Abstract
Introduction
Enterococcus faecium is a commensal organism commonly colonizing the human gastrointestinal tract. Although it is generally a non-virulent organism, E. faecium can cause significant morbidity and mortality due to its inherent and acquired resistances to commonly used antimicrobials. Patients who are immunosuppressed are particularly vulnerable.
Case presentation
A 65–75-year-old patient with a history of an orthotopic liver transplant for hepatitis C infection and diabetes was re-admitted to the hospital with abdominal pain and fever. The patient had several recent admissions related to the presentation reported here, which included treatment with a prolonged course of broad-spectrum antibiotics. The patient was found to have a recurrent liver abscess and blood cultures grew vancomycin-resistant E. faecium, non-susceptible to all tested agents: ampicillin, penicillin, vancomycin, daptomycin and linezolid. The patient was started initially on chloramphenicol intravenously while awaiting additional susceptibility testing, which ultimately revealed chloramphenicol non-susceptibility. Tigecycline was started but the patient ultimately decided to pursue hospice care.
Conclusion
Multi-drug-resistant organisms are increasingly being recognized and are associated with poorer outcomes, particularly in immunosuppressed patients. We describe a particularly resistant organism and discuss potential therapeutic options.
Keywords: bacteraemia, liver abscess, chloramphenicol, tigecycline, solid organ transplant
Introduction
Enterococcus species, including Enterococcus faecium, are frequent colonizers of the human gastrointestinal tract and are frequently viewed as commensal organisms [1]. Although not typically considered to be virulent organisms, Enterococcus species, particularly E. faecium, can have significant pathogenic potential due to both inherent and acquired resistances to many commonly used antibiotics used today [1, 2]. Despite advances in clinical care, including newer antimicrobials, outcomes for infections caused by Enterococcus species are poor, and are considerably worse for vancomycin-resistant E. faecium (VRE) compared to susceptible strains [2]. Multi-drug-resistant organisms are increasingly recognized as problems for immunocompromised patients, particularly VRE [3]. Having a deeper understanding of the antimicrobials available, as well as their potential toxicities and drug–drug interactions, is vitally important to improving patient outcomes.
Case report
Our patient was a 65–75-year-old with a history of orthotopic liver transplant in 2000 for hepatitis C infection, on chronic immunosuppression with cyclosporine, pancreatic adenocarcinoma status post-Whipple procedure in 2015, and diabetes mellitus, who was admitted for fever and worsening abdominal pain. The patient had several recent admissions in the preceding 3 months for perihepatic abscess and bacteraemia. Three months prior to the admission reported here, liver abscess cultures obtained during drain placement grew Rothia mucilaginosa and α-haemolytic Streptococcus species, with Streptococcus parasanguinis growing in two sets of blood cultures. The patient was sent home on ceftriaxone 2 grams (gm) intravenously (IV) every 24 h and metronidazole 500 milligrams (mg) by mouth (PO) every 8 h but was readmitted 1 month later with Candida parapsilosis fungaemia, felt to be related to the peripherally inserted central catheter (PICC). The PICC was removed and antimicrobials were changed to moxifloxacin 400 mg PO once daily and a 2 week course of fluconazole 400 mg PO every 24 h. The patient was readmitted 1 month before the admission reported here while still on the moxifloxacin, and was found to have worsening abdominal pain and an enlarging perihepatic abscess on a computerized tomography (CT) scan of the abdomen. A new drain was placed into the liver abscess, and cultures from this drain grew VRE. Antibiotics were then adjusted to daptomycin (dosed at 10 mg kg−1 every 24 h) and moxifloxacin 400 mg PO every 24 h for presumed polymicrobial infection, and the patient was discharged with a new PICC to complete a tentative 4–6-week course.
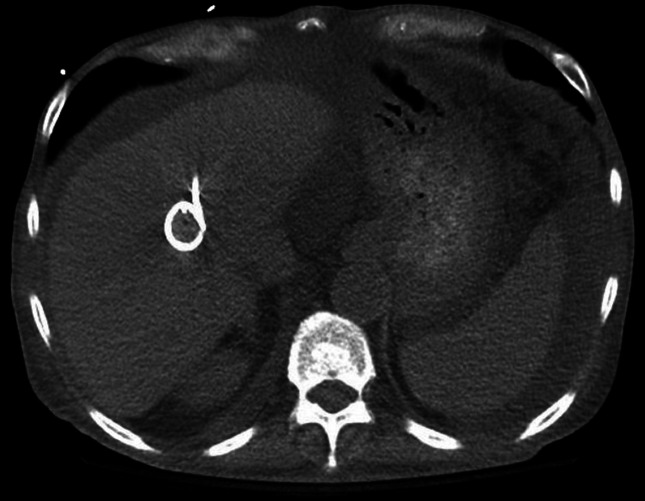
At the beginning of the patient’s admission reported here, approximately 2 weeks after the most recent discharge, the antibiotics were changed to daptomycin 10 mg/kg every 24 h and meropenem 500 mg every 8 h (adjusted for his renal impairment) for improved Gram-negative and anaerobic coverage. A CT scan of the patient’s abdomen and pelvis was obtained (Fig. 1). Blood cultures returned with VRE, non-susceptible to ampicillin, penicillin, vancomycin, daptomycin and linezolid on initial susceptibilities performed by the MicroScan WalkAway-96 plus system with the Pos Combo 33 panel (Beckman Coulter Diagnostics). The daptomycin MIC was confirmed to be 8 µg ml−1 with ETEST (bioMérieux).
Fig. 1.
CT scan of the liver abscess with the drain in place.
Additional susceptibilities were requested for chloramphenicol and tigecycline by ETEST. Susceptibility testing for quinupristin/dalfopristin, telavancin, dalbavancin and oritavancin was not performed because these agents were not on formulary and access to these therapeutics was not possible at the time. Quinupristin/dalfopristin was not used because it interacts with calcineurin inhibitors leading to supratherapeutic drug levels of cyclosporine and tacrolimus. The patient’s PICC was removed and the patient was started on intravenous chloramphenicol while awaiting further workup. This decision was based on the patient’s resistance pattern, as well as greater serum concentrations of chloramphenicol compared to tigecycline. Additional testing revealed non-susceptibility to chloramphenicol (MIC=16 µg ml−1). The isolate was susceptible to tigecycline (MIC ≤ 0.25 µg ml−1), so the decision was made to transition to tigecycline monotherapy, as it was felt that adequate serum concentrations were achievable with such a low MIC. The patient initially tolerated this change well and was discharged home with a new PICC. Unfortunately, the patient was later admitted with persistent failure to thrive including nausea, diarrhoea and weakness 1 month later. Palliative care was considered and the patient decided to pursue hospice care, declining further antimicrobial treatment.
Discussion
Multi-drug-resistant E. faecium treatment is an increasing challenge, confronting clinicians globally. Although current recommendations encourage the use of daptomycin or linezolid as the first-line treatment options for VRE [4, 5], data is lacking for solid organ transplant recipients on immunosuppressive agents or in cases where the first-line antimicrobials are ineffective. Alternative therapeutic agents for the treatment of VRE in the presence of resistance to ampicillin, penicillin, vancomycin, daptomycin and linezolid are limited. Quinupristin/dalfopristin is active against E. faecium but not Enterococcus faecalis, but it is poorly tolerated, requires central venous access for administration, and increases serum concentrations of calcineurin inhibitors and mTOR inhibitors, which are commonly used in solid organ transplant recipients [3]. Tigecycline also possesses activity against VRE, but achieves very low serum concentrations and there is concern for increased mortality compared to other agents [6]. Telavancin, a lipoglycopeptide, is active against VRE strains possessing van B but not van A. Oritavancin, a long-acting lipoglycopeptide, is active against VRE strains possessing both van A and van B [7, 8], but clinical data for VRE bacteraemia is sparse.
Chloramphenicol possesses activity against VRE, but its toxicities limit widespread use today. Chloramphenicol is associated with both a reversible, dose-dependent bone marrow suppression, as well as dose-independent, irreversible aplastic anaemia [9, 10]. Although the mechanisms are not fully clear, the irreversible aplastic anaemia is primarily seen with oral administration of the agent, but not with intravenous administration. It is felt that enteric bacteria may play a role by degrading chloramphenicol, releasing toxic metabolites that are then absorbed enterally. The p-nitrosulfathiazole group, which inhibits DNA synthesis, is believed to be the causative metabolite, supported by the fact that thiamphenicol, available in Europe but not currently in the USA, does not possess this group and is not associated with aplastic anaemia [9, 10].
It was surprising to find chloramphenicol resistance despite a lack of prior exposure to the agent. Chloramphenicol resistance has been described previously and appears to be related to recent exposure to fluoroquinolones, as was the case for our patient [11]. Resistance is typically mediated through a multi-drug efflux pump, conveying resistance to fluoroquinolones, tetracyclines and chloramphenicol [12]. It is likely that our patient’s recent prolonged exposure to moxifloxacin upregulated this efflux pump, conveying resistance to chloramphenicol despite the patient’s lack of prior exposure.
Multi-drug resistant and extensively drug resistant organisms are becoming more common with the increased use of broad-spectrum antimicrobials, especially in the immunocompromised host [3]. As resistance develops to the preferred agents, it is imperative to understand the strengths and limitations of alternative agents, along with emerging resistance mechanisms.
Funding information
N. A. S. is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health (grant numbers UL1 TR002378 and TL1 TR002382).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CT, computerized tomography; PICC, peripherally inserted central catheter; VRE, vancomycin-resistant Enterococcus faecium.
References
- 1.Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prematunge C, MacDougall C, Johnstone J, Adomako K, Lam F, et al. VRE and VSE bacteremia outcomes in the era of effective vre therapy: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37:26–35. doi: 10.1017/ice.2015.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel G, Snydman DR, AST Infectious Diseases Community of Practice Vancomycin-resistant Enterococcus infections in solid organ transplantation. Am J Transplant. 2013;13 (Suppl. 4):59–67. doi: 10.1111/ajt.12099. [DOI] [PubMed] [Google Scholar]
- 4.Arias CA, Contreras GA, Murray BE. Management of multidrug-resistant enterococcal infections. Clin Microbiol Infect. 2010;16:555–562. doi: 10.1111/j.1469-0691.2010.03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009. Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA. FDA Warns of Increased Risk of Death with IV Antibacterial Tygacil (Tigecycline) and Approves New Boxed Warning. Silver Spring, MD:: U.S. Food and Drug Administration;; 2013. [Google Scholar]
- 7.Arias CA, Mendes RE, Stilwell MG, Jones RN, Murray BE. Unmet needs and prospects for oritavancin in the management of vancomycin-resistant enterococcal infections. Clin Infect Dis. 2012;54:S233–S238. doi: 10.1093/cid/cir924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stryjewski ME, Graham DR, Wilson SE, O'Riordan W, Young D, et al. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis. 2008;46:1683–1693. doi: 10.1086/587896. [DOI] [PubMed] [Google Scholar]
- 9.Barnhill AE, Brewer MT, Carlson SA. Adverse effects of antimicrobials via predictable or idiosyncratic inhibition of host mitochondrial components. Antimicrob Agents Chemother. 2012;56:4046–4051. doi: 10.1128/AAC.00678-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yunis AA. Chloramphenicol toxicity: 25 years of research. Am J Med. 1989;87:44n–48n. [PubMed] [Google Scholar]
- 11.Gould CV, Fishman NO, Nachamkin I, Lautenbach E. Chloramphenicol resistance in vancomycin-resistant enterococcal bacteremia: impact of prior fluoroquinolone use? Infect Control Hosp Epidemiol. 2004;25:138–145. doi: 10.1086/502365. [DOI] [PubMed] [Google Scholar]
- 12.Lynch C, Courvalin P, Nikaido H. Active efflux of antimicrobial agents in wild-type strains of enterococci. Antimicrob Agents Chemother. 1997;41:869–871. doi: 10.1128/AAC.41.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]