Abstract
Background
VIS410, a broadly neutralizing monoclonal antibody that binds the hemagglutinin stem of influenza A viruses, was safe and efficacious in a human H1N1 virus challenge study. This study evaluated the safety and tolerability of VIS410 in non-hospitalized adult patients with uncomplicated influenza A.
Methods
Patients 18 to 65 years of age with symptom onset within 72 h were randomized 1:1:1 to receive a single intravenous infusion of VIS410 4000 mg, 2000 mg, or placebo. Neuraminidase inhibitor therapy was prohibited. Treatment-emergent adverse events (TEAEs) were evaluated up to 100 days post-infusion. Influenza symptoms were assessed daily for 10 days using the FLU-PRO tool. Nasopharyngeal virus shedding was assessed by quantitative reverse-transcription PCR (qRT-PCR) and viral culture through Day 7.
Findings
Of the 150 patients randomized, 148 received study drug, and 138 were confirmed influenza A positive. Median age was 42 years; median time from symptom onset to treatment was 42 h; 93% had influenza A subtype H3N2.
Safety
TEAEs, most commonly diarrhea of mild severity, were dose-related, occurring in 55%, 35%, and 24% of the 4000 mg, 2000 mg, and placebo patients, respectively. Two serious adverse events occurred, both in placebo patients.
Symptom analyses
Baseline FLU-PRO symptom scores were balanced among groups. Mean scores were lower by Days 3 and 4 in the pooled VIS410 treatment group versus placebo (p < 0.023), with a tendency toward faster resolution by Kaplan-Meier analysis.
Virology analyses
VIS410 was associated with reduced median nasopharyngeal viral load TCID50 AUCDay7 (days × log10 TCID50/mL) (3.66 pooled VIS410 vs 4.78 placebo, p = 0.08) and in the subset of patients with baseline hemagglutination inhibition (HAI) titer ≤40 (overall, 74% of patients) was significantly reduced vs placebo (4.218 pooled VIS410 vs 6.152 placebo, p = 0.009). Kaplan-Meier estimated time to resolution of viral shedding was reduced (1.9 vs 3.6 days, p = 0.03) in VIS410 treated patients. There was a trend toward greater proportion of culture-negative patients by Day 3 (66.7% vs 51.1%, p = 0.11); when this analysis was limited to the subset of patients with positive baseline cultures, this difference became more pronounced (63.2% vs 42.5%, p = 0.053). No differences were observed in nasopharyngeal influenza qRT-PCR profiles, which represent both live and neutralized virus.
Interpretation: VIS410 was safe and well tolerated in adults with uncomplicated influenza A, with favorable effects on symptom resolution and virus replication.
Trial registration: Clinical Trials: NCT02989194.
Funding
This project was funded in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under Contract No. HHSO100201500018C.
Research in context.
Evidence before this study
We searched PubMed for citations over the prior ten-year period using the terms influenza A and human clinical trials, linked to monoclonal antibody therapy, polyclonal antibody therapy, plasma and serum therapy. In addition, studies posted on Clinicaltrials.gov were reviewed for status updates. Publications describing use of convalescent plasma and serum (hyperimmune globulin) as well as investigational monoclonal antibodies targeting hemagglutinin stem and influenza-A M protein were identified. Studies of convalescent plasma and serum containing high HAI titers provided evidence of antiviral efficacy, with survival benefit in the case of patients with H1N1 infection treated in Hong Kong. Evidence for antiviral efficacy of monoclonal antibodies targeting the hemagglutinin stem in treatment of natural infection was limited. Collectively, these exploratory studies provide strong evidence for potential benefit of convalescent plasma or serum therapy, although they are underpowered for statistical proof of benefit. In contrast, monoclonal antibody trials targeting HA-stem have not demonstrated compelling antiviral or symptom relief benefit, to date.
Added value of this study
Conserved HA stem epitopes are targets not only for mAb therapy, but also for universal influenza vaccine strategies. Exploratory demonstration that HA stem binding antibodies may influence the influenza A disease course is meaningful to development efforts for novel therapies as well as universal vaccination research efforts.
Implications of all the available evidence
Further evaluation of HA-stem binding mAb VIS410 in hospitalized patients with severe influenza infection is warranted.
Alt-text: Unlabelled Box
1. Introduction
Annual influenza epidemics cause significant world-wide morbidity and mortality despite vaccine and antiviral drug development efforts, with an ever-present risk of emergent or zoonotic virus pandemics. Neuraminidase inhibitors including oseltamivir, zanamivir, laninamivir, and peramivir have been approved for treatment of uncomplicated influenza, and have also become de facto ‘standard of care’ therapy for hospitalized patients [1]. The cap-dependent endonuclease inhibitor, baloxavir marboxil, is a potent inhibitor of influenza A virus replication [2] and has been approved for use in uncomplicated influenza patients in Japan and the U.S., with application for approval pending in Europe. Despite these advances, no influenza antiviral has been approved to date for treatment of hospitalized patients with severe disease. In the U. S., it is estimated that up to 600,000 seasonal influenza patients may be hospitalized each year, with up to 27,000 deaths [3].
There is a long history of therapeutic antibody treatment for influenza, particularly in high-mortality pandemics. During the 1918 influenza pandemic, physicians resorted to administration of convalescent blood products (serum, plasma, or whole blood), interventions that a modern meta-analysis of 8 published studies involving 1074 patients suggests may have halved mortality rates [4]. During the 2009/2010 H1N1 pandemic, investigators in Hong Kong treated patients requiring intensive care with convalescent plasma containing a neutralizing H1N1 titer of ≥1:160, and observed lower mortality rates as well as lower influenza viral loads [5]. The investigators continued these studies over the following years using fractionated IVIG from H1N1 convalescent patients, again reporting a mortality benefit [6]. In an open-label Phase 2 randomized and controlled trial, Beigel and colleagues treated hospitalized patients in the U.S. with severe influenza A (principally H1N1) or influenza B with anti-influenza plasma containing hemagglutination inhibition (HAI) titers of ≥1:80 to the infecting strain [7]. In that study, 61% of patients had received neuraminidase inhibitor therapy prior to randomization (increasing to 99%, post-randomization); 58% of participants were in the Intensive Care Unit and 43% were already on mechanical ventilation at enrolment. This small study did not demonstrate significant benefits of plasma treatment, but the investigators reported encouraging trends in reduction in mortality and days on mechanical ventilation. Operational challenges to the use of patient-derived convalescent blood products in these trials were addressed, but clearly limit the scalability of this approach. Monoclonal antibody therapy has the potential to address the scalability challenge.
VIS410 is a broadly neutralizing IgG1 monoclonal antibody with demonstrated anti-viral activity in vitro and in animal models against influenza strains in Group 1 (including H1 and H5) and Group 2 (including H3 and H7). VIS410 has been studied in 2 Phase 1 trials [8] and a Phase 2a influenza virus challenge study in healthy volunteers. In these studies, VIS410 was associated with GI adverse events, including diarrhea, nausea, and vomiting. These events were mitigated with a pre-treatment regimen of aspirin or ibuprofen in combination with diphenhydramine. In a Phase 2a H1N1 influenza viral challenge study, single dose intravenous VIS410 administration 24 h after virus-inoculation was associated with a statistically significant decrease in viral load area under the curve (VL-AUC) compared with placebo, demonstrating its potential benefit in treatment of patients with influenza A infection [9]. In this study, the safety and efficacy of a single infusion of VIS410 (at two dose levels) versus placebo were evaluated in patients with uncomplicated influenza A infection.
2. Methods
2.1. Study design
This randomized, double-blind, placebo-controlled trial (NCT02989194) was initiated in January 2017 at 58 sites across the Northern and Southern hemispheres with 28 sites enrolling patients in 5 countries (United States, Bulgaria, Estonia, Latvia, and South Africa). The first and last patients were enrolled in January and July of 2017, respectively, with the final follow-up visit conducted in October of 2017. The study was designed to compare the safety and tolerability of a single infusion of a high dose (4000 mg) or low dose (2000 mg) of VIS410 versus placebo (saline) in the treatment of uncomplicated influenza infection. An independent Data Safety Monitoring Board (DSMB) reviewed all available safety data after 30 and again after 75 patients had completed visits on Day 5 and 28, respectively. This trial was approved by relevant regulatory agencies and local institutional review boards and was conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. All patients provided written informed consent.
2.2. Study population
Eligible patients were between 18 and 65 years of age, tested positive for influenza A by a Rapid Antigen Test (Quidel, Sofia®), had at least one respiratory symptom (cough, sore throat, or nasal symptoms) of moderate to severe intensity, or had presence of at least one constitutional symptom (myalgia [aches and pains], headache, feverishness, or fatigue) of moderate to severe intensity, with onset of symptoms no >72 h before the start of infusion. Patients were excluded from the study if they had received any dose of influenza antiviral therapy in the 7 days prior to screening, had used NSAIDs or antihistamines within 6 h of study drug dosing, had hypoxemia requiring oxygen support, or were considered to be immunocompromised. A completed list of inclusion and exclusion criteria is included in the study protocol (Appendix 1, Supplemental Materials).
2.3. Randomization and masking
Patients were randomly assigned in a 1:1:1 ratio to receive a single intravenous infusion of VIS410 of 2000 mg, 4000 mg, or placebo, using an interactive voice response system with permuted-block randomization, without stratification (block size was 3). Patients, clinical trial site and sponsor staff were blinded to treatment status, with the following exceptions: study pharmacists, unblinded study CRAs assigned to study drug management, PK analysts, and the unblinded statistician whose responsibilities were limited to support of the study Data Safety Monitoring Board. All patients (regardless of randomization arm) received a pre-treatment regimen of a single dose of diphenhydramine plus ibuprofen or acetylsalicylic acid 1 h prior to study drug administration. Concomitant use of antiviral therapy for influenza was prohibited. The use of symptom-modifying drugs such as NSAIDs, antihistamines, or pseudoephedrine was discouraged but if a symptom-modifying agent was needed, paracetamol/acetaminophen was encouraged as the drug of choice.
2.4. Analysis populations
Assessments of drug safety were performed for the safety population, which included all randomized patients who received intravenous study drug (VIS410 or placebo). Virologic and symptom response outcomes were assessed in the modified intent-to-treat (mITT) population which included all patients who received intravenous study drug and in whom influenza A infection was confirmed by qRT-PCR at the central virology laboratory. Pharmacokinetic (PK) analyses were performed in the PK population which included all patients who received intravenous study drug and had at least 1 measured VIS410 concentration.
2.5. Assessments
A complete physical examination and a nasopharyngeal swab to confirm influenza A infection by rapid antigen test were mandatory prior to randomization. All patients completed the FLU-PRO patient reported outcome symptom assessment at baseline and daily for 10 days following study drug administration [10,11]. The FluPRO questionnaire is a 32-question instrument that assesses the occurrence and intensity of influenza associated symptoms. FluPro data were analysed by mean total symptom scores for all questions, and by composite scores for related symptom complexes, as described in the supplemental data linked to this manuscript. Nasopharyngeal swabs were collected from all patients prior to study drug administration (screening and baseline) and after study drug administration on Days 3 (±1 day), 5 (±1 day), and 7 (±1 day) for viral load assessment by qRT-PCR and culture (TCID50) at a central laboratory (Viroclinics Biosciences B.V, Rotterdam, Netherlands). Influenza A subtype was determined from the baseline sample using a PCR-based method. Virus culture titers were determined using previously described methods [34]: red blood cell agglutination method for H1N1 viruses and an ELISA-based viral nucleoprotein (NP) detection method for H3N2 viruses which poorly agglutinate red blood cells. Serum samples were collected for PK and anti-drug antibody (ADA) assessment (Syneos Health, formerly inVentiv Health Clinical Lab; Princeton, NJ, USA) through Day 100. Nasal VIS410 concentration was also assessed through Day 100 in the first 50 subjects. All subjects had serum anti-influenza A antibody measured by HAI assay (against representative H1N1 and H3N2 strains) at baseline and 28 days following study drug administration.
Safety was assessed by review of adverse events, vital signs, physical examination findings, and clinical laboratory results through Day 100. Subjects were monitored for worsening of influenza symptoms and influenza-related complications.
Sanger sequencing of full-length hemagglutinin (HA) sequences was performed to monitor the emergence of genotypic resistance to VIS410. Sequences were derived from all study subjects for the baseline sample and all post-baseline samples that contained sufficient viral load for this method. Phenotypic testing was performed on samples with variant HA genotypes, including changes at VIS410 epitope residues, and a sub-set of samples with culturable virus post-treatment. Phenotypic methods included IC50 analysis of virus after initial expansion in culture.
2.6. Statistical analysis
The primary objective of this trial was to assess the safety and tolerability of a single intravenous dose of VIS410, at 2 dose levels, in subjects with uncomplicated influenza A infection. No formal sample size calculations were done for this study; a sample size of 50 patients per treatment arm was considered appropriate for a Phase 2a study to assess safety and tolerability of VIS410. Secondary objectives evaluated efficacy of VIS410 compared with placebo in the time to resolution of clinical symptoms and viral shedding. In addition, immunogenicity of VIS410 and PK were evaluated. All virologic and symptom endpoints were assessed in the mITT population, with ad hoc assessment of selected endpoints in rational subpopulations, as described. Resolution of influenza symptoms was defined as a total mean symptom score of ≤1.0 on the FLU-PRO questionnaire. Standard non-compartmental approaches using Phoenix WinNonlin (Pharsight Corporation, Princeton, NJ, USA; Version 7.0 or higher) were used to calculate PK, peak viral load, and VL-AUC. VIS410 endpoints compared aggregated VIS410 data and each dose level of VIS410 to the placebo arm. For PK parameters, the geometric mean and coefficient of variance (CV) were calculated. All statistical comparisons were performed using 2-sided tests at the 0.05 significance level. All p-values are presented for informational purposes only; there were no adjustments for multiple comparisons. Chi-square methods were used for virologic endpoints to compare differences in treatment groups relative to placebo for the proportion of subjects with negative results on Day 3, Day 5, and Day 7 study visits. Kaplan-Meier analyses were evaluated with log-rank testing. Statistical analyses were performed using SAS (version 9.4 or higher).
2.7. Role of the funding source
The study was sponsored by Visterra Inc. and supported with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under Contract No. HHSO100201500018C. The study sponsor was involved in study design; data collection, analysis, and interpretation; and writing and review of the manuscript. All authors had access to study data. The corresponding author had final responsibility for the decision to submit for publication.
3. Results
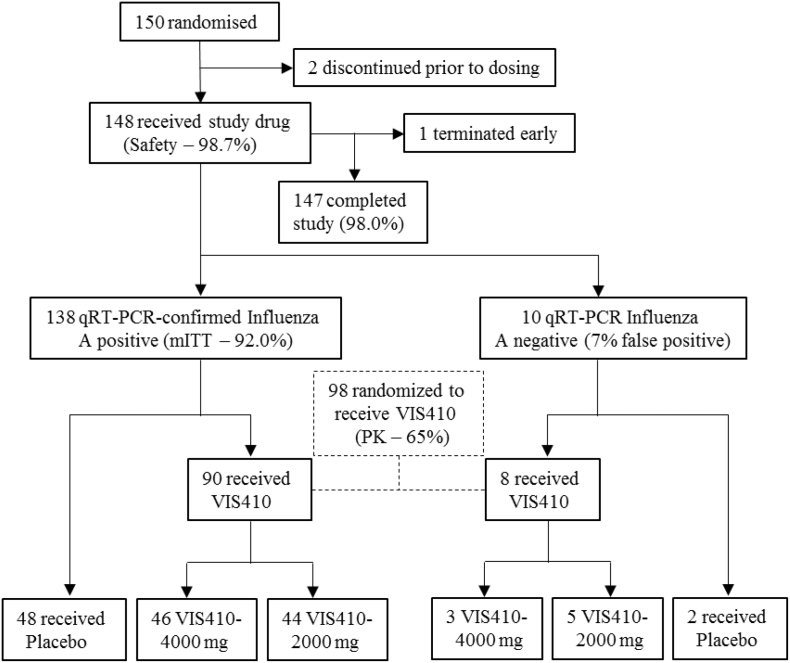
A total of 150 patients underwent study randomization; 148 (98.7%) received study drug (Safety population), and 138 (92.0%) tested positive for influenza A by qRT-PCR at the central laboratory (mITT population) (Fig. 1). Ninety-eight patients were randomized to receive VIS410. The study completion rate was 98.0%; 2 patients discontinued prior to receiving study drug (1 withdrew consent and one was not dosed due to temporary hold by the Sponsor to conduct an ad hoc DSMB meeting per protocol) and 1 patient was lost to follow-up. Disposition data are summarized in Supplemental Table 1.
Fig. 1.
Trial Profile and Patient Disposition.
qRT-PCR = quantitative reverse-transcription PCR; mITT = modified intent-to-treat population.
The demographic and baseline disease characteristics were generally comparable among treatment groups (Table 1). Overall, the majority of participants were White with a mean (SD) age of 41 (13.2) years. Slightly more females were enrolled than males. Body mass index was similar across groups with median of 28.6 kg/m [2]. All patients were enrolled within 72 h from onset of influenza symptoms with the majority of subjects enrolled within 24 to 48 h of symptom onset. More patients in the VIS410 4000 mg group were enrolled within 48 to 72 h after onset of influenza symptoms than in the with VIS410 2000 mg or placebo groups.
Table 1.
Demographic and baseline disease characteristics (ITT population).
| Characteristics | VIS410 4000 mg (N = 50) | VIS410 2000 mg (N = 50) | VIS410 Total (N = 100) | Placebo (N = 50) |
|---|---|---|---|---|
| Age (years), mean (SD) | 40.3 (13.13) | 39.6 (13.79) | 40.0 (13.40) | 43.1 (12.77) |
| Race, white, n (%) | 38 (76.0) | 38 (76.0) | 76 (76.0) | 36 (72.0) |
| Sex, male, n (%) | 23 (46.0) | 21 (42.0) | 44 (44.0) | 22 (44.0) |
| Mean Body Mass Index (BMI), kg/m2, (SD) | 29.01 (7.24) | 30.80 (7.89) | 29.91 (7.58) | 28.82 (5.63) |
| Time since onset of influenza, mean (SD) | 43.31 (16.48) | 40.57 (11.56) | 41.94 (14.23) | 38.19 (13.86) |
| < 24 h | 7 (14.0) | 5 (10.0) | 12 (12.0) | 11 (22.0) |
| 24 - < 48 h | 23 (46.0) | 31 (62.0) | 54 (54.0) | 27 (54.0) |
| 48 - < 72 h | 19 (38.0) | 13 (26.0) | 32 (32.0) | 12 (24.0) |
| Vaccination within past 6 months), n (%) Yes |
5 (10.0) | 1 (2.0) | 6 (6.0) | 2 (4.0) |
| Influenza Subtype, n (%) | ||||
| H3N2 | 42 (84.0) | 40 (80.0) | 82 (82.0) | 45 (90.0) |
| H1N1_2009 | 4 (8.0) | 2 (4.0) | 6 (6.0) | 2 (4.0) |
As expected, the percentage of participants who received vaccination for influenza in the prior 6 months was low. The most common influenza subtype was H3N2; only a few subjects were infected with H1N1 virus.
3.1. Safety and tolerability
Overall, 58/148 patients (39.2%) reported at least one adverse event (Table 2). There were 103 treatment-emergent adverse events (TEAEs) in 56/148 patients (37.8%). The proportion of subjects experiencing TEAEs did not differ significantly between VIS410 2000 mg (34.7%) and placebo (24.0%) recipients (p = 0.326), but was significantly higher among recipients of VIS410 4000 mg (55.1%) versus placebo (p = 0.014). The most commonly reported adverse events were diarrhea (26.5%, 16.3%, and 12.0%), vomiting (8.2%, 0%, and 2.0%), and headache (6.1%, 4.1%, and 4.0%) for the VIS410 4000 mg, VIS410 2000 mg, and placebo recipients, respectively (Table 2). The majority of TEAEs were mild to moderate in severity and resolved rapidly: overall, 11 subjects reported moderate TEAEs: 10.2% (5/49 subjects) in the VIS410 4000 mg group, 6.1% (3/49 subjects) in the VIS410 2000 mg group, and 6.0% (3/50 subjects) in the placebo group. Three adverse events were graded severe: one non-SAE episode of gastritis in the VIS410 4000 mg group, and 2 serious adverse events (SAEs) (upper gastrointestinal [GI] haemorrhage and cerebrovascular accident), both of which occurred in placebo recipients. The VIS410 recipient with an episode of severe gastritis, a 24-year old male with an unremarkable medical history, reported vomiting (of mild severity), diarrhea (of mild severity) and gastritis (considered severe, and treated with proton pump inhibitor therapy) following study drug administration. The placebo recipient with GI haemorrhage was determined to have a pre-pyloric gastric ulcer and H. pylori infection; it is possible that the protocol assigned single dose of ibuprofen prior to placebo administration contributed to this episode. No SAEs occurred in VIS410-treated patients in this study. There were no treatment-limiting adverse events, and no deaths occurred during the study. The overall rates of influenza complications (otitis media and sinusitis) were low and similar across groups, 4.4% and 6.3% in the pooled VIS410 and placebo groups, respectively. Additional adverse event details are summarized in (Supplemental Table 2).
Table 2.
Summary of overall rates of adverse event occurrence and specific TEAEs occurring in >2% of patients in any treatment group (safety population).
| Treatment-emergent adverse event | VIS410 pooled N = 98 n (%) P-value vs Placebo⁎ | VIS410 4000 mg N = 49 n (%) P-value vs Placebo⁎ | VIS410 2000 mg N = 49 n (%) P-value vs Placebo_⁎ | Placebo N = 50 N (%) |
|---|---|---|---|---|
| Number of Subjects with Any Adverse Event | 45 (45.9) 0.067 |
28 (57.1) 0.016 |
17 (34.7) 0.432 |
13 (26.0) |
| Number of Subjects with Any TEAE | 44 (44.9) 0.051 |
27 (55.1) 0.014 |
17 (34.7) 0.326 |
12 (24.0) |
| Number of Subjects with Any TEAE Considered to be Treatment Related | 22 (22.4) 0.167 |
15 (30.6) 0.44 |
7 (14.3) 0.754 |
6 (12.0) |
| TEAEs occurring in >2% of patients in any treatment group | ||||
| Diarrhea | 21 (21.4) 0.204 |
13 (26.5) 0.099 |
8 (16.3) 0.567 |
6 (12.0) |
| Vomiting | 4 (4.1) >0.50 |
4 (8.2) 0.204 |
0 | 1 (2.0) |
| Headache | 5 (5.1) >0.50 |
3 (6.1) >0.50 |
2 (4.1) 0.242 |
2 (4.0) |
| Bronchitis | 2 (2.0) >0.50 |
0 | 2 (4.1) | 0 |
| Urinary Tract Infection | 2 (2.0) >0.50 |
0 | 2 (4.1) | 0 |
| Muscle spasms | 2 (2.0) >0.50 |
2 (4.1) 0.242 |
0 | 0 |
| Myalgia | 2 (2.0) >0.50 |
0 | 2 (4.1) 0.242 |
0 |
| Hypertension | 3(3.1) >0.50 |
2 (4.1) 0.242 |
1 (2.0) 0.495 |
0 |
Note: P-values are based on a chi-square test for cells with counts of 5 or greater and the Fisher's exact test for cells with <5 events.
No patient reported worsening of influenza symptoms or had a relapse of infection following VIS410 infusion that might suggest antibody-dependent enhancement of infectivity.
The incidence of adverse events related to laboratory test results was low and comparable across treatment groups. Mean changes from baseline in laboratory parameters were small and not clinically meaningful.
The DSMB was convened twice to review the safety data following enrolment of 30 and 75 patients per protocol. In addition, the DSMB reviewed each of the SAEs to ensure subject safety and well-being. In all cases, the DSMB recommended that the study continue as planned.
3.2. Symptom analyses
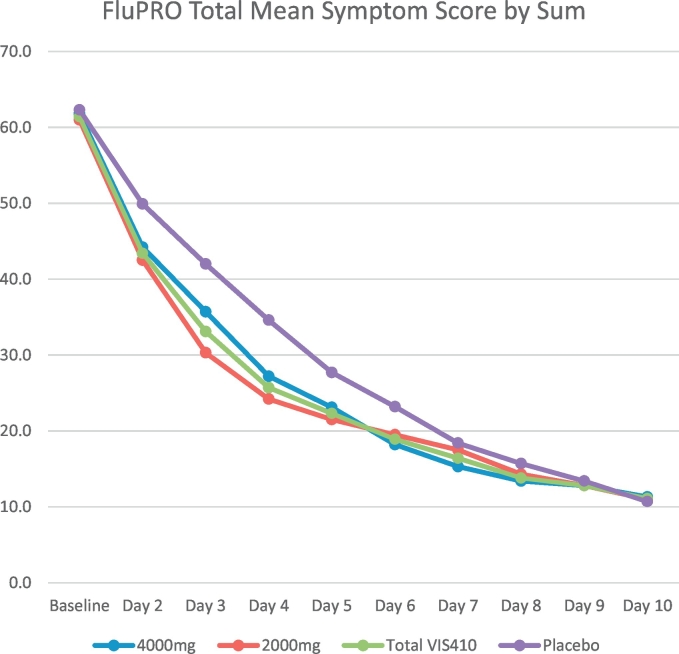
Mean total FLU-PRO symptom scores at baseline were balanced across all the treatment groups with mean scores of <2.0 (scale of 0–4) across groups, indicating that symptoms were generally of mild to moderate severity at baseline; the change in mean total symptom scores over time by treatment arm is presented in Fig. 2. Absolute and percentage reduction from baseline in mean total symptom scores was greater in VIS410-treated subjects through Day 6, and similar between VIS410 and placebo groups thereafter through Day 10 (Table 3). In the VIS410 2000 mg treated subjects, greater percentage change from baseline in comparison with placebo was evident on Day 2 and achieved significance for Day 3 (p = 0.002) through Day 5 (p = 0.030). Across pooled VIS410-treated patients (2000 and 4000 mg cohorts) the percentage reduction from baseline was statistically greater at Day 3 (p = 0.025) and Day 4 (p = 0.007). This is presumed to be a consequence of reduced viral replication, as presented in viral culture data below.
Fig. 2.
Mean Total FLU-PRO Symptom Scores by Treatment Group and Day.
Table 3.
Summary of percentage change from baseline in mean FLU-PRO total symptom scores by treatment group and visit in the mITT (confirmed influenza) population.
| Total symptom score parameter, by visit | VIS410 Total (N = 90) | VIS410 4000 mg (N = 46) | VIS410 2000 mg (N = 44) | Placebo (N = 48) |
|---|---|---|---|---|
| Baseline Mean (SD) | 1.92 (0.62) | 1.93 (0.65) | 1.91 (0.59) | 1.95 (0.70) |
| Percent Change from Baseline to Day 2, mean (SD) | −28.01 (28.18) | −26.12 (27.00) | −29.98 (29.55) | −19.53 (26.51) |
| Difference vs Placebo (p-value) | 0.158 | 0.429 | 0.101 | |
| Percent Change from Baseline to Day 3, mean (SD) | −43.89 (29.53) | −38.65 (28.78) | −49.37 (29.62) | −33.63 (23.60) |
| Difference vs Placebo (p-value) | 0.025 | 0.436 | 0.002 | |
| Percent Change from Baseline to Day 4, mean (SD) | −56.21 (28.67) | −53.46 (26.16) | −59.08 (31.12) | −44.55 (28.20) |
| Difference vs Placebo (p-value) | 0.007 | 0.097 | 0.003 | |
| Percent Change from Baseline to Day 5, mean (SD) | −62.69 (27.66) | −60.85 (25.52) | −64.61 (29.91) | −56.75 (24.31) |
| Difference vs Placebo (p-value) | 0.061 | 0.290 | 0.030 | |
| Percent Change from Baseline to Day 6, mean (SD) | −68.08 (27.61) | −69.31 (20.90) | −66.80 (33.42) | −64.01 (23.35) |
| Difference vs Placebo (p-value) | 0.107 | 0.256 | 0.100 | |
| Percent Change from Baseline to Day 7, mean (SD) | −72.43 (25.71) | −74.03 (19.31) | −70.76 (31.18) | −71.51 (20.61) |
| Difference vs Placebo (p-value) | 0.237 | 0.406 | 0.228 | |
| Percent Change from Baseline to Day 8, mean (SD) | −77.02 (23.13) | −77.98 (20.76) | −76.03 (25.53) | −76.16 (21.01) |
| Difference vs Placebo (p-value) | 0.497 | 0.569 | 0.552 | |
| Percent Change from Baseline to Day 9, mean (SD) | −79.27 (22.91) | −80.05 (19.78) | −78.48 (25.95) | −79.52 (19.84) |
| Difference vs Placebo (p-value) | 0.704 | 0.958 | 0.547 | |
| Percent Change from Baseline to Day 10, mean (SD) | −81.85 (20.14) | −82.30 (20.07) | −81.38 (20.43) | −84.35 (17.52) |
| Difference vs Placebo (p-value) | 0.585 | 0.504 | 0.793 |
The median Kaplan-Meier estimate of time to symptom resolution (based on total mean symptom score ≤ 1.0) was lower (2.1 days) in the Total VIS410 group compared with 2.6 days for the placebo group (p = 0.173). The Kaplan-Meier plot of time to symptom resolution for total symptom score based on mean score ≤ 1.0 is presented in Supplemental Fig. 1. Mean FluPro domain and composite symptom score percentage change from baseline values by treatment group and timepoint are presented in Supplemental Table 3, and presented graphically in Supplemental Fig. 2.
The number of subjects with documented fever at baseline (oral temperature > 38 °C) was 17/46 (37.0%) in the VIS410 4000 mg group, 18/44 (40.9%) in the VIS410 2000 mg group, and 20/48 (41.7%) in the placebo group. Median time to normal temperature post-treatment for patients with fever was 2.2 days across all treatment groups.
3.3. Virology analyses
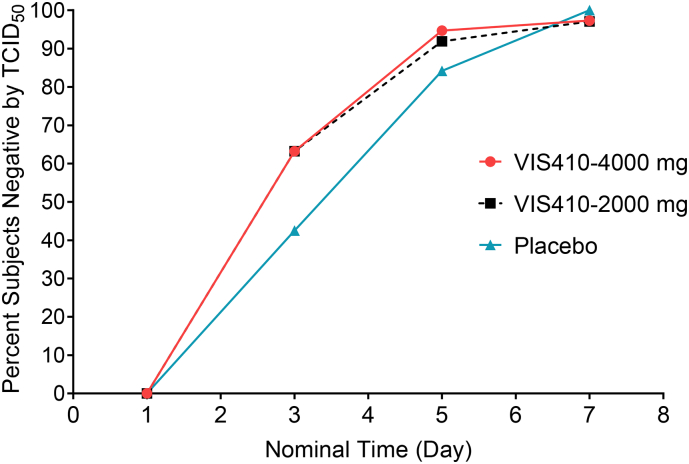
VIS410 was associated with reduced median nasopharyngeal viral load TCID50 AUCDay7 (days × log10 TCID50/mL) (3.66 pooled VIS410 vs 4.78 placebo, p = 0.08), and in the subset with baseline HAI titer ≤40 (overall, 74% of patients), the effect was more pronounced, with a significant reduction vs placebo (4.22 pooled VIS410 vs 6.15 placebo, p = 0.009). Kaplan-Meier estimated time to resolution of viral shedding was reduced (1.9 vs 3.6 days, p = 0.03) (Fig. 3). There was a trend in the pooled VIS410 treatment group toward a greater proportion of culture-negative patients by Day 3 (66.7% vs 51.1%, p = 0.11); when this analysis was limited to the subset of patients with positive baseline cultures, the difference became more pronounced (63.2% vs 42.5%, p = 0.053) (Fig. 4).
Fig. 3.
Time to resolution of viral shedding measured by TCID50 from the end of infusion in days (mITT population).
Time to resolution of viral shedding from the end of infusion until resolution of viral load by TCID50 was determined using Kaplan-Meier methods for the pooled VIS410 arms and placebo. Resolution of viral load is considered achieved at the first timepoint with virus BLQ by culture with no subsequent culture result >BLQ. If viral load was still above level of detection at the end of the study, then the last day of viral collection through Day 7 was used. The median Kaplan-Meier estimate of time to resolution of viral shedding by TCID50 from the end of infusion was 1·9 days for the VIS410 total group and 3·6 days in the placebo group (p = 0·03).
Fig. 4.
Percentage of subjects with negative virology (TCID50) by study day (subset of subjects with positive cultures at baseline).
The analysis subset includes subjects in the mITT group with a positive Baseline TCID50 measurement (>BLQ). Negative viral load by TCID50 is defined as the first timepoint with a TCID50 measurement of BLQ or lower with no samples following that are TCID50-positive. Percentages are calculated based on the number of subjects with a TCID50 measurement at each visit. The percent subjects negative for virus culture by Study Day 3 in the analysis subset was 63·2% for the VIS410 total group and 42·5% for the placebo group (p = 0·053).
Peak viral load occurred at baseline prior to dosing for the majority of subjects. Accordingly, the median peak viral load based on qRT-PCR from nasopharyngeal swabs was similar in the total VIS410 (6.75 log10 vp/mL) and placebo (7.11 log10 vp/mL) groups at baseline. The time profile of mean qRT-PCR viral shedding between treatment groups did not differ (Supplemental Fig. 3). No difference was observed in the median VL-AUC between VIS410 (26.39) and placebo (27.74) treated subjects by qRT-PCR.
All treatment groups demonstrated similar increases in anti-influenza A antibodies (HAI titers) from baseline (Day 1) to Day 28, indicating that treatment with VIS410 did not diminish the native humoral immune response (Supplemental Fig. 4).
3.4. Pharmacokinetic and anti-drug antibody analyses
A total of 96 subjects received VIS410 and were included in the PK analysis population. Following a single intravenous infusion of VIS410, dose-proportional PK was observed (as measured by AUC and Cmax). The nasal-to-serum ratio was consistent with expectations for partitioning of a monoclonal antibody to the nasal cavity, with ~3–4% VIS410 penetration to the nasal cavity (Supplemental Table 4).
Overall, 20/92 VIS410 recipients (23%) developed a measurable anti-VIS410 antibody (ADA) response with an increase of >4-fold over time. The maximum observed titer was 1:64, with a total of 4 (4.1%) VIS410 recipients with an ADA titer of 1:32 or 1:64 on Day 56, and 3 (3.1%) meeting this criterion on Day 100. There was minimal impact of ADA response on VIS410 PK through 28 days following treatment.
3.5. Assessment of viral resistance
HA sequence was determined for 131/138 influenza A-confirmed subjects [123 H3N2; 8 H1N1] including 107 paired baseline and post-baseline sequences [from 33 subjects that received VIS410–4000 mg, 36 subjects that received VIS410–2000 mg, and 38 Placebo-treated subjects.] Compared with the subject-matched baseline sequences, only 15 amino acid changes were observed post-baseline, including 6 in the VIS410–4000 mg group (18%), 6 in the VIS410–2000 mg group (17%), and 3 in the Placebo group (8%). None of the post-baseline changes occurred within the 25 HA residues that comprise the VIS410 epitope. Isolates from 129 subjects had VIS410 epitope amino acids that were identical to the H1N1 or H3N2 vaccine virus strains. Two patients harbored H3N2 viruses at baseline with rare polymorphisms at VIS410 contact residues in the HA2 subunit: HA2 N53D and HA2 G57R. Bioinformatics analysis of 40,000 influenza A HA sequences in the Global Initiative on Sharing All Influenza Data (GISAID) database demonstrated that HA2 D53 and HA2 R57 are rarely observed (HA2 D53 = 0.06% of influenza A HA sequences; HA2 R57 = 0.13% of all Group 2 HA sequences). Infection resolved in both subjects without evidence of delayed response or viral rebound. IC50 analysis of these isolates revealed reduced VIS410 susceptibility in the HA2 N53D virus [IC50 39.4 μg/mL] while HA2 G57R virus was fully VIS410 sensitive [IC50 2.4 μg/mL]. All other pre- and post-treatment viral isolates tested were VIS410 sensitive [IC50 range: 0.1–3.1 μg/mL].
4. Discussion
Despite advances in vaccine identification, manufacturing, and distribution and the development of novel antiviral therapies, influenza A continues to be a major cause of morbidity and mortality. Seasonal epidemics all too predictably lead to severe infection, secondary pneumonias, hospitalizations, and deaths, particularly in vulnerable populations such as infants and the elderly. The recent 2017–2018 Northern Hemisphere influenza season has been identified as one of the worst in recent years with the estimated U.S. national rate of hospitalization for influenza-like illness rising to an alarming 106.6 per 100,000 cases, with over 15,000 deaths considered influenza-related, tragically including 171 children with confirmed influenza-associated mortality [12,13]. Continual influenza virus evolution, manifest in antigenic drift and pandemic shifts, contributes to this challenging profile, exacerbated by the paucity of available influenza therapies for hospitalized patients. In this 100th anniversary year of the globally devastating 1918 influenza pandemic, it is well recognized that the stakes are very high, with a compelling need for new and more effective therapies, better vaccines, and greater surveillance [14,15].
As a broadly neutralizing IgG1 monoclonal antibody with in vitro and in vivo activity against both influenza Group 1 strains (including H1 and avian H5) and Group 2 strains (including H3 and avian H7), VIS410 may have the potential to help address the medical need for new therapies for treatment of severe influenza A infection. This Phase 2a study was designed to assess the safety and tolerability of a single infusion of two dose levels of VIS410 compared with placebo in uncomplicated influenza infection, as a prelude to trials in hospitalized patients with more severe disease. Despite the small sample size and relatively mild presentation of illness in this study population, there was clear evidence of the impact of a single infusion of VIS410 on the pace of symptom improvement and time to resolution of culturable virus shedding. A favorable effect of VIS410 in comparison with placebo on viral replication was seen in all patients, but was more obvious in the subset that did not have an H3N2 neutralizing antibody titer (as measured by HAI) of >1:40 at baseline. It seems reasonable to speculate that this circumstance (presentation with low HAI antibody titer to one's infecting influenza strains) may occur more frequently in hospitalized patients. Importantly, there was no evidence that VIS410 enhanced influenza infectivity, based on viral clearance profiles and the absence of influenza relapse or recrudescence in the treated population. VIS410 also did not appear to reduce the ability to elaborate an antibody response to influenza A virus as anti-influenza A HAI profiles were similar between treatment groups, consistent with the fact that VIS410 targets the stem of influenza HA, distinct from sites predominantly targeted by the majority of endogenous neutralizing antibodies [16].
VIS410, administered as a single infusion of 2000 or 4000 mg over 2 h following pre-medication with ibuprofen or aspirin and diphenhydramine, was demonstrated to be safe and well tolerated in patients with uncomplicated influenza infection. VIS410 demonstrated dose-related GI adverse events (diarrhea, nausea, and vomiting), but these events, with pre-medication, were typically mild and self-limited. GI adverse events occurred more frequently in recipients of the VIS410 4000 mg dose, while among recipients of 2000 mg, the rates of these events were generally comparable to those observed in placebo recipients. In light of the comparable antiviral activity of the two dose levels and the reduced adverse event rate associated with 2000 mg versus 4000 mg dosing, the lower dose appears most favorable for further development, although this conclusion will be tested by observations from an ongoing trial of severe influenza in hospitalized patients.
It is important to acknowledge that administration of single doses of ibuprofen or aspirin and diphenhydramine was required to mitigate dosing-related GI adverse events. The mechanism responsible for the occurrence of gastrointestinal adverse events has not been determined. Unpublished studies confirmed that VIS410 does not induce mast cell degranulation or histamine release under in vitro assessment. The side effect ameliorating profile of the combination of ibuprofen or aspirin plus diphenhydramine was an empirical observation, and is evident in comparing rates of GI side effects between Phase 1 studies [8] and this trial. Occasionally, should the drug move into clinical practice, clinicians may determine that administration of single doses of these pre-medications does not satisfy benefit/risk assessment, or may require additional prophylactic medications. The important conclusion from this trial, however, is that this relatively simple measure reduced the AE profile of the 2000 mg dose to one comparable to placebo.
VIS410 demonstrated dose-proportional pharmacokinetics, with a half-life of approximately 10 days in serum and the nasopharynx. Nasopharyngeal VIS410 concentrations were approximately 3–4% of serum drug levels, consistent with previously observed partitioning of monoclonal IgG [17]. Nasopharyngeal VIS410 exposures exceeded the EC50 of the majority of global isolates tested to date, maintaining those exposures through the expected period of viral shedding. Importantly, in naturally occurring severe influenza, lung concentrations of therapeutic antibodies should be far more relevant than nasal concentrations, particularly given that lower lung involvement is the hallmark of severe infection [18,19]. In general, monoclonal antibody partitioning to the lung has been estimated to be approximately 15% relative to plasma [17]. Accordingly, VIS410 concentration at the site of action (lung) is predicted to be higher than that measured in the nasal compartment in this study. Lung concentrations of VIS410 will be more relevant in the hospitalized patient population with lower respiratory tract infection, the intended target population for VIS410 development.
Reduced susceptibility to VIS410 was rarely observed in this Phase 2a study in subjects with uncomplicated influenza, and no treatment-emergent resistant viruses were observed based on genotypic assessment. Most HA sequences (129 of 131) were identical to the vaccine strain at VIS410 epitope positions, with two variants identified at baseline. Infection in the two subjects harboring HA variants at VIS410 epitope positions resolved without evidence of delayed response or viral rebound. Notably, the HA2 N53D and HA2 G57R virus isolates demonstrated slower replication kinetics when cultured, consistent with possible reduced viral fitness for these variants. One variant, HA2 N53D, which has been identified in 0.06% of HA sequences in the GISAID database, demonstrated reduced susceptibility to VIS410 in vitro.
This study may also have broader significance. Development of a universal influenza vaccine is a critical priority of global health authorities, including the U.S. National Institute of Allergy and Infectious Diseases and the World Health Organization [15,20]. Development of vaccine candidates that elicit a broadly protective immune response targeting conserved and constrained HA stalk domain epitopes has been a major objective in these ongoing efforts [[21], [22], [23], [24]]. Preclinical studies have identified that such antibodies, if of sufficiently high affinity and potency, can be protective [25,26]; however, depending on the epitope targeted and binding affinity, some antibodies may lack protective capacity or potentially even exacerbate disease [27]. In support of efforts to identify a universal or nearly universal vaccine strategy, we have demonstrated in both a previously conducted H1N1 challenge study and in this study of uncomplicated influenza that predominantly enrolled H3N2 infected patients, that an anti-stem antibody can have a measurable anti-viral effect during active infection, with evidence of disease suppression and faster resolution of symptoms.
The results presented here also provide support for the pursuit of broadly neutralizing antibodies to treat viral infections, a strategy that is being pursued in HIV [28] and Ebola [29], among others. The evidence in influenza has been mixed at best, with polyclonal preparations demonstrating disease-modifying activity [7] and HA-stalk binding monoclonal antibodies such as MEDI8852 [30,31] and MHAA4549A [32] suffering setbacks in clinical trials. MEDI8852 failed to advance into a Phase 2 trial in hospitalized patients (NCT03028909; ClinicalTrials.gov) and a Phase 2b trial of MHAA4549A in combination with oseltamivir for treatment of severe influenza in hospitalized patient was terminated following an interim analysis [33]. In this context, the preliminary evidence for efficacy of VIS410, a unique HA-stalk antibody, provides support that biologics-based therapeutics may be a viable treatment option for severe influenza infection, and that the influenza A HA-stem remains a rational target for universal vaccine development efforts.
Overall, data from this trial raise important questions that remain unanswered. Ongoing and future studies must determine if the antiviral effect observed in faster resolution of culturable nasopharyngeal virus in uncomplicated influenza translates into clinically meaningful outcomes when targeting virus replication in the lungs in more severe disease. Similarly, it must be determined if the persistence of influenza RNA in the nasopharynx (with resolution at the same rate as observed in placebo recipients) reflects failure of efficacy, or is simply a consequence of the mechanism of action of the mAb, binding and preventing viral replication in new cell targets while leaving neutralized but intact virions and sloughed respiratory mucosal cells available for collection by nasopharyngeal aspirate for RNA extraction and detection by RT-PCR. Most importantly, new influenza therapeutics must be demonstrated to have a meaningful impact on clinical parameters, particularly patients' functional status, in the setting of severe influenza.
This study set the stage for this evaluation, demonstrating the general safety and tolerability of a single intravenous dose of VIS410 in subjects with uncomplicated influenza A infection with evidence for more rapid symptom improvement and resolution of infectious virus shedding. Supported by these observations, a global Phase 2b evaluation of VIS410 in combination with oseltamivir in hospitalized patients with severe influenza A infection is ongoing at this time and will provide important additional data regarding the potential utility of VIS410 for the treatment of influenza.
5. Research in context
5.1. Systematic review
We identified references for this study by searching PubMed and Embase for articles published before July 2018 with the search terms “influenza” and “monoclonal antibody” with “clinical trials” and “treatment guidelines”. Results of this search suggested that [1]: influenza A is associated with significant morbidity, mortality, and hospital admissions [2]; treatment options are limited in this population. These search results emphasize the need for novel agents to treat severe influenza A infections. VIS410 has promising pharmacologic properties that make it a suitable candidate for clinical development in this setting.
5.2. Interpretation
Severe influenza infection is an unmet medical need and targeted monoclonal antibodies in combination with the standard-of-care therapy may play a role in management of hospitalized patients.
The results from this study are promising in terms of both safety and antiviral activity of a monoclonal antibody and the potential role this class of drugs can play in the management of severe influenza A infection.
Contributors
EH, SS, KN, CH, PS, FE, JT, and ZS contributed to study conception and/or design. EH, JT, CH, and DO were responsible for active trial management and patient safety review. SLS and REJ are employees of Viroclinics Biosciences B.V. and conducted virology assays. All authors contributed to data analysis, interpretation of the results, and preparation of the manuscript. All authors read and approved the final version of the manuscript.
Declaration of interests
All authors are or were employees of or consultants to Visterra at the time the trial was conducted.
Acknowledgments
We thank the trial participants and investigators who made this study possible. Additional investigators are listed in the appendix. Staff from Pharm-Olam International (Houston, TX, USA) provided invaluable global operational support for the trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.12.051.
Appendix A. Supplementary data
Supplementary material
References
- 1.WHO . Feb. 2010. Guidelines for pharmacological management of Pandemic influenza A(H1N1) 2009 and other influenza viruses. Revised. [PubMed] [Google Scholar]
- 2.Hayden F.G., Sugaya N., Hirotsu N. Baloxavir Marboxil for Uncomplicated Influenza in adults and Adolescents. N Engl J Med. 2018;379(10):913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 3.Reed C., Chaves S.S., Daily Kirley P. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 5.Hung I.F., To KK, Lee C.K. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza a (H1N1) 2009 virus infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung I.F.N., To KKW, Lee C.K. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144(2):464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- 7.Beigel J.H., Tebas P., Elie-Turenne M.C. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med. 2017;5(6):500–511. doi: 10.1016/S2213-2600(17)30174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wollacott A.M., Boni M.F., Szretter K.J. Safety and Upper respiratory Pharmacokinetics of the Hemagglutinin Stalk-Binding Antibody VIS410 support Treatment and Prophylaxis based on Population Modeling of Seasonal Influenza a Outbreaks. EBioMedicine. 2016;5:147–155. doi: 10.1016/j.ebiom.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trevejo J.M., SEKS Sloan, Bedard S., Hay C.A. International Conference of Respiratory and Critical Care Medicine. American Thoracic Society; San Francisco, CA: 2016. Safety and efficacy of the monoclonal antibody VIS410 in a human volunteer challenge model of infection with an H1N1 Influenza A virus. [Google Scholar]
- 10.Powers J.H., 3rd, Bacci E.D., Guerrero M.L. Reliability, Validity, and Responsiveness of InFLUenza Patient-Reported Outcome (FLU-PRO(c)) scores in Influenza-positive patients. Value Health J Int Soc Pharmacoeconomics Outcome Res. 2018;21(2):210–218. doi: 10.1016/j.jval.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers J.H., Guerrero M.L., Leidy N.K. Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza. BMC Infect Dis. 2016;16:1. doi: 10.1186/s12879-015-1330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garten R., Blanton L., Elal A.I.A. Update: Influenza activity in the United States during the 2017-18 season and Composition of the 2018-19 Influenza Vaccine. MMWR Morb Mortal Wkly Rep. 2018;67(22):634–642. doi: 10.15585/mmwr.mm6722a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammond A., Laurenson-Schafer H., Marsland M. World Health Organization Weekly Epidemiological Record. Vol. 93. 2018. Review of the 2017-2018 influenza season in the Northern Hemisphere; pp. 429–444. [Google Scholar]
- 14.The Lancet Infectious D How to be ready for the next influenza pandemic. Lancet Infect Dis. 2018;18(7):697. doi: 10.1016/S1473-3099(18)30364-5. [DOI] [PubMed] [Google Scholar]
- 15.The Lancet Infectious D Plotting a route to a universal influenza vaccine. Lancet Infect Dis. 2018;18(5):475. doi: 10.1016/S1473-3099(18)30235-4. [DOI] [PubMed] [Google Scholar]
- 16.Popova L., Smith K., West A.H. Immunodominance of antigenic site B over site a of hemagglutinin of recent H3N2 influenza viruses. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0041895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah D.K., Betts A.M. Antibody biodistribution coefficients: inferring tissue concentrations of monoclonal antibodies based on the plasma concentrations in several preclinical species and human. MAbs. 2013;5(2):297–305. doi: 10.4161/mabs.23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbo L., Quartin A., Morris M.I. Pulmonary imaging of pandemic influenza H1N1 infection: relationship between clinical presentation and disease burden on chest radiography and CT. Br J Radiol. 2010;83(992):645–651. doi: 10.1259/bjr/53692814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taubenberger J.K., Morens D.M. The pathology of influenza virus infections. Annu Rev Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erbelding E.J., Post D.J., Stemmy E.J. A Universal Influenza Vaccine: the Strategic Plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis. 2018;218(3):347–354. doi: 10.1093/infdis/jiy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coughlan L., Palese P. Overcoming Barriers in the Path to a Universal Influenza Virus Vaccine. Cell Host Microbe. 2018;24(1):18–24. doi: 10.1016/j.chom.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Nachbagauer R., Palese P. Development of next generation hemagglutinin-based broadly protective influenza virus vaccines. Curr Opin Immunol. 2018;53:51–57. doi: 10.1016/j.coi.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Andrews S.F., Graham B.S., Mascola J.R., AB McDermott. Is it possible to develop a "Universal" Influenza virus vaccine? immunogenetic considerations underlying B-cell biology in the development of a pan-subtype influenza a vaccine targeting the Hemagglutinin stem. Cold Spring Harb Perspect Biol. 2018:10(7). doi: 10.1101/cshperspect.a029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowe J.E., Jr. Is it possible to develop a "Universal" Influenza virus vaccine? potential for a universal influenza vaccine. Cold Spring Harb Perspect Biol. 2018:10(7). doi: 10.1101/cshperspect.a029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyce M.G., Wheatley A.K., Thomas P.V. Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza a Viruses. Cell. 2016;166(3):609–623. doi: 10.1016/j.cell.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baranovich T., Jones J.C., Russier M. The Hemagglutinin Stem-Binding Monoclonal Antibody VIS410 Controls Influenza Virus-Induced Acute respiratory Distress Syndrome. Antimicrob Agents Chemother. 2016;60(4):2118–2131. doi: 10.1128/AAC.02457-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khurana S., Loving C.L., Manischewitz J. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med. 2013;5(200) doi: 10.1126/scitranslmed.3006366. [DOI] [PubMed] [Google Scholar]
- 28.Caskey M., Schoofs T., Gruell H. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23(2):185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Group PIW, Multi-National PIIST, Davey R.T., Jr. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med. 2016;375(15):1448–1456. doi: 10.1056/NEJMoa1604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali S.O., Takas T., Nyborg A. Evaluation of MEDI8852, an anti-influenza a monoclonal antibody, in treating acute uncomplicated influenza. Antimicrob Agents Chemother. 2018 Oct 24;62(11) doi: 10.1128/AAC.00694-18. (Print 2018 Nov, pii: e00694-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kallewaard N.L., Corti D., Collins P.J. Structure and function analysis of an antibody recognizing all influenza a subtypes. Cell. 2016;166(3):596–608. doi: 10.1016/j.cell.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride J.M., Lim J.J., Burgess T. Phase 2 randomized trial of the safety and efficacy of MHAA4549A, a broadly neutralizing monoclonal antibody, in a human Influenza A virus challenge model. Antimicrob Agents Chemother. 2017:61(11). doi: 10.1128/AAC.01154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim J.L., Nilsson A.C., Silverman M., Mironova N., Kulkarni P., McBride J.M. 28th ECCMID; 2018. The CRANE study: A phase 2b randomized, double-blind, placebo-controlled trial of monoclonal antibody MHAA4549A combined with oseltamivir versus oseltamivir alone for treatment of severe influenza A infection; p. 2018. [Google Scholar]
- 34.Van Baalen C.A., Els C., Sprong L., van Beek R., van der Vries E., Osterhaus A.D. Detection of nonhemagglutinating influenza a(h3) viruses by enzyme-linked immunosorbent assay in quantitative influenza virus culture. J Clin Microbiol. 2014 May;52(5):1672–1677. doi: 10.1128/JCM.03575-13. 24622097 Epub 2014 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material