Abstract
Both systemic and local production contribute to the concentration of steroids measured in the brain. This idea was originally based on rodent studies and was later extended to other species including humans and birds. In quail, a widely used model in behavioral neuroendocrinology, it was demonstrated that all enzymes needed to produce sex steroids from cholesterol are expressed and active in the brain but the actual concentrations of steroids produced were never investigated. We carried out a steroid profiling in multiple brain regions and serum of sexually mature male and female quail by gas chromatography coupled to mass spectrometry. Concentrations of some steroids (e.g., corticosterone, progesterone, testosterone) were in equilibrium between brain and periphery but other steroids (e.g., pregnenolone, 5α/β-dihydroprogesterone and estrogens) were more concentrated in the brain. In brain regions investigated, pregnenolone sulfate, progesterone and estrogen concentrations were higher in the hypothalamus-preoptic area (POA). Progesterone and its metabolites were more concentrated in the female than the male brain while testosterone, its metabolites and dehydroepiandrosterone (DHEA) were more concentrated in males suggesting that sex steroids present in quail brain mainly depend on their specific steroidogenic pathways in ovaries and testes. However, castration experiments suggested that sex steroids could also be produced in the brain independently of peripheral source. Treatment with testosterone or estradiol restored the concentrations of most androgens or estrogens, respectively, although penetration of estradiol in the brain appeared more limited. These studies illustrate the complex interaction between local brain synthesis and the supply from the periphery for the steroids present in the brain that are either directly active or represent the substrate of centrally located enzymes.
Keywords: brain steroid concentrations, brain aromatization, preoptic area, gas chromatography, mass spectrometry
Introduction
It was originally believed that the brain is only a target for steroid action and that steroid hormones (androgens, estrogens, progestagens, corticoids,…) are produced exclusively by peripheral specialized organs (gonads, adrenals, …). The discovery of steroid synthesizing enzymes in the brain and demonstration that the concentration of some steroids in the brain (e.g., pregnenolone) does not change following removal of their suggested peripheral source (e.g., after castration) challenged this notion. This led to the concept of neurosteroids, i.e., steroids directly synthesized in the brain from cholesterol, for example, progesterone and its 5α-reduced metabolites, and producing their physiological effects at the site of synthesis (1–6). Other studies further showed that some steroids found in the brain can be derived from precursors produced in the periphery that are metabolized in the brain by a variety of enzymes. This is for example the case of estradiol (E2) that can be synthesized by the female ovary but can also be derived from testosterone (T) produced by the testes and aromatized in specific brain loci under the catalytic action of the aromatase enzyme (7–9). These steroids are usually called neuroactive steroids and directly act on neural cells either via membrane or nuclear signaling mechanisms (10, 11).
These studies were initially performed in mammals and little was known until recently in other taxa, in particular in birds. The Japanese quail (Coturnix japonica) has become a widely used model in (behavioral) neuroendocrinology (12, 13). Extensive work in the laboratory of Kazuyoshi Tsutsui demonstrated during the past 10 years that the RNAs corresponding to all enzymes involved in the synthesis of sex steroids are expressed in the quail brain and that they are active (14, 15). Similar studies in a songbird species, the zebra finch Taeniopygia guttata performed in the laboratory of Barney Schlinger identified expression of the RNAs for steroid synthesizing and metabolizing enzymes in the brain and gathered evidence for their functional significance (16–19). Although it was shown in several cases by in vitro radioenzymatic assays that the corresponding enzymes are active, i.e., that brain homogenates can metabolize radioactive steroid substrates into their products (see for review (15)), the actual concentrations of steroids present in the quail brain are in most cases unknown. This is partly due to technical difficulties associated with measuring these compounds present in small amounts in a highly lipophilic tissue.
In quail like in many other vertebrate species, extensive experimental work demonstrated the key role of brain steroid metabolism in the control of male sexual behavior (20, 21). In particular, the activation of male copulatory behavior depends on E2 derived from the central aromatization of T (22–24). Systemic treatment or stereotaxic implantation in the preoptic area of aromatase inhibitors indeed very efficiently block male sexual behavior activated by exogenous T (25–27). Surprisingly, peripheral administration of E2 is, however, not very efficient in restoring male sexual behavior in castrates. Low doses of E2 are inactive and behavioral activation is only observed at supraphysiological concentrations that display toxic effects (e.g., liver cirrhosis) and are sometimes lethal (20, 22, 28–30).
The apparent discrepancy between effects of locally synthesized E2 and the relative lack of action of E2 injected in the periphery could have multiple reasons (28). It is possible that very high local concentrations of E2 in anatomically discrete areas of the brain, only reached by in situ synthesis, are needed to activate the male behavior. Alternatively, it is also possible that E2 injected in the periphery does not readily cross the blood brain barrier and thus only reaches the brain in low amounts. Finally, systemically injected E2 is likely subjected to the intense catabolic activity of the liver rapidly reducing the active concentration of the hormone. These possibilities are not mutually exclusive.
The present studies were therefore initiated to investigate some of these unanswered questions concerning the concentrations of steroids in the quail brain and more specifically to: 1) describe the profile of steroid concentrations in different regions of the male and female quail brain, 2) determine to what extent these steroids are present in the extra-cellular space, 3) analyze how these brain concentrations of steroids are affected by castration and by systemic treatments with T or E2 and in particular assess whether peripheral E2 can freely enter the brain as commonly accepted for all steroids.
Material and Methods
Three independent experiments were performed on male and female Japanese quail (Coturnix japonica) that were either obtained from local breeders in Belgium and maintained at the quail colony of the GIGA Neurosciences, University of Liège or were derived from the quail breeding colony at the INRA Pôle d’Expérimentation Avicole de Tours (PEAT) in Nouzilly, Tours, France (Part of experiment 1 and experiment 3). Birds always had food and water available ad libitum and were kept in a long photoperiod (16 hours of light: 8 hours of dark per day). We first describe the methods that are common to all experiments and we will in a second step describe the specific protocols of each experiment.
All experimental procedures were in agreement with the Belgian and French laws on the “Protection and Welfare of Animals” and on the “Protection of experimental animals” and were approved by the Ethics Committee for the Use of Animals at the University of Liège.
Brain collection and dissection
Birds were rapidly killed by decapitation without anesthesia to avoid potential changes in steroid concentrations. Trunk blood was collected and, after centrifugation, serum was stored at −80°C until assayed for steroid content (0.5 to 1ml used in the assays). Brains were immediately extracted from the skull and dissected into four great subdivisions: the hypothalamus-preoptic area (POA, 30–60 mg), the telencephalon (TEL, 300–450 mg), the optic lobes (OL, 120–180 mg), and the cerebellum (CB, 90–110 mg). To dissect the POA, the brain was placed with its ventral surface up and the POA was dissected by two coronal cuts at the level of the tractus septopallio-mesencephalicus (rostral edge of the POA) and of the oculomotor nerves (caudal edge of the hypothalamus), two parasagittal cuts placed approximately 2 mm lateral to the brain midline and a final horizontal cut 2 mm below the floor of the brain. This isolated a block of tissue that contains most of, if not all, the neurons that show a dense expression of aromatase in the quail brain (31). The OL and CB were then separated from the rest of the brain with one scissor cut in each case and finally the caudal part containing the brain stem and base of the mesencephalon was separated from the TEL by a final cut. Each sample was immediately frozen on dry ice, weighed, wrapped in aluminum foil and stored at −80°C until assayed. The entire procedure was usually completed in less than 3 min. The list of steroids assayed and the different brain parts that were considered varied however from one experiment to another as described in the results.
Specific experimental protocols
Experiment 1: Steroid profiles in brain areas and serum in adult male and female quail
This experiment was carried out with 4 successive batches (three from Liege and one from Tours, #3) of sexually mature adult males and females as attested by the presence of a fully developed cloacal gland in males and the fact that females were regularly laying eggs. The cloacal gland is an androgen-sensitive measure that provides a reliable estimate of the circulating concentrations of androgens (32, 33).
This experiment thus included a total of 21 males and 21 females although not every steroid was assayed in each subject or brain sample due to the fact that our capacity to detect steroids increased in time and we progressively focused on avian-typical steroids such as the 5β-reduced androgens. The final number of data points for each steroid, sex and brain region is indicated by the degrees of freedom in the table summarizing the results of statistical analyzes.
Experiment 2: In vivo microdialysis in the hypothalamus and preoptic area of males
Five adult males were anesthetized with isoflurane, placed in a stereotaxic frame and implanted with a guide cannula (22g plastic tubing) aimed at the medial preoptic nucleus (coordinates: X=+1.6, Y=+2.5 and Z=±0.5 mm), a key site for the activation by T of male copulatory behavior (34, 35). The cannula was fixed to the skull with dental cement and then obturated with a dummy insert that was 300 μm longer than the cannula until the microdialysis experiment began. Animals were allowed a 2 weeks recovery period after surgery.
The probe (Microbiotech SE, MAB 4.15.IC) was initially perfused with artificial cerebrospinal fluid, aCSF (199 mM NaCl, 26.2 mM NaHCO3, 2.5 mM KCl, 1 mM NaH2PO4, 1.3 mM MgSO4, 2.5 mM CaCl2, 11 mM glucose, 1% bovine serum albumin, pH = 7.4) during several minutes at a speed of 2 μl/min. After that time, the dummy insert was removed and the probe was inserted into the guide cannula. The awake bird was placed in a glass chamber (60 cm × 30 cm × 35 cm aquarium). The bird was attached by a harness and a tether encased the in- and out-flow tubing (FEP Tubing). The tubing was attached to a counter-balanced lever arm that prevented addition of extra weight on the head of the animal and to a swivel to avoid torsion of the tubing. The dialysis membrane (Microbiotech MAB 4.15.2. PES) had an outer diameter of 0.2 mm, an active dialyzing length of 1.8 mm and a 6 kDa cutoff. The freely moving bird was then perfused with aCSF overnight at a rate of 1 μl/min and the eluate was collected in a single vial that was frozen in the morning and stored at −80°C until assayed for steroid content. Steroids were assayed in a 500 μl aliquot (corresponding to 500 min of perfusion) of these dialysates collected from 5 different birds.
At the end of the experiments, birds were injected with ink through the cannula, euthanized by decapitation, brains were collected and directly frozen on dry ice. Brains were cut on a cryostat in 30 μm-thick coronal sections that were stained with Toluidine blue by standard procedures (see (36)). The location of the tip of the probes was then determined under the microscope based on the Nissl staining and localization of the ink spot.
Experiment 3: Effects of castration and replacement with T or E2
This experiment was performed with 31 male quail obtained from the breeding colony established at the INRA station in Nouzilly, Tours (France) in order to test the effect of castration on brain and serum steroid content and whether T had an easier access to the brain than E2 as potentially suggested by previous experiments (see introduction).
All birds were castrated at three weeks of age under Ketamine/Xylazine anesthesia (38.75 and 2.45 mg/kg respectively). The two testes were removed through a unilateral incision behind the last rib as described previously (37, 38). Two weeks later, birds were assigned to one of 4 experimental groups matched on body weight and were implanted with either two 20 mm Silastic™ capsules filled with T (CX+T40; n=9), or two 20 mm Silastic™ capsules filled with E2 (CX+E40; n=8), or four 20 mm Silastic™ capsules filled with E2 (CX+E80; n=7) or two 20 mm Silastic™ capsules left empty as a control (CX; n=9). Implants were made of Silastic™ tubing (catalog #602–252; Dow Corning, Midland, MI) (1.57 mm inner diameter; 2.41 mm outer diameter) filled with crystalline T or E2 and closed at both ends with Silastic™ glue. Implants were introduced under skin via a small incision through the apterium located at the side of the neck and pushed under the skin in the back of the bird.
Seven days later, all birds were weighed, their cloacal gland area was measured to the nearest tenth of millimeter with a caliper (width and length) to determine the surface of the rectangle enveloping the gland, called here the cloacal gland area (CGA). They were then killed by decapitation, trunk blood was collected and brains were dissected and frozen on dry ice as described in the general procedures.
Steroid assay by gas chromatography and mass spectrometry (GC/MS)
Pregnenolone, 20α-dihydropregnenolone, progesterone (PROG) and its 3α/β, 5α/β, 20α/β reduced metabolites, 11-deoxycorticosterone (DOC), glucocorticoids (corticosterone, cortisone and cortisol), androgens (dehydroepiandrosterone, androstenediol, androstenedione and its 3α/β, 5α/β reduced metabolites, T and its 3α/β, 5α/β reduced metabolites), estrogens (estrone, E2, Estriol, 2-methoxy estradiol [2-ME]) and steroid sulfates levels were determined by GC/MS according to the protocol described by Liere et al. (39) with minor modifications. Briefly, steroids were extracted from individual plasmas (1 ml), brain tissues or dialysates by adding 10 volumes of methanol. Internal standards were introduced for steroid quantification: 2 ng of 2H4-pregnenolone sulfate (for steroid sulfates), 2 ng of 2H6-5α-dihydroprogesterone (for 5α/β-dihydroprogesterone and 5α/β-dihydroandrostanedione), 5 ng of 19-nor progesterone (for progesterone (PROG), 20α/β-dihydroprogesterone, 3α/β, 5α/β-tetrahydroprogesterone, 5α20α-tetrahydroprogesterone, 3α5α20α-hexahydroprogesterone, androstenedione), 2 ng of epietiocholanolone (for pregnenolone, 5α/β-dihydroT, 3α/β,5α/β-tetrahydroT, dehydroepiandrosterone, etiocholanolone, epiandrosterone), 2 ng of 2H5-T (for T), 2 ng of 2H5-17β-E2 (for 17β-E2, estrone, estriol, 2-ME), 2 ng of 2H8-DOC (for DOC), 10 ng of 2H8-corticosterone (for corticosterone), 10 ng of 2H7-cortisone (for cortisone) and 10 ng of 2H3-cortisol (for cortisol).
Samples were purified and fractionated by solid-phase extraction with the recycling procedure (40). The steroid sulfates-containing fraction was directly derivatized with 20 μl heptafluorobutyric anhydride (HFBA) in 100 μl anhydrous acetone for 30 min at 20°C. This reaction cleaves the sulfate moiety and the resulting free steroid is then analyzed by GC/MS..
The unconjugated steroid-containing fraction was filtered and further purified and fractionated by high performance liquid chromatography (HPLC) as previously described (41). Three fractions were collected from the HPLC system: 5α/β-dihydroprogesterone and 5α/β-dihydroandrostanedione were eluted in the first HPLC fraction (3–10 min) and were silylated with 50 μl MSTFA (N-methyl-N-trimethylsilyltrifluoroacetamide)/NH4I/DTE (1000:2:5 vol/vol/vol) for 15 min at 70°C. The second fraction (10–31 min) containing pregnenolone, progestagens, androgens, estrone and E2 was derivatized with 25 μl HFBA and 25 μl anhydrous acetone for 1h at room temperature. Corticosterone, cortisone, cortisol and estriol were eluted in the third HPLC fraction (31–45 min) and derivatized with 25 μl HFBA and 25 μl anhydrous hexane for 1h at 80°C. All fractions were dried under a stream of N2 and resuspended in hexane for GC/MS analysis.
Calibration and biological samples were analyzed by GC/MS with an AS 3000 autosampler (ThermoFisher Scientific, USA). The Focus GC gas chromatograph is coupled with a DSQII mass spectrometer (ThermoFisher Scientific, USA). Injection was performed in the splitless mode at 250°C (1 min of splitless time) and the temperature of the gas chromatograph oven was initially maintained at 50°C for 1 min and ramped between 50 to 200°C at 20°C/min, then ramped to 285°C at 10°C/min and finally ramped to 350°C at 30°C/min. The helium carrier gas flow was maintained constant at 1 ml/min during the analysis. The transfer line and ionization chamber temperatures were 300°C and 220°C, respectively. Ionization was performed by electronic impact with electron energy of 70 eV. Derivatized steroids were identified by their retention time and two diagnostic ions in single ion monitoring (SIM) (Table 1). Quantification was performed according to the major diagnostic ion, called quantification ion. The detection thresholds for all the screened steroids in plasma, POA and TEL are reported in Table 2.
Table 1.
Parameters used for steroid identifications and measurements by GC-MS
| Steroids (Molecular weight) | Derivatized steroids (molecular weight) |
Retention time (min.) |
Diagnosticions (m/z) |
|---|---|---|---|
| Sulfates fraction | |||
| DHEA-S, Na+ (390) | DHEA-3-HFB (484) | 15.08 | 255 and 270 |
| * 2H4-PREG-S, Na+ (422) | 2H4-PREG-3-HFB (516) | 16.08 | 301–302 |
| PREG-S, Na+ (418) | PREG-3-HFB (512) | 16.12 | 283 and 298 |
| Fraction I-HPLC | |||
| 5β-dihydroandrostanedione (288) | 5β-dihydroandrostanedione-TMS2 (432) | 14.17 | 417 and 432 |
| 5α-dihydroandrostanedione (288) | 5α-dihydroandrostanedione-TMS2 (432) | 15.68 | 417 and 432 |
| 5β-dihydroprogesterone (316) | 5β-dihydroprogesterone-3,20-TMS2 (460) | 16.29 | 445 and 460 |
| * 2H6-5α-dihydroprogesterone (322) | 2H6-5α-dihydroprogesterone-3,20-TMS2 (466) | 18.20 | 449–453 |
| 5α-dihydroprogesterone (316) | 5α-dihydroprogesterone-3,20-TMS2 (460) | 18.24 | 445 and 460 |
| Fraction II-HPLC | |||
| 3α5α-tetrahydroT (292) | 3α5α- tetrahydroT −3,17- HFB2 (684) | 12.54 | 455 and 470 |
| * 2H5-T (293) | 2H5-T, 17-HFB2 (685) | 13.16 | 682–685 |
| T (288) | T-3,17-HFB2 (680) | 13.20 | 665 and 680 |
| 3β5α- tetrahydroT (292) | 3β5α- tetrahydroT −3,17- HFB2 (684) | 13.41 | 455 and 470 |
| 2H5-17β-estradiol (277) | 2H5-17β-estradiol-3,l7-HFB2 (669) | 13.57 | 667–669 |
| 17β-estradiol (272) | 17β-estradiol-3,17- HFB2 (664) | 13.60 | 451 and 664 |
| 3α5α20α-hexahydroprogesterone (320) | 3α5α20α-hexahydroprogesterone-3,20-HFB2 (712) | 14.18 | 697 and 712 |
| 20α-dihydropregnenolone (318) | 20α-dihydropregnenolone −3,20-HFB2 (710) | 14.63 | 481 and 496 |
| * Epietiocholanolone (290) | Epietiocholanolone-HFB (486) | 14.90 | 442 and 486 |
| 20α-dihydroprogesterone (316) | 20α-dihydroprogesterone-3,20-HFB2 (708) | 14.99 | 693 and 708 |
| Etiocholanolone (290) | Etiocholanolone-3-HFB (486) | 15.15 | 442 and 486 |
| 3β5α20α-hexahydroprogesterone (320) | 3β5α20α-hexahydroprogesterone-3,20-HFB2 (712) | 15.22 | 697 and 712 |
| 2-méthoxyestradiol (302) | 2-méthoxyestradiol-HFB2 (694) | 15.41 | 481 and 694 |
| Dehydroepiandrosterone (288) | Dehydroepiandrosterone-3-HFB (484) | 15.71 | 255 and 270 |
| Androstenedione (286) | androstenedione-3-HFB (482) | 15.74 | 467 and 482 |
| Epiandrosterone (290) | Epiandrosterone-3-HFB (486) | 16.06 | 442 and 486 |
| Estrone (270) | Estrone-3-HFB (466) | 16.17 | 422 and 466 |
| 5α-dihydroT (290) | 5α- dihydroT-17-HFB (486) | 16.50 | 414 and 486 |
| 3α5α-tetrahydroprogesterone (318) | 3α5α- tetrahydroprogesterone-3-HFB (514) | 16.51 | 496 and 514 |
| 3α5β-tetrahydroprogesterone (318) | 3α5β- tetrahydroprogesterone-3-HFB (514) | 16.62 | 496 and 514 |
| 3α5α-tetrahydroDOC (334) | 3α5α- tetrahydroDOC-3-HFB (726) | 16.73 | 497 and 512 |
| * 19 nor-progesterone (300) | 19 nor-progesterone-HFB (496) | 17.25 | 481 and 496 |
| Pregnenolone (316) | Pregnenolone-3-HFB (512) | 17.35 | 283 and 298 |
| Progesterone (314) | Progesterone-3-HFB (510) | 17.43 | 495 and 510 |
| * 2H8-DOC (338) | 2H8-DOC-HFB2 (730) | 17.58 | 727–730 |
| 3β5α- tetrahydroprogesterone (318) | 3β5α- tetrahydroprogesterone-3-HFB (514) | 17.68 | 496 and 514 |
| DOC (330) | DOC (722) | 17.68 | 707 and 722 |
| 5α20α- tetrahydroprogesterone (318) | 5α20α- tetrahydroprogesterone-3-HFB (514) | 18.83 | 499 and 514 |
| Fraction III-HPLC | |||
| Estriol (E3) (288) | E3-HFB3 (876) | 14.85 | 663 and 876 |
| * 2H4-Cortisol (365) | 2H4-Cortisol-3,21-HFB3 – H2O (935) | 16.87 | 680–681 |
| Cortisol (362) | Cortisol-3,21-HFB3 – H2O (932) | 16.91 | 463 and 677 |
| * 3α6α-diol-5β-pregnan-20-one (334) | 3α6α-diol-5β-pregnan-20-one-3,6-HFB2(708) | 18.04 | 708 |
| * 2H8-Corticosterone (354) | 2H8-Corticosterone-3,21-HFB2-H2O (728) | 20.52 | 709–713 |
| Corticosterone (346) | Corticosterone-3,21-HFB2-H2O (720) | 20.62 | 705 and 720 |
| * 2H7-Cortisone (367) | 2H7-Cortisone-3,21-HFB3 (955) | 22.04 | 738–741 |
| Cortisone (360) | Cortisone-3,21-HFB3 (948) | 22.05 | 684 and 734 |
The diagnostic ions in bold face served for quantification
: Internal standards
Table 2.
GC/MS detection threshold in quail brain and plasma
| Steroids | Plasma (1 ml) | TEL (400 mg) | POA (40 mg) |
|---|---|---|---|
| ng/ml | ng/g | ||
| DHEA-S, Na+ | 0.002 | 0.005 | 0.05 |
| PREG-S, Na+ | 0.002 | 0.005 | 0.05 |
| 5β-dihydroandrostanedione | 0.005 | 0.010 | 0.10 |
| 5α-dihydroandrostanedione | 0.005 | 0.010 | 0.10 |
| 5β-dihydroprogesterone | 0.010 | 0.050 | 0.50 |
| 5α-dihydroprogesterone | 0.010 | 0.050 | 0.50 |
| 3α5α-tetrahydroT | 0.001 | 0.002 | 0.02 |
| T | 0.002 | 0.005 | 0.05 |
| 3β5α- tetrahydroT | 0.005 | 0.010 | 0.10 |
| 17β-estradiol | 0.002 | 0.005 | 0.05 |
| 3α5α20α-hexahydroprogesterone | 0.010 | 0.020 | 0.20 |
| 20α-dihydropregnenolone | 0.010 | 0.020 | 0.20 |
| 20α-dihydroprogesterone | 0.005 | 0.010 | 0.10 |
| Etiocholanolone | 0.005 | 0.010 | 0.10 |
| 3β5α20α-hexahydroprogesterone | 0.010 | 0.020 | 0.20 |
| 2-méthoxyestradiol | 0.002 | 0.005 | 0.05 |
| Dehydroepiandrosterone | 0.010 | 0.020 | 0.20 |
| Δ4-androstene 3,17-dione | 0.010 | 0.020 | 0.20 |
| Epiandrosterone | 0.005 | 0.010 | 0.10 |
| Estrone | 0.020 | 0.050 | 0.50 |
| 5α-dihydroT | 0.005 | 0.010 | 0.10 |
| 3α5α-tetrahydroprogesterone | 0.020 | 0.050 | 0.50 |
| 3α5β-tetrahydroprogesterone | 0.020 | 0.050 | 0.50 |
| 3α5α-tetrahydroDOC | 0.020 | 0.050 | 0.50 |
| Pregnenolone | 0.010 | 0.020 | 0.20 |
| Progesterone | 0.005 | 0.010 | 0.10 |
| 3β5α- tetrahydroprogesterone | 0.005 | 0.010 | 0.10 |
| DOC | 0.010 | 0.020 | 0.20 |
| 5α20α- tetrahydroprogesterone | 0.050 | 0.100 | 1.00 |
| Estriol (E3) | 0.002 | 0.005 | 0.05 |
| Cortisol | 0.050 | 0.100 | 1.00 |
| Corticosterone | 0.005 | 0.010 | 0.10 |
| Cortisone | 0.050 | 0.100 | 1.00 |
POA: Hypothalamus-preoptic area
Statistical analysis
All data were analyzed by one or two-way analyses of variance or by t tests performed with Prism 7.0 on MacIntosh computers. When a steroid was below the detection limit in a sample, it was assigned for statistical and graphing purposes a concentration equal to the threshold of detection that varied between brain areas as a function of the amount of tissue available.
In experiment 1, data for the 4 or 2 brain areas (POA, TEL, CB and OL or only POA and TEL when steroids were assayed in these 2 brain regions only) were compared by two way ANOVA (sex and area as independent factors) followed by Bonferroni’s multiple comparisons post hoc test when an effect of brain areas was detected or followed by the Tukey’s multiple comparisons post-hoc test when an interaction between brain areas and sex were detected. Data for plasma assays were compared by t-test between males and females. Systemic and central concentrations of steroids are only compared qualitatively in this study. Pooled results for all subjects from the 4 batches of birds are presented in the results. Qualitative inspection did not reveal major batch differences even if a few steroids in some brain areas were present in different concentrations in different batches. However, including this variability in the general pattern never created overall effects and could only potentially lead to missing some sex or brain area differences (type 2 error). Effects presented here appeared despite the limited batch differences and are therefore more robust.
In experiment 3, data for two brain areas were compared across treatments by two-way ANOVAs (treatment and areas as independent factors). Data for serum or TEL alone were compared across treatments by one-way ANOVAs. These analyses when significant were followed by Tukey’s multiple comparisons post-hoc tests.
Differences or effects were considered significant for p<0.05. All data are presented by their mean ± Standard Error of the Mean (SEM).
Results
Experiment 1. Steroid profile and sex differences
A total of 25 steroids were reliably detected in the brain (4 [POA, TEL, CB and OL] or 2 [POA and TEL] brain regions assayed) and plasma of sexually mature males and females. Means ± SEM of concentrations are presented in figures 1 to 4 and the results of the corresponding statistical analyses are summarized in Table 3 that also indicates the degrees of freedom of all analyses and thus the number of samples that were analyzed in each case.
Figure 1.
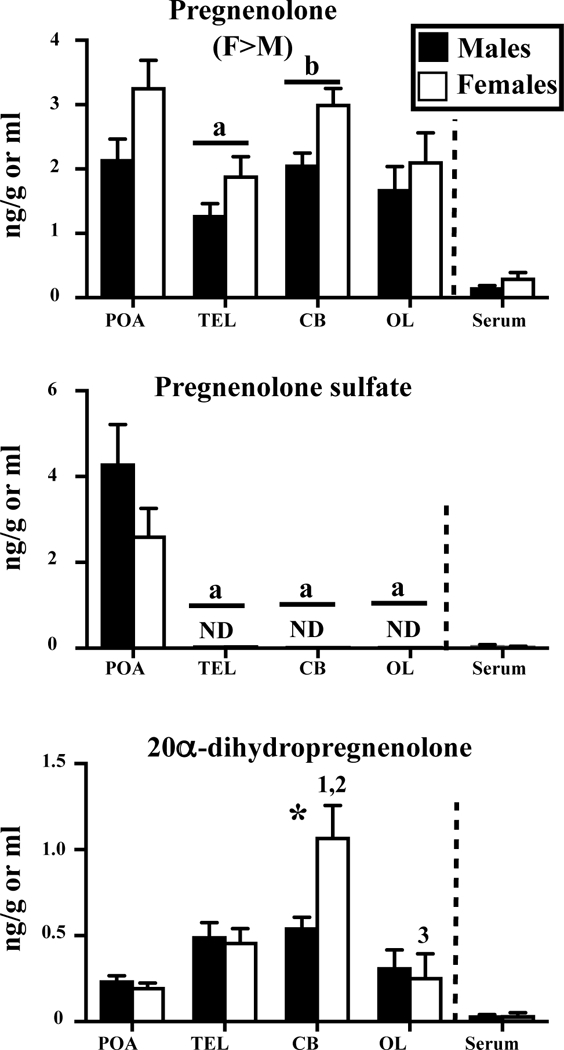
Concentrations of pregnenolone and its metabolites pregnenolone sulfate and 20α-dihydropregnenolone measured by GC/MS in the serum, hypothalamus-preoptic area (POA), whole telencephalon (TEL), cerebellum (CB) and optic lobes (OL) of adult sexually mature male and female Japanese quail. Data for brain areas were analyzed by a two-way ANOVA for each steroid with the brain area and sex as independent factors. Overall effects of sex are indicated under the name of the steroid considered (M>F or F>M). Results of Bonferroni post-hoc tests performed when a significant effect of brain areas was detected without interaction with sex are reported by a letter above the corresponding pairs of bars corresponding to the area of interest in both males and females (a=p<0.05 compared to the POA). Results of Tukey’s post-hoc tests performed when there was a significant interaction between sex and brain areas are reported by numbers above the bar associated with the sex and nucleus considered as follows: 1= p<0.05 compared to POA in the same sex, 2= p<0.05 compared to TEL in the same sex and 3= p<0.05 compared to CB in the same sex. Asterisks indicate sex differences within an area as detected by Tukey’s post hoc tests following a significant interaction between sex and area in the brain or by t-tests for the serum. ND above a bar indicates that the steroid was not detectable in any sample of the tissue and sex considered. These samples were for statistical and graphing purposes assigned a concentration equal to the threshold of detection.
Figure 4.
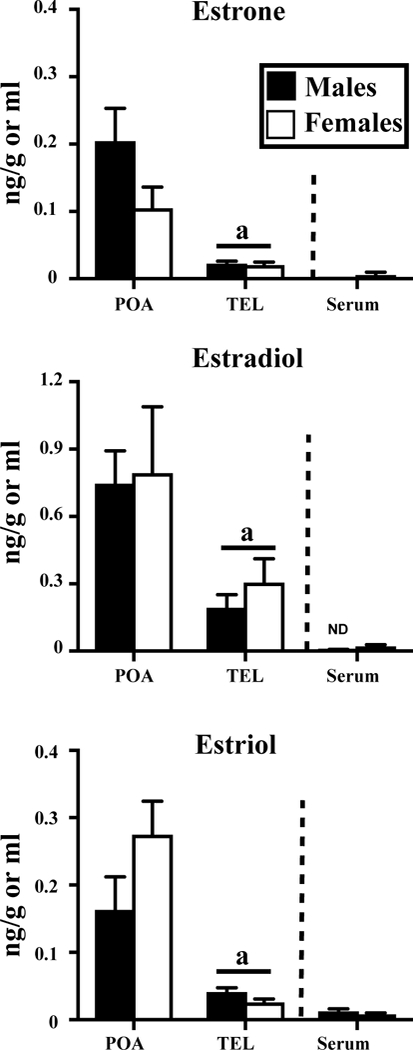
Concentrations of the estrogens measured by GC/MS in the serum, hypothalamus-preoptic area (POA), and whole telencephalon (TEL of adult sexually mature male and female Japanese quail. See also figure 1 for the detail of statistical analyses.
Table 3:
Summary of the statistical analyses performed on 25 steroids measured in 4 brain areas and in the serum of adult male and female quail. Data for brain areas were analyzed by a two-way ANOVA for each steroid with the area considered and sex of the subjects as independent factors. Concentrations in the serum were compared in males and females by a t test for each steroid. Df= degrees of freedom.
| Steroid | Sex | Nucleus | Interaction | Sex in | serum | |||
|---|---|---|---|---|---|---|---|---|
| F (df) | P | F(df) | P | F(df) | P | t(df) | p | |
| Pregnenolone | F1,51=7.961 |
0.007 ** |
F3,51=5.787 |
0.002 ** |
F3,51=0.373 | 0.773 | T14=1.869 | 0.083 |
| Pregnenolone-sulfate | F1,79=0.361 | 0.550 | F3,79=13.36 |
0.001 *** |
F3,79=0.800 | 0.497 | T26=1.226 | 0.231 |
| 20α-dihydropregnenolone | F1,39=2.650 | 0.112 | F3,39=16.74 |
0.001 *** |
F3,39=4.169 |
0.012 * |
T14=0.577 | 0.573 |
| Progesterone | F1,90=10.78 |
0.002 ** |
F3,90=11.80 |
0.001 *** |
F3,90=0.424 | 0.736 | T34=3.405 |
0.002 ** |
| Corticosterone | F1,58=0.249 | 0.62 | F3,58=1.948 | 0.132 | F3,58=0.767 | 0.517 | T19=1.141 | 0.268 |
| 5α-dihydroprogesterone | F1,32=11.02 |
0.002 ** |
F3,32=5.068 |
0.006 ** |
F3,32=2.238 | 0.103 | T6=2.521 |
0.045 * |
| 5β-dihydroprogesterone | F1,12=20.75 |
0.001 *** |
F1,12=0.022 | 0.884 | F1,12=0.238 | 0.634 | ND | ---- |
| 3α,5α-THP | F1,12=2.462 | 0.143 | F1,12=30.65 |
0.001 *** |
F1,12=2.462 | 0.143 | ND | --- |
| 3α,5β-THP | F1,12=29.29 |
0.001 *** |
F1,12=44.82 |
0.001 *** |
F1,12=29.28 |
0.001 *** |
T6=3.418 |
0.014 * |
| Dehydroepiandrosterone | F1,46=5.110 |
0.029 * |
F3,46=1.653 | 0.190 | F3,46=0.187 | 0.905 | T14=8.252 |
0.001 *** |
| Androstenediol | F1,12=6.839 |
0.023 * |
F1,12=53.77 |
0.001 *** |
F1,12=0.760 | 0.400 | T6=1,765 | 0.128 |
| T | F1,50=97.92 |
0.001 *** |
F3,50=5.99 |
0.002 ** |
F3,50=5.932 |
0.002 ** |
T14=4.352 |
0.001 *** |
| Androstenedione | F1,36=60.62 |
0.001 *** |
F3,36=2.145 | 0.111 | F3,36=2.100 | 0.117 | T14=3.442 |
0.004 ** |
| 5α-dihydrotestosterone | F1,38=1.073 | 0.307 | F3,38=27.41 |
0.001 *** |
F3,38=10.34 |
0.001 *** |
T14=0.863 | 0.403 |
| 5β-dihydrotestosterone | F1,12=39.87 |
0.001 *** |
F1,12=2.296 | 0.156 | F1,12=0.985 | 0.340 | T6=3.007 |
0.024 * |
| 3α,5α-THT | F1,64=29.33 |
0.001 *** |
F3,64=0.311 | 0.817 | F3,64=1.394 | 0.253 | T6=10.05 |
0.001 *** |
| Etiocholanolone | F1,23=33.53 |
0.001 *** |
F3,23=15.85 |
0.001 *** |
F3,23=6.679 |
0.003 ** |
T6=4.531 |
0.004 ** |
| 5α-dihydroandrostanedione | F1,12=0.001 | 0.988 | F1,12=266.3 |
0.001 *** |
F1,12=0.031 | 0.863 | T6=1.458 | 0.195 |
| 5β-dihydroandrostanedione | F1,12=12.73 |
0.004 ** |
F1,12=4.804 |
0.049 * |
F1,12=1.774 | 0.208 | T6=2.086 | 0.082 |
| 3α,5β-THT | F1,12=0.231 |
0.002 ** |
F1,12=2.310 | 0.154 | F1,12=0.011 | 0.920 | T6=3.842 |
0.009 ** |
| 3β,5α-THT | F1,12=2.026 | 0.180 | F1,12=7.606 |
0.018 * |
F1,12=1.204 | 0.2941 | T6=1.945 | 0.099 |
| Androstenediol | F1,12=6.839 |
0.023 * |
F1,12=53.77 |
0.001 *** |
F1,12=0.760 | 0.400 | T6=1,765 | 0.128 |
| Epiandrosterone | F1,12=0.763 | 0.400 | F1,12=14.98 |
0.002 ** |
F1,12=1.325 | 0.272 | T6=0.637 | 0.548 |
| Estrone | F1,21=0.688 | 0.416 | F1,21=4.730 |
0.041 * |
F1,12=0.642 | 0.432 | T6=1.344 | 0.227 |
| E2 | F1,56=0.127 | 0.722 | F1,56=5.501 |
0.023 * |
F1,56=0.021 | 0.883 | T28=1.769 | 0.088 |
| Estriol | F1,23=1.471 | 0.238 | F1,23=21.96 |
0.001 *** |
F1,23=2.565 | 0.123 | T6=1.322 | 0.234 |
Data concerning pregnenolone, the precursor of all steroid hormones and its metabolites are summarized in figure 1 and table 3. A few noticeable, statistically significant results are immediately obvious, namely:
-Pregnenolone was present in high concentrations in all brain samples and higher in females but its concentration in serum samples was comparatively very low,
-Surprisingly, pregnenolone sulfate was detectable exclusively in the preoptic area,
-20α-dihydropregnenolone was also more concentrated in the brain, especially in the cerebellum of females, as compared to serum.
Brain and serum levels of PROG, its reduced metabolites and corticosterone are shown in figure 2 and table 3. PROG and its metabolites 5α-dihydroprogesterone, 5β-dihydroprogesterone and 3α,5β-THP, were present in higher concentrations in females than in males in all brain samples. A similar sex difference was also detected in the serum for PROG, 5α-dihydroprogesterone and 3α,5β-THP.
Figure 2.
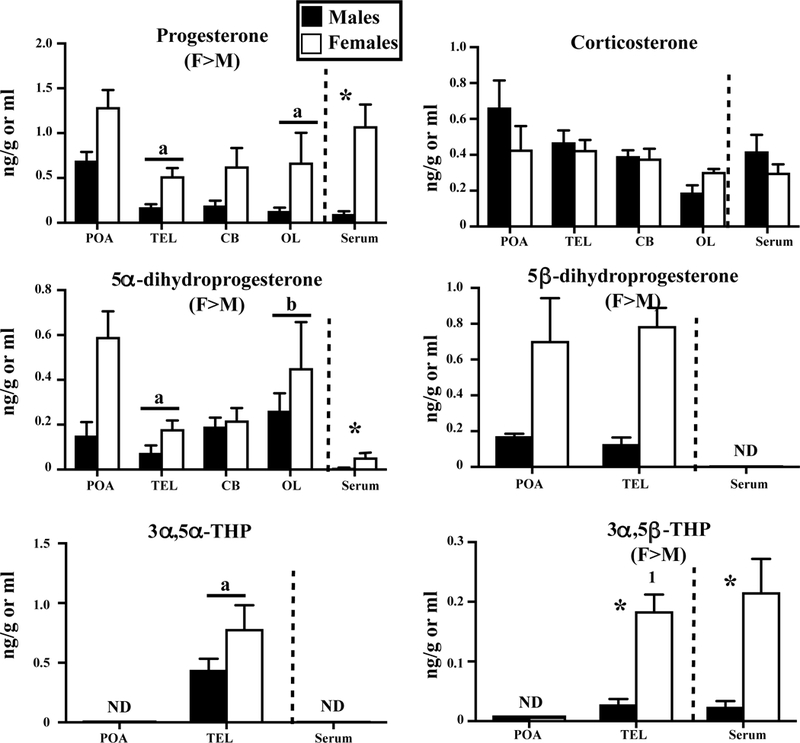
Concentrations of progesterone (PROG) and its reduced metabolites and corticosterone measured by GC/MS in the serum, hypothalamus-preoptic area (POA), whole telencephalon (TEL), optic lobes (OL) and cerebellum (CB) of adult sexually mature male and female Japanese quail. See also figure 1 for the detail of statistical analyses.
PROG levels were highest in POA and 5α-dihydroprogesterone levels were highest in POA and OL. Both 5α-dihydroprogesterone and 5β-dihydroprogesterone were more concentrated in brain as compared to serum. A few global differences between brain areas were also observed for 3α,5α-THP and 3α,5β-THP but it is difficult to determine whether these differences are genuine. 3α,5α-THP and 3α,5β-THP were detected in the TEL only, but this might relate to the larger size of the TEL that increases the assay sensitivity.
Corticosterone was detected in similar concentrations in all brain samples and in the serum of both sexes.
In contrast, the pattern of concentrations of androgens, their metabolites and their precursor DHEA as presented in figure 3 was quite different. First there was generally an overall sex difference in brain concentration in favor of males. This overall sex difference was statistically significant for DHEA and for 8 of the 12 other steroids presented in figure 3. These androgens were also often more concentrated in the serum of males compared to females (7 out of 13 steroids).
Figure 3.
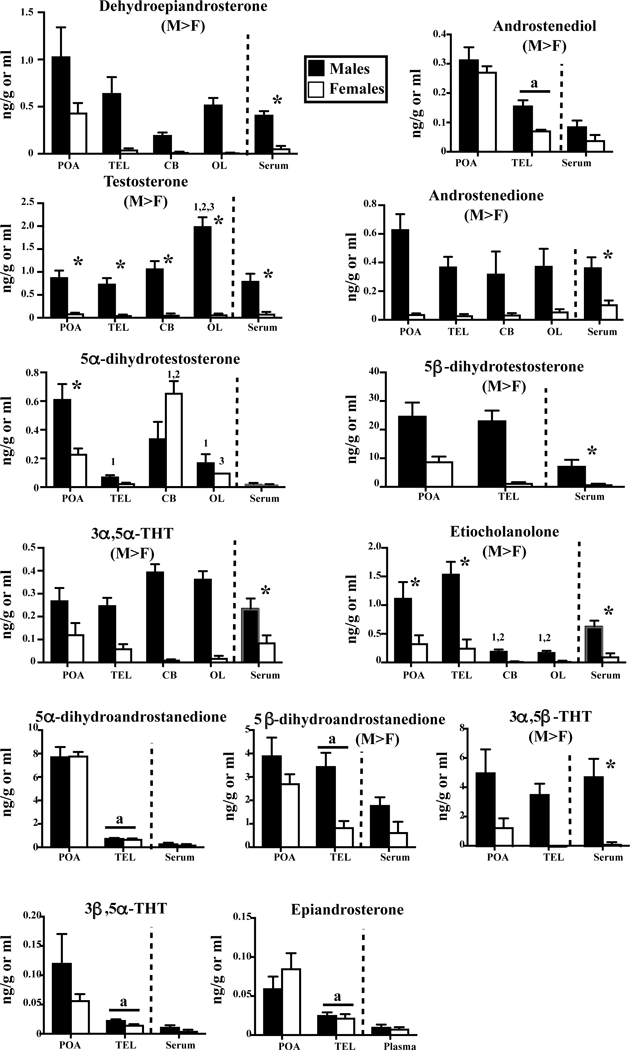
Concentrations of DHEA, androstenediol, T and its metabolites measured by GC/MS in the serum, hypothalamus-preoptic area (POA), whole telencephalon (TEL), optic lobes (OL) and cerebellum (CB) of adult sexually mature male and female Japanese quail. See also figure 1 for the detail of statistical analyses.
Some tissue specificity was also present here with higher concentrations, for T in OL in males, for 5α-dihydrotestosterone in the POA (and cerebellum) in males, for 5α-dihydrotestosterone in CB in females. Concentrations in both sexes were higher in the POA than in other regions for androstenediol, 5α-and 5β-dihydroandrostanedione, 3β,5α-THT and epiandrosterone (see details in Figure 3).
Some steroids were in apparent equilibrium between the brain and the serum (e.g., DHEA, T, androstenedione, 3α,5α-THT, 3α,5β-THT) but this was not the case for others, suggesting they are produced mainly in the brain (e.g., androstenediol, 5α- and 5β-dihydrotestosterone, 3β,5α-THT, 5α- and 5β-dihydroandrostenedione, epiandrosterone). Finally note that 5β-reduced androgens, in particular 5β-dihydrotestosterone, 5β-dihydroandrostanedione and 3α,5β-THT were present in very high concentrations in the brain compared to the “main” androgens T and androstenedione. 5β-dihydrotestosterone in particular, which is a direct metabolite of T was at least 10–20 times more concentrated than its parent steroid.
Three estrogens, estrone, estradiol and estriol could also be detected in quail brain and serum but no trace of 17α-estradiol could be identified (See Figure 4 and table 3). Their concentrations were higher in the brain as compared to serum, especially in POA of both males and females. Interestingly, no significant sex difference could be detected. It must be noted that concentrations of E2 in serum were below threshold in all 10 male samples while this was the case in only one of the 20 female samples. The 19 other female samples were above threshold but barely. As a consequence, no sex difference was detected by t test (t28= 1.769, p=0.088) but a non-parametric Mann Whitney test suggested a highly significant difference (U=18.5, p<0.001).
Experiment 2. Steroid concentrations in dialysates from the male medial preoptic area
We searched in dialysates of male POA for all steroids that had been assayed in the male POA. Many of them were detected (see figure 5A) but several of them were below the detection level. This was namely the case for PROG, 5α-dihydroprogesterone, 3α,5α-THP, 3α,5β-THP, androstenedione, epiandrosterone, estrone, estriol and most surprisingly pregnenolone sulfate that was present in high concentrations in the male POA. The concentrations of steroids in dialysates are shown in figure 5A and compared with the concentrations previously detected during experiment 1 in the male POA (figure 5B).
Figure 5.
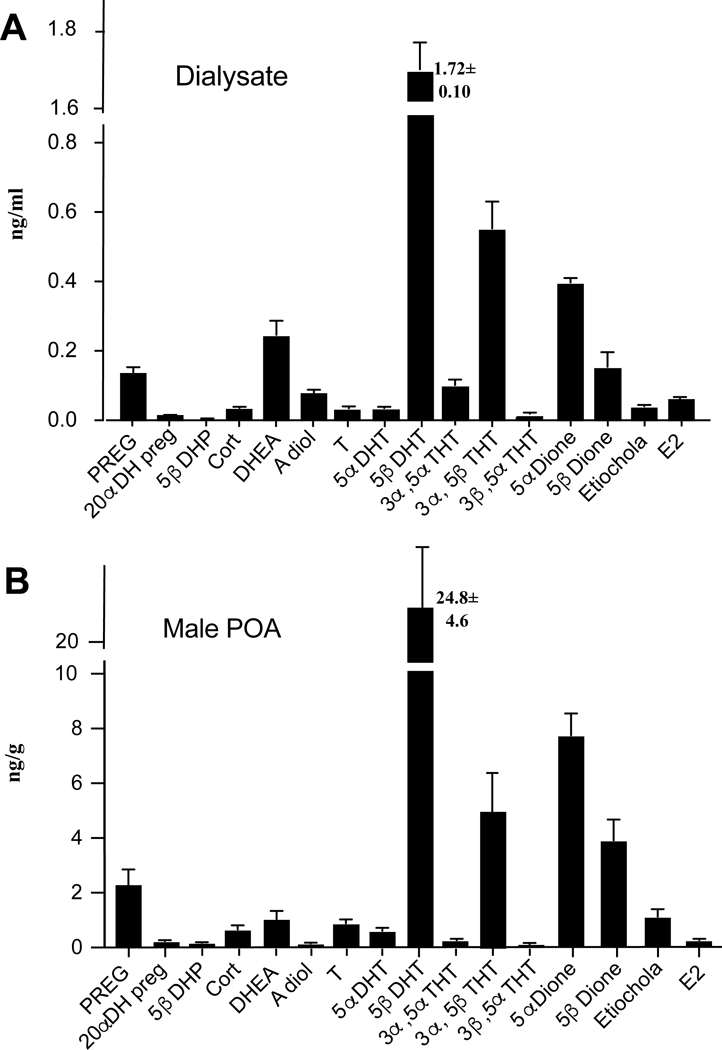
Comparison of the steroid concentrations measured in dialysate collected in vivo from the male preoptic area (A) with the tissue concentrations in the same general brain region (POA) of males (B).
Histological analysis confirmed that all cannulae were located in the rostral half of the hypothalamus although there was some variability in location at the fine level. One cannula was in the medial preoptic nucleus, one was immediately ventral to this nucleus, two were in the ventromedial nucleus of the hypothalamus and one was located in between but more dorsally in the bed nucleus of the stria terminalis.
As can easily be seen, the concentrations measured by in vivo dialysis (which represents a fraction of what was present in the extracellular milieu) reflected the concentrations measured in the entire tissue. In particular, the higher concentrations of 5β-dihydrotestosterone, 5α- and 5β-dihydroandrostanedione, 3α,5β-THT, DHEA and of pregnenolone were found in the dialysate as in the entire tissue. Noticeably this list includes a large number of 5β-reduced metabolites of T and the amount of steroids in the dialysate reflects their tissue concentration. Note however that concentrations in the dialysate were roughly one order of magnitude lower than in the entire tissue.
Experiment 3. Effects of castration and replacement with T or E2
Morphological data
The body weight of the four groups of males did not differ when birds received their Silastic™ implants (F3,27=0.0927, p=0.9634) nor one week later when brains were collected (F3,27=1.079, p=0.2389). Note that overall the birds from the INRA French colony were smaller that the birds from Belgium used in previous experiments (e.g. 149.8 ± 1.8 g here vs. a range of 200–240 g for birds from Liege).
As expected, the cloacal gland area (CGA) was significantly affected by the endocrine treatments (F3,27=215.7, p=0.0114). It was markedly increased in T-treated birds as compared to CX birds (190.7 ± 1.8 vs. 46.1 ± 2.9 mm2, p<0.001 by Tukey post hoc tests). Some increase in CGA was also observed in birds treated with E2 (E40: 127.1 ± 3.6 mm2, E80: 152.3 ± 6.7 mm2, p<0.001 vs CX birds in both cases) but this relates more to the E2-induced enlargement of the cloacal diameter than to a true swelling in the gland itself (28).
Steroid concentrations
Pregnenolone, T, androstenedione and E2 were detected in the serum, POA and TEL of all subjects and corresponding results are presented in figure 6. Given the lower endogenous amounts of steroids as a result of the castration, the concentration of several steroids was below the detection threshold in the POA or serum. By contrast, several steroids were detected in the TEL only (figures 6–7) probably because of its relative large size.
Figure 6.
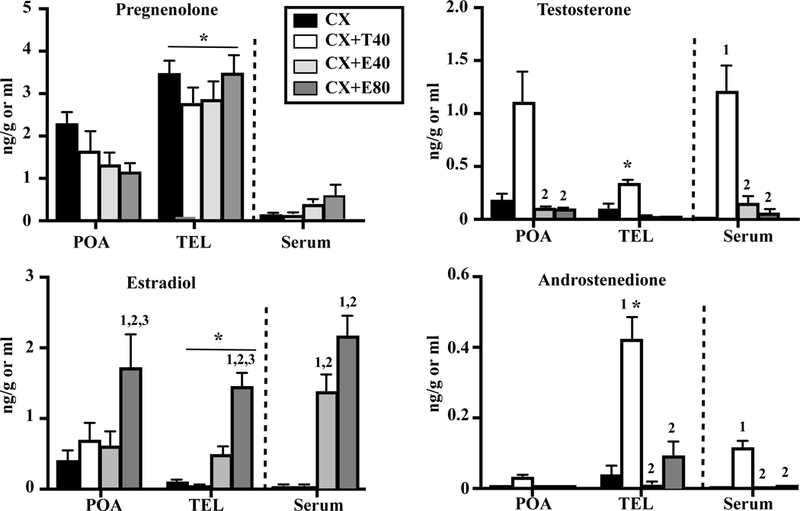
Concentrations of pregnenolone, T, E2 and androstenedione in the hypothalamus-preoptic area (POA), telencephalon (TEL) and serum of castrated male Japanese quail or castrated male quail treated with Silastic implants filled with T (T40) or with two doses of E2 (E40 and E80). Data for each steroid were analyzed by a two-way ANOVA with the two brain area considered (2 levels) and treatment of the subjects (4 levels) as independent factors or by a one-way ANOVA for the serum concentrations (4 levels). Results of Bonferroni post-hoc tests performed when a significant effect of brain areas was detected without interaction with treatment (PREG, E2) are reported by an underlined asterisk above the corresponding bars or by an asterisk above the bar if there was an interaction between treatment and brain area (T, androstenedione). Results of Tukey’s post-hoc tests performed when there was a significant overall effect of treatment are reported by numbers above the bar associated with the area and treatment considered as follows: 1= p<0.05 compared to CX same area, 2= p<0.05 compared to CX+T same area, 3= p<0.05 compared to CX+E40 same area and *= p<0.05 compared to POA same treatment.
Figure 7.
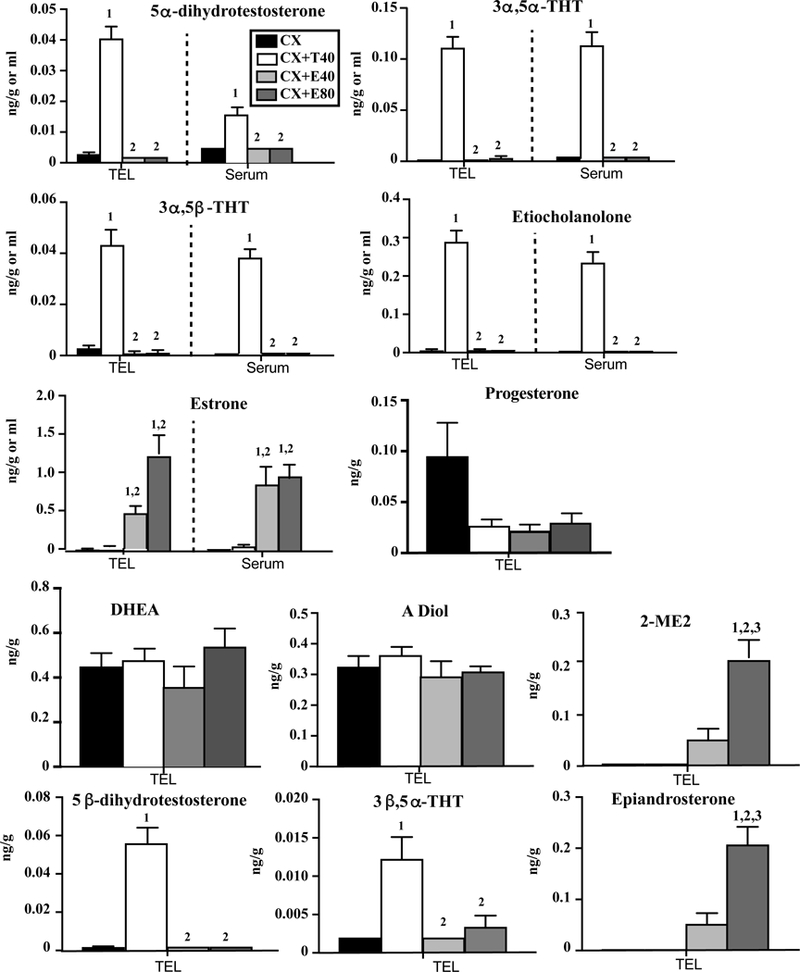
Concentrations of 5 steroids detected in the telencephalon (TEL) and serum (first three lines) and of 7 steroids detected in the TEL only (last three lines) in castrated males or castrated Japanese quail treated with Silastic implants filled with T (T40) or with two doses of E2 (E40 and E80). Data for each steroid were analyzed by a one-way ANOVA for each type of samples (TEL or Serum). Results of Tukey post-hoc tests performed when a significant effect of treatment was detected are reported by numbers above the bar associated with the treatment (and tissue) considered as follows: 1= p<0.05 compared to CX, 2= p<0.05 compared to CX+T40 and 3= p<0.05 compared to CX+E40.
Like in experiment 1, pregnenolone concentration was low in the serum but again high in the POA and TEL despite the fact that birds were castrated. Concentrations were significantly higher in the TEL than in the POA but they were not affected by the hormonal treatments. Average data suggested an interaction between treatment and areas (decrease following steroid treatment in POA, no clear effect in TEL) but this interaction did not reach statistical significance (p=0.051, see table 4).
Table 4:
Summary of the statistical analyses performed on 4 steroids measured in 2 brain areas and in the serum of adult castrated males or castrates treated with Silastic implants filled with T (T40) or with two doses of E2 (E40 and E80). Data for brain areas were analyzed by a two-way ANOVA for each steroid with the area considered and treatment of the subjects as independent factors. Concentrations in the serum were compared in the 4 treatment group by a one-way ANOVA for each steroid. Df= degrees of freedom.
| Steroid | Treatment | Area | Interaction | Treatment in | serum | |||
|---|---|---|---|---|---|---|---|---|
| F (df) | P | F(df) | P | F(df) | P | F (df) | p | |
| Pregnenolone | F3,52=0.805 | 0.497 | F1,52=40.46 |
0.001 *** |
F3,53=2.764 | 0.051 | T3,26=3.544 | 0.028 |
| T | F3,53=20.57 |
0.001 *** |
F1,53=14.47 |
0.001 *** |
F3,53=5.666 |
0.002 ** |
T3,26=27,79 |
0.001 *** |
| Androstenedione | F3,51=21.90 |
0.001 *** |
F1,51=41.91 |
0.001 *** |
F3,51=17.30 |
0.001 *** |
T3,27=42.42 |
0.001 *** |
| E2 | F3,52=17.55 |
0.001 *** |
F1,52=5.333 |
0.025 * |
F3,52=0.532 | 0.662 | T3,24=39,12 |
0.001 *** |
T was extremely low in the serum of CX males and markedly increased in T-treated birds while E2 implants only had a very low non significant effect on plasma T. Concentrations in the brain reflected these systemic changes suggesting that T freely enters the brain, although these concentrations remained much lower in the TEL than in the POA. The lower concentration of T in the TEL was possibly explained by its metabolism into androstenedione. A higher concentration of this androgen was indeed found specifically in the TEL suggesting that the 17β-hydroxysteroid dehydrogenase 2 is particularly active in this brain region.
In contrast the transfer of E2 from the periphery to the brain seemed less effective. While the two doses of E2 increased serum concentration in a dose-dependent manner, there was no significant increase in E2 concentration in the POA or TEL at the lowest dose and only the high dose was able to significantly increase the concentrations in the POA and TEL. Unexpectedly, the T implants did not significantly increase the POA concentration of E2 even if an average slight increase was detected. Mean E2 levels were however higher in POA as compared to TEL and serum in T-treated CX birds suggesting a specific higher aromatase activity in POA.
All metabolites of T were present in extremely low concentration in the plasma and TEL of castrated males and they were very significantly increased in males treated with exogenous T but not with exogenous E2. This pattern was observed for 5α-dihydrotestosterone, 3α,5α-THT, 3α,5β-THT, etiocholanolone, 5β-dihydrotestosterone and 3β,5α-THT (not detected in the serum for the last two of these steroids). Conversely, the two groups treated with exogenous E2 displayed a large increase in two estrogenic metabolites in the TEL (estrone, 2 methoxy-estradiol or 2-ME) and also in the serum for estrone while these steroids were not detected in castrates or castrates treated with T. Surprisingly, epiandrosterone levels were only increased in the E2-treated birds. DHEA, PROG and androstenediol (ADIOL) were only present in the TEL and unaffected by the systemic hormonal treatments. Average PROG concentrations decreased after treatments with T or E2 but the effect just failed to reach statistical significance (p=0.051; see table 5). This weak effect might result from an inhibitory cross-talk between the hypothalamo-pituitary-gonadal and hypothalamo-pituitary-adrenal axes (42).
Table 5:
Summary of the statistical analyses performed on steroids measured in the telencephalon and serum of adult castrated males or castrates treated with Silastic implants filled with T (T40) or with two doses of E2 (E40 and E80). Data for each steroid and tissue were analyzed by a one-way ANOVA with the treatments of the subjects as independent factor. Df= degrees of freedom.
| Steroid | TEL | Serum | ||||
|---|---|---|---|---|---|---|
| F (df) | P | F(df) | P | |||
| 5α-dihydrotestosterone | F3,27=115.3 |
0.001 *** |
F3,27=29.21 |
0.001 *** |
||
| 3α,5α-THT | F3.27=53.49 |
0.001 *** |
F3,27=67.88 |
0.001 *** |
||
| 3α,5β-THT | F3,27=53.49 |
0.001 ** |
F3,27=134.6 |
0.001 *** |
||
| Etiocholanolone | F3,27=112.1 |
0.001 *** |
F3,27=96.37 |
0.001 *** |
||
| Estrone | F3,27=23.06 |
0.001 *** |
F3,27=12.32 |
0.001 *** |
||
| Progesterone | F3,26=2.96 | 0.051 | ||||
| DHEA | F3,27=1.03 | 0.394 | ||||
| Androstenediol | F3,27=0.691 | 0.566 | ||||
| 2-ME | F3,27=26.15 |
0.001 *** |
||||
| 5β-dihydrotestosterone | F3,27=52.28 |
0.001 *** |
||||
| 3β,5α-THT | F3,27=11.50 |
0.001 *** |
||||
| Epiandrosterone | F3,27=26.15 |
0.001 *** |
||||
Discussion
The studies described in this paper provide new information concerning steroid concentrations in the quail brain and their potential responses to changes in circulating sex steroids. These data articulate around two main themes: the sex and tissue differences in concentrations of multiple steroids and the effects on the brain concentrations of castration and treatments with exogenous T or E2, with special reference to the brain concentration of E2.
Steroid profiles in male and female serum and brain
GC/MS allowed us to measure a large number of steroids in individual samples and revealed the presence in most brain areas of PROG, T, E2, corticosterone and many of their precursors and metabolites (see Figure 8).
Figure 8.
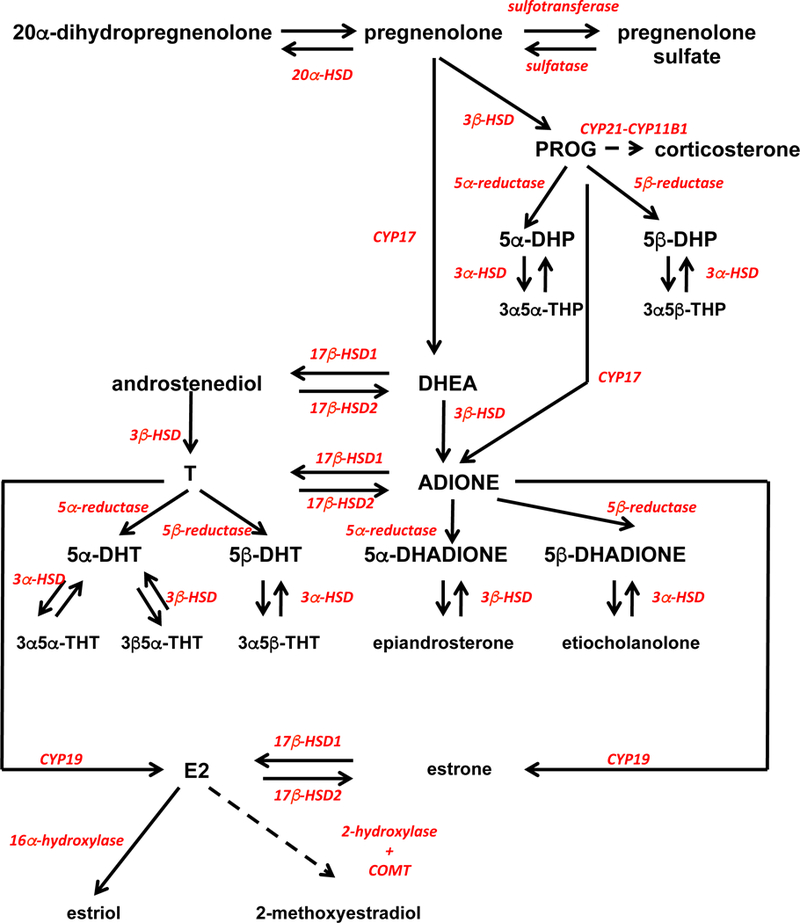
Metabolic pathways describing the putative synthesis pathways of steroids that could be reliably identified in the quail brain and serum.
These data are consistent with the existence in quail of metabolic pathways for sex steroid synthesis and metabolism that are very similar to the pathways described in mammals and other avian species although a few species-specific results were observed. Previous work had identified in the quail and zebra finch brain the messenger RNA coding for most, if not all, enzymes responsible for synthesis of sex steroids from cholesterol and their transformation into a variety of metabolites (14, 15, 17–19). The presence of these mRNA does not, however, necessarily demonstrate that the corresponding enzymes are active. Translation of the mRNA into protein might be low and the enzymatic activity of the protein could be low namely because the corresponding substrates are not present in sufficient concentration. In addition, a number of in vitro studies had previously analyzed the metabolism of radioactive T or of its precursors in the brain of male and female quail studied in a variety of endocrine conditions (14, 15, 43–48) but again these studies did not necessarily demonstrate that the corresponding metabolites are produced in vivo since a) substrate may be a limiting factor and b) enzymatic activities are analyzed in optimal conditions (e.g. presence of cofactors in saturating concentrations) when studied in vitro and this might not reflect the in vivo situation. This paper therefore provides new data describing with more detail in the quail brain and serum the endogenous concentrations of a variety of sex steroids, their precursors and metabolites in males and females as well as the contribution of circulating steroids, especially T and E2, to their respective brain concentrations.
Central vs. systemic concentrations and local differences in the brain
Sex steroids are lipophilic and classically supposed to enter freely in lipophilic tissues such as the brain (49, 50) despite their association with low to moderate affinity to circulating binding proteins Therefore it could have been expected that their concentration would be similar in the serum and in the two or four brain areas that were sampled. This was indeed the case for some hormonal steroids such as progesterone (in females more than in males), corticosterone or testosterone but not for many others that were generally more concentrated in the brain. In contrast, we never detected serum concentrations that were higher than brain concentrations, as could have been expected if a peripheral source (gonads, adrenals) was present but the steroid could not freely enter the brain (see however discussion of experiment 3 for estradiol). As a note of caution, it should be reminded here that due to the absence of some individual data points, all data were analyzed considering the different brain regions as an independent factor in the ANOVAs and we did not statistically compare serum and brain concentrations. All conclusions presented below therefore refer to mean concentrations of steroids in a given sex or experimental group and do not exclude that a different pattern of distribution between the periphery and specific brain areas could be observed at the individual level. These observations also should not be construed to suggest that all steroids that were more concentrated in the brain than in the serum are actually synthesized locally. Systemic steroids produced in the gonads or adrenal glands certainly contribute to these brain concentrations and they could accumulate in the very lipophilic brain tissue or via binding to specific receptors or binding proteins with a higher capacity but lower affinity (51, 52).
Although all steroids are supposed to diffuse freely in the entire organisms, very important differences were observed between the steroids concentrations across brain areas and serum. Three types of average distributions were essentially detected: 1) steroids present in roughly similar concentrations in all samples, 2) steroids present in higher concentrations in all brain regions than in the serum and finally 3) steroids present in high concentration specifically in some brain areas but not in others.
As already mentioned the first type of steroids includes corticosterone and testosterone but also multiple other androgens including DHEA, androstenedione, 3α5α-THT and 3α5β-THT. These steroids are presumably secreted in periphery and highly diffusible. This is clearly the case for corticosterone secreted by the adrenal glands and testosterone by the testes. Androstenedione and 5α- or 5β-reduced androgens could also be formed in the brain; there is indeed a very high 17β-HSD activity in the TEL (see experiment 3), converting T into androstenedione, and previous work with radioactive T had identified an extremely high 5β-reductase activity in the brain (43, 46). It is therefore conceivable that a fraction at least of these steroids are produced in the brain by metabolism of the parent steroid (T) and secondarily released in the periphery as shown previously for circulating estrogens that are produced in the brain of zebra finches (53, 54).
Progesterone has also some but not all features of this first group. It is secreted by the ovaries and adrenals and was roughly in equilibrium between periphery and the POA. It was also present in all other areas but concentrations were significantly lower in the TEL and OL than in the POA. Either progesterone did not reach these areas from the periphery at the same rate or it was rapidly metabolized in a region-specific manner. The high concentrations of 3α,5α-THP and of 3α,5β-THP detected in the TEL but not the POA would be consistent with this interpretation.
It should also be noted that corticosterone concentrations measured here were lower (0.5 ng/ml) than in previous studies in quail based on enzyme imunoassays (1–5 ng/ml)(55, 56). This might reflect differences between assay methods as well as biological effects. It must indeed be recalled that here trunk blood was collected from birds rapidly killed by decapitation while in previous studies, concentrations were measured in blood samples collected from the wing or jugular vein, and these values already doubled after 5 min of restraint stress. It is therefore conceivable that values observed here are truly basal concentrations while those measured in previous studies were already affected by stress even if the samples were collected within 3 min, which is broadly considered as acceptable for getting baseline concentrations.
The second type of steroids includes pregnenolone, 20α-dihydropregnenolone, 5α- and 5β-dihydroprogesterone and possibly 5β-dihydrotestosterone (more concentrated in the brain although present in the periphery). The presence of a high 5β -reductase activity in the brain presumably explains the preferential distribution of 5β-reduced progesterone and testosterone in the brain. It must be emphasized that incubation of quail brain homogenates with radioactive T leads within minutes to the formation of extensive amounts of 5β-reduced metabolites (43–46). For example one study showed that within 15 min, radioactive T had been metabolized in substantial amounts of E2, 5α- and 5β-dihydrotestosterone but that the 5β-reduced metabolite is at least 10 times more concentrated than the others (57). The relative affinity (Km) of the 5β-reductase for T is lower (473 nM) than for aromatase and 5α-reductase (respectively 15 and 114 nM) but its maximum velocity is by far much higher (485 fmol/mg Fresh Weigh/15 min) than for the two other enzymes (54.6 and 37.2 fmol/mg Fresh Weigh/15 min for aromatase and 5α-reductase, respectively (58)). This probably explains why the concentration of 5β-dihydrotestosterone in the brain was found to be 10 to 20 times larger than the concentration of T and also much higher than 5α-dihydrotestosterone and E2. Other secondary 5β-reduced metabolites (5β-dihydroandrostanedione, 3α5β-THT) were similarly present in high concentration in quail brain.
Pregnenolone, that is derived from cholesterol, and its metabolite 20α-dihydropregnenolone are also present in the brain in higher concentrations than in the serum and data strongly suggest that pregnenolone and its metabolite are primarily synthesized in the brain. Accordingly we also found that brain concentrations of pregnenolone are not diminished following castration (experiment 3) thus confirming the independence of brain pregnenolone concentrations from gonadal activity as previously shown in mammalian species (41). The synthesis of radioactive pregnenolone from radioactive cholesterol has indeed been previously demonstrated in quail brain homogenates (15).
Finally, the third type of steroids concerned mostly compounds that were present in high concentration in the POA and in much lower concentration or absent in other brain regions and/or in the serum. This was mainly the case for pregnenolone sulfate (detected only in POA) and to a lower extent for androstenediol, 5α-dihydroandrostanedione, 3β5α-THT, epiandrosterone, the three estrogens in both sexes, 5α-dihydroprogesterone and 5β-dihydroandrostanedione in females and, 5α-dihydrotestosterone in males. The enzyme catalyzing the production of pregnenolone sulfate from pregnenolone thus seems to be specifically expressed and active exclusively in the POA that is closely associated with the control of all instinctive behaviors and with autonomic control. Thus, quail seem to have a highly active pregnenolone sulfotransferase in their POA that should obviously play a physiological role. In human and non-human primates but not in rodents (40), pregnenolone sulfate is a steroid produced in the zona reticularis of adrenals (59) and is secreted in high amounts in blood (≂ 300 nM) (60). Pregnenolone sulfate is known to increase activity in neuronal networks by inhibiting GABAergic and by stimulating glutamatergic neurotransmission (61). These neuromodulatory effects of pregnenolone sulfate are consistent with their promnesic actions (62). The functional implications of pregnenolone sulfate synthesis in the POA and its control mechanisms should thus be investigated.
The 5α-reductase also seems extremely active towards androgens in the POA compared to the TEL. But the specific function of this enzyme in the POA is poorly defined although it is clear that 5α-dihydrotestosterone synergizes with estrogens to activate male copulatory behavior (20, 28), an action that takes place to a large extent in the POA (26). Similarly estrogens are specifically detected in high amounts in the POA. E2 and estrone are formed by aromatization of testosterone or androstenedione, respectively, a reaction catalyzed by aromatase or estrogen synthase (9). This enzyme is well known to be expressed and active at a high level in the quail POA (31, 46, 57). It is also extensively documented that the production of high concentrations of estrogens at this site plays a key role in the control of appetitive and consummatory aspects of male sexual behavior (24).
It must be mentioned that the serum concentrations of the estrogens were extremely low, below the detection level in many samples. These concentrations are much lower than previously reported in studies based on RIA (29, 63). A similar discrepancy for blood concentrations of testosterone was previously identified between RIA and GC/MS in canaries (64) and between RIA and Liquid chromatography-MS/MS in zebra finches (65). Future work should determine whether these discrepancies reflect technical problems associated with RIA measurements. Note finally that both 3α5α-THP and 3α5β-THP were present only in the TEL and not in the POA (or serum for 3α5α-THP) but this could be explained by the amount of tissue available for the assay and thus the assay sensitivity
This study therefore demonstrates a great heterogeneity in the distribution of steroids in the brain. Multiple processes potentially contribute to the establishment of these differences: secretion by the gonads or adrenals, penetration in the brain, synthesis from steroids originating from cholesterol or from the periphery (e.g., 5α-reduction or aromatization of T) and metabolism/catabolism in specific brain area. This creates a patchwork of neuroendocrine situations whose functional significance is obviously difficult to apprehend.
Sex differences
These analyses also revealed or confirmed the existence of multiple average sex differences in steroid concentrations in quail brain and serum. PROG and its reduced metabolites were shown to be significantly more concentrated in females than in males in all brain areas as well as in the serum (except for 5β-dihydroprogesterone and 3α5α-THP that were not detected in serum).
Conversely, as could be predicted based on previous research in quail and other species (63, 66), T, its precursors DHEA and androstenediol, and its main metabolites (5β-dihydrotestosterone, 3α5β-THT, 3α5α-THT, androstenedione, 5β-dihydroandrostanedione, etiocholanolone) were more concentrated in the serum of males than in females. Since steroids seem to freely enter the brain (see above) this difference was reflected in all brain areas thus resulting in an overall sex difference in the two way ANOVAs (figure 3). Quite surprisingly however, the T concentrations detected here in females were extremely low (< 0.1 ng/ml). This strikingly contrasts with previous studies that either found no significant difference of T levels (measured by RIA) between males and females after three weeks of exposure to long days (43) or found a significant sex difference in circulating T concentrations in favor of males but with average values in females that were about half of those in males and with individual high female values overlapping with low male values (63). In the present study based on GC/MS assays, there was absolutely no overlap between serum T concentrations in females and in males and a 10 fold difference in mean values (0.803±0.159 vs. 0.089±0.038 ng/ml respectively in males and females, means ± SEM). This suggests that the RIA used in previous studies was affected in females by a non-identified cross-reaction. A number of androgens such as 5α-dihydrotestosterone are known to cross-react to various extents with T antibodies and it is likely that this is also the case for 5β-dihydrotestosterone. However, to our knowledge, this was never formally tested since this steroid is largely specific to birds and assay kits are usually developed for mammals. The abnormally high T values previously reported in females could also be due to a non specific interference in the assay of the high concentrations of fat that are known to circulate in egg laying females.
Overall, pregnenolone and PROG (and metabolites) were thus significantly more concentrated in the brain of females compared to males, and an opposite sex difference was seen for DHEA (M>F) and a large number of androgens (T, androstenedione and their metabolites). This suggests that males produce T via mainly PREG, DHEA and androstenedione by successive catalysis by CYP17, 3β-HSD and 17β-HSD1 in the testes (figure 8). The main metabolism pathway of androstenedione and T is the 5β-reduction but they could also be aromatized to estrone and E2, respectively. In contrast in females, E2 could be produced from PROG via androstenedione and estrone by CYP17, aromatase and 17β-HSD1 enzymes in ovaries, respectively.
Interestingly, an active synthesis of sex steroids from DHEA has been previously reported in another avian species, the song sparrow (Melospiza melodia). This species maintains territorial and aggressive behaviors throughout the year even when plasma T and E2 concentrations are basal during the winter. But these behaviors are sex steroid-dependent since they are blocked by antiandrogens combined with aromatase inhibitors (67, 68). Elegant work from Kiran Soma and collaborators clearly showed that these behaviors are activated during the winter by sex steroids produced in the brain from DHEA of adrenal origin (69, 70).
Steroids are present in the extracellular space
The analysis of brain concentrations of steroids is critical for the understanding of their physiological and behavioral actions. This approach is however limited by the fact that a single measure per subject can be performed post-mortem so that it is impossible to assess in detail the dynamics of concentration changes. The technique of in vivo microdialysis makes it possible to assess “on line” changes in concentration of chemical compounds present in the extracellular fluid in the brain. This approach is often limited by the low concentration of compounds recovered in the dialysates and the limited sensitivity of the assays. However, it was demonstrated that this approach allowed quantifying dynamic changes in E2 concentration taking place in auditory brain areas of zebra finches during social interactions (71) and this initiated an entire line of research (18, 72–74). We recently showed that changes in E2 concentrations can similarly be detected in the POA of Japanese quail during sexual interactions by ultrasensitive RIA based on an iodinated ligand (75). We therefore wondered whether this approach would allow us to identify a full steroid profile by the GC/MS technique.
Experiment 2 allowed us to detect a substantial number of steroids in dialysate that had been collected in freely moving male quail implanted with a dialysis probe aimed at their POA although not all steroids were detectable by this technique. A few observations are important in this context. First, the relative concentrations in the dialysate and in the entire tissue are similar for many steroids and the highest concentrations in both types of samples always concerned the same steroids (e.g., 5β-dihydrotestosterone, 3α5β-THT, 5α- and 5β-dihydroandrostanedione). This suggests that the relative polarity of steroids does not influence their relative extracellular concentration. The only exception is the absence of pregnenolone sulfate in dialysate in spite of the high amounts found in POA. This apparent discrepancy is puzzling and should be confirmed. If it proved to be true, the mechanistic bases of this difference (difference in polarity, binding to an endogenous compound preventing access to the probe) should then be researched
Secondly, absolute concentrations in the dialysate were 10 to 50 times lower than in the whole tissue. It is important to remember that these measures in dialysates only allow detecting relative changes in extracellular concentrations, which are clearly much higher. Amount of compounds in dialysates are limited by the rate of diffusion through the membrane and the flow rate through the probe. The no-net-flux approach must be performed to estimate the exact extracellular concentration but this technique can only target a specific steroid and is time-consuming: various concentrations of the target steroid must be tested in the perfusion liquid until no increase is found after the flux has passed through the brain (76, 77). It would therefore be of fundamental interest to assess in a few selected cases the real distribution of steroids in a tissue: how much of the total concentration seen in experiment 1 reflects intra- versus extra-cellular steroids?
Finally the present experiment represents a proof of concept indicating that it is now technically possible to follow in a dynamic manner the changes in steroid concentrations within the brain. The assay sensitivity of our GC/MS technique allowed detection of 16 steroids in 500 μl samples that correspond to 500 min of collection at a flow rate of 1 μl/min. This sensitivity is therefore not sufficient for studies that would try to follow acute steroid changes in response to short-lived stimuli (e.g. during the course of sexual interactions) unless major improvements in the sensitivity of the detection methods take place. However, the current state of the technique already clearly permits analyses of the brain responses to global changes in physiological condition such as castration, treatment with exogenous steroids or exposure to different photoperiods. We know in many cases the consequences of these manipulations on the blood concentrations of several steroids but given the steroidogenic capacity of the brain, it would be important to determine whether concentrations in specific brain regions are similarly affected
Effects of exogenous steroids on brain concentrations of steroids
We also analyzed the effects of castration and treatments with exogenous T or E2 on the concentrations of steroids in quail brain and serum. These experiments were prompted by behavioral neuroendocrinology experiments showing that although the activation by T of copulatory behavior in male quail critically depends on the action in the preoptic area of E2 derived from local aromatization, treatment of castrated males with E2 only produce a very limited activation of this behavior (see introduction). Furthermore the presence of steroid synthesizing enzymes in the brain (14, 15, 17) raised the question of why functional responses to steroids (e.g., male copulatory behavior) disappear after castration when the potential to produce sex steroids in the brain is still present.
We show here that in castrated males, circulating and brain concentration of most steroids are very low as compared to intact quail and/or undetectable, thus confirming previously published reports concerning systemic concentrations in quail (63) and mice (78). Treatment of castrated males with T or E2 markedly increased the circulating concentrations of these steroids. On average, this increased circulating concentration of T rapidly (within 7 days, probably sooner) equilibrated with concentrations in the POA, the brain area where T action is sufficient and necessary to activate male copulatory behavior (34, 35, 38). These data suggest that circulating T enters into the brain by easily crossing the blood-brain barrier and equally diffuses to all brain areas. The lower T concentration in TEL presumably relates to the high 17β-HSD2 activity in this brain region, which metabolizes T into androstenedione.
The changes in brain E2 concentration in birds implanted with Silastic™ capsules filled with E2 were more subtle. The serum concentrations of E2 increased in a dose-dependent manner. However, the low dose (40 mm implant) produced no increase in the POA nor in the TEL. Only the highest dose (80 mm implant) of E2 induced a significant increase of E2 brain concentration in both brain areas. This discrepancy between systemic and central E2 concentration could be the result of an extensive metabolism of the steroid by a variety of enzymatic processes such as 2- and 4-hydroxylation (production of catecholestrogens (79), sulfonation, glucuronidation or O-methylation (80, 81) eventually catalyzed at least in part by the aromatase enzyme itself that has been shown to support a variety of enzymatic processes beside the transformation of androgens into estrogens (82). Alternatively, systemic E2 might not penetrate the brain as efficiently as T does. A similar control of the entry of progesterone into the ewe brain has been reported that is modulated by the photoperiod animals are exposed to (83). This peripheral sequestration could relate to the presence of a compound that binds E2 in the blood, even if no specific E2 binding protein is known to occur in birds, or to the existence of a selective permeability of the blood-brain barrier. Thus, although T is known to activate male sexual behavior via its preoptic aromatization into an estrogen (23, 25–27), systemic treatment with exogenous E2 has little effect on this behavior unless extremely high doses are administered (see introduction)(22, 28, 29). These apparent paradoxical observations could possibly be explained by assuming that preoptic aromatization produces high local concentrations of E2 that are nearly impossible to reach by systemic administration of E2 without producing systemic toxic effects or because locally produced estrogens reach sub-cellular targets (e.g. synaptic clefts) that are not easily reached by the steroid coming from the periphery. This interpretation is consistent with the finding that treatment of rat hippocampal slice cultures with an aromatase inhibitor produces a dose-dependent decrease in E2 concentration associated with a significant inhibition of spine synapses and synaptophysin expression. These two estrogen-dependent responses were however not affected by treatment of the cultures with levels of E2 equivalent to peripheral concentrations in males (10−12M) or in females (10−10M). A much higher concentration (10−7M), as could be possibly obtained by local aromatization, was needed to affect these processes (84). Testing this interpretation is however difficult since measurement of E2 concentrations in the close vicinity of aromatase-positive sites such as the presynaptic boutons is technically difficult if not impossible with the available technology.
Previous work has very reliably shown that T treatment increases the preoptic aromatase activity (46) as well as the expression of the corresponding mRNA (85, 86) and protein (87, 88) (see for review: (89)). There was here a doubling of the average E2 concentration in POA following T treatment (0.301±0.091 vs. 0.698±0.240 ng/g), which did not reach statistical significance possibly due to the large individual variance and limited number of data points (the power of the analysis based on these sample estimates was only 0.31). Thus, either a more powered study would detect an increase in E2 consistent with the established increase in aromatase activity with a T treatment or this increase in enzymatic activity demonstrated during ex vivo assays does not result in an increased E2 concentration in vivo. This could be because the enzyme does not behave in vivo as it does in vitro (absence of critical cofactors, inadequate pH…) or an increased catabolism compensates for the increased production of E2. In this latter option, one should then explain why the in vivo inhibition of preoptic aromatase activity drastically decreases male sexual behavior. Importantly, note however that the E2 concentration was higher in the POA than in the TEL and serum in the T-treated CX birds in agreement with a specific higher aromatase activity/expression in the POA.
Interestingly, this experiment also demonstrated that treatment of castrates with T or E2 results in a clear increase of their respective metabolites in the TEL and in some cases in the serum suggesting that they are produced via metabolism of gonadal T or E2 by active endogenous enzymes including, 17β-HSD2, 5α- and 5β-reductase, 3α- and 3β-HSD, 2-hydroxylase and catechol-o-methyl-transferase (production of 2-ME).
The concentrations of T were similar in the TEL and serum of T-treated CX quail and in gonadally intact subjects and this was also the case for some of their metabolites such as androstenedione, 5α-dihydrotestosterone, 3α,5α-THT and 3β,5α-THT (quantified in the TEL only for the last 3). This suggests that T can be metabolized into androstenedione by 17β-HSD2 and into 5α-dihydrotestosterone by 5α−reductase in the TEL and the testis does not contribute in an important manner to the concentration of these metabolites in the brain. By contrast, 5β-dihydrotestosterone was at least 200 times more concentrated in the TEL of gonadally intact males than in T-treated castrated males and this was also the case, to a lower extent, for 3α,5β-THT. These results might suggest that in gonadally intact subjects the testes markedly contribute to the circulating and brain concentrations of these metabolites. This conclusion is however in apparent conflict with the observations that a) 5β-reduced metabolites of T can be produced in large amounts in the quail brain, as previously shown by in vitro radioenzymatic assays (43, 46, 57) and b) concentrations of 5β-DHT were shown here to be lower in the serum than in the brain of gonadally intact birds, The origins of this discrepancy should be investigated by more causal experiments.
It is finally important to note that brain pregnenolone concentrations were high in both intact male and female quail and unaffected by castration despite the fact that average concentrations of this steroid were in both cases low in the serum. Previous work has demonstrated in several animal species including quail (15) that the enzymes catalyzing the synthesis of sex steroids from pregnenolone are expressed in the brain. We actually found that T and its main reduced metabolites and E2 were detectable in the brain of CX quail. As a consequence, castration should not entirely remove steroids from the brain. DHEA and androstenediol, the precursors of T, were also found in the brain and were almost unaffected by castration. We can thus speculate that sex steroids, T and E2, could be produced in the brain independently of the peripheral source, via the pregnenolone – DHEA - androstenediol enzymatic pathway. However, the lower levels of E2 induced by castration are clearly insufficient for the sex steroid-dependent activation of copulatory behavior (43).
In conclusion, this study provides new information on the brain vs. systemic concentrations of multiple steroids in a broadly used model for neuroendocrine studies, the Japanese quail and on the possible reasons explaining the limited behavioral activity of estrogens administered systemically in comparison with estrogens produced centrally by T aromatization. These data highlight the sex differences in steroidogenesis and indicate interaction between local brain synthesis and the supply from the periphery for the steroids present in brain that are either directly active or represent the substrate of centrally located enzyme.
Acknowledgments
This research was supported by grants from NIH/NIMH (R01MH50388) to JB. CAC is Senior Research Associate from the F.R.S.-FNRS.
References
- 1.Baulieu EE, Robel P, Vatier O, Haug M, Le Goascogne C, Bourreau E. Neurosteroids: pregnenolone and dehydroepiandrosterone in the brain In: Fuxe K, Agnati LF, eds. Receptor interactions. Basingstoke: MacMillan; 1987: 89–104. [Google Scholar]
- 2.Le Goascogne C, Robel P, Gouezou M, Sananès N, Baulieu EE, Waterman M. Neurosteroids: cytochrome P450 scc in rat brain. Science. 1987; 237: 1212–1215. [DOI] [PubMed] [Google Scholar]
- 3.Robel P, Baulieu EE. Neurosteroids. Biosynthesis and function. Trends Endocrinol Metab. 1994; 5:1–8. [DOI] [PubMed] [Google Scholar]
- 4.Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Rev. 2001; 37: 3–12. [DOI] [PubMed] [Google Scholar]
- 5.Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends in endocrinology and metabolism: Trends Endocrinol Metab. 2002; 13: 35–43. [DOI] [PubMed] [Google Scholar]
- 6.Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Rec Prog Horm Res. 1997; 52:1–32. [PubMed] [Google Scholar]
- 7.Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the diencephalon. J. Clin Endo Metab. 1971; 33: 368–370. [DOI] [PubMed] [Google Scholar]
- 8.Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975; 31:295–319. [DOI] [PubMed] [Google Scholar]
- 9.Balthazart J, Ball GF. Brain Aromatase, estrogens and behavior New York: Oxford University Press, 2013. [Google Scholar]
- 10.Paul SM, Purdy RH. Neuroactive steroids. FASEB journal : official publication of the Fed Am Socr Exp Biol. 1992; 6: 2311–2322. [PubMed] [Google Scholar]
- 11.Schumacher M, Guennoun R, Mattern C, Oudinet JP, Labombarda F, De Nicola AF, Liere P. Analytical challenges for measuring steroid responses to stress, neurodegeneration and injury in the central nervous system. Steroids. 2015; 103:42–57. [DOI] [PubMed] [Google Scholar]
- 12.Mills AD, Crawford LL, Domjan M, Faure JM. The behavior of the Japanese or domestic quail Coturnix japonica. Neurosci Biobehav Rev. 1997; 21:261–281. [DOI] [PubMed] [Google Scholar]
- 13.Ball GF, Balthazart J. Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR J. 2010; 51: 310–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsutsui K, Matsunaga M, Miyabara H, Ukena K. Neurosteroid biosynthesis in the quail brain: a review. J Exp Zool 2006; 305: 733–742. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui K Neurosteroid biosynthesis and function in the brain of domestic birds. Front Endocrinol. 2011; 2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006; 147: 5975–5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.London SE, Schlinger BA. Steroidogenic enzymes along the ventricular proliferative zone in the developing songbird brain. J Comp Neurol. 2007; 502: 507–521. [DOI] [PubMed] [Google Scholar]
- 18.London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front Neuroendocrinol. 2009; 30: 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remage-Healey L, London SE, Schlinger BA. Birdsong and the neural production of steroids. J Chem Neuroanat. 2010; 39: 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adkins EK, Pniewski EE. Control of reproductive behavior by sex steroids in male quail. J Comp Physiol Psychol. 1978; 92:1169–1178. [Google Scholar]
- 21.Balthazart J Steroid metabolism and the activation of social behavior In: Balthazart J, ed. Advances in Comparative and Environmental Physiology, vol 3 Berlin: Springer Verlag; 1989: 105–59. [Google Scholar]
- 22.Adkins EK, Nock BL. The effects of the antiestrogen CI-628 on sexual behavior activated by androgen and estrogen in quail. Horm Behav. 1976; 7:417–29. [DOI] [PubMed] [Google Scholar]
- 23.Adkins EK, Boop JJ, Koutnik DL, Morris JB, Pniewski EE. Further evidence that androgen aromatization is essential for the activation of copulation in male quail. Physiol Behav. 1980; 24:441–6. [DOI] [PubMed] [Google Scholar]
- 24.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol. 1993; 44: 521–540. [DOI] [PubMed] [Google Scholar]
- 25.Watson JT, Adkins-Regan E. Testosterone implanted in the preoptic area of male Japanese quail must be aromatized to activate copulation. Horm Behav. 1989; 23:432–47. [DOI] [PubMed] [Google Scholar]
- 26.Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol Behav. 1990; 48:599–609. [DOI] [PubMed] [Google Scholar]
- 27.Balthazart J, Evrard L, Surlemont C. Effects of the nonsteroidal inhibitor R76713 on testosterone-induced sexual behavior in the Japanese quail (Coturnix coturnix japonica). Horm Behav. 1990; 24: 510–531. [DOI] [PubMed] [Google Scholar]
- 28.Balthazart J, Schumacher M, Malacarne G. Interaction of androgens and estrogens in the control of sexual behavior in male Japanese quail. Physiol Behav. 1985; 35:157–166. [DOI] [PubMed] [Google Scholar]
- 29.Watson JT, Abdelnabi M, Wersinger S, Ottinger MA, Adkins-Regan E. Circulating estradiol and the activation of male and female copulatory behavior in Japanese quail (Coturnix japonica). Gen Comp Endocrinol. 1990; 77:229–238. [DOI] [PubMed] [Google Scholar]
- 30.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006; 166: 110–123. [DOI] [PubMed] [Google Scholar]
- 31.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995; 8:267–282. [DOI] [PubMed] [Google Scholar]
- 32.Sachs BD. Photoperiodic control of the cloacal gland of the Japanese quail. Science. 1967; 157:201–203. [DOI] [PubMed] [Google Scholar]
- 33.Delville Y, Hendrick JC, Sulon J, Balthazart J. Testosterone metabolism and testosterone-dependent characteristics in Japanese quail. Physiol Behav. 1984; 33: 817–823. [DOI] [PubMed] [Google Scholar]
- 34.Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: A key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol 1996; 17: 51–125. [DOI] [PubMed] [Google Scholar]
- 35.Balthazart J, Ball GF. Topography in the preoptic region: Differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007; 28: 161–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aste N, Panzica GC, Aimar P, Viglietti-Panzica C, Harada N, Foidart A, Balthazart J. Morphometric studies demonstrate that aromatase-immunoreactive cells are the main target of androgens and estrogens in the quail medial preoptic nucleus. Exp Brain Res. 1994; 101: 241–252. [DOI] [PubMed] [Google Scholar]
- 37.Balthazart J, Schumacher M. Estradiol contributes to the postnatal demasculinization of female Japanese quail (Coturnix coturnix japonica). Horm and Behav. 1984; 18: 287–297. [DOI] [PubMed] [Google Scholar]
- 38.Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behavior in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998; 18:6512–6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liere P, Akwa Y, Weill-Engerer S, Eychenne B, Pianos A, Robel P, Sjovall J, Schumacher M, Baulieu EE. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. J chromatography B, Biomedical sciences and applications. 2000; 739: 301–312. [DOI] [PubMed] [Google Scholar]
- 40.Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjovall J, Baulieu EE. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J Lipid Res. 2004; 45: 2287–2302. [DOI] [PubMed] [Google Scholar]
- 41.Labombarda F, Pianos A, Liere P, Eychenne B, Gonzalez S, Cambourg A, De Nicola AF, Schumacher M, Guennoun R. Injury elicited increase in spinal cord neurosteroid content analyzed by gas chromatography mass spectrometry. Endocrinology. 2006; 147: 1847–1859. [DOI] [PubMed] [Google Scholar]
- 42.Viau V Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002; 14: 506–513. [DOI] [PubMed] [Google Scholar]
- 43.Balthazart J, Schumacher M, Ottinger MA. Sexual differences in the Japanese quail: Behavior, morphology, and intracellular metabolism of testosterone. Gen Comp Endocrinol. 1983; 51: 191–207. [DOI] [PubMed] [Google Scholar]
- 44.Balthazart J, Ottinger MA. 5β-Reductase activity in the brain and cloacal gland of male and female embryos in the Japanese quail (Coturnix coturnix japonica). J Endocrinol. 1984; 102: 77–81. [DOI] [PubMed] [Google Scholar]
- 45.Ottinger MA, Balthazart J. Altered endocrine and behavioral responses with reproductive aging in the male Japanese quail. Horm Behav. 1986; 20: 83–94. [DOI] [PubMed] [Google Scholar]
- 46.Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986; 370: 285–293. [DOI] [PubMed] [Google Scholar]
- 47.Deviche P, Delville Y, Balthazart J. Central and peripheral metabolism of 5 alpha-dihydrotestosterone in the male Japanese quail: biochemical characterization and relationship with reproductive behavior. Brain Res. 1987; 421: 105–116. [DOI] [PubMed] [Google Scholar]
- 48.Balthazart J, Schumacher M, Evrard L. Sex differences and steroid control of testosterone-metabolizing enzyme activity in the quail brain. J Neuroendocrinol. 1990; 2:675–683. [DOI] [PubMed] [Google Scholar]
- 49.Lovejoy DA. Neuroendocrinology: An integrated approach West Sussex, England: John Wiley and Sons Ltd, 2005. [Google Scholar]
- 50.Barabas K, Godo S, Lengyel F, Ernszt D, Pal J, Abraham IM. Rapid non-classical effects of steroids on the membrane receptor dynamics and downstream signaling in neurons. Horm Behav. 2018, 104:183–191. [DOI] [PubMed] [Google Scholar]
- 51.Wade GN, Feder HH. (1,2 3 H)progesterone uptake by guinea pig brain and uterus: differential localization, time-course of uptake and metabolism, and effects of age, sex, estrogen-priming and competing steroids. Brain Res. 1972; 45: 525–543. [DOI] [PubMed] [Google Scholar]
- 52.Wade GN, Feder HH. Uptake of (1,2– 3 H)20 -hydroxypregn-4-en-3-one, (1,2– 3 H)corticosterone, and (6,7– 3 H)estradiol-17 by guinea pig brain and uterus: comparison with uptake of (1,2– 3 H)progesterone. Brain Res. 1972; 45: 545–554. [DOI] [PubMed] [Google Scholar]
- 53.Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sc iUSA. 1992; 89:7650–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlinger BA, Arnold AP. Estrogen synthesis in vivo in the adult zebra finch: Additional evidence that circulating estrogens can originate in brain. Endocrinology. 1993; 133:2610–2616. [DOI] [PubMed] [Google Scholar]
- 55.Dickens MJ, Cornil CA, Balthazart J. Acute stress differentially affects aromatase activity in specific brain nuclei of adult male and female quail. Endocrinology. 2011; 152: 4242–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dickens MJ, Balthazart J, Cornil CA. Brain Aromatase and Circulating Corticosterone are Rapidly Regulated by Combined Acute Stress and Sexual Interaction in a Sex-Specific Manner. J Neuroendocrinol. 2012; 24: 1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone-metabolizing enzymes in the Japanese quail. Brain Res. 1987; 422: 137–148. [DOI] [PubMed] [Google Scholar]
- 58.Schumacher M, Contenti E, Balthazart J. Partial characterization of testosterone-metabolizing enzymes in the quail brain. Brain Res. 1984; 305: 51–59. [DOI] [PubMed] [Google Scholar]
- 59.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine Rev. 2011; 32: 81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N, Lefevre G, Uzan S, Rondeau E, Rozenberg P, Rafestin-Oblin ME. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. Am J Obstetrics Gynecol. 2010; 203: 477 e1–9. [DOI] [PubMed] [Google Scholar]
- 61.Gibbs TT, Russek SJ, Farb DH. Sulfated steroids as endogenous neuromodulators. Pharm Biochem Behavior. 2006; 84: 555–567. [DOI] [PubMed] [Google Scholar]
- 62.Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligands. Prog Neuropsychopharmacol Biol Psychiatry. 1997; 21: 69–102. [DOI] [PubMed] [Google Scholar]
- 63.Balthazart J, Delville Y, Sulon Y, Hendrick JC. Plasma levels of luteinizing hormone and of five steroids in photostimulated, castrated and testosterone-treated male and female Japanese quail (Coturnix coturnix japonica). General Endocrinol (Life SciAdv). 1987; 5:31–36. [Google Scholar]
- 64.Shevchouk OT, Ghorbanpoor S, Smith E, Liere P, Schumacher M, Ball GF, Cornil CA, Balthazart J. Behavioral evidence for sex steroids hypersensitivity in castrated male canaries. Horm Behav. 2018; 103:80–96. [DOI] [PubMed] [Google Scholar]
- 65.Prior NH, Yap KN, Mainwaring MC, Adomat HH, Crino OL, Ma C, Guns ES, Griffith SC, Buchanan KL, Soma KK. Sex steroid profiles in zebra finches: Effects of reproductive state and domestication. Gen Comp Endocrinol. 2017; 244:108–17. [DOI] [PubMed] [Google Scholar]
- 66.Balthazart J, Arnold AP, Adkins-Regan E. Sexual differentiation of brain and behavior in birds In: Pfaff D, Joels M, eds. Hormones, Brain and Behavior 3rd Ed New York: Academic Press; 2017: pp185–224. [Google Scholar]
- 67.Soma KK, Sullivan K, Wingfield J. Combined aromatase inhibitor and antiandrogen treatment decreases territorial aggression in a wild songbird during the nonbreeding season. Gen Comp Endocrinol. 1999; 115: 442–453. [DOI] [PubMed] [Google Scholar]
- 68.Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: Seasonal regulation and relationship to territorial aggression. Gen Comp Endocrinol. 2001; 123: 144–155. [DOI] [PubMed] [Google Scholar]
- 69.Soma KK, Wissman AM, Brenowitz EA, Wingfield JC. Dehydroepiandrosterone (DHEA) increases territorial song and the size of an associated brain region in a male songbird. Horm Behav. 2002; 41: 203–212. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt KL, Pradhan DS, Shah AH, Charlier TD, Chin EH, Soma KK. Neurosteroids, immunosteroids, and the Balkanization of endocrinology. Gen Comp Endocrinol. 2008; 157: 266–274. [DOI] [PubMed] [Google Scholar]
- 71.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008; 11: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Nat Acad Sci USA. 2010; 107: 3852–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012; 107: 1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Remage-Healey L, Jeon SD, Joshi NR. Recent evidence for rapid synthesis and action of oestrogens during auditory processing in a songbird. J Neuroendocrinol. 2013; 25: 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Bournonville MP, de Bournonville C, Ball GF, Balthazart J, Cornil CA. Rapid changes in preoptic estradiol concentration during male sexual behavior. Abst Soc Neurosci, Washington DC. 201767.04/HH10. [Google Scholar]
- 76.Le Quellec A, Dupin S, Genissel P, Saivin S, Marchand B, Houin G. Microdialysis probes calibration: gradient and tissue dependent changes in no net flux and reverse dialysis methods. J Pharmacological Toxicological Methods. 1995; 33: 11–16. [DOI] [PubMed] [Google Scholar]
- 77.Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis. Current protocols in neuroscience. 2009; Chapter 7 Unit7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nilsson ME, Vandenput L, Tivesten A, Norlen AK, Lagerquist MK, Windahl SH, Borjesson AE, Farman HH, Poutanen M, Benrick A, Maliqueo M, Stener-Victorin E, Ryberg H, Ohlsson C. Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology. 2015; 156: 2492–2502. [DOI] [PubMed] [Google Scholar]
- 79.Balthazart J, Stoop R, Foidart A, Granneman JCM, Lambert JGD. Distribution and regulation of estrogen-2-hydroxylase in the quail brain. Brain Res Bull. 1994; 35:339–45. [DOI] [PubMed] [Google Scholar]
- 80.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006; 29: 241–249. [DOI] [PubMed] [Google Scholar]
- 81.Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006; 49: 45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osawa Y, Higashiyama T, Shimizu Y, Yarborough C. Multiple functions of aromatase and the active site structure; Aromatase is the placental estrogen 2-hydroxylase. J Steroid Biochem Mo lBiol. 1993; 44:469–480. [DOI] [PubMed] [Google Scholar]
- 83.Thiery JC, Robel P, Canepa S, Delaleu B, Gayrard V, Picard-Hagen N, Malpaux B. Passage of progesterone into the brain changes with photoperiod in the ewe. Eur J Neurosci. 2003; 18: 895–901. [DOI] [PubMed] [Google Scholar]
- 84.Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004; 24: 5913–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harada N, Yamada K, Foidart A, Balthazart J. Regulation of aromatase cytochrome P-450 (estrogen synthetase) transcripts in the quail brain by testosterone. Mol Brain Res. 1992; 15:19–26. [DOI] [PubMed] [Google Scholar]
- 86.Harada N, Abe-Dohmae S, Loeffen R, Foidart A, Balthazart J. Synergism between androgens and estrogens in the induction of aromatase and its messenger RNA in the brain. Brain Res. 1993; 622: 243–256. [DOI] [PubMed] [Google Scholar]
- 87.Foidart A, De Clerck A, Harada N, Balthazart J. Aromatase-immunoreactive cells in the quail brain: effects of testosterone and sex dimorphism. Physiol Behav. 1994; 55: 453–464. [DOI] [PubMed] [Google Scholar]
- 88.Balthazart J, Tlemçani O, Harada N. Localization of testosterone-sensitive and sexually dimorphic aromatase-immunoreactive cells in the quail preoptic area. J Chem Neuroanat. 1996; 11:147–71. [DOI] [PubMed] [Google Scholar]
- 89.Absil P, Baillien M, Ball GF, Carlo Panzica G, Balthazart J. The control of preoptic aromatase activity by afferent inputs in Japanese quail. Brain Res Rev. 2001; 37: 38–58. [DOI] [PubMed] [Google Scholar]


