Abstract
Bisphenol A (BPA) is a ubiquitous industrial chemical that has been identified as an endocrine disrupting compound (EDC). There is growing concern that early life exposures to EDCs, such as BPA, can adversely affect the male reproductive tract and function. This study was conducted as part of the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA) to further delineate the toxicities associated with continuous exposure to BPA from early gestation, and to comprehensively examine the elicited effects on testes and sperm. NCTR Sprague Dawley rat dams were gavaged from gestational day (GD) 6 until parturition, and their pups were directly gavaged daily from postnatal day (PND) 1 to 90 with BPA (2.5, 25, 250, 2500, 25000, 250000 μg/kg/d) or vehicle control. At PND 90, the testes and sperm were collected for evaluation.
The testes were histologically evaluated for altered germ cell apoptosis, sperm production, and altered spermiation. RNA and DNA isolated from sperm were assessed for elicited changes in global mRNA transcript abundance and altered DNA methylation. Effects of BPA were observed in changes in body, testis and epididymis weights only at the highest administered dose of BPA of 250000 μg/kg/d. Genome-wide transcriptomic and epigenomic analyses failed to detect robust alterations in sperm mRNA and DNA methylation levels. These data indicate that prolonged exposure starting in utero to BPA over a wide range of levels has little, if any, impact on the testes and sperm molecular profiles of 90 day old rats as assessed by the histopathologic, morphometric, and molecular endpoints evaluated.
Introduction
Bisphenol A (BPA) is a high production volume chemical widely used in manufacturing polycarbonate plastics and epoxy resins used in nearly every industry. Its widespread use in products ranging from food packaging materials to thermal receipt papers to dental sealants has resulted in trace levels in >90% of the United States population (Calafat et al., 2005; Vandenberg et al., 2010). The Food and Agriculture Organization of the United Nations and World Health Organization estimated that the average adult human exposure to BPA is <0.01 – 0.4 µg/kg/day (World Health Organization, 2011).
BPA is a recognized endocrine disrupting chemical (EDC). The male reproductive tract undergoes a complex sequence of developmental morphological and cellular maturation events, many of which are susceptible to hormonal disruption. In the rat, testosterone-producing fetal Leydig cells are replaced postnatally during puberty by a proliferating pool of adult Leydig cells that are needed to support spermatogenesis (Ge et al., 2008). Sertoli cells, the somatic cells that support germ cells within the seminiferous epithelium, continue to proliferate into the third postnatal week in the rat, at which time they mature and support the development of a blood-testis barrier (Franca et al., 2016) and germ cells. Soon after birth in male rats, germ cells begin to proliferate and mature, with the first wave of spermatogenesis filling the seminiferous tubules with spermatogonia, spermatocytes, and spermatids by 8 weeks of age.
Many of these developmental events can be altered by exposures to estrogenic and anti-androgenic chemicals. A 90-day feeding and one-generation reproduction study of exposure to 17 beta-estradiol showed degeneration of the seminiferous epithelium, atrophy of the testis and accessory sex glands in both the parents and progeny at high levels of exposure, with delayed sexual maturation of the progeny at lower levels of exposure (Biegel et al., 1998). Prenatal exposure to the androgen receptor antagonist flutamide resulted in a persistent reduction in anogenital distance (AGD) and persistent areola/nipple retention, sexually-dimorphic androgen-mediated endpoints, along with increased cryptorchidism, hypospadias, and decreased weights of androgen-dependent tissues, including testes and epididymides (McIntyre et al., 2001). Detailed perinatal studies in the rat have demonstrated persistent effects of flutamide exposure on reproductive hormones (Miyata et al., 2002) and a monotonic dose-response (Fussell et al., 2015)
An expert panel convened in 2008 by the National Toxicology Program (NTP) Center for the Evaluation of Risks to Human Reproduction (CERHR) examined BPA research related to human reproduction and development. Based largely on the panel’s assessment (Chapin et al., 2008), the NTP reported negligible concern for reproductive effects in non-occupationally exposed adults and minimal concern for occupationally exposed workers, but identified some concern for effects on the brain, behavior, and prostate gland in fetuses, infants, and children at current levels of human BPA exposure (National Toxicology Program, 2008). Mouse and rat multi-generational studies gestationally and lactationally exposed to BPA did not cause changes in reproductive endpoints at environmentally relevant levels; changes in testes and epididymis weights were observed only at systemically toxic levels (Tyl et al., 2002; Tyl et al., 2008). However, it is reported that there is poor lactational transfer of BPA from the exposed dams to their pups, which limited the perinatal exposure in these studies (Doerge et al., 2010b). The CLARITY-BPA study addresses this issue by directly dosing the pups from the day after birth to ensure that perinatal exposure to BPA is maintained.
Since the release of the CERHR assessment (Chapin et al., 2008), male reproductive effects of BPA have been reported in animal studies suggesting that BPA induced Leydig and germ cell apoptosis in mouse testes (Li et al., 2009), and decreased sperm counts and motility in rats (Salian et al., 2009). Furthermore, BPA was reported to partially inhibit meiotic germ cell progression in rat seminiferous tubule cultures (Ali et al., 2014). Two recent well-powered studies in rats across a wide range of doses showed different effects on sperm counts with BPA exposure, either no effect ((Delclos et al., 2014) or a decrease only at a lowest dose tested, suggesting of a non-monotonic dose-response (Hass et al., 2016). In humans, epidemiologic studies identified associations between higher urinary BPA levels with increased serum testosterone levels (Galloway et al., 2010) and decreased sperm quality (Li et al., 2011). The literature on associations between BPA exposure and male reproductive effects continues to expand (Vandenberg and Prins, 2016), including a recent report of human fetal testis in vitro and xenotransplant assays showing BPA-induced germ cell loss (Eladak et al., 2018).
Exposure to BPA and other environmental contaminants has been associated with changes in epigenetic status in humans and animals (Dolinoy et al., 2007; Rusiecki et al., 2008; Bromer et al., 2010; Doshi et al., 2011; Manikkam et al., 2013), raising concern about their effects on the reproductive system, and transmission to subsequent progeny. For example, in utero exposure to 5 mg/kg BPA in mice on days 9–16 of pregnancy resulted in epigenetic reprogramming of uterine estrogen response (Bromer et al., 2010). Parallel effects in mouse testes have been reported wherein neonatal exposure to BPA resulted in hypermethylated promoters of estrogen receptors α and β (Doshi et al., 2011). In humans, women with elevated serum BPA levels were associated with lower methylation at promoter CpG islands of the testis specific protease 50 gene (TSP50) (Hanna et al., 2012). These findings suggest that BPA has the potential to alter the epigenetic status in the reproductive systems.
This study was performed as part of the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA) (Birnbaum et al., 2012; Schug et al., 2013). The design and intent of the consortium (Heindel et al., 2015), and findings of a preliminary dose-range finding study have been published (Delclos et al., 2014). The experiments performed here supplemented the standard toxicological evaluations conducted in the core study (to be reported elsewhere) by implementing additional histopathological and morphometric examinations of the testes, and by assessing sperm mRNA transcripts and DNA methylation.
Materials and Methods
This study was part of the CLARITY-BPA consortium program (Birnbaum et al., 2012; Schug et al., 2013) and details of the CLARITY-BPA study design are provided in brief. The comprehensive details of the animal husbandry, treatment and dosing procedures have been previously published (Heindel et al., 2015). All animal use and procedures were approved in advance by the National Center for Toxicological Research (NCTR) and Brown University Institutional Animal Care and Use Committees, and were conducted in an Association for Assessment and Accreditation of Laboratory Animal Care (AALAC)-accredited facility. Experiments were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” by the National Research Council.
Animal Husbandry
Animal rooms were maintained at 23 ± 3ºC with a relative humidity of 50 ± 20% and a 12:12h light/dark cycle. A low phytoestrogen diet (5K96-verified casein diet 10 IF, round pellets, γ-irradiated, Test Diets, Purina Mills, Richmond, IN) and Millipore-filtered water in glass water bottles were available ad libitum. Diet lots and other study materials were monitored for BPA by liquid chromatography/mass spectrometry as described previously (Delclos et al., 2014). None of the assayed lots of diet contained BPA above the protocol-specified limit of 5 ppb. No study material, including cage leachates, drinking water, and bedding extracts, were found to have BPA detectable above the analytical method blank (Heindel et al., 2015).
Dose Preparation and Administration
The in-life portion of the CLARITY-BPA consortium study was performed at the FDA’s National Center for Toxicological Research (NCTR) and included 6 dose groups (n=10 per group; 1 pup per litter); vehicle and 2.5, 25, 250, 2500 and 25000 μg/kg/d BPA (Heindel et al., 2015). Additionally, a 250000 μg/kg/d BPA and vehicle group (n=20 per group; 2 pups per litter) were included in the current study to identify changes in sperm mRNA transcript and DNA methylation levels due to exaggeration of the potential endocrine disrupting effects of BPA (herein designated as “high-dose study”). The high-dose study started two-weeks prior to the CLARITY-BPA consortium study. For both studies, approximately two weeks prior to mating, female NCTR-Sprague Dawley breeders were randomized to exposure groups stratified by body weight to give approximately equivalent mean body weights in each group. Neither sibling nor mating of adult offspring of dams arising from the same litter were permitted. In the CLARITY-BPA consortium study, rats were mated in five loads (cohorts) spaced 4 weeks apart; animals from the first two cohorts were used in the current study. Mating was conducted as previously described (Delclos et al., 2014), except that solid-bottomed polysulfone cages with hardwood chip bedding were used in place of wire bottom cages. Dosing via oral gavage began with dams on gestational day (GD) 6 up to parturition, and continued with pups on postnatal day (PND) 1 to PND 90 (dose volume determined by that day’s body weight). Two pups from the 250000 μg/kg/d BPA dose group were omitted from the study due to gavage injuries.
BPA (CAS no. 80-05-7, TCI America, Portland, OR; catalog no. B0494, Lot no. 111909/AOHOK [air-milled], >99% purity) was prepared in the vehicle, 0.3% aqueous carboxymethyl cellulose (Sigma-Aldrich, St. Louis, MO; catalog no. C5013, Lot no. 041M0105V), and administered by gavage daily at a volume of 5 mL/kg body weight using a modified Hamilton Microlab ML511C programmable 115V pump (Hamilton Co., Reno, NV). Prior to the start of the study, homogeneity and stability analyses of the dose preparations were conducted. Doses were administered within the stability window and the preparations were analyzed at the start and end of dosing, and periodically over the course of the study. This ensured that the preparations were ±10% of the target dose and the study animals were dosed with the correct dosing formulation.
Tissue Collection and Histological Examination
Animals were asphyxiated by carbon dioxide and exsanguinated before being necropsied ±10 days of PND90, and the body and reproductive organ weights were recorded. The average age of each litter is provided in Table 1. Left testes were fixed in modified Davidson’s and transferred to 10% neutral-buffered formalin after 24 hours for histological examination, and a portion of each animal’s right testis was detunicated and snap frozen in liquid nitrogen for the automated determination of homogenization resistant spermatid head (HRSH) counts (Pacheco et al., 2012). The epididymides were weighed and sperm from the caudal regions of the epididymides were used to isolate RNA and DNA.
Table 1:
Body, testis and epididymis weightsa at postnatal day 90 following continuous BPA exposure from early gestation
| Dose (µg/kg/d) |
Average Age (days) | Body (g) |
Testisb (g) |
Epididymisb (mg) |
||||
|---|---|---|---|---|---|---|---|---|
| CLARITY-BPA doses (n=10)c | vehicle | 84 | 432.3 ± 39.8 | 1.662 ± 0.101 | 502.2 ± 49.1 | |||
| 2.5 | 84 | 404.4 ± 35.0 | 1.635 ± 0.223 | 461.8 ± 25.1 | ||||
| 25 | 85 | 433.6 ± 25.7 | 1.729 ± 0.117 | 514.8 ± 38.7 | ||||
| 250 | 86 | 409.7 ± 57.7 | 1.649 ± 0.143 | 499.2 ± 63.9 | ||||
| 2,500 | 83 | 429.0 ± 34.1 | 1.611 ± 0.101 | 474.2 ± 46.1 | ||||
| 25,000 | 84 | 415.0 ± 42.6 | 1.688 ± 0.135 | 494.6 ± 45.1 | ||||
| High-dosed | Vehicle (n=10) |
97 | 474.3 ± 33.2 | 1.794 ± 0.092 | 579.9 ± 44.7 | |||
| 250,000 (n=9) |
96 | 439.4 ± 41.8* | 1.554 ± 0.115 **** | 528.8 ± 32.9** | ||||
weights are reported as the mean ± SD; n represents number of litters analyzed
organ weights are the average of the left and right testis or epididymis
data were analyzed using one-way ANOVA relative to the CLARITY-BPA vehicle group followed by Dunnett’s multiple correction test
data were analyzed using a one-way unpaired t-test relative to the high-dose study vehicle group
p < 0.05
p < 0.0
p < 0.0001
Two cross sections from the center of the fixed testes were embedded in glycol methacrylate (Technovit 7100; Heraeus Kulzer GmBH, Wehrheim, Germany) for histological examination of stage-specific retained spermatid heads (RSH) or embedded in paraffin for detection of apoptosis by TUNEL staining. The Aperio ScanScope (Aperio Technologies, Vista, CA) was used to create digital images of the microscope slides, and all histological endpoints were analyzed using ImageScope software.
Enumeration of RSH was performed using two cross sections (3 μm) of testes from six randomly selected rats per treatment group stained with periodic acid-Schiff’s reagent followed by hematoxylin counter stain (PASH). Each section was evaluated for seminiferous tubules in spermatogenesis stages IX-XI, each of which was required to be nearly round (ratio of major axis to minor axis length of less than 1.5:1) (Bryant et al., 2008). RSH were identified as condensed spermatid heads in the basal epithelium in stages IX-XI, and the counts were averaged together on an individual rat basis. The counts were log-transformed to ensure normally distributed errors prior to statistical analysis.
For the evaluation of apoptosis, paraffin sections (5 μm) of testes from the subset of rats utilized above were stained using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (EMD Millipore, Burlington, MA) following the manufacturer’s protocol and were counterstained with methyl green. Apoptotic cells were counted in a minimum of 50 seminiferous tubules, systematically selected using a grid, having a ration of major axis to minor axis length of less than 1.5:1. The percentage of seminiferous tubules containing TUNEL positive cells was assessed.
Sperm Isolation and RNA Extraction
Sperm from the cauda epididymides were isolated as previously described (Pacheco et al., 2012; Dere et al., 2016). Briefly, the cauda epididymides were punctured repeatedly with 26 and 30 gauge needles, placed into micro-centrifuge tubes containing phosphate buffered saline (PBS, Life Technologies, Grand Island, NY), and incubated in a water bath at 37 °C for 10 minutes to allow sperm release. Epididymal tissue and debris were pelleted by centrifugation for 3 minutes at 300 ☓ g, and the supernatant was removed and centrifuged for 5 minutes at 2000 ☓ g to pellet the sperm. Somatic cell contaminants were removed by resuspending the sperm in a somatic cell lysis buffer (0.15 M ammonium chloride, 10 mM potassium bicarbonate, 0.1 mM EDTA) (Thermo Fischer Scientific Inc., Pittsburgh, PA) and incubating for 30 seconds prior to centrifugation at 16,100 ☓ g for 1 minute, leaving the sperm intact. The pelleted sperm were subsequently washed with PBS and centrifuged again at 16,100 ☓ g for 1 minute and RNA was extracted using the mirVana miRNA Isolation Kit (Applied Biosystems, Austin, TX). The isolated RNA was further purified and concentrated using Qiagen’s RNase-free DNase and RNeasy MinElute Cleanup kits (Qiagen Sciences, Germantown, MD), following the manufacturer’s protocol. RNA yields and concentrations were measured on a NanoDrop 1000 spectrophotometer (Thermo Scientific).
Sperm mRNA Transcript Analysis
RNA samples from all the BPA treatment groups (n=10 for the CLARITY-BPA consortium doses, and n=20 and n=18 from the vehicle and 250000 μg BPA/kg/d from the high-dose study, respectively) were used to perform whole-genome microarray profiling of sperm mRNA using Affymetrix Rat Gene 1.0 ST GeneChips (Affymetrix, Santa Clara, CA). Microarray data were processed in the R software environment (R Core Team) and Bioconductor (Gentleman et al., 2004) using the RMA algorithm in the oligo package (Carvalho and Irizarry, 2010) for probe-level summarization and analyzed using the limma package (Ritchie et al., 2015). Gene annotation information was joined to the transcriptomic data using the ragene10sttranscriptcluster.db package. Significantly altered transcripts were defined as having a q-value < 0.05 with a |fold change| > 1.5. This analysis used the litter as the unit of replication for the consortium dose groups and the individual pups as the unit for the high-dose study.
A Monte Carlo method implemented in R that was previously used to analyze the robustness of –omic signatures (Dere et al., 2016) was used to repeatedly identify significantly altered sperm mRNA transcripts from five randomly selected BPA and vehicle treated samples from the CLARITY-BPA consortium dose groups using 100 iterations, and significantly altered mRNA transcripts were identified for each iteration. The analysis of the high-dose study dose groups was conducted with 1000 iterations, where five randomly selected litters from the 250000 μg BPA/kg/d and concurrent vehicle control groups were chosen, and a pup from each of those selected litters was randomly picked for the analysis. This selection process ensured that pups from the same litter were not chosen, thereby maintaining the litter as the unit of replication.
RRBS Library Construction
Reduced representation bisulfite sequencing (RRBS) libraries were constructed using previously published protocols from sperm samples with sufficient amounts of DNA (Gu et al., 2011; Boyle et al., 2012). The number of samples for each dose group with sufficient amounts of DNA library construction are provided in Figure 2. A modified guanidine thiocyanate DNA extraction method was utilized to extract highly compacted DNA from sperm (Griffin, 2013), and the quality and quantity were assessed using a NanoDrop 1000 (Thermo Scientific). Briefly, for each sample, 500 ng of DNA were digested with MspI (New England Biolabs, Ipswich, MA) and then purified using QIAquick Nucleotide Removal Kit (Qiagen, Germantown, MD). Subsequently, end-repair A-tailing was performed using Klenow fragment (3′−5′ exonuclease; New England Biolabs), and TruSeq methylated indexed adaptors (Illumina, San Diego, CA) were ligated with T4 DNA ligase (New England Biolabs). Fragments were size selected using Agencourt AMPure XP beads (Beckman Coulter, Indianapolis, IN) and then followed with two rounds of bisulfite conversion to ensure complete conversion of the methylated cytosines using the EpiTect kit (Qiagen). Following bisulfite treatment, the DNA was purified as directed and amplified using Pfu Turbo Cx Hotstart DNA polymerase (Agilent Technologies, Santa Clara, CA). Library quantification was performed using the Quant-iT high sensitivity DNA assay kit (Invitrogen, Grand Island, NY) and the Bioanalyzer DNA 1000 kit (Agilent Technologies). Single-end 50 bp sequence runs were performed for each constructed multiplexed library on an Illumina HiSeq 2500 (Illumina). The data from the sequence runs were processed using trim_galore (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/), and then aligned to the rat reference genome RGSC Rnor_6.0 using Bismark (Krueger and Andrews, 2011).
Figure 2:
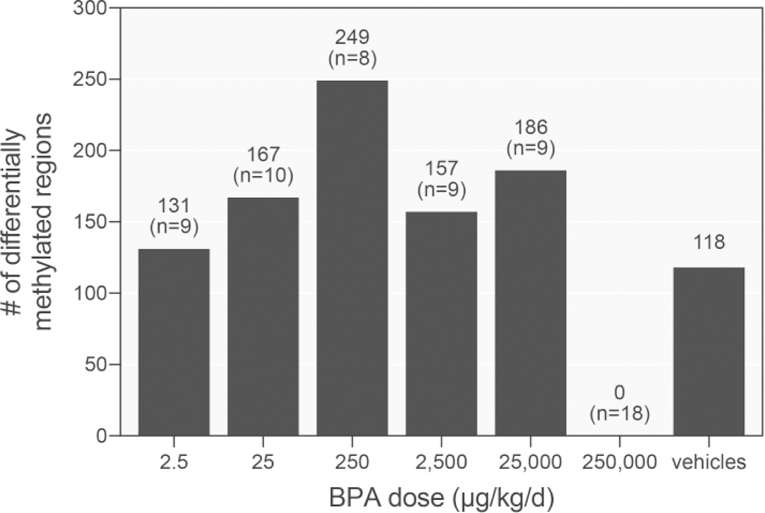
Quantification of BPA-elicited DMRs in sperm across doses. Sperm DNA methylation in the consortium and 250000 μg/kg/d high-dose groups were compared against their corresponding vehicle controls. Litters were used as the unit of replication for the analysis of the consortium dose groups, and pups as unit of replication for the high-dose study (n=8 and n=19, respectively; n represents the number of pups/litters analyzed). Further comparison between the two separate vehicle control groups was used to identify the background frequency of DMRs due to biological variability.
RRBS Methylation Analysis
Differentially methylated CpGs and 100 bp tiles were identified using the methylKit package (Akalin et al., 2012) for R (R Core Team) as previously described (Dere et al., 2016). Logistic regression analysis was used to compare the methylation means of the BPA and vehicle treated groups to calculate methylation levels and p-values, and the p-values were corrected to genomic-wide false discovery rate (FDR)-based q-values by using the SLIM method (Wang et al., 2011). The analysis was performed both on an individual CpG and 100 bp stepwise tiling window level. The methylation level of a tile represents the average of all the individual CpGs within the tile, and averaged across replicates. Differentially methylated CpGs or 100 bp tiles elicited by BPA treatment were defined as having >10% methylation difference relative to vehicle controls and a q-value < 0.05. This analysis used the litter as the unit of replication for the consortium dose groups and the individual pups as the unit for the high-dose study.
The Monte Carlo method was applied to repeatedly identify differentially methylated CpGs and tiles from randomly selected BPA and vehicle treated samples from both the CLARITY-BPA consortium dose groups, and the 250000 μg/kg/d high-dose group. The random selection process was conducted as described in the sperm mRNA transcript analysis methodology to ensure that the litter was treated as the unit of replication. Additionally, Monte Carlo analysis was performed with the two vehicle groups, using the litter as the unit of replication. For this analysis, five randomly selected samples per group were selected for 100 or 1000 iterations, and differentially methylated regions were identified for each iteration.
Data Decoding
All the blinded raw data for the CLARITY-BPA consortium dose groups were submitted to the NTP Chemical Effects in Biological Systems (CEBS) database. Data were then independently verified to account for all expected data sets and data points, and “locked” such that data could not be altered. The researchers at Brown University were then provided with the exposure code for data analysis. Unblinded data for the high-dose study dose groups were also uploaded into the CEBS database.
Statistical Analysis
Body and organ weights, and quantitative RSH, HRSH and TUNEL data from the CLARITY-BPA consortium dose groups, and the sperm mRNA transcript data from all dose groups were analyzed using one-way ANOVA followed by the Dunnett’s multiple correction test to identify significant changes relative to vehicle control. For the high-dose study groups, there were two male pups per dam and the litter was used as the unit of replication for comparing body and organ weights, and quantitative RSH, HRSH and TUNEL data. These data were analyzed using a one-tailed unpaired t-test relative to the high-dose group’s concurrent vehicle control. The statistical analyses of the data, with the exception of the microarray and RRBS data, were performed using Prism 6 by GraphPad (GraphPad Software, La Jolla, CA). Dose-response modeling of the sperm DNA methylation data was performed using the drc package for R (Ritz et al., 2015).
Results
Body, Testis, and Epididymis Weights
NCTR-Sprague Dawley rats were orally gavaged with BPA from GD 6 to PND 90 over a wide dose range that included concentrations that approached the estimated human exposure levels (~0.2 μg/kg/d BPA) and were below the rat no-observed-adverse-effect-level (NOAEL) of 5 mg/kg/d BPA (Tyl et al., 2002; Tyl et al., 2008; World Health Organization, 2011). Terminal body, testis, and epididymis weights were recorded immediately following euthanasia (Table 1). None of the CLARITY-BPA consortium doses (vehicle, 2.5, 25, 250, 2500 and 25000 μg/kg/d) resulted in any significant changes in either body, testis or epididymis weights, in agreement with a previous study conducted by Delclos et al. (2014). Statistically significant decreases in body, testis, and epididymis weights were observed following treatment with 250000 μg/kg/d BPA in the high-dose group (Table 1), representing a 7.4%, 13.4% and 8.8% decrease relative to vehicle control, respectively. It should be noted that necropsies were performed ±10 days of PND90 where the average age of CLARITY-BPA consortium dose groups and high-dose study groups were 84 and 97 days, respectively, resulting in an age difference between the two vehicle cohorts of 13 days (Table 1).
Quantitative Histological and Morphometric Assessment
The effect of BPA on spermiation, sperm production and germ cell apoptosis was evaluated in the testes of exposed rats. Quantification of retained spermatid heads (RSH) in the basal compartment of the seminiferous tubules provided an assessment of disrupted spermiation, while sperm production was evaluated by quantifying homogenization resistant spermatid heads (HRSH) in testis homogenates. TUNEL-positive cell nuclei provided a measure of apoptotic germ cells. Quantitative measurement of these endpoints did not identify any statistically significant effects due to BPA exposure at any dose (Figure 1). The individual pup variability is consistent with the litter variability for each of these endpoints (data not shown).
Figure 1:

Quantification of retained spermatid heads (RSH), homogenization resistant spermatid heads (HRSH) and TUNEL-positive germ cell nuclei at postnatal day 90 following continuous exposure to BPA from early gestation. Values indicated by the boxes are expressed as the mean ± SEM and data points for each litter are represented by the circles. Data for the consortium were analyzed using one-way ANOVA relative to the CLARITY-BPA vehicle group followed by Dunnett’s multiple correction test and data from the high-dose group were analyzed using a one-tailed unpaired t-test relative to the concurrent vehicle group.
Sperm mRNA Analysis
Global sperm transcriptomic analysis was performed to identify BPA treatment effects on sperm mRNA content levels across all doses. Whole genome microarray analysis with a multiple correction test identified 1,551 and 29 significantly altered probes following exposures to 25, 250 and 2500 μg/kg/d BPA, respectively (|fold change| ≥ 1.5 and q-value < 0.05). Neither the 2.5, 25000 nor the 250000 μg/kg/d BPA dose groups resulted in a significant perturbation in sperm mRNA transcript levels. Hierarchical clustering of the normalized probe signal intensities demonstrated no discernable difference in the BPA treatment groups relative to vehicle controls (Supplemental Figure 1). A Monte Carlo-based method approach was used to iteratively analyze the dose with the greatest number of significantly altered probes, 250 μg/kg/d BPA, as previously described (Dere et al., 2016). Five samples were randomly selected from the 250 μg/kg/d dose and concurrent vehicle control groups and 100 iterations were performed and analyzed to generate a distribution profile of dysregulated probes. Interestingly, no probes were dysregulated (|fold change| ≥ 1.5 and q-value < 0.05) in any of the 100 iterations, demonstrating that BPA exposure did not robustly alter sperm mRNA levels.
The initial analysis of the high-dose study group (250000 μg/kg/d BPA) microarray data used the individual pups as the unit of replication to provide greater statistical power in identifying dysregulated probes. A complementary analysis using a Monte Carlo-based approach and the litter as the unit of replication was used to test the robustness of the response by generating the distribution profile for the number of significant probes. The Monte Carlo analysis was conducted with 1000 iterations and a randomly selected pup from each of five randomly selected high-dose and corresponding vehicle control group litters. The analysis failed to identify any dysregulated probes (q-value < 0.05 and |fold change| ≥ 1.5) in any of the iterations. This finding raised questions as to whether the multiple correction method that was used influenced the calculated q-values. To evaluate this concern, the Monte Carlo analysis was performed based on the unadjusted p-values < 0.05 and |fold change| ≥ 1.5, and identified 44 different iterations with only a single dysregulated probe. Furthermore, in each of these 44 iterations, a different probe was identified, demonstrating the lack of a robust transcriptomic response. These collective findings from the sperm transcriptome analyses indicate that a continuous exposure to BPA from gestation until PND 90 had minimal impact on sperm mRNA content.
Sperm DNA Methylation Analysis
Global changes in sperm CpG methylation were assessed using RRBS across 100 bp tiling windows. Differentially methylated regions (DMRs) were defined as 100 bp windows with >10% methylation difference in the BPA dose groups relative to the concurrent vehicle control and a q-value < 0.05. From the CLARITY-BPA consortium doses, the greatest number of DMRs, 249, were identified at 250 μg/kg/d and the fewest, 131, at the lowest dose of 2.5 μg/kg/d (Figure 2). Additionally, since there were two vehicle control cohorts, their methylation levels were compared and 118 DMRs were identified between the two vehicle control groups. These DMRs are a representation of the biological variability in the methylation levels in the vehicle control samples and provide an estimate of the background frequency of DMRs. Across the consortium dose levels, 7 DMRs were common to all doses. However, each of these DMRs was identified in the vehicle cohort comparison as well, suggesting that these DMRs cannot be attributable to BPA exposure. Analysis of the high-dose BPA group compared to its concurrent vehicle control did not identify any DMRs. An important factor is that the high-dose BPA group comparison was by design able to identify DMRs with greater certainty and power because of the larger number of biological replicates and by using the pup as the unit of replication in the comparison (n=18 for BPA exposure; n=20 for vehicle control).
The dose-responsiveness of the DNA methylation signals was investigated. Although no BPA-induced DMRs were common across all the consortium dose levels, 68 DMRs were shared by the 25 and 250 μg/kg/d doses and, of these 68 DMRs, 32 had a full complement of methylation data across all the consortium dose groups. The dose-dependent nature of these 32 DMRs was examined by fitting the data to 4 and 5 parameter Brain-Cousens and log-logistic models. The data for two of the 32 DMRs investigated were modeled appropriately with the 4 and 5 parameter Brain-Cousens model for non-monotonic dose-responses (chr4: 157,585,001–157,585,100 and chr12: 22,137,001–22,137,100), while the data for the remaining DMRs were either forced to fit a model poorly or were not able to fit a specific model (and thus no fitted line is shown) (Supplemental Figures 2–5). The DMR located at chr12: 22,137,001–22,137,100 is located within the genic region of the cytoskeletal scaffolding protein-encoding leucine rich repeats and calponin homology domain containing 4 (Lrch4) gene, while the other DMR is located within intergenic DNA.
The robustness of the detected DMRs across doses was assessed by applying a Monte Carlo approach. Briefly, five vehicle and five BPA treated samples were randomly selected and iteratively analyzed to generate a frequency distribution of the number of DMRs (Figure 3 and Table 2). This approach qualitatively demonstrated that the distributions were highly skewed to the left, indicating that few DMRs were identified repeatedly with high frequency (Figure 3). A quantitative summary (Table 2) demonstrated the lack of a robust DNA methylation difference at any dose level. Additionally, the background incidence of DNA methylation differences, as determined by the analysis of the two vehicle cohorts, demonstrated that the incidence of BPA-induced DMRs was similar to that of the vehicle controls (Table 2).
Figure 3:
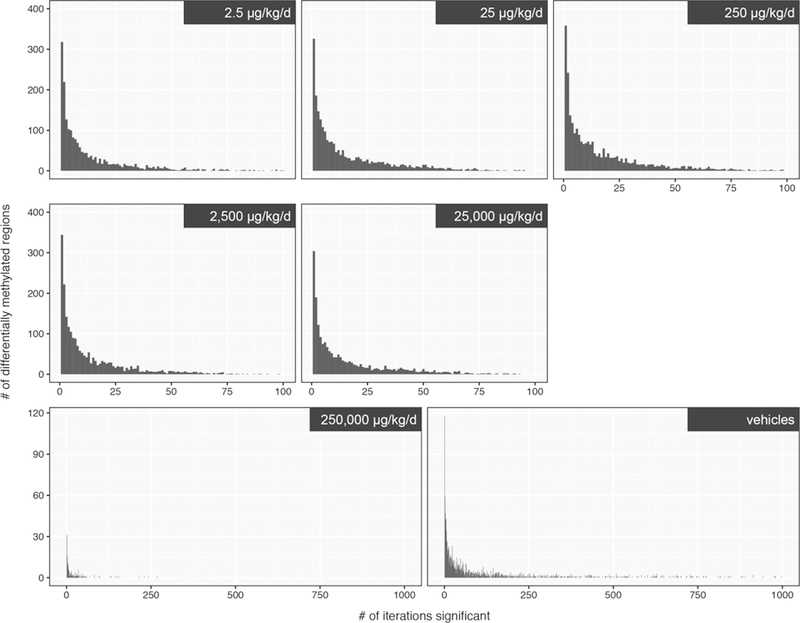
Monte Carlo analysis of sperm DNA methylation following continuous exposure to BPA from early gestation through PND 90. Randomly selected BPA-treated and vehicle controls (n=5 litters per group) were iteratively analyzed to identify aberrant DNA methylation; 100 iterations were conducted for the consortium dose groups, and 1000 iterations were conducted for the high-dose group (>10% methylation difference relative to concurrent vehicle control and q-value < 0.05).
Table 2:
Summary of the Monte Carlo analysis providing an assessment of the robustness of the BPA-induced changesa in sperm DNA methylation. Values in the table represent the total number of unique DMRs that occur at a given frequency relative to number of iterative analyses (i.e. # of unique DMRs / # of iterations).
| Frequency of occurrenceurrence | Dose (µg/kg/d) |
||||||
|---|---|---|---|---|---|---|---|
| 2.5b | 25b | 250b | 2500b | 25,000b | 250,000b | Vehiclesc | |
| ≥ 0 | 1,925 | 2,311 | 2,574 | 2,133 | 1,939 | 173 | 1,532 |
| > 0.75 | 23 | 22 | 44 | 13 | 24 | 0 | 25 |
| > 0.8 | 17 | 14 | 26 | 8 | 15 | 0 | 20 |
| > 0.9 | 7 | 7 | 14 | 3 | 4 | 0 | 9 |
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
>10% methylation difference relative to concurrent vehicle control and q-value < 0.05; litters used as the unit of replication
Monte Carlo analysis performed with 100 iterations
Monte Carlo analysis performed with 1000 iterations
Discussion
There is a growing concern that exposure to EDCs, such as BPA, can negatively impact the male reproductive system (Manfo et al., 2014; Minguez-Alarcon et al., 2016; Vandenberg and Prins, 2016) and alter the epigenetic status of sperm (Chang et al., 2006; Manikkam et al., 2013). The primary goal of this study was to provide enhanced testis histopathology and morphometry, as well as sperm molecular assessment, of BPA-induced male reproductive effects as part of the CLARITY-BPA consortium study (Birnbaum et al., 2012; Heindel et al., 2015). Multiple studies have reported on the non-monotonic dose-responsive nature of BPA and concluded that low-level exposures elicit the greatest response, and are of greatest concern to humans (Palanza et al., 2002; Vandenberg et al., 2010; Angle et al., 2013; Kim et al., 2014; Faulk et al., 2015; Hass et al., 2016; Zhou et al., 2017). To address these concerns, the CLARITY-BPA consortium study investigated a wide BPA dose range from 2.5 to 25000 μg/kg/d in 10-fold increments. Additionally, a high-dose BPA group of 250000 μg/kg/d was included to identify potential endocrine disrupting effects of BPA on testes histological and sperm epigenetic endpoints. BPA is known to elicit its effects in a species- and strain-dependent manner (Richter et al., 2007; Beronius et al., 2013; Vrooman et al., 2015). The CLARITY-BPA consortium study used the NCTR Sprague-Dawley rat, a strain previously used to assess the effects of ethinyl estradiol, genistein, and BPA (Delclos et al., 2009; Latendresse et al., 2009; Delclos et al., 2014) and for which the pharmacokinetics of BPA across life stages has been well characterized (Doerge et al., 2010a; Doerge et al., 2010b). . Our analysis of sensitive testicular endpoints failed to identify any low-dose responses and provided evidence that, under our experimental conditions, the testis in the NCTR Sprague-Dawley rat is not a sensitive target of BPA-induced toxicities.
Body weight loss can be used as a surrogate indicator of overt toxicity. In this study, only the 250000 μg/kg/d dose of BPA resulted in a 7.4% decrease in body weights at PND 90, suggestive of mild growth retardation and potential for impaired spermatogenesis. Similarly, decreases in the absolute testis weights are a strong indicator of injury to the male reproductive system. Although both testis and epididymal weights were decreasedby 13.4% and 8.8%, respectively, in the highest dose group (250000 μg/kg/d) at PND90, there were no disruption in testis function (i.e. spermiation, sperm production and germ cell apoptosis). These results are in contrast to studies in mice exposed gestationally to BPA through drinking water (10 µg/mL), where a marked increase in germ cell apoptosis was reported (Krementsov et al., 2013). Collectively, our findings demonstrate that continuous exposure to BPA from early gestation over a wide range of levels did not impact testis function under our experimental conditions. Furthermore, no effects on the male reproductive system were observed during the initial assessment of the effects of BPA on the male reproductive system in a study that used the same animal model and dosing regimen as the current study (Delclos et al., 2014), although another well-powered study of pre- and postnatal BPA exposure in the rat reported decreased sperm counts at the lowest dose tested (Hass et al., 2016). A recent study in male Wistar rats exposed to 50 μg BPA/kg/d via diet for 35 weeks failed to observe overt BPA-elicited toxicities or increases in germ cell apoptosis (Chen et al., 2017).
Epigenetic factors, such as DNA methylation, and sperm RNAs have been explored as potentially sensitive indicators of toxicity in different tissues (Dong et al., 2008; Boellmann et al., 2010; Chapman et al., 2014; Chappell et al., 2014; Conti et al., 2014; Koczor et al., 2015; Ozden et al., 2015; Chen et al., 2016; Dere et al., 2016). Transmission of epigenetic marks in the germ line has garnered attention recently due to increasing evidence of transgenerational inheritance and increased risk factors of developing diseases associated with such alterations (Guerrero-Bosagna et al., 2010; Manikkam et al., 2013; Skinner et al., 2013; Tracey et al., 2013). Our initial analysis of mRNA abundance and DNA methylation in sperm following continuous BPA exposure from early gestation suggested a possible non-monotonic dose-response, with the middle dose of 250 μg BPA/kg/d eliciting the greatest number of dysregulated genes and DMRs. Taken at face value, these results agree with previous studies reporting non-monotonic dose-relationships with BPA. However, using additional modeling approaches, we concluded that these responses lacked robustness and reproducibility, and should not be considered BPA-elicited responses. This conclusion is based on our analysis of: (1) iterative (Monte Carlo) analyses with randomly selected subsets of samples, which failed to consistently identify dysregulated transcripts or a common set of DMRs, (2) a relatively high background frequency of DMRs detected in an analysis comparing the two groups of vehicle controls, and (3) dose-response modeling of BPA-elicited DMRs, which poorly fit the DNA methylation data of both linear and non-linear dose-response models. The collective findings from these independent analyses strongly suggest that BPA did not alter sperm DNA methylation. A limitation in bisulfite sequencing used in this study to assess DNA methylation status is that it cannot distinguish between 5-methylcytosine (5-mC) and 5-hydroxymethylcytosine (5-hMC). This technical limitation is important and highlighted by a recent study which reported a significant increase in sperm DNA hydroxymethylation by BPA in occupationally-exposed men (Zheng et al., 2017). Histone modification is another form of epigenetic regulation that has been reported to be sensitive to BPA exposure; however, BPA-mediated histone modifications were not assessed in this current study. A recent study with adult Wistar male rats that were exposed to dietary 50 μg/kg BPA daily for 35 weeks reported a marked decrease in H3K9, H3K27 and H4K12 acetylation in the testes (Chen et al., 2017), raising the possibility that alterations to the histone code may be more sensitive to the effects of BPA.
Overall, the study results indicate that NCTR Sprague-Dawley rat testes and sperm were insensitive to oral BPA exposure over a wide-range of doses. No non-monotonic responses were identified with statistical confidence for the histopathologic, morphometric, and molecular endpoints evaluated. A strength of this study was the use of multiple BPA dose levels and multiple approaches to increase confidence in the identification of potential treatment effects.
Supplementary Material
Supplemental Figure 1: Hierarchical clustering of 10,000 randomly selected normalized probe intensities from the sperm transcriptomic analyses.
Supplemental Figure 2: Dose-response modeling of the 32 DMRs identified using the 4-parameter Brain-Cousens model.
Supplemental Figure 3: Dose-response modeling of the 32 DMRs identified using the 5-parameter Brain-Cousens model.
Supplemental Figure 4: Dose-response modeling of the 32 DMRs identified using the 4-parameter log-logistic model.
Supplemental Figure 5: Dose-response modeling of the 32 DMRs identified using the 5-parameter log-logistic model.
Acknowledgements
The authors would like to thank Melinda Golde and Paula Weston for preparing the testes for histopathological analyses, Dr. Christoph Schorl of the Brown University Genomics Core Facility for preparing the Affymetrix GeneChip and running the Illumina HiSeq 2500 sequence runs, Dr. Mel Anderson for his comments that led to the Monte Carlo analysis of the -omic data sets, and Dr. K. Barry Delclos for his contributions to the design and conduct of the study. Part of this research was conducted using computational resources and services at the Center for Computation and Visualization, Brown University. The contents of this manuscript should not be interpreted as current or future policy of the FDA or NIEHS. The mention of any manufacturers or trade names is only for clarity and does not constitute endorsement.
Funding
National Institute of Environmental Health Sciences CLARITY-BPA (U01ES020913) and Training Grant (T32 ES007272). The animal portion of this study was supported by NIEHS Interagency Agreement AES12013 (FDA IAG 224-12-0003).
References
- Chemical Effects in Biological Systems (CEBS). National Toxicology Program (NTP), Research Triangle Park, NC, pp. [Google Scholar]
- Akalin A, Kormaksson M, Li S, Garrett-Bakelman FE, Figueroa ME, Melnick A, Mason CE, 2012. methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol 13, R87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Steinmetz G, Montillet G, Perrard MH, Loundou A, Durand P, Guichaoua MR, Prat O, 2014. Exposure to low-dose bisphenol A impairs meiosis in the rat seminiferous tubule culture model: a physiotoxicogenomic approach. PLoS One 9, e106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P, Parmigiani S, vom Saal FS, Taylor JA, 2013. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol 42, 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronius A, Johansson N, Ruden C, Hanberg A, 2013. The influence of study design and sex-differences on results from developmental neurotoxicity studies of bisphenol A: implications for toxicity testing. Toxicology 311, 13–26. [DOI] [PubMed] [Google Scholar]
- Biegel LB, Flaws JA, Hirshfield AN, O’Connor JC, Elliott GS, Ladics GS, Silbergeld EK, Van Pelt CS, Hurtt ME, Cook JC, Frame SR, 1998. 90-day feeding and one-generation reproduction study in Crl:CD BR rats with 17 beta-estradiol. Toxicol Sci 44, 116–142. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Bucher JR, Collman GW, Zeldin DC, Johnson AF, Schug TT, Heindel JJ, 2012. Consortium-based science: the NIEHS’s multipronged, collaborative approach to assessing the health effects of bisphenol A. Environ Health Perspect 120, 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boellmann F, Zhang L, Clewell HJ, Schroth GP, Kenyon EM, Andersen ME, Thomas RS, 2010. Genome-wide analysis of DNA methylation and gene expression changes in the mouse lung following subchronic arsenate exposure. Toxicol Sci 117, 404–417. [DOI] [PubMed] [Google Scholar]
- Boyle P, Clement K, Gu H, Smith ZD, Ziller M, Fostel JL, Holmes L, Meldrim J, Kelley F, Gnirke A, Meissner A, 2012. Gel-free multiplexed reduced representation bisulfite sequencing for large-scale DNA methylation profiling. Genome Biol 13, R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS, 2010. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J 24, 2273–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant BH, Yamasaki H, Sandrof MA, Boekelheide K, 2008. Spermatid head retention as a marker of 2,5-hexanedione-induced testicular toxicity in the rat. Toxicol Pathol 36, 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL, 2005. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect 113, 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho BS, Irizarry RA, 2010. A framework for oligonucleotide microarray preprocessing. Bioinformatics 26, 2363–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H-S, Anway MD, Rekow SS, Skinner MK, 2006. Transgenerational epigenetic imprinting of the male germline by endocrine disruptor exposure during gonadal sex determination. Endocrinology 147, 5524–5541. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE, Hayward SW, Lees PSJ, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, Vandenbergh JG, Woskie SR, 2008. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol 83, 157–395. [DOI] [PubMed] [Google Scholar]
- Chapman VL, Zollinger T, Terranova R, Moggs J, Kimber I, Dearman RJ, 2014. Chemical allergen induced perturbations of the mouse lymph node DNA methylome. Toxicol Sci 139, 350–361. [DOI] [PubMed] [Google Scholar]
- Chappell G, Kobets T, O’Brien B, Tretyakova N, Sangaraju D, Kosyk O, Sexton KG, Bodnar W, Pogribny IP, Rusyn I, 2014. Epigenetic events determine tissue-specific toxicity of inhalational exposure to the genotoxic chemical 1,3-butadiene in male C57BL/6J mice. Toxicol Sci 142, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yan W, Duan E, 2016. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet 17, 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zuo X, He D, Ding S, Xu F, Yang H, Jin X, Fan Y, Ying L, Tian C, Ying C, 2017. Long-term exposure to a ‘safe’ dose of bisphenol A reduced protein acetylation in adult rat testes. Sci Rep 7, 40337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti A, Kobets T, Escudero-Lourdes C, Montgomery B, Tryndyak V, Beland FA, Doerge DR, Pogribny IP, 2014. Dose- and time-dependent epigenetic changes in the livers of Fisher 344 rats exposed to furan. Toxicol Sci 139, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, Davis KJ, Patton RE, Gamboa da Costa G, Woodling KA, Bryant MS, Chidambaram M, Trbojevich R, Juliar BE, Felton RP, Thorn BT, 2014. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci 139, 174–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos KB, Weis CC, Bucci TJ, Olson G, Mellick P, Sadovova N, Latendresse JR, Thorn B, Newbold RR, 2009. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod Toxicol 27, 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Wilson SK, Anderson LM, Boekelheide K, 2016. Sperm Molecular Biomarkers Are Sensitive Indicators of Testicular Injury following Subchronic Model Toxicant Exposure. Toxicol Sci 153, 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW, 2010a. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol 247, 158–165. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Vanlandingham M, Twaddle NC, Delclos KB, 2010b. Lactational transfer of bisphenol A in Sprague-Dawley rats. Toxicol Lett 199, 372–376. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL, 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A 104, 13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Nelson M, Grayson DR, Costa E, Guidotti A, 2008. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc Natl Acad Sci U S A 105, 13614–13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G, 2011. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology 289, 74–82. [DOI] [PubMed] [Google Scholar]
- Eladak S, Moison D, Guerquin MJ, Matilionyte G, Kilcoyne K, N’Tumba-Byn T, Messiaen S, Deceuninck Y, Pozzi-Gaudin S, Benachi A, Livera G, Antignac JP, Mitchell R, Rouiller-Fabre V, Habert R, 2018. Effects of environmental Bisphenol A exposures on germ cell development and Leydig cell function in the human fetal testis. PLoS One 13, e0191934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk C, Kim JH, Jones TR, McEachin RC, Nahar MS, Dolinoy DC, Sartor MA, 2015. Bisphenol A-associated alterations in genome-wide DNA methylation and gene expression patterns reveal sequence-dependent and non-monotonic effects in human fetal liver. Environ Epigenet 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca LR, Hess RA, Dufour JM, Hofmann MC, Griswold MD, 2016. The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology 4, 189–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussell KC, Schneider S, Buesen R, Groeters S, Strauss V, Melching-Kollmuss S, van Ravenzwaay B, 2015. Investigations of putative reproductive toxicity of low-dose exposures to flutamide in Wistar rats. Arch Toxicol 89, 2385–2402. [DOI] [PubMed] [Google Scholar]
- Galloway T, Cipelli R, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, Money C, McCormack P, Melzer D, 2010. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ Health Perspect 118, 1603–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R, Chen G, Hardy MP, 2008. The role of the Leydig cell in spermatogenic function. Advances in experimental medicine and biology 636, 255–269. [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J, 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J, 2013. Methods of sperm DNA extraction for genetic and epigenetic studies. Methods Mol Biol 927, 379–384. [DOI] [PubMed] [Google Scholar]
- Gu H, Smith ZD, Bock C, Boyle P, Gnirke A, Meissner A, 2011. Preparation of reduced representation bisulfite sequencing libraries for genome-scale DNA methylation profiling. Nat Protoc 6, 468–481. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK, 2010. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna CW, Bloom MS, Robinson WP, Kim D, Parsons PJ, vom Saal FS, Taylor JA, Steuerwald AJ, Fujimoto VY, 2012. DNA methylation changes in whole blood is associated with exposure to the environmental contaminants, mercury, lead, cadmium and bisphenol A, in women undergoing ovarian stimulation for IVF. Hum Reprod 27, 1401–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass U, Christiansen S, Boberg J, Rasmussen MG, Mandrup K, Axelstad M, 2016. Low-dose effect of developmental bisphenol A exposure on sperm count and behaviour in rats. Andrology 4, 594–607. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Newbold RR, Bucher JR, Camacho L, Delclos KB, Lewis SM, Vanlandingham M, Churchwell MI, Twaddle NC, McLellen M, Chidambaram M, Bryant M, Woodling K, Gamboa da Costa G, Ferguson SA, Flaws J, Howard PC, Walker NJ, Zoeller RT, Fostel J, Favaro C, Schug TT, 2015. NIEHS/FDA CLARITY-BPA research program update. Reprod Toxicol 58, 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Sartor MA, Rozek LS, Faulk C, Anderson OS, Jones TR, Nahar MS, Dolinoy DC, 2014. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics 15, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczor CA, Ludlow I, Hight RS 2nd, Jiao Z, Fields E, Ludaway T, Russ R, Torres RA, Lewis W, 2015. Ecstasy (MDMA) Alters Cardiac Gene Expression and DNA Methylation: Implications for Circadian Rhythm Dysfunction in the Heart. Toxicol Sci 148, 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krementsov DN, Katchy A, Case LK, Carr FE, Davis B, Williams C, Teuscher C, 2013. Studies in experimental autoimmune encephalomyelitis do not support developmental bisphenol A exposure as an environmental factor in increasing multiple sclerosis risk. Toxicol Sci 135, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Andrews SR, 2011. Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27, 1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, Newbold RR, Delclos KB, 2009. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reprod Toxicol 28, 342–353. [DOI] [PubMed] [Google Scholar]
- Li DK, Zhou Z, Miao M, He Y, Wang J, Ferber J, Herrinton LJ, Gao E, Yuan W, 2011. Urine bisphenol-A (BPA) level in relation to semen quality. Fertil Steril 95, 625–630 e621–624. [DOI] [PubMed] [Google Scholar]
- Li Y-J, Song T-B, Cai Y-Y, Zhou J-S, Song X, Zhao X, Wu X-L, 2009. Bisphenol A exposure induces apoptosis and upregulation of Fas/FasL and caspase-3 expression in the testes of mice. Toxicol Sci 108, 427–436. [DOI] [PubMed] [Google Scholar]
- Manfo FP, Jubendradass R, Nantia EA, Moundipa PF, Mathur PP, 2014. Adverse effects of bisphenol A on male reproductive function. Rev Environ Contam Toxicol 228, 57–82. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK, 2013. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 8, e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Foster PM, 2001. Androgen-mediated development in male rat offspring exposed to flutamide in utero: permanence and correlation of early postnatal changes in anogenital distance and nipple retention with malformations in androgen-dependent tissues. Toxicol Sci 62, 236–249. [DOI] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Hauser R, Gaskins AJ, 2016. Effects of bisphenol A on male and couple reproductive health: a review. Fertil Steril 106, 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata K, Yabushita S, Sukata T, Sano M, Yoshino H, Nakanishi T, Okuno Y, Matsuo M, 2002. Effects of perinatal exposure to flutamide on sex hormones and androgen-dependent organs in F1 male rats. J Toxicol Sci 27, 19–33. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program, 2008. NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. NTP CERHR MON, i-III1 [PubMed]
- Ozden S, Turgut Kara N, Sezerman OU, Durasi IM, Chen T, Demirel G, Alpertunga B, Chipman JK, Mally A, 2015. Assessment of global and gene-specific DNA methylation in rat liver and kidney in response to non-genotoxic carcinogen exposure. Toxicol Appl Pharmacol 289, 203–212. [DOI] [PubMed] [Google Scholar]
- Pacheco SE, Anderson LM, Sandrof MA, Vantangoli MM, Hall SJ, Boekelheide K, 2012. Sperm mRNA transcripts are indicators of sub-chronic low dose testicular injury in the Fischer 344 rat. PLoS One 7, e44280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS, 2002. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect 110 Suppl 3, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, R: A Language and Environment for Statistical Computing, Vienna, Austria, pp. [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS, 2007. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 24, 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK, 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz C, Baty F, Streibig JC, Gerhard D, 2015. Dose-Response Analysis Using R. PLoS One 10, e0146021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC, 2008. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect 116, 1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G, 2009. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci 85, 742–752. [DOI] [PubMed] [Google Scholar]
- Schug TT, Heindel JJ, Camacho L, Delclos KB, Howard P, Johnson AF, Aungst J, Keefe D, Newbold R, Walker NJ, Thomas Zoeller R, Bucher JR, 2013. A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program. Reprod Toxicol 40, 35–40. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque M, Nilsson EE, 2013. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med 11, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey R, Manikkam M, Guerrero-Bosagna C, Skinner MK, 2013. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod Toxicol 36, 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, Seely JC, Dimond SS, Van Miller JP, Shiotsuka RN, Beyer D, Hentges SG, Waechter JM Jr., 2008. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol Sci 104, 362–384. [DOI] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Thomas BF, Keimowitz AR, Brine DR, Veselica MM, Fail PA, Chang TY, Seely JC, Joiner RL, Butala JH, Dimond SS, Cagen SZ, Shiotsuka RN, Stropp GD, Waechter JM, 2002. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol Sci 68, 121–146. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G, 2010. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118, 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Prins GS, 2016. Clarity in the face of confusion: new studies tip the scales on bisphenol A (BPA). Andrology 4, 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrooman LA, Oatley JM, Griswold JE, Hassold TJ, Hunt PA, 2015. Estrogenic exposure alters the spermatogonial stem cells in the developing testis, permanently reducing crossover levels in the adult. PLoS Genet 11, e1004949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HQ, Tuominen LK, Tsai CJ, 2011. SLIM: a sliding linear model for estimating the proportion of true null hypotheses in datasets with dependence structures. Bioinformatics 27, 225–231. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2011. Toxicological and health aspects of bisphenol A: Joint FAO/WHO expert meeting 2–5 November 2010 and stakeholder meeting on bisphenol A, Ottawa, Canada World Health Organization, Geneva, Switzerland, pp. [Google Scholar]
- Zheng H, Zhou X, Li DK, Yang F, Pan H, Li T, Miao M, Li R, Yuan W, 2017. Genome-wide alteration in DNA hydroxymethylation in the sperm from bisphenol A-exposed men. PLoS One 12, e0178535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Z, Xia M, Zhuang S, Gong X, Pan J, Li C, Fan R, Pang Q, Lu S, 2017. Neurotoxicity of low bisphenol A (BPA) exposure for young male mice: Implications for children exposed to environmental levels of BPA. Environ Pollut 229, 40–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Hierarchical clustering of 10,000 randomly selected normalized probe intensities from the sperm transcriptomic analyses.
Supplemental Figure 2: Dose-response modeling of the 32 DMRs identified using the 4-parameter Brain-Cousens model.
Supplemental Figure 3: Dose-response modeling of the 32 DMRs identified using the 5-parameter Brain-Cousens model.
Supplemental Figure 4: Dose-response modeling of the 32 DMRs identified using the 4-parameter log-logistic model.
Supplemental Figure 5: Dose-response modeling of the 32 DMRs identified using the 5-parameter log-logistic model.


