Abstract
Hemispheric integration and specialization are two prominent organizational principles for macroscopic brain function. Impairments of interhemispheric cooperation have been reported in schizophrenia patients, but whether such abnormalities should be attributed to effects of illness or familial risk remains inconclusive. Moreover, it is unclear how abnormalities in interhemispheric connectivity impact hemispheric specialization. To address these questions, we performed magnetic resonance imaging (MRI) in a large cohort of 253 participants, including 84 schizophrenia patients, 106 of their unaffected siblings and 63 healthy controls. Interhemispheric connectivity and hemispheric specialization were calculated from resting-state functional connectivity, and compared across groups. Results showed that schizophrenia patients exhibit lower interhemispheric connectivity as compared to controls and siblings. In addition, patients showed higher levels of hemispheric specialization as compared to siblings. Level of interhemispheric connectivity and hemispheric specialization correlated with duration of illness in patients. No significant alterations were identified in siblings relative to controls on both measurements. Furthermore, alterations in interhemispheric connectivity correlated with changes in hemispheric specialization in patients relative to controls and siblings. Taken together, these results suggest that lower interhemispheric connectivity and associated abnormalities in hemispheric specialization are features of established illness, rather than an expression of preexistent familial risk for schizophrenia.
Keywords: Schizophrenia, Unaffected relative, Interhemispheric connectivity, Hemispheric specialization, Resting-state fMRI
Highlights
-
•
Schizophrenia patients show lower interhemispheric connectivity (IC) compared to siblings and controls.
-
•
Lower IC is associated with higher hemispheric specialization (HS) in patients.
-
•
Siblings do not show changes on IC and HS relative to controls.
-
•
Connectivity metrics (IC, HS) correlate with duration of illness in patients.
1. Introduction
Two prominent features of macroscopic brain organization are hemispheric integration, characterized as a high level of collaboration between bilateral brain regions, and hemispheric specialization, referring to the association of behavioral traits with a particular side of brain (Hervé et al., 2013; Serrien et al., 2006). Proper integration and segregation of the two cerebral hemispheres are crucial for a broad repertoire of cognitive functions including language, visuospatial attention, and manual preference (Cai et al., 2013; Gotts et al., 2013; Liu et al., 2009). Conversely, disturbances of hemispheric coordination may impact cognitive and behavioral functioning (Gazzaniga, 1995; Toga and Thompson, 2003) and contribute to neuropsychiatric disorders including schizophrenia (Bleich-Cohen et al., 2012; Crow, 1998; Ribolsi et al., 2009).
Multiple lines of evidence suggest that schizophrenia may involve abnormalities in interhemispheric connectivity and cooperation, which may contribute to central features of the illness such as auditory verbal hallucinations (Chang et al., 2015) and cognitive deficits (Liu et al., 2018). Behavioral studies indicate that a bilateral processing advantage that is normally present during language tasks is compromised in schizophrenia patients (Lohr et al., 2006; Mohr et al., 2000) and studies using event-related potentials (ERP) show evidence of impaired information transfer between the hemispheres during cognitive tasks in schizophrenia (Barnett and Kirk, 2005; Endrass et al., 2002; Mohr et al., 2008). Moreover, structural neuroimaging studies report that schizophrenia patients have decreased volume and fiber integrity of the corpus callosum, the major fiber bundle connecting the two hemispheres (Hulshoff Pol et al., 2004; Knöchel et al., 2012; Kubicki et al., 2008; Patel et al., 2011), and fMRI studies show impaired levels of functional synchronization between homotopic brain regions (i.e., corresponding areas in the left and right hemisphere) at rest (Hoptman et al., 2012; Li et al., 2015).
However, important open questions regarding interhemispheric connectivity in schizophrenia remain. First, it is unclear whether interhemispheric connectivity deficits are primarily related to the effects of the illness or a reflection of (genetic) risk for the disorder (Boos et al., 2012; MacDonald et al., 2009). Unaffected first-degree relatives of schizophrenia patients are a valuable population to study this question as they share the genetic predisposition and environmental factors for the illness, but are not clinically affected. Previous studies have reported abnormalities in unaffected relatives in large-scale brain regional interactions during tasks (Whitfield-Gabrieli et al., 2009; Woodward et al., 2009) and rest (Collin et al., 2011; Oertel-Knöchel et al., 2013, Oertel-Knöchel et al., 2014; Repovs et al., 2011), and a limited number of studies have shown subtle abnormalities in functional (Guo et al., 2014), and structural (Knöchel et al., 2012) connectivity between homotopic brain regions in patients and relatives. In the current study, we examine homotopic interhemispheric functional connectivity in a large cohort of schizophrenia patients and their nonpsychotic siblings to assess how interhemispheric connectivity alterations relate to risk for schizophrenia.
Another open question is what the consequence of reduced interhemispheric connectivity may be for hemispheric specialization characterized by intrinsic connectivity. Traditionally, hemispheric specialization studies have focused on functional or anatomical asymmetry of homotopic brain regions (Ocklenburg et al., 2013; Oertel-Knöchel and Linden, 2011; Sommer et al., 2001). More recently, studies have extended the concept of hemispheric specialization to include connectivity asymmetry, by investigating functional interaction patterns of brain regions in the two hemispheres (Ribolsi et al., 2014; Stephan et al., 2007). One such measurement is the hemispheric autonomy index (Wang et al., 2014), which calculates the difference in connectivity strength of a brain region with ipsilateral areas versus contralateral areas during rest. Studies have shown that regions with high autonomy values (i.e. preferably connecting to ipsilateral areas) in the left hemisphere overlap with regions showing asymmetrical activation during language tasks (Wang et al., 2014), and that regional autonomy index values correlate with verbal and visuospatial behavioral scores in healthy subjects (Gotts et al., 2013). We hypothesize that impaired interhemispheric connectivity may cause the two hemispheres to function more autonomously, favoring within over between-hemisphere interregional cooperation. To test this hypothesis, we examine how interhemispheric connectivity deficits in schizophrenia patients and their unaffected siblings relate to hemispheric specialization as quantified by the hemispheric autonomy index (Gotts et al., 2013; Mueller et al., 2015; Wang et al., 2014).
2. Material and methods
2.1. Participants
A total of 253 participants, including 84 schizophrenia patients, 106 of their unaffected siblings and 63 healthy controls, were recruited at the University Medical Center Utrecht between September 2004 and April 2008, as part of the Genetic Risk and Outcome of Psychosis (GROUP) study (Korver et al., 2012). The GROUP study was conducted by four university psychiatric centers and their affiliated mental health care institutions in the Netherlands. The medical ethics committee of the University Medical Center Utrecht approved the current study. All subjects provided written informed consent prior to participation.
For all participants, presence or absence of current and lifetime psychopathology was established using the Comprehensive Assessment of Symptoms and History (CASH) (Andreasen et al., 1992). This semi-structured interview is designed to obtain comprehensive information on current and past signs and symptoms of major psychiatric disorders, premorbid functioning, sociodemographic status, treatment, and course of illness. Patients were included if they met Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM-IV) criteria for schizophrenia or schizoaffective disorder. Symptom severity was assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). The dosage of antipsychotic medication patients were taking at the time of scanning were converted to a chlorpromazine equivalent dosage (Kroken et al., 2009).
Patients and siblings originated from 120 unique families and included 48 patient-sibling pairs or triplets. The control group comprised 63 subjects from 57 families. A family identification code (family ID) was assigned to every subject to keep track of their pedigrees. Siblings and healthy controls were excluded for any current or previous psychotic disorder. In addition, controls were examined with the Family Interview for Genetic Studies (Maxwell, 1992), to exclude a family history of psychotic disorders (in first- or second-degree relatives). Exclusion criteria for all participants included a history of head trauma or major medical or neurological illness. All participants were between 18 and 60 years of age at the time of inclusion. Demographics and clinical information are described in Table 1.
Table 1.
Demographic and clinical information of the three groups of participants (controls, siblings, schizophrenia N = 63, 106, 84).
| Controls | Siblings | Patients | Statisticsa | |
|---|---|---|---|---|
| Age | 29.16 ± 7.59 | 29.50 ± 7.65 | 29.86 ± 5.27 | F(2,250) = 0.18, p = .83 |
| Sex (M / F) | 29/34 | 49 / 57 | 70/14 | χ(2)2 = 31.95, p < .001 |
| IQ | 113.76 ± 15.66 | 105.96 ± 17.10 | 95.99 ± 15.21 | F(2,250) = 20.54, p < .001 |
| Handedness (right / left / mixed) | 44/8/1 | 94/6/6 | 70/5/4 | χ(2)2 = 5.63, p = .23 |
| Scanner (#1 / #2) | 28 / 28 | 59 / 47 | 49 / 33 | χ(2)2 = 1.28, p = .53 |
| Illness duration (years)b | – | – | 5.91 (4.12) | – |
| Chlorpromazine dosage (mg/day) | – | – | 300 (200, 600) | – |
| PANSS total | – | – | 48.38 ± 14.89 | – |
| PANSS positive | – | – | 11.58 ± 5.42 | – |
| PANSS negative | – | – | 12.23 ± 4.35 | – |
| PANSS general psychopathology | – | – | 24.57 ± 7.05 | – |
Group comparison of continuous variables (Age, IQ) are tested with one-way analysis of variance (ANOVA). Nominal variables (sex, handedness, scanner) were compared using chi-square statistics.
Distribution of illness duration and medication is skewed, therefore median and inter-quartile range was reported.
2.2. Data acquisition
MRI scans were acquired on two 1.5 T Philips Achieva scanners at the University Medical Center Utrecht, with the same acquisition protocol. The scanner information was included as a covariate in further group-comparisons. For each subject, resting-state functional magnetic resonance imaging (fMRI) time series were acquired with a three dimensional (3D) echo shifting with a train of observations (PRESTO) acquisition scheme (Liu et al., 1993). The PRESTO protocol combines the echo shifting technique with multiple gradient echoes per excitation to allow for a repetition time shorter than echo time. The acquisition parameters were: TR / TE = 21.1 / 31.1 ms; flip angle = 90°; field of view = 256 × 256 mm2, voxel size = 4 × 4 × 4 mm3, 900 time frames, consisting of 32 slices covering whole brain. In addition, a T1-weighted scan was collected as anatomical reference with the following scanning parameters: TR/TE = 30/4.6 ms; flip angle = 30°; field of view = 256 × 256 mm2, voxel size = 1 × 1 × 1.2 mm3.
2.3. Data preprocessing
Resting-state fMRI data were preprocessed using the Data Processing and Analysis for Brain Imaging (DPABI) toolbox (Yan et al., 2016) on MATLAB v. R2016a (Mathworks). For every subject, the first 10 functional volumes were removed to allow for signal stabilization. The rest of the functional images were realigned, co-registered with their individual anatomic image, and corrected for nuisance variables (six rigid realignment parameters, signal drift, averaged signal from white matter and cerebrospinal fluid) using a linear regression model. Band-pass filtering (0.01–0.1 Hz) was applied to fMRI time-series to reduce effects of low-frequency drifts and high-frequency noise. Subsequently, the functional scans were spatially normalized to MNI space using a symmetric group-specific T1 template, and resampled to 3 × 3 × 3 mm3 voxels. Spatial smoothing was applied to normalized images with a 4 mm full-width half-maximum (FWHM) Gaussian kernel. In view of recent findings on the influence of in-scanner head motion on fMRI signals (Power et al., 2012; Satterthwaite et al., 2013; Yan et al., 2013), images with excessive movement (frame-wise displacement >0.5 mm) and 1 backward and 2 forward scans relative to the marked frame, were scrubbed (Power et al., 2012). The scrubbing procedure negated group-differences in head motion (before scrubbing χ(2)2 = 6.20, p = .04 and after scrubbing χ(2)2 = 4.09, p = .13, Supplementary Table 1), and did not lead to differences on the number of images rejected among the three subject groups (χ(2)2 = 3.54, p = .17).
The Harvard-Oxford atlas (Goldstein et al., 2007; Makris et al., 2006) was applied to whole-brain functional data in order to parcellate the brain into 112 bilateral regions (48 cortical and 8 subcortical regions per hemisphere). Region names, abbreviations, anatomic classification (frontal, temporal, parietal, occipital lobe, cingulate cortex and subcortical regions) and functional hierarchy (primary sensorimotor regions, unimodal, heteromodal association areas, (para)limbic and subcortical regions) are referenced in Supplementary Table 2. The functional hierarchy subdivision scheme is based on previous studies (Bassett et al., 2008; Mesulam, 1998; Stark et al., 2008). Regional time-series were averaged across voxels, and cross-correlated for each pair of brain regions using Pearson's correlation. Correlation coefficients were converted to z scores using Fisher's z transformation, serving as a measure of functional connectivity strength between two regions.
2.4. Interhemispheric connectivity
The level of functional connectivity between 56 regions in one hemisphere and their corresponding areas in the other hemisphere is used here as the measure of interhemispheric connectivity. Interhemispheric connections between homotopic regions are among the strongest functional associations in cortical-cortical interactions (Stark et al., 2008) and have reliably been identified in both healthy and clinical populations (Anderson et al., 2011; Guo et al., 2013; Wei et al., 2014; Zhou et al., 2013). As a supplementary analysis, group-differences in heterotopic interhemispheric connectivity and left and right intrahemispheric connectivity were examined in the same manner as homotopic interhemispheric connectivity. Moreover, to assess the consistency of interhemispheric connectivity measurements between studies, we performed an additional analysis examining the correlation in regional interhemispheric connectivity in controls between this study and the study of Stark et al. (2008).
2.5. Hemispheric specialization
Hemispheric specialization is calculated here as the difference in connectivity strength between intrahemispheric and heterotopic interhemispheric connections of each brain region. Homotopic interhemispheric connections were not used in the calculation of hemispheric specialization so that we could examine relationship between hemispheric specialization and interhemispheric connectivity in the following analysis. A positive value of hemispheric specialization indicates that a region or hemisphere is preferentially connected to other regions within its own hemisphere, whereas a negative value suggests that a region or hemisphere is more strongly connected to regions in the contralateral hemisphere. The method used to compute hemispheric specialization using intrinsic functional connectivity has been described in previous studies using a voxel-based analysis (Mueller et al., 2015; Wang et al., 2014).
2.6. Statistical analysis
Effects of group and covariates on interhemispheric connectivity and hemispheric specialization were assessed using linear-mixed effect models. For interhemispheric connectivity, fixed-effects include: group status (controls, siblings, patients), age, sex, handedness (right / left / mixed), scanner (#1 / #2) and head motion. To control for potential group-differences in global connectivity, mean connectivity strength of all non-homotopic connections (i.e., all intrahemispheric and heterotopic interhemispheric connections) was included as a fixed-effect term (global FC). Family ID, encoding the subjects' pedigree information, was included as random-effect term to control for family ties between subjects (Eq. (1)). The model has 237 observations (number of subjects with complete information), and 10 fixed term predictors (constant, group_sibling, group_patient, age, gender_female, handedness_left, handedness_mixed, scanner_#2, motion, global FC), leaving = 237–10 = 227 residual degrees of freedom.
Equation 1:
| (1) |
For hemispheric specialization, group status, age, sex, handedness, scanner and head motion were again included as fixed-effect terms. Additionally, we included hemisphere as a fixed factor to examine potential differences in connectivity patterns between the two hemispheres. Random effect terms include family ID and subject ID nested within family ID. The number of fixed term predictors is 10 (constant, group_sibling, group_patient, age, gender_female, handedness_left, handedness_mixed, scanner_#2, motion, hemisphere_RH), and residual degrees of freedom is 237 × 2 (hemisphere) – 10 = 464.
Equation 2:
| (2) |
Linear-mixed effects analysis was first applied to overall interhemispheric connectivity and hemispheric specialization (p < .05), and then to each of 56 regional measurements, controlling for multiple comparisons (p < .05, Bonferroni corrected). Overall and regional measurements with significant group effects were subjected to post-hoc bivariate comparisons between each pair of subject groups (p < .05).
2.7. Relationship between interhemispheric connectivity and hemispheric specialization
To test whether schizophrenia-related changes in interhemispheric connectivity co-vary with alterations in hemispheric specialization, we calculated the mean difference between controls and patients on both measurements for each brain region. Pearson's correlation analysis was performed on group-differences of interhemispheric connectivity and hemispheric specialization across regions (p < .05). The same analysis was performed for siblings as compared to patients, and for controls compared to siblings.
2.8. Correlation between connectivity alteration and clinical factors
To examine the potential clinical relevance of connectivity changes in patients, the level of interhemispheric connectivity and hemispheric specialization were correlated with illness duration and PANSS total scores in patients (p < .05). Moreover, antipsychotic medication was examined as a potential confounder of functional connectivity measurements by correlating daily medication in chlorpromazine equivalent dose with interhemispheric connectivity and hemispheric specialization.
3. Results
3.1. Interhemispheric connectivity
Group mean homotopic interhemispheric connectivity strength was plotted on a brain surface for each subject group (Fig. 1). Regional variation in interhemispheric connectivity indicated higher bilateral synchrony in primary sensorimotor, auditory cortex and midline regions as compared to heteromodal association cortex. Regional interhemispheric connectivity in healthy controls correlated with values reported in a previous study (Stark et al., 2008) in healthy subjects (r = 0.41, p = .002, Supplementary Fig. 1).
Fig. 1.
Regional homotopic interhemispheric connectivity strength (z score) were plotted on brain volume for the three subject groups. The lower right boxplot depicts global interhemispheric connectivity for every subject. Patients had lower interhemispheric connectivity strength than controls (p = .04) and siblings (p = .004).
Linear mixed-effects analysis showed a significant main effect of group on homotopic interhemispheric connectivity (F(2,227) = 4.31, p = .01, Fig. 1), such that interhemispheric connectivity was lower in patients as compared to both controls (t(227) = 2.03, p = .04) and siblings (t(227) = 2.90, p = .004). Group-differences for heterotopic interhemispheric connectivity or left and right intra-hemispheric functional connectivity was not significant after controlling for global connectivity differences (F(2,227) = 0.61, 0.11, 0.14, p = .54, 0.89, 0.87 respectively, Supplementary Fig. 2).
Regional homotopic interhemispheric connectivity showed a significant group-effect in pre- and postcentral gyrus, subcallosal cortex and anterior supramarginal gyrus (p < .05, Bonferroni corrected, Fig. 2). Post-hoc analyses revealed significant reductions in interhemispheric connectivity in patients relative to both controls and siblings for all these regions (p < .05, detailed statistics are provided in the Supplementary Table 3). There were no significant group-differences in regional interhemispheric connectivity between controls and siblings.
Fig. 2.
Regions showing a significant group effect on interhemispheric connectivity (p < .05, Bonferroni corrected). Post-hoc analyses revealed significant reductions in interhemispheric connectivity in patients relative to both controls and siblings for all these regions (p < .05, detailed statistics were provided in the Supplementary Table 3). Abbreviation: CON, healthy controls; SIB, unaffected siblings; PAT, schizophrenia patients.
3.2. Hemispheric specialization: group effects
Regional variation of hemispheric specialization showed a distinct pattern from interhemispheric connectivity (Fig. 3). Heteromodal regions showed higher levels of hemispheric specialization than primary sensorimotor areas, indicating a tendency of heteromodal regions to interact preferentially with ipsilateral brain areas.
Fig. 3.
The color of brain volume represents regional hemispheric specialization (z score) in the three subject groups. The boxplot shows individual global hemispheric specialization. Patients with schizophrenia had higher global hemispheric specialization than siblings (p = .01).
There was a significant main effect of group on global hemispheric specialization (F(2,464) = 3.73, p = .02). Post-hoc comparisons showed that hemispheric specialization is higher in patients as compared to siblings (t(464) = 2.73, p = .01), indicating that functional connectivity in schizophrenia patients is more confined within their own hemispheres.
Ten out of 56 whole-brain areas showed significant group effects of regional hemispheric specialization (p < .05, Bonferroni corrected), including parietal, temporal and occipital association cortices, sensorimotor regions and limbic areas, mainly belonging to unimodal and heteromodal association cortices (Fig. 4A). In these regions, hemispheric specialization was increased in patients as compared to both controls and siblings (p < .05, Supplementary Table 4). The only exception was the posterior supramarginal gyrus which showed marginally higher hemispheric specialization in patients relative to controls (t(464) = 1.90, p = .058), but lower hemispheric specialization in siblings relative to controls (t(464) = −1.73, p = .084). There were no significant group-differences between siblings and controls in hemispheric specialization.
Fig. 4.
Regional hemispheric specialization showing significant (A) group differences; (B) hemispheric effects (p < .05, Bonferroni corrected, Supplementary Tables 4, 5). For group effects (A), patients exhibited increased hemispheric specialization in unimodal and heteromodal association cortices, sensorimotor cortices and limbic areas. The hemispheric effects (B) indicate higher specialization in frontal and temporal regions and subcortical structures in the left hemisphere, whereas higher specialization was observed in the right hemisphere for parietal, occipital, auditory, subcortical and anterior cingulate cortex. For illustration purpose, regional hemispheric specialization values were averaged over the two hemispheres (A) and over the three groups (B). Abbreviation: LH, left hemisphere; RH, right hemisphere; brain regional abbreviation and classification please refer to Supplementary Table 2.
3.3. Hemispheric specialization: hemispheric effects
There were no significant hemispheric effects of global hemispheric specialization (F(1,464) = 0.02, p = .89). On the regional level, significant main effects of hemisphere were observed for a range of cortical and subcortical brain (p < .05, Bonferroni corrected, Fig. 4B). Higher left-hemispheric specialization was found predominantly in superior/inferior frontal and inferior/middle temporal gyrus, as well as subcortical regions and posterior cingulate cortex. Regions with higher right-hemispheric specialization included parietal and occipital areas, central opercular cortex, heschls gyrus, planum temporale, caudate nucleus and accumbens, anterior and paracingulate gyrus (Supplementary Table 5).
3.4. Relationship between interhemispheric connectivity and hemispheric specialization
Comparing patients to controls, group-differences in regional interhemispheric connectivity correlated with changes of regional hemispheric specialization averaged for the left and right hemisphere (r = −0.54, p < .001, Fig. 5). Co-variation of group-differences in the two measurements was also significant for the sibling-patient comparison (r = −0.54, p < .001), and marginally significant for the control-sibling comparison (r = −0.26, p = .053). These correlations indicate that regions showing a larger decrease in interhemispheric connectivity in patients as compared to controls or siblings show higher level of hemispheric specialization, indicating that they are preferentially collaborating with regions within the same hemisphere.
Fig. 5.
Correlation between group-differences in homotopic interhemispheric connectivity and differences in hemispheric specialization. Decreased regional interhemispheric connectivity is negatively correlated with increased hemispheric specialization in patients as compared to controls (r = −0.54, p < .001) and siblings (r = −0.54, p < .001). For the control-sibling comparison, co-variation of group-differences in the two connectivity measurements is marginally significant (r = −0.26, p = .053).
3.5. Correlation between connectivity alterations and clinical factors
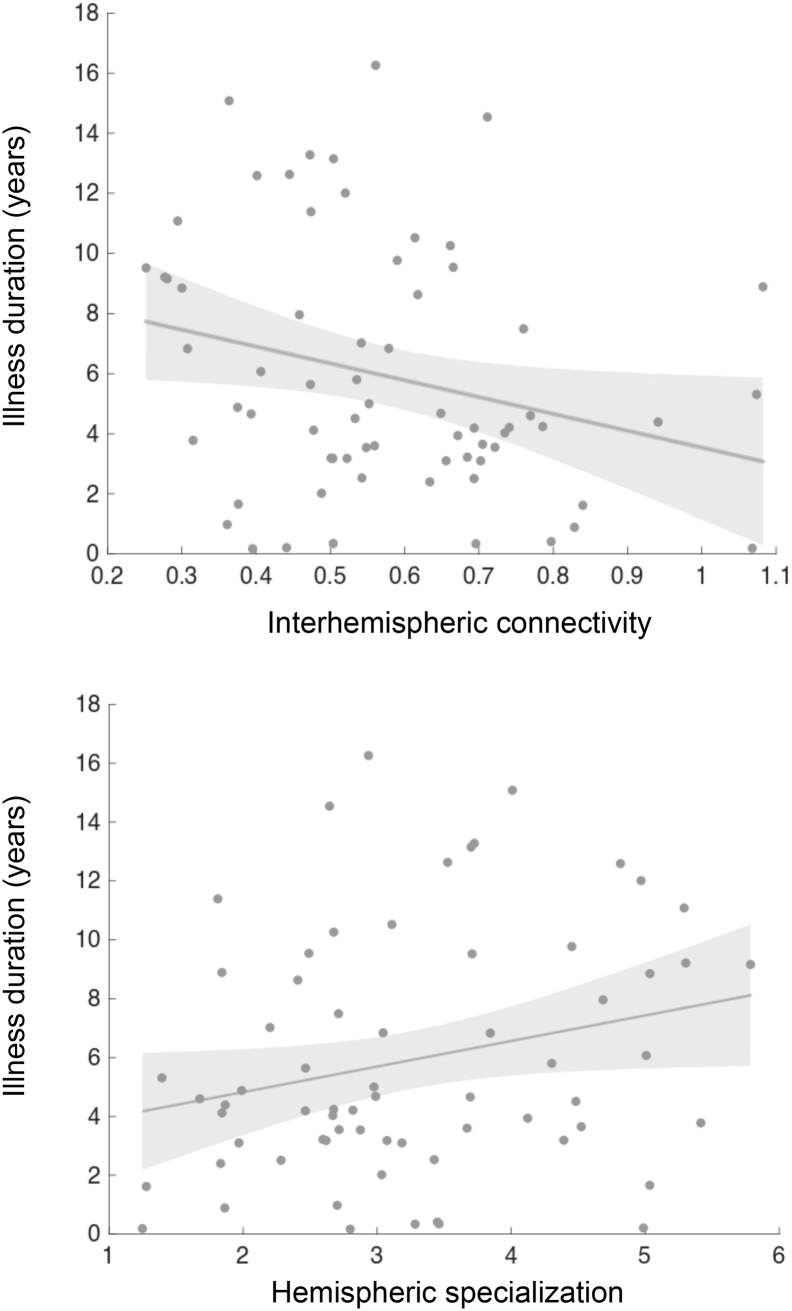
Duration of illness was negatively correlated with interhemispheric connectivity (r = −0.26, p = .03), and marginally associated with hemispheric specialization (r = 0.24, p = .049) in patients (Fig. 6). With longer course of illness, patients have more reduced interhemispheric connectivity and a higher level of segregation between the hemispheres. These connectivity alterations are not likely to be attributable to age, as for controls and siblings, we found no correlations between age and interhemispheric connectivity (controls: r = −0.05, p = .69, siblings: r = −0.07, p = .50) or between age and hemispheric specialization (controls: r = −0.03, p = .84, siblings: r = 0.04, p = .70). We found no correlations between PANSS total scores and connectivity measurements in patients (interhemispheric connectivity: r = 0.12, p = .34, hemispheric specialization r = −0.07, p = .60). Dosage of antipsychotic medication did not show significant associations with interhemispheric connectivity (r = −0.05, p = .70) or hemispheric specialization (r = 0.09, p = .49).
Fig. 6.
Duration of illness is negatively correlated with global homotopic connectivity (r = −0.26, p = .03) and marginally associated with hemispheric specialization (r = 0.24, p = .049) in the patient group.
4. Discussion
The current study examined interhemispheric connectivity and hemispheric specialization in a large cohort of schizophrenia patients, their unaffected siblings, and healthy controls. Compared to controls and siblings, patients showed marked decreases in interhemispheric connectivity, as well as increased hemispheric specialization on a global and regional level. No major deficits in interhemispheric connectivity or hemispheric specialization were found in unaffected siblings of patients. Moreover, interhemispheric connectivity and hemispheric specialization were found to correlate with duration of illness in patients. Taken together, our study shows impaired interhemispheric connectivity and a more segregated hemispheric connectivity profile in schizophrenia patients and suggests that these abnormalities are likely to be characteristics of established disease, rather than an expression of preexistent risk for the disorder.
The first question we set out to answer is whether impairments in interhemispheric connectivity are present in patients with schizophrenia and, if so, whether these impairments relate to preexistent familial risk for the illness. To answer this question, we examined a group of unaffected siblings of schizophrenia patients, who share 50% of their genetic information with affected probands on average. In contrast to a previous study reporting a subtle decline of regional interhemispheric connectivity strength in siblings as compared to controls (Guo et al., 2014), our current study does not find significant reductions in interhemispheric connectivity in unaffected siblings, suggesting that interhemispheric connectivity impairments may be an overt clinical phenotype of schizophrenia. Alternatively, interhemispheric connectivity impairments may be present from a young age in patients and siblings, but normalize with development in unaffected siblings due to a compensatory mechanism, as has been shown for siblings of childhood-onset schizophrenia patients (Moran et al., 2013; Ordóñez et al., 2016; Zalesky et al., 2015).
Another interest of the current study was to examine disturbances of interhemispheric connectivity in relation to putative abnormalities in hemispheric specialization. We find that schizophrenia patients exhibit increased hemispheric specialization in several unimodal and heteromodal association regions, as well as sensorimotor cortex and limbic areas (Fig. 4A), indicating an excessive reduction in cross-hemisphere connectivity relative to within-hemisphere connectivity in these brain regions. The unimodal and heteromodal association cortices have reciprocal connectivity with lower level brain regions, and are involved in the integration of sensory information from multiple modalities (Mesulam, 1998). Previous studies have shown that the cortical network of multimodal brain regions is disproportionately affected in schizophrenia (Bassett et al., 2008; Pearlson et al., 1996; van den Heuvel et al., 2013) and abnormalities in association cortices such as reduced cortical thickness and fiber integrity have been found in schizophrenia patients (Cannon et al., 2002; Sprooten et al., 2013; Zalesky et al., 2011). Extending previous findings, we suggest that unimodal and heteromodal areas more preferentially connected to ipsilateral brain areas in schizophrenia, at the expense of facilitating cross-hemisphere interactions with a broader range of brain regions.
In terms of the relationship between interhemispheric connectivity and hemispheric specialization, we find a significant correlation between lower interhemispheric connectivity and higher hemispheric specialization index in patients as compared to both controls and siblings (Fig. 5). These connectivity abnormalities may relate to reduced lateralization in schizophrenia. Previous studies have found that schizophrenia patients exhibit a loss of asymmetry in the auditory cortex in task-induced activation (Bleich-Cohen et al., 2009; Oertel et al., 2010; Spaniel et al., 2007), and its functional connectivity with other regions (Ke et al., 2010; Oertel-Knöchel et al., 2013; Oertel-Knöchel et al., 2014; Rotarska-Jagiela et al., 2010). In addition, reduced asymmetry in brain morphology (Barta et al., 1997; Bilder et al., 1994) and white matter connectivity (Kubicki et al., 2002; Miyata et al., 2012) have also been reported (for review and meta-analysis please refer to Ocklenburg et al., 2013; Oertel-Knöchel and Linden, 2011; Ribolsi et al., 2014; Sommer et al., 2001). The identified abnormalities of intrinsic interhemispheric connectivity and hemispheric specialization suggest impairments of synchronization between the two hemispheres, and a preference of intra- versus inter-hemispheric connectivity in patients, which is consistent with a lack of efficient lateralized processing in the dominant hemisphere during tasks (Bleich-Cohen et al., 2012; Innocenti et al., 2003; Ribolsi et al., 2014).
A strong main effect of hemisphere was found for hemispheric autonomy of many cortical and subcortical regions (Fig. 4B). Higher hemispheric specialization in the left hemisphere was found predominately in language-related regions, as well as subcortical regions and posterior cingulate cortex. Regions with higher autonomy in the right hemisphere mainly included parietal and occipital areas, as well as a few frontal, temporal, cingulate, and subcortical regions. This lateralization pattern is consistent with previous findings of a dissociation between left hemisphere specialization in language areas and a right hemisphere dominance in visuospatial and attentional areas (Cai et al., 2013; Gotts et al., 2013; Wang et al., 2014). Interestingly, we found that the hemispheric specialization of Heschl's gyrus and the temporal plane is lower in the left than the right hemispheric regions, indicating that in these primary auditory cortices, left hemispheric regions are more bilaterally connected (low hemispheric specialization), whereas right-sided regions are more ipsilaterally connected (high hemispheric specialization). This connectivity profile may indicate a more important role of the left primary auditory cortex in integration and processing of auditory information (Tervaniemi and Hugdahl, 2003). Connectivity of the caudate nucleus tends to be more ipsilateral in the right hemisphere as compared to the left hemisphere, which is different from the results of a previous study (Mueller et al., 2015), but is consistent with a right-over-left dopamine receptor availability in this region in healthy subjects (Laakso, 2000; Vernaleken et al., 2007). Other subcortical regions such as thalamus, putamen, pallidum, and amygdala were all found to exhibit higher hemispheric autonomy for the right hemisphere, in line with previous findings of a more bilaterally connected pattern in the right hemisphere (Gotts et al., 2013; Iturria-Medina et al., 2011; Zhong et al., 2017).
Several points need to be taken into consideration when interpreting our results. First, we preformed region-based rather than voxel-based analyses of interhemispheric connectivity and hemispheric specialization. Hence, our definition of hemispheric specialization is different from a previous study based on voxel-wise measurements (Mueller et al., 2015). Region-based and voxel-based approaches each have their benefits and limitations (Hayasaka and Laurienti, 2010; Joliot et al., 2015). We adopted a region-based approach for better sensitivity and interpretability of the results, and to allow us to correlate measurements of interhemispheric connectivity and hemispheric specialization across brain regions. Second, the proportion of male and female subjects are not balanced across groups. To address this issue, we included sex as a covariate when performing group-comparisons. Previous studies have produced mixed results in terms of the effects of sex on interhemispheric connectivity strength (Gracia-Tabuenca et al., 2018; Guo et al., 2014; Zuo et al., 2010). Future studies with a balanced sex distribution would be better suited to examine potential sex differences on interhemispheric connectivity and hemispheric specialization in schizophrenia. Third, we did not find a significant correlation between regional interhemispheric disconnectivity and symptom severity in patients as some previous studies have reported (Hoptman et al., 2012; Li et al., 2015). A possible reason for the lack of association may be that the patients recruited in this study were relatively stable at the time of participation (PANSS total scores 48.38 ± 14.89, see Table 1). Therefore, we cannot rule out the possibility that an association exists between disconnectivity and symptoms in patients with more pronounced clinical symptoms.
5. Conclusion
This study explored interhemispheric connectivity and hemispheric specialization in a large cohort of schizophrenia patients, their unaffected siblings and healthy controls. Our results indicate that patients exhibit decreased interhemispheric connectivity as compared to both healthy controls and unaffected siblings, and these impairments were found to correlate with increases in the level of hemispheric specialization. These connectivity alterations appear to be primarily related to illness manifestation, as siblings did not show significant changes compared to controls, and connectivity changes in patients correlated with duration of illness. In summary, the current study suggests that decreased interhemispheric connectivity and associated abnormalities in hemispheric specialization are features of established schizophrenia rather than an expression of preexistent risk for the disorder.
The following are the supplementary data related to this article.
Correlation of regional homotopic connectivity strength between the current study and those reported in a previous study (Stark et al., 2008). A significant association was observed (r = 0.41, p = .002) despite different MRI processing and analysis methods.
Functional connectivity strength of inter- and intra-hemispheric connectivity strength in schizophrenia patients, unaffected siblings and healthy controls. Patients have a pronounced global connectivity decrease (p < .001). After controlling for this global effect, only homotopic interhemispheric connectivity showed a significant group-effect (F(2, 250) = 4.31, p = .01).
Supplementary material
Funding statement
The infrastructure for the GROUP study is funded through the Geestkracht programme of the Dutch Health Research Council (Zon-Mw, grant number 10-000-1001), and matching funds from participating pharmaceutical companies (Lundbeck, AstraZeneca, Eli Lilly, Janssen Cilag) and universities and mental health care organizations (Amsterdam: Academic Psychiatric Centre of the Academic Medical Center and the mental health institutions: GGZ Ingeest, Arkin, Dijk en Duin, GGZ Rivierduinen, Erasmus Medical Centre, GGZ Noord Holland Noord. Groningen: University Medical Center Groningen and the mental health institutions: Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht and Parnassia Psycho-Medical Center The Hague. Maastricht: Maastricht University Medical Centre and the mental health institutions: GGzE, GGZ Breburg, GGZ Oost-Brabant, Vincent van Gogh voor Geestelijke Gezondheid, Mondriaan, Virenze riagg, Zuyderland GGZ, MET ggz, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem. Utrecht: University Medical Center Utrecht and the mental health institutions Altrecht, GGZ Centraal and Delta).
Conflict of interest
All authors declare no conflict of interest.
Acknowledgement
XC would like to acknowledge the Sino-Dutch Bilateral Exchange Scholarship for financial support. GC was supported by an EU Marie Curie Global Fellowship (Grant no. 749201).
References
- Anderson J.S., Druzgal T.J., Froehlich A., DuBray M.B., Lange N., Alexander A.L., Abildskov T., Nielsen J.A., Cariello A.N., Cooperrider J.R., Bigler E.D., Lainhart J.E. Decreased interhemispheric functional connectivity in autism. Cereb. Cortex. 2011;21:1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., Flaum M., Arndt S. The comprehensive assessment of symptoms and history (CASH). An instrument for assessing diagnosis and psychopathology. Arch. Gen. Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- Barnett K.J., Kirk I.J. Lack of asymmetrical transfer for linguistic stimuli in schizophrenia: an ERP study. Clin. Neurophysiol. 2005;116:1019–1027. doi: 10.1016/j.clinph.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Barta P.E., Pearlson G.D., Brill L.B., Royall R., McGilchrist I.K., Pulver A.E., Powers R.E., Casanova M.F., Tien A.Y., Frangou S., Petty R.G. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am. J. Psychiatry. 1997;154:661–667. doi: 10.1176/ajp.154.5.661. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E., Verchinski B.A., Mattay V.S., Weinberger D.R., Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder R.M., Wu H., Bogerts B., Degreef G., Ashtari M., Alvir J.M.J., Snyder P.J., Lieberman J.A. Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am. J. Psychiatry. 1994;151:1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- Bleich-Cohen M., Hendler T., Kotler M., Strous R.D. Reduced language lateralization in first-episode schizophrenia: an fMRI index of functional asymmetry. Psychiatry Res. Neuroimaging. 2009;171:82–93. doi: 10.1016/j.pscychresns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Bleich-Cohen M., Sharon H., Weizman R., Poyurovsky M., Faragian S., Hendler T. Diminished language lateralization in schizophrenia corresponds to impaired inter-hemispheric functional connectivity. Schizophr. Res. 2012;134:131–136. doi: 10.1016/j.schres.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Boos H.B.M., Cahn W., van Haren N.E.M., Derks E.M., Brouwer R.M., Schnack H.G., Hulshoff Pol H.E., Kahn R.S. Focal and global brain measurements in siblings of patients with schizophrenia. Schizophr. Bull. 2012;38:814–825. doi: 10.1093/schbul/sbq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Van der Haegen L., Brysbaert M. Complementary hemispheric specialization for language production and visuospatial attention. Proc. Natl. Acad. Sci. 2013;110:E322–E330. doi: 10.1073/pnas.1212956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon T.D., Thompson P.M., van Erp T.G.M., Toga A.W., Poutanen V.-P., Huttunen M., Lonnqvist J., Standerskjold-Nordenstam C.-G., Narr K.L., Khaledy M., Zoumalan C.I., Dail R., Kaprio J. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc. Natl. Acad. Sci. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X., Xi Y.-B., Cui L.-B., Wang H.-N., Sun J.-B., Zhu Y.-Q., Huang P., Collin G., Liu K., Xi M., Qi S., Tan Q.-R., Miao D.-M., Yin H. Distinct inter-hemispheric dysconnectivity in schizophrenia patients with and without auditory verbal hallucinations. Sci. Rep. 2015;5(11218) doi: 10.1038/srep11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G., Hulshoff Pol H.E., Haijma S.V., Cahn W., Kahn R.S., van den Heuvel M.P. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front. Psychiatry. 2011;2(73) doi: 10.3389/fpsyt.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T.J. Schizophrenia as a transcallosal misconnection syndrome. Schizophr. Res. 1998;30:111–114. doi: 10.1016/s0920-9964(97)00139-4. [DOI] [PubMed] [Google Scholar]
- Endrass T., Mohr B., Rockstroh B. Reduced interhemispheric transmission in schizophrenia patients: evidence from event-related potentials. Neurosci. Lett. 2002;320:57–60. doi: 10.1016/s0304-3940(02)00032-0. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M.S. Principles of human brain organization derived from split-brain studies. Neuron. 1995;14:217–228. doi: 10.1016/0896-6273(95)90280-5. [DOI] [PubMed] [Google Scholar]
- Goldstein J.M., Seidman L.J., Makris N., Ahern T., O'Brien L.M., Caviness V.S., Kennedy D.N., Faraone S.V., Tsuang M.T. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol. Psychiatry. 2007;61:935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Gotts S.J., Jo H.J., Wallace G.L., Saad Z.S., Cox R.W., Martin A. Two distinct forms of functional lateralization in the human brain. Proc. Natl. Acad. Sci. 2013;110:E3435–E3444. doi: 10.1073/pnas.1302581110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Tabuenca Z., Moreno M.B., Barrios F.A., Alcauter S. Hemispheric asymmetry and homotopy of resting state functional connectivity correlate with visuospatial abilities in school-age children. NeuroImage. 2018;174:441–448. doi: 10.1016/j.neuroimage.2018.03.051. [DOI] [PubMed] [Google Scholar]
- Guo S., Kendrick K.M., Zhang J., Broome M., Yu R., Liu Z., Feng J. Brain-wide functional inter-hemispheric disconnection is a potential biomarker for schizophrenia and distinguishes it from depression. NeuroImage Clin. 2013;2:818–826. doi: 10.1016/j.nicl.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Jiang J., Xiao C., Zhang Z., Zhang J., Yu L., Liu J., Liu G. Decreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patients. Schizophr. Res. 2014;152:170–175. doi: 10.1016/j.schres.2013.11.030. [DOI] [PubMed] [Google Scholar]
- Hayasaka S., Laurienti P.J. Comparison of characteristics between region-and voxel-based network analyses in resting-state fMRI data. NeuroImage. 2010;50:499–508. doi: 10.1016/j.neuroimage.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé P.-Y., Zago L., Petit L., Mazoyer B., Tzourio-Mazoyer N. Revisiting human hemispheric specialization with neuroimaging. Trends Cogn. Sci. 2013;17:69–80. doi: 10.1016/j.tics.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Hoptman M.J., Zuo X.-N., D'Angelo D., Mauro C.J., Butler P.D., Milham M.P., Javitt D.C. Decreased interhemispheric coordination in schizophrenia: a resting state fMRI study. Schizophr. Res. 2012;141:1–7. doi: 10.1016/j.schres.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol H.E., Schnack H.G., Mandl R.C.W., Cahn W., Collins D.L., Evans A.C., Kahn R.S. Focal white matter density changes in schizophrenia: reduced inter-hemispheric connectivity. NeuroImage. 2004;21:27–35. doi: 10.1016/j.neuroimage.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Innocenti G.M., Ansermet F., Parnas J. Schizophrenia, neurodevelopment and corpus callosum. Mol. Psychiatry. 2003;8:261–274. doi: 10.1038/sj.mp.4001205. [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y., Fernández A.P., Morris D.M., Canales-Rodríguez E.J., Haroon H.A., Pentón L.G., Augath M., García L.G., Logothetis N., Parker G.J.M., Melie-García L. Brain hemispheric structural efficiency and interconnectivity rightward asymmetry in human and nonhuman primates. Cereb. Cortex. 2011;21:56–67. doi: 10.1093/cercor/bhq058. [DOI] [PubMed] [Google Scholar]
- Joliot M., Jobard G., Naveau M., Delcroix N., Petit L., Zago L., Crivello F., Mellet E., Mazoyer B., Tzourio-Mazoyer N. AICHA: an atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods. 2015;254:46–59. doi: 10.1016/j.jneumeth.2015.07.013. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Ke M., Zou R., Shen H., Huang X., Zhou Z., Liu Z., Xue Z., Hu D. Bilateral functional asymmetry disparity in positive and negative schizophrenia revealed by resting-state fMRI. Psychiatry Res. Neuroimaging. 2010;182:30–39. doi: 10.1016/j.pscychresns.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Knöchel C., Oertel-Knöchel V., Schönmeyer R., Rotarska-Jagiela A., van de Ven V., Prvulovic D., Haenschel C., Uhlhaas P., Pantel J., Hampel H., Linden D.E.J. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. NeuroImage. 2012;59:926–934. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- Korver N., Quee P.J., Boos H.B.M., Simons C.J.P., De Haan L., Kahn R.S., Linszen D.H. Genetic Risk and Outcome of Psychosis (GROUP), a multi site longitudinal cohort study focused on gene-environment interaction: Objectives, sample characteristics, recruitment and assessment methods. Int. J. Methods Psychiatr. Res. 2012;21:205–221. doi: 10.1002/mpr.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroken R.A., Johnsen E., Ruud T., Wentzel-Larsen T., Jørgensen H.A. Treatment of schizophrenia with antipsychotics in Norwegian emergency wards, a cross-sectional national study. BMC Psychiatry. 2009;9(24) doi: 10.1186/1471-244X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M., Westin C.-F., Maier S.E., Frumin M., Nestor P.G., Salisbury D.F., Kikinis R., Jolesz F.A., McCarley R.W., Shenton M.E. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am. J. Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M., Styner M., Bouix S., Gerig G., Markant D., Smith K., Kikinis R., McCarley R.W., Shenton M.E. Reduced interhemispheric connectivity in schizophrenia-tractography based segmentation of the corpus callosum. Schizophr. Res. 2008;106:125–131. doi: 10.1016/j.schres.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso A. Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am. J. Psychiatry. 2000;157:269–271. doi: 10.1176/appi.ajp.157.2.269. [DOI] [PubMed] [Google Scholar]
- Li H.-J., Xu Y., Zhang K.-R., Hoptman M.J., Zuo X.-N. Homotopic connectivity in drug-naïve, first-episode, early-onset schizophrenia. J. Child Psychol. Psychiatry. 2015;56:432–443. doi: 10.1111/jcpp.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Sobering G., Duyn J., Moonen C.T.W. A. Functional MRI technique combining principles of echo-shifting with a train of observations (PRESTO) Magn. Reson. Med. 1993;30:764–768. doi: 10.1002/mrm.1910300617. [DOI] [PubMed] [Google Scholar]
- Liu H., Stufflebeam S.M., Sepulcre J., Hedden T., Buckner R.L. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc. Natl. Acad. Sci. 2009;106:20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Guo W., Zhang Y., Lv L., Hu F., Wu R., Zhao J. Decreased resting-state interhemispheric functional connectivity correlated with neurocognitive deficits in drug-naive first-episode adolescent-onset schizophrenia. Int. J. Neuropsychopharmacol. 2018;21:33–41. doi: 10.1093/ijnp/pyx095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr J.B., Hellige J.B., Cherry B.J., Lulow L., Kwok W., Caligiuri M.P. Impaired hemispheric communication in schizophrenia: a study using the consonant–vowel–consonant task. Schizophr. Res. 2006;87:279–288. doi: 10.1016/j.schres.2006.05.018. [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., Thermenos H.W., Barch D.M., Seidman L.J. Imaging genetic liability to schizophrenia: systematic review of fMRI studies of patients' nonpsychotic relatives. Schizophr. Bull. 2009;35:1142–1162. doi: 10.1093/schbul/sbn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Goldstein J.M., Kennedy D., Hodge S.M., Caviness V.S., Faraone S.V., Tsuang M.T., Seidman L.J. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr. Res. 2006;83:155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Maxwell E. Clinical Neurogenetics Branch, Intramural Research Program, National Institute of Mental Health; 1992. Family Interview for Genetic Studies (FIGS): A Manual for FIGS. [Google Scholar]
- Mesulam M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Miyata J., Sasamoto A., Koelkebeck K., Hirao K., Ueda K., Kawada R., Fujimoto S., Tanaka Y., Kubota M., Fukuyama H., Sawamoto N., Takahashi H., Murai T. Abnormal asymmetry of white matter integrity in schizophrenia revealed by voxelwise diffusion tensor imaging. Hum. Brain Mapp. 2012;33:1741–1749. doi: 10.1002/hbm.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr B., Pulvermüller F., Cohen R., Rockstroh B. Interhemispheric cooperation during word processing: evidence for callosal transfer dysfunction in schizophrenic patients. Schizophr. Res. 2000;46:231–239. doi: 10.1016/s0920-9964(00)00020-7. [DOI] [PubMed] [Google Scholar]
- Mohr B., Pulvermüller F., Rockstroh B., Endrass T. Hemispheric cooperation-a crucial factor in schizophrenia? Neurophysiological evidence. NeuroImage. 2008;41:1102–1110. doi: 10.1016/j.neuroimage.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Moran M.E., Hulshoff Pol H., Gogtay N. A family affair: brain abnormalities in siblings of patients with schizophrenia. Brain. 2013;136:3215–3226. doi: 10.1093/brain/awt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Wang D., Pan R., Holt D.J., Liu H. Abnormalities in hemispheric specialization of caudate nucleus connectivity in schizophrenia. JAMA Psychiatry. 2015;72:552. doi: 10.1001/jamapsychiatry.2014.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocklenburg S., Westerhausen R., Hirnstein M., Hugdahl K. Auditory hallucinations and reduced language lateralization in schizophrenia: a meta-analysis of dichotic listening studies. J. Int. Neuropsychol. Soc. 2013;19:410–418. doi: 10.1017/S1355617712001476. [DOI] [PubMed] [Google Scholar]
- Oertel V., Knöchel C., Rotarska-Jagiela A., Schönmeyer R., Lindner M., van de Ven V., Haenschel C., Uhlhaas P., Maurer K., Linden D.E.J. Reduced laterality as a trait marker of schizophrenia--evidence from structural and functional neuroimaging. J. Neurosci. 2010;30:2289–2299. doi: 10.1523/JNEUROSCI.4575-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knöchel V., Linden D.E.J. Cerebral asymmetry in schizophrenia. Neuroscientist. 2011;17:456–467. doi: 10.1177/1073858410386493. [DOI] [PubMed] [Google Scholar]
- Oertel-Knöchel V., Knöchel C., Matura S., Prvulovic D., Linden D.E.J., van de Ven V. Reduced functional connectivity and asymmetry of the planum temporale in patients with schizophrenia and first-degree relatives. Schizophr. Res. 2013;147:331–338. doi: 10.1016/j.schres.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Oertel-Knöchel V., Knöchel C., Matura S., Stäblein M., Prvulovic D., Maurer K., Linden D.E.J., van de Ven V. Association between symptoms of psychosis and reduced functional connectivity of auditory cortex. Schizophr. Res. 2014;160:35–42. doi: 10.1016/j.schres.2014.10.036. [DOI] [PubMed] [Google Scholar]
- Ordóñez A.E., Luscher Z.I., Gogtay N. Neuroimaging findings from childhood onset schizophrenia patients and their non-psychotic siblings. Schizophr. Res. 2016;173:124–131. doi: 10.1016/j.schres.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Mahon K., Wellington R., Zhang J., Chaplin W., Szeszko P.R. A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophr. Res. 2011;129:149–155. doi: 10.1016/j.schres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Pearlson G.D., Petty R., Ross C., Tien A. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G., Csernansky J.G., Barch D.M. Brain Network Connectivity in individuals with Schizophrenia and their Siblings. Biol. Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribolsi M., Koch G., Magni V., Di Lorenzo G., Rubino I.A., Siracusano A., Centonze D. Abnormal brain lateralization and connectivity in Schizophrenia. Rev. Neurosci. 2009;20:61–70. doi: 10.1515/revneuro.2009.20.1.61. [DOI] [PubMed] [Google Scholar]
- Ribolsi M., Daskalakis Z.J., Siracusano A., Koch G. Abnormal asymmetry of brain connectivity in schizophrenia. Front. Hum. Neurosci. 2014;8(1010) doi: 10.3389/fnhum.2014.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A., van de Ven V., Oertel-Knöchel V., Uhlhaas P.J., Vogeley K., Linden D.E.J. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010;117:21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E., Eickhoff S.B., Hakonarson H., Gur R.C., Gur R.E., Wolf D.H. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien D.J., Ivry R.B., Swinnen S.P. Dynamics of hemispheric specialization and integration in the context of motor control. Nat. Rev. Neurosci. 2006;7:160–166. doi: 10.1038/nrn1849. [DOI] [PubMed] [Google Scholar]
- Sommer I., Aleman A., Ramsey N., Bouma A., Kahn R. Handedness, language lateralisation and anatomical asymmetry in schizophrenia. Br. J. Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Spaniel F., Tintera J., Hajek T., Horacek J., Dezortova M., Hajek M., Dockery C., Kozeny J., Höschl C. Language lateralization in monozygotic twins discordant and concordant for schizophrenia. A functional MRI pilot study. Eur. Psychiatry. 2007;22:319–322. doi: 10.1016/j.eurpsy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Sprooten E., Papmeyer M., Smyth A.M., Vincenz D., Honold S., Conlon G.A., Moorhead T.W.J., Job D., Whalley H.C., Hall J., McIntosh A.M., Owens D.C.G., Johnstone E.C., Lawrie S.M. Cortical thickness in first-episode schizophrenia patients and individuals at high familial risk: a cross-sectional comparison. Schizophr. Res. 2013;151:259–264. doi: 10.1016/j.schres.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Stark D.E., Margulies D.S., Shehzad Z.E., Reiss P., Kelly A.M.C., Uddin L.Q., Gee D.G., Roy A.K., Banich M.T., Castellanos F.X., Milham M.P. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J. Neurosci. 2008;28:13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Fink G.R., Marshall J.C. Mechanisms of hemispheric specialization: insights from analyses of connectivity. Neuropsychologia. 2007;45:209–228. doi: 10.1016/j.neuropsychologia.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaniemi M., Hugdahl K. Lateralization of auditory-cortex functions. Brain Res. Rev. 2003;43:231–246. doi: 10.1016/j.brainresrev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.P., Sporns O., Collin G., Scheewe T., Mandl R.C.W., Cahn W., Goñi J., Hulshoff Pol H.E., Kahn R.S. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- Vernaleken I., Weibrich C., Siessmeier T., Buchholz H.-G., Rösch F., Heinz A., Cumming P., Stoeter P., Bartenstein P., Gründer G. Asymmetry in dopamine D2/3 receptors of caudate nucleus is lost with age. NeuroImage. 2007;34:870–878. doi: 10.1016/j.neuroimage.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Wang D., Buckner R.L., Liu H. Functional specialization in the human brain estimated by intrinsic hemispheric interaction. J. Neurosci. 2014;34:12341–12352. doi: 10.1523/JNEUROSCI.0787-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Tian Y., Yu Y., Zhang F., Hu X., Dong Y., Chen Y., Hu P., Hu X., Wang K. Modulation of interhemispheric functional coordination in electroconvulsive therapy for depression. Transl. Psychiatry. 2014;4:e453. doi: 10.1038/tp.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., Tsuang M.T., Faraone S.V., McCarley R.W., Shenton M.E., Green A.I., Nieto-Castanon A., LaViolette P., Wojcik J., Gabrieli J.D.E., Seidman L.J. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward N.D., Waldie B., Rogers B., Tibbo P., Seres P., Purdon S.E. Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophr. Res. 2009;109:182–190. doi: 10.1016/j.schres.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Yan C.-G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di Martino A., Li Q., Zuo X.-N., Castellanos F.X., Milham M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.-G., Wang X.-D., Zuo X.-N., Zang Y.-F. DPABI: Data processing & analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14:339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Seal M.L., Cocchi L., Westin C.-F., Bullmore E.T., Egan G.F., Pantelis C. Disrupted axonal fiber connectivity in schizophrenia. Biol. Psychiatry. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Pantelis C., Cropley V., Fornito A., Cocchi L., McAdams H., Clasen L., Greenstein D., Rapoport J.L., Gogtay N. Delayed development of brain connectivity in adolescents with schizophrenia and their unaffected siblings. JAMA Psychiatry. 2015;72:900–908. doi: 10.1001/jamapsychiatry.2015.0226. [DOI] [PubMed] [Google Scholar]
- Zhong S., He Y., Shu H., Gong G. Developmental changes in topological asymmetry between hemispheric brain white matter networks from adolescence to young adulthood. Cereb. Cortex. 2017;27:2560–2570. doi: 10.1093/cercor/bhw109. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Milham M., Zuo X.-N., Kelly C., Jaggi H., Herbert J., Grossman R.I., Ge Y. Functional homotopic changes in multiple sclerosis with resting-state functional MR imaging. Am. J. Neuroradiol. 2013;34:1180–1187. doi: 10.3174/ajnr.A3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.-N., Kelly C., Di Martino A., Mennes M., Margulies D.S., Bangaru S., Grzadzinski R., Evans A.C., Zang Y.-F., Castellanos F.X., Milham M.P. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J. Neurosci. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation of regional homotopic connectivity strength between the current study and those reported in a previous study (Stark et al., 2008). A significant association was observed (r = 0.41, p = .002) despite different MRI processing and analysis methods.
Functional connectivity strength of inter- and intra-hemispheric connectivity strength in schizophrenia patients, unaffected siblings and healthy controls. Patients have a pronounced global connectivity decrease (p < .001). After controlling for this global effect, only homotopic interhemispheric connectivity showed a significant group-effect (F(2, 250) = 4.31, p = .01).
Supplementary material