. For the molecular analyses, partial sequences of the COI, 16 S rRNA, and 28 S rRNA gene fragments were obtained and afterwards checked using a sequence alignment editor (BioEdit v. 7.2.5)37 as described in Bolotov et al.8 The PCR primers are provided in Supplementary Table 4.
Phylogenetic analyses
Muscle algorithm implemented in MEGA638 was used for sequence alignment of COI, 16 S rRNA and 28 S rRNA gene fragments. To get alignments with final lengths (Supplementary Table 5) we used GBlocks v. 0.91b39 as described in Bolotov et al.8 Through an online FASTA sequence toolbox (FaBox 1.41)40 we joined aligned data sets into combined nucleotide sequence alignments and collapsed them into unique haplotypes. Combined data set (3 codons of COI + 16 S rRNA + 28 S rRNA) of unique haplotypes was used for phylogenetic analyses. The best evolution models for each partition were selected based on the corrected Akaike Information Criterion (AICc) of MEGA638 (Supplementary Table 6). Bayesian inference analysis (BI) was performed in MrBayes v. 3.2.641 with four runs, each with three heated (temperature = 0.1) and one cold Markov chain, during 30 million generations and sampling every 1000th generation. The first 15% of trees were discarded as burn-in. All calculations were performed at San Diego Supercomputer Center through the CIPRES Science Gateway42. Trace analysis tool (Tracer v. 1.6)43 was used to check a convergence of the MCMC chains to a stationary distribution. The effective sample size (ESS) for each parameter was recorded as > 2000. The maximum likelihood (ML) analysis was performed in RAxML GUI v. 1.3 with 1000 bootstrap replications44. We used a unique GTR + G model for all the partitions.
Morphological and morphometric analyses
We studied type series of nominal taxa and other shell lots in the collections of the BMNH – Natural History Museum, London, UK, NMNH – National Museum of Natural History, Smithsonian Institution, Washington, USA, MCZ – Museum of Comparative Zoology, Harvard University, Cambridge, USA, NCSM – North Carolina Museum of Natural Sciences, Raleigh, USA, UF – Florida Museum of Natural History, Gainesville, USA, SMF – Naturmuseum Senckenberg, Frankfurt, Germany, MNHN – Muséum national d’histoire naturelle, Paris, France, as well as RMBH – Russian Museum of Biodiversity Hotspots, Federal Center for Integrated Arctic Research, Russian Academy of Sciences, Arkhangelsk, Russia. The images of the nominal taxa from MUSSELp Database were also analyzed45. The comparative analysis of shell morphology was carried out with regard to the main distinguishing traits, such as shell shape, umbo position, structures of pseudo-cardinal and lateral teeth, as well as muscle attachment scars8,14. Three shell dimensions at each specimen of the studied taxa, i.e., the length, height, and width of the shell (all at the maximum diameter), were measured using calipers (±0.1 mm). Shell shape of Trapezoideus foliaceus and the Yaukthwa species were analyzed through Fourier coefficients using software package SHAPE v. 1.346 as described in Konopleva et al.14. We used 139 individuals, from 8 to 12 shells per each species, depending on the number of available specimens. Photographs were obtained for mussels from our collections and were processed using GIMP v. 2.8.2 (www.gimp.org).
Nomenclatural acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature (ICZN), and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank (http://zoobank.org), the online registration system for the ICZN. The LSID for this publication is: urn:lsid:zoobank.org:pub:01AE2C5D-6857-4F76-B0FF-A15AAF76DDC8. The electronic edition of this paper was published in a journal with an ISSN, and has been archived and is available from PubMed Central.
Supplementary information
Acknowledgements
This work was partly funded by grants from the Russian Ministry of Education and Science (Project No. 6.2343.2017/4.6), Federal Agency for Scientific Organizations (Project No. 0409-2015-0143), National Geographic Society (Project No. NGS-274R-18), U.S. National Science Foundation Doctoral Dissertation Improvement Grant (DEB 1701901), Russian Foundation for Basic Research (Project Nos. 16-34-00638 and 18-34-20033), and Northern Arctic Federal University. The authors would like to thank Dr. Robert Hershler and Dr. Ellen Strong (NMNH, Washington, DC, USA) for the opportunity to work with mussel collection and for photographs of the lectotype of Unio foliaceus as well as Zachary S. Randall for the photos of type locality of Y. paiensis and habitat of T. foliaceus. We would like to express our sincerest gratitude to the Department of Fisheries of the Ministry of Agriculture, Livestock and Irrigation (Myanmar) for the permission and personally to Dr. Myint Than Soe for participating in the field works and sampling in Myanmar (survey permission No. 5/6000/MOLFRD-3103/2016 and export permission No. Nga La/Nga Tha Hta-Phont Thu/2016-5856). Finally, we are grateful for the information on the Unionidae type collection in SMF provided to us by Dr. Maxim Vinarski (Saint Petersburg State University, Russia).
Author Contributions
I.N.B., E.S.K. and J.M.P. developed the concept of the study. I.V.V. coordinated field works and sampling. J.M.P., E.S.K and I.V.V. studied the type series of the nominal taxa. I.N.B., I.V.V., J.M.P., Z.L., N.C. and E.S.K. collected samples. A.V.K. designed and carried out molecular analyses, with contribution from E.S.K. M.Y.G. created the map. E.S.K. performed phylogenetic and morphometric analysis. E.S.K., I.N.B. and J.M.P. wrote the paper, with input from A.V.K., I.V.V., M.Y.G., Z.L. and N.C. All authors discussed the manuscript.
Data Availability
The type series of the new species are available in the malacological collections of the Russian Museum of Biodiversity Hotspots (RMBH), Federal Center for Integrated Arctic Research, Russian Academy of Sciences, Arkhangelsk, Russia (Yaukthwa inlenensis sp. nov.) and Florida Museum of Natural History (UF), Gainesville, USA (Y. paiensis sp. nov.). The sequences obtained in this study are available from the NCBI GenBank database. Species locality, accession numbers and vouchers for each specimen are presented in Supplementary Table 1 as well as Table 1.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39365-1.
References
- 1.Graf DL, Cummings KS. Review of the systematics and global diversity of freshwater mussel species (Bivalvia: Unionoida) Journal of Molluscan Studies. 2007;73:291–314. doi: 10.1093/mollus/eym029. [DOI] [Google Scholar]
- 2.Bogan AE. Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia. 2008;595:139–147. doi: 10.1007/s10750-007-9011-7. [DOI] [Google Scholar]
- 3.Graf DL. Patterns of freshwater bivalve global diversity and the state of phylogenetic studies on the Unionoida, Sphaeriidae, and Cyrenidae. American Malacological Bulletin. 2013;31:135–153. doi: 10.4003/006.031.0106. [DOI] [Google Scholar]
- 4.Zieritz A, et al. Diversity, biogeography and conservation of freshwater mussels (Bivalvia: Unionida) in East and Southeast Asia. Hydrobiologia. 2018;810:29–44. doi: 10.1007/s10750-017-3104-8. [DOI] [Google Scholar]
- 5.Pfeiffer JM, III, Graf DL. Evolution of bilaterally asymmetrical larvae in freshwater mussels (Bivalvia: Unionoida: Unionidae) Zoological Journal of the Linnean Society. 2015;175:307–318. doi: 10.1111/zoj.12282. [DOI] [Google Scholar]
- 6.Lopes-Lima M, et al. Phylogeny of the most species-rich freshwater bivalve family (Bivalvia: Unionida: Unionidae): Defining modern subfamilies and tribes. Molecular Phylogenetics and Evolution. 2017;106:174–191. doi: 10.1016/j.ympev.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Bolotov IN, et al. Ancient river inference explains exceptional Oriental freshwater mussel radiations. Scientific Reports. 2017;7:2135. doi: 10.1038/s41598-017-02312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolotov IN, et al. New taxa of freshwater mussels (Unionidae) from a species-rich but overlooked evolutionary hotspot in Southeast Asia. Scientific Reports. 2017;7:11573. doi: 10.1038/s41598-017-11957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolotov IN, et al. A new genus and tribe of freshwater mussel (Unionidae) from Southeast Asia. Scientific Reports. 2018;8:10030. doi: 10.1038/s41598-018-28385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zieritz A, et al. Factors driving changes in freshwater mussel (Bivalvia, Unionida) diversity and distribution in Peninsular Malaysia. Science of the Total Environment. 2016;571:1069–1078. doi: 10.1016/j.scitotenv.2016.07.098. [DOI] [PubMed] [Google Scholar]
- 11.Zieritz A, et al. Changes and drivers of freshwater mussel diversity and distribution in northern Borneo. Biological Conservation. 2018;219:126–137. doi: 10.1016/j.biocon.2018.01.012. [DOI] [Google Scholar]
- 12.Do VT, Tuan LQ, Bogan AE. Freshwater mussels (Bivalvia: Unionida) of Vietnam: diversity, distribution, and conservation status. Freshwater Mollusk Biology and Conservation. 2018;21:1–18. doi: 10.31931/fmbc.v21i1.2018.1-18. [DOI] [Google Scholar]
- 13.Pfeiffer, J. M., Graf, D. L., Cummings, K. S., & Page, L. M. Molecular phylogeny and taxonomic revision of two enigmatic freshwater mussel genera (Bivalvia: Unionidae incertae sedis: Harmandia and Unionetta) reveals a diverse clade of Southeast Asian Parreysiinae. Journal of Molluscan Studies, eyy028; 10.1093/mollus/eyy028 (2018).
- 14.Konopleva ES, Bolotov IN, Vikhrev IV, Gofarov MY, Kondakov AV. An integrative approach underscores the taxonomic status of Lamellidens exolescens, a freshwater mussel from the Oriental tropics (Bivalvia: Unionidae) Systematics and Biodiversity. 2017;15:204–217. doi: 10.1080/14772000.2016.1249530. [DOI] [Google Scholar]
- 15.Brandt RAM. The non-marine aquatic mollusca of Thailand. Archiv für Mollusckenkunde. 1974;105:1–423. [Google Scholar]
- 16.Subba Rao, N. V. Handbook of freshwater molluscs of India (Calcutta, 1989).
- 17.Gould AAD. Gould had examined the shells not long since announced as having been received from the Rev. Francis Mason, missionary at Tavoy, in British Burmah. Proceedings of the Boston Society of Natural History. 1843;1:139–141. [Google Scholar]
- 18.Simpson CT. Synopsis of the naiades, or pearly fresh-water mussels. Proceedings of the United States National Museum. 1900;22:501–1044. doi: 10.5479/si.00963801.22-1205.501. [DOI] [Google Scholar]
- 19.Deshayes GP. & Jullien. Mémoire sur les mollusques nouveaux du Cambodge envoyés au Muséum, par M. le docteur Jullien. Nouv. Arch. Mus. Hist. Nat. Paris. 1874;10:115–162. [Google Scholar]
- 20.Rochebrune A-T. Documents sur la faune malacologique de la Cochinchine et du Cambodge. Bulletin de la Société philomathique de Paris. 1881;6:35–74. [Google Scholar]
- 21.Haas F. Superfamilia Unionacea. Das Tierreich. 1969;88:1–663. [Google Scholar]
- 22.Sowerby GB. Monograph of the Genus Anodon. Conchologica Iconica. 1867;17:pls. 2–19. [Google Scholar]
- 23.Morelet A. Rectifications et additions à la faune malacologique de l’Indo-Chine. Journal de Conchyliologie. 1865;13:19–23. [Google Scholar]
- 24.Frierson LS. Some criticisms on Dr. F. Haas’ monograph of the Unionidæ. Nautilus. 1913;26:141–142. [Google Scholar]
- 25.Haas, F. Die Unioniden. [in] H. C. Küster, Systematisches Conchylien-Cabinet von Martini und Chemnitz 9 (pt. 2, h. 46), 113–136, pls. 30–35 (1912).
- 26.Haas, F. Die Unioniden. [in] H. C. Küster. Systematisches Conchylien-Cabinet von Martini und Chemnitz 9 (pt. 2, h. 51), 257–288, pls. 60–63 (1919).
- 27.Srinivasa Rao H. The aquatic and amphibious Mollusca of the northern Shan States, Burma. Records of the Indian Museum. 1928;30:399–468. [Google Scholar]
- 28.Preston HB. A catalogue of the Asiatic naiades in the collection of the Indian Museum, Calcutta, with descriptions of new species. Records of the Indian Museum. 1912;7:279–308. [Google Scholar]
- 29.Preston, H.B. Mollusca (Freshwater Gastropoda & Pelecypoda). Fauna of British India, including Ceylon and Burma (London, Taylor & Francis, 1915).
- 30.Anthony JG. Descriptions of new species of shells. American Journal of Conchology. 1865;1:351. [Google Scholar]
- 31.Benson WH. Descriptions of Indian and Burmese species of the genus Unio, Retz. Annals and Magazine of Natural History (Third Series) 1862;10:184–195. doi: 10.1080/00222936208681307. [DOI] [Google Scholar]
- 32.Martens Ev. Binnen-Conchylien aus Ober-Birma. Archiv für Naturgeschichte. 1899;65:30–48. [Google Scholar]
- 33.Theobald W. Descriptions of some new land and freshwater shells from India and Burmah. Journal of the Asiatic Society of Bengal. 1876;45:184–189. [Google Scholar]
- 34.Haas F. Eine neue indische Najade, Trapezoideus prashadi. Senckenbergiana Biologica. 1922;4:101–102. [Google Scholar]
- 35.Prashad B. Notes on lamellibranchs in the Indian Museum. Records of the Indian Museum. 1920;19:165–173. [Google Scholar]
- 36.Bolotov IN, et al. Ecology and conservation of the endangered Indochinese freshwater pearl mussel, Margaritifera laosensis (Lea, 1863) in the Nam Pe and Nam Long rivers, Northern Laos. Tropical Conservation Science. 2014;7:706–719. doi: 10.1177/194008291400700409. [DOI] [Google Scholar]
- 37.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- 38.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talavera G, Castresana J. Improvement of phylogenies afer removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 40.Villesen P. FaBox: an online toolbox for fasta sequences. Molecular Ecology Notes. 2007;7:965–968. doi: 10.1111/j.1471-8286.2007.01821.x. [DOI] [Google Scholar]
- 41.Ronquist F, et al. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, M., Pfeiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Gateway Computing Environments Workshop (GCE). 1–8 (IEEE, 2010).
- 43.Rambaut, A., Suchard, M. & Drummond, A. J. Tracer v1.6http://beast.bio.ed.ac.uk/sofware/tracer/ (2013).
- 44.Silvestro D, Michalak I. RaxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution. 2012;12:335–337. doi: 10.1007/s13127-011-0056-0. [DOI] [Google Scholar]
- 45.Graf, D. L. & Cummings, K. S. The freshwater mussels (Unionoida) of theWorld (and other less consequential bivalves), updated 9 August 2018. MUSSEL Project Web Sitehttp://www.mussel-project.net (2018).
- 46.Iwata H, Ukai Y. SHAPE: a computer program package for quantitative evaluation of biological shapes based on elliptic fourier descriptors. The Journal of Heredity. 2002;93:384–385. doi: 10.1093/jhered/93.5.384. [DOI] [PubMed] [Google Scholar]
- 47.Wessel P, Smith WHF. A. Global Self-consistent, Hierarchical, High-resolution Shoreline Database. J. Geophys. Res. 1996;101:8741–8743. doi: 10.1029/96JB00104. [DOI] [Google Scholar]
- 48.Lehner B, Grill G. Global river hydrography and network routing: baseline data and new approaches to study the world’s large river systems. Hydrological Processes. 2013;27(15):2171–2186. doi: 10.1002/hyp.9740. [DOI] [Google Scholar]
- 49.Lehner B, Verdin K, Jarvis A. New global hydrography derived from spaceborne elevation data. Eos, Transactions, AGU. 2008;89(10):93–94. doi: 10.1029/2008EO100001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The type series of the new species are available in the malacological collections of the Russian Museum of Biodiversity Hotspots (RMBH), Federal Center for Integrated Arctic Research, Russian Academy of Sciences, Arkhangelsk, Russia (Yaukthwa inlenensis sp. nov.) and Florida Museum of Natural History (UF), Gainesville, USA (Y. paiensis sp. nov.). The sequences obtained in this study are available from the NCBI GenBank database. Species locality, accession numbers and vouchers for each specimen are presented in Supplementary Table 1 as well as Table 1.