Abstract
Background
Endometrial cancer is one of the most common gynecological malignancies and has exhibited an increasing incidence rate in recent years. Cancer stem cells (CSCs), which are responsible for tumor growth and chemoresistance, have been confirmed in endometrial cancer. However, it is still challenging to identify endometrial cancer stem cells to then target for therapy.
Methods
Flow cytometry was used to identify the endometrial cancer stem cells. Sphere formation assay, western blotting, qRT-PCR assay, cell viability assay, xenograft assay and immunohistochemistry staining analysis were utilized to evaluate the effect of SPARC-related modular calcium binding 2 (SMOC-2) on the cells proliferation and drug resistance. Cell viability assay, qRT-PCR assay, immunofluorescence staining, Co-IP assay and luciferase reporter gene assay were performed to explore the possible molecular mechanism by which SMOC-2 activates WNT/β-catenin pathway.
Findings
We found the expression of SPARC-related modular calcium binding 2 (SMOC-2), a member of SPARC family, was higher in endometrial CSCs than that in non-CSCs. SMOC-2 was also more highly expressed in spheres than in monolayer cultures. The silencing of SMOC-2 suppressed cell sphere ability; reduced the expression of the stemness-associated genes SOX2, OCT4 and NANOG; and enhanced chemosensitivity in endometrial cancer cells. By co-culture IP assay, we demonstrated that SMOC-2 directly interacted with WNT receptors (Fzd6 and LRP6), enhanced ligand-receptor interaction with canonical WNT ligands (Wnt3a and Wnt10b), and finally, activated the WNT/β-catenin pathway in endometrial cancer. SMOC-2 expression was closely correlated with CSC markers CD133 and CD44 expression in endometrial cancer tissue.
Interpretation
Taken together, we conclude that SMOC-2 might be a novel endometrial cancer stem cell signature gene and therapeutic target for endometrial cancer.
Fund
National Natural Science Foundation of China, Scientific and Technological Innovation Act Program of Shanghai Science and Technology Commission, Scientific and Technological Innovation Act Program of Fengxian Science and Technology Commission, Natural Science Foundation of Shanghai.
Keywords: SMOC-2, Endometrial carcinoma, Cancer stem cells, Chemoresistance, WNT/β-catenin pathway
Research in context.
Evidence before this study
Endometrial cancer stem cells have been prospectively enriched by use of markers such as CD133, CD44 and ALDH. However, there is no conclusive evidence showing that none of these markers is the universal marker for endometrial cancer stem cells. Previously, SPARC-related modular calcium binding 2 (SMOC-2), an extracellular matrix proteins, has been found highly expressing during embryogenesis and wound healing. SMOC-2 mediated cell type–specific differentiation during gonad and reproductive tract development. It was also suggested as a stem cell signature gene in intestine.
Added value of this study
This study identifies SMOC-2 as a cancer stem cell signature gene in endometrial cancer. The expression of SMOC-2 was higher in CD133+/CD44+ cancer stem cells (CSCs) than that in CD133−/CD44− non-CSCs. SMOC-2 expression is greatly increased in spheres compared to monolayer cultures. Using the sphere assay, silencing SMOC-2 reduced the clonogenic potential of endometrial cancer cells and downregulated the expression of SOX2, OCT4 and NANOG, the stemness-associated genes. SMOC-2 enhanced the chemoresistance of endometrial cancer cells. Immunohistochemical staining revealed that the expression of SMOC-2 was positively correlated with CSCs markers expression (CD133 and CD44) in patient tissues. Furthermore, we found that SMOC-2 activated WNT/β-catenin pathway through directly interacting with WNT receptors (Fzd6 and LRP6) in stem cells and enhanced the interaction between receptors (Fzd6 and LRP6) and WNT ligands (Wnt3a and Wnt10b).
Implication of all the available evidence
Together, SMOC-2 may be a signature gene for enrich endometrial cancer stem cells. SMOC-2-Wnt/β-catenin axis may play a crucial role in cell self-renewing and chemoresistance of endometrial CSCs. And SMOC-2-Wnt/β-catenin axis might be a novel target for endometrial cancer therapy.
Alt-text: Unlabelled Box
1. Introduction
Endometrial cancer is the second most common gynecological malignancy among women worldwide with an estimated 61,380 new cases per year in the United States alone [1,2]. Recently, endometrial cancer was classified into four subtypes, POLE ultramutated, microsatellite instability hypermutated (MSI), copy number low, and copy number high, through an integrated analysis of genomic, transcriptomic, and proteomic characteristics of 373 endometrial carcinomas [3]. Among of the four subgroups, POLE proofreading mutant endometrial cancers have a favorable prognosis despite a strong association with high-grade cancer cells [4]. Patients with MSI tumors were more likely to present with early-stage disease [5,6]. Further, most endometrioid tumors have few somatic copy number alterations (SCNAs) when most serous and serous-like tumors exhibit extensive SCNAs with significantly worse progression-free survival than other groups [3]. Although most patients present with early-stage disease, 15–20% of these tumors still recur after primary surgery in metastatic disease [7,8], which require novel biomarkers or targets identified for diagnosing or treating.
The human endometrium is a highly regenerative tissue that undergoes a steroid-induced monthly cycle of proliferation, differentiation and shedding [9,10]. Evidence showed that endometrial stem cells were present in the endometrium and responsible for the cyclical regeneration of the endometrium each month [11]. The endometrium undergoes regenerative alterations under the influence of circulating ovarian steroid hormones, estrogen and progesterone [12]. CD15 appears to be a marker suitable for the enrichment of basal epithelial progenitor cells demonstrating classic adult stem cell properties [13]. Endometrial cancer was also confirmed to involve stem-like cells, self-renewing cancer stem cells (CSCs) [14]. These cells with stem cell properties are responsible for tumor growth and treatment resistance [[15], [16], [17]]. Furthermore, the vast majority of endometrial cancer is estrogen- and progestin-related [18,19]. A variety of cell surface proteins have been successfully identified as surrogate markers for these cancer stem cells. In endometrial cancer, the surface markers, CD133 and CD44, have been used to enrich CSCs [20,21]. Recently, epithelial membrane protein-2 (EMP2) has been clearly demonstrated as an endometrial cancer stem cell-associated gene [22].
SPARC-related modular calcium binding 2 (SMOC-2), a member of the SPARC family, is highly expressed during embryogenesis and wound healing [[23], [24], [25]]. The gene product is a matricellular protein that can stimulate endothelial cell proliferation and migration, as well as angiogenic activity [24,26,27]. Furthermore, SMOC-2 has been identified as the intestinal stem cell signature gene that is required for L1-mediated colon cancer progression [28]. It has been suggested that SMOC-2 may mediate intercellular signaling and cell type–specific differentiation during gonad and reproductive tract development [23]. Thus, we wonder if SMOC-2 has similar characteristics in the CSCs of endometrial cancer.
In this study, we compared the CSCs (CD133+/CD44+) with non-CSCs (CD133−/CD44−) flow-sorted from endometrial cancer cells and found the expression of SMOC-2 was significantly higher in CD133+/CD44+ cells than in CD133−/CD44− cells. Silencing SMOC-2 suppressed the ability of the cells to form spheres and enhanced paclitaxel and cisplatin sensitivity in endometrial cancer cells. We further demonstrated that SMOC-2 physically interacted with Fzd6 and LRP6, enhanced their interaction with canonical WNT ligands and thus activated the WNT/β-catenin pathway in endometrial CSCs. Furthermore, SMOC-2 was high expression in endometrial cancer tissues and was closely associated with CSC markers expression in endometrial cancer tissue.
2. Materials and methods
2.1. Cell culture and reagents
Human endometrial cancer cells AN3CA, HEC-1A, ECC-1, Ishikawa and HEK293T were obtained as gifts from Shanghai Cancer Institute. All these cells were cultured according to American Type Culture Collection (ATCC) instructions. Antibodies used in this study were against SMOC-2 (ab78068, Abcam) for western blotting (WB) and Immunohistochemistry (IHC). CD133 (372805, Biolegend), CD44 (103008, Biolegend) for Flow Cytometry. CD133 (ab216323, Abcam), CD44 (ab51037, Abcam) and PCNA (13110, Cell Signaling) for IHC. β-actin (M1210–2, Huabio), β-Tubulin (66240–1-Ig) and Lamin A/C (2032s, cell signaling) for western blotting (WB), β-catenin (ab32572, Abcam) for WB, immunofluorescence (IF). Wnt3a (ab28472, Abcam), Wnt10b (ab70816, Abcam), Frizzled3 (sc-68334, Santacruz), Frizzled6 (5158, cell signaling), Frizzled8 (sc-33503, Santacruz) Frizzled6 (5158, cell signaling), LRP1 (ab92544, Abcam), LRP6 (ab134146, Abcam), LRP7 (ab36121, Abcam), HA-tag (3724, cell signaling), Flag-tag (8146, cell signaling), and Myc-tag (2278, cell signaling) for CO-IP. Secondary antibodies were purchased from Jackson. TUNEL assay was purchased from Sigma (11684817910). Chemicals and biochemicalused were Paclitaxel (EY1353, Amquar), cisplatin (EY0024, Amquar), FGF (091608, PeproTech), EGF (0816AFC05, PeproTech), B27 (17504044, Gibco) and XAV-939 (S1180, Selleck).
2.2. Clinical samples
Human endometrial cancer and tissues normal endometrium were obtained from the Department of Obstetrics and Gynecology, Fengxian Hospital, Southern Medical University and the Department of Gynecology, Changzhou Maternal and Child Care Hospital. None of them had received radiotherapy, chemotherapy and other related anti-tumor therapies before surgery. All human tissues were obtained with informed consent and the study was approved by the Research Ethics Committee of Fengxian Hospital, Southern Medical University.
2.3. Immunohistochemical staining
Immunohistochemical staining were performed as described [29]. The following primary antibodies were used: SMOC-2 (1:200), CD133 (1:1000), CD44 (1:100) and PCNA (1:5000). After treatment with diaminobenzidine and counterstaining with hematoxylin, all the sections were observed and photographed with amicroscope (Axio Imager: Carl Zeiss). Scoring was conducted according to the ratio and intensity of positive-staining cells: 0–5% scored 0; 6–35% scored 1; 36–70% scored 2; >70% scored 3. The final scores of SMOC-2, CD133 and CD44 expression were designated as low or high expression group as follows: low expression: score 0–1; high expression: score 2–3. These scores were assigned independently and in a blinded manner by two senior pathologists.
2.4. Sphere formation assay
The spheres of endometrial cancer cells were cultured in DMEM: F12 medium containing 2% B27, 20 ng/ml bFDF, 20 ng/ml EGF and plated in 6-well Ultra-Low Attachment Plates. The growth factors were replenished every 3 days. 14-day-old spheres as primary sphere were harvested using 40 μm cell strainers, dissociated to single cells with trypsin, and then regrown for 7 days, defined as secondary sphere. The diameter and number (>50 μm) for primary and secondary spheres were evaluated and quantified [30].
2.5. Flow cytometry
Cell were prepared as single cell suspension for FACS staining. To identify endometrial CSCs, the following antibodies were used: FITC-CD133, PE -CD44 for 30 min at 4 °C and second antibody AF647 goat anti-mouse IgG for 30 min at 4 °C. The stained cells were acquired for analysis or sorting on LSRFortessa or AriaII (BD). Flow cytometry data was analyzed with FlowJo software (Tree Star Inc.).
2.6. Lentivirus constructs
shRNA sequences targeting SMOC-2 (SMOC-2-shRNA-1: 5′-GCUGAAAGUACGUCUAAUA -3′, SMOC-2-shRNA-2: 5′-GUGGCCGAAAGGAAGUAUA -3′, SMOC-2-shRNA-3: 5′-GACGUGAAUAAUGACAAAU -3′ and a negative control sequence(control: 5′-TTCTCCGAACGTGTCACGT-3′) were synthesized and inserted into pGLVU6/Puro vector (GenePharma, Shanghai, China). Virus packaging was performed in 293 T cells using Lipofectamine 3000 (Invitrogen). Viruses were harvested at 72 h after transfection. The AN3CA cells (1 × 105) were infected with the filtered lentivirus in the presence of 2 μg/ml polybrene (Sigma-Aldrich). The transfected cells were cultured in the presence of 2 μg/ml puromycin (Sangon, Shanghai) and the silencing effects were verified by western blotting analysis.
2.7. Cell transfection
Cells were plated at 60–70% confluence in 60 mm dishes. AN3CA, Ishikawa and HEC-1A Cells were transfected with si-SMOC-2 or with a non-targeted siRNA as a control. The sequences of the siRNA used were as follows: si-SMOC-2-1, GCUGAAAGUACGUCUAAUA; si-SMOC-2-2, GUGGCCGAAAGGAAGUAUA, LRP6, GCAGCCAAATGCCACAAATCC; Fzd6, GCTTCTTGCTATGAACAAAGT. siRNA oligos were produced by Gene Pharma (Shanghai, China). Transfection steps were performed according to the manufacturer's protocols. The human SMOC-2 ORF (NM_001166412) was subcloned into the pcDNA3.1 (+) vector (Obio, Shanghai, China). Target cells (1 × 105) AN3CA, HEC-1A and ECC-1 were infected with plasmids using Lipofectamine 3000.
2.8. Cell viability assay (CCK8 assay)
The cells were plated in 96-well plates at a density of 4000 cells per well with 100 μl of complete culture medium and cultured for 2–5 days. Each group contains five wells. 10 μl Cell Counting Kit-8 (CCK-8, WST-8, Dojindo, Japan) solution was added to each well after 48 h and 120 h. The cell cultures were sparse after 48 h or confluent after 120 h. CCK8 was metabolized to produce a colorimetric dye that was read at 450 nm using a microplate reader.
2.9. Western blotting
Total cellular protein and nuclear-cytosol protein were extracted using a total protein extraction buffer (Beyotime, China) and Nuclear-cytosol extraction kit (Applygen Technologies Inc. #1200). Cell lysates were separated by SDS-PAGE followed by blocking in 1% BSA (Bovine Serum Albumin), then incubated with primary antibodies against SMOC-2 (1:1000), β-catenin (1:1000), β-actin (1:2000), β-Tubulin (1:100000), Lamin A/C (1:1000) and species-specific secondary antibodies. Bound secondary antibodies were detected with the Odyssey imaging system (LI-COR Biosciences, Lincoln, NE).
2.10. RNA isolation and real-time qPCR
Total cellular RNA was extracted using Trizol reagent (Takara). PrimeScript RT-PCR kit (Takara) was used to perform the RT according to the protocol. SYBR Premix Ex Taq (Takara) on a 7500 real-time PCR system (Applied Biosystems) was used to determine the SMOC-2 mRNA expression at the following cycling settings: one initial cycle at 95 °C for 10 s followed by 40 cycles of 5 s at 95 °C and 31 s at 60 °C. Data were normalized to 18 s expression and represent the average of three repeated experiments. Prime sequences used for SMOC-2, MYC, CyclinD1, SOX2, OCT4, NANOG and 18 s detection are showed in Supplementary Table 2.
2.11. Immunofluorescence (IF)
We cultured the cells in 8-well chambers (Ibidi, Germany) for IF staining. The cells were fixed with 4% polyformaldehyde (15 min), permeabilized with 0.1% TritonX-100 (2 min) and blocked with 10% BSA (60 min) at room temperature. Cells were incubated with primary antibodies against β-catenin (1:200) at room temperature (60 min) then labeled with Alexa 488-conjugated secondary antibody (1:400) for 1 h at room temperature. The nuclei were counterstained for 2 min with DAPI (Sigma, USA). Images were acquired using confocal microscopy (LSM 510, METALaser Scanning Microscope, Zeiss).
2.12. In vivo tumor xenograft model
Six-week-old female athymic nude (nu/nu) mice (SLAC, Shanghai, China) were randomly divided into two groups and injected subcutaneously in the right flank with the stable single cell clones of AN3CA-sh1 and control cells at 5 × 106 cells in 100 μl serum-free DMEM medium for each nude mouse. After 6 weeks, mice were killed. The tumors were dissected and fixed with phosphate-buffered neutral formalin for standard histologic examination. Then paraffin embedded tumor samples were cut into 4-μm-thick sections for apoptosis detection. In situ tissue apoptosis was detected according to the protocol of TUNEL kit. Mice were manipulated and housed according to protocols approved by the East China Normal University Animal Care Commission.
2.13. Transcriptional reporter gene assay
SMOC-2 silenced cells and control cells were cultured in 96-well plates at a concentration of 7000 cells per well. 200 ng TCF Reporter Plasmid (WNT/β-catenin signaling), GLI Reporter Plasmid (Hedgehog signaling) or RBP-JK Reporter Plasmid (Notch signaling) (Millipore) and 10 ng pRL-TK (Renilla-TK-luciferase vector, Promega) were co-transfected into the cells. After 48 h, Dual-Glo Luciferase reporter Assay System (E1910, Promega) was used to measure the luciferase activities following the manufacturer's instructions. The ratio of reporter plasmids was determined, each normalized to the luciferase activities of the Renilla-TK-luciferase vector.
2.14. Paclitaxel, cisplatin and XAV-939 treatment
The stock solution of paclitaxel and cisplatin were prepared in dimethyl sulfoxide (DMSO) at 1 mM and 50 mM. Cells were treated with 0.01 nM, 0.1 nM, 0.5 nM, 2.5 nM, 12.5 nM, 200 nM, 500 nM, 1000 nM paclitaxel and 0.1 μM, 0.5 μM, 2.5 μM, 12.5 μM, 50 μM, and 200 μM, 500 μM, 1000 μM cisplatin for 48 h. Drugs in the medium were replaced every 24 h. Control cells were incubated with the same volume of DMSO. Cells in the combined paclitaxel/cisplatin with XAV-939 treatment group were cultured in the presence of 5 μM XAV-939 and drugs for 48 h. For in vivo tumor xenograft, cisplatin (5 mg/kg) or normal saline were injected intraperitoneally once every 3 days.
2.15. Co-immunoprecipitation (CO-IP) assay and co-culture immunoprecipitation assay
For co-immunoprecipitation assay, AN3CA cell lysates transfected with Flag-tagged SMOC-2 or vector control were subjected to immunoprecipitation with Flag antibody (1:1000) or control IgG for 2 h at 4 °C. All immunoprecipitations were performed with protein A/G sepharose (Santa Cruz Biotechnology) on a spinning wheel at 4 °C overnight. The beads were collected by centrifugation at 3000 rpm, then washed three times with lysis buffer. The immunoprecipitates were subjected to WB.
For the co-culture IP assay, the plasmids of Fzd6-HA, LRP6-HA, Wnt3a-myc, and Wnt10b-myc were all purchased from Shanghai Generay Biotech Co., Ltd. HEK293T cells were cultured in 6-well plates and individually transfected with 0.5 μg expression plasmids for SMOC-2-Flag or WNT receptor expression plasmids: LRP6-HA or Fzd6-HA, ligand expression plasmids: Wnt3a-myc or Wnt10b-myc using Lipofectamine 3000. After 24 h of co-culture, cell lysates with Fzd6 or LRP6 receptors and cell lysates with Wnt3a or Wnt10b ligands were prepared in a ratio of 1:2:2 to detect the interaction of one receptor (Fzd6 or LRP6) and two ligands (Wnt3a and Wnt10b). The consequent steps were the same as the IP assay mentioned above. Membranes were probed using anti-HA (1:1000), anti-myc (1:1000), or anti-Flag (1:1000) monoclonal antibodies.
2.16. In situ proximith ligation assay
Duolink PLA assay was performed to detect the interactions between SMOC-2 and LRP6/Fzd6 in stem cells and non-stem cells (DUO92102, Sigma). After fixation and permeabilization, cells were incubated with DuoLink blocking buffer for 60 min at 37 °C, followed by incubation with primary antibodies (SMOC-2, 1:200, Abcam, ab78069; LRP6, 1:200, Abcam, ab134146; Fzd6 1:100, Bioss, bs-13218R) for overnight at 4 °C. Cells were incubated with Duolink PLA Probe (anti-rabbit PLA probe PLUS and anti-mouse PLA probe MINUS)) for 1 h at 37 °C in a pre-heated humidity chamber, followed by incubation with Duolink Ligation buffer and Amplification buffer according to manufacturer's protocol. Finally, mounted the slides with Duolink In Situ Mounting Medium with DAPI, and analyzed in a fluorescence microscope.
2.17. Statistical analysis
Data are presented as means ± standard deviation (SD). Statistical analyses were done using GraphPad Prism 6 for windows. Chi-square test or student's t-test were used to compare the results from different groups. The relationship between SMOC-2 expression and CSCs markers (CD133 and CD44) was analyzed using Chi-square test (χ2 test) and Pearson's correlation test. The relationship between SMOC-2 expression and nuclear localization of β-catenin was used Spearman correlation analysis. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. SMOC-2 is a signature gene of endometrial cancer stem cells
To determine whether SMOC-2 is related to the CSCs of endometrial cancer, CD133+/CD44+ cells, which have been previously described as cells possessing CSC characteristics, were isolated from AN3CA and Ishikawa cells by flow cytometry. As shown in Fig. 1a, CD133+/CD44+ was heterogeneously expressed in tested endometrial cells, 1.57% in Ishikawa and 1.66% in AC3CA cells. Then, we measured the SMOC-2 expression level in CD133+/CD44+ and CD133−/CD44− cells by quantitative RT-PCR and western blotting. Our data revealed that SMOC-2 expression was much higher in sorted CD133+/CD44+ cells than that in CD133−/CD44− cells (Fig. 1b-c). It is well-known that CSCs have the ability to proliferate as spheres when cultured under non-adherent conditions [31]. Thus, we applied CSC-rich spheroids as a second model to confirm our results. Quantitative RT-PCR was conducted to analyze the SMOC-2 expression in AN3CA and Ishikawa cells grown either as monolayers or as spheres. The expression of SMOC-2 was significantly increased in spheres compared to monolayer cultures (Fig. 1d). We further investigated whether SMOC-2 have any effects on the clonogenic potential of endometrial cancer. The results showed that silencing of SMOC-2 greatly reduced the clonogenic potential of endometrial cancer by sphere assay in both primary and secondary sphere (Fig. 1e, f and g). Moreover, the expression of stemness-associated genes (SOX2, OCT4, NANOG) were downregulated in SMOC-2-silenced cells; overexpressioning of SMOC-2 upregulated the expression of SOX2, OCT4 and NANOG (Fig. 1h, i and Fig. S1a, 1b). These results collectively demonstrated that SMOC-2 might be a potential cancer stem cell marker for endometrial cancer.
Fig. 1.
SMOC-2 is a signature gene of endometrial cancer stem cells.
(a) Flow cytometry analysis of CD133+/CD44+ and CD133−/CD44− cells sorted from AN3CA and Ishikawa.
(b) The expression of SMOC2 in CD133+/CD44+ and CD133−/CD44− cells from AN3CA and Ishikawa was detected by western blotting and normalized by tubulin expression.
(c) Relative mRNA expression levels of SMOC2 in AN3CA/ CD133+/CD44+ and Ishikawa/ CD133+/CD44+ cells compared with AN3CA/ CD133−/CD44− and Ishikawa/ CD133−/CD44− cells. (mean ± SD, **P < 0.01, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
(d) Relative mRNA expression levels of SMOC2 in spherical cultures of AN3CA and Ishikawa cells compared with those in monolayer cultures. (mean ± SD, *P < 0.05, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
(e) Silencing efficacy of SMOC-2 in AN3CA and Ishikawa cells was detected by real-time PCR.
(f) Silencing efficacy of SMOC-2 in AN3CA and Ishikawa cells was detected by western blotting.
(g) Sphere assay was performed with siSMOC2–1-, siSMOC2–2- or siCT-transfected AN3CA and Ishikawa cells and passage cells. Scale bars, 50 μm. Quantification of sphere number was shown. (mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
(h) The expression of SOX2, OCT4, and NANOG in SMOC-2-silenced cells AN3CA/Ishikawa were detected by western blotting.
(i) Overexpression efficacy of SMOC-2 in HEC-1A and ECC-1 cells was determined by western blotting. The expression of SOX2, OCT4, and NANOG in SMOC-2-overexpressioning cells HEC-1A/ECC-1 were detected by western blotting.
3.2. SMOC-2 promotes endometrial cancer cell growth in vitro and in vivo
We further investigated whether SMOC-2 has any effect on the cell proliferation of endometrial cancer. The results showed that silencing SMOC-2 had no effect on the proliferation of cells grown at low density (sparse culture). However, interestingly, we found silencing SMOC-2 significantly suppressed the proliferation of cells grown at high density (confluent culture) (Fig. 2a). Normally, cancer cells lose their contact inhibition, a phenomenon that restricts the in vitro growth of normal cells at confluence, so they still grow in confluent condition. Furthermore, we found that the overexpression of SMOC-2 promoted cell proliferation in confluent culture but had no effect on cell proliferation in sparse monolayers (Fig. 2b). These data indicated that SMOC-2 might confer endometrial cancer cells with the ability to overcome their contact inhibition.
Fig. 2.
SMOC-2 promotes endometrial cancer cell growth in vitro and in vivo.
(a) The cell proliferation of siCT and siSMOC-2-1, siSMOC-2-2 groups in AN3CA/ Ishikawa cells was determined by CCK8 assay at low or high density. (mean ± SD, **P < 0.01, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
(b) The cell proliferation of the vector and overexpressing SMOC-2 groups in HEC-1A/ECC-1 cells was determined by CCK8 assay in low or high density. (mean ± SD, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
(c and d) Morphologic characteristics of tumors from mice inoculated with Lenti-vector and Lenti-shSMOC-2/AN3CA cells. Tumor weights and volumes of Lenti-vector and Lenti-shSMOC-2 groups were shown, n = 5. (mean ± SD, *P < 0.05. Experiments were statistically analyzed using two-tailed Student's t-test).
To further confirm the effects of SMOC-2 on endometrial cancer cell growth in vivo, stable cell lines were established that were transduced by the lentivirus carrying SMOC-2-short hairpin RNA in endometrial cancer cells (Fig. S1c). Then, Lenti-shSMOC-2/AN3CA cells and Lenti-vector cells were inoculated subcutaneously into nude mice. The weight and size of the tumors formed by Lenti-sh-SMOC-2 cells were significantly decreased in comparison with the tumors formed by Lenti-vector cells. The average volume and weight of the tumors in Lenti-sh-SMOC-2 mice were 0.309 ± 0.079 cm3 and 0.329 ± 0.008 g in contrast to 0.554 ± 0.191 cm3 and 0.590 ± 0.176 g in control mice (P = 0.042 and 0.025, (Statistical analysis was performed by Student's t-test) (Fig. 2c, d). In short, silencing SMOC-2 in endometrial cancer cells suppressed cell growth at high density in vitro and reduced xenograft tumor growth in vivo.
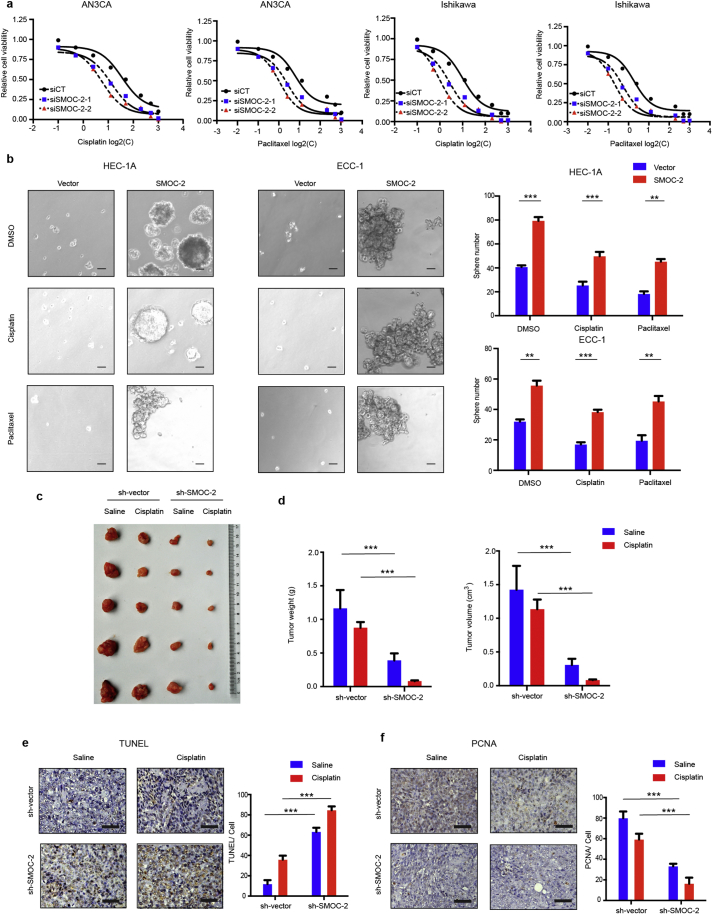
3.3. SMOC-2 enhances paclitaxel- and cisplatin-resistance in endometrial cancer cell
As chemotherapeutic resistance is another feature of CSCs, we next determined whether there is an association between SMOC-2 expression and resistance to two chemotherapeutic agents, paclitaxel and cisplatin, in endometrial cancer. To this end, we treated SMOC-2-silenced cells grown at high density with a series of concentrations paclitaxel or cisplatin. After 48 h treatment, cells survival rates were measured by CCK8 assay and half maximal inhibitory concentration (IC50) of paclitaxel/cisplatin against cell survivability were analyzed. The results showed that IC50 value of siSMOC-2 groups were less than siCT groups. Silencing SMOC-2 enhanced the cytotoxic effects on AN3CA and Ishikawa cells (Fig. 3a and Table 1). Moreover, overexpressioning of SMOC-2 reduced the cytotoxic effects on HEC-1A and ECC-1 cells in sphere assay (Fig. 3b). This result indicated that SMOC-2 enhanced paclitaxel- and cisplatin-resistance.
Fig. 3.
Silencing SMOC-2 enhanced paclitaxel and cisplatin sensitivity.
(a) siCT, siSMOC-2-1, and siSMOC-2-2 groups of AN3CA and Ishikawa cells were treated with a series of concentrations paclitaxel/cisplatin to obtain half maximal inhibitory concentration (IC50).
(b) Sphere assay was performed with vector, SMOC-2 DNA transfected HEC-1A and ECC-1 cells and SMOC-2 overexpressioning cells treated with paclitaxel or cisplatin. Scale bars, 50 μm. Quantification of sphere number was shown. (mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
(c) Morphologic characteristics of tumors from mice inoculated with sh-vector/Saline, sh-vector/Cisplatin, sh-SMOC-2/Saline and sh-SMOC-2/Cisplatin cells.
(d) Tumor volumes of 4 groups from c. n = 5. (mean ± SD, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
(e) TUNEL assay was detected in 4 groups from c. Scale bars, 50 μm. (mean ± SD, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
(f) PCNA staining was detected in 4 groups from c. Scale bars, 50 μm. (mean ± SD, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
Table 1.
Half maximal inhibitory concentration (IC50) of three groups cells treated with Paclitaxel or Cisplatin.
| Paclitaxel (nM) |
Cisplatin (μM) |
|||
|---|---|---|---|---|
| AN3CA | Ishikawa | AN3CA | Ishikawa | |
| siCT | 6.308 | 1.857 | 35.76 | 10.04 |
| siSMOC-2-1 | 2.404 | 0.5394 | 13.1 | 2.705 |
| siSMOC-2-2 | 1.05 | 0.2058 | 5.526 | 0.9221 |
To further confirm the results in vivo, Lenti-shSMOC-2/AN3CA cells or Lenti-vector cells were subcutaneously inoculated into nude mice. Athymic mice bearing subcutaneous tumors were treated with cisplatin (3 mg/kg per mouse) or saline once a week for four weeks (four injections in total) when the tumors first became palpable and lasted for 1 week. A significant reduction in the tumor weight and volume was recorded in the Lenti-shSMOC-2 group treated with saline compared to that in the Lenti-vector group treated with saline (P = 0.0004, P = 0.001. Statistical analysis was performed by Student's t-test). Further, tumors derived from the Lenti-shSMOC-2 group treated with cisplatin were significantly smaller than those derived from Lenti-vector group treated with cisplatin (P < 0.0001. Statistical analysis was performed by Student's t-test) (Fig. 3c-d). Apoptosis was significantly increased, and proliferation was suppressed in Lenti-shSMOC-2 tumors versus Lenti-vector tumors and cisplatin treated versus Lenti-vector tumors as detected by TUNEL assay and PCNA staining, respectively. Moreover, SMOC-2 knockdown enhanced cisplatin-induced tumor cell apoptosis and anti-proliferation (Fig. 3e -f). Taken together, these data demonstrated that silencing SMOC-2 enhanced paclitaxel and cisplatin sensitivity in endometrial cancer cells.
3.4. SMOC-2 activates the WNT/β-catenin pathway
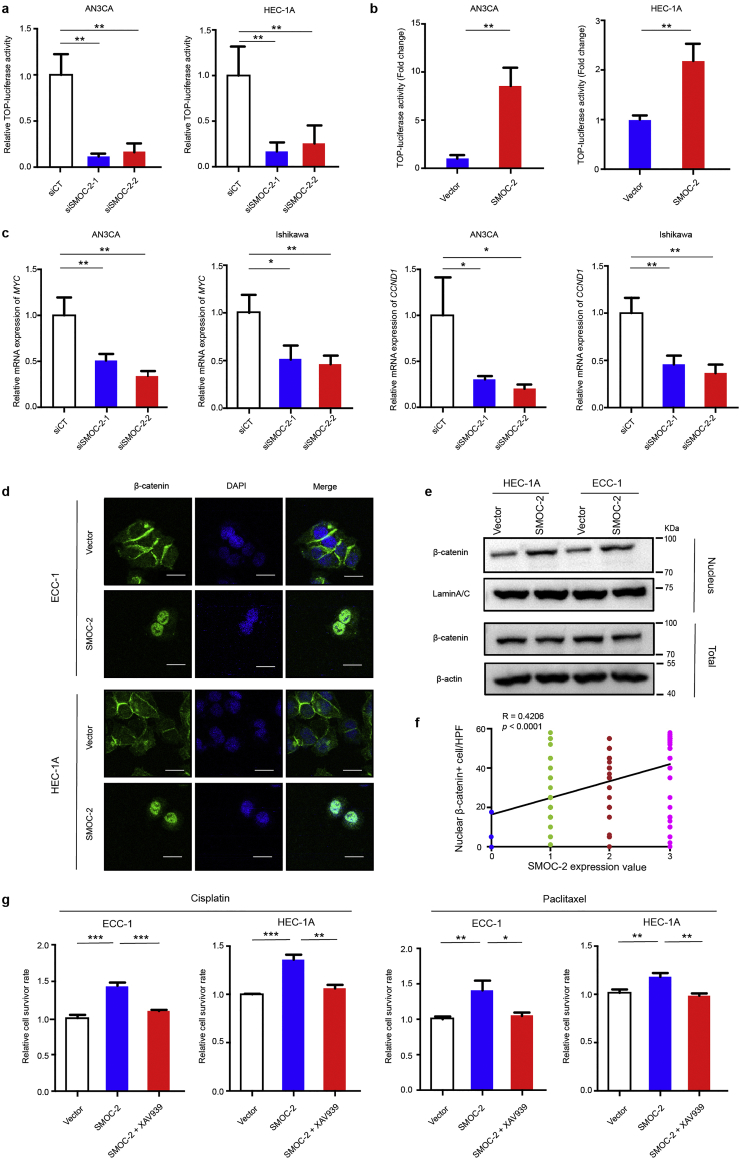
Having shown the effects of SMOC-2 on endometrial cancer cell proliferation and chemoresistance, we next explored the mechanism by which SMOC-2 alters the function of CSCs. It has been reported that related signaling pathways, such as WNT/β-catenin, Hedgehog and Notch, played essential roles in cancer stem cells. Here, we examined which pathway is involved in the function of SMOC-2 in endometrial cancer cells using a dual-luciferase reporter gene assay. A WNT/β-catenin reporter plasmid (TCF/catenin plasmid), Hedgehog reporter plasmid (GLI plasmid), Notch reporter plasmid (RBP plasmid) and Renilla were transfected into SMOC-2 silencing cells AN3CA and HEC-1A. The results showed that WNT/β-catenin signaling was significantly inhibited in SMOC-2 silenced groups, while the other signaling pathways experienced no significant change (Fig. 4a, Figs. S1d and S2a). We further confirmed that WNT/β-catenin signaling was activated by overexpressing SMOC-2 (Fig. 4b and Fig. S1e). Moreover, the expression of downstream target genes of WNT/β-catenin signaling (cyclin-D1 and MYC) were downregulated in SMOC-2-silenced cells compared with control cells (Fig. 4c). Immunofluorescence (IF) staining of β-catenin showed that the nuclear localization of β-catenin was more evident in SMOC-2 overexpressing cells than that in control cells (Fig. 4d). Western blotting also showed nuclear β-catenin was significantly increased in SMOC-2 overexpressing cells compared to that in control cells (Fig. 4e). Moreover, immunohistochemical staining of 151 endometrial cancer tissues also showed a positive association between SMOC-2 expression and nuclear localization of β-catenin, R = 0.4266, P < 0.001 (Spearman correlation analysis) (Fig. 4f and Fig. S2b). To further illustrate that SMOC-2 activates WNT/β-catenin signaling in endometrial cancer cells, XAV-939, a small molecule inhibitor of WNT/β-catenin signaling was used for further experiments. We treated HEC-1A and ECC-1 cells with XAV-939 and 50 nM paclitaxel or 1 μM cisplatin at the same time. The data showed that the overexpression of SMOC-2 enhanced chemoresistance in both HEC-1A and ECC-1 cells. These effects were completely abrogated by XAV-939 (Fig. 4g). These results indicated that SMOC-2 activates the WNT/β-catenin pathway by promoting the nuclear translocation of β-catenin. Obstructing the WNT/β-catenin signaling can reverse the effects of SMOC-2 on endometrial cancer cell chemoresistance.
Fig. 4.
SMOC-2 activates the WNT/β-catenin pathway.
(a) Luciferase reporter gene assay of AN3CA and HEC-1A cells transfected with siSMOC-2-1, siSMOC-2-2, or siCT. The results shown are the mean ± SD of the relative firefly/Renilla ratio. (mean ± SD, **P < 0.01. Experiments were statistically analyzed using two-tailed Student's t-test).
(b) Luciferase reporter gene assay of AN3CA and HEC-1A cells transfected with SMOC-2 plasmid or vector. The results shown are the mean ± SD of the relative firefly/Renilla ratio. (mean ± SD, **P < 0.01. Experiments were statistically analyzed using two-tailed Student's t-test).
(c) Silencing SMOC2 induced a reduction of Wnt/β-catenin target gene (MYC and CCND1) mRNA expression. (mean ± SD, *P < 0.05, **P < 0.01. Experiments were statistically analyzed using two-tailed Student's t-test).
(d) Representative images of the distribution of β-catenin in HEC-1A and ECC-1 cells transfected with vector or SMOC-2 plasmid by immunofluorescence staining. Scale bars: 20 μm.
(e) The expression of β-catenin from nuclear and whole cell lysates of SMOC-2-overexpression cell lines (HEC-1A and ECC-1) and vector cells were detected by western blotting.
(f) Correlation between SMOC-2 expression and number of nuclear β-catenin+ cells was determined based on the IHC staining (R = 0.4206, P < 0.0001. HPF, high power field. Spearman correlation analysis was used).
(g) Cell survivor rate was determined by CCK8 in HEC-1A and ECC-1 cells treated with vector, SMOC-2 or SMOC-2 + 5 μM XAV-939 and 50 nM paclitaxel or 1 μM cisplatin (mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test).
3.5. SMOC-2 enhances ligand-receptor interaction of WNT/β-catenin pathway
Next, to investigate how the SMOC-2 activate WNT/β-catenin pathway, we examined the interaction between SMOC-2 and WNT proteins. Co-immunoprecipitation (CO-IP) results showed that SMOC-2 had no direct interaction with the canonical WNT proteins (Wnt3a and Wnt10b). However, we observed an interaction between SMOC-2 and the WNT pathway co-receptors Fzd6 and LRP6 (Fig. 5a). SMOC-2 had no direct interaction with WNT pathway co-receptors Fzd3, Fzd8, LRP1 and LRP7 (Fig. S3). We further investigated the molecular mechanism of SMOC-2 modulating the WNT ligand–receptor interaction. To this end, an interaction assay was performed, which was based on the immunoprecipitation of tagged proteins from the lysates of co-cultured HEK293T cells. Co-cultured HEK293T cells were transfected with the expression plasmids of SMOC-2-Flag, Fzd6-HA, LRP6-HA, Wnt3a-myc and Wnt10b-myc (co-culture IP) (Fig. 5b-e). Cell lysates were immunoprecipitated with an HA antibody and immunoblotted with anti-Flag, anti-HA or anti-Myc antibodies. As shown in Fig. 5b, Fzd6 can co-precipitate with SMOC-2, Wnt3a and Wnt10b, and SMOC-2 overexpression can enhance the binding between Wnt3a and Fzd6 (lanes 6 and 8). A similar enhancement of the WNT-FZD interaction by SMOC-2 was also observed in combinations of Wnt10b and Fzd6 (Fig. 5b, lanes 7 and 9). Next, the effect of SMOC-2 on the interaction between Wnt3a/Wnt10b and LRP6 was examined. Similarly, SMOC-2 also enhanced the interaction between Wnt3a/Wnt10b and LRP6 (Fig. 5d-e). Moreover, we tested if the interaction of SMOC-2 with Fzd6/LRP6 occurs differently in stem versus non-stem cells. Duolink in situ proximity ligation assay showed that non-stem cells showed only a few PLA foci, whereas many PLA foci were found in stem cells (Fig. 5f). To further confirm that SMOC-2 direct interacted with Fzd6/LRP6 in activating WNT/β-catenin pathway, we treated SMOC-2 overexpressioning cells with a series of concentrations paclitaxel or cisplatin and silenced Fzd6/LRP6 at the same time. The results showed that overexpression of SMOC-2 reduced the cytotoxic effects on both HEC-1A and ECC-1 cells. Silencing of Fzd6/LRP6 reversed the chemoresistance effects of SMOC-2 on endometrial cancer cells (Fig. 5g,Table 2). These results suggest that SMOC-2 directly interacted with Fzd6/LRP6 and enhances the interaction between the canonical WNT ligands and receptors in endometrial cancer CSCs.
Fig. 5.
SMOC-2 enhances ligand-receptor interaction of WNT/β-catenin pathway.
(a) Co-IP assay between SMOC-2 and canonical WNT components (ligand: Wnt3a or Wnt10b; receptor: Fzd6 or LRP6). AN3CA cells were transfected with SMOC-2-Flag or a control vector. The input on the right panel shows the levels of transfected Flag-SMOC-2 and endogenous WNT components (Wnt3a, Wnt10b, Fzd6 and LRP6) in Flag-tagged SMOC-2 or vector control.
(b) SMOC-2 enhanced the binding between canonical WNT protein (Wnt3a or Wnt10b) and Fzd6. HA-tagged Fzd6-expressing cells were co-cultured with Myc-tagged WNT ligand-expressing (Wnt3a or Wnt10b) cells and Flag-tagged SMOC-2 expressing cells both separately and in combination. The HA-tagged Fzd6 was immunoprecipitated in this experiment.
(c and d) Densitometric analysis showed the relative amounts of precipitated WNT ligand (Wnt3a or Wnt10b) interacted with Fzd6 or LRP6 affected by SMOC-2. Values are normalized to intensities without SMOC-2 as 1.
(e) SMOC-2 enhanced the binding between the canonical WNT protein (Wnt3a or Wnt10b) and LRP6. Similarly, HA-tagged LRP6-expressing cells were co-cultured with WNT ligands-expressing cells and Flag-tagged SMOC-2-expressing cells both separately and in combination.
(f) The interactions of SMOC-2 and LRP6/Fzd6 were detected by in situ proximity ligation assay (red dots, n = 3, mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001. Experiments were statistically analyzed using two-tailed Student's t-test). Scale bars: 20 μm.
(g) Vector, SMOC-2, SMOC-2 + siLRP6 and SMOC-2 + siFzd6 groups of HEC-1A and ECC-1 cells were treated with a series of concentrations paclitaxel/cisplatin to obtain half maximal inhibitory concentration (IC50). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Half maximal inhibitory concentration (IC50) of four groups cells treated with Paclitaxel or Cisplatin.
| Paclitaxel (nM) |
Cisplatin (μM) |
|||
|---|---|---|---|---|
| AN3CA | Ishikawa | AN3CA | Ishikawa | |
| Vector | 0.8369 | 2.404 | 11.69 | 8.978 |
| SMOC-2 | 19.3 | 28.79 | 123.8 | 156.3 |
| SMOC-2 + siLRP6 | 3.02 | 7.585 | 40.17 | 76.77 |
| SMOC-2 + siFzd6 | 8.123 | 10.32 | 27.52 | 23.09 |
3.6. SMOC-2 and CSCs markers are co-expressed in endometrial cancer
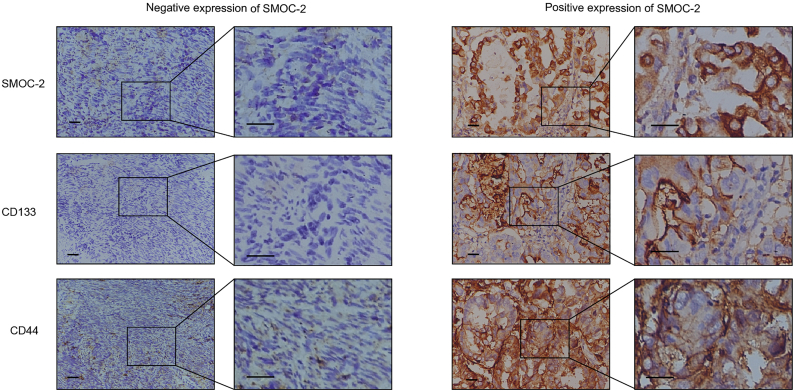
To further evaluate whether SMOC-2 is a marker for CSCs in endometrial cancer, we investigated the expression level of SMOC-2, CD133 and CD44 in 151 endometrial cancer patients, and analyzed the relevance with clinicopathological parameters. The high expression rate of SMOC-2, CD133 and CD44 were 70.19%, 50.99% and 58.27%, respectively. The results also demonstrated that SMOC-2 expression correlated with pathological grade, depth of myometrial invasion and age (P = 0.000, 0.0469 and 0.0046, Statistical analysis was performed by Student's t-test) (Table 3). CD133 expression correlated with pathological grade, depth of myometrial invasion and vascular invasion while CD44 expression correlated with FIGO stage, pathological grade and lymph node metastasis (Table 3, Table 4, Table 5). Moreover, based on the IHC staining, a positive correlation was found between the SMOC-2 expression and the CD133 expression (R = 0.4122, P < 0.0001, Pearson correlation analysis). The expression of SMOC-2 also had a positively correlation with the expression of CD44 (R = 0.4358, P < 0.0001. Pearson correlation analysis) (Table 6). Obviously, SMOC-2 expression was closely associated with risk factors of poor prognosis and CSC marker expression (Fig. 6).
Table 3.
Correlation of SMOC-2 expression with patient's clinical characteristics.
| SMOC-2 |
Total | X2 | P | ||
|---|---|---|---|---|---|
| High | Low | ||||
| FIGO stage | |||||
| I | 66 (64.71%) | 36 (35.29%) | 102 | 4.64 | 0.098 |
| II | 15 (78.95%) | 4 (21.05%) | 19 | ||
| III + IV | 25 (83.33%) | 5 (16.67%) | 30 | ||
| Grade | |||||
| 1 | 14 (43.75%) | 18 (56.25%) | 32 | 16.212 | 0.000 |
| 2 | 47 (71.21%) | 19 (28.79%) | 66 | ||
| 3 | 45 (84.91%) | 8 (15.09%) | 53 | ||
| Depth of Myometrial Invasion | |||||
| ≤ 50% | 52 (63.41%) | 30 (36.59%) | 82 | 3.948 | 0.0469 |
| > 50% | 54 (78.26%) | 15 (21.74%) | 69 | ||
| Lymph node metastasis | |||||
| Present | 16 (80%) | 4 (20%) | 20 | 1.059 | 0.3035 |
| Absent | 90 (68.71%) | 41 (31.29%) | 131 | ||
| Vascular Invasion | |||||
| Present | 20 (76.92%) | 6 (23.08%) | 26 | 0.5824 | 0.4454 |
| Absent | 86 (68.8%) | 39 (31.2%) | 125 | ||
| Age | |||||
| ≤ 60 y | 59 (62.11%) | 36 (37.89%) | 95 | 8.021 | 0.0046 |
| > 60 y | 47 (83.93%) | 9 (16.07%) | 56 | ||
Table 4.
Correlation of CD133 expression with patient's clinical characteristics.
| CD133 |
Total | X2 | P | ||
|---|---|---|---|---|---|
| High | Low | ||||
| FIGO stage | |||||
| I | 51 (50%) | 51 (50%) | 102 | 0.527 | 0.769 |
| II | 9 (47.4%) | 10 (52.6%) | 19 | ||
| III + IV | 17 (56.67%) | 13 (43.33%) | 30 | ||
| Grade | |||||
| 1 | 7 (21.88%) | 25 (78.12%) | 32 | 20.115 | 0.000 |
| 2 | 32 (48.49%) | 34 (51.51%) | 66 | ||
| 3 | 38 (71.69%) | 15 (28.31%) | 53 | ||
| Depth of Myometrial Invasion | |||||
| ≤ 50% | 35 (42.68%) | 47 (57.32%) | 82 | 4.959 | 0.0260 |
| > 50% | 42 (60.87%) | 27 (39.13%) | 69 | ||
| Lymph node metastasis | |||||
| Present | 11 (55%) | 9 (45%) | 20 | 0.1481 | 0.7004 |
| Absent | 66 (50.38%) | 65 (49.62%) | 131 | ||
| Vascular Invasion | |||||
| Present | 18 (69.23%) | 8 (30.77%) | 26 | 4.180 | 0.0409 |
| Absent | 59 (47.2%) | 66 (52.8%) | 125 | ||
| Age | |||||
| ≤ 60 y | 50 (52.63%) | 45 (47.37%) | 95 | 0.2751 | 0.5999 |
| > 60 y | 27 (48.21%) | 29 (51.79%) | 56 | ||
Table 5.
Correlation of CD44 expression with patient's clinical characteristics.
| CD44 |
Total | X2 | P | ||
|---|---|---|---|---|---|
| High | Low | ||||
| FIGO stage | |||||
| I | 52 (50.98%) | 50 (49.02%) | 102 | 8.242 | 0.016 |
| II | 12 (63.16%) | 7 (36.84%) | 19 | ||
| III + IV | 24 (80%) | 6 (20%) | 30 | ||
| Grade | |||||
| 1 | 7 (21.88%) | 25 (78.13%) | 32 | 24.285 | 0.000 |
| 2 | 41 (62.12%) | 25 (37.88%) | 66 | ||
| 3 | 40 (75.47%) | 13 (24.53%) | 53 | ||
| Depth of Myometrial Invasion | |||||
| ≤50% | 42 (51.22%) | 40 (48.78%) | 82 | 0.5968 | 0.4398 |
| >50% | 46 (66.67%) | 23 (33.33%) | 69 | ||
| Lymph node metastasis | |||||
| Present | 17 (85%) | 3 (15%) | 20 | 6.770 | 0.0093 |
| Absent | 71 (54.19%) | 60(45.81%) | 131 | ||
| Vascular Invasion | |||||
| Present | 16 (61.54%) | 10 (38.46%) | 26 | 0.1373 | 0.7110 |
| Absent | 72 (57.6%) | 53 (42.2%) | 125 | ||
| Age | |||||
| ≤ 60 y | 53 (55.79%) | 42 (44.21%) | 95 | 0.6525 | 0.4192 |
| > 60 y | 35 (62.5%) | 21 (37.5%) | 56 | ||
Table 6.
The association between SMOC-2, CD133 and CD44 expression.
| Total |
SMOC-2 expression(n = 151) |
Ra | P-Valueb | ||
|---|---|---|---|---|---|
| n | High (%) | Low (%) | |||
| CD133 | |||||
| Low | 74 | 33 (44.59) | 41 (55.41) | 0.4122 | 0.000 |
| High | 77 | 73 (94.81) | 4 (5.19) | ||
| CD44 | |||||
| Low | 63 | 29(46.03) | 34(53.97) | 0.4358 | 0.000 |
| High | 88 | 77(87.5) | 11(12.5) | ||
Values evaluated by the Pearson correlation analysis.
Evaluated by x2 test.
Fig. 6.
SMOC-2 and CSCs markers are co-expressed in endometrial cancer.
151 endometrial cancer tissues were stained with SMOC-2, CD44 and CD133 antibodies using serial sections. The left images represented a typical case of low expression of SMOC-2, and CD44 and CD133. The right images represented a typical case with high expression of SMOC-2, CD44 and CD133. Scale bars, 100 μm.
4. Discussion
Cancer stem cells (CSCs) have distinct characteristics including self-renewal, drug resistance, and expression of specific markers that enable their isolation [32]. In endometrial cancer, it is still challenging to identify CSCs. A number of cell surface markers, including CD133, CD44, etc., can identify endometrial CSCs [20,21,33,34]. However, the markers of endometrial CSCs remain controversial. Here, we identified SMOC-2 as a novel signature gene of endometrial cancer stem cells. First, the expression of SMOC-2 was higher in CD133+/CD44+ cells than that in CD133−/CD44− cells. Second, SMOC-2 expression is greatly increased in spheres compared to monolayer cultures. Third, using the sphere assay, silencing SMOC-2 reduced the clonogenic potential of endometrial cancer cells and downregulated the expression of SOX2, OCT4, and NANOG, the stemness-associated genes. Fourth, SMOC-2 enhanced the chemoresistance of endometrial cancer cells. Fifth, immunohistochemical staining revealed that the expression of SMOC-2 was positively correlated with CD133 and CD44 expression in patient tissues. A previous study has also elaborately identified SMOC-2 as a colon cancer stem cell signature gene [28].
It has been reported that SMOC-2 executed its biological function through integrin-dependent signaling [35]. In this study, we demonstrated that SMOC-2 activated WNT/β-catenin signaling in endometrial cancer, which is supported by the following evidence. 1) Using the luciferase reporter assay system, it was revealed that overexpressing SMOC-2 promoted the activation of Wnt/β-catenin signaling, while silencing SMOC-2 reduced the activation of Wnt/β-catenin signaling. 2) Silencing SMOC-2 could significantly inhibit the expression of cyclinD1 and MYC, the important target genes of the Wnt/β-catenin signaling. 3) Both western blotting and immunofluorescence staining revealed that overexpressing SMOC-2 significantly increased the nuclear translocation of β-catenin, which led to activate a downstream pathway. 4) It was discovered through CO-IP assay that SMOC-2 may bridge the Fzd6 and LRP6 proteins through trimer formation, further enhancing Wnt ligand-receptor interactions. Therefore, we concluded that SMOC-2 may participate in the progression of endometrial cancer through the Wnt/β-catenin signaling pathway.
The Wnt/β-catenin signaling pathway has a crucial function in cell proliferation, differentiation, growth, survival, development and fate determination in adult organisms [36]. Moreover, Wnt signaling supports the formation and maintenance of stem and cancer stem cells [37]. In the intestine, downstream of Wnt/β-catenin, the Tcf4-driven target gene program is essential to maintain intestinal crypt stem cells [38]. In the mammary gland, a Wnt ligand, Wnt3a, functions as a rate-limiting, self-renewal factor to clonally expand mammary stem cells [39]. Several studies in skin tumors, intestinal cancer and mammary tumors have demonstrated that Wnt signaling mediates stem cell renewal and proliferation by promoting β-catenin translocation to the nucleus [[40], [41], [42], [43], [44]]. In endometrial cancer, targeting Wnt signaling inhibited the proliferation, migration, invasiveness and tumorigenicity of endometrial cancer stem cells [45]. Our findings identify the extracellular matrix protein SMOC-2 as a unique signaling node that drives self-renewal and therapeutic resistance through the Wnt/β-catenin signal pathway, which provides novel evidence for targeting Wnt signaling in endometrial cancer.
Our study revealed that SMOC-2 may be a stem cell signature gene in endometrial cancer, and we discovered a novel regulator of WNT/β-catenin signaling. However, a key limitation of this study is that we only examined the biological functions of SMOC-2 in an in vitro cell model and xenograft mouse model. We are establishing a patient-derived xenograft (PDX) model and genetically engineered mice for further studies.
In conclusion, SMOC-2 is a cancer stem cell-associated matricellular protein in endometrial cancer and promoted cell proliferation, enhanced resistance toward chemotherapy both in vitro and in vivo. This effect was associated with the activation of WNT/β-catenin signaling. These findings suggest that the SMOC-2-Wnt/β-catenin axis may be a novel target for endometrial cancer therapy.
Acknowledgments
Acknowledgments
Not applicable.
Funding sources
This work was supported by the National Natural Science Foundation of China [grant numbers 81472445 and 81672587 to R. Zhang], the Scientific and Technological Innovation Act Program of Shanghai Science and Technology Commission (grant number 14411973100 to R. Zhang) and the Scientific and Technological Innovation Act Program of Fengxian Science and Technology Commission (grant number 20160908 to H. Lu), the Natural Science Foundation of Shanghai (17ZR1428300 to J. Li).
Declaration of interest
The authors declare no conflict of interest.
Author contributions
H.L. and DD.J. performed the in vitro experiments and wrote the original manuscripts. R.Z. and ZG.Z supervised the experimental work and were involved in the analysis of the experimental data and the preparation of the manuscript. GD.Y. and LY.Z. conducted the in vivo tumor formation assay. WW.S., JH. W and CC.Z. performed the rest of the experiments. J.L and XM.Y. revised manuscripts. All authors contributed to the discussion and approved the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.12.044.
Contributor Information
Zhi-gang Zhang, Email: zzhang@shsci.org.
Rong Zhang, Email: rongzhang1965@163.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017 doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research N., Kandoth C., Schultz N., Cherniack A.D., Akbani R., Liu Y. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Church D.N., Stelloo E., Nout R.A., Valtcheva N., Depreeuw J., ter Haar N. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015;107(1):402. doi: 10.1093/jnci/dju402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basil J.B., Goodfellow P.J., Rader J.S., Mutch D.G., Herzog T.J. Clinical significance of microsatellite instability in endometrial carcinoma. Cancer. 2000;89(8):1758–1764. doi: 10.1002/1097-0142(20001015)89:8<1758::aid-cncr16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Lax S.F., Kendall B., Tashiro H., Slebos R.J.C., Ellenson L.H. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma—Evidence of distinct molecular genetic pathways. Cancer. 2000;88(4):814–824. [PubMed] [Google Scholar]
- 7.Nout R.A., Smit V.T., Putter H., Jurgenliemk-Schulz I.M., Jobsen J.J., Lutgens L.C. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet. 2010;375(9717):816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 8.Group AES, Blake P., Swart A.M., Orton J., Kitchener H., Whelan T. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373(9658):137–146. doi: 10.1016/S0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mihm M., Gangooly S., Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci. 2011;124(3–4):229–236. doi: 10.1016/j.anireprosci.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Gargett C.E., Nguyen H.P., Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13(4):235–251. doi: 10.1007/s11154-012-9221-9. [DOI] [PubMed] [Google Scholar]
- 11.Gargett C.E. Uterine stem cells: What is the evidence? Hum Reprod Update. 2007;13(1):87–101. doi: 10.1093/humupd/dml045. [DOI] [PubMed] [Google Scholar]
- 12.Gargett C.E., Chan R.W., Schwab K.E. Hormone and growth factor signaling in endometrial renewal: Role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288(1–2):22–29. doi: 10.1016/j.mce.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Valentijn A.J., Palial K., Al-Lamee H., Tempest N., Drury J., Von Zglinicki T. SSEA-1 isolates human endometrial basal glandular epithelial cells: Phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod. 2013;28(10):2695–2708. doi: 10.1093/humrep/det285. [DOI] [PubMed] [Google Scholar]
- 14.Hubbard S.A., Friel A.M., Kumar B., Zhang L., Rueda B.R., Gargett C.E. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009;69(21):8241–8248. doi: 10.1158/0008-5472.CAN-08-4808. [DOI] [PubMed] [Google Scholar]
- 15.Miller S.J., Lavker R.M., Sun T.T. Interpreting epithelial cancer biology in the context of stem cells: Tumor properties and therapeutic implications. Biochim Biophys Acta. 2005;1756(1):25–52. doi: 10.1016/j.bbcan.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Nagaraj A.B., Joseph P., Kovalenko O., Singh S., Armstrong A., Redline R. Critical role of Wnt/beta-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget. 2015;6(27):23720–23734. doi: 10.18632/oncotarget.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiechert A., Saygin C., Thiagarajan P.S., Rao V.S., Hale J.S., Gupta N. Cisplatin induces stemness in ovarian cancer. Oncotarget. 2016;7(21):30511–30522. doi: 10.18632/oncotarget.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan J., Gao Y., Zeng K., Yin Y., Zhao M., Wei J. The levels of the sex hormones are not different between type 1 and type 2 endometrial cancer. Sci Rep. 2016;6:39744. doi: 10.1038/srep39744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lax S.F. Pathology of Endometrial Carcinoma. Adv Exp Med Biol. 2017;943:75–96. doi: 10.1007/978-3-319-43139-0_3. [DOI] [PubMed] [Google Scholar]
- 20.Mizrak D., Brittan M., Alison M. CD133: Molecule of the moment. J Pathol. 2008;214(1):3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 21.Mirantes C., Espinosa I., Ferrer I., Dolcet X., Prat J., Matias-Guiu X. Epithelial-to-mesenchymal transition and stem cells in endometrial cancer. Hum Pathol. 2013;44(10):1973–1981. doi: 10.1016/j.humpath.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Kiyohara M.H., Dillard C., Tsui J., Kim S.R., Lu J., Sachdev D. EMP2 is a novel therapeutic target for endometrial cancer stem cells. Oncogene. 2017;36(42):5793–5807. doi: 10.1038/onc.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pazin D.E., Albrecht K.H. Developmental expression of Smoc1 and Smoc2 suggests potential roles in fetal gonad and reproductive tract differentiation. Dev Dyn. 2009;238(11):2877–2890. doi: 10.1002/dvdy.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocnik E.F., Liu P., Sato K., Walsh K., Vaziri C. The novel SPARC family member SMOC-2 potentiates angiogenic growth factor activity. J Biol Chem. 2006;281(32):22855–22864. doi: 10.1074/jbc.M513463200. [DOI] [PubMed] [Google Scholar]
- 25.Christian Vannahme S.G., Paulsson Mats, Maurer Patrik, Hartmann Ursula. Characterization of SMOC-2, a modular extracellular calcium-binding protein. Biochem. J. 2003;(373):805–814. doi: 10.1042/BJ20030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimoto S.H.Y., Toda Y., Toyoda H., Kitamura K., Komurasaki T. Identification of a novel smooth muscle associated protein, smap2, upregulated during neointima formation in a rat carotid endarterectomy model. Biochim Biophys Acta. 2002;1576(1–2):225–230. doi: 10.1016/s0167-4781(02)00345-7. [DOI] [PubMed] [Google Scholar]
- 27.Maier S., Paulsson M., Hartmann U. The widely expressed extracellular matrix protein SMOC-2 promotes keratinocyte attachment and migration. Exp Cell Res. 2008;314(13):2477–2487. doi: 10.1016/j.yexcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Shvab A., Haase G., Ben-Shmuel A., Gavert N., Brabletz T., Dedhar S. Induction of the intestinal stem cell signature gene SMOC-2 is required for L1-mediated colon cancer progression. Oncogene. 2016;35(5):549–557. doi: 10.1038/onc.2015.127. [DOI] [PubMed] [Google Scholar]
- 29.Schatton T., Murphy G.F., Frank N.Y., Yamaura K., Waaga-Gasser A.M., Gasser M. Identification of cells initiating human melanomas. Nature. 2008;451(7176):345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patrizia C., Ylenia L., Maria G.F., Sebastino B., Matilde T., Giorgio S. Isolation and culture of colon cancer stem cells. Method Cell Biol. 2008;86:311–324. doi: 10.1016/S0091-679X(08)00014-9. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J., Zhang Y. Cancer stem cells: Models, mechanisms and implications for improved treatment. Cell Cycle. 2008;7(10):1360–1370. doi: 10.4161/cc.7.10.5953. [DOI] [PubMed] [Google Scholar]
- 32.Clarke M.F., Dick J.E., Dirks P.B., Eaves C.J., Jamieson C.H., Jones D.L. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 33.Rutella S., Bonanno G., Procoli A., Mariotti A., Corallo M., Prisco M.G. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res. 2009;15(13):4299–4311. doi: 10.1158/1078-0432.CCR-08-1883. [DOI] [PubMed] [Google Scholar]
- 34.Saygin C., Wiechert A., Rao V.S., Alluri R., Connor E., Thiagarajan P.S. CD55 regulates self-renewal and cisplatin resistance in endometrioid tumors. J Exp Med. 2017;214(9):2715–2732. doi: 10.1084/jem.20170438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peijun Liu J.L., Wellington V. Cardoso,and cyrus vaziri. The SPARC-related factor SMOC-2 promotes growth factor-induced cyclin D1 expression and DNA synthesis via integrin-linked kinase. Mol Biol Cell. 2008;19(1):248–261. doi: 10.1091/mbc.E07-05-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi J., Chi S., Xue J., Yang J., Li F., Liu X. Emerging role and therapeutic implication of Wnt signaling pathways in autoimmune diseases. J Immunol Res. 2016;2016:9392132. doi: 10.1155/2016/9392132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peter Wend J.D.H. Ulrike ZIEBOLD, Walter Birchmeier. Wnt signaling in stem and cancer stem cell. Semin Cell Dev Biol. 2010;21:855–863. doi: 10.1016/j.semcdb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Grigoryan T.W.P., Klaus A., Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: Conditional loss- and gain-of-function mutations of beta-catenin in mice. Gene Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng Y.A.N.R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- 41.O'Brien C.A.P.A., Gallinger S., Dick J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 42.Sangiorgi E.C.M. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;407:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sreekumar A., Toneff M.J., Toh E., Roarty K., Creighton C.J., Belka G.K. WNT-mediated regulation of FOXO1 constitutes a critical axis maintaining pubertal mammary stem cell homeostasis. Dev Cell. 2017;43(4):436–448. doi: 10.1016/j.devcel.2017.10.007. [e6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sansom O.J., Meniel V.S., Muncan V., Phesse T.J., Wilkins J.A., Reed K.R. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446(7136):676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 45.Kusunoki S., Kato K., Tabu K., Inagaki T., Okabe H., Kaneda H. The inhibitory effect of salinomycin on the proliferation, migration and invasion of human endometrial cancer stem-like cells. Gynecol Oncol. 2013;129(3):598–605. doi: 10.1016/j.ygyno.2013.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material