Abstract
Background
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease, which exhibits multiple B cell abnormalities including expanded populations of memory B cells and elevated levels of autoantibodies. Belimumab is a monoclonal antibody targeting the B cell cytokine BAFF (a.k.a. BLyS), approved for the treatment of SLE.
Methods
In this prospective cohort study, B cells from peripheral blood of 23 SLE patients initiating belimumab treatment and followed longitudinally for up to three years, were assessed using mass cytometry.
Findings
B cells decreased during the study period, with a rapid decrease of both naïve and CD11c+CD21− B cells at the first follow-up visit, followed by a continuous reduction at subsequent follow-ups. In contrast, plasma cells and switched memory B cells remained stable throughout the study. The observed immunological changes correlated with early, but not late, clinical improvements. Moreover, high baseline B cell counts were predictive of failure to attain low disease activity. In summary, our data unveiled both rapid and gradual later therapy-associated alterations of both known and unforeseen B cell phenotypes.
Interpretation
Our results suggest that evaluation of B cell counts might prove useful prior to initiation of belimumab treatment and that early treatment evaluation and discontinuation might underestimate delayed clinical improvements resultant of late B cell changes.
Keywords: Systemic lupus erythematosus, Biologics, BLyS, Mass cytometry, CyTOF
Research in context.
Evidence before this study
Belimumab is a recent biologic therapy, approved in 2011 for treating the autoimmune disease SLE. This agent neutralises the B cell cytokine BAFF and has been shown to primarily impact on naïve B cells. Different B cell subsets have been implicated in SLE and lately the so-called age (or autoimmunity)-associated B cells (ABCs) have been associated to autoantibody production and disease manifestations.
Added value of this study
We studied the B cell composition of SLE patients undergoing belimumab treatment, using a technique called mass cytometry where we measured ~30 cell markers and performed hypothesis-free analyses to gain an unbiased view of B cell alterations longitudinally (up to three years) after treatment start. We observed both rapid and slow changes in the B cell composition. Two types of B cell clusters tracked the pace of positive therapy response: surface IgA-positive B cells and CD11c+CD21−, and it is likely that they represent the so-called age (or autoimmunity)-associated B cells (ABCs).
Implications of all the available evidence
It may be possible to predict therapy response by studying B cells at base line, but therapy response may develop gradually. It is reassuring that the SLE-associated ABCs are also affected by BAFF-blockade given their implication in disease pathogenesis.
Alt-text: Unlabelled Box
1. Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous systemic autoimmune disease, characterised by considerable morbidity and premature mortality [1,2,3]. B cells are central in the pathogenesis and autoantibody formation to nuclear antigens is a hallmark of the disease [4]. Genetic associations also underline the importance of B cells [5], and levels of the B cell survival and maturation cytokine B lymphocyte stimulator (BLyS), also known as B cell activating factor belonging to the TNF family (BAFF) or TNFSF13B, are elevated in patients with SLE [6,7].
Belimumab (Benlysta, GlaxoSmithKline) is a recombinant human IgG1-λ monoclonal antibody that specifically binds to soluble BAFF and is approved for the treatment of active SLE despite ongoing standard of care treatment. Its efficacy has been demonstrated in two phase III randomised, placebo-controlled clinical trials [8,9], with anti-double stranded (ds)DNA antibody positive patients showing better responses [10].
Previous studies have demonstrated, by flow cytometry, that treatment with belimumab reduced CD20+ B cells, but did not affect T cells or pre-existing antibodies to pneumococcal and tetanus vaccines [11,12], and provide a first insight into the immunological effects of this biologic agent. In the current real-life prospective observational study, we sought to perform a deeper characterisation of B cell alterations following therapy by studying both earlier time points as well as a longer time span as compared to the clinical trials. Hereby we could discriminate early and late B cell alterations in patients with SLE during belimumab treatment and analyse these changes in relation to clinical responses. For this, we made use of mass cytometry (CyTOF), a multi-parametric, single-cell approach allowing the redefinition of cell subsets based on unbiased clustering and dimensionality reduction.
2. Material and methods
2.1. Subjects
Twenty-three patients with moderately active SLE despite standard of care treatment from the Karolinska University Hospital, in whom treatment with belimumab was initiated upon clinical judgement of the treating physician, were enrolled between 2011 and 2015. The patients received belimumab intravenously at weeks 0, 2, 4 and every fourth week thereafter, and were followed longitudinally with visits at baseline and at months 3, 6, 12, 24, 36 and 48, or more frequently if clinically indicated. All patients fulfilled the 1982 revised criteria [13] and the Systemic Lupus International Collaborating Clinics criteria [14] for classification of SLE. Baseline characteristics are presented in Table 1.
Table 1.
Patient characteristics.
| Sex; n = 23 | |
| Female; n (%) | 19 (82.6%) |
| Ethnicity; n = 23 | |
| Caucasian; n (%) | 22 (95.7%) |
| Black; n (%) | 1 (4.3%) |
| Age (years); M (IQR); n = 23 | 38.4 (30.4–50.3) |
| SLE disease duration (years); M (IQR); n = 23 | 7.7 (4.3–14.4) |
| SLEDAI-2K; M (IQR); n = 23 | 9 (7–15) |
| Prednisone equivalent dose (mg/day); M (IQR); n = 23 | 10.0 (5.0–10.0) |
| Previous exposure to corticosteroids (years); M (IQR); n = 23 | 6.8 (4.2–9.9) |
| Previous mean prednisone equivalent dose (mg/day); M (IQR); n = 23 | 10.0 (7.5–15.0) |
| Number of DMARDs at baseline; M (IQR); n = 23 | 1 (0–1) |
| Number of DMARDs ever; M (IQR); n = 23 | 3 (1–4) |
| Patients on antimalarials at baseline; M (IQR); n = 23 | 17 (73.9%) |
SLE: systemic lupus erythematosus; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; DMARDs: disease-modifying antirheumatic drugs (excluding antimalarials); M: median; IQR: interquartile range.
In addition, 19 patients with RRMS and ten healthy controls were enrolled between 2014 and 2015. The RRMS group consisted both of patients switching from a previous therapy (n = 11) and treatment naïve patients (n = 8). All treatment experienced patients switched from interferon-beta, except for one who switched from fingolimod with a 6 months washout. All patients were sampled both at baseline immediately before starting DMF and after six months. DMF was initiated with 120 mg BID for two weeks and then 240 mg BID for the remaining study period.
The regional ethics review board at Karolinska Institutet, Stockholm, Sweden approved the study protocol. Written informed consent in accordance with the declaration of Helsinki was obtained from all participants prior to enrolment.
2.2. Clinical evaluation
Disease activity was assessed by the SLE Disease Activity Index (SLEDAI) 2000 (SLEDAI-2K) [20], British Isles Lupus Assessment Group (BILAG) index [15,16], and Physician's Global Assessment (PGA) on a 100 mm visual analogue scale (VAS) [17] and according to the Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)-SLEDAI PGA (scored 0–3; [18]).
Response to treatment was defined in line with the SLE responder index (SRI; [19] as (i) a reduction of ≥4 points in SLEDAI-2K, (ii) no new BILAG A or no >1 new BILAG B, and (iii) no deterioration in PGA by ≥30 mm. Low disease activity was defined according to the Lupus Low Disease Activity State (LLDAS) as a SLEDAI-2K ≤ 4, no activity in major organ systems, no haemolytic anaemia or gastrointestinal activity, no new SLE activity compared with the previous assessment, a SELENA-SLEDAI PGA ≤ 1, a current prednisone equivalent dose ≤ 7.5 mg/day, and well tolerated standard maintenance doses of immunosuppressive drugs and approved biologic agents [20].
2.3. Cell analyses
Heparinised blood samples were prospectively collected at baseline and at all follow-up visits and peripheral blood mononuclear cells (PBMCs) were cryopreserved following Ficoll density gradient centrifugation. The PBMC samples were analysed by a CyTOF2 (Fluidigm Inc., South San Francisco, CA, USA) mass cytometer (cytometry by time-of-flight, CyTOF) and the CyTOF software version 6.0.626 with noise reduction using a lower convolution threshold of 200, event length limits of 10–150, a Sigma value of 3 and a flow rate of 0.045 mL/min. Two million thawed PBMCs from each sample were stained directly ex vivo using a panel of 30 different metal-tagged antibodies, the majority of them against B cell related proteins (Table S1). The procedure comprised two CyTOF2 runs: a pilot run including baseline and follow-up PBMC samples from five patients and a second run including samples from 18 patients. Cell counts were corrected by the absolute lymphocyte count at the respective visit by dividing with the number of informative B cells and T cells and multiplying with the number of informative cells for the cell type of interest.
Bead-based normalisation of CyTOF data was applied for correction of signal fluctuations [21]. Cells were gated by event length, DNA (0.125 μM Iridium 191/193 or MaxPar Intercalator-Iridium, Fluidigm), beads and viability (Cisplatin, Fluidigm). B cells were gated as CD20+CD3e−, plasma cells as CD19+CD38+CD27+CD20−, T cells as CD3e+CD20−, and monocytes as CD14+CD20−CD3e−.
Flow cytometry was performed for confirmatory purposes. Cryopreserved PBMC samples from one of the SLE patients (baseline) and a healthy control were thawed, and the cell suspensions were stained for 30 min at 4 °C in PBS containing 0.5% human serum with mouse anti-human monoclonal antibodies. The complete panel of antigens is presented in Table S2. Dead cells were excluded using 7-aminoactinomycin D (BioLegend Inc., San Diego, CA, USA). Flow cytometric analysis was carried out using an LSRFortessa cell analyser (BD Biosciences, San Jose, CA, USA), and data were processed using FlowJo software (FlowJo LLC, Ashland, OR, USA). To distinguish cells expressing an antigen from cells lacking expression of the respective antigen, the cut-off was determined by fluorescent minus one (FMO) controls [22].
2.4. Serologic markers
Anti-dsDNA antibodies were determined by the Crithidia luciliae substrate based immunofluorescence technique [23] and by addressable laser bead immunoassay (ALBIA), using the Connective profile MX 117 FIDIS kit (Theradiag, Paris, France).
2.5. Dimensionality reduction and cell subset clustering
For phenotypic B cell subset separation based on marker distributions, we performed Barnes-Hut t-distributed stochastic neighbour embedding (t-SNE) reducing high-dimensional phenotypes into a two-dimensional space, using the Automatic Classification of Cellular Expression by Nonlinear Stochastic Embedding (ACCENSE) software, with a perplexity value of 30 [24]. The PhenoGraph algorithm was used for clustering [25]. Each dot in the resulting t-SNE plot corresponds to one cell, and is coloured according to the expression of the indicated markers. Colour channels were assigned the value 0.2 + expression value (v)·0.8/maxv if v > 0, or 0.05 if v = 0 (maxv: the largest v for the marker in the plot). CMY colour space was converted to RGB using R = round(255·(1-C)), G = round(255·(1-M)) and B = round(255·(1-Y)). To perform principal component analysis, we added 0.1 to all values, log-transformed them and applied the R function prcomp.
2.6. Correlations of marker expression with time
Expression values were transformed to a new value (nv) using 2 + log2(min(0.25, original value)). For marker combinations, we calculated a combination value using nv(M1)·nv(M2) for the marker combination M1+M2+, and nv(M1)/nv(M2) for M1+M2−. Correlations with time on treatment were calculated using the Spearman's rank correlation coefficient (ρ). For the two-marker heat maps, we calculated |ρ(X+Y+,time)|-max(|ρ(X+Y−,time)|, |ρ(X−Y+,time)|). Hierarchical clustering for these heat maps used complete linkage based on 1-the difference calculated above as distance metric. We tested for differences in correlations between |ρ(X+Y+,time)| and max(|ρ(X+Y−,time)|, |ρ(X−Y+,time)|) using the paired.r function from the R psych package, with a P-value of 0.05 as the level of significance. The reason for subtracting the correlation for cells expressing only one of the markers in the pair was to avoid the clustering of many markers with the ones showing the strongest changes, e.g. to avoid that IgD+CD123+ inherits a strong change due to the expression of IgD rather than the combination. Benjamini-Hochberg correction for multiple comparisons was applied.
2.7. Statistical analyses
For comparisons of baseline cell counts between patient subgroups with regard to treatment response, we used the Mann-Whitney U test, and as a control for multiple testing, we randomised the patient-to-value assignment and ascertained that the resulting P-values were higher. Missing data were addressed by exclusion of the respective occasion from analysis; no assumption principle was applied. In cases where patients were lost to follow-up, they contributed in the analyses up until the last occasion. The SPSS Statistics 23 software (IBM Corp., Armonk, New York, USA) was used for baseline characteristics and receiver operating characteristic (ROC) curve analyses, the python package SciPy for correlation analyses, and the paired.r function from the R psych package for comparisons of correlations.
2.8. Data and software availability
Mass cytometry data and code which was used for analysis will be deposited at: https://github.com/danielramskold/B_cell_alterations_during_BAFF_inhibition_in_SLE (Github) and http://dx.doi.org/10.17632/7894wb6yxh.1 (Mendeley Data).
3. Results
3.1. Visualisation and clustering of B cell subsets
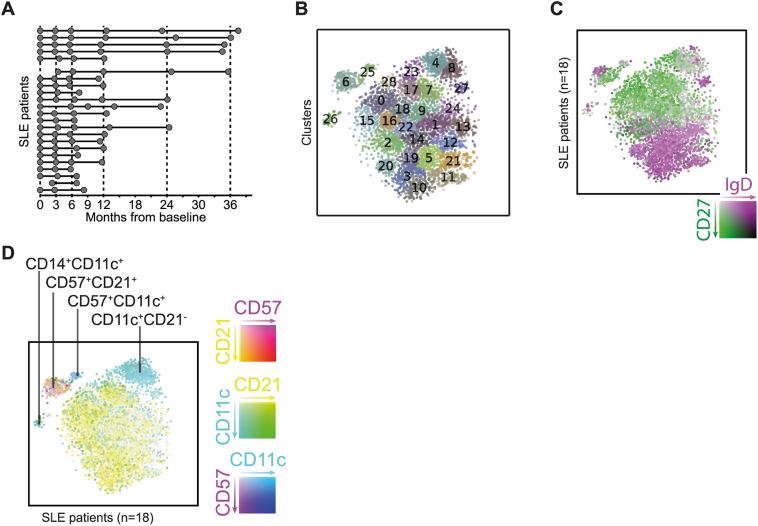
Longitudinally collected peripheral blood samples (n = 96) from 23 SLE patients undergoing BAFF neutralising treatment with belimumab were included in our study (Fig. 1A). We utilised mass cytometry and focused our analyses on CD20+ B cells. As a first step, we visualised the entire data set following t-SNE dimensionality reduction into two-dimensional space, which clustered cells with similar phenotypes in an unbiased manner and displayed the diversity of the B cells (Fig. 1B). The same dataset was also visualised based on the expression of IgD and CD27 (Fig. 1C), corresponding to naïve and memory B cells respectively. As expected, the SLE patient samples comprised of a large proportion of memory phenotype (CD27+) B cells (in green). This was strikingly different as compared to the healthy control sample (Fig. S1A) and again as expected. Overall and importantly, this first analysis illustrates how the clusters from Fig. 1B represent variations in expression patterns and not necessarily cell subsets.
Fig. 1.
B cell subset distribution. (A) Sampling timepoints, divided into two groups of 5 and 18 SLE patients by date of data generation. (B) t-distributed stochastic neighbour embedding (t-SNE) clustering of B cells from 18 SLE patients and all follow-up occasions (100 cells per sample). (C–D) Distribution of naïve (purple) and memory (green) B cells in the SLE patients (all follow-up occasions; B) and a healthy control (C). (E) A predominant CD57 and CD11c expression in two distinct clusters (upper right and upper left) is apparent. (F) The CD57+ cluster is absent in the healthy control. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The clusters characterised by a predominant CD11c expression evoked our interest from the t-SNE plots based on their position outside of the main naïve and memory subsets. One of these (#4 + 8; Fig. 1A and D, S1, S2 and S3) most probably corresponds to the B cell subtype designated as age-associated B cells [26,27,28], as it lacked CD21 expression and displayed low expression of CD27. Another small but distinct and novel B cell cluster co-expressed CD14 and CD11c (#26, Fig. 1B and D, S1E). Furthermore, yet another cluster was characterised by expression of CD57 (#6, Fig. 1B and D, S1C, S4A), a terminal differentiation marker commonly studied in the context of T cells [29,30,31] and NK cells [32]. This CD57 cluster was enriched for CD27 expression but otherwise not characterised by any specific combination of other markers (Fig. S4C). We used flow cytometry to confirm this unexpected B cell phenotype in baseline samples from five of the SLE patients, whereby we consistently saw a small but distinct CD57+ B cell population (Fig. S4B). Moreover, we used a cohort of patients with relapsing-remitting multiple sclerosis (RRMS) to replicate our data. This cohort (n = 19) consisted of patients who initiated treatment with dimethyl fumarate (DMF; Tecfidera, Biogen) with sampling at baseline and at 6 months after start of therapy. The cell samples were analysed with the same B cell panel in the same CyTOF facility as our SLE cohort. As shown in Fig. S5, a significant expansion of CD57+ B cells compared to healthy controls (n = 10; P = .000013, Mann-Whitney U test)) was evident also in this autoimmune disease, which was diminished upon therapy with DMF (P = .00084, Wilcoxon signed rank test).
Given the concerns that t-SNE may form false clusters [33], we set out to replicate the observed clusters by principal component analysis (PCA) (Fig. S4). We could recreate both the CD14+CD11c+ and the CD11c+CD21− clusters, as well as the separation between naïve and memory B cells, although the CD57+ cells did not form a cluster here, consistent with the lack of other marker expression differences for these cells (Fig. S4C).
3.2. Leucocyte subset composition following treatment
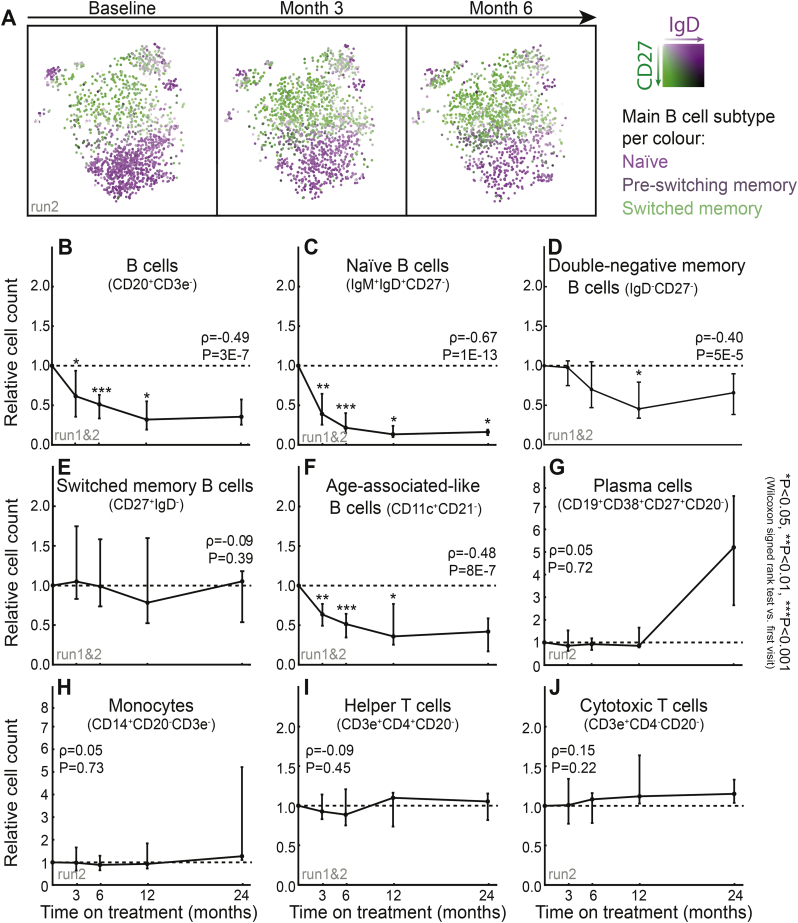
To exploit the longitudinal design of our study, we next investigated changes during follow-up using t-SNE plots for B cells from baseline samples and the first two follow-up visits (three and six months), and hereby a gradual loss of naïve IgD+CD27− B cells (Fig. 2A, depicted in purple) could be observed. This loss of naïve B cells also continued during the later time points, as visualised in t-SNE plots from five SLE patients (Fig. S3A).
Fig. 2.
Cell population and subset alterations over time. (A) Changes in naïve (purple) and memory (green) B cells within the first 6 months of belimumab treatment visualised by t-SNE clustering of CD20+ B cells. (B–K) Relative cell count alterations in predefined cell subsets on each follow-up occasion compared to baseline values, with lines representing medians and error bars representing quartiles. Spearman's rank correlation coefficients (ρ) for correlations of cell populations with time from baseline and the corresponding P-values are shown in the respective graphs. See Fig. 1A for the number of datapoints. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We further investigated alterations of well-described leucocyte populations for the entire study period (Fig. 2B–J, S3A–B). We observed a consistent overall decrease of B cells (Fig. 2B), significant already at the first follow-up visit, replicating observations from previous clinical trials [11,12]. To increase the granularity of our data, we subdivided the B cells into subsets with distinct maturation and differentiation markers; namely naïve B cells (IgM+IgD+CD27−), switched (IgD−CD27+) and double-negative (IgD−CD27−) memory B cells, CD11c+CD21− B cells resembling age-associated B cells (ABCs). At month three, significant decreases were observed for naïve (Fig. 2C; P = 1·10−13) and CD11c+CD21− (Fig. 2F; P = 8·10−7) B cells, whereas double-negative (Fig. 2D; P = .033) memory B cells showed a more modest decline, which occurred at later time points. The two CD57+ clusters observed in the t-SNE plots (Fig. 1D; clusters #6 and #25) showed a continuous decrease over time (Fig. S3B; P = .02). In contrast, switched memory B cells showed no decline (Fig. 2E). Despite their scarcity in peripheral blood, we also investigated plasma blasts (CD19+CD38+CD27+CD20−), without detecting any significant alterations (Fig. 2G; P = .72). Finally, no significant changes were observed in monocytes (Fig. 2H; P = .73), helper T cells (Fig. 2I; p = .45), or cytotoxic T cells (Fig. 2J; P = .22). In addition to this hypothesis-based analysis, we also visualised B cell subset changes for each one of the t-SNE clusters in Fig. 1B and noted similar trajectories (Fig. S3B).
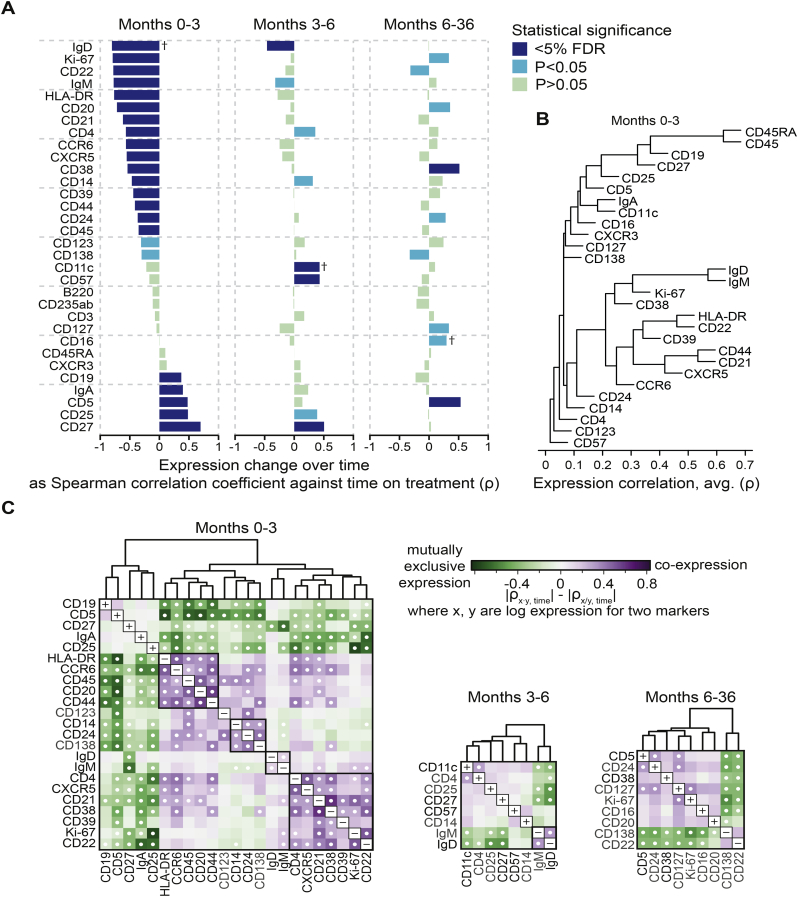
Next, we returned to unbiased analyses and explored the correlation between protein expression levels and time on treatment, divided into early (months 0–3), intermediate (months 3–6) and late (months 6–36) time intervals (Fig. 3A). In order to detect expression grade changes, we also analysed the geometric mean marker expression rather than positive cell counts (Fig. S6). The changes were distinctly different across the three time-intervals. Several markers were affected by treatment during early time points, showing moderate to strong correlations with time, while only a few markers showed changes in the later time intervals. More specifically, IgD+ (naïve) B cells continued to decrease within the intermediate time interval while CD27 showed a positive correlation. It is worth noting that mean expressions were calculated over the background of decreasing overall numbers of B cells; therefore, a positive correlation at late time points do not necessarily correspond to an increase in total CD27+ B cell counts. No significant changes were detected in the same analysis for T cells (Fig. S7A).
Fig. 3.
Changes in protein expression on B cells during follow-up time points. (A) Spearman's rank correlation coefficients for correlations of each antigen with time on treatment are grouped into early (months 0–3), intermediate (months 3–6) and late (months 6–36) alterations. Colour intensity represents statistical significance. Positive correlations represent relative marker expression increases, and negative correlations represent relative decreases. As examples, correlations marked with † are further explained in Fig. S6. (B) Hierarchical clustering (average linkage) of protein expression by Spearman's rank correlation coefficient. (C) Paired marker expression for the same time intervals arranged by hierarchical clustering. Only antigens showing significant changes (P < .05) in (A) were included in the heat maps. Colour intensity represents the difference between correlations of co-expression and mutually exclusive expression of antigen pairs with time on treatment. Dots denote significant differences (P < .05) of cell subsets co-expressing or mutually lacking the respective pair of markers.
Since changes in marker expression over time might in several cases represent a phenotypic alteration within a cell type rather than transition into another cell type, we next sought to identify marker combinations representing B cell subtypes. We did this in two ways. The first approach was hierarchical clustering based on expression level (Fig. 3B). This analysis indicated that the decrease of IgD, IgM, Ki-67 and CD38 at months 0–3 represents the loss of naïve and proliferating B cells, which are BAFF dependent. This is followed by a parallel increase of CD27, CD25 and CD19. Such an increase of CD19, but not CD20 further supports the preferential loss of naïve B cells and the relative resilience of memory B cells to belumimab. Additionally, we also found a cluster of B cell expressing activation markers (HLA-DR, CD22, CD39), and a cluster of B cells expressing CD21, CXCR5 and CD44, both of which displayed an apparent decrease during BAFF inhibition as seen in Fig. 3A. Overall, the analysis exhibits a decrease of markers which are associated with naïve, activated and proliferating B cells, indicating that those B cells were most sensitive to the action of belumimab.
In the second approach, we created heat maps illustrating time correlations for pairs of markers, in order to identify cell subset alterations over time (Fig. 3C, S8A–C). Decreasing numbers of naïve B cells were visualised as a clustering of IgM and IgD at both early (months 0–3) and intermediate (months 3–6) time points, and CD21 and CXCR5 still displayed co-expression in this analysis. Of note, some B cell subsets did not appear as a separate cluster in this analysis, e.g. transitional B cells since CD24, CD38 and IgM were neither strongly co-expressed on the same cells nor contiguous in hierarchical clustering of the markers. In the individual marker analysis (Fig. 3A), CD38 displayed an increase during late time points, despite an initial decrease during months 0–3. We further explored this in the pair analysis, and CD38 was strongly co-expressed with CD21 and CD22 at early time points (Fig. 3C), whereas it showed a mutually exclusive expression pattern with CD21 and CD22 at later time points (Fig. S8C). Thus, the early decrease of CD38 presumably corresponds to the changes in naïve B cells, and the late increase corresponds to a CD38+IgM+IgD+CD22−CD21−CD27− B cell subtype, most likely transitional B cells (Fig. S8D), which was resistant to belimumab treatment (Fig. S8E). Together with the cell type time plots in Fig. 2B–J, these results delineate a stepwise change in the B cell composition during belimumab treatment, with less differentiated cell types being affected early, presumably resulting in cascade effects of cell subtypes of later differentiation stages decreasing subsequently.
Combining unbiased and targeted approaches, we were also able to distinguish changes in cell phenotypes from cell subset alterations. E.g. CD57 and CD11c showed moderate increases during months 3–6 (Fig. 3A), while the CD57+CD11c+ cluster observed in t-SNE (Fig. 1D; cluster #25, Fig. 1B) displayed a decrease during the same time interval (Fig. S3B), and CD57 and CD11c were not co-expressed at either early or later time points (Fig. S8A–C), altogether implying a relative increase in the expression of these markers rather than numerical alterations in the CD57+CD11c+ B cell cluster.
3.3. B cell alterations and SLE disease activity
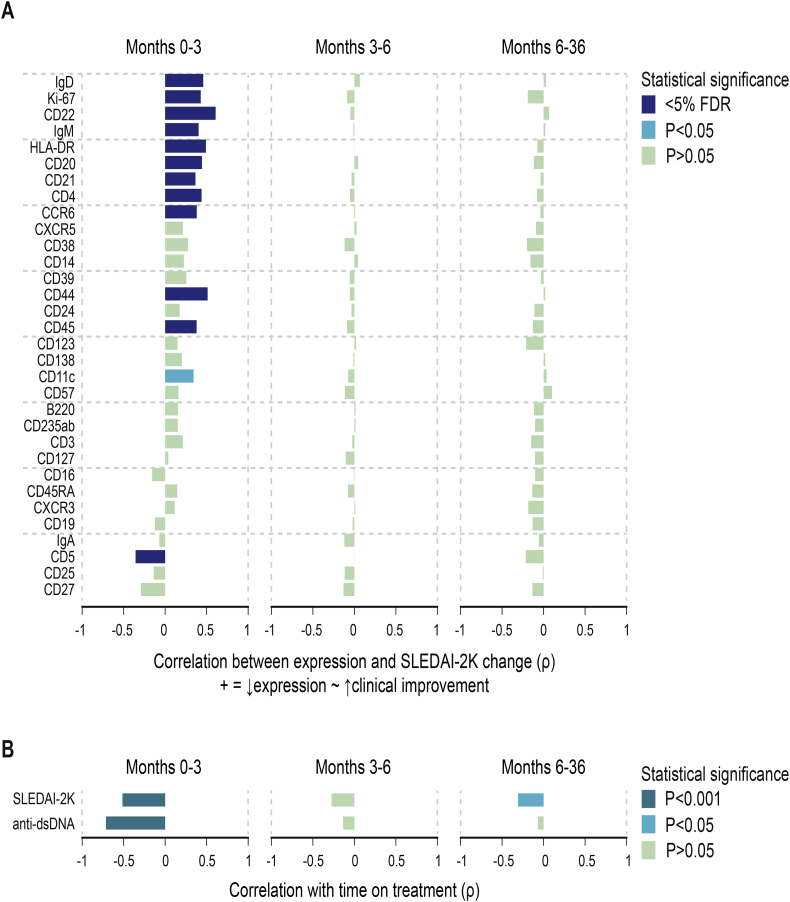
To investigate B cell alterations in the context of disease activity, we next repeated the correlation analysis of individual markers, this time with SLE Disease Activity Index 2000 (SLEDAI-2K; [34]) scores normalised for baseline values (Fig. 4A). Disease activity showed a rapid early and a continuous gradual decrease during follow-up (Fig. 4B). Early immunological changes correlated with clinical improvements, but not immune changes during the later time points. Interestingly, despite only moderate alterations in CD11c expression during months 0–3 (Fig. 3A), decreasing CD11c expression correlated with decreasing SLEDAI-2K (Fig. 4A).
Fig. 4.
Correlations between changes in protein expression and changes in disease activity. (A) Spearman's rank correlation coefficients for early (months 0–3), intermediate (months 3–6) and late (months 6–36) alterations. Colour intensity represents statistical significance. Positive correlations represent relative marker expression decreases concomitant with clinical improvements, and negative correlations represent relative marker expression increases concomitant with clinical improvements. (B) Spearman's rank correlations of SLE Disease Activity Index 2000 (SLEDAI-2K) scores and anti-double stranded (ds) DNA antibody levels as assessed by addressable laser bead immunoassay (ALBIA) with time on treatment.
Concurrently with decreasing SLEDAI-2K and decreasing levels of markers for naive B cells (Fig. 3A), levels of anti-dsDNA autoantibodies also decreased at early, but not at later time points (Fig. 4B). The changes in anti-dsDNA levels also correlated with the immunological changes observed at early time points (Fig. 4B).
3.4. B cell alterations and clinical response to treatment
In addition to disease activity, we analysed the observed changes in cell subsets in relation to clinical response to treatment. It is worth noting that essentially all patients responded to treatment immunologically; e.g. all but one patient showed immediate reductions of naïve B cells.
Most patients attained a clinical response (Fig. 5A). Of the 23 patients, 19 patients met the SLE Responder Index (SRI; [19]) criteria (median time from baseline: 3.6 months; IQR: 2.9–12.2 months), 3 patients did not, and in one case the SRI was not applicable (baseline SLEDAI-2K score: 2). Similarly, 18 of 23 patients attained Lupus Low Disease Activity State (LLDAS; [20]) during the study period, though after a longer time from treatment initiation (median: 12.0 months; IQR: 6.3–24.1 months).
Fig. 5.
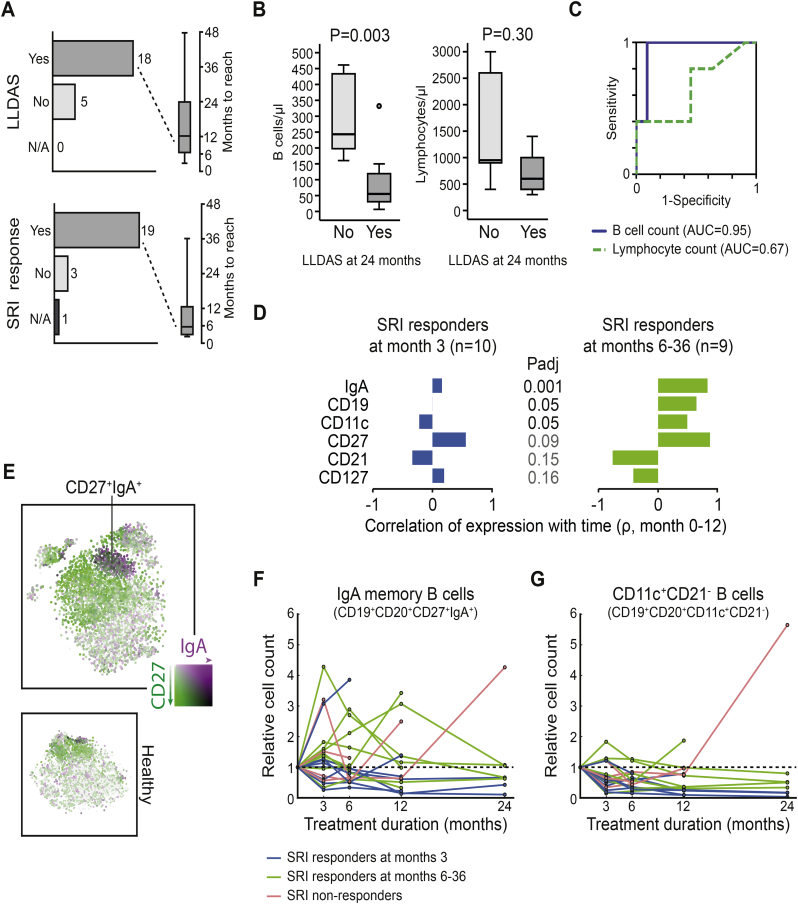
Baseline B cell counts as predictors of treatment response and B cell subset alterations in responders versus non-responders. (A) Response rates according to the SLE responder index (SRI) and lupus low disease activity state (LLDAS). (B) Comparisons of baseline B cell and lymphocyte counts between patients who had attained LLDAS at month 24 and patients who had not. Box plots represent baseline cell count distributions. Lines in the boxes denote medians, bounds denote quartiles, whiskers denote ranges and circles denote out or extreme values. (C) Receiver operating characteristic (ROC) curves for baseline B cell and lymphocyte counts by LLDAS at month 24. High baseline B cell counts performed better as a predictor of non-attaining LLDAS (blue line; area under the curve (AUC): 0.95; 95% confidence interval (CI): 0.83–1.0; P = .006) compared with total lymphocyte counts (green line; AUC: 0.66; 95% CI: 0.35–0.97; P = .308). (D) Spearman's rank correlations of expression levels with time on treatment (months 0–12), and comparisons of these correlations between early responders, late responders and non-responders according to SRI. Antigens were selected based on a false discovery rate (FDR) of <0.2 for the comparison between early and late responders. Benjamini-Hochberg multiple testing correction was applied for P-value adjustment. (E) CD27 and IgA co-expression is apparent in a distinct cluster representing IgA+ switched memory B cells in the t-SNE plot of B cells from all follow-up occasions. (F–G) Alterations of IgA+ switched memory B cells (F) and presumed age-associated B cells (G) (G) during follow-up in early responders (blue), late responders (green) and non-responders (red) according to the SRI. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At the 24-month follow-up, 11 of the 17 patients still being on treatment fulfilled the LLDAS criteria. We observed lower baseline B cell counts in patients who attained LLDAS at month 24 compared to patients who did not (P = .003), whereas baseline total lymphocyte counts did not differ between the two groups (P = .301) (Fig. 5B). ROC-curve analysis confirmed that higher baseline B cell counts were predictive of non-attaining LLDAS at month 24, with an area under the curve (AUC) of 94.5% (95% confidence interval (CI) 0.830–1.00; P = .006). The optimal threshold value was 155 cells/μL and yielded a specificity of 90.9% and a sensitivity of 100% for predicting failure to attain LLDAS through month 24. The respective AUC for baseline lymphocyte counts was 66.7% (95% CI 0.388–0.946; P = .269) (Fig. 5C). Although baseline B cell counts were superior in distinguishing patients attaining LLDAS from non-responding patients, naïve B cells (P = .027) and switched memory B cells (P = .027) were also found to be lower in patients who attained LLDAS, whereas levels of double-negative memory B cells (P = .090), CD11c+CD21− B cells (P = .320), plasma blasts (P = .497) and total T cell counts (P = .583) did not differ between the two groups (Fig. S9A).
We further compared correlations of individual markers with time on treatment between early SRI responders, late responders and non-responders. As treatment outcomes may influence the time on treatment, we restricted this analysis to samples from the first year of treatment. Several surface markers appeared to differ between early and late SRI responders, with late responders showing greater changes, especially in IgA expression. IgA was primarily co-expressed with CD27 in a distinct t-SNE region (Fig. 5E; Fig. 1B, clusters #7 and #17), representing IgA+ switched memory B cells. We found prominent differences in how this B cell subset was altered during follow-up between early (blue) and later (green) clinical responders, delineated in Fig. 5F.
Due to the significant difference of CD11c expression change between early and late responders, we also plotted the CD11c+CD21− B cells over time in the different SRI response groups. Indeed, these age-associated-like B cells decreased rapidly and remained low in early responders, decreased more gradually in late responders, and were unaltered in non-responders (Fig. 5G). This pattern was absent in the same analysis for total B cell counts, implicating B cell subtype specificity (Fig. S9C).
4. Discussion
We investigated alterations in leucocyte populations and subsets during anti-BAFF treatment with belimumab in 23 patients with SLE using mass cytometry (CyTOF). Patients with SLE represent a heterogeneous group where the peripheral blood composition can deviate considerably from that of healthy individuals [35]. Indeed, the SLE patients in our cohort displayed a large fraction of memory B cells and pronounced cell differentiation. These features became even more prominent following treatment, as belimumab had rapid deleterious effects on B cells of earlier developmental stages, especially naïve B cells, while later stage B cells were more resilient to alterations and were only affected at late time points.
The combination of CyTOF data and longitudinal clinical assessments using validated SLE measures enabled us to analyse immunological changes during BAFF inhibition in relation to not only disease activity, but also several well-defined clinical outcomes. We combined unbiased with hypothesis-based approaches to analyse the data and identified both unexpected B cell phenotypes and B cell subsets associated to outcome measures following treatment with belimumab.
In previous studies, belimumab treatment led to decreased numbers of CD20+ B cells but did not significantly affect T cells [9,11,12]. Subdividing the B cells, naïve and double-negative memory B cells were reported to continuously decrease in numbers [11,12] while plasmablasts decreased at later time points and switched memory B cells showed no changes in a study by Jacobi et al. [12] and an initial expansion with a subsequent return to baseline levels in a study by Stohl et al. [11]. In the same study, Stohl et al. demonstrated preservations of pre-existing antibodies to pneumococcal and tetanus vaccines, but decreasing plasma cell numbers [11]. We similarly observed a rapid decrease of naïve B cells, a gradual decrease in double-negative memory B cells, and no significant impact of belimumab on T cells. In contrast, the initial expansion of class-switched memory B cells was not replicated in our cohort, and we found no significant changes in plasmablast numbers, in contrast to previous reports of decreases in plasmablasts/plasma cells. While plasma blasts in other studies have been gated as CD138+CD20−, CD27brightCD20−, and CD19+CD27brightCD38bright [11] or CD27brightCD38bright cells for both plasma cells and plasmablasts [12], in our study we used the marker combination CD19+CD27+CD38+CD3e−CD20−, which possibly could explain this discrepancy.
In addition to replicating some previous data sets in our real-life clinical prospective study we also report novel data with regard to the early time point of diminished naïve B cells and the sensitivity to belumimab therapy of several additional B cell phenotypes and subsets. We were also interested in studying age-associated B cells (ABCs), a recently described B cell subset, characterised by a gradual accumulation with age, in chronic inflammatory diseases, or following repeated viral infections [26,27]. Recently, Wang et al. [28] demonstrated that the majority of the CD11chi B cells in SLE express the transcription factor T-bet and can hence be assigned to the ABC subset. Intriguingly, these cells were implicated in disease manifestations as they were found present in affected kidneys and produced autoantibodies upon stimulation. Our panel only included some ABC-related markers (unfortunately not T-bet). We observed decreases in ABC-like cells already at early time points, with continuous gradual and significant decreases over time, which is consistent with previous reports of age-associated B cells expressing cell surface receptors for BLyS/BAFF [27,28]. Interestingly, these cells were affected differently in patients with different clinical outcomes, showing an early and sustained decrease following treatment in early responders, but being more resistant to change in non-responding patients and patients with delayed clinical improvements. Moreover, IgA+ memory B cells showed similar response to what was seen in ABC-like B cells, suggesting that IgA-expressing and CD11c+CD21− memory B cells likely represent an overlapping subset within the age-associated B cells. We also saw additional B cell clusters which may be related to ABCs, i.e. CD11c + CD14+ and CD11c + CD57+ B cells. These may represent different facets of terminally differentiated B cells.
The unbiased t-SNE and hierarchical clustering analyses revealed cell types that we were unable to relate to the literature. Here, we demonstrated a distinct cluster of B cells expressing CD57, a marker of final stage differentiation in the context of T cells and NK cells [29,30,32,36]. In the context of B cells, CD57 expression has to date only been implicated in specific types of B cell lymphomas [37,38,39,40], which together with the findings in the current study renders support for a role in disease state. Notably, expansion of a CD57+ B cell cluster was not restricted solely to SLE patients as this finding was replicated in a cohort of RRMS patients. Furthermore, the CD57 B cell cluster was diminished in RRMS patients starting treatment with DMF, a disease modulatory treatment for RRMS associated with activation of transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) [41], as well as with B cell subset alterations [42]. Knowing that CD57 expression increases with age and chronic infections, at least in the context of NK cells [32], these B cells might share common characteristics with age-associated B cells. We also distinguished a group of cells expressing CD11c, CD14 and low levels (compared to T cells) of CD4 among the CD20+ B cells, however we did not investigate these sufficiently to exclude that they instead are monocytes. In line with the previously reported decrease in activated B cell counts [11], we found decreasing expression of HLA-DR and CD22, common activation markers.
B cell alterations occurred in two phases, a rapid early and a more gradual late phase. In contrast, anti-dsDNA levels showed mostly rapid decreases following treatment initiation, whereas SLE activity decreased rapidly and continued to decrease during later time points. Interestingly, clinical improvements showed correlations with immunological changes at early time points only. Thus, clinical improvements observed at later time points might reflect preceding immunological alterations. This might have direct implications in the clinical use of belimumab; since immunological changes continued to occur during later stages, especially regarding late stage B cells, early treatment evaluation and discontinuation might result in underestimation of delayed clinical effects as a possible consequence of these late B cell changes.
High baseline B cell counts were found to predict unfavourable treatment outcomes, unlike total lymphocyte counts. In clinical praxis, it is common to test for lymphocyte counts, but B cell counts are seldom assessed. Our results suggest that evaluation of B cell counts might prove useful prior to initiation of belimumab treatment, and, as previously speculated upon [11,43,44], they also favour the notion that B cell depletion preceding belimumab might be an effective therapeutic strategy in cases of high B cell counts. Our belimumab cohort has also been clinically evaluated as part of a larger Swedish SLE cohort (without cellular studies) whereby cigarette smoking and organ damage predicted reduced treatment efficacy [45].
The low number of patients and the observational nature of our study were among its main limitations. Additionally, the study population was mainly of Caucasian origin; our findings may therefore not be applicable in SLE populations of other ethnic origins. We admittedly lack a straightforward validation cohort. Still, since our samples needed to be run on two separate occasions given the large number of them (n = 96) we could sub-analyse the two runs for consistency; as can be seen in Fig. S10, most of the changes we observed in the entire cohort were also visible in the sub-runs. Moreover, we had access to a cohort of multiple sclerosis patients undergoing therapy with dimethyl fumarate and we could here validate the CD57 phenotype in another inflammatory autoimmune disease setting. With regard to other weaknesses in our study, belimumab is indicated as an add-on drug to standard of care therapy, and the patients were on concomitant treatments with other drugs, including corticosteroids, which might have contributed to the patients' aberrant leucocyte subset composition. It is worth nothing that corticosteroid dosages were actively reduced during treatment with belimumab. Major strengths were the long observation time, the systematic clinical assessment with validated indices at regular visits, and the unbiased principles in several of our analyses. Utilisation of mass cytometry facilitated the use of a broad antigen panel for B cells, resulting in deeper understanding of cell subtype alterations during treatment with belimumab, and also in the identification of B cell subsets with novel attributes.
To conclude, belimumab treatment had rapid effects on naïve B cells and B cells of earlier developmental stages while B cells of later stages showed delayed or no responses. The immunological changes betided in two distinct phases, a rapid early and a gradual late phase, whereas SLE activity showed a continuous decrease. Early treatment evaluation and discontinuation might underestimate delayed clinical improvements resultant of late B cell changes.
Author contributions
The study was conceived, designed and coordinated by IP, IG and VM. IP, AZ and IG contributed to SLE sample collection and the clinical assessments. MK and FP contributed to MS sample collection. TL, JM, AA and PB handled the cell samples and acquired the mass cytometry data. DR, YC and PB performed the bioinformatics analyses. NS and KA acquired the flow cytometry data. IP and DR performed the statistical analyses. IP, DR and VM drafted the manuscript.
Funding source
The study was supported by grants from the Swedish Research Council, Swedish Rheumatism Association, King Gustaf V's 80-year Foundation, Swedish Heart-Lung Foundation, Foundation in memory of Clas Groschinsky, Professor Nanna Svartz Foundation, Stockholm County Council, and Karolinska Institutet Foundations. The SLE study was independent of pharmaceutical sponsors, while the MS cohort used for the CD57 replication was covered by an unrestricted academic research grant from Biogen. The funders had no role in the study design, data collection, analyses, interpretation nor writing of this study.
Declaration of interests
The authors declare no conflicts of interest.
Acknowledgements
The authors thank Eleanor Gullström, Eva Jemseby, Julia Norkko and Gull-Britt Almgren for administrating and preparing the samples, as well as all participating patients.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.12.035.
Appendix A. Supplementary data
Supplementary material
References
- 1.Chambers S.A., Allen E., Rahman A., Isenberg D. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford) 2009;48(6):673–675. doi: 10.1093/rheumatology/kep062. [DOI] [PubMed] [Google Scholar]
- 2.Mok C.C., Kwok C.L., Ho L.Y., Chan P.T., Yip S.F. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum. 2011;63(5):1182–1189. doi: 10.1002/art.30277. [DOI] [PubMed] [Google Scholar]
- 3.Urowitz M.B., Gladman D.D., Tom B.D., Ibanez D., Farewell V.T. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol. 2008;35(11):2152–2158. doi: 10.3899/jrheum.080214. [DOI] [PubMed] [Google Scholar]
- 4.Liossis S.N., Melissaropoulos K. Molecular abnormalities of the B cell in systemic lupus erythematosus are candidates for functional inhibition treatments. Expert Opin Pharmacother. 2014;15(6):833–840. doi: 10.1517/14656566.2014.894976. [DOI] [PubMed] [Google Scholar]
- 5.Farh K.K., Marson A., Zhu J., Kleinewietfeld M., Housley W.J., Beik S. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518(7539):337–343. doi: 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stohl W., Metyas S., Tan S.M., Cheema G.S., Oamar B., Xu D. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003;48(12):3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- 7.Parodis I., Zickert A., Sundelin B., Axelsson M., Gerhardsson J., Svenungsson E. Evaluation of B lymphocyte stimulator and a proliferation inducing ligand as candidate biomarkers in lupus nephritis based on clinical and histopathological outcome following induction therapy. Lupus Sci Med. 2015;2(1):e000061. doi: 10.1136/lupus-2014-000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarra S.V., Guzman R.M., Gallacher A.E., Hall S., Levy R.A., Jimenez R.E. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 9.Furie R., Petri M., Zamani O., Cervera R., Wallace D.J., Tegzova D. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Vollenhoven R.F., Petri M.A., Cervera R., Roth D.A., Ji B.N., Kleoudis C.S. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis. 2012;71(8):1343–1349. doi: 10.1136/annrheumdis-2011-200937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stohl W., Hiepe F., Latinis K.M., Thomas M., Scheinberg M.A., Clarke A. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64(7):2328–2337. doi: 10.1002/art.34400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobi A.M., Huang W., Wang T., Freimuth W., Sanz I., Furie R. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2010;62(1):201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan E.M., Cohen A.S., Fries J.F., Masi A.T., McShane D.J., Rothfield N.F. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 14.Petri M., Orbai A.M., Alarcon G.S., Gordon C., Merrill J.T., Fortin P.R. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hay E.M., Bacon P.A., Gordon C., Isenberg D.A., Maddison P., Snaith M.L. The BILAG index: a reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. Q J Med. 1993;86(7):447–458. [PubMed] [Google Scholar]
- 16.Isenberg D.A., Gordon C., Group BGBILA From BILAG to BLIPS—disease activity assessment in lupus past, present and future. Lupus. 2000;9(9):651–654. doi: 10.1191/096120300672904669. [DOI] [PubMed] [Google Scholar]
- 17.Petri M., Kim M.Y., Kalunian K.C., Grossman J., Hahn B.H., Sammaritano L.R. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 18.Buyon J.P., Petri M.A., Kim M.Y., Kalunian K.C., Grossman J., Hahn B.H. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142(12):953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. Pt 1. [DOI] [PubMed] [Google Scholar]
- 19.Furie R.A., Petri M.A., Wallace D.J., Ginzler E.M., Merrill J.T., Stohl W. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. 2009;61(9):1143–1151. doi: 10.1002/art.24698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franklyn K., Lau C.S., Navarra S.V., Louthrenoo W., Lateef A., Hamijoyo L. Definition and initial validation of a lupus low disease activity state (LLDAS) Ann Rheum Dis. 2016;75(9):1615–1621. doi: 10.1136/annrheumdis-2015-207726. [DOI] [PubMed] [Google Scholar]
- 21.Finck R., Simonds E.F., Jager A., Krishnaswamy S., Sachs K., Fantl W. Normalization of mass cytometry data with bead standards. Cytometry A. 2013;83(5):483–494. doi: 10.1002/cyto.a.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perfetto S.P., Chattopadhyay P.K., Roederer M. Seventeen-colour flow cytometry: unravelling the immune system. Nat Rev Immunol. 2004;4(8):648–655. doi: 10.1038/nri1416. [DOI] [PubMed] [Google Scholar]
- 23.Aarden L.A., de Groot E.R., Feltkamp T.E. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann N Y Acad Sci. 1975;254:505–515. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- 24.Shekhar K., Brodin P., Davis M.M., Chakraborty A.K. Automatic classification of cellular expression by nonlinear stochastic embedding (ACCENSE) Proc Natl Acad Sci U S A. 2014;111(1):202–207. doi: 10.1073/pnas.1321405111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine J.H., Simonds E.F., Bendall S.C., Davis K.L., Amir El A.D., Tadmor M.D. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell. 2015;162(1):184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubtsov A.V., Rubtsova K., Fischer A., Meehan R.T., Gillis J.Z., Kappler J.W. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118(5):1305–1315. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubtsova K., Rubtsov A.V., Cancro M.P., Marrack P. Age-associated B cells: a T-bet-dependent effector with roles in protective and pathogenic immunity. J Immunol. 2015;195(5):1933–1937. doi: 10.4049/jimmunol.1501209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S., Wang J., Kumar V., Karnell J.L., Naiman B., Gross P.S. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun. 2018;9(1):1758. doi: 10.1038/s41467-018-03750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenchley J.M., Karandikar N.J., Betts M.R., Ambrozak D.R., Hill B.J., Crotty L.E. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Verges S., Milush J.M., Pandey S., York V.A., Arakawa-Hoyt J., Pircher H. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116(19):3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Focosi D., Bestagno M., Burrone O., Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87(1):107–116. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen C.M., White M.J., Goodier M.R., Riley E.M. Functional significance of CD57 expression on human NK cells and relevance to disease. Front Immunol. 2013;4:422. doi: 10.3389/fimmu.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orlova D.Y., Herzenberg L.A., Walther G. Science not art: statistically sound methods for identifying subsets in multi-dimensional flow and mass cytometry data sets. Nat Rev Immunol. 2017;18(1):77. doi: 10.1038/nri.2017.150. [DOI] [PubMed] [Google Scholar]
- 34.Gladman D.D., Ibanez D., Urowitz M.B. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–291. [PubMed] [Google Scholar]
- 35.Kaminski D.A., Wei C., Qian Y., Rosenberg A.F., Sanz I. Advances in human B cell phenotypic profiling. Front Immunol. 2012;3:302. doi: 10.3389/fimmu.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kared H., Martelli S., Ng T.P., Pender S.L., Larbi A. CD57 in human natural killer cells and T-lymphocytes. Cancer Immunol Immunother. 2016;65(4):441–452. doi: 10.1007/s00262-016-1803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delsol G., Lamant L., Mariame B., Pulford K., Dastugue N., Brousset P. A new subtype of large B-cell lymphoma expressing the ALK kinase and lacking the 2; 5 translocation. Blood. 1997;89(5):1483–1490. [PubMed] [Google Scholar]
- 38.Matsubara K., Yura K., Hirata T., Nigami H., Harigaya H., Nozaki H. Acute lymphoblastic leukemia with coexpression of CD56 and CD57: case report. Pediatr Hematol Oncol. 2004;21(7):677–682. doi: 10.1080/08880010490501105. [DOI] [PubMed] [Google Scholar]
- 39.Reichard K.K., McKenna R.W., Kroft S.H. ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature. Mod Pathol. 2007;20(3):310–319. doi: 10.1038/modpathol.3800742. [DOI] [PubMed] [Google Scholar]
- 40.Chiu A., Frizzera G., Mathew S., Hyjek E.M., Chadburn A., Tam W. Diffuse blastoid B-cell lymphoma: a histologically aggressive variant of t(14;18)-negative follicular lymphoma. Mod Pathol. 2009;22(11):1507–1517. doi: 10.1038/modpathol.2009.106. [DOI] [PubMed] [Google Scholar]
- 41.Linker R.A., Lee D.H., Ryan S., van Dam A.M., Conrad R., Bista P. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–692. doi: 10.1093/brain/awq386. Pt 3. [DOI] [PubMed] [Google Scholar]
- 42.Li R., Rezk A., Ghadiri M., Luessi F., Zipp F., Li H. Dimethyl fumarate treatment mediates an anti-inflammatory shift in B cell subsets of patients with multiple sclerosis. J Immunol. 2017;198(2):691–698. doi: 10.4049/jimmunol.1601649. [DOI] [PubMed] [Google Scholar]
- 43.Vallerskog T., Heimburger M., Gunnarsson I., Zhou W., Wahren-Herlenius M., Trollmo C. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2006;8(6):R167. doi: 10.1186/ar2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cambridge G., Stohl W., Leandro M.J., Migone T.S., Hilbert D.M., Edwards J.C. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum. 2006;54(3):723–732. doi: 10.1002/art.21650. [DOI] [PubMed] [Google Scholar]
- 45.Parodis I., Sjowall C., Jonsen A., Ramskold D., Zickert A., Frodlund M. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev. 2017;16(4):343–351. doi: 10.1016/j.autrev.2017.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Mass cytometry data and code which was used for analysis will be deposited at: https://github.com/danielramskold/B_cell_alterations_during_BAFF_inhibition_in_SLE (Github) and http://dx.doi.org/10.17632/7894wb6yxh.1 (Mendeley Data).