Abstract
Although structural and functional neuroimaging techniques have recently been used to investigate the mechanisms of sexual attraction to children, a hallmark of pedophilic disorder, the differences in the processing of child sexual stimuli between men attracted to children and those attracted to adults remain unclear. Here, our purpose was to identify through positron emission tomography the brain responses of 15 male outpatients with pedophilic disorder to validated visual sexual stimuli depicting children (VSSc) and to compare them with 15 male healthy controls matched for sexual orientation (to female or male adults), age, and handedness. The patients' sample comprised both offenders and non-offenders. In response to VSSc, the between-groups analysis showed that activation in the right inferior temporal cortex [Brodmann area (BA) 20] was lower in patients than in controls. Moreover, in patients but not in controls, the presentation of VSSc induced an activation in a more caudal region of the right inferior temporal gyrus (BA 37) and in the left middle occipital gyrus (BA 19). In addition, in patients the level of activation in the caudal right inferior temporal gyrus was positively correlated with ratings of sexual arousal elicited by VSSc, whereas this correlation was negative in BA 20. These results implicate the right inferior temporal gyrus as a possible candidate area mediating sexual arousal in patients with pedophilic disorder and suggest that two of its areas play opposite, i.e., activating and inhibitory, roles.
Keywords: Pedophilic disorder, Positron Emission Tomography, Visual sexual stimuli, Brain, Temporal cortex
Abbreviations: Ch, children; Ad, adults; DSA, Desire for Sexual Activity; PE, Perceived Erection; SA, sexual arousal; VSSc, visual sexual stimuli depicting children; VSSa, visual sexual stimuli depicting adults
Highlights
-
•
Images of children activate the pedophilic patients' right Brodmann area (BA) 37.
-
•
Images of children (IC) activate the pedophilic patients' left BA 19.
-
•
Right BA37 activation correlates with pedophilic patients' rating of sexual arousal.
-
•
IC induce lower right BA 20 activation in pedophilic patients than in controls.
1. Introduction
In their review of the rates of child sexual abuse (CSA), Barth et al. (2012) concluded that the prevalence of CSA in children and adolescents under 18 years of age ranged from 8 to 31% for females and from 3 to 17% for males. Several studies of the consequences of CSA on the victims' mental health show that a history of CSA is associated with a substantially higher prevalence of various psychiatric disorders in adulthood (Kendler et al., 2000; Nelson et al., 2002; Pérez-Fuentes et al., 2013). It is, therefore, justified to consider CSA as a public health problem.
Importantly, not all men with histories of sexual offenses against children meet the diagnostic criteria for pedophilic sexual disorder. As pointed out by Mohnke et al. (2014), failure to distinguish child sexual offending from pedophilic sexual interest may have biased earlier studies of the neural correlates of pedophilia. In addition, recent studies have shown that clinical characteristics as well as both structural and functional neuroimaging findings differed across offending and non-offending patients with pedophilia (Gerwinn et al., 2018; Kärgel et al., 2017; Schiffer et al., 2017).
At least half of the perpetrators of CSA do meet the diagnostic criteria of pedophilia (Seto, 2008). Although its causes are poorly known, pedophilic orientation has repeatedly been found associated with a history of CSA (Seto, 2008). In addition, evidence supports a neurodevelopmental etiology, at least for pedophilic child sexual offending. Compared with teleiophilic controls, men with pedophilia with a history of child sexual offenses are more likely to report head injuries before the age of 13 (Blanchard et al., 2003), obtain lower IQ scores on average (Cantor et al., 2004; Cantor et al., 2005), show increased rate of non-right-handedness (Cantor et al., 2004) and alterations in fronto-executive and/or temporal verbal skills (Fabian, 2012; Joyal et al., 2007). Given the major role of the brain in sexual arousal (SA) (Stoléru et al., 2012), several recent studies have investigated the possible implication of structural and/or functional brain abnormalities in the pathophysiology of pedophilic disorder. The identification of specific dysfunctions of brain areas or brain networks might lead to novel approaches to the prevention of first sexual offenses and of recidivism.
Several studies using voxel-based morphometry have reported widely diverging results. As reviewed by Mohnke et al. (2014), the only replicated finding is a reduction in amygdalar volume (Poeppl et al., 2013; Schiltz et al., 2007). Although there may not be any consistent structural brain abnormality in pedophilia, sexual attraction to children may have functional neural correlates. In seven functional magnetic resonance imaging (fMRI) studies, brain responses to erotic pictures were compared across patients and teleiophilic controls (for a review, Mohnke et al., 2014). Within-group analyses have shown that regions activated in men with pedophilic disorder in response to VSSc were similar to those activated in teleiophiles in response to VSSa (Poeppl et al., 2011; Schiffer et al., 2008a, Schiffer et al., 2008b). A preliminary meta-analysis (Polisois-Keating and Joyal, 2013) confirmed this result by demonstrating robust activations in both groups within the fusiform gyrus, occipital cortex, cerebellum, anterior cingulate cortex, and substantia nigra in response to the subjects' preferred sexual stimuli. Activated regions were distributed over the four components of the neurophenomenological model of SA, i.e., the cognitive, motivational, emotional and bodily components (Stoléru et al., 2012).
The second category of analyses has directly compared patients and controls (between-groups analyses). In response to pictures representing children, the right medial frontal gyrus (Poeppl et al., 2011; Schiffer et al., 2008a), the hippocampus, and the thalamus (Poeppl et al., 2011; Schiffer et al., 2008b) were repeatedly found more strongly activated in men with pedophilic disorder than in teleiophiles. However, locations of maximal activation in the activated clusters were divergent across studies, except for the hippocampus. For some other regions, as reviewed by Mohnke et al. (2014), diverging results were reported; for instance, Schiffer et al. (2008b) found a greater anterior cingulate cortex activation in response to images of children in patients than in teleiophiles, a result not replicated by Poeppl et al. (2011).
In a study comparing patients attracted to boys with heterosexual healthy controls, patients exhibited more activation in the right amygdala in response to pictures of both boys and girls wearing swimsuits or underwear, with the highest activation observed for boys (Sartorius et al., 2008).
In response to VSSa, the findings in teleiophiles of a higher activation in the insula (Poeppl et al., 2011; Walter et al., 2007) and in the right cuneus (Schiffer et al., 2008b; Walter et al., 2007) were replicated.
Finally, Ponseti et al. (2012) showed that it was possible to diagnose pedophilic attraction on the sole basis of fMRI responses to child versus adult stimuli. They found preference-specific activity in areas involved in processing sexually arousing stimuli (Stoléru et al., 2012), such as the caudate nucleus, cingulate cortex, insula, and fusiform gyrus.
Reviews of the literature have suggested some methodological improvements, including larger sample sizes, control for the recruitment setting (in-patient vs. out-patient status), stricter inclusion/exclusion criteria to better limit potential confounds, control of sexual orientation (gender orientation and/or exclusivity of pedophilic preference), and validation of stimulus sets (Mohnke et al., 2014; Tenbergen et al., 2015). In the present study, we incorporated several of these recommendations. In addition, we used penile plethysmography as an objective index of SA in response to visual sexual stimuli (VSS).
Our objective was to study male outpatients with pedophilic disorder in order (i) to identify through Positron Emission Tomography (PET) their brain responses to validated VSS, and (ii) to compare them with healthy controls matched on sexual orientation (to female or male adults), age, and handedness.
In the neurophenomenological model developed by Stoléru et al. (2012), the cognitive component of SA comprises a process of appraisal through which specific stimuli, e.g., adults and/or children, males and/or females, are categorized as sexual incentives and quantitatively evaluated as such. Our main hypothesis was that brain activation in men with pedophilic disorder in response to VSSc would comprise regions mediating the cognitive component of SA, including the lateral part of the right orbitofrontal cortex and the caudal part of the fusiform gyri (for a review, Stoléru et al., 2012). Conversely, we hypothesized that VSSc would elicit lower, or no, activation in these regions in controls. In addition, as our proposed model of SA posits that the activation of the right lateral orbitofrontal cortex is the starting point of “downstream” components, we hypothesized that a higher activation would be found in patients than in controls in the insula bilaterally (emotional component), the left anterior cingulate gyrus (motivational component), and the hypothalamus (bodily component).
2. Methods
2.1. Participants
Fifteen patients were recruited through centers specialized in the treatment of persons with sexual offense histories and of patients with paraphilias. The latter included patients who had not committed CSA, but used child pornography or remained totally abstinent. Fifteen controls were recruited through a web platform dedicated to informing potential participants about current investigations in psychology and neuroscience.
Inclusion criteria common to the two groups were male gender and age between 18 and 65. Specific inclusion criteria for patients were: (i) to meet the criteria for pedophilia according to the DSM-IV-TR (American Psychiatric Association, 2000) and/or according to the International Classification of Diseases (World Health Organization, 1992); the latter classification allows for the inclusion of patients attracted to children at the early stage of puberty; patients who had not committed CSA could be included as they met the second clause of the second criterion of the DSM IV-R, “The person has acted on these urges, or the sexual urges or fantasies cause marked distress or interpersonal difficulty” and/or the corresponding clause of the ICD-10 definition, “Acts on the urges or is markedly distressed by them”; (ii) to acknowledge sexual attraction to prepubescent children; (iii) willingness to participate in treatment to prevent first, or further, sexual offenses. As a specific inclusion criterion, each control had to match an already recruited patient on age (± 13 years), on the Edinburgh Handedness Inventory score (± 50 points), and on sexual orientation (to adult women, men, or to women and men for controls matching patients attracted to girls, to boys, or to children of both genders, respectively).
Exclusion criteria common to both groups were: (i) IQ < 70; (ii) brain disorders; and (iii) current detention. Specific exclusion criteria for patients were: (i) current treatment with antihormonal therapy or with antidepressant drug of the selective serotonin reuptake inhibitor family; (ii) endocrine disorder affecting the hypophysis, the hypothalamo-pituitary-gonadal axis or the adrenal glands. Specific exclusion criteria for controls were: (i) psychiatric disorders, including sexual and substance-related disorders; (ii) psychotropic medication; (iii) drug treatment with potential sexual side-effects.
Protection of subjects' intimacy was ensured following guidelines (Rosen and Beck, 1988). Subjects received financial compensation for participating. The study was approved by the local ethics committee, and written informed consent was obtained after complete description of the study. All procedures performed in the study were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments.
2.2. Assessment of participants
A psychiatrist interviewed each patient to confirm the diagnosis of pedophilia and each control to assess potential psychiatric disorders or medication. A psychologist conducted a standardized psychological assessment with all participants. Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971), and IQ was evaluated with a seven-subtest short form of the Wechsler Adult Intelligence Scale-III (Axelrod et al., 2001). Sexual function was assessed as follows in all participants, unless otherwise specified: (1) to further characterize the sexual orientation of all participants and identify potential interest in children in controls, all subjects were presented with standardized questions, redacted by our team, on the age and sex of persons to whom they felt attracted; (2) to assess the severity of pedophilia and identify potential comorbidity of several paraphilias (Raymond et al., 1999), patients were presented with the Sex History Questionnaire Revised (SHQ-R; Langevin and Paitich, 2002); (3) to obtain a quantitative assessment of the current level of sexual interest, two instruments were used: (i) the questionnaire on Sexual Fantasies, Desires and Activity (SFDAQ; Stoléru et al., 2011) which focuses on the last 30 days; while its first part assesses the levels of fantasies, of desires and of sexual activity whatever their targets (adults, adolescents and/or children), the second focuses on the levels of fantasies, of desires and of sexual activity exclusively directed to prepubescent children; Fantasies and Desires scores range theoretically from 1 to 18, Activities scores from 1 to 24, and the means from 1 to 20; (ii) the Sexual Interest Score (SIS, Bancroft et al., 1974), which does not specify the targets of sexual interest; (4) in order to assess sexual function directed to adults during the last month, all participants were presented with the Brief Sexual Function Questionnaire (BSFQ; Reynolds et al., 1988); (5) finally, to compare the controls with those of previously published studies, control subjects were presented with the Sexual Arousal Inventory (SAI; Hoon et al., 1976), adapted to men.
Other aspects of psychopathology were assessed using the Symptom Checklist-90-Revised (SCL-90R; Derogatis, 1977), the 21-item Beck Depression Inventory version (Beck, 1978), and the Barratt Impulsiveness Scale (BIS-10; Patton et al., 1995).
A physician performed a medical examination and screened participants for exclusion criteria. A blood sample was obtained for hormonal investigation. Testosterone, sex-hormone binding globulin (SHBG), bioavailable testosterone (not bound to SHBG), and estradiol were measured by using previously reported methods (Déchaud et al., 1989) and commercially available kits were used for routine measurements of LH, FSH, prolactin, and cortisol.
The above-mentioned assessments were distributed over two to three sessions in patients and over one or two sessions in controls. On the following session, functional images were acquired. At the end of each PET scan, subjects were presented with a series of rating scales, ranging from 1 (null or extremely low) to 9 (extremely high), to assess seven kinds of subjective responses to each of the picture categories: desire for sexual activity (DSA), perceived erection (PE), beauty of presented characters, pleasure, displeasure, attention, tenderness. Participants indicated their answers through an audio system connected to the acquisition room. The functional imaging acquisitions were coupled with penile response measurements performed by using a volumetric penile plethysmograph (described in Mouras et al., 2008).
2.3. Positron Emission Tomography
2.3.1. Stimuli and design
Six categories of pictures were presented: (i) undressed, or lightly dressed, female children; (ii) undressed, or lightly dressed, male children; (iii) ordinarily dressed female children; (iv) ordinarily dressed male children; (v) undressed, or lightly dressed, adults of the participant's preferred gender; and (vi) ordinarily dressed adults of the preferred gender. Pictures of adults of the non-preferred gender were not presented for radiation exposure limitation considerations.
All pictures showed only one person. Most were obtained free of rights, or, for some pictures representing children, from a photographic agency. Most photographs of undressed or lightly dressed children showed children in swimming suits, with two back-view photographs showing a nude boy, and two back-view photographs a nude girl. Pictures of adult women were the same as those used in our previous studies (e.g., Mouras et al., 2008). Pictures of children and those of adult males were selected out of a large series of photographs by 15 control subjects and 11 men with histories of sexual offenses against children, and not otherwise involved in the experiment. They rated the level of SA and of negative emotion induced by each picture on scales ranging from 1 to 9. Then, the sets of selected pictures of undressed or lightly dressed adults and children were matched on the average SA ratings, level of nudity and view of the portrayed person (front, back, profile or three-quarter front views). Pictures of dressed adults and children were also matched in the same manner, except for nudity degree. Pictures retained as stimuli did not induce negative emotions.
Subjects were scanned in six experimental conditions, each of which was defined by the category of presented pictures. For each condition, two scans were performed to ameliorate the signal/noise ratio (12 acquisitions per subject). Conditions were presented in a pseudorandom order across participants, as each patient and matched control had the same assigned order. Each condition comprised 9 photographs, each presented for 10 s. Pictures were displayed through a mirror and a screen disposed behind the scanner. For each scan, the following procedure was followed: presentation of three pictures (30 s) in order to induce SA; start of PET acquisition simultaneously with the presentation of six photographs (60 s). To ensure participants paid attention to the stimuli, they were requested to press a button of a keypad when a brief orange screen (0.5 s) appeared, once for each scan and at a random time. A ten-minute interval separated successive PET scans to allow for sufficient decay of radioactivity, for the collection of subjective ratings, and for the presentation of a two-minute emotionally neutral documentary to help participants regain their baseline emotional state.
2.3.2. Neuroimaging data acquisitions
Prior to functional acquisitions, a structural MRI examination was performed in order both to identify potential brain abnormalities and to record the anatomical volume used for PET data processing. Structural MRIs were acquired on a Siemens Magnetom Sonata 1.5 T scanner (Siemens, Erlangen, Germany), using a 3D anatomic T1-weighted sequence covering the whole brain, with the following parameters: 176 slices; slice thickness = 1 mm; TE = 3.93 ms; TR = 1970 ms; 1 mm × 1 mm pixel size, 256 × 256 matrix).
PET data were acquired on a PET-CT tomograph (Siemens Biograph mCT/S 64, Erlangen, Germany). Subjects were surrounded by a curtain isolating them from staff's view. Head position was maintained by a vacuum cushion. To check that anxiety levels were low before beginning data acquisition, a short version of the State-Trait Anxiety Inventory (Van Knippenberg et al., 1990) was presented. At the beginning of the session, a low-dose CT scan (<0.3 mSv) was carried out in order to estimate tissue attenuation. For each scan, the regional cerebral blood flow (rCBF) was measured by recording the radioactivity distribution following a bolus injection of 333 MBq (9 mCi) of [15O] H2O. Emission data were collected in 3D list-mode.
The first 60 s of each 90 s PET scan corresponding to visual stimulation were then reconstructed using the 3D OPSEM iterative algorithm, incorporating point spread function and time of flight, with a Gaussian filter of 3 mm and after correction for scatter and attenuation. Reconstructed volumes consisted in 109 contiguous slices (2.03-mm thickness) of 200 × 200 voxels in-plane (1.36 × 1.36 mm2). The actual resolutions for reconstructed images were approximately 2.6 mm in full width at half maximum in the axial direction and 3.1 mm in full width at half maximum in the transaxial direction measured for a source located 1 cm from the field of view (Jakoby et al., 2011; Martí-Climent et al., 2013).
2.3.3. PET data preprocessing
Statistical Parametric Mapping software (SPM12, Wellcome Department of Cognitive Neurology, London, UK) was used for image preprocessing and subsequent analysis. The PET rCBF volumes of each subject were realigned with the first acquired volume, in order to correct for subject's motion during acquisition. The T1 structural MRI image was then coregistered with PET volumes, using the mean functional image as a reference, and spatially normalized into the standard MNI space (Montreal Neurological Institute/International Consortium for Brain Mapping stereotactic space) using the segment function of SPM12. Spatial normalization parameters were applied to the realigned PET volumes, and finally, images were smoothed using an isotropic 8 mm full width at half maximum Gaussian kernel to reduce the variance due to inter-individual anatomical variability and to improve the signal/noise ratio in individual data.
2.3.4. Contrast analyses
Preprocessed PET volumes were analyzed with SPM using flexible factorial designs, defined by a Subject factor, a six-level Condition factor and, for between-groups comparisons, a Group (Patients vs. Controls) factor. The models were adjusted using the Global Mean (ANCOVA).
In order to increase statistical power, we pooled into the same group heterosexual and homosexual patients on the one hand, and heterosexual and homosexual controls on the other hand. Pooling was conducted as heterosexual and homosexual patients did not show any differential responses to the pictures of their respective preferred gender [(Girlsundressed − Girlsdressed)heterosexual patients – (Boysundressed − Boysdressed)homosexual patients, and opposite contrast].
Contrasts are listed in Table 1. Regional responses to the Dressing style factor (Dressed versus Undressed) were analyzed on a voxel-wise basis for pictures showing prepubescent girls (i.e., Girlsresponse = Girlsundressed − Girlsdressed), prepubescent boys, as well as adult females or adult males. All contrasts were based on stimuli with the preferred gender.
Table 1.
Statistical contrasts tested both for behavioral variables and PET data.
| Contrast number | Contrast |
|---|---|
| C1 | (Chresponse)patients – (Chresponse)controls |
| C2 | (Adresponse)controls – (Adresponse)patients |
| C3 | (Chresponse)patients – (Adresponse)controls |
| C4 | (Chresponse)patients – (Adresponse)patients |
| C5 | (Chresponse)offender_patients – (Chresponse)non-offender_patients |
Note. Ch: children; Ad: adults; “Response” refers to the effect of dressing style, i.e., undressed or lightly dressed minus ordinarily dressed. For every “(X-Y)” contrast, its opposite, “(Y-X)”, was also tested.
As planned before acquiring the data, we compared patients and controls on both their behavioral and brain responses (1) to pictures of children (Ch) of the patients' preferred gender; (2) to pictures of adults (Ad) of the patients' preferred gender; (3) to pictures of characters with both the preferred age and gender. Other analyses were performed post hoc. (4) We compared patients' responses to images of prepubescent children with their responses to images of adults: [(Chresponse)patients − (Adresponse)patients]. (5) In line with Ponseti et al. (2012), we subtracted in each group the responses to female (resp., male) VSSc from the responses to female (resp., male) VSSa and then compared patients with controls on the obtained differences. (6) Finally, in an exploratory analysis, we compared patients who had committed child sexual offenses to patients with no histories of child sexual offenses on their responses to the nudity of preferred children.
To further compare brain responses across groups, contrasts obtained in one group were used as masks for contrasts obtained in the other group. Exclusive masks reveal clusters in one group that do not overlap with those from the other group.
2.3.5. Correlational analyses
In order to study the correlation between psychological (DSA, PE, beauty of presented characters, pleasure, displeasure, attention, tenderness) or physiological (induced erection) responses of subjects and the PET signal, we conducted regression analyses by introducing each of these parameters as covariates in the SPM statistical model (Flexible factorial design, subject factor, 1 or 2-level group factor, 1 covariate, adjusted on global mean).
Regarding plethysmographic data, for each participant presenting an erectile response and each PET acquisition, the Area Under the Curve (AUC) was computed from the corresponding dataset, using as a baseline the mean plethysmographic value recorded during the first 15 s of visual stimuli presentation.
For all these regression analyses, within and between-groups analyses were performed, assessing both positive and negative correlations.
While correlational analyses between hormonal levels, in particular those of hormones of the pituitary-gonadal axis, and brain activity would have been of great interest, we could not perform such exploration for the following reasons: (i) PET analyses are performed following a fixed-effects methodology (Group factor). As we acquired 12 scans per subject (2 repetitions per condition * 6 conditions), a random-effects approach, i.e., a second-level analysis as performed with fMRI data, is impossible because of lack of statistical power at the individual level; (ii) exploring the correlation between any hormonal level and brain activity in such a fixed-effects design would require one hormone measurement for each PET scan entered in the model, i.e., 12 hormonal assays, but in our experiment, we performed one hormonal assay per subject.
2.3.6. PET data exploratory and hypothesis-driven analyses
Regarding statistical significance, inferences were made based on cluster-wise significance (p < 0.05 FWE-corrected). Accordingly, although peak MNI coordinates and T-value are reported in the tables, it can only be inferred that there is signal somewhere within the cluster and no inferences can be made about the statistical significance of specific locations within the cluster (Woo et al., 2014). To minimize the risk of false-positive (type I) errors (Eklund et al., 2016), the cluster-forming height threshold was set at P = 0.001, uncorrected, as recommended in Roiser et al. (2016) and Woo et al. (2014). This also applied to results of masking procedures, with masks thresholded at p < 0.001, uncorrected. All analyses were conducted in the following two spaces.
Exploratory analyses. We performed traditional exploratory analyses in the whole brain.
Hypothesis-driven analyses. We also used a set of regions previously found to show activation or deactivation in response to VSS in healthy adult males in two meta-analyses (Poeppl et al., 2014; Stoléru et al., 2012). Those regions were hypothesized to respond to images of children in pedophiles. For each region, we specified its shape, center, radius, and dimensions based on information available in the two meta-analyses (Poeppl et al., 2014; Stoléru et al., 2012) and thus defined a sphere or a box centered on a peak voxel. In order to apply SPM12 small-volume correction procedure, we used WFU PickAtlas software (Maldjian et al., 2003) to define two masks of activated and deactivated regions of interest, respectively. These masks were then used to apply small-volume corrections (SVC) on activation and deactivation results obtained in the whole brain analyses. The same procedure was used to define two masks and perform SVC analyses for brain regions positively or negatively correlated with indices of SA.
T-tests or chi-square tests were performed to compare patients and controls on sociodemographic, clinical and behavioral variables. Repeated measures analyses of variance of subjective ratings and of erectile responses were performed using SPSS® 19 software (Armonk, NY). Regarding erectile responses, the dependent measure was the mean area under the two curves obtained for each category of pictures (mean of the two acquisitions performed for each category of pictures).
3. Results
3.1. Sociodemographic and clinical characteristics
Sociodemographic and sexological characteristics of participants are presented in Table 2. Ten patients had sexually offended children, while five had used child pornography or remained totally abstinent. Seven patients were attracted to prepubescent girls and five to prepubescent boys; these patients are called heterosexual and homosexual patients, respectively. Three were attracted to children of both genders; these patients were assigned to the subgroup corresponding to the children of their preferred gender, which was the homosexual subgroup for all three. Twelve patients were non-exclusive, i.e., were also attracted to adults. Six heterosexual patients and two bisexual patients were attracted to adult women; four homosexual patients were attracted to adult men; and three patients – one heterosexual, one homosexual and one bisexual - declared no sexual attraction to adults. Ten patients were attracted to children outside of the family (six with a history of child sexual offenses), one was incestuous (with a history of child sexual offenses), and four belonged to both categories (three with a history of child sexual offenses). Among controls, eight were heterosexual (here “heterosexual” refers to adult women), four were homosexual, and three bisexual. One bisexual control was predominantly homosexual and was presented with male VSSa, the two other bisexual controls predominantly heterosexual and presented with female VSSa. For the three bisexual controls the gender of their “preferred” children was male, in accordance with the orientation of their matched patients. It should be noted that one heterosexual control was matched with a bisexual patient (predominantly attracted to boys) who was more attracted to adult women than to adult men and was presented with female VSSa; in PET contrast analyses (see 3.3.1. below) where patients' brain responses to children of their preferred gender were compared to those of controls, the brain responses of this heterosexual control were those to male children, in accordance with the orientation of his matched patient.
Table 2.
Sociodemographic and sexological characteristics of subjects.
| Variable | Patients n = 15 |
Controls n = 15 |
P |
|---|---|---|---|
| Age | 42.0 ± 12.6 | 41.2 ± 16.2 | NS |
| Years of education | 16.6 ± 5.5 | 14.9 ± 4.1 | NS |
| Sexual partnership with adult (M/PS/PNS/NoP) | 4/2/2/7 | 3/6/3/3 | NS |
| SHQ-R | |||
| Female Child Frequency (heterosexual patients) | 57.5 ± 27.5 | NP | NA |
| Male Child Frequency (homosexual patients) | 65.5 ± 26.6 | NP | NA |
| SFDAQ | |||
| Fantasies, any target | 7.9 ± 6.6 | 4.3 ± 2.5 | NS |
| Desires, any target | 15.5 ± 5.0 | 16.7 ± 3.1 | NS |
| Sexual activity, any target | 9.1 ± 4.7 | 8.6 ± 1.5 | NS |
| Mean score, any target | 10.8 ± 3.3 | 9.9 ± 1.7 | NS |
| Fantasies, children | 5.5 ± 4.7 | 1.0 ± 0.0 | <0.01 |
| Desires, children | 10.9 ± 7.3 | 1.0 ± 0.0 | <0.001 |
| Sexual activity, children | 5.4 ± 3.3 | 1.0 ± 0.0 | <0.001 |
| Mean score, children | 7.3 ± 4.6 | 1.0 ± 0.0 | <0.001 |
| Sexual interest score | 3.0 ± 1.1 | 2.8 ± 0.6 | NS |
| BSFQ factors | |||
| Sexual activity | 40.4 ± 31.3 | 85.3 ± 28.5 | <0.001 |
| Sexual interest | 37.8 ± 29.7 | 45.8 ± 27.2 | NS |
| Sexual satisfaction | 3.9 ± 2.7 | 7.5 ± 2.8 | <0.01 |
| Physiological component | 12.7 ± 6.2 | 20.5 ± 6.7 | <0.01 |
Note. Figures are means ± SDs, except for sexual partnership where figures are frequencies; BSFQ: Brief Sexual Function Questionnaire; M: Married or living together; NA: not applicable; NP: not presented; NoP: no current adult partner; NS: Not significant; PNS: Partner, Not Stable; PS: lasting relationship, Same Partner, not living together; SFDAQ: Sexual Fantasies, Desires and Activities Questionnaire; SHQ-R: Sex History Questionnaire-Revised; figures are percentiles referring to a sample of sexual offenders gathered by the SHQ-R developers. “Female/Male Child Frequency” refer to the overall frequency of sexual activities of various types with children over the lifetime of the patients.
On the BSFQ, patients had lower scores than controls on Sexual Activity, Sexual Satisfaction, and Physiological Component, while Sexual Interest did not differ significantly across groups. On the BDI and on the SCL-90R, patients had significantly higher scores than controls (see Inline Supplementary Table 1). Twelve patients were receiving some form of psychological treatment at the time of the study, mostly individual supportive psychotherapy, for their pedophilic disorder.
Patients and controls did not differ significantly on intelligence quotient (Table 2 and see Inline Supplementary Table 1). Regarding hormonal measurements (see Inline Supplementary Table 2), the plasma levels of total testosterone and of bioavailable testosterone, i.e., testosterone unbound to the Sex-Hormone Binding Globulin, were lower in patients.
3.2. Measures of sexual arousal during PET scans
We first provide the results of the analyses of the behavioral indexes of SA, based on the same contrasts (Table 1) as those subsequently reported for the neural responses.
3.2.1. Ratings of stimuli
Here we detail the results of analyses focused on two rating scales, DSA and PE (Table 3). Analyses related to the other scales are presented in Inline Supplementary Table 3.
Table 3.
Comparison of patients and controls on desire for sexual activity and perceived erection ratings of stimuli of their preferred gender.
| Variable | Patients |
Controls |
Group effect | Condition effect | Group by condition |
|---|---|---|---|---|---|
| n = 15 | n = 15 | ||||
| Desire for sexual activity | |||||
| Children, undressed | 4.8 ± 1.9 | 1.0 ± 0.1 | <0.01E-4 | <0.01E-5 | <0.01E-5 |
| Children, normally dressed | 2.2 ± 1.3 | 1.0 ± 0.0 | |||
| Adults, undressed | 5.0 ± 2.5 | 5.9 ± 1.7 | NS | <0.01E-7 | NS |
| Adults, normally dressed | 1.2 ± 0.5 | 1.8 ± 0.8 | |||
| Characters with preferred age, undressed | 4.8 ± 1.9 | 5.9 ± 1.7 | NS | <0.01E-9 | <0.05 |
| Characters with preferred age, dressed | 2.2 ± 1.3 | 1.8 ± 0.8 | |||
| Perception of erection | |||||
| Children, undressed | 3.1 ± 2.1 | 1.0 ± 0.0 | <0.01 | <0.001 | <0.001 |
| Children, normally dressed | 1.4 ± 1.0 | 1.0 ± 0.0 | |||
| Adults, undressed | 2.9 ± 2.1 | 3.5 ± 2.0 | NS | <0.01E-3 | NS |
| Adults, normally dressed | 1.0 ± 0.0 | 1.2 ± 0.5 | |||
| Characters with preferred age, undressed | 3.1 ± 2.1 | 3.5 ± 2.0 | NS | <0.01E-3 | NS |
| Characters with preferred age, dressed | 1.4 ± 1.0 | 1.2 ± 0.5 | |||
Note. Figures are means ± SDs. Condition effect: Pictures of undressed vs. dressed characters.
For both DSA and PE, compared with controls patients showed a higher difference between ratings for undressed children and for normally dressed children (Contrast 1). This Group by Condition interaction is important as it represents the behavioral correlate of the C1 contrast tested on PET data.
The comparison of patients and controls on their ratings of the pictures of characters with both the age and the gender preferred by each group (Contrast 3) showed a Group by Condition interaction only for DSA, with a lower difference between the conditions in patients.
Finally, the comparison of patients who had committed child sexual offenses to patients with no history of child sexual offenses on their ratings of pictures of preferred children did not reveal any significant differences (Contrast 5).
3.2.2. Penile plethysmography
One homosexual patient could not be included in analyses due to technical problems affecting plethysmographic data collection. Results are presented in Inline Supplementary Table 4. The comparison of the two groups on their responses to pictures showing children of the patients' preferred gender showed a Group by Condition interaction, with a higher difference in patients between responses to images of undressed and dressed children. Finally, the comparison of patients who had committed child sexual offenses to those with no history of child sexual offenses on their responses to pictures of preferred children did not reveal any significant difference (Contrast 5).
3.3. PET imaging
3.3.1. Contrast analyses
Contrast 1. In response to preferred VSSc, patients showed an activation in the right inferior temporal gyrus and in the left middle occipital gyrus (Table 4; Fig. 1). After masking (exclusive mask) the previous contrast with activations observed in patients in response VSSa, the two clusters were no longer present, as they were also activated in response to images of nude adults. Thus, these activations were not specifically related to the age of characters displayed. By contrast, when the same contrast was masked (exclusively) by the controls' response to children's nudity, the two clusters persisted. In other words, these responses to images of children's nudity were characteristic of the patients' group. Finally, when Contrast 1 was masked (exclusively) by the controls' response to adults' nudity, the clusters were no longer apparent.
Table 4.
Brain responses of patients and controls to pictures of children.
| Brain region | BA | k | Side | MNI coordinates |
T | Pcorrected | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Undressed > Dressed | ||||||||
| Patients | ||||||||
| Inf. Temporal Gyr. (SVC+) | 37 | 331 | R | 50 | −68 | −8 | 4.81 | <0.001 |
| Middle Occipital Gyr. | 19 | 159 | L | −40 | −88 | 0 | 4.59 | <0.05 |
| Controls | ||||||||
| Middle Occipital Gyr.a, (SVC+) | 18 | 162 | R | 24 | −98 | 2 | 4.77 | <0.05 |
| Fusiform Gyr. a, SVC- | 18 | 66 | L | −22 | −90 | −18 | 4.21 | <.05SVC |
| Undressed > Dressed: Controls – Patients | ||||||||
| Inferior Temporal Gyr. | 20 | 146 | R | 58 | −34 | −16 | 3.86 | <0.05 |
| Dressed > Undressed | ||||||||
| Patients | ||||||||
| Lingual Gyr. | 18 | 172 | R | 2 | −70 | 0 | 4.45 | <0.05 |
| Controls | ||||||||
| Superior Frontal Gyr. | 6 | 557 | R | 18 | −14 | 72 | 5.15 | <0.001 |
| Paracentral Lobule | 5 | 212 | L | −4 | −42 | 64 | 4.75 | <0.01 |
| Postcentral Gyr. | 43 | 176 | L | −64 | −6 | 24 | 4.04 | <0.05 |
Note. Contrast 1: ChUndressed – ChDressed. All probabilities are corrected for multiple comparisons, based on the Family-Wise Error (FWE) rate control. Pcorrected refers to cluster-wise significance (cluster-forming threshold: 0.001, unc.; cluster-extent thresholds: for (Undressed – Dressed), FWEc = 159 for patients and FWEc = 162 for controls; for (Dressed – Undressed), FWEc = 172 for patients and FWEc = 176 for controls; for between-groups analysis, FWEc = 146). k: Cluster-size; SVC+, SVC- Small Volume Correction, based on areas showing activation or deactivation, respectively, in response to VSSa in healthy males; (SVC+) Region identified both within whole mask and with small volume correction; for these regions, unless otherwise noted, Pcorr refers to analysis in whole mask. Gyr.: gyrus; BA: Brodmann area.
Cluster that survives exclusive masking by regions activated in response to images of nude adults (exploratory analysis).
Fig. 1.
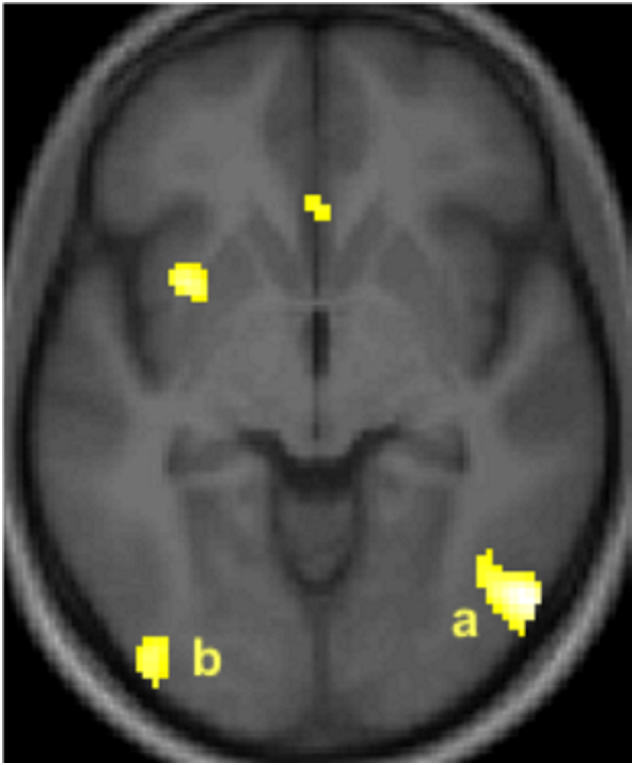
Patients' brain activation in response to pictures of undressed children of their preferred gender in (a) the right inferior temporal (Brodmann area 37) and (b) the left middle occipital (Brodmann area 19) gyri.
Note. Activated clusters (PFWE < 0.05, corrected) are rendered on the normalized whole sample mean structural MRI in an axial section, 6 mm below the bicommissural plane; the anterior part of the brain is in the upper part of the figure and the right hemisphere is on the right. For display, height threshold: T = 3.14; extent threshold: k = 0. Rostral clusters failed to reach statistical significance threshold.
Regarding controls, analysis of contrast 1 showed an activation in the right middle occipital gyrus. In addition, an SVC analysis, limited to the space of regions that get deactivated in healthy subjects in response to VSSa, showed a cluster in the left fusiform gyrus (Table 4). Thus, it is only in controls that a region considered as inhibitory in the neurophenomenological model was found activated in response to images of undressed children. After masking (exclusive mask) the previous contrast with activations observed in controls in response to nude adults of the preferred gender, both clusters survived. Thus, these clusters of activation characterized the controls' responses to images of children's nudity, and not to adults' nudity. Similarly, when the contrast was masked exclusively by the patients' response to adults' nudity, both clusters survived. Finally, when the same contrast was masked exclusively by the patients' response to children's nudity, both clusters persisted. In other words, these controls' responses were both stimulus-specific and group-specific. Inline Supplementary Table 5 shows which regions showed both group- and stimulus-specific activations. Conversely, regarding “deactivations” (Dressed – Undressed contrast), in patients there was a deactivated cluster in the right lingual gyrus in response to pictures of undressed children (Table 4).
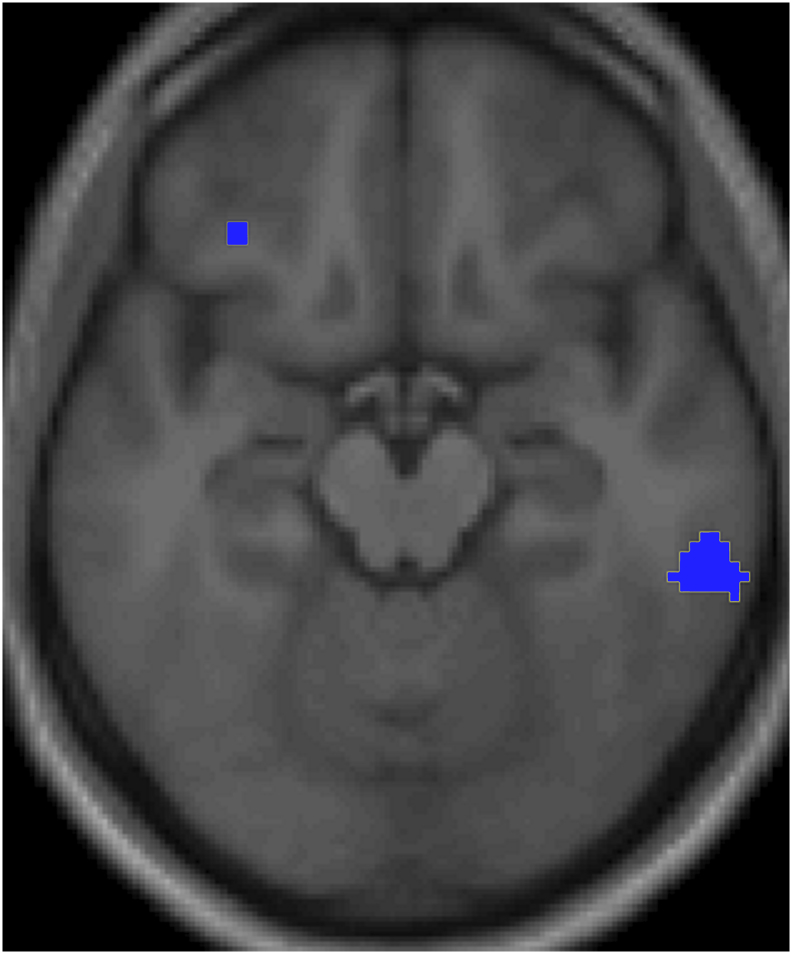
The between-group analysis of the contrast [(Chresponse)Controls − (Chresponse)Patients] showed a cluster in the right inferior temporal gyrus (p < 0.05, corrected) (Fig. 2 and Table 4). This cluster in the right inferior temporal lobe lay in a distinctly more rostral position (BA 20) than the above-mentioned cluster found activated in patients (BA 37).
Fig. 2.
Compared with controls patients' Brodmann area 20 in the right inferior temporal gyrus shows a lower response to undressed children of their preferred gender.
Note. The lesser-activated cluster (PFWE < 0.05, corrected) is rendered on the normalized whole sample mean structural MRI in an axial section, 16 mm below the bicommissural plane; the anterior part of the brain is in the upper part of the figure and the right hemisphere is on the right. For display, height threshold: T = 3.86, extent threshold: k = 0. The rostral cluster failed to reach statistical significance threshold. The blue color, instead of the yellow, is used to show that activation was lower in patients than in controls.
Contrast 2. Regarding the within-group analyses, in response to VSSa, patients showed a significant activation in the right and left middle occipital gyri (Table 5). When this contrast was masked with activations of patients in response to VSSc (Chresponse), activation in the left middle occipital gyrus persisted. Thus, this cluster characterized the patients' responses to images of adults' nudity, and not to children's nudity. In addition, when the patients' response to adults' nudity was masked (exclusively) by the controls' response to adults' nudity, the bilateral activation in the middle occipital gyri persisted. Finally, when the patients' response to adults' nudity was masked (exclusively) by the controls' response to children's nudity, these two clusters survived. In other words, the response in the left middle occipital gyrus characterized the response of patients only (group-specificity) to images of undressed adults only (stimulus-specificity).
Table 5.
Brain responses of participants to pictures of adults of preferred gender.
| Brain regions | BA | k | Side | MNI coordinates |
T |
Pcorr |
||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Undressed > Dressed | ||||||||
| Patients | ||||||||
| Middle Occipital Gyr. (SVC+) | 19 | 808 | R | 50 | −66 | −8 | 5.75 | <0.001 |
| Middle Occipital Gyr. a, (SVC+) | 37 | 349 | L | −38 | −66 | −2 | 4.64 | <0.001 |
| Controls | ||||||||
| Middle Occipital Gyr. a(SVC+) | 19 | 1481 | R | 46 | −78 | −8 | 6.13 | <0.001 |
| Inferior Occipital Gyr. a, (SVC+) | 19 | 527 | L | −46 | −72 | −10 | 4.72 | <0.001 |
| Anterior Insula/Inf. Frontal Gyr.a | 47 | 307 | R | 28 | 14 | −16 | 4.40 | <0.01 |
| Medial Frontal Gyr.a | 10 | 250 | L | −8 | 50 | 4 | 4.94 | <0.01 |
| Dressed > Undressed | ||||||||
| Patients | ||||||||
| Inferior Temporal Gyr. | 20 | 223 | R | 62 | −30 | −20 | 4.55 | <0.01 |
| Inferior Temporal Gyr.SVC- | 39 | 51 | L | −54 | −68 | 28 | 4.22 | <0.05SVC |
| Inferior Frontal Gyr. | 45 | 161 | L | −46 | 24 | 16 | 4.77 | <0.05 |
| Dressed > Undressed: Patients– Controls | ||||||||
| Olfactory Sulcus: Gyr. Rectus/Medial Orbital Gyr. (SVC-) | 11 | 200 | R | 8 | 50 | −24 | 4.13 | <0.05 |
Note. Contrast 2: AdUndressed − AdDressed, and opposite contrast. Probability threshold and abbreviations: see Note of Table 4. Cluster-forming threshold: 0.001, unc.; cluster-extent thresholds: for (Undressed – Dressed), FWEc = 349 for patients and FWEc = 250 for controls; for (Dressed – Undressed), FWEc = 161 for patients; for between-groups analysis, FWEc = 200. (SVC+), (SVC-) Region identified both within whole mask and with small volume correction; for these regions, Pcorr refer to analysis in whole mask.
Cluster that survives exclusive masking by regions activated in response to images of nude children (exploratory analysis).
Conversely, in patients the analysis of the opposite contrast [Addressed – Adundressed] showed a deactivation in both inferior temporal gyri and in the left inferior frontal gyrus (Table 5).
The between-groups analysis of the contrast (Adresponse)controls– (Adresponse)patients showed a cluster in the medial orbitofrontal cortex (Table 5), with a peak in the olfactory sulcus.
Contrast 3. In response to their preferred stimuli, no cluster was more activated in patients than in controls. The same was true for the opposite contrast.
Contrast 4. The analysis of the patients' responses to children's versus adults' nudity showed a cluster in the posterior cingulate cortex [x, y, z = 2, −56, 20; PFWE-corr. <0.05; k = 145 voxels; t = 4.63); it was related to a higher deactivation in response to VSSa than to VSSc.
Contrast 5. Analysis did not show any brain regions responding differentially to VSSc in patients who had committed child sexual offenses (n = 10) compared to those with no history of child sexual offenses (n = 5). The same was true for the comparisons between each subgroup of patients and the controls.
3.3.2. Correlational analyses
Ratings of pictures. In patients the ratings of DSA (whatever the age and gender of characters in pictures) was positively correlated with the level of activation in widespread areas including (i) the right inferior temporal gyrus and the left inferior occipital gyrus, (ii) two clusters located bilaterally in the insula and claustrum, (iii) a cluster at the junction of the anterior insula and the orbital operculum, (iv) the pregenual anterior cingulate gyrus, (v) and a cluster in the cerebellar uvula (Table 6; Fig. 3). The ratings of PE were positively correlated with the level of activation in similar areas, including the right inferior temporal gyrus (BA 37), as well as in the supplementary motor area of the right medial prefrontal gyrus. Conversely, ratings were negatively correlated with rCBF in the right inferior temporal gyrus (BA 20) for Desire for Sexual Activity and with rCBF in the middle temporal gyrus (BA 20) for Perception of Erection.
Table 6.
Areas where responses were correlated with desire for sexual activity in patients.
| Brain region | BA | k | Side | MNI coordinates |
T | Pcorr | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Positive | ||||||||
| Inferior Temporal Gyr. | 37 | 1246 | R | 48 | −68 | −6 | 6.46 | <0.001 |
| Claustrum/insula(SVC+) | – | 256 | R | 28 | 16 | −10 | 5.01 | <0.01 |
| Anterior Insula/Orbital Operculum | 13/47 | 210 | R | 36 | 32 | 4 | 4.83 | <0.01 |
| Inferior Occipital Gyr. | 19 | 480 | L | −44 | −72 | −12 | 4.78 | <0.001 |
| Cerebellum, uvula | – | 150 | L | −10 | −68 | −42 | 4.63 | <0.05 |
| Cerebellum, central lobule SVC+ | NA | 37 | L/R | 0 | −48 | −20 | 4.53 | <0.05 SVC |
| Anterior cingulate Gyr. (SVC+) | 24 | 246 | R | 4 | 34 | 16 | 4.56 | <0.01 |
| Claustrum/Insula (SVC+) | 13 | 210 | L | −34 | 8 | −8 | 4.53 | <0.01 |
| Negative | ||||||||
| Middle frontal Gyr. (SVC+) | 8 | 348 | R | 32 | 26 | 56 | 5.62 | <0.001 |
| Inferior Parietal Lobule | 40 | 220 | R | 54 | −58 | 44 | 4.66 | <0.01 |
| Supramarginal Gyr. | 40 | 157 | L | −60 | −56 | 26 | 4.23 | <0.05 |
| Inferior Temporal Gyr. SVC- | 20 | 81 | R | 56 | −16 | −32 | 4.59 | <0.05SVC |
All probabilities are corrected for multiple comparisons, based on the Family-Wise Error (FWE) rate control. Unless P is associated with “SVC”, Pcorr. refers to cluster-wise significance (cluster-forming threshold: 0.001 unc.; cluster-extent threshold: FWEc = 150 and FWEc = 157 for positive and negative correlations, respectively). SVC+, SVC-: Small Volume Correction, based on areas showing a positive or a negative correlation with markers of sexual arousal in healthy males. (SVC+) Region identified both within whole brain and with Small Volume Correction; for these regions, Pcorr refer to analysis in whole mask. k: Cluster extent; when no k-value is provided, the region belongs to the cluster indicated on the line above. Abbreviations: see Note of Table 4.
Fig. 3.
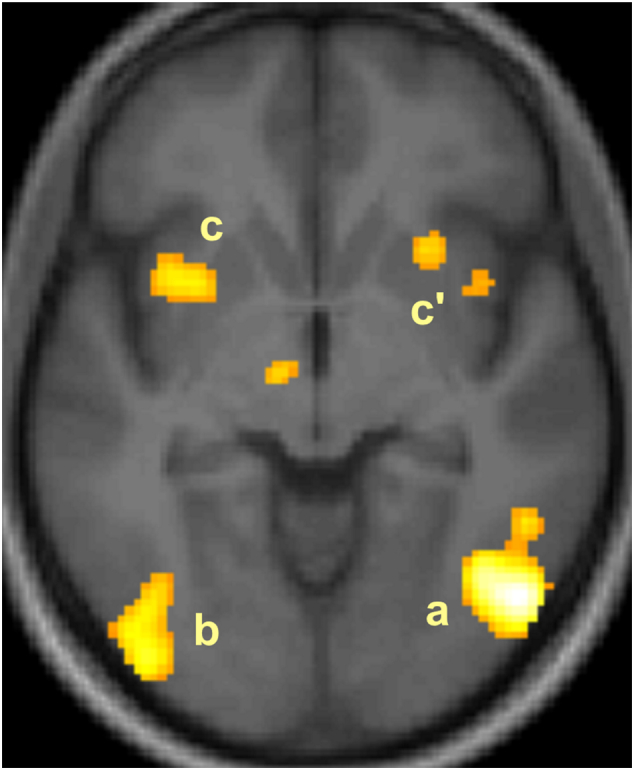
Positive correlation between the patients' Desire for Sexual Activity ratings and the regional cerebral blood flow in the right inferior temporal gyrus (a), left inferior occipital gyrus (b), and bilateral claustrum/insula (c, c’).
Note. Activated clusters (PFWE < 0.05, corrected) are rendered on the normalized whole sample mean structural MRI in an axial section, 6 mm below the bicommissural plane; the anterior part of the brain is in the upper part of the figure and the right hemisphere is on the right. For display, height threshold: T = 3.14; extent threshold: k = 0. Cluster on the left of third ventricle failed to reach statistical significance threshold.
Correlations of DSA ratings with rCBF found in controls are presented in Inline Supplementary Table 6. The results of the correlational analysis of ratings of PE were similar to those for DSA.
The between-groups analysis showed that the ratings of DSA were differentially correlated across groups in the anterior cingulate gyrus (BA 24; x, y, z = −8, 10, 34; PFWE-corr. < 0.05, k = 195), with a higher correlation in patients; conversely, in the cerebellar pyramis (x, y, z = 14, −76, −34; PFWE-corr. < 0.05, k = 163) and in the right gyrus rectus (BA 11; x, y, z = 4, 42, −24; PFWE-corr. < 0.01, k = 319), the correlation was lower in patients than in controls. Similarly, the between-groups analysis showed that the ratings of PE were differentially correlated across groups in the anterior cingulate gyrus (BA 32; x, y, z = −2, 6, 44; PFWE-corr. < 0.001, k = 889), and in the central sulcus (BA 3/BA 4; x, y, z = −52, −12, 46; PFWE-corr. < 0.05, k = 207), with a higher correlation in patients; conversely, in the cerebellar declive (x, y, z = 16, −76, −32; PFWE-corr. < 0.01, k = 255), the correlation was higher in controls.
Penile plethysmography. Twelve patients and 10 controls presented penile responses and were included in the analyses. The regions where the rCBF showed a correlation with the variations of patients' penile volume are presented in Inline Supplementary Table 7. Positive correlation was found in the right inferior frontal gyrus, precentral and postcentral gyri, claustrum and putamen, midbrain as well as in the left supplementary motor area, and right anterior cingulate gyrus. By contrast, in the 10 controls with an erectile response, no region showed a correlation between rCBF and penile volume. The between-groups comparison of correlations showed that in patients there was a higher correlation between rCBF and penile volume in the left anterior cingulate cortex (x, y, z = −8, 14, 40; k = 312; PFWE-corr. < 0.001, corrected) and in the supplementary motor area bilaterally (x, y, z = 0, −8, 58; k = 150; PFWE-corr. < 0.05, corrected).
4. Discussion
Visual stimuli induced the behavioral effects that we intended and whose correlates were investigated through PET analyses. For instance, compared with controls, patients showed a higher difference between DSA ratings for undressed and for normally dressed children. The same Group by Condition interaction was found for erectile responses to VSSc.
The lower plasma levels of testosterone in patients was unexpected. To our knowledge, decreased basal plasma testosterone has rarely been reported in pedophilia (Shostakovich et al., 1991). Most studies focus on pharmacologically induced decreased testosterone as a means to control sexual desire, but do not compare its basal levels in patients and healthy controls (e.g., Rösler and Witztum, 1998). Although lower than in controls, testosterone levels were not pathological. They could not be caused by antihormonal therapy as such treatment was a criterion for non-inclusion. In normal circumstances, aging males show a progressive decrease in testosterone levels with some increase in SHBG that contributes to lower the bioavailable testosterone, as reported by several groups, including ours (Déchaud et al., 1989). However, age could not explain this finding as it was similar in both groups and the difference between groups remained significant both for total and for bioavailable testosterone when age was included as a covariate in the comparison.
4.1. Subtractive analyses
Activations found in patients in response to VSSc were located in lateral temporal and occipital areas that respond in healthy males to VSSa (Stoléru et al., 2012), which was consistent with one of our hypotheses and with the patients' higher behavioral responses to pictures of undressed children than to pictures of dressed children (DSA and PE ratings, erectile responses; Table 3 and Inline Supplementary Table 4). Observed activations are also consistent with some previous reports (Poeppl et al., 2011; Schiffer et al., 2008a and Schiffer et al., 2008b). However, by contrast with Poeppl et al. (2011), we did not observe any significant activation in the right middle frontal gyrus, the posterior cingulate gyrus, the right hippocampus-amygdala complex, the right postcentral gyrus, the right precentral gyrus and the right calcarine sulcus. However, the comparability of analyses is limited as Poeppl et al. (2011) used neutral scrambled images as reference conditions whereas we used images of dressed characters.
Unlike the patients, controls did not show any significant activation of lateral occipital and temporal areas in response to pictures of undressed children. This is consistent with the non-significant difference between their ratings of pictures of undressed and normally dressed children (Table 3). Activation was located at the occipital pole, right above the calcarine fissure. We speculate that the location of this activation in controls, i.e., near their primary visual cortex, could correspond to the processing of the low-level visual features distinguishing dressed from undressed children, whereas the more lateral location of activation in patients could correspond to their attribution of a sexually arousing character to images of undressed children. However, in contrast with our results, bilateral occipital activations have previously been reported in controls (Schiffer et al., 2008a).
The activation in the caudal part of the fusiform gyrus (BA 18) of controls in response to images of nude children was located in a region where healthy subjects show a deactivation in response to VSSa (Redouté et al., 2000); such deactivation in response to VSSa occurring in the context of unfolding SA has been understood as the release of an inhibition of SA. Hence, the activation of the fusiform gyrus in response to nude children could be a correlate of the ongoing inhibition of SA in controls.
As regards the between-groups analysis, the analysis of the [(Chresponse)Controls − (Chresponse)Patients] can theoretically be related to higher activations in controls than in patients and/or to higher deactivations in patients than in controls. In order to investigate these alternative mechanisms regarding the response in the right inferior temporal gyrus (BA 20), we examined the responses of this area in within-group analyses performed separately in controls and in patients. While no significant responses were observed in controls, a deactivation was found in patients (Undressed < Dressed), albeit at an uncorrected threshold (p < .001, unc.). This maintained activation in the right inferior temporal gyrus (BA 20) of controls in response to images of nude children also occurred in a region that plays an inhibitory role on SA according to the neurophenomenological model (Stoléru et al., 2012). This part of the right inferior temporal gyrus (BA 20; y = −34) is distinct from the activated cluster found in patients in a more caudal location (BA 37; y = −68). According to the neurophenomenological model, while the more caudal part is positively related to SA, the more rostral part is inhibitory. The between-group difference is related to the fact that, whereas the level of activation in right BA 20 decreased in patients in response to undressed children (at an uncorrected threshold), it did not change significantly in controls. Thus, the between-group difference appeared clearly for an inhibitory region (no need for masking operations), while it was less obvious and needed masking procedures for regions that mediate SA: in other words, according to the neurophenomenological model, patients may be said to have “released the brake”, but not the controls. This finding can be related to the fact that in supposedly nonpedophilic samples of male participants, the prevalence of sexual attraction to children has been reported to be non-negligible (Briere and Runtz, 1989). We speculate that in the men of these samples who report attraction to children, the level of activation in right BA 20 in response to the sight of children may be weaker than in men who do not feel such attraction.
Surprisingly, we did not find any higher activations in patients than in controls in response to VSSc despite the significant Condition by Group interaction on ratings of DSA and of PE and on erectile responses. This is in line with results reported by Poeppl et al. (2011) when, like in the present study, they used a corrected probability threshold. In heterosexual pedophiles, Schiffer et al. (2008b) reported a higher activation of the left hippocampus (PFDR-corrected < 0.05, > 10 voxels) in response to nude female children, which we did not observe at the same probability threshold.
Regarding the patients' responses to VSSa, the bilateral activation in the right and left lateral occipital and temporal cortices is consistent with previous findings in heterosexual and/or homosexual patients (Schiffer et al., 2008a; Schiffer et al., 2008b). These regions are also activated in healthy males in response to VSSa (Stoléru et al., 2012). In the control group, these regions were activated in response to VSSa. Conversely, the “deactivation” observed in patients in the right and left inferior temporal gyri (BA 20 and BA 39, respectively), i.e., the higher activation in response to images of dressed than of undressed adults, is consistent with findings in healthy males who showed a negative correlation between markers of SA and activation in these areas (Redouté et al., 2000). These findings are likely to be related with the fact that most patients (80%) were non-exclusive, i.e., were also attracted to adults.
As regards the between-groups analysis of responses to adults' nudity (Table 5), the analysis of the [(Adresponse)Controls − (Adresponse)Patients] can theoretically be related to higher activations in controls than in patients and/or to higher deactivations in patients than in controls. In order to investigate these alternative mechanisms regarding the response in the medial orbital gyrus (BA 11), we examined the responses of this area in within-group analyses performed separately in controls and in patients. While no significant responses were observed in controls, a deactivation was found in patients (Undressed < Dressed), albeit at an uncorrected threshold (p < 0.001, unc.). The lack of deactivation in controls stands in contrast to the neurophenomenological model (Stoléru et al., 2012). In contrast with Walter et al. (2007) and using the same threshold as in their study (p < 0.005, uncorrected, k > 10 voxels), we did not find a higher activation in healthy males in the dorsal midbrain, the left dorsolateral prefrontal cortex, the right parietal cortex, the right ventrolateral frontal cortex, the right occipital cortex and the left insula. However, the comparability of analyses is limited as Walter et al. (2007) compared groups on the contrast “sexual arousal > emotional arousal”.
Whereas Poeppl et al. (2011) reported that several regions showed higher activation (using an uncorrected threshold) in men with pedophilia than in controls in response to their respective age-preferred VSS (Contrast 3), we found no such region (also using uncorrected threshold). However, in Poeppl et al. (2011) control subjects were men with nonsexual offense histories, which limits the comparability with our study. A meta-analysis comparing men with pedophilia to non-pedophilic men (mostly non clinical subjects) on their brain responses to their age-preferred VSS failed to reveal any significant difference in either direction (men with pedophilia > non-pedophilic men, or non-pedophilic men > men with pedophilia) (Polisois-Keating and Joyal, 2013).
4.2. Correlational analyses
Several regions where rCBF was found positively correlated with the patients' erectile response have previously been identified as neural correlates of erection in healthy men (Kühn and Gallinat, 2011; Poeppl et al., 2014). This is the case for the right and left anterior cingulate cortex, right putamen/claustrum, and the left insula. In addition, the cluster observed in the right inferior frontal gyrus is consistent with a similar finding reported by Mouras et al. (2008), also in a study of healthy men.
Such replication of results previously reported in healthy subjects was also observed for regions where activation was negatively correlated with penile tumescence, i.e., the bilateral middle temporal gyri, left inferior and superior temporal gyri, and left medial orbitofrontal cortex (Redouté et al., 2000). By contrast, and surprisingly, a negative correlation was also found in the middle part of the right orbitofrontal cortex, a region where rCBF was reported as positively correlated with penile volume in healthy subjects (Moulier et al., 2006; Mouras et al., 2008).
As regards ratings of stimuli, in patients the clusters showing a positive correlation between rCBF and ratings of DSA were located in the lateral occipital and temporal cortices as well as in the anterior cingulate gyrus. This is consistent with previous meta-analyses which found these regions positively related to SA in healthy men (Poeppl et al., 2014; Stoléru et al., 2012). Similarly, the negative correlation of the rCBF in the right inferior temporal gyrus (BA 20) and in the left supramarginal gyrus (BA 40) is consistent with similar negative correlation found previously in healthy men in response to VSSa (Poeppl et al., 2014; Redouté et al., 2000; Stoléru et al., 2012).
The rCBF in the left anterior cingulate gyrus was positively correlated with the three markers of the patients' SA, i.e., the erectile response, and ratings of both DSA and PE.
The scarcity of statistically significant between-groups differential responses to VSSc found in this study may be related to a true lack of differential responses or to methodological limitations. Regarding limitations, it is possible that some patients felt embarrassment and even some guilt while watching pictures of undressed children. Twelve (80%) were engaged in psychotherapy and may have had ambivalent feelings during these presentations, including the fear of potential relapse because of sexual stimulation. In addition, it is also possible that the moderate level of SA induced by pictures of children in swimming suits (only four photographs presented naked children) limited the level of activation. While the level of DSA induced by their preferred stimuli in the undressed condition was similar in patients and controls, the difference between ratings induced by undressed and dressed characters was higher in controls, with a significant Condition by Group interaction. Here, the experimental need to induce strong SA in patients was conflicting with the ethical and clinical concerns to limit such arousal, especially because the outpatients recruited in the present study were left unaccompanied after the experimental sessions. In addition, although the two groups did not differ significantly on age, it should be noted that the age range used for matching was wide (± 13 years), due to the difficulty to match simultaneously on three variables (age, handedness, and sexual orientation). Finally, from a statistical point of view, the sizes of the two groups may be insufficient to demonstrate limited effect sizes.
5. Conclusion
Several lines of evidence implicate the right inferior temporal gyrus (BA 37) as a possible candidate area mediating SA of patients with pedophilic disorder in response to VSSc. Firstly, this region was more activated in response to pictures of undressed than of dressed children of the preferred gender. Secondly, in patients the rCBF in this area was positively correlated with the ratings of Desire for Sexual Activity and of Perceived Erection. Thirdly, this area of the right inferior temporal cortex was activated by pictures of undressed children in patients but not in controls. This temporal area is distinct from another area, BA 20, also located in the right temporal lobe but more rostrally, which is thought to exert an inhibitory function on SA in healthy males and whose rCBF was (i) lower in patients than in controls and (ii) negatively correlated with the ratings of Desire for Sexual Activity and of Perceived Erection in the patients of the present study. Altogether, these results implicate the right inferior temporal gyrus as a possible candidate region mediating sexual arousal in patients with pedophilic disorder and suggest that two of its areas play opposite, i.e., activating and inhibitory, roles.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any personal, professional or financial relationships that could be construed as a potential conflict of interest.
Funding
This research project has been made possible by a grant from the Programme Hospitalier de Recherche Clinique, France (PHRC 2008 n° 27.42; Promoter: Hospices Civils de Lyon; Principal Investigator: Michel Pugeat).
Acknowledgments
We thank V. Berthier, F. Cazala, H. Dechaud, H. Leca, A. Maurin, E. Nischelwitzer, C. Orset, C. Pelletier, F. Vey, and C. Vighi for their help in data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.101647.
Appendix A. Supplementary data
Supplementary material
References
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Desk Reference to the Diagnostic Criteria from DSM-IV-TR. [Google Scholar]
- Axelrod B., Ryan J., Ward L. Evaluation of seven-subtest short forms of the Wechsler Adult Intelligence Scale-III in a referred sample. Arch. Clin. Neuropsychol. 2001;16:1–8. [PubMed] [Google Scholar]
- Bancroft J., Tennent G., Loucas K., Cass J. The control of deviant sexual behaviour by drugs: I. Behavioural changes following oestrogens and anti-androgens. Br. J. Psychiatry. 1974;125:310–315. doi: 10.1192/bjp.125.3.310. [DOI] [PubMed] [Google Scholar]
- Barth J., Bermetz L., Heim E., Trelle S., Tonia T. The current prevalence of child sexual abuse worldwide: a systematic review and meta-analysis. Int. J. Public Health. 2012;7:469–483. doi: 10.1007/s00038-012-0426-1. [DOI] [PubMed] [Google Scholar]
- Beck A.T. Center for Cognitive Therapy; Philadelphia, PA: 1978. Depression Inventory. [Google Scholar]
- Blanchard R., Kuban M.E., Klassen P., Dickey R., Christensen B.K., Cantor J.M., Blak T. Self-reported head injuries before and after age 13 in pedophilic and nonpedophilic men referred for clinical assessment. Arch. Sex. Behav. 2003;32:573–581. doi: 10.1023/a:1026093612434. [DOI] [PubMed] [Google Scholar]
- Briere J., Runtz M. University males' sexual interest in children: predicting potential indices of “pedophilia” in a non-forensic sample. Child Abuse Negl. 1989;13:65–75. doi: 10.1016/0145-2134(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Cantor J.M., Blanchard R., Christensen B.K., Dickey R., Klassen P.E., Beckstead A.L., Blak T., Kuban M.E. Intelligence, memory, and handedness in pedophilia. Neuropsychology. 2004;18:3–14. doi: 10.1037/0894-4105.18.1.3. [DOI] [PubMed] [Google Scholar]
- Cantor J.M., Blanchard R., Robichaud L.K., Christensen B.K. Quantitative reanalysis of aggregate data on IQ in sexual offenders. Psychol. Bull. 2005;131:555–568. doi: 10.1037/0033-2909.131.4.555. [DOI] [PubMed] [Google Scholar]
- Déchaud H., Lejeune H., Garoscio-Cholet M., Mallein R., Pugeat M. Radioimmunoassay of testosterone not bound to sex-steroid-binding protein in plasma. Clin. Chem. 1989;35:1609–1614. [PubMed] [Google Scholar]
- Derogatis L.R. 1977. The SCL-90-R Manual. I: Scoring, Administration and Procedures for the SCL-90. Clinical Psychometrics. Baltimore. [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U S A. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian J.M. Neuropsychology, neuroscience, volitional impairment and sexually violent predators: a review of the literature and the law and their application to civil commitment proceedings. Aggress. Violent Behav. 2012;17:1–15. [Google Scholar]
- Gerwinn H., Weiß S., Tenbergen G., Amelung T., Födisch C., Pohl A., Massau C., Kneer J., Mohnke S., Kärgel C., Wittfoth M., Jung S., Drumkova K., Schiltz K., Walter M., Beier K.M., Walter H., Ponseti J., Schiffer B., Kruger T.H.C. Clinical characteristics associated with paedophilia and child sex offending–Differentiating sexual preference from offence status. Eur. Psychiatry. 2018;51:74–85. doi: 10.1016/j.eurpsy.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Hoon E.F., Hoon P.W., Wincze J.P. An inventory for the measurement of female sexual arousability. Arch. Sex. Behav. 1976;5:291–300. [PubMed] [Google Scholar]
- Jakoby B.W., Bercier Y., Conti M., Casey M.E., Bendriem B., Townsend D.W. Physical and clinical performance of the mCT time-of-flight PET/CT scanner. Phys. Med. Biol. 2011;56:2375–2389. doi: 10.1088/0031-9155/56/8/004. [DOI] [PubMed] [Google Scholar]
- Joyal C.C., Black D.N., Dassylva B. The neuropsychology and neurology of sexual deviance: a review and pilot study. Sex. Abus. 2007;19:155–173. doi: 10.1177/107906320701900206. [DOI] [PubMed] [Google Scholar]
- Kärgel C., Massau C., Weiß S., Walter M., Borchardt V., Krueger T.H., Tenbergen G., Kneer J., Wittfoth M., Pohl A., Gerwinn H., Ponseti J., Amelung T., Beier K.M., Mohnke S., Walter H., Schiffer B. Evidence for superior neurobiological and behavioral inhibitory control abilities in non-offending as compared to offending pedophiles. Hum. Brain Mapp. 2017;38:1092–1104. doi: 10.1002/hbm.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Bulik C.M., Silberg J.S., Hettema J.M., Myers J., Prescott C.A. Childhood sexual abuse and adult psychiatric and substance use disorders in women. An epidemiological and cotwin control analysis. Arch. Gen. Psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- Kühn S., Gallinat J. A quantitative meta-analysis on cue-induced male sexual arousal. J. Sex. Med. 2011;8:2269–2275. doi: 10.1111/j.1743-6109.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- Langevin R., Paitich D. Multi-Health Systems; Toronto, Canada: 2002. Sex History Questionnaire for Males – Revised. Technical Manual. [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.B., Kraft R.A. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martí-Climent J.M., Prieto E., Domínguez-Prado I., García-Velloso M.J., Rodríguez-Fraile M., Arbizu J., Vigil C., Caicedo C., Penuelas I., Richter J.A. Contribution of time of flight and point spread function modeling to the performance characteristics of the PET/CT Biograph mCT scanner. Rev. Esp. Med. Nucl. Imagen Mol. (English Edition) 2013;32:13–21. doi: 10.1016/j.remn.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Mohnke S., Müller S., Amelung T., Krüger T.H., Ponseti J., Schiffer B., Walter M., Beier K.M., Walter H. Brain alterations in paedophilia: a critical review. Prog. Neurobiol. 2014;122:1–23. doi: 10.1016/j.pneurobio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Moulier V., Mouras H., Pélégrini-Issac M., Glutron D., Rouxel R., Grandjean B., Bittoun J., Stoléru S. Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. NeuroImage. 2006;33:689–699. doi: 10.1016/j.neuroimage.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Mouras H., Stoléru S., Moulier V., Pélégrini-Issac M., Rouxel R., Grandjean B., Glutron D., Bittoun J. Activation of mirror-neuron system by erotic video clips predicts degree of induced erection: an fMRI study. NeuroImage. 2008;42:1142–1150. doi: 10.1016/j.neuroimage.2008.05.051. [DOI] [PubMed] [Google Scholar]
- Nelson E.C., Heath A.C., Madden P.A., Cooper M.L., Dinwiddie S.H., Bucholz K.K., Glowinski A., McLaughlin T., Dunne M.P., Statham D.J., Martin N.G. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch. Gen. Psychiatry. 2002;59:139–145. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Pérez-Fuentes G., Olfson M., Villegas L., Morcillo C., Wang S., Blanco C. Prevalence and correlates of child sexual abuse: a national study. Compr. Psychiatry. 2013;54:16–27. doi: 10.1016/j.comppsych.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl T.B., Nitschke J., Dombert B., Santtila P., Greenlee M.W., Osterheider M., Mokros A. Functional cortical and subcortical abnormalities in pedophilia: a combined study using a choice reaction time task and fMRI. J. Sex. Med. 2011;8:1660–1674. doi: 10.1111/j.1743-6109.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- Poeppl T.B., Nitschke J., Santtila P., Schecklmann M., Langguth B., Greenlee M.W., Osterheider M., Mokros A. Association between brain structure and phenotypic characteristics in pedophilia. J. Psychiatr. Res. 2013;47:678–685. doi: 10.1016/j.jpsychires.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Poeppl T.B., Langguth B., Laird A.R., Eickhoff S.B. The functional neuroanatomy of male psychosexual and physiosexual arousal: a quantitative meta-analysis. Hum. Brain Mapp. 2014;35:1404–1421. doi: 10.1002/hbm.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisois-Keating A., Joyal C.C. Functional neuroimaging of sexual arousal: a preliminary meta-analysis comparing pedophilic to non-pedophilic men. Arch. Sex. Behav. 2013;42:1111–1113. doi: 10.1007/s10508-013-0198-6. [DOI] [PubMed] [Google Scholar]
- Ponseti J., Granert O., Jansen O., Wolff S., Beier K., Neutze J., Deuschl G., Mehdorn H., Siebner H., Bosinski H. Assessment of pedophilia using hemodynamic brain response to sexual stimuli. Arch. Gen. Psychiatry. 2012;69:187–194. doi: 10.1001/archgenpsychiatry.2011.130. [DOI] [PubMed] [Google Scholar]
- Raymond N.C., Coleman E., Ohlerking F., Christenson G.A., Miner M. Psychiatric comorbidity in pedophilic sex offenders. Am. J. Psychiatry. 1999;156:786–788. doi: 10.1176/ajp.156.5.786. [DOI] [PubMed] [Google Scholar]
- Redouté J., Stoléru S., Grégoire M.C., Costes N., Cinotti L., Lavenne F., Le Bars D., Forest M.G., Pujol J.-F. Brain processing of visual sexual stimuli in human males. Hum. Brain Mapp. 2000;11:162–177. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C.F., Frank E., Thase M.E., Houck P.R., Jennings J.R., Howell J.R., Lilienfeld S.O., Kupfer D.J. Assessment of sexual function in depressed, impotent, and healthy men: factor analysis of a Brief Sexual Function Questionnaire for men. Psychiatry Res. 1988;24:231–250. doi: 10.1016/0165-1781(88)90106-0. [DOI] [PubMed] [Google Scholar]
- Roiser J.P., Linden D.E., Gorno-Tempinin M.L., Moran R.J., Dickerson B.C., Grafton S.T. Minimum statistical standards for submissions to Neuroimage: clinical. NeuroImage: Clinical. 2016;12:1045–1047. doi: 10.1016/j.nicl.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen R.C., Beck J.G. Concerns involving human subjects in sexual psychophysiology. In: Rosen R.C., Beck J.G., editors. Patterns of Sexual Arousal. Psychophysiological Processes and Clinical Applications. The Guilford Press; New York: 1988. pp. 345–355. [Google Scholar]
- Rösler A., Witztum E. Treatment of men with paraphilia with a long-acting analogue of gonadotropin-releasing hormone. N. Engl. J. Med. 1998;338:416–422. doi: 10.1056/NEJM199802123380702. [DOI] [PubMed] [Google Scholar]
- Sartorius A., Ruf M., Kief C., Demirakca T., Bailer J., Ende G., Henn F.A., Meyer-Lindenberg A., Dressing H. Abnormal amygdala activation profile in pedophilia. Eur. Arch. Psychiatry Clin. Neurosci. 2008;258:271–277. doi: 10.1007/s00406-008-0782-2. [DOI] [PubMed] [Google Scholar]
- Schiffer B., Krueger T., Paul T., de Greiff A., Forsting M., Leygraf N., Schedlowski M., Gizewski E. Brain response to visual sexual stimuli in homosexual pedophiles. J. Psychiatry Neurosci. 2008;33:23–33. [PMC free article] [PubMed] [Google Scholar]
- Schiffer B., Paul T., Gizewski E., Forsting M., Leygraf N., Schedlowski M., Kruger T.H.C. Functional brain correlates of heterosexual paedophilia. NeuroImage. 2008;41:80–91. doi: 10.1016/j.neuroimage.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Schiffer B., Amelung T., Pohl A., Kaergel C., Tenbergen G., Gerwinn H., Mohnke S., Massau C., Matthias W., Weiß S., Marr V. Gray matter anomalies in pedophiles with and without a history of child sexual offending. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz K., Witzel J., Northoff G., Zierhut K., Gubka U., Fellmann H., Kaufmann J., Tempelmann C., Wiebking C., Bogerts B. Brain pathology in pedophilic offenders: evidence of volume reduction in the right amygdala and related diencephalic structures. Arch. Gen. Psychiatry. 2007;64:737–746. doi: 10.1001/archpsyc.64.6.737. [DOI] [PubMed] [Google Scholar]
- Seto M.C. American Psychological Association; Washington, DC: 2008. Pedophilia and Sexual Offending against Children: Theory, Assessment, and Intervention. [Google Scholar]
- Shostakovich B.V., Smirnova L.K., Tkachenko A.A., Ushakova I.M., Kartelishev A.V., Nikolaeva T.N. A comparative evaluation of the biochemical and psychopathological characteristics in subjects with signs of pedophilia. Zhurnal nevropatologii i psikhiatrii imeni SS Korsakova. 1991;92:83–88. (Moscow, Russia: 1952) [PubMed] [Google Scholar]
- Stoléru S., Moulier V., Fonteille V., Cazala F. Paper presented at the Congrès International Francophone sur l'Agression Sexuelle, Montreux, September. 2011. Développement du Questionnaire sur l'intensité des Fantasmes, des Désirs et des Comportements Sexuels chez les auteurs de violences sexuelles sur enfants. [Google Scholar]
- Stoléru S., Fonteille V., Cornélis C., Joyal C., Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci. Biobehav. Rev. 2012;36:1481–1509. doi: 10.1016/j.neubiorev.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Tenbergen G., Wittfoth M., Frieling H., Ponseti J., Walter M., Walter H., Beier K.M., Schiffer B., Kruger T.H. The neurobiology and psychology of pedophilia: recent advances and challenges. Front. Hum. Neurosci. 2015;9:344. doi: 10.3389/fnhum.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Knippenberg F.C.E., Duivenvoorden H.J., Bonke B., Passchier J. Shortening the state-trait anxiety inventory. J. Clin. Epidemiol. 1990;43:995–1000. doi: 10.1016/0895-4356(90)90083-2. [DOI] [PubMed] [Google Scholar]
- Walter M., Witzel J., Wiebking C., Gubka U., Rotte M., Schiltz K., Bermpohl F., Tempelmann C., Bogerts B., Heinze H.J., Northoff G. Pedophilia is linked to reduced activation in hypothalamus and lateral prefrontal cortex during visual erotic stimulation. Biol. Psychiatry. 2007;62:698–701. doi: 10.1016/j.biopsych.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Woo C.W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 1992. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material