Abstract
Dysfunction in cortico-limbic circuitry is implicated in internalizing disorders (i.e., depressive and anxious disorders), but less is known about whether structural variations precede frank disorder and thus potentially mark risk. We therefore examined associations between white matter (WM) tract microstructure in cortico-limbic circuitry at age 7 and concurrent and longitudinal patterns of internalizing symptoms in 42 typically developing girls using Diffusion Tensor Imaging (DTI). Girls' internalizing symptoms were concurrently associated with reduced fractional anisotropy (FA) in segments of the cingulum bundle (CB) and the uncinate fasciculus (UF), bilaterally. Moreover, latent profile analysis showed that girls with increasing internalizing symptoms, based on assessments at ages 3, 6, 7, and 8, had reduced FA in these segments compared to girls with stably low symptoms. These results point to a putative neural mechanism underlying the course of childhood internalizing symptoms.
Keywords: Internalizing symptoms, White matter microstructure, Diffusion Tensor Imaging (DTI), Latent profile analysis
Highlights
-
•
Early internalizing symptoms are linked to lower FA in the cingulum bundle and uncinate fasciculus.
-
•
Girls with increasing symptoms across childhood also show reduced FA in these tracts.
-
•
Lower structural connectivity of cortico-limbic circuitry may confer risk for later disorder.
1. Introduction
1.1. Childhood internalizing symptoms
Internalizing problems (i.e., depressive and anxious symptoms) can appear early in development and mark increased risk for depressive and anxiety disorders in later life (Clark et al., 2007; Copeland et al., 2009; Reinke and Ostrander, 2008; Toumbourou et al., 2011). The impact of early internalizing symptoms, even when subthreshold, is significant. For example, children with these symptoms have poor academic performance, low emotional competence, and problematic peer and family relationships (Ialongo et al., 1994; Masten et al., 2005; Masten and Cicchetti, 2010; Mathews et al., 2016). Moreover, they are at especially high risk for persistent and severe mental health outcomes, such as depression and early onset substance use (Kaplow et al., 2002; Luby, 2010; Luby et al., 2009), which exact a substantial economic burden. Indeed, annual estimates of the burden due to depression alone is as high as USD $53 billion (Greenberg et al., 2003), with more recent studies reporting indirect costs as high as USD $210.5 billion (Greenberg et al., 2015). Given this steep toll, research that speaks to the early origins of risk is vital for informing early prevention and intervention efforts.
1.2. Neural mechanisms
Deficits in affect processing and regulation characterize an array of internalizing problems (Pagliaccio et al., 2014; Zahn-Waxler et al., 2016), implicating brain structures that govern these functions as mediators of risk for disorder. Indeed, extant literature on adult internalizing psychopathology points to structural and functional abnormalities in the neural networks that subserve affective processing, particularly cortico-limbic circuitry (Phillips et al., 2003b). For instance, magnetic resonance imaging (MRI) studies demonstrate volumetric reductions in prefrontal cortex (PFC), orbitofrontal cortex, anterior cingulate cortex (ACC), amygdala, and the hippocampus, in adults with current or remitted depression (Bremner et al., 2002; Hastings et al., 2004; MacQueen et al., 2003). The precise causal mechanisms that account for these brain-disorder associations are unclear; however, work on healthy adults at risk for depression, indexed by family history, suggests that these structural factors may be a precursor to illness, rather than a consequence (Amico et al., 2011). It is also possible that these structural characteristics interact with environmental factors that serve as more proximal triggers of disorder; for example, reduced hippocampal volume has been found in depressed women with a history of childhood sexual abuse (Vythilingam et al., 2002). Volumetric reductions in the amygdala and PFC have also been reported in anxiety disorders (van Tol et al., 2010), although findings are less consistent (Shin and Liberzon, 2010).
Functional MRI studies of clinical populations also implicate altered activity of cortico-limbic circuitry in depression and anxiety (Etkin and Wager, 2007; Leppänen, 2006; Price and Drevets, 2012). For instance, depressed patients show greater amygdalar activation to sad stimuli and reduced activity in response to positively valenced stimuli (Price and Drevets, 2012). In addition, regions of the ACC and the PFC associated with reward and positive emotions show reduced activity in response to reward in depression, and this reduced activity is further associated with subjective ratings of anhedonia (Price and Drevets, 2012). Hyperactivation of the amygdala and dorsal ACC has also been implicated in anxiety disorders, and is theorized to underlie the threat hypersensitivity and elevated fear response that characterize these disorders (Duval et al., 2015; Shin and Liberzon, 2010).
Similarly, and consistent with women's well-established increased risk for internalizing disorders (McLean et al., 2011a; Nolen-Hoeksema, 2001), extant literature demonstrates normative sex differences in the activity of cortico-limbic structures during emotion processing (Stevens and Hamann, 2012). In particular, relative to men, women exhibit greater activation of the amygdala, hippocampus, medial PFC, and ACC in response to negatively valenced emotional stimuli, which is in turn related to heightened emotional and physiological responding (Bradley et al., 2001; Stevens and Hamann, 2012). Moreover, activity of cortico-limbic structures underlies depression and anxiety specific cognitive processes that have clear sex differences favoring women, such as rumination and heightened memory for emotional stimuli (Canli et al., 2002; Leach et al., 2008). Together, studies of clinical populations illustrate the role of cortico-limbic circuitry in internalizing psychopathology, and are supported by the normative sex differences in neural structure and function that may serve as mechanisms of women's increased risk (Cahill, 2006; Madeira and Lieberman, 1995; Mechelli, 2005). Thus, studying this neural system and its association with early emerging internalizing symptoms may shed light on the neural processes that underlie the development of internalizing disorders, particularly for women.
1.3. Developmental neurobiology of internalizing problems
Development of cortico-limbic structures differs in the first two decades of life, with limbic structures maturing earlier than cortical areas (Mills et al., 2014). Considering the well-established regulatory role of the PFC on the amygdala (Banks et al., 2007; Jackson and Moghaddam, 2001), asynchronous development of cortico-limbic circuitry is thought to mark a sensitive period in adolescence that coincides with an increase in internalizing problems, and the emergence of sex differences in their prevalence (Jones et al., 2017; Zahn-Waxler et al., 2008). For instance, reduced fronto-limbic functional connectivity is associated with increases in depressive symptoms across adolescence, and this atypical functional connectivity at baseline differentiated symptom-free controls from adolescents who developed symptoms later on (Scheuer et al., 2017). Moreover, Burghy et al. (2012) report an association between early life stress and elevated stress reactivity in infants that prospectively predicted altered prefrontal-amygdala connectivity in adolescence, which was further related to concurrent anxious and depressive symptoms. Of note, these associations were found for females only (Burghy et al., 2012), underscoring the need to consider sex when studying the developmental neurobiology of internalizing psychopathology. Similarly, altered connectivity and function of cortico-limbic circuitry has been demonstrated in pediatric anxiety disorders (Swartz and Monk, 2013). In contrast, increasing functional connectivity of cortico-limbic connectivity in the course of normative development is associated with normative reductions in anxiety and better emotion regulation (Gee et al., 2013). Together, these lines of evidence highlight the importance of understanding the developmental trajectory of cortico-limbic circuitry as a potential risk marker for internalizing psychopathology, and point to specific mechanisms by which atypical development confers risk. However, work thus far has been limited to adolescence, a period in which many youth have already developed significant symptoms (Copeland et al., 2014; Moilanen et al., 2010). Examining neural structure and function in cortico-limbic circuitry in childhood may speak more clearly to neural precursors of disorder.
1.4. Structural connectivity
Contemporary models of brain function emphasize the highly connected and interactive organization of the brain, whereby distinct regions communicate and influence each other (Sporns et al., 2004). Indeed, bidirectional axonal connections exist between elements of cortico-limbic circuitry, such that the limbic system relays information to cortical regions which in turn regulate limbic activation, influencing emotional reactivity (Banks et al., 2007; Ghashghaei et al., 2007). Structural connectivity then refers to white matter (WM) tracts, which are highly organized fiber bundles composed primarily of myelinated axons, that mediate communication between grey matter structures (Honey et al., 2009). Given the continuous reconfiguration of neuronal activity (Honey et al., 2009), especially in children whose brains are undergoing rapid change, methods that speak to structural connectivity provide a practical and robust approach to studying brain networks (Van Den Heuvel et al., 2009). One such method, Diffusion Tensor Imaging (DTI), allows in-vivo investigation of the microstructure of WM tracts, yielding fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD) parameters (Alexander et al., 2007; Feldman et al., 2010; Soares et al., 2013). While FA provides a global index of tract microstructure, AD and RD provide more specific information about the axonal factors driving FA; increased RD has been associated with reduced myelination and diffuse packing of axons within a bundle while lower AD has been related to changes in intercellular fluid that may result from axonal degeneration (Alexander et al., 2011a; Aung et al., 2013).
With respect to major tracts that may be relevant, the cingulum bundle (CB) and the uncinate fasciculus (UF) govern communication within cortico-limbic circuity. The CB runs from the anterior to the posterior of the brain, and is thought to mediate communication between components of the limbic system and the cingulate gyrus, as well as between regions of the cingulate gyrus (Catani and Thiebaut de Schotten, 2008). The UF is a hook-shaped bundle that connects the limbic system to regions of the PFC (Catani and Thiebaut de Schotten, 2008). Given their anatomical projections, structural integrity of these tracts is crucial to emotion processing and regulation (Schmahmann et al., 2008).
Consistent with developmental changes in cortico-limbic circuitry structure and function, studies demonstrate normative increases in FA of the UF and CB that peak in adulthood (Johnson et al., 2014a; Lebel et al., 2012; Swartz et al., 2014). Higher FA is thought to reflect greater integrity of the WM tracts, and therefore more efficient communication between grey matter structures; indeed, increasing FA of cortico-limbic tracts across development is associated with better emotion regulation (Kim and Whalen, 2009). In addition, sex differences in the rate of WM development have been noted in the UF and CB, particularly in childhood (Johnson et al., 2014b), further demonstrating the need to consider sex when studying structural connectivity in this age window.
Past work using DTI with clinical populations implicates dysfunctional connectivity and alterations in the WM microstructure of major tracts within cortico-limbic circuitry in adult depressive and anxiety disorders (Ayling et al., 2012; Sexton et al., 2009). The limited work on depressed adolescents and young adults also shows reduced FA of WM in prefrontal areas, cingulum, limbic system and thalamic projection tracts, and the superior longitudinal fasciculus (SLF; Bessette et al., 2014; Cullen et al., 2010; Murphy and Frodl, 2011). Conversely, depressed women were found to have increased FA in segments of the corticospinal tract, compared to controls, which was hypothesized to be related to the psychomotor symptoms of depression given connectivity of this tract to cortical motor centers (Sacchet et al., 2014). Similarly, young adults with social anxiety and generalized anxiety disorder exhibit reduced volume and FA of the UF compared to controls (Baur et al., 2013; Baur et al., 2012; Hilbert et al., 2014; Phan et al., 2009). However, Han et al. (2008) reported increased FA of the anterior and posterior regions of the cingulate gyrus in adults with panic disorder, which may account for the increased attention to interoceptive cues seen in this disorder, given that the cingulate is involved in learning and selective attention (Hayden and Platt, 2009).
Emerging research suggests that altered WM microstructure may also mark vulnerability to internalizing disorders. For instance, healthy young women with parental history of depression and subclinical anhedonia were found to have lower FA of both left and right cingulum bundles (Keedwell et al., 2012). Using voxel-wise analyses, Huang et al. (2011a) report reduced FA in localized segments of the left cingulum, corpus callosum, bilateral SLF, UF, and fronto-occipital fasciculi in at-risk adolescents with parental history of depression, suggesting that identification of specific loci within WM tracts may be of unique relevance to at-risk populations. Indeed, using Automated Fiber Quantification (AFQ; Yeatman et al., 2012), Ho and colleagues (2017) found that reduced FA in a frontal segment of the right UF predicted sensitivity to early life stress and subsequent anxiety symptoms in a community sample of adolescents.
1.5. Research objectives
In summary, extant literature supports cross-sectional associations between WM structure and both subthreshold and clinical manifestations of internalizing psychopathology in adolescents and adults. However, it is unclear how early such variations in WM appear in life, and how they relate to other markers of risk in childhood. Early emerging internalizing symptoms, even when subthreshold in severity, are clearly related to increased risk for later disorder (W. Copeland et al., 2009; Roza et al., 2003; Toumbourou et al., 2011), particularly when such symptoms are persistently elevated across childhood. Typically developing children show variation in symptom manifestations such that some have consistently low symptoms while others show stably elevated symptoms over time, and are hence at greater risk for later disorder (Fernandez Castelao and Kröner-Herwig, 2013; Sterba et al., 2007). If specific patterns of structural connectivity in cortico-limbic circuitry are associated with elevated early symptoms, this would provide additional support for the notion that these structural patterns serve as a mechanism of risk for internalizing symptoms.
We therefore used DTI to examine the integrity of the WM of the CB and UF in a community sample of 42 seven-year-old girls, looking at cross-sectional associations with internalizing symptoms. In addition to its aforementioned value as a tool for investigating structural connectivity, DTI also has the key benefit of facilitating the study of neural structures in children as acquisition time is considerably shorter compared to other neuroimaging methods. Given that the sex differences in childhood WM development could introduce undesirable variability (Johnson et al., 2014b), as well as females' elevated risk for internalizing psychopathology (McLean et al., 2011b; Nolen-Hoeksema, 2001), we included only young girls in this study. Moreover, to see whether any cross-sectional associations between FA and symptoms were found when considering more long-term symptom presentations, we capitalized on the availability of multiple waves of symptom scores to examine whether variations in WM microstructure were associated with stably elevated symptoms during early childhood, a pattern of symptoms known to mark risk for later internalizing disorder (Clark et al., 2007; W. Copeland et al., 2009; Roza et al., 2003; Toumbourou et al., 2011). We anticipated that elevated internalizing symptoms concurrent to DTI data collection would be associated with reduced FA in the CB and UF, and that girls with stably elevated symptoms would also show reduced FA in these tracts compared to girls with other symptom patterns.
2. Methods
2.1. Participants
Participants were 46 seven-year-old girls drawn from a larger study of 409 community-dwelling children, recruited to this study based on their responses to a stress task designed to elicit cortisol reactivity (described further below). At the larger study baseline, eligible three-year-old children had at least one biological parent and no significant medical and psychological problems (e.g., Kryski et al., 2011; Kryski et al., 2013). As a further screen at baseline, children were administered the Peabody Picture Vocabulary Test (PPVT; Dunn and Dunn, 1997), and were of average cognitive ability (M = 112.0, SD = 14.05). The larger sample was predominantly White (93.2%) and over half of families were middle-class, reporting a family income between CAD $40,000 and CAD $100,000. These characteristics are comparable to that of the population of London, ON area from which they were recruited (Statistics Canada, 2017). In addition to the baseline assessment at child age three, children and families in the larger study participated in additional waves of data collection when children were six and eight years old, with girls in the current study participating in a DTI session at age 7 (M = 6.7, SD = 0.68). This study was approved by the University of Western Ontario Health Sciences Research Ethics Board.
2.2. Study procedure
Of the 409 children comprising the longitudinal sample, only right-handed girls (N = 210) were eligible to participate in this follow-up, given that the aim of this study is to investigate early neural risk factors in girls, and to control for sex differences in neural development. The eligible participant pool was refined further based on girls' cortisol reactivity to a stress task conducted at baseline (Kryski et al., 2011), such that only girls at the extreme ends of cortisol reactivity (i.e., using quartile scores based on mean cortisol level over time and cortisol change from baseline in response to the stress task) were eligible (N = 117). The effect of cortisol reactivity on neural factors was part of a different planned analysis from the one examined in the present study, and for the present purposes all children were considered in a single group irrespective of cortisol reactivity data.1 Parents gave consent for 58 girls, who were then invited to participate in a 1-h mock scanning session to determine whether girls were likely to be compliant with imaging data collection procedures. Forty-six girls completed both the mock-scanner training and the DTI session. To further increase compliance at the DTI session, the assessment was described as a “space mission” and girls were given an astronaut suit to wear during the visit. Girls also watched a developmentally appropriate film during image acquisition. The primary caregiver completed the Child Behavior Check List (CBCL; Achenbach, 1991) during either the mock scanner or DTI visit.
2.3. Longitudinal latent profile analysis of symptoms
The CBCL was also completed by the child's primary caregiver when girls were three (M = 3.5, SD = 0.30), six (M = 6.0, SD = 0.31), and eight (M = 8.7, SD = 0.71) years old. The CBCL asks the parent to rate the frequency and intensity of their child's emotional and behavioral problems over the past 6 months. As we were interested in internalizing symptoms common in middle childhood, scores from the anxious/depressed subscale were used in this study (12 items; range of Cronbach's αs = 0.68–0.80). Early internalizing symptoms show homotypic continuity with later clinically significant manifestations of anxiety and depression (Toumbourou et al., 2011; Zahn-Waxler et al., 2016), particularly when stably elevated over time. To support our analyses using cross-sectional measures of symptoms, and to better capture symptom presentation over time, we used longitudinal Latent Profile Analysis (LPA; Mplus v.8, Muthén and Muthén, 2017) to characterize our participants in terms of internalizing symptom patterns across early childhood (obtained concurrent to the DTI session as well as at ages 3, 6, and 8). LPA allows one to examine individuals based on shared patterns of observations that can then be related to other variables of interest (Berlin et al., 2014). As a data reduction method, LPA identifies the smallest number of homogenous groups that account for maximum variation in the indicator variables (Lazarsfeld and Henry, 1968; Oberski, 2016), symptom scores at each time point in this case. The optimal number of profile groups is determined by estimating a series of a priori models that can then be compared against each other using relative goodness-of-fit indices. Each individual is assigned probability scores that reflect the likelihood that they belong to each of the profile groups in each model based on their similarity to others in the group.
Past work on childhood internalizing symptom patterns suggests some youth exhibit low-stable symptoms, others show high variability, and other youth show stably elevated symptoms (Sterba et al., 2007). Accordingly, and given our relatively small sample, we tested 3- and 2-profile group models. To determine the best fitting model, the Vuong-Lo-Mendell-Rubin and Lo-Mendell-Rubin adjusted LRT goodness-of-fit indices were used, which test whether an additional profile group significantly improves the model's ability to explain variation in the indicator variables. t-tests were then conducted to compare mean FA of previously identified segments within the CB and UF between the groups resulting from the best fitting model.
2.4. Diffusion tensor image acquisition
MRI scans were conducted at Western's Centre for Functional and Metabolic Mapping (CFMM), on a 3 T Siemens TIM Trio scanner equipped with a 32-channel head coil. Using echo planar imaging, a non-diffusion weighted b0 scan was first acquired in the axial plane, followed by diffusion-weighted scans in 30 directions (b1 = 700 s/mm2; iPAT GRAPPA acceleration factor = 2; TR = 9100 ms; TE = 91 ms; voxel size = 2 × 2 × 2 mm; 62 slices; in-plane FOV = 192 mm2). Additionally, a T1-weighted anatomical MRI scan was collected using a T1 MPRAGE sequence (iPAT GRAPPA acceleration factor = 2; TR = 2300 ms; TE = 3.01 ms; voxel size = 1 × 1 × 1 mm; 192 slices; in-plane FOV = 256 mm2).
2.5. Data preprocessing and analysis
Diffusion MRI data were preprocessed using the mrDiffusion package available as part of the open-source VISTA Lab MATLAB toolbox (http://web.stanford.edu/group/vista/cgi-bin/wiki/index.php/MrDiffusion). The raw diffusion weighted images were first corrected for eddy current distortions and movement by co-registration to the non-diffusion weighted (b0) image, using a rigid body transformation algorithm. Raw images were then resampled, combining the eddy current-correction, motion-correction and anatomical alignment parameters using the 7th-order b-spline algorithm available in the SPM8 toolbox, and rotating b-vectors accordingly. Four girls were excluded from the sample as over 20% of their acquired volumes contained motion and eddy current artifacts, yielding a final sample of 42 participants. For each of the remaining participants, translational and rotational movement parameters were combined to produce measures of absolute mean displacement (M = 0.83, S.D. = 0.43) and maximum displacement (M = 1.23 mm, S.D. = 0.78). Mean and maximum displacement did not differ significantly between the high and low risk groups (p = 0.86 and p = 0.67, respectively). Diffusion tensors were fit using a least-squares algorithm, which also removed outliers from the tensor estimation. The eigenvalue decomposition of the diffusion tensor at each voxel was then computed, which represent the magnitude of diffusion, and directions of maximal and minimal diffusion. Eigenvalues were then used to calculate the following at each voxel: (1) axial diffusivity (AD), which represents diffusion along the long axis of a fascicle of fibers; (2) radial diffusivity (RD), which describes diffusion perpendicular to the long axis of a fascicle of fibers; and (3) fractional anisotropy (FA), which is the normalized standard deviation of the three eigenvalues and indicates the degree of anisotropy, (i.e., the overall directionality of diffusion).
Analysis of the processed DTI data and identification of major fiber tracts was done individually for each participant using Automated Fiber Quantification (AFQ) (Yeatman et al., 2012). AFQ is a novel method for the analysis of DTI data that automates the identification of regions of interest (ROIs) across subjects, and quantifies diffusion properties at multiple nodes along a tract's length (Yeatman et al., 2012). Relative to other DTI analysis methods, AFQ circumvents errors that may arise from manual identification of ROIs and gives weight to normative variations in WM microstructure that occur along a tract's trajectory that may be obscured by mean measures (Yeatman et al., 2012). As such, AFQ allows fine-grained identification of specific loci in WM tracts where microstructure variations may be driving observed behavioral differences. The AFQ pipeline consists of 3 main steps: (1) whole-brain tractography, (2) region-of-interest (ROI)-based tract segmentation and cleaning, and (3) fiber tract quantification. First, a deterministic streamline tracking algorithm (STT) is applied to estimate fiber tracts, and tracking is initiated from voxels with FA values >0.3. Streamlines are traced in both directions along the principal diffusion axes, and propagation is terminated if the FA estimated at the current position is below 0.2 in addition to the angle between the last path segment and next step direction being >30° (Yeatman et al., 2012).
In the second step, the resulting tracts are segmented into anatomically defined fascicles using the waypoint ROI procedure (Wakana et al., 2007). Briefly, this procedure categorizes tracts as belonging to the same fascicle if they pass through two waypoint ROIs that are based on group-averaged DTI data in MNI space. Each resulting fiber is then compared to a standard fiber tract probability map (Hua et al., 2008) and tracts with high scores, reflecting higher probability of belonging to a fascicle, are retained. Outlier fibers are removed if their length is >4 standard deviations above the mean or if they are >5 standard deviations from the fiber tract core, resulting in coherently bundled fibers at the center of the tract's trajectory. The third step, quantification, is limited to the tract's core which spans the portion bounded by the ROIs. This limits variability between subjects due to anatomical differences between brains. Diffusion properties (FA, AD, and RD) were quantified at 100 equidistant nodes along the core of the CB and UF bilaterally, yielding a tract profile for each tract. Cross-sectional associations between concurrent CBCL score on the Anxious/Depressed subscale and FA were examined using bivariate correlations, which were used to determine whether region-wise brain-behavior associations were localized to specific segments within each tract. We corrected for multiple comparisons using permutation-based suprathreshold cluster tests outlined by Nichols and Holmes (2002); this approach employs a Monte Carlo method to assess a false positive rate for each tract, which can then be used to determine the number of spatially contiguous nodes that must meet an alpha of 0.05 in order to be considered significant. Cluster threshold was set at p = 0.01, and yielded the following cluster sizes for each tract: left CB = 7; right CB = 7; left UF = 8; right UF = 9. As FA is a global index of WM microstructure, we were interested in investigating which axonal properties influenced FA in these regions. To this end, we computed correlations between concurrent symptoms and mean RD, and mean AD, for each segment. Finally, because WM tracts are highly correlated, associations between the tracts of interest and internalizing symptoms may reflect a global WM phenomenon, rather than functional specificity of the CB and UF. As such, we examined whether the FA in the inferior longitudinal fasciculus (ILF), which is not hypothesized to be related to affective processing (Ashtari, 2012), was also associated with internalizing symptoms.
3. Results
3.1. Cross-sectional analyses
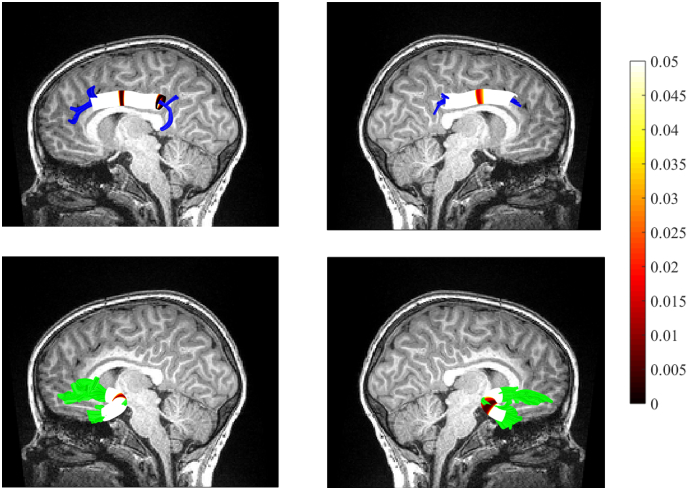
Table 1 presents means, standard deviations, and bivariate correlations between all major study variables. FA at each node along the tracts of interest were used as our primary DVs. Neither FA nor internalizing symptoms were related to demographic variables. FA in two distinct segments of the left CB, a posterior and medial segment (shown in Fig. 1a), was significantly associated with concurrent internalizing symptoms. Similarly, concurrent symptoms and FA of a medial segment of the right CB were negatively correlated, shown in Fig. 1b. Only mean RD of the posterior segment of the left CB was positively correlated with concurrent symptoms (r(38) = 0.351, p = 0.031), suggesting that increased diffusion in the direction perpendicular to the fascicle axis may be driving FA reductions. Significant negative associations were found between concurrent symptoms and medial segments in the left UF (Fig. 1c), and right UF (Fig. 1d). Axial diffusivity in the medial segment of the left UF was found to be negatively correlated with concurrent symptom scores; r(39) = −0.338, p = 0.035, suggesting that reduced FA in this region can be explained by impeded diffusion along the long axis of the fascicle. In the right UF, radial diffusivity in the medial segment was found to be positively associated with concurrent symptoms; r(39) = 0.400, p = 0.012, indicating that reductions in mean FA can be accounted for by impeded diffusion in the direction perpendicular to the fascicle axis. No significant associations were found between internalizing symptoms and FA at individual nodes in the left or right ILF (all ps > 0.05).
Table 1.
Bivariate correlations between symptoms and mean FA of segments of interest of the bilateral CB and UF.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. T1 CBCL Anxious/Depressed Symptoms | – | |||||||||||||
| 2. T2 CBCL Anxious/Depressed Symptoms | 0.33⁎ | |||||||||||||
| 3. T3 CBCL Anxious/Depressed Symptoms | 0.27 | 0.72⁎⁎ | ||||||||||||
| 4. T4 CBCL Anxious/Depressed Symptoms | 0.37⁎ | 0.45⁎⁎ | 0.42⁎ | |||||||||||
| 5. FA of the posterior segment of the left CB | −0.33⁎ | −0.55⁎⁎ | −0.51⁎⁎ | −0.34⁎ | ||||||||||
| 6. FA of the medial segment of the left CB | 0.11 | −0.33⁎ | −0.45⁎⁎ | −0.17 | 0.36⁎ | |||||||||
| 7. FA of the medial segment of the right CB | −0.05 | −0.39⁎ | −0.43⁎ | −0.33 | 0.47⁎⁎ | 0.62⁎⁎ | ||||||||
| 8. FA of the medial segment of the left UF | −0.16 | −0.29 | −0.38⁎ | −0.09 | 0.31 | 0.19 | 0.09 | |||||||
| 9. FA of the medial segment of the right UF | −0.09 | −0.42⁎⁎ | −0.45⁎⁎ | −0.09 | 0.54⁎⁎ | 0.42⁎⁎ | 0.20 | 0.40⁎⁎ | ||||||
| 10. Age at DTI (years) | −0.20 | −0.19 | −0.12 | −0.18 | −0.04 | −0.05 | 0.09 | 0.18 | 0.03 | |||||
| 11. Risk group | 0.44⁎⁎ | 0.68⁎⁎ | 0.85⁎⁎ | 0.51⁎⁎ | −0.44⁎⁎ | −0.39⁎ | −0.32 | −0.36⁎ | −0.38⁎ | −0.23 | ||||
| 12. PPVT | −0.25 | −0.01 | −0.01 | 0.14 | −0.03 | −0.28 | −0.24 | 0.20 | 0.09 | 0.33⁎ | −0.09 | |||
| 13. Family Income | −0.13 | −0.10 | −0.25 | 0.09 | −0.10 | 0.26 | −0.14 | 0.01 | 0.21 | −0.17 | −0.24 | 0.22 | ||
| 14. Ethnicity | −0.13 | 0.01 | −0.24 | −0.06 | 0.14 | 0.05 | 0.09 | 0.25 | 0.15 | 0.28 | −0.23 | 0.20 | −0.23 | – |
| Mean | 2.23 | 2.53 | 2.26 | 3.16 | 0.40 | 0.51 | 0.49 | 0.44 | 0.39 | 6.72 | 0.24 | 112.00 | 3.85 | 1.14 |
| SD | 2.21 | 2.12 | 2.26 | 3.01 | 0.06 | 0.07 | 0.08 | 0.06 | 0.05 | 0.68 | 0.43 | 14.43 | 1.09 | 0.35 |
CBCL = Child Behavior Checklist; FA = Fractional Anisotropy; CB = Cingulum Bundle; UF = Uncinate Fasciculus; PPVT Peabody Picture Vocabulary Test; DTI = Diffusion Tensor Imaging; Risk group: Low = 0; High = 1; Family income:1 < $20,000, 2 = $20,000—$40,000, $40,001—$70,000 = 3, $70,001—$100,000 = 4, and > $100,001 = 5; Ethnicity: 1 = Caucasian, 2 = Other.
p < 0.05.
p < 0.01.
Fig. 1.
Tract profiles of a) left cingulum bundle, b) right cingulum bundle (both shown in blue), c) left uncinate fasciculus, and d) right uncinate fasciculus (both shown in green), with red color indicating segments where FA was significantly correlated with internalizing symptoms. The range of p-values is shown on the color bar. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Longitudinal analyses
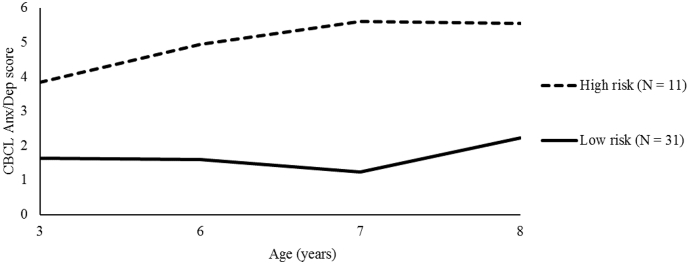
As a supplement to the cross-sectional symptom measures, we used LPA to examine whether similar associations with structural connectivity would be found with any high-risk groups that emerged based on symptom manifestations over time. The Vuong-Lo-Mendell-Rubin and Lo-Mendell-Rubin adjusted LRT tests comparing a 2- vs. a 3-profile group model were non-significant (p = 0.17 and p = 0.19, respectively), indicating that a more parsimonious, 2-profile group model was sufficient to represent the symptom patterns during childhood in this sample (Fig. 2). The first profile group consisted of girls whose symptoms remained consistently low over time (referred to as the low-risk group; N = 31, 74.8%), while girls in the second profile group had symptoms that increased over time and then stabilized (referred to as the high-risk group; N = 11, 26.2%). These group sizes were deemed acceptable as each represented >10% of the sample, and similar group sizes have been reported in other studies using LPA (Au et al., 2013; Brinkman et al., 2013; Cohan et al., 2008; Hill et al., 2006). Moreover, given past work on the prevalence of internalizing problems in this age group (Furniss et al., 2006; Slemming et al., 2010), we did not expect the two groups to be equal in size. The groups did not differ in age at each data collection time point, family income, or ethnicity (all ps > 0.05). Not surprisingly, the high-risk group exhibited significantly higher symptoms than the low-risk group at T1 (t(40) = −2.22, p = 0.004), T2 (t(38) = −6.52, p < 0.001), T3 (t(37) = −9.37, p < 0.001), and T4 (t(36) = −3.53, p = 0.001).
Fig. 2.
Latent Profile Analysis plot for CBCL Anxiety/Depression symptom profiles (2-class model).
3.3. Risk group comparisons
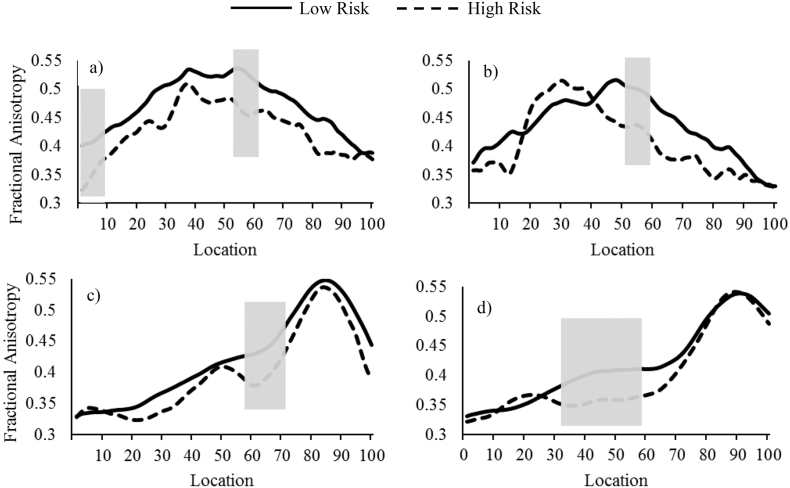
Next, we used independent group t-tests to compare the high- and low-risk groups on mean FA of segments previously identified; i.e., the medial and posterior segment of the left CB, medial segment of the right CB, medial segment of the left UF, and medial segment of the right UF. The high-risk group had significantly lower FA of the left medial and posterior CB segments (t(39) = 3.46, p = 0.001; t(39) = 2.97, p = 0.005, respectively), as well as the right CB (t(34) = 2.13, p = 0.04) compared to the low-risk group. This difference was marginal for the medial left UF segment (t(40) = 1.92, p = 0.062). The high-risk group also had lower FA in the medial segment of the right UF (t(40) = 2.55, p = 0.015), compared to the low-risk group. Regions of significance along each tract profile are shown in Fig. 3. These findings are consistent with cross-sectional associations with symptoms reported here, implicating reduced FA in segments of the left and right CB, and left and right UF in girls at high-risk for internalizing disorders, suggesting that these brain patterns mark the presence of persistent behavioral manifestations of internalizing disorders risk, rather than just concurrent symptoms.
Fig. 3.
Tract FA profiles for the a) left cingulum bundle, b) right cingulum bundle, c) left uncinate fasciculus, and d) right uncinate fasciculus showing regions where mean FA differed significantly between risk groups. Location of cingulum bundle nodes are shown posterior to anterior, and those of the uncinate fasciculus are shown temporal to prefrontal.
4. Discussion
We investigated associations between early emerging internalizing symptoms and microstructure of WM tracts involved in emotion processing and regulation in a community sample of girls. Consistent with previous literature on at-risk adolescents and adults (Huang et al., 2011b; Keedwell et al., 2012; Whalley et al., 2013), concurrent internalizing symptoms in childhood were associated with reduced integrity of two major WM tracts within cortico-limbic circuitry, the cingulum bundle and uncinate fasciculus. Further, girls with elevated internalizing symptoms across childhood exhibited reduced integrity of WM in segments of these tracts, compared to girls with stably low symptoms. In line with past work with clinical populations demonstrating altered structural connectivity in affective processing networks (Kaiser et al., 2015), reduced integrity within the CB and UF appearing in childhood may underlie aberrant emotion processing patterns that manifest as early emerging anxious and depressed symptoms. Overall, altered structural connectivity of the fronto-limbic circuitry may be a mechanism by which early emerging symptoms confer elevated risk for later internalizing psychopathology.
Our finding that reduced integrity of WM in the CB and UF is associated with internalizing symptoms in childhood sheds light on specific brain-behavior relationships that may put children at risk for later anxious and depressive symptoms. Internalizing disorders are characterized by maladaptive emotion processing, which is governed by cortico-limbic circuitry (Drevets et al., 2008; Phillips et al., 2003a; Price and Drevets, 2012). Phillips et al. (2003b) proposed that emotion perception occurs through processes that are determined by activity of specific neural structures within this circuitry. In particular, the amygdala is involved in modulating attention and immediate response to emotionally evocative stimuli, suggesting that its activity governs identification of the emotional significance of a stimulus, information that is relayed to frontal regions such as the cingulate gyrus and PFC (Phillips et al., 2003a; Stein et al., 2007). Activity of the cingulate and PFC is associated with affective and behavioral responding, as well as reward anticipation and modulation of autonomic nervous system functioning, which, in turn, underlies neurovegetative processes, such as arousal, sleep, and appetite known to go awry in internalizing psychopathology (Koenigs and Grafman, 2009; Phillips et al., 2003a). Finally, appraisal and production of affective state processes are regulated by activity in the dorsal regions of the PFC and anterior cingulate gyrus, and the hippocampus (Phillips et al., 2003a). In particular, activity of these regions is correlated with inhibition of the stress response, effortful control, and active allocation of attentional resources (Ahmed et al., 2015).
Taken together, this model highlights the significance of bidirectional pathways between frontal regions and the limbic system in determining both adaptive and maladaptive emotion expression and regulation (Stein et al., 2007). Accordingly, the reduced WM integrity in the CB and UF we found in young girls may mark impairments in the capacity to effectively regulate responses to stress, potentially laying the groundwork for internalizing vulnerability in the context of negative life events. Indeed, previous work from our lab revealed reduced FA in tracts adjacent to the thalamus, right ACC and superior frontal gyrus in girls with elevated cortisol reactivity, that was moderated by early parenting (Sheikh et al., 2014). Furthermore, Dufford and Kim (2017) recently demonstrated lower FA in portions of the UF and CB in children from lower-income families who had greater exposure to cumulative stress. Further underscoring the role of the environment, Adluru et al. (2017) report that negative associations between FA and anxious symptoms in adolescence may be better explained by unshared environmental influences relative to genetic or unshared environmental factors. Alternatively, reduced FA may reflect differences in the maturation of the CB and UF, especially considering that WM maturation in these tracts exhibits rapid change in the age window studied (Lebel et al., 2012; Lebel and Beaulieu, 2011; Olson et al., 2015). Longitudinal studies are needed to more clearly understand interactions between the developmental trajectories of these tracts and environmental factors as they relate to the development of risk for internalizing disorders.
Our findings suggest a shared neural vulnerability for internalizing problems, consistent with the well-established etiological overlap between anxiety and depressive disorders, especially in childhood and adolescence (Cummings et al., 2014). However, some neural activation patterns in cortico-limbic structures may differ between anxiety and depression (Liotti et al., 2000; Thomas et al., 2001). Given the young age of our sample, depression in particular is quite rare (Luby et al., 2009), leaving little opportunity to examine processes that may be specific to it versus anxiety. Investigating whether specific WM tracts differentiate youth with anxiety versus depression later in development is an important direction for future research.
We identified reduced FA in localized segments of the CB and UF using an automatic fiber alignment, identification and quantification procedure that also permitted us to identify behavioral associations at specific points along each tract (Yeatman et al., 2012). Specifically, reduction in WM integrity was limited to medial and posterior segments of the left CB, medial segment of the right CB, and medial segments of the left and right UF. The CB runs within the WM of the cingulate gyrus, which is a key structure implicated in controlling arousal and visceral states associated with emotion and social behavior (Hadland et al., 2003). In particular, the CB mediates communication between distal portions of the cingulate gyrus, and links the prefrontal regions with the temporal cortex by way of the cingulate gyrus (Catani and Thiebaut de Schotten, 2008; Jellison et al., 2004; Kier et al., 2004; Schmahmann et al., 2008). Accordingly, the CB is hypothesized to play an integral role in organizing perception of emotional valence associated with memory, motivation and somatic sensations (Schmahmann et al., 2008). Consistent with studies indicating substantial variation in anatomical properties within the CB (Bubb et al., 2018), our analyses were focused on the core of this tract. It is likely that the segments with reduced WM integrity identified in the current sample are those where the CB receives input from the medial cingulate gyrus and projects to the rostral cingulate gyrus, reflecting dysfunction in communication within this structure. This may in turn contribute to the deficits in emotional arousal and social withdrawal associated with internalizing symptoms.
The UF is a local association fiber that links the PFC with structures within the temporal lobe, specifically the amygdala (Catani and Thiebaut de Schotten, 2008; Kier et al., 2004). Given the top-down regulatory influence of the PFC on the temporal lobe (Banks et al., 2007; Ghashghaei et al., 2007), the UF is implicated in regulating emotional responsivity to auditory and visual stimuli, as well as cognitive processes underlying emotion (Schmahmann et al., 2008). Our findings tie elevated symptom profiles in girls with reduced WM integrity in the medial segments of the left and right UF, which may correspond to the region that marks the tract's entry into the temporal lobe (Catani et al., 2002; Kier et al., 2004). Reduced WM integrity of this segment may indicate disruptions in regulatory control by the PFC on the amygdala, accounting for elevated negative emotional responsivity seen in girls with elevated internalizing symptom profiles. Essentially, reduced WM integrity localized to specific segments, rather than the entire tract, may be related to the subthreshold nature of the internalizing symptoms in our sample. It is possible that reductions in WM integrity in the CB and UF becomes increasingly prominent as symptoms become more pronounced and impairing.
We did not find associations between FA of the ILF and internalizing symptoms in our sample. Structural integrity of the ILF has been implicated in visual perceptual functioning, given that it mediates communication between the occipital and temporal lobes (Ashtari, 2012). As such, altered organization of WM microstructure in relation to internalizing symptoms may be specific to the CB and UF, rather than reflecting a global WM phenomenon.
By examining specific diffusion indices, we found that elevated symptoms were associated with higher RD in the left posterior CB and right medial UF, and reduced AD in the left medial UF. Directionality and degree of water diffusion is a passive process that is determined by the boundaries that restrict motion. Generally, radial diffusivity is restricted by myelin that occupies intra-axonal space within a bundle, whereas diffusivity along the axis of the tract is influenced by changes in the extracellular water due to inflammation and degree of axonal maturation that impedes motion of water molecules, and is higher in healthy axonal bundles (Alexander et al., 2011b, 2007; Aung et al., 2013). Thus, reduced AD may indicate delayed maturation in that region of the tract, or potential degeneration of the axonal bundle, whereas higher RD indicates less restricted diffusivity of water molecules perpendicular to the tract axis and may be suggestive of demyelination. Future studies should examine RD and AD in clinical populations to determine which axonal properties account for altered WM microstructure in internalizing disorders toward the goal of replicating and extending our findings.
The present study has several strengths: we capitalized on multiple waves of data collection across childhood in girls not yet experiencing clinically significant symptoms, providing novel information regarding structural connectivity patterns that may be important in driving vulnerability to disorder. However, DTI data were obtained at one time point only, precluding our ability to make conclusions about the temporal association between symptoms and variations in brain structure. Additionally, we focused only on subthreshold symptoms, a known marker of risk for later disorder (Clark et al., 2007; Copeland et al., 2009; Toumbourou et al., 2011). Nevertheless, we cannot conclude that the pattern of effects obtained predicts clinically significant disorder. Furthermore, our sample size was relatively small. Thus, further longitudinal work that follows a larger sample well into the age of risk for frank disorder is needed to provide more conclusive information on brain-disorder associations over time. Our investigation was limited to girls, which is a reasonable first step toward uncovering mechanisms that contribute to the increased risk of internalizing psychopathology in women. However, further research is needed to determine if similar patterns are observable in young boys. Moreover, the use of a primarily Caucasian, low-risk sample limits the generalizability of our findings, and extending this work to more heterogeneous and higher-risk samples is needed. While our study is focused specifically on internalizing symptoms, work that investigates shared and unshared neural correlates of both internalizing and externalizing symptoms is needed, considering the heterotypic continuity between internalizing and externalizing psychopathology (Costello and Mustillo, 2003).
Our findings dovetail with past work on the neural correlates of adolescent and adult internalizing disorders by demonstrating similar patterns in childhood, highlighting aspects of structural connectivity of cortico-limbic circuitry in girls that may put them at risk for later internalizing psychopathology. Findings suggest that WM alterations constitute a putative mechanism that underlies the maintenance of internalizing symptoms across childhood, potentially laying the groundwork for the onset of depressive and anxiety disorders later in development.
Funding sources
This research was supported by grants from Canadian Institutes of Health Research (CIHR) (MOP 86458), the Academic Development Fund (UWO), and Children's Health Research Institute. The Center for Functional and Metabolic Mapping (CFMM) is funded by Brain Canada and Canada Foundation for Innovation (CFI).
Conflicts of interest
The authors have no conflicts of interest to report.
Acknowledgements
The authors would like to that all the families who participated in this study, especially for their continued involvement in the Child Development Project.
Footnotes
While cortisol reactivity was not a variable of interest in the current study, we conducted further analyses to ensure that high- vs. low-cortisol reactivity did not influence any current findings. A chi-square test showed that latent profile membership was not associated with cortisol reactivity group χ(1) = 0.120, p = .729. Moreover, cortisol reactivity groups did not differ on demographics, symptom scores, or white matter microstructure measures reported below (all ps > 0.05).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.101650.
Appendix A. Supplementary data
leftCC
leftUFfig
rightCC
rightUF
References
- Achenbach T.M. 1991. Manual for the Child Behavior Checklist/ 4-18 and 1991 Profile. (Burlington) [Google Scholar]
- Adluru N., Luo Z., Van Hulle C.A., Schoen A.J., Davidson R.J., Alexander A.L., Goldsmith H.H. Anxiety-related experience-dependent white matter structural differences in adolescence: a monozygotic twin difference approach. Sci. Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-08107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.P., Bittencourt-Hewitt A., Sebastian C.L. Neurocognitive bases of emotion regulation development in adolescence. Dev. Cognitive Neurosci. 2015;15:11–25. doi: 10.1016/j.dcn.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A.L., Hurley S.A., Samsonov A.A., Adluru N., Hosseinbor A.P., Mossahebi P., Tromp D.P.M., Zakszewski E., Field A.S. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity. 2011;1(6):423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A.L., Hurley S.A., Samsonov A.A., Adluru N., Hosseinbor A.P., Mossahebi P., Tromp D.P.M., Zakszewski E., Field A.S. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity. 2011;1(6):423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico F., Meisenzahl E., Koutsouleris N., Reiser M., Moller H.-J., Frodl T. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J. Psychiatry Neurosci. 2011;36(1):15–22. doi: 10.1503/jpn.090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M. Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Dev. Med. Child Neurol. 2012;54(1):6–7. doi: 10.1111/j.1469-8749.2011.04122.x. [DOI] [PubMed] [Google Scholar]
- Au T.M., Dickstein B.D., Comer J.S., Salters-Pedneault K., Litz B.T. Co-occurring posttraumatic stress and depression symptoms after sexual assault: a latent profile analysis. J. Affect. Disord. 2013;149(1–3):209–216. doi: 10.1016/j.jad.2013.01.026. [DOI] [PubMed] [Google Scholar]
- Aung W.Y., Mar S., Benzinger T.L. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. 2013;5(5):427–440. doi: 10.2217/iim.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayling E., Aghajani M., Fouche J. Diffusion tensor imaging in anxiety disorders. Curr. Psychiatry Rep. 2012;14:197–202. doi: 10.1007/s11920-012-0273-z. [DOI] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Luan Phan K. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V., Hänggi J., Jäncke L. Volumetric associations between uncinate fasciculus, amygdala, and trait anxiety. BMC Neurosci. 2012;13(1):4. doi: 10.1186/1471-2202-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur V., Brühl A.B., Herwig U., Eberle T., Rufer M., Delsignore A., Jäncke L., Hänggi J. Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: a quantitative fiber tractography study. Hum. Brain Mapp. 2013;34(2):437–446. doi: 10.1002/hbm.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin K.S., Parra G.R., Williams N.a. An introduction to latent variable mixture modeling (part 1): Overview and Cros-Sectional Latent Class and Latent Profile analyses. J. Pediatr. Psychol. 2014;39(2):174–187. doi: 10.1093/jpepsy/jst084. [DOI] [PubMed] [Google Scholar]
- Bessette K.L., Nave A.M., Caprihan A., Stevens M.C. White matter abnormalities in adolescents with major depressive disorder. Brain Imaging Behavior. 2014;8(4):531–541. doi: 10.1007/s11682-013-9274-8. [DOI] [PubMed] [Google Scholar]
- Bradley M.M., Codispoti M., Sabatinelli D., Lang P.J. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1(3):300–319. [PubMed] [Google Scholar]
- Bremner J.D., Vythilingam M., Vermetten E., Nazeer A., Adil J., Khan S., Staib L.H., Charney D.S. Reduced volume of orbitofrontal cortex in major depression. Biol. Psychiatry. 2002;51(4):273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Brinkman T.M., Zhu L., Zeltzer L.K., Recklitis C.J., Kimberg C., Zhang N., Muriel A.C., Stovall M., Srivastava D.K., Robison L.L., Krull K.R. Longitudinal patterns of psychological distress in adult survivors of childhood cancer. Br. J. Cancer. 2013;109(5):1373–1381. doi: 10.1038/bjc.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubb E.J., Metzler-Baddeley C., Aggleton J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018;92(January):104–127. doi: 10.1016/j.neubiorev.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy C.A., Stodola D.E., Ruttle P.L., Molloy E.K., Armstrong J.M., Oler J.A., Fox M.E., Hayes A.S., Kalin N.H., Essex M.J., Davidson R.J., Birn R.M. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Canli T., Desmond J.E., Zhao Z., Gabrieli J.D.E. Sex differences in the neural basis of emotional memories. Proc. Natl. Acad. Sci. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Clark C., Rodgers B., Caldwell T., Power C., Stansfeld S. Childhood and adulthood psychological ill health as predictors of midlife affective and anxiety disorders: the 1958 British Birth Cohort. Arch. Gen. Psychiatry. 2007;64(6):668–678. doi: 10.1001/archpsyc.64.6.668. [DOI] [PubMed] [Google Scholar]
- Cohan S.L., Chavira D.A., Shipon-Blum E., Hitchcock C., Roesch S.C., Stein M.B. Refining the classification of children with selective mutism: a latent profile analysis. J. Clin. Child Adoles. Psychol. 2008;37(4):770–784. doi: 10.1080/15374410802359759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W., Shanahan L., Costello E., Angold A. Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Arch. Gen. Psychiatry. 2009;66(7):764–772. doi: 10.1001/archgenpsychiatry.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland W.E., Angold A., Shanahan L., Costello E.J. Longitudinal patterns of anxiety from childhood to adulthood: the great smoky mountains study. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(1):21–33. doi: 10.1016/j.jaac.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E., Mustillo S. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives General. 2003;60 doi: 10.1001/archpsyc.60.8.837. http://archneur.jamanetwork.com/article.aspx?articleid=207725 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Cullen K.R., Klimes-Dougan B., Muetzel R., Mueller B.A., Camchong J., Houri A., Kurma S., Lim K.O. Altered white matter microstructure in adolescents with major depression: a preliminary study. J. Am. Acad. Child Adolesc. Psych. 2010;49(2):173–183. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings C.M., Caporino N.E., Kendall P.C. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychol. Bull. 2014;140(3):816–845. doi: 10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Furey M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufford A.J., Kim P. Family income, cumulative risk exposure, and white matter structure in middle childhood. Front. Hum. Neurosci. 2017;11:547. doi: 10.3389/fnhum.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L., Dunn D. Third. Guidance Service Circle; 1997. PPVT III: Peabody Picture Vocabulary Test. [Google Scholar]
- Duval E.R., Javanbakht A., Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther. Clin. Risk Manag. 2015;11:115–126. doi: 10.2147/TCRM.S48528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H.M., Yeatman J.D., Lee E.S., Barde L.H.F., Gaman-Bean S. Diffusion Tensor Imaging: a Review for Pediatric researchers and Clinicians. J. Dev. Behav. Pediatr. 2010;31(4):346–356. doi: 10.1097/DBP.0b013e3181dcaa8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Castelao C., Kröner-Herwig B. Different trajectories of depressive symptoms in children and adolescents: predictors and differences in girls and boys. J. Youth Adolesc. 2013;42(8):1169–1182. doi: 10.1007/s10964-012-9858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furniss T., Beyer T., Guggenmos J. Prevalence of behavioural and emotional problems among six-years-old preschool children: Baseline results of a prospective longitudinal study. Soc. Psychiatry Psychiatr. Epidemiol. 2006;41(5):394–399. doi: 10.1007/s00127-006-0045-3. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Hare T.A., Bookheimer S.Y., Tottenham N. A developmental shift from positive to negative connectivity in human Amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H.T., Hilgetag C.C., Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P.E., Kessler R.C., Birnbaum H.G., Leong S.A., Lowe S.W., Berglund P.A., Corey-Lisle P.K. The economic burden of depression in the United States. J. Clin. Psych. 2003;64(12):1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Greenberg P.E., Fournier A.-A., Sisitsky T., Pike C.T., Kessler R.C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J. Clin. Psych. 2015;2010(February):155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- Hadland K.A., Rushworth M.F.S., Gaffan D., Passingham R.E. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia. 2003;41(8):919–931. doi: 10.1016/s0028-3932(02)00325-1. [DOI] [PubMed] [Google Scholar]
- Han D.H., Renshaw P.F., Dager S.R., Chung A., Hwang J., Daniels M.A., Lee Y.S., Lyoo I.K. Altered cingulate white matter connectivity in panic disorder patients. J. Psychiatr. Res. 2008;42(5):399–407. doi: 10.1016/j.jpsychires.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hastings R.S., Parsey R.V., Oquendo M.A., Arango V., Mann J.J. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29(5):952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- Hayden B.Y., Platt M.L. Encyclopedia of Neuroscience. Elsevier; 2009. Cingulate cortex; pp. 887–892. [Google Scholar]
- Hilbert K., Lueken U., Beesdo-baum K. Neural structures, functioning and connectivity in Generalized Anxiety Disorder and interaction with neuroendocrine systems: A systematic review. J. Affect. Disord. 2014;158:114–126. doi: 10.1016/j.jad.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Hill A.L., Degnan K.A., Calkins S.D., Keane S.P. Profiles of externalizing behavior problems for boys and girls across preschool: the roles of emotion regulation and inattention. Dev. Psychol. 2006;42(5):913–928. doi: 10.1037/0012-1649.42.5.913. [DOI] [PubMed] [Google Scholar]
- Ho T.C., King L.S., Leong J.K., Colich N.L., Humphreys K.L., Ordaz S.J., Gotlib I.H. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Soc. Cogn. Affect. Neurosci. 2017;12(9):1460–1469. doi: 10.1093/scan/nsx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey C.J., Sporns O., Cammoun L., Gigandet X., Thiran J.P., Meuli R., Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. U. S. A. 2009;106(6):2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D.S., Calabresi P.A., Pekar J.J., van Zijl P.C.M., Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Fan X., Williamson D.E., Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36(3):684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Fan X., Williamson D.E., Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36(3):684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ialongo N., Edelsohn G., Werthamer-Larsson L., Crockett L., Kellam S. The significance of self-reported anxious symptoms in first-grade children. J. Abnorm. Child Psychol. 1994;22(4):441–455. doi: 10.1007/BF02168084. [DOI] [PubMed] [Google Scholar]
- Jackson M.E., Moghaddam B. Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J. Neurosci. 2001;21(2):676–681. doi: 10.1523/JNEUROSCI.21-02-00676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellison B.J., Field A.S., Medow J., Lazar M., Salamat M.S., Alexander A.L. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. Am. J. Neuroradiol. 2004;25(3):356–369. [PMC free article] [PubMed] [Google Scholar]
- Johnson R.T., Yeatman J.D., Wandell B.A., Buonocore M.H., Amaral D.G., Nordahl C.W. Diffusion properties of major white matter tracts in young, typically developing children. NeuroImage. 2014;88:143–154. doi: 10.1016/j.neuroimage.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.T., Yeatman J.D., Wandell B.A., Buonocore M.H., Amaral D.G., Nordahl C.W. Diffusion properties of major white matter tracts in young, typically developing children. NeuroImage. 2014;88(November 2013):143–154. doi: 10.1016/j.neuroimage.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.A., Morales A.M., Lavine J.B., Nagel B.J. Convergent neurobiological predictors of emergent psychopathology during adolescence. Birth Defects Res. 2017;109(20):1613–1622. doi: 10.1002/bdr2.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psych. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplow J.B., Curran P.J., Dodge K.A., Bierman K.L., Coie J.D., Greenberg M.T., Lochman J.E., McMahon R.J., Pinderhughes E.E. Child, parent, and peer predictors of early-onset substance use: a multisite longitudinal study. J. Abnorm. Child Psychol. 2002;30(3):199–216. doi: 10.1023/a:1015183927979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell P.A., Chapman R., Christiansen K., Richardson H., Evans J., Jones D.K. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol. Psychiatry. 2012;72(4):296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Kier E.L., Staib L.H., Davis L.M., Bronen R.A. MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. Am. J. Neuroradiol. 2004;25(5):677–691. [PMC free article] [PubMed] [Google Scholar]
- Kim M.J., Whalen P.J. The structural integrity of an Amygdala – prefrontal pathway predicts trait anxiety. J. Neurosci. 2009;29(37):11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009;201(2):239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryski K.R., Smith H.J., Sheikh H.I., Singh S.M., Hayden E.P. Assessing stress reactivity indexed via salivary cortisol in preschool-aged children. Psychoneuroendocrinology. 2011;36:1127–1136. doi: 10.1016/j.psyneuen.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Kryski K.R., Smith H.J., Sheikh H.I., Singh S.M., Hayden E.P. HPA axis reactivity in early childhood: Associations with symptoms and moderation by sex. Psychoneuroendocrinology. 2013;38(10):2327–2336. doi: 10.1016/j.psyneuen.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Lazarsfeld P.F., Henry N.W. Houghton Mifflin; Boston, MA: 1968. Latent Structure Analysis: Psychology A Study of Science. [Google Scholar]
- Leach L.S., Christensen H., Mackinnon A.J., Windsor T.D., Butterworth P. Gender differences in depression and anxiety across the adult lifespan: the role of psychosocial mediators. Soc. Psychiatry Psychiatr. Epidemiol. 2008;43(12):983–998. doi: 10.1007/s00127-008-0388-z. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal Development of Human Brain Wiring Continues from Childhood into Adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Leppänen J.M. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr. Opin. Psych. 2006;19(1):34–39. doi: 10.1097/01.yco.0000191500.46411.00. [DOI] [PubMed] [Google Scholar]
- Liotti M., Mayberg H.S., Brannan S.K., McGinnis S., Jerabek P., Fox P.T. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: Implications for affective disorders. Biol. Psychiatry. 2000;48(1):30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- Luby J.L. Preschool depression: the importance of identification of depression early in development. Curr. Dir. Psychol. Sci. 2010;19(2):91–95. doi: 10.1177/0963721410364493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J.L., Si X., Belden A.C., Tandon M., Spitznagel E. Preschool Depression: Homotypic continuity and course over 24 months. Arch. Gen. Psychiatry. 2009;66(8):897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G.M., Campbell S., McEwen B.S., Macdonald K., Amano S., Joffe R.T., Nahmias C., Young L.T. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. U. S. A. 2003;100(3):1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira M.D., Lieberman A.R. Sexual dimorphism in the mammalian limbic system. Prog. Neurobiol. 1995;45(4):275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Masten A.S., Cicchetti D. Developmental cascades. Dev. Psychopathol. 2010;22(3):491–495. doi: 10.1017/S0954579410000222. [DOI] [PubMed] [Google Scholar]
- Masten A.S., Roisman G.I., Long J.D., Burt K.B., Obradović J., Riley J.R., Boelcke-Stennes K., Tellegen A. Developmental cascades: linking academic achievement and externalizing and internalizing symptoms over 20 years. Dev. Psychol. 2005;41(5):733–746. doi: 10.1037/0012-1649.41.5.733. [DOI] [PubMed] [Google Scholar]
- Mathews B.L., Koehn A.J., Abtahi M.M., Kerns K.A. Emotional competence and anxiety in childhood and adolescence: a meta-analytic review. Clin. Child. Fam. Psychol. Rev. 2016;19(2):162–184. doi: 10.1007/s10567-016-0204-3. [DOI] [PubMed] [Google Scholar]
- McLean C.P., Asnaani A., Litz B.T., Hofmann S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011;45(8):1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C.P., Asnaani A., Litz B.T., Hofmann S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011;45(8):1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A. Structural covariance in the human cortex. J. Neurosci. 2005;25(36):8303–8310. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Clasen L.S., Giedd J.N., Blakemore S.J. The developmental mismatch in structural brain maturation during adolescence. Dev. Neurosci. 2014;36(3–4):147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Moilanen K.L., Shaw D.S., Maxwell K.L. Developmental cascades: Externalizing, internalizing, and academic competence from middle childhood to early adolescence. Dev. Psychopathol. 2010;22(3):635–653. doi: 10.1017/S0954579410000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.L., Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol. Mood Anxiety Disord. 2011;1(1):3. doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. In: Mplus User's Guide. Eighth. Muthén L.K., Muthén B.O., editors. 2017. Los Angeles, CA. [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in depression. Curr. Dir. Psychol. Sci. 2001;10(5):173–176. [Google Scholar]
- Oberski D. Mixture models: Latent profile and latent class analysis. Modern Statist. Methods HCI. 2016:275–287. [Google Scholar]
- Olson I.R., Von Der Heide R.J., Alm K.H., Vyas G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev. Cognitive Neurosci. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Luking K.R., Belden A.C., Barch D.M. Brain-behavior relationships in the experience and regulation of negative emotion in healthy children: Implications for risk for childhood depression. Dev. Psychopathol. 2014;26:1289–1303. doi: 10.1017/S0954579414001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Orlichenko A., Boyd E., Angstadt M., Coccaro E.F., Liberzon I., Arfanakis K. Preliminary evidence of White Matter Abnormality in the Uncinate Fasciculus in Generalized Social anxiety Disorder. Biol. Psychiatry. 2009;66(7):691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol. Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Reinke W.M., Ostrander R. Heterotyic and homotypic continuity: the moderating effects of age and gender. J. Abnorm. Child Psychol. 2008;36(7):1109–1121. doi: 10.1007/s10802-008-9236-6. [DOI] [PubMed] [Google Scholar]
- Roza S.J., Hofstra M.B., Van Der Ende J., Verhulst F.C. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence, and young adulthood. Am. J. Psychiatr. 2003;160(12):2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- Sacchet M.D., Prasad G., Foland-Ross L.C., Joshi S.H., Hamilton J.P., Thompson P.M., Gotlib I.H. Structural abnormality of the corticospinal tract in major depressive disorder. Biol. Mood Anxiety Disorders. 2014;4(1):8. doi: 10.1186/2045-5380-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer H., Alarcón G., Demeter D.V., Earl E., Fair D.A., Nagel B.J. Reduced fronto-amygdalar connectivity in adolescence is associated with increased depression symptoms over time. Psych. Res. 2017;266(October 2016):35–41. doi: 10.1016/j.pscychresns.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D., Smith E.E., Eichler F.S., Filley C.M. Cerebral white matter: Neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann. N. Y. Acad. Sci. 2008;1142 doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton C.E., Mackay C.E., Ebmeier K.P. A systematic review of diffusion tensor imaging studies in affective disorders. Biol. Psychiatry. 2009;66(9):814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Sheikh H.I., Joanisse M.F., Mackrell S.M., Kryski K.R., Smith H.J., Singh S.M., Hayden E.P. Links between white matter microstructure and cortisol reactivity to stress in early childhood: evidence for moderation by parenting. NeuroImage. 2014;6:77–85. doi: 10.1016/j.nicl.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol. Rev. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemming K., Sørensen M.J., Thomsen P.H., Obel C., Henriksen T.B., Linnet K.M. The association between preschool behavioural problems and internalizing difficulties at age 10-12 years. Eur. Child Adolesc. Psychiatry. 2010;19(10):787–795. doi: 10.1007/s00787-010-0128-2. [DOI] [PubMed] [Google Scholar]
- Soares J.M., Marques P., Alves V., Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front. Neurosci. 2013;7(7 MAR):1–14. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Chialvo D.R., Kaiser M., Hilgetag C.C. Organization, development and function of complex brain networks. Trends Cogn. Sci. 2004;8(9):418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Stein J.L., Wiedholz L.M., Bassett D.S., Weinberger D.R., Zink C.F., Mattay V.S., Meyer-Lindenberg A. A validated network of effective amygdala connectivity. NeuroImage. 2007;36(3):736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Sterba S.K., Printein M.J., Cox M.J. Trajectories of internalizing problems across childhood: Heterogeneity, external validity, and gender differences. Dev. Psychopathol. 2007;19(2):345–366. doi: 10.1017/S0954579407070174. [DOI] [PubMed] [Google Scholar]
- Stevens J.S., Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50(7):1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Swartz J.R., Monk C.S. Current Topics Behav Neurosci. Vol. 32. 2013. The role of corticolimbic circuitry in the development of anxiety disorders in children and adolescents; pp. 133–148. [DOI] [PubMed] [Google Scholar]
- Swartz J.R., Carrasco M., Wiggins J.L., Thomason M.E., Monk C.S. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. NeuroImage. 2014;86:212–220. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K.M., Drevets W.C., Dahl R.E., Ryan N.D., Birmaher B., Eccard C.H., Axelson D., Whalen P.J., Casey B.J. Amygdala response to fearful faces in anxious and depressed children. Arch. Gen. Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Toumbourou J.W., Williams I., Letcher P., Sanson A., Smart D. Developmental trajectories of internalising behaviour in the prediction of adolescent depressive symptoms. Aust. J. Psychol. 2011;63(4):214–223. [Google Scholar]
- Van Den Heuvel M.P., Mandl R.C.W., Kahn R.S., Hulshoff Pol H.E. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum. Brain Mapp. 2009;30(10):3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol M.-J., van der Wee N.J.A., van den Heuvel O.A., Nielen M.M.A., Demenescu L.R., Aleman A., Renken R., van Buchem M.A., Zitman F.G., Veltman D.J. Regional Brain volume in Depression and anxiety Disorders. Arch. Gen. Psychiatry. 2010;67(10):1002. doi: 10.1001/archgenpsychiatry.2010.121. [DOI] [PubMed] [Google Scholar]
- Vythilingam M., Heim C., Newport J., Miller A.H., Anderson E., Bronen R., Brummer M., Staib L., Vermetten E., Charney D.S., Nemeroff C.B., Bremner J.D. Childhood trauma associated with smaller Hippocampal volume in women with major depression. Am. J. Psychiatry. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L., Hua K., Zhang J., Jiang H., Dubey P., Blitz A., van Zijl P., Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley H.C., Sprooten E., Hackett S., Hall L., Blackwood D.H., Glahn D.C., Bastin M., Hall J., Lawrie S.M., Sussmann J.E., McIntosh A.M. Polygenic risk and white matter integrity in individuals at high risk of mood disorder. Biol. Psychiatry. 2013;74(4):280–286. doi: 10.1016/j.biopsych.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Myall N.J., Wandell B.A., Feldman H.M. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0049790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C., Shirtcliff E.A., Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annu. Rev. Clin. Psychol. 2008;4(1):275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C., Klimes-Dougan B., Slattery M.J. Internalizing problems of childhood and adolescence: prospects, pitfalls, and progress in understanding the development of anxiety and depression. Dev. Psychopathol. 2016;12:443–466. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
leftCC
leftUFfig
rightCC
rightUF