Abstract
Military personnel are often exposed to multiple instances of various types of head trauma. As a result, there has been increasing concern recently over identifying when head trauma has resulted in a brain injury and what, if any, long-term consequences those brain injuries may have. Efforts to develop equipment to protect soldiers from these long-term consequences will first require understanding the types of head trauma that are likely responsible. In this study, we sought to identify the types of head trauma most likely to lead to the deposition of tau, a protein identified as a likely indicator of long-term negative consequences of brain injury. To define the types of head trauma in a military population, we applied a factor analysis to interviews from a larger cohort of 428 Veterans enrolled in the Translational Research Center for Traumatic Brain Injury and Stress Disorders. Three factors were identified: Blast Exposure, Symptom Duration, and Blunt Concussion. Sixteen male Veterans from this study and one additional male civilian (aged 25–69, mean 35.2 years) underwent simultaneous positron emission tomography/magnetic resonance imaging using a tracer that binds to tau protein, the ligand T807/AV-1451 (Flortaucipir). Standard uptake value ratios to the isthmus of the cingulate were calculated from a 20-minute time frame 70 min post-injection. We found that tracer uptake throughout the brain was associated with Blast Exposure factor beta weights, but not with either Symptom Duration or Blunt Concussion. Associations with uptake were located primarily in the cerebellar, occipital, inferior temporal and frontal regions. The data suggest that in this small, relatively young cohort of Veterans, elevated T807/AV-1451 uptake is associated with exposure to blast neurotrauma. These findings are unanticipated, as they do not match histopathological descriptions of tau pathology associated with head trauma. Continued work will be necessary to understand the nature of the regional T807/AV-1451 uptake and any associations with clinical symptoms.
Keywords: Blast, Positron emission tomography, Tau, Traumatic brain injury, Veteran
Abbreviations: 3D OP-OSEM, 3D Ordinary Poisson ordered-subset expectation maximization; BAT-L, Boston Assessment of TBI- Lifetime; CAPS, Clinician-Administered PTSD Scale; CT, Computed Tomography; CTE, Chronic Traumatic Encephalopathy; DASS, Depression Anxiety Stress Scales; DRRI, Deployment Risk and Resilience Inventory; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICA, Independent Components Analysis; LDH, Lifetime Drinking History; MEMPRAGE, Multi-echo Magnetization-Prepared Rapid Acquisition Gradient-Echo; MR/PET, Magnetic Resonance/Positron Emission Tomography; MSVT, Medical Symptom Validity Test; OEF/OIF/OND, Operation Enduring Freedom/Operation Iraqi Freedom/Operation New Dawn; PCL, PTSD Checklist; PHF, paired helical filament; PTSD, Posttraumatic Stress Disorder; SCID, Structured Clinical Interview for DSM Disorders; SUV, Standard Uptake Value; SUVR, Standard Uptake Value Ratio; RR&D, Rehabilitation Research and Development; TBI, Traumatic Brain Injury; TE, Echo Time; TR, Repetition Time; TRACTS, Translational Research Center for TBI and Stress Disorders; VA, Department of Veterans Affairs
Highlights
-
•
Veterans of Iraq and Afghanistan were scanned with a tau-binding PET tracer.
-
•
Number of blast exposures, but not concussions, were associated with tracer uptake.
-
•
Anatomy of tracer uptake did not match other trauma-related neurodegeneration.
-
•
Uptake distribution confirmed with independent components analysis.
-
•
Results not attributable to comorbid neuropsychiatric conditions, such as PTSD
1. Introduction
Service members who have been deployed overseas are likely to encounter many forms of head trauma as a result of their military service. Immediate, severe consequences of these head traumas are relatively rare, with most events leading to either no acute symptoms, or to symptoms that resolve within days to weeks (e.g., a classic concussion). Over the last decade or so, there has been increasing interest in developing objective methods for identifying when a brain injury has occurred, and gauging the potential long-term consequences of such injuries. Although military head trauma is common, and often occurs more than once for a single person (Hoge et al., 2008), there is much left to learn regarding which traumas produce brain injuries, and how such injuries may affect the function of the brain in the short- and long-term.
It has been hypothesized that moderate and severe traumatic brain injuries (as defined by the symptoms present at the time of the event) may increase the risk for individuals to present with Alzheimer's dementia in late life, (Mortimer et al., 1991; Sivanandam and Thakur, 2012) and that repetitive milder injuries (concussive or even subconcussive) may produce a different disorder, known as chronic traumatic encephalopathy, or CTE (McKee et al., 2013). While trauma-related neurodegeneration has been most extensively studied for blunt traumas, there are suggestions that blast trauma may also result in issues later in life (Huber et al., 2013; McKee and Robinson, 2014; Omalu et al., 2011; Trotter et al., 2015). Histopathological studies, when available, have identified the protein tau as a key feature of these trauma-related neurodegenerative pathologies, and so for this study, we utilize increased uptake of a PET tracer for tau (T807/AV-1451) as evidence of potential long-term consequences of head trauma.
A difficulty for studying military head trauma is that deployed individuals are simultaneously at high risk for many types of head trauma, of various etiologies and severities. Thus, in cases where evidence of trauma-related neurodegeneration appears for an individual, it is likely that they have been exposed to many types of head trauma throughout their life. While the blunt traumas encountered by military personnel (e.g., motor vehicle accidents, falls, fights, etc.) are quite comparable to those encountered in civilian life, the wars in Afghanistan and Iraq have been notable for the extensive use of explosive weaponry (Belmont Jr et al., 2012), and the resulting blast injuries are, for the most part, unique to the military. Neuroimaging studies of Veterans with blast exposure suggest that blunt and blast traumas may have different effects on brain structure and function (Fischer et al., 2014; Robinson et al., 2015a; Trotter et al., 2015). For each of these etiologies, the complete cascade of biochemical and neurobiological events resulting in acute brain injuries are unclear, and the threshold at which head trauma may initiate this cascade is not known. Most diagnostic guidelines utilize duration of acute symptoms (such as loss-of-consciousness, altered mental state, and post-traumatic amnesia) to categorize the severity of brain injuries, (The Management of Concussion/mTBI Working Group; 2009), however, these acute symptoms may not be predictive of long-term neuropsychiatric problems. Neuroimaging studies of individuals following head trauma have demonstrated that notable changes in immediate mental status may not be necessary to produce changes in brain physiology following blast exposure (Bazarian et al., 2012a; Robinson et al., 2015a; Taber et al., 2015; Trotter et al., 2015) or blunt trauma (Abbas et al., 2014; Bazarian et al., 2012b; Breedlove et al., 2012; Koerte et al., 2012; Poole et al., 2014; Robinson et al., 2015b).
The current study seeks to understand if one or more of the various aspects of military-related head trauma are associated with evidence of tau accumulation in the brain. We utilized [18F]-T807/AV1451 (Flortaucipir) (Chien et al., 2013; Xia et al., 2013), a tau-binding PET tracer in a sample of primarily military Veterans (n = 17) drawn from those enrolled in TRACTS (McGlinchey et al., 2017). TRACTS is a large prospective longitudinal cohort study of Veterans who served in OEF/OIF/OND. Using data from comprehensive structured interviews of head trauma suffered across the lifespan (Fortier et al., 2014) from the larger cohort, we identified types of head trauma and then sought to determine which, if any, of these types of head trauma were associated with tracer uptake.
2. Materials and methods
2.1. Participants
Nineteen male participants who served in OEF/OIF/OND were recruited from TRACTS, an ongoing longitudinal VA RR&D National Network Research Center located at VA Boston Healthcare System. Participants were recruited via flyers in the VA hospital advertising the study, as well as through calls to participants in TRACTS. On average, PET study visits were 1.05 ± 0.89 years (range: 72 days – 2.96 years) removed from participation in a TRACTS study visit. One additional civilian participant was also recruited. After excluding for excessive motion during scanning (3 participants), data from 17 participants were retained for this study. Participants were all male aged 25–69 years old (mean: 35.2 years).
2.2. Neuroimaging – magnetic resonance
Images were acquired during simultaneous MR/PET imaging on a TIM Trio 3 Tesla MR scanner with a BrainPET insert (Siemens Healthcare, Erlangen, Germany). MEMPRAGE (TR = 2350 ms, TE = 1.63/3.49/5.35/7.21 ms, flip angle 700, 1 mm isotropic, 280x280x200 matrix) anatomical images were acquired for registration to a volume-based template (TT_N27) using AFNI (Cox, 1996) and cortical reconstruction and registration using FreeSurfer. These images were also used in the pseudo-CT procedure for attenuation correction. (Izquierdo-Garcia et al., 2014; Ladefoged et al., 2017)
2.3. Neuroimaging – positron emission tomography
Participants were injected with approximately 370 MBq (10 mCi, 9.41 ± 1.29 mCi) of [18F]-T807/AV-1451. Images were acquired dynamically after injection for 1.5–2 h (depending on the participant). We utilized a combination of in-house and vendor-provided software for MR/PET data processing and image reconstruction. The images were reconstructed using a 3D OP-OSEM algorithm accounting for variations in detector efficiency, photon attenuation, and scatter. Output volumes were 1.25 mm isotropic with a matrix of 256x256x153 with one 70-minute frame immediately post-injection and 4 × 5-minute frames following that. The five-minute PET images were registered to the MRI anatomical image with the 70-minute frame as a mid-point using AFNI (Cox, 1996). An average frame was generated from the 4 five-minute frames (70–90 min post-injection) and registered to both surface-based and volume-based template spaces using FreeSurfer (Fischl et al., 1999) and AFNI respectively. Participants who moved >2.5 mm (2 voxels) over the 20-minute frame were excluded. The isthmus of the cingulate, as defined by FreeSurfer's aparc parcellation (Dale et al., 1999), was used as a reference region rather than the more commonly utilized cerebellum (Wooten et al., 2017), as many participants with significant blast exposure had high uptake in the cerebellum, making it inappropriate as a reference region. Conversely, the cingulate was found to have consistently low uptake across our sample (relative to other brain regions). Activity in the isthmus cingulate was calculated by sampling within the cortex and averaging the two hemispheres. SUVRs were calculated at the regional, voxel-wise and vertex-wise levels. With a young cohort, levels of tracer uptake are not expected to be high, so regional averages were employed first to detect small changes over larger areas. Surface-based alignment (vertex-wise) was utilized to assess if there were changes relative to curvature of the cortical folds, as these have been implicated in CTE, or near grey-white boundaries, as implicated in histological studies of Veterans with blast injury (Shively et al., 2016). As surface-based analyses do not include sub-cortical regions, volume-based analyses were employed as well. Images were smoothed at 4mm3 in the volume or at 5mm2 along the surface.
2.4. Head trauma assessment
Head trauma was assessed with the BAT-L (Fortier et al., 2014) semi-structured interview, which assessed lifetime history of head trauma events. In the larger cohort of TRACTS baseline visits (n = 500), participants were excluded if they were flagged by consensus of doctoral-level psychologists for neurologic, cognitive, or psychiatric reasons, or if they failed the MSVT, indicating their self-report may not be accurate. This resulted in n = 428 participants being included in the factor analysis. Certain measures within the BAT-L were not normally distributed (i.e. skewed) and were thus transformed via square root or logarithmic transform prior to factor analysis (see Table 2). Factors were computed using SAS, with no a priori determination of number of factors. Factors with eigenvalues >1.0 were kept for further analysis. Each participant in the PET study completed a BAT-L interview during a visit that was either part of a TRACTS baseline, follow-up or specific to this study. Within 30 days of the acquisition of PET data, a questionnaire was administered to assess for any new head trauma that would require updating their BAT-L interview. PET study participants were assigned factor score beta weights based on the factor weightings generated from the larger cohort and their head trauma assessment collected with their PET scan. For participants in the PET study, these scores were still skewed, and so they were transformed to ranks for all statistical tests to mitigate influential points.
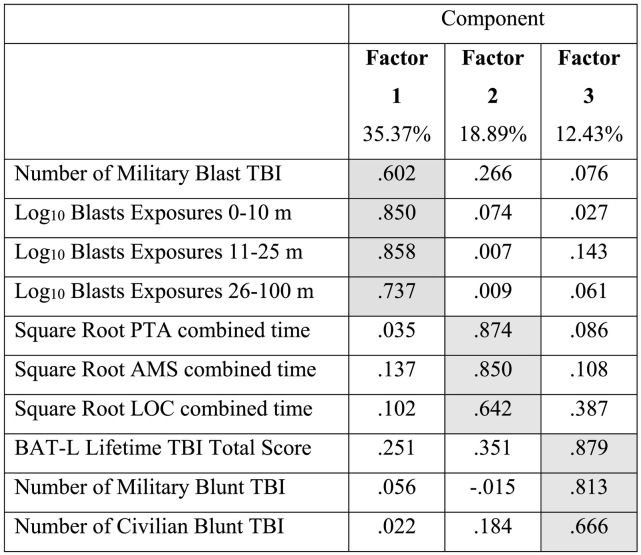
Table 2.
Component matrix from factor analysis of BAT-L assessments from 428 participants in the TRACTS study. Components are reported after varimax rotation. Heavily loaded weightings are shaded grey.
2.5. Clinical assessment
Participants underwent a clinical assessment as described by McGlinchey et al. (2017) as part of their enrollment in the TRACTS study. This assessment included a CAPS IV (Blake et al., 2006) to assess their current severity of PTSD symptoms. The CAPS interview was also used to determine if participants met criteria for a current diagnosis of PTSD, using DSM IV criteria. Additionally, PCL ratings (Weathers et al., 1993) were acquired for each participant if their last PTSD assessment was >30 days old. Clinical assessments also included the DASS (Henry and Crawford, 2005) and the SCID (First, et al.; 1996), which addressed depression, anxiety, and substance use; the LDH (Skinner and Sheu, 1982) to assess history of alcohol use; and the DRRI (King et al., 2006) to assess combat experience.
2.6. Statistical analysis
Associations between T807 uptake and head trauma history were assessed in multiple ways. First, at the regional, voxel-wise, and vertex-wise levels, SUVRs were compared to head trauma factor scores using general linear models. At the vertex-wise and voxel-wise levels (that is, on the cortical surface and in the volume, respectively), potentially relevant covariates, including age, PCL, DASS-Depression score, and weight-corrected LDH were also included in the model to assess if any other prevalent clinical or demographic factor in this population was better able to explain the effects than the head trauma measures.
The reference region we utilized to generate the SUVRs was non-standard, and so we performed an additional analysis for validation. We confirmed the spatial patterns of uptake identified in SUVR analyses through the use of ICA. SUVs from each subject (without taking a ratio) were concatenated into a participant series and submitted to FSL's melodic tool for ICA (Beckmann and Smith, 2004), resulting in a set of maps describing each component, and a component score for each participant associated with each component. After the initial ICA analysis, components likely to be noise were identified by assessing correlations between component scores and known nuisance factors including age, administered tracer dose, motion during the scan, and specific activity of the dose. Components identified as likely noise were then regressed from the participant series and the resulting ‘denoised’ series was resubmitted to the ICA and compared to factors of head trauma. This approach is commonly used in functional MRI analyses, and it has also been used to assess subject-level variation in PET data (Di, and Biswal and Alzheimer's Disease Neuroimaging Initiative, B.B., 2012).
For each analysis, corrections for multiple comparisons were applied. In the cortical region analysis, rates of significant associations were compared to the expected rate of chance associations (3.2 cortical regions out of 64). For the vertex-wise and voxel-wise analysis, false detection rate corrections were applied using AFNI. Finally, in the ICA, Bonferroni corrections were applied to the 12 head trauma comparisons (4 components × 3 factors).
3. Results
3.1. Participant characterization
As expected based on findings in the larger TRACTS cohort, (McGlinchey et al., 2017) the Veterans in our study with (and without) military-related head trauma also presented with other psychiatric and behavioral conditions, and multiple instances of blunt or blast trauma were common. Participants who completed the PET session are described in Table 1.
Table 1.
Characterization of the participants included in this study, including head trauma history, as assessed by the BAT-L, and other relevant clinical and psychiatric variables assessed near the time of their PET scan or during their participant in the TRACTS study. The visit where the assessment was collected is indicated in the table.
| Traumatic brain injury/Head trauma | Mean ± St.Dev. (Range) | % Endorsed |
|---|---|---|
| Number of Moderate/Severe TBI | 0.06 ± 0.24 (0–1) | 5.88% |
| Number of Military Blunt TBI | 0.59 ± 0.79 (0–2) | 41.18% |
| Number of Civilian Blunt TBI | 0.71 ± 0.91 (0–3) | 47.06% |
| Number of Military Blast TBI | 0.53 ± 0.62 (0–2) | 47.06% |
| Number of Blast Exposures within 0–10 m | 3.47 ± 8.35 (0−30) | 41.18% |
| Number of Blast Exposures within 11–25 m | 3.76 ± 12.27 (0–51) | 29.41% |
| Number of Blast Exposures within 26–100 m | 56.53 ± 153.60 (0–501) | 70.59% |
| PTA combined time (minutes) | 735 ± 2430 (0–10,020) | 58.82% |
| AMS combined time (minutes) | 861 ± 2431 (0–10,020) | 76.47% |
| LOC combined time (minutes) | 1 ± 1.96 (0–7) | 29.41% |
| BAT-L Lifetime TBI Total Score | 3.24 ± 2.56 (0–9) | – |
| Behavioral/Psychiatric | Mean ± St.Dev. (Range) | % Diagnosed |
|---|---|---|
| CAPS Score (TRACTS Visit) | 40.47 ± 27.90 (7–93) | 47.06% |
| PCL (Within 30 days of PET scan) | 35.35 ± 13.81 (17–65) | – |
| DASS/SCID Depression/Mood (TRACTS Visit) | 9.13 ± 10.30 (0–28) | 29.41% |
| DASS/SCID Anxiety (TRACTS Visit) | 4.25 ± 4.72 (0–14) | 5.88% |
| DASS Stress (TRACTS Visit) | 12.13 ± 7.67 (0–28) | |
| SCID Substance Use Disorder (TRACTS Visit)⁎ | – | 0% |
| Lifetime Drinking History (TRACTS Visit) | 1227 ± 1258 (64–3947) | – |
| DRRI Combat Score (TRACTS Visit) | 16.73 ± 11.73 (2–46) | – |
Note: Many participants in the PET study were interested in completing other study-associated procedures that required a drug screen. Participants were informed of the drug screen prior to enrollment, and so participants from the TRACTS study with Substance Use Disorder were less likely to enroll.
3.2. Head trauma factors
The sample of participants able to enroll in the PET study was not large enough to examine all aspects of the complex lifetime history of head trauma assessed in the BAT-L interview. Thus, a factor analysis was employed for dimension reduction. Three factors resulted in an eigenvalue >1.0. We named these factors as follows: Factor 1 - Blast Exposure, Factor 2 - Symptom Duration, and Factor 3 - Blunt Concussion. Weightings of each input variable from the BAT-L (Fortier et al., 2014) on each factor are described in Table 2. These factor weightings, which were calculated in the larger TRACTS cohort, were applied to the assessment of head trauma history from the BAT-L nearest to the PET scan. This resulted in each PET study participant having a beta weight for each of the head trauma factors.
3.3. T807 measurements
T807/AV-1451 has been most commonly utilized in studies of older adults in order to study dementia (Johnson et al., 2016; Marquié et al., 2017). Few studies (Schöll et al., 2016) have examined the tracer's behavior in individuals in the age range of the participants in our study, and so we present images of the data collected for two representative young participants, with different levels of head trauma history (Fig. 1).
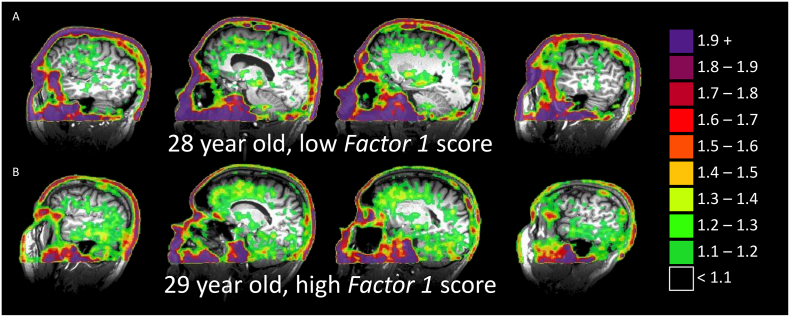
Fig. 1.
SUVR (using isthmus cingulate as reference) of two representative participants. Note high uptake in skin and eyes, similar to that reported in older adults, but strong nonspecific binding in midbrain is not observed. Representative participants with low score (A) and a high score (B) on Factor 1- Blast Exposure. Uptake for the participant in (A) is generally consistent with that presented for participants aged 20–26 by Schöll et al. (2016).
3.4. Association between SUVR of T807 and head trauma
Ratios of standard uptake values, using the isthmus of the cingulate as a reference, were averaged in grey matter parcellations as defined by FreeSurfer. No cortical regions had significant associations with age, probably due to the relatively young age of the cohort. Cortical SUVRs were then compared to the three head trauma factors. We found that 47 out of 66 (71%) of cortical regions defined by FreeSurfer had significant (r > 0.483, p < .05) correlations to Factor 1 – Blast Exposure beta weights, which is many more regions than would be expected due to chance alone. For the remaining two factors (Factor 2 - Symptom Duration and Factor 3 – Blunt Concussion), associations were not observed above the level expected by chance. None of the 66 regions showed significant correlation between T807 uptake and Symptom Duration beta weights, and 3 out of 66 regions (4.5%) showed correlation with Blunt Concussion beta weights. Regions that were correlated with the Blast Exposure factor are displayed in Fig. 2.
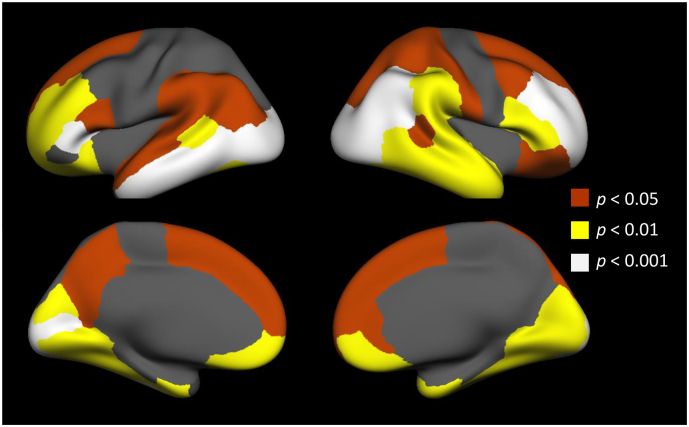
Fig. 2.
SUVR was sampled in the cortex and evaluated for associations with the head trauma factors. Of the 66 cortical regions defined, 47 were correlated with Factor 1- Blast Exposure. Significant regions are displayed, with colors indicating significance level.
As previous work has demonstrated blast-associated histological findings specific to the grey-white matter boundary (Shively et al., 2016), we explored this association further by additionally sampling activity over thinner layers of the cortex at three different cortical depths: in white matter 0.5–0.2 mm below the grey-white boundary, in grey matter from 0 to 30% through the cortical ribbon (that is, in grey matter near the grey-white boundary), and in grey matter from 50 to 80% through the surface. We assessed correlations to Factor 1 – Blast Exposure and found that correlations in grey matter were stronger near the pial surface than near the white matter surface (paired t-test, t67 = 10.27 p < .0001) and stronger in the cortex near the white matter than in the white matter just below the surface (paired t-test, t67 = 7.16, p < .0001). The mean correlation to the Blast Exposure factor in all regions was 0.391 in the white matter, 0.4461 in the deep grey matter, and 0.541 in the superficial grey matter. Due to the resolution of our acquisition, measurement of uptake at these levels may by strongly affected by partial volume averaging, although there is no reason to expect partial volume effects to be correlated with head trauma.
At the voxel- and vertex-level (that is, registered in the volume and to the cortical surface, respectively), we find similar correlation patterns, with occipital and frontal cortical uptake, as well as with uptake in the cerebellar grey matter. Maps of associations (while co-varying for age) are shown in Fig. 3. Similar to the regional analysis, only Factor 1 – Blast Exposure predicted T807 uptake, with the other two factors showing no associations above the false detection rate-corrected threshold (q ≥ 0.9999 for Factor 2 – Symptom Duration and q ≥ 0.9998 for Factor 3 – Blunt Concussion). A noteworthy feature of the statistical map showing the association between Factor 1 – Blast Exposures and ligand uptake is that statistical peaks appear to be concentrated at the crests of the gyri. Similar to the analysis of cortical depth, because this tracer binds strongly to skin, there may be partial volume effects at the crests of the gyri, but there is no reason to expect those effects to be associated with history of head trauma.
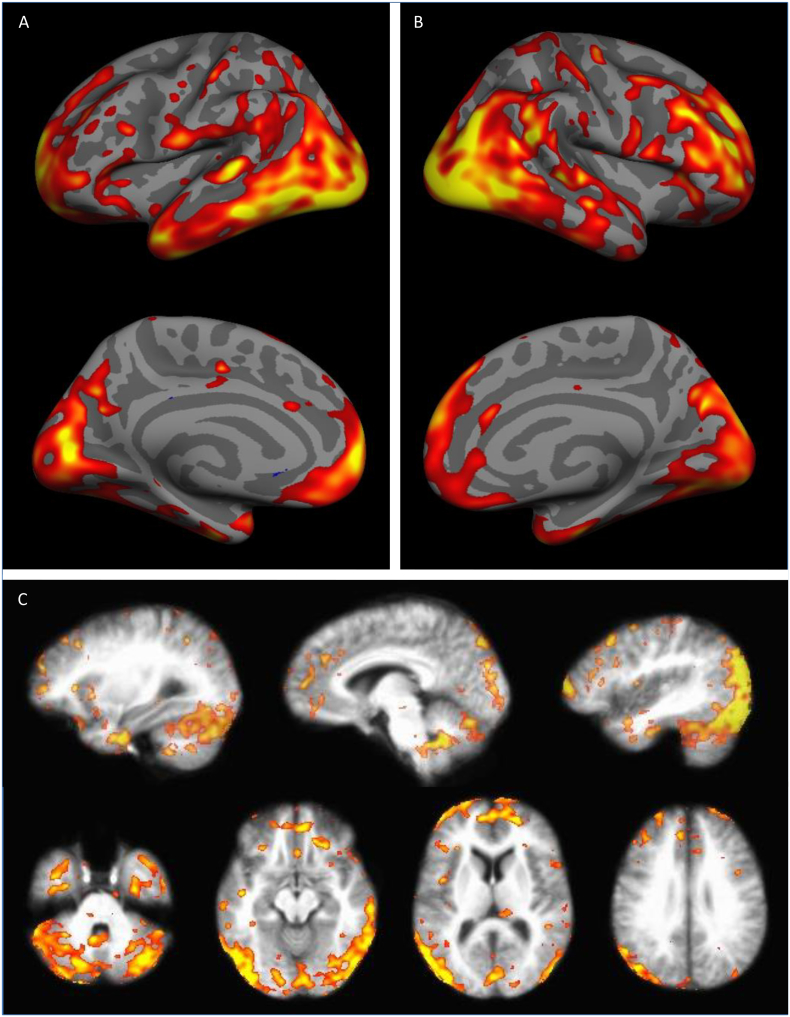
Fig. 3.
Vertex-wise (A and B) and Voxel-wise (C) correlations between T807 uptake and Factor 1 – Blast Exposure. Images displayed are t-statistics thresholded at q = 0.05 (FDR correction). Maximum (yellow) values are 5.0 for all images. Minimum values are as follows: left hemisphere = 2.239; right hemisphere = 2.149; volume = 3.010. For the remaining two factors (Factor 2 – Symptom Duration and Factor 3 – Blunt Concussion) no voxels/vertices survived the correction for multiple comparisons.
3.5. Military-related comorbidities
Military service is associated with not only high risk for head trauma, but additionally, multiple comorbid medical, behavioral, and neuropsychiatric conditions. One of the strengths of the TRACTS study is the extensive characterization of the Veteran participants, which allows us to assess the potential influence of other moderating or mediating conditions on our results. We compared T807 SUVRs to Factor 1- Blast Exposure beta weights while controlling for PCL score to assess the potential contribution of PTSD symptoms on the associations described above. We found that PCL was not significantly correlated, and that Factor 1 was still correlated with a similar map to that shown. Similarly, DASS-Depression score was not significantly correlated, and there were still significant associations to Factor 1 when Depression was included in the model. Factor 1 and weight-corrected LDH score were correlated (r = 0.538, p < .039), and when the general linear model includes both LDH and Factor 1, neither is significant, but the blast exposure factor has a larger effect size than LDH. Thus, no military-related psychiatric factors were better able to explain T807 uptake than Factor 1 – Blast Exposure.
3.6. Independent components analysis
Scans from all participants were submitted to an ICA using FSL's melodic tool (Beckmann and Smith, 2004). This technique identifies brain areas that covary across participants, and importantly, does not require a reference region. The purpose of this analysis is largely confirmatory, as we utilized a non-standard reference region for our SUVR analyses described above. Using this technique, we identified 9 components that described 75% of the participant-level variation. Each participant was assigned a weighting for each component, and these correlations between weightings and nuisance variables were then used to identify components associated primarily with variance that is irrelevant to our questions. Five of the nine components were identified as noise, and were regressed from the participant series using FSL's fsl_regfilt. These components were removed because they correlated significantly to one or more of the following variables: age, dose of tracer administered, or total motion over the frame. Correlations to specific activity of the administered radiotracer were also tested, but no components were found to be significantly associated. The resulting series, after regression of noise components, was resubmitted to the ICA analysis and four components explaining 60% of the variance were calculated to be optimal (see Fig. 4 and Supplemental Figs. 1 and 2). Post-hoc analysis, while controlling for age, revealed that the first of these components (which accounted for 17.85% of total variance after denoising) was associated with the Factor 1- Blast Exposure (GLM β = 0.13, z = 3.96, p < .00004). Similar post-hoc analyses showed no significant relationships between the four ICA-derived components and the Symptom Duration or Blunt Concussion factors after correcting for multiple comparisons (Bonferroni, threshold p < .0042). Component 2 was associated with Factor 3 - Blunt Concussion at p < .03, and thus may merit further study in a larger sample.
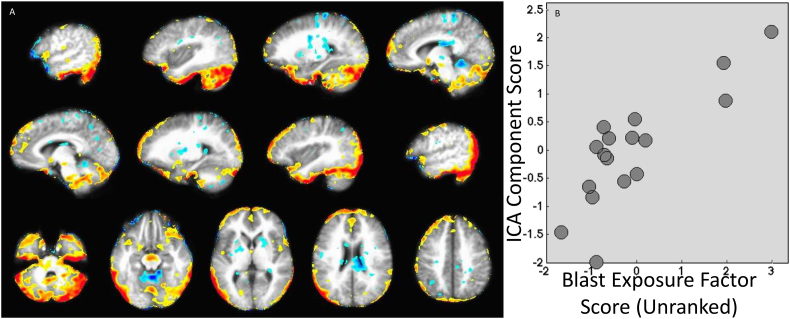
Fig. 4.
Results of Independent Components Analysis (ICA). After denoising, four components were an ideal fit for the data. The first component and its significant associations are shown here. Results indicate that, with increasing beta weight on Factor 1 – Blast Exposure, tracer uptake appears more like the map shown (e.g., with increasing numbers of blasts, more tracer uptake is expected in the inferior cerebellum and lateral occipital regions, but less in the anterior lobe of the cerebellum). Panel B shows a scatter plot of ICA component scores and beta weights for Factor 1 – Blast Exposure. The remaining three components from the ICA analysis and the remaining 11 scatter plots comparing component scores to factor beta weights are included in the supplemental materials. Note: The scatter plot displays the unranked factor beta weights, although statistics were completed using rank transforms.
The ICA component was then used to create a mask of regions with a z-score of 2.0 or higher, and the average SUVR was taken for each participant within the mask. For most participants, the average SUVR was between 0.8 and 1.1. For those participants with the highest beta weights in Factor 1 – Blast Exposure, SUVRs were between 1.1 and 1.35. As expected, the correlation between Factor 1 – Blast Exposure and SUVR from the masked region was strong (p < .0001).
4. Discussion
The data presented in this report suggest that in this small, relatively young cohort of Veterans, elevated T807/AV-1451 PET signal is associated with exposure to blast-related head trauma, but not to blunt traumas or duration of TBI symptoms. These elevated signals were primarily found in frontal, occipital and cerebellar brain regions. Although tau has been observed in post-mortem brains of young adults with history of blunt head trauma, (Geddes et al., 1999; Williams and Tannenberg, 1996) to our knowledge, this is the first study to report in vivo evidence of elevated tau associated with history of blast trauma.
4.1. Head trauma
Our analyses revealed that, of the three independent factors optimally accounting for variance in the BAT-L assessment of lifetime head trauma exposure, the Blast Exposure factor had a substantial relationship with T807 uptake, while the other two (Symptom Duration and Blunt Concussion) did not. Given our relatively modest sample size, it is possible that we were simply underpowered to detect associations between T807 and Blunt Concussion or Symptom Duration factors, however, we can say that the sizes of those effects, if they exist, are considerably smaller than the effect of Blast Exposure.
The observation that the effects of blast trauma are different than that of blunt trauma is consistent with other findings in the neuroimaging literature. Studies of military Veterans have suggested that blunt concussions produce different effects on the brain than blast concussions (Fischer et al., 2014) or even blast exposures (Bazarian et al., 2012a, Bazarian et al., 2012b; Robinson et al., 2015a; Taber et al., 2015; Trotter et al., 2015). While it may have been expected that number of symptomatic TBIs or the severity of TBI symptoms would be related to tracer uptake, studies in contact sport cohorts demonstrate that acute concussion symptoms are poor predictors of MR-visible brain changes as well (Abbas et al., 2014; Bazarian et al., 2012b; Breedlove et al., 2012; Johnson et al., 2014;Bailes et al., 2013; Poole et al., 2014; Robinson et al., 2015b; Talavage et al., 2014). We note that in our PET study cohort, Factor 1 – Blast Exposure is correlated at 0.90 or above with (log transformed) total number of military blasts, total number of close-range blasts (that is, self-report of blasts within 10 m) and close-to-mid-range blasts (self-report of blasts within 25 m). Thus, any of these elements of the blast exposure history are equally likely to be driving the findings of our study. While it may be instructive to more precisely determine which aspects of blast trauma history predict T807 uptake, that is not possible in our sample at this time. Another interesting analysis would be the comparison between blast-exposed participants and controls without blast exposure or history of TBI. However, this is relatively rare among OEF/OIF/OND Veterans and that is reflected in our sample. Only two such controls were able to be recruited into the study.
4.2. Anatomic distribution
Of note, the anatomical distribution of the significant associations between blast exposures and ligand uptake does not match those described in histopathology studies for either CTE or Alzheimer's Disease. It also does not appear to match that reported in a PET study of individuals at high risk for CTE (Barrio et al., 2015). However, it does correspond relatively well to reports of diffusion imaging changes associated with blast neurotrauma (Davenport et al., 2012; Mac Donald et al., 2011). We do not know that blast-related trauma would be expected to lead to a condition that is strictly analogous to CTE as identified in post-mortem studies of contact sports athletes (McKee and Robinson, 2014); it is difficult to know if the biological processes arising from repetitive subconcussive injuries (such as those implicated in CTE) (Baugh et al., 2012) are similar to those arising from blasts (Salat et al., 2017). Further, total exposure to neurotrauma may differ across in these cohorts: contact athletes may encounter over 1000 head impacts over a year (Bailes et al., 2013) and continue this for many years, while exposure to >100 blasts is relatively rare over the course of a military career (McGlinchey et al., 2017).
The mechanisms that might precipitate different outcomes from blunt and blast injuries are unclear. One possible mechanism is cavitation (Nakagawa et al., 2011), where a blast pressure wave compresses bubbles of blood gasses that re-expand explosively as the pressure wave subsides. Computer simulations of head-on blasts predict distributions of cavitation injuries (Goeller et al., 2012) similar to the distributions of PET ligand uptake we have observed. However, we do not know that the blasts experienced by this cohort were head-on. We find that the statistical power of the association is stronger when the images from each participant are aligned by matching sulcal patterns (that is, while using surface-based registration), which could indicate a role for local anatomy in determining the distribution of tau. Further, Blast Exposure-T807 associations are generally more prevalent on the gyri than in the sulci, which is inconsistent with histopathology studies of CTE (McKee et al., 2015), and appear to be strongest in cortical layers most distant from white matter. If cavitation is a prevalent mechanism driving the distribution of effects, that may explain why associations were more likely to be observed nearer to the CSF (on the gyri and at the superficial layers of the cortex) although our image resolution may not be sufficient to make a strong claim to that effect. There is also some correspondence between distribution of Blast Exposure–T807 associations and territories served by the anterior and posterior, but not middle, cerebral arteries. While the reasons for this are unclear, work by (Zarrinkoob et al., 2015) notes that these territories differ in blood volume and redundant collateralization. Future studies will be necessary to understand the role of vascular anatomy in the distribution of tau accumulation.
4.3. Considerations for PET tracer
To date, the tracer utilized in this study, T807/AV-1451 (Flortaucipir) has been primarily utilized in the study of neurodegenerative processes in older adults with and without clinical evidence of neurological disorder (Johnson et al., 2016). Although the behavior of this tracer in younger adults is not as well characterized, uptake of the tracer in young adults has been published previously (Schöll et al., 2016). Consistent with this previous work, we generally see non-specific uptake in the thalamus and basal ganglia only in our older participants and in nuisance maps of age effects (data not shown). Importantly, in older adults there has been histopathological confirmation that increased T807 binding corresponds to deposition of tau, and not to other proteins that may also bind to the tracer (Marquié et al., 2015); such work has not, however, been done in a younger cohort such as that studied here. Thus, we cannot rule out the possibility that increased uptake is related to phenomena other than accumulation of tau, such as binding with other chemicals (e.g., MAO-A or MAO-B), changes in the clearance rate of the tracer, or blood brain barrier compromise. Off-target labeling in the dural venous sinuses has been reported (Lemoine et al., 2018), and is of particular concern given the distribution of tracer uptake we observe. Additionally, individual differences in blood flow related to head trauma could produce systematic changes in tracer uptake. We briefly explored this possibility using arterial spin labeling data collected in the same session as the PET data, and present our findings in the supplemental materials. We find no evidence that tracer uptake is being driven by differences in perfusion. However, this and other possibilities must be evaluated in future work utilizing corroborating biomarkers to identify if tau, specifically, is responsible for observed uptake differences. Also of note, even in areas that were identified as being most likely associated with blast neurotrauma, SUVR values were modest at 1.1–1.35, and considerably less than those seen in cases of neurodegenerative disorders. As such, we cannot confidently state that the increase in T807 uptake observed in our study is indicative of CTE or CTE-like pathology. Rather, we can state that there are neurophysiological brain changes associated with blast neurotrauma (and independent of blunt trauma) that merit further study.
One caveat in the interpretation of these results is the type of tau protein we may be able to detect. T807 has been shown to have different binding potential for PHF tau and straight filament tau (Marquié et al., 2015). While much of the characterization of the tracer has taken place in Alzheimer's disease, which has prevalent PHF tau, we do not know that trauma-induced tau accumulation would have similar prevalence of PHF tau over straight filament tau, so we do not know the extent to which the sensitivity of the tracer to PHF versus straight tau affects our results. However, this would serve to decrease the likelihood of finding associations with head trauma or underestimate our effect size, and is unlikely to result in false positives.
In other applications, the cerebellum has been validated as a reference region for T807. (Wooten et al., 2017) In our cohort, we saw high uptake in the cerebellum, making it a poor choice for a reference. There is no a priori reason to expect the cerebellum to be free of tau in trauma-related neurodegenerations—in fact, cerebellar involvement is consistent with other neuroimaging findings in military blast populations (Mac Donald et al., 2011). Instead we conducted SUVR analyses using the isthmus cingulate as a reference region. While the isthmus cingulate appears to be a robust reference region in this sample, it is known to show tau accumulation in many neurodegenerative disorders seen in older adults. Thus, it may not be a suitable reference region for similar studies in older cohorts. For our study, if the isthmus cingulate has tau, this would be likely to underestimate our effects rather than create spurious findings. The isthmus cingulate was chosen because it is relatively spared in post-mortem studies of CTE (McKee et al., 2013; McKee et al., 2015) and appeared to be among the regions with the lowest uptake across participants. While there may be some question to the appropriateness of using the isthmus cingulate as a reference, the ICA analysis confirmed the spatial patterns of uptake associations that we saw. The ICA analysis identified spatial patterns of T807 uptake variability that were consistent across individuals. Similar techniques have been used in other PET studies, (Di, and Biswal and Alzheimer's Disease Neuroimaging Initiative, B.B., 2012; Vogel et al., 2017) and are common in functional MRI (Beckmann et al., 2009; Calhoun et al., 2009). Future studies utilizing T807 in older Veterans with blast trauma, where age-related tau accumulation in the cingulate may prove problematic, could potentially utilize a similar strategy.
4.4. Conclusions
In summary, we present evidence of tau accumulation associated with blast neurotrauma in OEF/OIF/OND Veterans. Of note, T807 uptake was associated with number of blast exposures, and not associated with number of blunt TBIs or duration of concussion symptoms. Further work will be necessary to confirm these findings as the tracer used in this study is relatively new and its use in participants of this age group is sparse. Investigation of changes in tracer uptake across time in relation to the evolution of functional deficits may also be informative.
Acknowledgments
Acknowledgements
The authors would like to acknowledge Jennifer Fonda, PhD for her statistical expertise; Ariye Krassner, Grae Arabasz, Shirley Hsu, and Regan Butterfield for their assistance with imaging data acquisition; Pedram Parva, MD, Catherine Fortier, PhD, and Colleen Jackson, PhD for clinical evaluations; and the TRACTS team for all their hard work. The contents do not represent the views of the U.S. Department of Veterans Affairs of the United States Government.
Funding
This work was supported by Merit Review Award (RX000991) and the TRACTS National Network Research Center for TBI research (B9254-C) from the United States Department of Veterans Affairs Rehabilitation Research and Development Service.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101651.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
References
- Abbas K., Shenk T., Poole V., Breedlove E., Breedlove K., Leverenz L., Nauman E., Talavage T., Robinson M. Alteration of default mode network in high school football athletes due to repetitive sub-concussive mTBI. Brain Connect. 2014;5:91–1014. doi: 10.1089/brain.2014.0279. [DOI] [PubMed] [Google Scholar]
- Bailes Julian E., Petraglia Anthony L., Omalu Bennet I., Nauman Eric, Talavage Thomas. Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 2013;119:1235–1245. doi: 10.3171/2013.7.JNS121822. [DOI] [PubMed] [Google Scholar]
- Barrio J.R., Small G.W., Wong K.-P., Huang S.-C., Liu J., Merrill D.A., Giza C.C., Fitzsimmons R.P., Omalu B., Bailes J. In vivo characterization of chronic traumatic encephalopathy using [F-18] FDDNP PET brain imaging. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E2039–E2047. doi: 10.1073/pnas.1409952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh C.M., Stamm J.M., Riley D.O., Gavett B.E., Shenton M.E., Lin A., Nowinski C.J., Cantu R.C., McKee A.C., Stern R.A. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6:244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- Bazarian J., Donnelly K., Peterson D., Warner G., Zhu T., Zhong J. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J. Head Trauma Rehabil. 2012;28:1–12. doi: 10.1097/HTR.0b013e318256d3d3. [DOI] [PubMed] [Google Scholar]
- Bazarian J.J., Zhu T., Blyth B., Borrino A., Zhong J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn. Reson. Imaging. 2012;30:171–180. doi: 10.1016/j.mri.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Mackay C.E., Filippini N., Smith S.M. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. NeuroImage. 2009;47:S148. [Google Scholar]
- Belmont P.J., Jr., McCriskin B.J., Sieg R.N., Burks R., Schoenfeld A.J. Combat wounds in Iraq and Afghanistan from 2005 to 2009. J. Trauma Acute Care Surg. 2012;73:3–12. doi: 10.1097/TA.0b013e318250bfb4. [DOI] [PubMed] [Google Scholar]
- Blake D., Weathers F., Nagy L., Kaloupek D., Gusman F., Charney D., Keane T. The development of a clinician-administered PTSD scale. J. Trauma. Stress. 2006;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Breedlove E., Robinson M., Talavage T., Morigaki K., Yoruk U., O'Keefe K., King J., Leverenz L., Gilger J., Nauman E. Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J. Biomech. 2012;45:1265–1272. doi: 10.1016/j.jbiomech.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adali T.l. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien D.T., Bahri S., Szardenings A.K., Walsh J.C., Mu F., Su M.-Y., Shankle W.R., Elizarov A., Kolb H.C. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J. Alzheimers Dis. 2013;34:457–468. doi: 10.3233/JAD-122059. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davenport N.D., Lim K.O., Armstrong M.T., Sponheim S.R. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. NeuroImage. 2012;59:2017–2024. doi: 10.1016/j.neuroimage.2011.10.050. [DOI] [PubMed] [Google Scholar]
- Di X., Biswal and Alzheimer's Disease Neuroimaging Initiative, B.B Metabolic brain covariant networks as revealed by FDG-PET with reference to resting-state fMRI networks. Brain Connect. 2012;2:275–283. doi: 10.1089/brain.2012.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. 1996. Structured Clinical Interview for the DSM-IV Axis I Disorders. [Google Scholar]
- Fischer B.L., Parsons M., Durgerian S., Reece C., Mourany L., Lowe M.J., Beall E.B., Koenig K.A., Jones S.E., Newsome M.R. Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J. Neurotrauma. 2014;31:169–179. doi: 10.1089/neu.2013.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fortier C.B., Amick M.M., Grande L., McGlynn S., Kenna A., Morra L., Clark A., Milberg W.P., McGlinchey R.E. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: evidence of research utility and validity. J. Head Trauma Rehabil. 2014;29:89–98. doi: 10.1097/HTR.0b013e3182865859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes J., Vowles G., Nicoll J., Revesz T. Neuronal cytoskeletal changes are an early consequence of repetitive head injury. Acta Neuropathol. 1999;98:171–178. doi: 10.1007/s004010051066. [DOI] [PubMed] [Google Scholar]
- Goeller J., Wardlaw A., Treichler D., O'Bruba J., Weiss G. Investigation of cavitation as a possible damage mechanism in blast-induced traumatic brain injury. J. Neurotrauma. 2012;29:1970–1981. doi: 10.1089/neu.2011.2224. [DOI] [PubMed] [Google Scholar]
- Henry J.D., Crawford J.R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005;44:227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., Castro C.A. Mild traumatic brain injury in US soldiers returning from Iraq. N. Engl. J. Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Huber B.R., Meabon J.S., Martin T.J., Mourad P.D., Bennett R., Kraemer B.C., Cernak I., Petrie E.C., Emery M.J., Swenson E.R. Blast exposure causes early and persistent aberrant phospho-and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J. Alzheimers Dis. 2013;37:309. doi: 10.3233/JAD-130182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Garcia D., Hansen A.E., F√∂rster S., Benoit D., Schachoff S., F√°rst S., Chen K.T., Chonde D.B., Catana C. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. J. Nucl. Med. 2014;55:1825–1830. doi: 10.2967/jnumed.113.136341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B., Neuberger T., Gay M., Hallett M., Slobounov S. Effects of subconcussive head trauma on the default mode network of the brain. J. Neurotrauma. 2014;31:1907–1913. doi: 10.1089/neu.2014.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K.A., Schultz A., Betensky R.A., Becker J.A., Sepulcre J., Rentz D., Mormino E., Chhatwal J., Amariglio R., Papp K. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.A., King D.W., Vogt D.S., Knight J., Samper R.E. Deployment Risk and Resilience Inventory: a collection of measures for studying deployment-related experiences of military personnel and veterans. Mil. Psychol. 2006;18:89. [Google Scholar]
- Koerte I.K., Ertl-Wagner B., Reiser M., Zafonte R., Shenton M.E. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA. 2012;308:1859–1861. doi: 10.1001/jama.2012.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladefoged C.N., Law I., Anazodo U., Lawrence K.S., Izquierdo-Garcia D., Catana C., Burgos N., Cardoso M.J., Ourselin S., Hutton B. A multi-Centre evaluation of eleven clinically feasible brain PET/MRI attenuation correction techniques using a large cohort of patients. NeuroImage. 2017;147:346–359. doi: 10.1016/j.neuroimage.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine L., Leuzy A., Chiotis K., E R.V., A N. Tau positron emission tomography imaging in tauopathies: the added hurdle of off-target binding. Alzheimers Dement. 2018;10:232–236. doi: 10.1016/j.dadm.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S., Snyder A.Z., Raichle M.E., Witherow J.R., Fang R., Flaherty S.F., Brody D.L. Detection of blast-related traumatic brain injury in US military personnel. N. Engl. J. Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquié M., Normandin M.D., Vanderburg C.R., Costantino I.M., Bien E.A., Rycyna L.G., Klunk W.E., Mathis C.A., Ikonomovic M.D., Debnath M.L. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann. Neurol. 2015;78:787–800. doi: 10.1002/ana.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquié M., Normandin M.D., Meltzer A.C., Siao Tick Chong M., Andrea N.V., Antón-Fernández A., Klunk W.E., Mathis C.A., Ikonomovic M.D., Debnath M. Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann. Neurol. 2017;81:117–128. doi: 10.1002/ana.24844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey R., Milberg W., Fonda J., Fortier C. A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudinal prospective cohort study. Int. J. Methods Psychiatr. Res. 2017;26(3):e1556–e1558. doi: 10.1002/mpr.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.C., Robinson M.E. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10:S242–S253. doi: 10.1016/j.jalz.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Daneshvar D.H., Alvarez V.E., Lee H.-S., Hall G., Wojtowicz S.M., Baugh C.M., Riley D.O., Kubilus C.A., Cormier K.A., Jacobs M.A., Martin B.R., Abraham C.R., Ikezu T., Reichard R.R., Wolozin B.L., Budson A.E., Goldstein L.E., Kowall N.W., Cantu R.C. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.C., Stein T.D., Kiernan P.T., Alvarez V.E. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–364. doi: 10.1111/bpa.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer J., Van Duijn C., Chandra V., Fratiglioni L., Graves A., Heyman A., Jorm A., Kokmen E., Kondo K., Rocca W. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. Int. J. Epidemiol. 1991;20:S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- Nakagawa A., Manley G.T., Gean A.D., Ohtani K., Armonda R., Tsukamoto A., Yamamoto H., Takayama K., Tominaga T. Mechanisms of primary blast-induced traumatic brain injury: insights from shock-wave research. J. Neurotrauma. 2011;28:1101–1119. doi: 10.1089/neu.2010.1442. [DOI] [PubMed] [Google Scholar]
- Omalu B., Hammers J.L., Bailes J., Hamilton R.L., Kamboh M.I., Webster G., Fitzsimmons R.P. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg. Focus. 2011;31:E3. doi: 10.3171/2011.9.FOCUS11178. [DOI] [PubMed] [Google Scholar]
- Poole V., Abbas K., Shenk T., Breedlove E., Breedlove K., Robinson M., Leverenz L., Nauman E., Talavage T., Dydak U. MR Spectroscopic evidence of brain injury in the non-diagnosed collision sport athlete. J. Dev. Neuropsychol. 2014;39:459–473. doi: 10.1080/87565641.2014.940619. [DOI] [PubMed] [Google Scholar]
- Robinson M.E., Lindemer E.R., Fonda J.R., Milberg W.P., McGlinchey R.E., Salat D.H. Close-range blast exposure is associated with altered functional connectivity in Veterans independent of concussion symptoms at time of exposure. Hum. Brain Mapp. 2015;36:911–922. doi: 10.1002/hbm.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.E., Shenk T.E., Breedlove E.L., Leverenz L.J., Nauman E.A., Talavage T.M. The role of location of subconcussive head impacts in fMRI brain activation changes. J. Dev. Neuropsychol. 2015;40:74–79. doi: 10.1080/87565641.2015.1012204. [DOI] [PubMed] [Google Scholar]
- Salat D.H., Robinson M.E., Miller D.R., Clark D.C., McGlinchey R.E. Neuroimaging of deployment-associated traumatic brain injury (TBI) with a focus on mild TBI (mTBI) since 2009. Brain Inj. 2017;31(9):1204–1219. doi: 10.1080/02699052.2017.1327672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöll M., Lockhart S.N., Schonhaut D.R., O'Neil J.P., Janabi M., Ossenkoppele R., Baker S.L., Vogel J.W., Faria J., Schwimmer H.D. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively S.B., Horkayne-Szakaly I., Jones R.V., Kelly J.P., Armstrong R.C., Perl D.P. Characterisation of interface astroglial scarring in the human brain after blast exposure: a post-mortem case series. Lancet Neurol. 2016;15(9):944–953. doi: 10.1016/S1474-4422(16)30057-6. [DOI] [PubMed] [Google Scholar]
- Sivanandam T.M., Thakur M.K. Traumatic brain injury: a risk factor for Alzheimer's disease. Neurosci. Biobehav. Rev. 2012;36:1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Skinner H.A., Sheu W.J. Reliability of alcohol use indices. The Lifetime Drinking history and the MAST. J. Stud. Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Taber K., Hurley R., Haswell C., Rowland J., Hurt S., Lamar C., Morey R. White matter compromise in veterans exposed to primary blast forces. J. Head Trauma Rehabil. 2015;30:E15–E25. doi: 10.1097/HTR.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavage T.M., Nauman E.A., Breedlove E.L., Yoruk U., Dye A.E., Morigaki K.E., Feuer H., Leverenz L.J. Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma. 2014;31:327–338. doi: 10.1089/neu.2010.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Management of Concussion/mTBI Working Group . In: VA/DoD Clinical Practice Guildeline for Management of Concussion/Mild Traumatic Brain Injury (mTBI) Department of Veterans Affairs DoD, editor. 2009. Washington, DC. [Google Scholar]
- Trotter B.B., Robinson M.E., Milberg W.P., McGlinchey R.E., Salat D.H. Military Blast Exposure, Ageing, and White Matter Integrity. Brain. 2015;138:2278–2292. doi: 10.1093/brain/awv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.W., Mattsson N., Medina Y.I., Strandberg O., Schöll M., Dansereau C., Villeneuve S., van der Flier W.M., Scheltens P., Bellec P. Data-driven Tau-PET covariance networks enhance prediction of retrospective cognitive change in Alzheimer's disease. Alzheimers Dement. 2017;13:P1548–P1549. [Google Scholar]
- Weathers F.W., Litz B.T., Herman D.S., Huska J.A., Keane TM. 1993. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. annual convention of the international society for traumatic stress studies, San Antonio, TX. [Google Scholar]
- Williams D.J., Tannenberg A.E. Dementia pugilistica in an alcoholic achondroplastic dwarf. Pathology. 1996;28:102–104. doi: 10.1080/00313029600169653. [DOI] [PubMed] [Google Scholar]
- Wooten D.W., Guehl N.J., Verwer E.E., Shoup T.M., Yokell D.L., Zubcevik N., Vasdev N., Zafonte R.D., Johnson K.A., El Fakhri G. Pharmacokinetic evaluation of the tau PET radiotracer 18F-T807 (18F-AV-1451) in human subjects. J. Nucl. Med. 2017;58:484–491. doi: 10.2967/jnumed.115.170910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C.-F., Arteaga J., Chen G., Gangadharmath U., Gomez L.F., Kasi D., Lam C., Liang Q., Liu C., Mocharla V.P. [18 F] T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement. 2013;9:666–676. doi: 10.1016/j.jalz.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Zarrinkoob L., Ambarki K., W√•hlin A., Birgander R., Eklund A., Malm J. Blood flow distribution in cerebral arteries. J. Cereb. Blood Flow Metab. 2015;35:648–654. doi: 10.1038/jcbfm.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2