Abstract
Background
While androgen deprivation therapy (ADT) and radiotherapy (RT) are currently used together to treat locally advanced prostate cancer (PCa), RT might have the adverse effect of increasing the PCa androgen receptor (AR) protein expression, which might then increase the resistance to continued RT.
Methods
We used multiple assays for RT sensitivity, protein and RNA expression of AR and related DDR genes, ROS level, DNA damage/repair level, cell cycle and apoptosis. All statistical comparisons were analyzed with t-test or one-way ANOVA.
Findings
We demonstrated that RT induced AR expression in C4-2 and CWR22Rv-1 cells. We found that combining RT and ASC-J9®, but not the antiandrogen, Enzalutamide, could increase radiosensitivity via inducing DNA damage, altering the AR mediated and DNA repair pathways, and activating apoptosis. ASC-J9® had little effects on normal bladder cells.
Interpretation
Targeting ionizing radiation (IR)-increased AR with the AR degradation enhancer, ASC-J9®, could increase the radiosensitivity while sparing adjacent normal tissue. Mechanism dissection revealed that ASC-J9®, but not Enzalutamide, treatment could increase radiosensitivity via inducing DNA damage, altering DNA repair pathways, as well as activating the IR-induced apoptosis via suppressing the pATR-CHK1 signals. Importantly, results from preclinical studies using an in vivo mouse model also demonstrated that combining RT with ASC-J9® to target AR led to better therapeutic efficacy to suppress PCa progression.
Highlights
-
•
ASC-J9• enhances efficacy of radiotherapy (RT) in PCa through both AR-dependent and AR-independent mechanistic pathways.
-
•
In AR-independent pathway, ASC-J9• increases endogenous ROS and DNA damage and makes PCa cells more sensitive to RT
-
•
ASC-J9• could also reduce the DNA damage repair after RT via suppression of AR dependent DDR genes and apoptotic pathway.
-
•
From pre-clinical mouse model, we found that combining RT and ASC-J9• can provide better efficacy than RT only.
Research in context.
Evidence before this study
AR mediates 144 DDR genes and directly targets 32 of them, which may result in radiation resistance. ADT plus RT are currently used together to treat localized and locally advanced prostate cancer (PCa), and improve cause-specific survival.Clinically, nearly 20% of RT patients have rising serum levels of AR-regulated hK2 protein, which provides evidence of AR pathway upregulation after RT.
Added value of this study
Increasing DNA damage, suppression of DDR genes and induction of RT-mediated apoptosis are three important principles to enhance the therapeutic efficacy of radiotherapy for cancer. Here, we demonstrate that targeting RT-increased AR with ASC-J9® could increase the radiosensitivity via regulating these 3 important pathways in PCa with little effect on the neighboring bladder cells.
Implication of all the available evidence
Combination of RT and ASC-J9® treatment represents a new effective therapeutic strategy to suppress PCa progression.
Alt-text: Unlabelled Box
1. Introduction
Prostate cancer (PCa), a common cancer among men worldwide, has been increasing in the recent years with 1 out of 6 men being diagnosed during their lifetime [1]. Radiation therapy (RT) is a popular treatment choice among patients with localized or locally advanced PCa that is categorized as either low risk PCa (≤ T2a, PSA ≤ 10 ng/dL, and Gleason score < 7), intermediate risk PCa (PSA > 10–20, Gleason score = 7, or clinical stage T2b or T2c) or high-risk PCa (PSA > 20, Gleason score between 8 and 10 or clinical stage T3a). However, nearly 25% of intermediate and high-risk PCa tumors recur after RT.
Importantly, RT can be combined with a course of androgen deprivation therapy (ADT) using various antiandrogens together with RT [2,3]. The result has a proven overall survival advantage, thereby establishing it as standard-of-care for high-risk localized PCa. While ADT + RT provides therapeutic benefit for PCa patients, yet it may also be accompanied with some adverse effects, including depression, hot flashes, fatigue, and loss of bone/muscle mass, which seriously compromise the quality-of-life of patients. Several therapeutic approaches, including β-lapachone [4] or resveratrol (RSV) [5], were developed to enhance the RT efficacy to further suppress PCa progression with fewer adverse effects of urinary and/or erectile dysfunction.
Recent studies indicated that androgen effects might not be equal to the androgen receptor (AR) effects and ADT with antiandrogens [6] may only reduce the androgen biosynthesis or prevent androgen from binding to AR, with little effect on the AR expression [[7], [8], [9], [10], [11]]. Since more and more data indicated that AR, at the castration concentration (1–2 nM) of androgens, could also be transactivated by various growth factors, cytokines and kinases [[12], [13], [14], [15]], targeting the AR, instead of targeting androgens, may result in better efficacy to further suppress the PCa progression.
The recently developed ASC-J9® (5-hydroxy-1,7-bis(3,4-dimethoxyphenyl)-1,4,6-heptatrien-3-one), the first identified AR degradation enhancer, has been shown to effectively inhibit the growth of several AR-related tumors including prostate, liver, bladder and kidney cancers with low toxicity, minimal adverse effects and drug resistance [[16], [17], [18], [19], [20]].
We unexpectedly found that RT might have the adverse effect of increasing the AR expression, which could not be suppressed by the current ADT-antiandrogen treatment. We also found the RT-increased AR might increase the resistance to continued RT [21] or subsequent ADT, and combining RT with ASC-J9® could enhance RT efficacy through both AR dependent and independent mechanisms to better suppress the PCa progression, with little adverse effects or damage to the neighboring normal bladder cells.
2. Materials and methods
2.1. Cell lines and cell cultures
We used two different PCa cell lines (C4-2 and CWR22Rv-1; ATCC Cat# CRL-3315, RRID:CVCL_4782 and Cat# CRL-2505, RRID:CVCL_1045), one normal bladder epithelial cell line (SV-HUC; ATCC Cat# CRL-9520, RRID:CVCL_3798), and the 293T cell line. C4-2 and CWR22Rv-1 cells were maintained in RPMI 1640, SV-HUC cells in F-12K media, and 293T cells in DMEM media, all with penicillin (25 units/ml), streptomycin (25 g/ml), 1% L-glutamine, and 10% fetal bovine serum (FBS). For the castration resistant condition, the 10% FBS medium was replaced with phenol red free media containing 10% charcoal-depleted (CD) FBS and 1 nM DHT (this concentration simulates ADT, because it replicates the DHT concentration remaining in the tumors of PCa patients following castration).
2.2. Plasmids and lentivirus
A recombinant lentiviral vector containing AR-shRNA (pLKO-shAR) and a scramble lentiviral control vector (pLKO-Scr) were used to knock-down AR. To generate the recombinant lentivirus, lentiviral pLKO-shAR/pLKO-Scr with pMD2.G packaging and psPAX2 envelope plasmids (lentivirus:packaging:envelope, 2:1:1) were co-transfected into 293 T cells for 6 h, and then were changed to normal media for immediate use or frozen at −80 °C for later use. For the virus infection, viral supernatants were added to the target cells with 8 mg/ml polybrene (Millipore, Billerica, MA, USA) to prepare stable cell line clones.
2.3. Chemical compounds
ASC-J9® (PubChem CID: 6477182) was purchased from AndroScience Corp. (San Diego, CA, USA). Enzalutamide (PubChem CID: 15951529) was purchased from MedChem Express (Monmouth Junction, New Jersey, USA). Casodex (PubChem CID: 2375) was purchased from Toronto Research Chemicals (North York, ON, CAN). Drug stocks (10 mM) were dissolved in DMSO and stored at −20 °C.
2.4. Clonogenic survival assays
Cells were plated in 60-mm culture dishes at various densities of cells per plate, depending on the IR dosage used, and allowed to attach overnight. Media were changed to castration resistant condition (10% CD-FBS + 1 nM DHT) for 24 h. For assay of the long-term survival rate (Clonogenic forming assay), cells were treated with 1 μM or 2·5 μM ASC-J9® for 6 h, and then cells were irradiated (Cs137 radiator, URMC, Rochester, NY, USA). The drugs were removed as soon as possible after irradiation (the irradiation source is in a different area of the Medical Center away from the lab) and cells incubated for 14 days. The colonies were fixed with 10% methanol and stained with 1% Toluidine blue. Colonies containing >50 cells were counted using a stereomicroscope. The mean normalized surviving fraction from three similar independent experiments was calculated and the SEM reported.
2.5. TUNEL assay
Cells were plated on coverslips and allowed to attach overnight. Media was changed to castration resistant condition (10% CD-FBS + 1 nM DHT) for 24 h, cells were treated with 1 μM or 2·5 μM ASC-J9® for 6 h, and then cells were irradiated (Cs137 radiator). The drugs were removed as soon as possible after irradiation. Samples were harvested 24 h after irradiation, fixed with 4% paraformaldehyde, incubated with 0.5% Trition X-100 in PBS, and blocked with 3% H2O2 in methanol. Then cells were stained with Cell Death Detection Kit (Roche, No. 11684817910). Apoptotic cells were visualized by fluorescence microscope at 20× power magnification. The total number of cells (DAPI was used for counter-staining) and apoptotic cells were counted in 3 random fields to calculate the apoptosis percentage.
2.6. Alkaline comet assay
C4-2, CWR22Rv-1, and SV-HUC cells were grown, treated, and irradiated under the described conditions. DNA lesions, including total base damage, double strand breaks (DSBs) and single strand breaks (SSBs), were assessed using single-cell gel electrophoretic comet assays under alkaline conditions (TREVIGEN, Gaitherburg, MD, USA), according to the manufacturer's instructions. Slides were stained with SYBR Gold (S11494, Thermo Fisher Scientific, Waltham, MA, USA) and visualized using a fluorescence microscope. Digital photomicrographs were taken and comet tail lengths quantified using alpha-image 2000. Each datum point represents an average of 5 cells ± SEM, and data are representative of experiments performed in triplicate.
2.7. Analysis of ROS Levels
C4-2 cells were plated at 4 × 104 cells/well, allowed to attach overnight, and treated with a series of doses of ASC-J9® (1, 2·5, or 5 μM) or DMSO for 16 h. Cells were then washed with a PBS solution containing 0.14 g of CaCl2 and 0.1 g of MgCl2 in 1 l of DPBS. The cells were then incubated with 10 μM 2′,7′-dichlorofluorescein diacetate (DCF-DA) (#ab113851, Abcam, Cambridge, UK) for 1 h at 37 °C before irradiation. Test plates were irradiated at 10 Gy (control plates were taken to the source for the same time frame away from the lab as with test plates), and the fluorescence was measured for all the plates using an ELISA reader at an absorption wavelength of 485 nm and emission wavelength of 520 nm. The results were expressed as relative fluorescence, normalized to un-irradiated control.
2.8. Glutathione detection
Endogenous levels were measured according to instructions from the GSH-Glo™ Glutathione Assay Kit (#V6911, Promega, Madison, WI, USA). Briefly, PCa cells were grown under described conditions in 96-well plates, with approximately 5000 cells/well in 200 μl total volume. After 16 h ASC-J9® treatment, we carefully removed the culture media from the wells, added 100 μl of prepared 1× GSH-Glo™ Reagent to each well, and incubated at room temperature for 30 min. After incubation, 100 μl of reconstituted Luciferin Detection Reagent was added and luminescence measured by ELISA reader.
2.9. γ-H2AX assay
PCa cells were grown under described conditions for 2 days on coverslips, with approximately 2000 cells/well in 1 ml total volume. Following IR, coverslips were washed and cells fixed with 4% paraformaldehyde and 0.2% Triton X-100, blocked with 2% Bovine Serum Albumin (#A2058, Sigma-Aldrich,) and 0.5% Triton X-100, and then were incubated with γ-H2AX (Millipore Cat# 05–636, RRID:AB_309864) overnight at 4 °C. Coverslips were then washed, incubated with secondary antibody (Goat anti-Mouse IgG Alexa Fluor 488 Dye; #: R37120 ThermoFisher Scientific, St. Louis, MO, USA) for 1 h at room temperature, and stained with 4′,6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI; #D1306 ThermoFisher Scientific). Foci (green signal) number of each nuclei were compared under 60× power fields under a confocal fluorescence microscope.
2.10. Quantitative real-time PCR
Total RNA was isolated using the TRIzol® Reagent (#15596026 ThermoFisher Scientific) according to the manufacturer's instructions. Total RNA was reverse transcribed into cDNA using iScript™ cDNA Synthesis Kit (# 1708891, BioRad, Hercules, CA, USA). The primer sequences were listed in the Table S1.The qRT-PCR was performed using the Bio-Rad iQ5 real-time thermal cycler and iQ™ SYBR® Green Supermix (#1708880, Biorad). Relative mRNA expression levels were normalized against GAPDH (as an internal control) and determined by the 2 − ΔΔCt method. QPCR primer list in Supplementary Table S1.
2.11. Homologous recombination DR-GFP assay
2 × 106 293 T cells in 6-well plates were transfected with 0.7 μg of pDR-GFP plasmid (#26475, Addgene, Watertown, MA, USA) and 2 μg of I-SceI expression plasmid pCBASceI (#26477, Addgene) using lipofectamine® 2000 (#11668019, ThermoFisher Scientific). After 48 h, GFP positive and total cells were counted in 5 random high-power fields under a fluorescence microscope.
2.12. Protein analysis
For western blot analyses, protein extracts of each sample (50 μg/lane) were electrophoretically separated and transferred onto PVDF membranes that were incubated with antibodies against AR (Santa Cruz Biotechnology Cat# sc-816, RRID:AB_1563391), pATR (GeneTex Cat# GTX128145, RRID:AB_2687562), cleaved PARP, pCHK1 (Cell Signaling Technology Cat# 9546, RRID:AB_2160593, and Cat# 2348, RRID:AB_331212), and GAPDH (Santa Cruz Biotechnology Cat# sc-48166, RRID:AB_783595), followed by horseradish peroxidase-conjugated secondary antibody. Protein-antibody complexes were detected by SuperSignal™ West Femto Maximum Sensitivity Substrate (#34095, ThermoFisher Scientific) using the Bio-Rad imaging system.
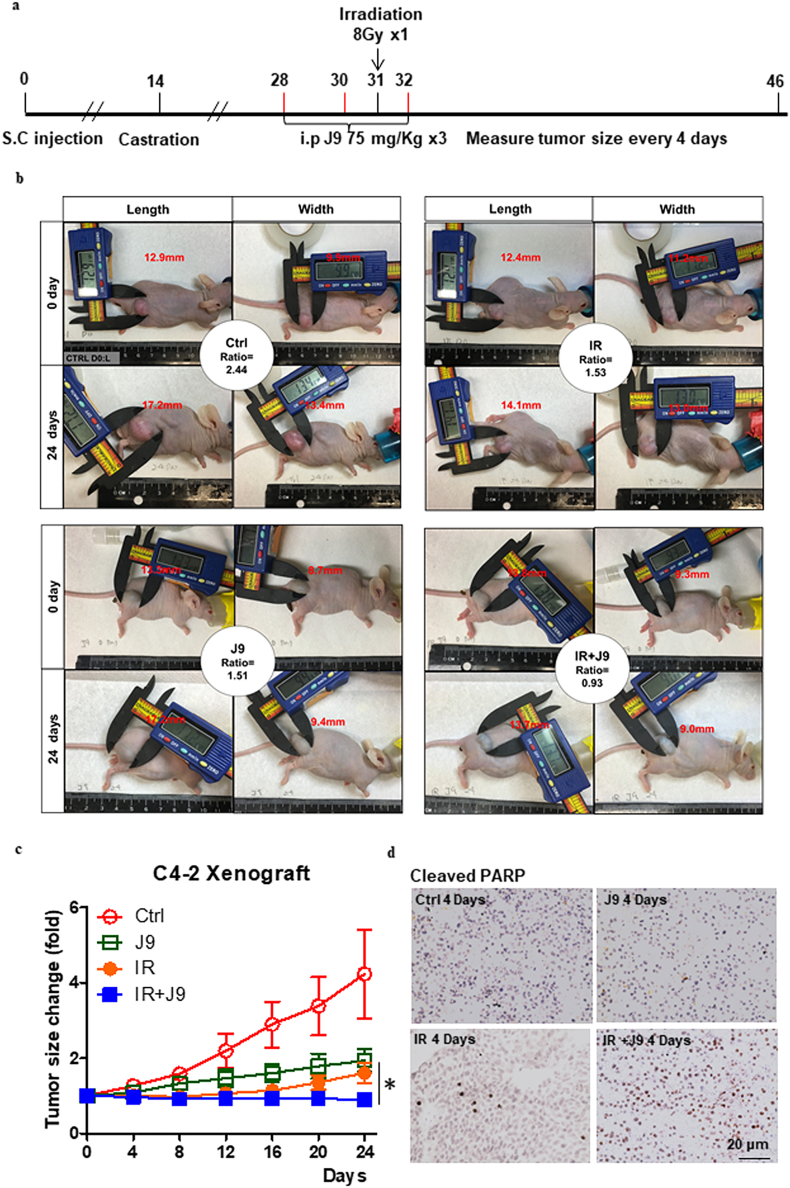
2.13. Formation of xenograft and γ-radiation
C4-2 cells (5 × 105) were injected s.c. into the right flank of 6–8 week-old nude mice (National Cancer Institute, Rockville, MD, USA). Mice were castrated 14 days after tumor cell inoculation. Xenografts reaching >100 mm3 were i.p. injected with vehicle or 75 mg/kg ASC-J9® every other day, for a total of 3 times. Mice were exposed to 8Gy γ-ray (IR) at 16 h after the second ASC-J9® treatment, but the mock control or ASC-J9® only group were not irradiated. Mice were anesthetized i.p. with Avertin (2,2,2, tribromoethanol, # T48402-25G, Sigma-Aldrich) (when irradiated) or isofurane (to measure tumor sizes) (NDC 11695-0500-2, Henry Schein, Melville, NY, USA). Xenografts were locally irradiated with a Model 8114 600 Ci Shepherd Cs137 irradiator (URMC), while other body parts were protected with lead shielding. Tumor regrowth curves of the mice were assessed for an additional 24 days. Tumor sizes were established with calipers (VWR, Radnor, PA, USA), and the volume of each xenograft calculated as follows: [(short axis2 × long axis)/2]. Ethics statement: The PCa xenograft mice study protocol was approved by the University Committee on Animal Resources (UCAR) and monitored by the Radiation Safety Unit. All the nude mice were ordered from NCI and were maintained in URMC vivarium facilities which is accredited by Accreditation of Laboratory Animal Care International (AAALAC).
2.14. Statistics
The data values were presented as the mean ± SEM. Differences in mean values between two groups were analyzed by two-tailed Student's t-test. The p ≤ 0·05 was considered statistically significant. Error bars represent SEM from three independent fields under microscopy and/or from at least 3 independent experiments. Limitation: multiple comparisons are made without adjustment for multiple-hypothesis testing (e.g. Bonferroni's correction).
3. Results
3.1. Radiation increased AR expression in castration resistant prostate cancer cells
According to NCCN Guidelines, RT combined with ADT with antiandrogens to either reduce androgen synthesis or prevent androgens from binding to AR are used currently as the standard therapy to treat advanced PCa [22]. While several studies indicated that ADT might have some adverse effects [23], the impact of RT on the AR expression remains unclear.
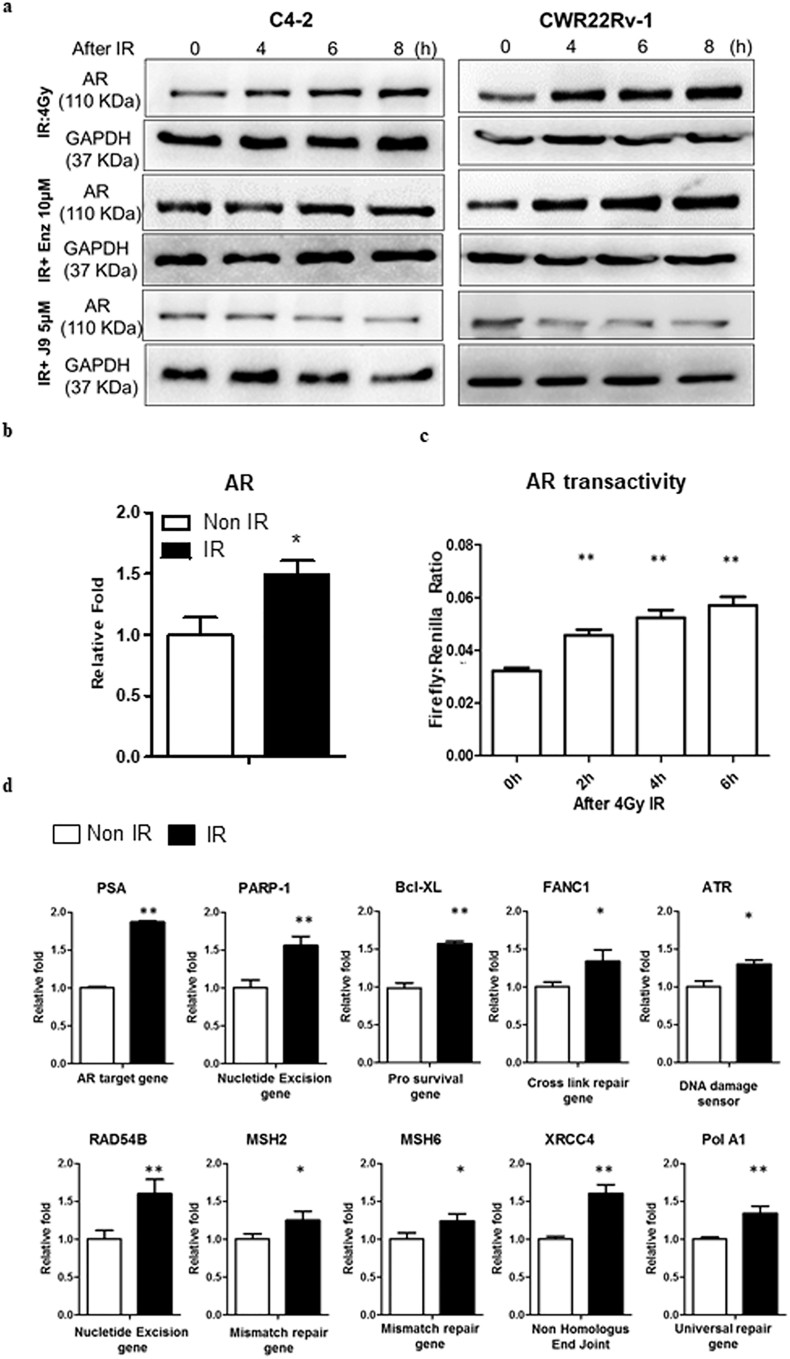
To study the potential RT effects on the AR expression, we first exposed the castration resistant prostate cancer (CRPC) C4-2 and CWR22Rv-1 cells to 4Gy IR under the castration conditions (1 nM DHT), and results revealed that radiation could increase AR expression at both mRNA and protein levels (Fig. 1a-b, respectively), which might then increase the AR transactivation (Fig. 1c) and AR downstream targets related to the DNA damage response (DDR) signals (Fig. 1d).
Fig. 1.
The AR protein, transactivity and AR downstream DDR gene expression levels are elevated after IR. (a) After IR the AR protein level increased in a time dependent-manner (upper panels) in C4-2 and CWR22Rv-1 cells. Treating cells with ASC-J9® (J9) and IR can inhibit AR increase (lower panels). Enz treatment does not show the similar effect (middle panels). (b) The qPCR results show AR RNA levels increased in C4–2 cells after IR. (c) Luciferase activity reporter assays demonstrate that IR increases AR transactivity in C4-2 cells. (d) The qPCR results of several AR target genes and downstream DDR genes expressions are elevated in C4–2 cells after IR.For b-d, data are presented as Mean ± SEM, *P < 0·05, **P < 0·01.
This unexpected finding showing radiation may increase AR protein may have significant clinical implications, since elevated AR may counteract effects of ADT with antiandrogens. Elevated AR indeed has many adverse effects on the continuation of RT or subsequent ADT treatment to suppress PCa progression.
We also confirmed our finding via targeting the radiation-increased AR with either the AR degradation enhancer ASC-J9® or AR-shRNA, or FDA-approved antiandrogen Enzalutamide (Enz, also named MDV3100). As expected, we found combining IR with ASC-J9® treatment suppressed the IR-induced AR protein expression, yet IR combined with Enz failed to suppress the IR-induced AR protein expression (Fig. 1a).
3.2. ASC-J9® increased radiosensitivity in CRPC cells with little injury to normal bladder cells
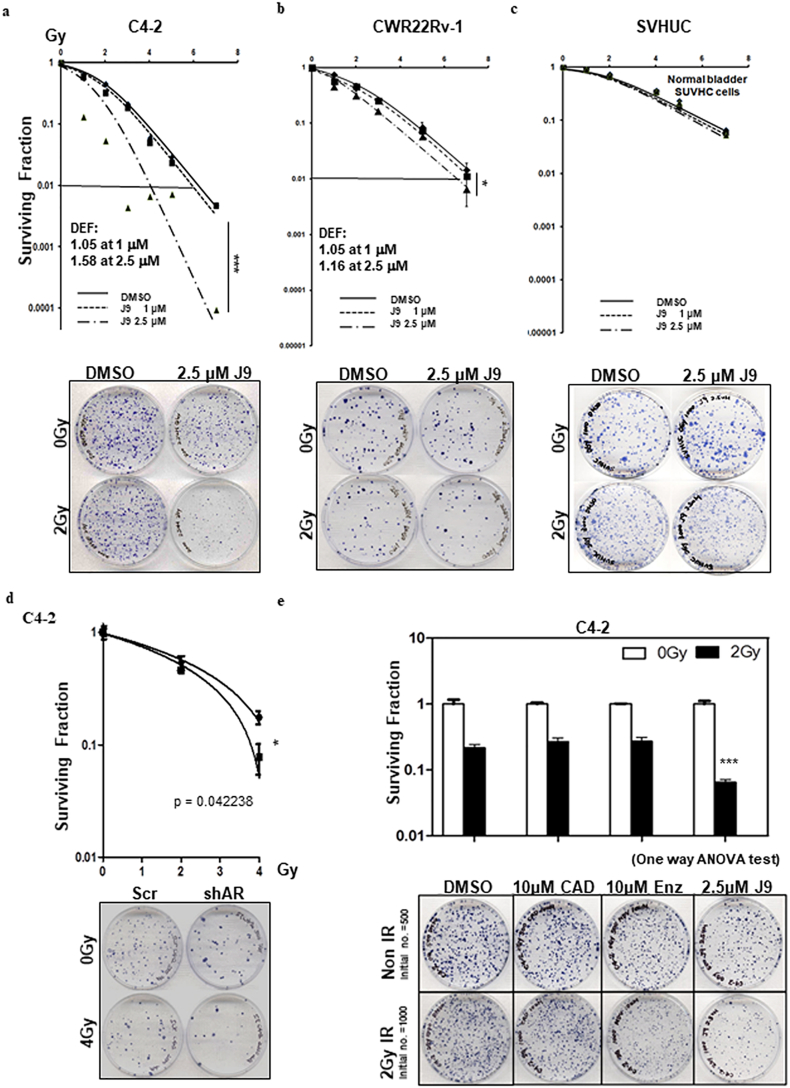
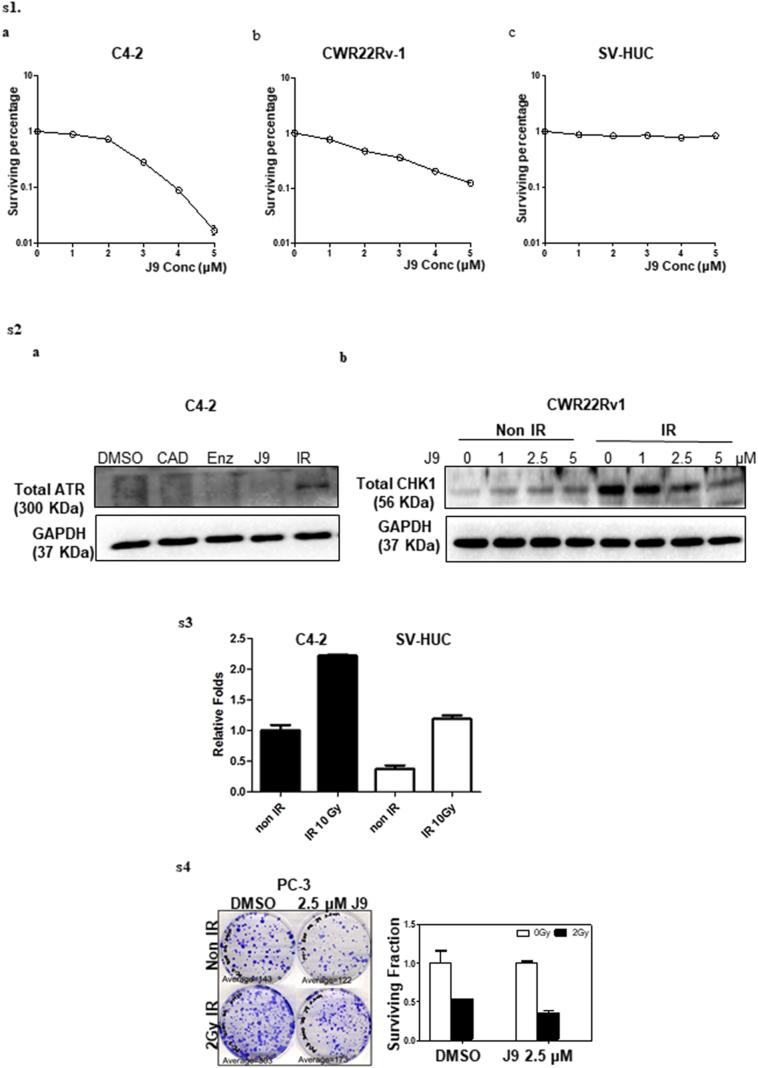
To examine the potential impacts ASC-J9® on the radiosensitivity, we applied the clonogenic forming assay to measure radiosensitivity on CRPC vs bladder epithelial SV-HUC cells to evaluate the possible adverse effects in bladder tissue, which is adjacent to the prostate during RT. We treated C4-2 cells with a sub-lethal dose of ASC-J9® (1 μM or 2·5 μM, see the sub-lethal dose condition test in Supplementary Fig. S1a-c) for 6 h and then irradiated with various doses (from 1 Gy–7 Gy) of IR, and results revealed that treating with 2·5 μM ASC-J9® followed by IR significantly reduced clonogenic survival, compared to the radiation alone or ASC-J9® treatment alone (Fig. 2a). Similar, but not as dramatic, results were observed when we replaced C4–2 with CWR22Rv-1 cells (Fig. 2b).
Fig. 2.
Differential radiosensitization effects of ASC-J9® in prostate cancer cells vs. normal bladder cells. (a-b) Clonogenic survival was reduced with the combined treatment of ASC-J9® (J9) + IR in C4-2 (a) and CWR22Rv1 (b) cells. (c) ASC-J9® + IR together results in only very minor effects in SV-HUC cells. (d) AR-shRNA + IR decreased clonogenic survival rate compared to scr control + IR. (e) Treatments with current antiandrogens could not enhance radio-sensitivity in C4–2 cells compared to ASC-J9® treatment. Three independent experiments were performed in triplicate and mean ± SEM has been plotted. Representative images are shown in bottom panels of each section. *P < 0·05, **P < 0·01, ***P < 0·001.
We want to know whether combining IR with ASC-J9® damaged normal bladder cells, may result in a potential adverse effect on the urinary bladder (radiation cystitis), since it is critical for radiosensitizers to selectively target tumor cells with little damage to the neighboring normal cells. We applied the in vitro assay of normal bladder cells to test this concept. The results revealed that ASC-J9® treatment resulted in little damage on the normal bladder SV-HUC cells (Fig. 2c). These results match well the early studies showing ASC-J9® could degrade AR with fewer adverse effects in various mouse models.
Similar results were also obtained when we replaced ASC-J9® with AR-shRNA to decrease AR expression in C4-2 cells (Fig. 2d), suggesting that targeting AR protein contributes to increased radiation sensitivity to better suppress PCa.
In contrast, we found treatment with Enz failed to decrease AR protein expression and could not provide the similar benefit to increase radiation sensitivity as 2·5 μM ASC-J9® (Fig. 2e).
Together, results from Fig. 2a-e and Supplementary Fig. S1 suggest that treating with ASC-J9® to decrease the IR-increased AR protein expression can increase radiosensitivity to better suppress PCa without damaging normal bladder cells.
3.3. Mechanism dissection of why ASC-J9®-decreased AR expression can increase RT sensitivity: inducing apoptosis via altering the AR dependent ATR-CHK1 pathway
To dissect the mechanisms of why ASC-J9®-decreased AR expression increases RT sensitivity, we focused on the following 3 different mechanisms: apoptosis, suppressing the DNA damage response (DDR) system, and inducing the DNA damage pathways [24].
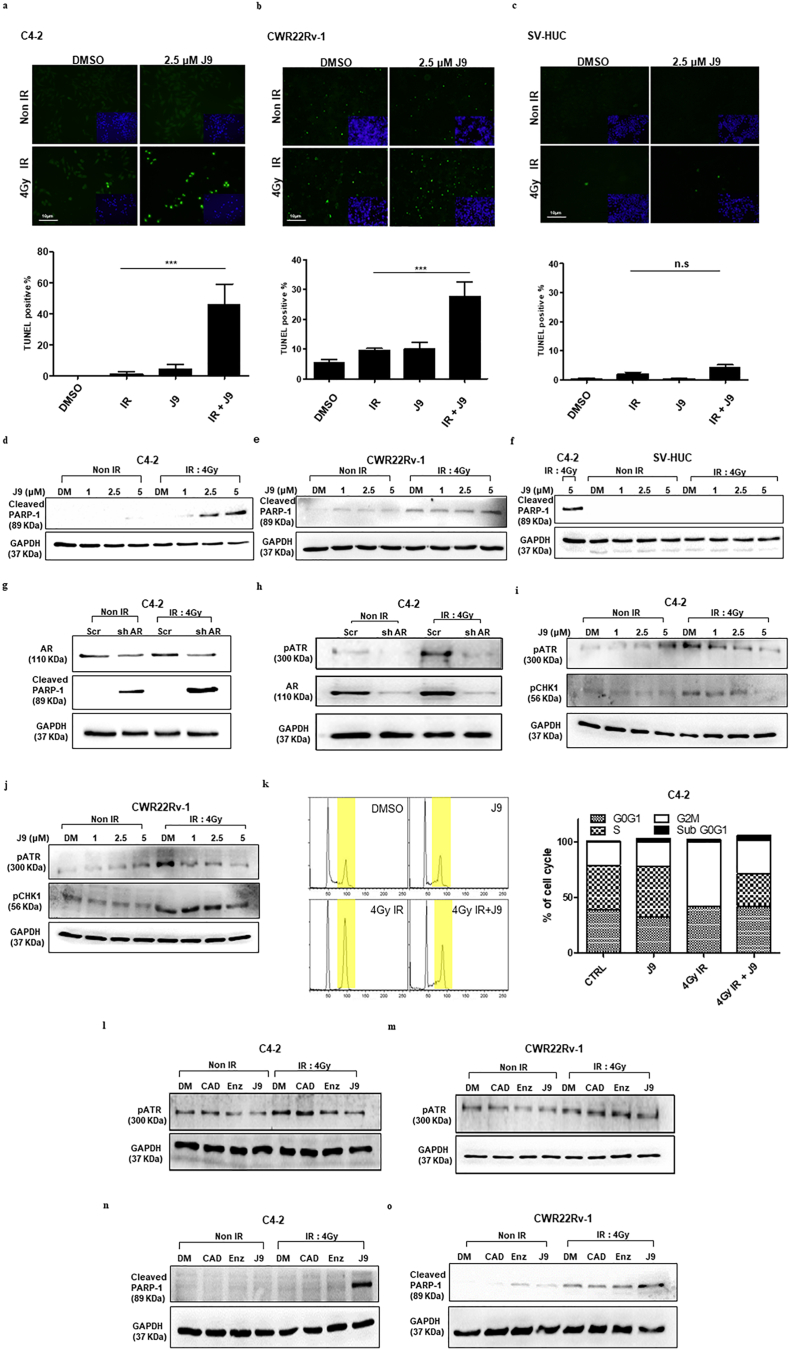
We first examined the impact of ASC-J9® on IR-induced apoptosis in PCa cells, since early studies indicated that PCa was highly resistant to γ-radiation-induced apoptosis [25]. Using the TUNEL assay, we found that treatment with ASC-J9® could overcome the resistance to IR via altering the PCa cell apoptosis. We found that 2·5 μM ASC-J9® plus 4Gy IR could significantly increase the TUNEL positive population in C4-2 (Fig. 3a) and CWR22Rv-1 (Fig. 3b) cells. Mechanism dissection indicated that ASC-J9® treatment might mediate IR-induced apoptosis via activation of the atypical PARP cleavage in C4-2 (Fig. 3d) and CWR22Rv-1 (Fig. 3e) cells. In contrast, we found little effects on normal bladder SV-HUC cell apoptosis (Fig. 3c and f). Similar results were also obtained when we replaced ASC-J9® with AR-shRNA in C4–2 cells to decrease AR, suggesting IR-induced apoptosis is AR protein dependent (Fig. 3g).
Fig. 3.
The TUNEL positive cells are increased and apoptosis is promoted via atypical PARP after combination treatment of ASC-J9® and IR in PCa cells. (a-b) TUNEL-positive cells were significantly increased in the ASC-J9® (J9) + IR treated C4-2 (a) and CWR22Rv-1 (b) cells. (c) Fewer TUNEL-positive cells were shown in IR + J9 treated SV-HUC cells. (d–e) Western blot shows cleaved PARP-1 increases in C4-2 (d) and CWR22Rv-1 (e) cells. (f) The cleaved PARP-1 does not show expression in IR + J9 treated SV-HUC cells. (g–h) Transducing C4–2 (g) cells with scr or shAR, then applying IR, can enhance atypical PARP-1 cleavage and can suppress pATR (h). (i–j) Treating with ASC-J9® can suppress IR induced pATR-CHK1 activation in C4–2 (i) and CWR22Rv1 (j) cells. (k) Treating cells with ASC-J9® for 16 h, then irradiating cells, can result in G2M phase arrest bypass (left) in C4–2 cells (quantitation at right). (l–m) ASC-J9® treatment could suppress IR-induced ATR phosphorylation, but not treatment with antiandrogen CAD or Enz in C4–2 (l) and CWR22Rv1 (m) cells compared to DMSO (DM) treatment group. (n-o) ASC-J9®, but not CAD or Enz treatment, could trigger IR-induced apoptosis in C4–2 (n) and CWR22Rv1 (m) cells. For A-C, data are presented as Mean ± SEM, *P < 0·05, **P < 0·01, ***P < 0·001, n.s = not significant.
To further dissect the mechanism why ASC-J9®-decreased AR could trigger the IR-induced apoptosis, we then focused on the ataxia telangiectasia and Rad3-related protein (ATR) kinase, the key damage sensors for detecting DNA breaks and DDR molecular recruitment [26], as well as Checkpoint kinase 1 (CHK1) [27]. Early studies indicated that ATR-CHK1 signals regulated the cell cycle G2M phase arrest after DNA damage and it was a key step for DNA repair and cell survival after IR [28]. Bypassing cell cycle arrest causes chromosome segregation without complete repair, which might lead to mitotic catastrophe and result in activation of cell apoptosis [29]. Importantly, recent studies indicated that agents that could target pATR-CHK1 signals (e.g. VE821) might trigger IR-induced apoptosis and further increase radio-sensitivity [30].
We found transducing AR-shRNA in C4-2 cells decreased AR protein expression, which might then suppress the expression of pATR (Fig. 3h). As expected, treating with ASC-J9® to degrade AR protein could also suppress pATR and its downstream target pCHK1 in C4-2 and CWR22Rv-1 cells (Fig. 3i-j; we demonstrated that after IR the total ATR and CHK1 were not ideal loading controls, see Supplementary Fig. S2a-b). The consequences of such AR-suppression might then bypass the G2M phase arrest in cells (Fig. 3k and Supplementary Table S2).
As expected, treating with the antiandrogens Casodex (CAD) and Enz failed to suppress pATR expression in C4-2 and CWR22Rv-1 cells (Fig. 3l-m, respectively), since these antiandrogens failed to decrease AR protein expression, and therefore also failed to trigger the radiation-induced PCa apoptosis in both cell lines (Fig. 3n-o).
Together, results from Fig. 3a-o, Supplementary Fig. S2, and Supplementary Table S2 suggest ASC-J9®, but not CAD or Enz, may function via degrading radiation-induced AR to increase radiation sensitivity via overcoming the resistance of radiation-induced PCa apoptosis that may involve altering the pATR-pCHK1 signals.
3.4. Mechanism dissection of why ASC-J9®-decreased AR expression can increase RT sensitivity: suppressing the DNA repair pathway
To evaluate the 2nd mechanism of how ASC-J9®-decreased AR expression increased radio-sensitivity, we focused measuring the γ-H2AX, an in situ marker, to check un-repaired DNA breaks since early studies indicated visualization of γ-H2AX allowed the assessment of DNA repair [31].
Our results in C4-2 and CWR22Rv-1 cells revealed that persistent γ-H2AX foci numbers (Fig. 4a-b) and amounts (Fig. 4c-d) increased after IR in an ASC-J9® dose-dependent manner after 6 h treatment with ASC-J9®, suggesting that ASC-J9® has the capacity of increasing radio-sensitivity through suppressing the DNA repair response. As expected in both cell lines, treating with CAD or Enz failed to suppress γ-H2AX amount (Fig. 4e–f) since these anti-androgens failed to decrease AR protein expression, and also failed to alter the DDR activation.
Fig. 4.
ASC-J9® treatment increases the persistence of γ-H2AX and suppresses DDR.(A-B) Immunofluorescence image of γ-H2AX (Ser139) persistence at 24 h after IR with/without ASC-J9® (J9) treatment under confocal microscope (60× field) in C4–2 (a) and CWR22Rv1 (b) cells. (c-d) Western blot of γ-H2AX persistence at 24 h after IR treatment with/without ASC-J9® in C4–2 (c) and CWR22Rv1 (d) cells. (e-f) Antiandrogens (10 μM CAD and 10 μM Enz) fail to maintain γ-H2AX persistence in C4–2 (e) and CWR22Rv1 (f) cells. (g) ASC-J9® treatment suppresses IR induced homologous recombination in 293 T cells, quantitation at right. (h) RNA-seq results showing AR downstream DDR genes were suppressed by ASC-J9® treatment. For G, data are presented as Mean ± SEM, *P < 0·05, **P < 0·01, ***P < 0·001.
We then applied the homologous recombination repair (HR) in the C4-2 cell line to confirm the above conclusions, and results revealed that IR triggered HR, and pre-treatment with ASC-J9® suppressed the HR effect (Fig. 4g).
Importantly, when we applied RNAseq assay to globally analyze AR downstream DDR genes based on previous study [32], we found that 50% had significant suppression after ASC-J9® treatment, and 47% had the suppression tendency (Fig. 4h and Supplementary Table S3).
Together, results from Fig. 4a-h and Supplementary Table S2–S3 suggest that ASC-J9® may also function through suppressing the radiation-induced DNA repair process to alter the radiosensitivity.
3.5. Mechanism dissection of why ASC-J9®-decreased AR expression can increase RT sensitivity: inducing DNA damage by ROS generation and altering GSH level
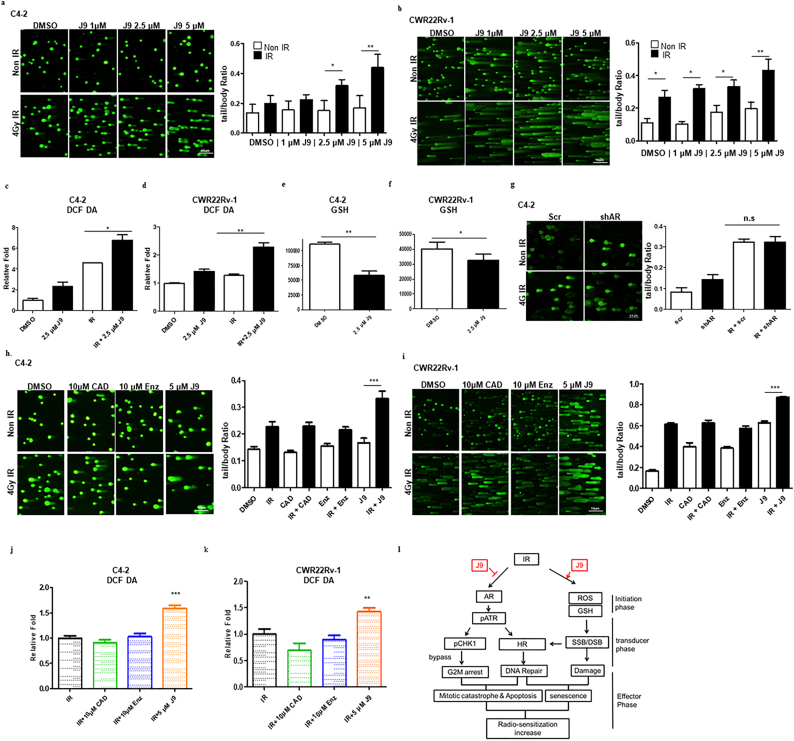
The 3rd mechanism of how ASC-J9®-decreased AR expression can increase radio sensitivity may involve altering the peroxidative damage with modulating the intra-cellular reactive oxygen species (ROS) to enhance the DNA damage [33]. We first applied the alkaline comet assay [34] to evaluate the overall level of DNA breaks (including SSB and DSB) after ASC-J9® treatment + IR. PCa cells were treated with ASC-J9® for 6 h and then exposed to 4 Gy IR. The results in Fig. 5a–b revealed that ASC-J9® with IR dramatically enhanced IR-induced DNA damage (increased tail/body ratio) as compared to control groups in both the C4-2 (Fig. 5a) and CWR22Rv-1 (Fig. 5b) cells. We then measured the ROS generation through detecting DCF-DA, an indicator for cellular ROS, since early studies indicated that the major source of RT-induced DNA damage is via ROS production [35]. Results in Fig. 5c–d revealed that treatment of C4-2 (Fig. 5c) and CWR22Rv-1 (Fig. 5d) cells with ASC-J9® for 6 h before IR dramatically increased ROS generation, compared to IR alone. Endogenous GSH, an important antioxidant that prevents oxidative damage was also obviously decreased in C4–2 and CWR22Rv-1 cells treated with ASC-J9® (Fig. 5e-f).
Fig. 5.
ASC-J9® could enhance the RT-induced DNA damage through elevating ROS and reducing GSH levels. (a-b) Alkaline Comet assay showing increased DNA damage when C4–2 (a) and CWR22Rv1 (b) cells were treated with ASC-J9® (J9) for 6 h prior to IR. Quantification of fluorescence intensity of body/tail ratio at right. (c-d) ROS level as measured by DCF-DA fluorescence in C4–2 (c) and CWR22Rv1 (d) cells with/without ASC-J9® treatment and 10 Gy IR. (e-f) Endogenous GSH after ASC-J9® treatment in C4–2 (e) and CWR22Rv1 (f) cells. (g) AR-shRNA (shAR) + IR does not dramatically enhance DNA damage in C4–2 cells, quantitation at right. (h-i) ASC-J9® (5 μM) treatment with antiandrogens (10 μM CAD and 10 μM Enz), could enhance IR induced DNA damage in C4–2 (h) and CWR22Rv-1 (i) cells, with quantitation at right. (j-k) ASC-J9® (5 μM), but not 10 μM CAD or Enz treatment, increases ROS level in C4–2 (j) and CWR22Rv1 (k) cells as measured by DCF-DA. (l) Schematic representation of the roles of ASC-J9® in RT. For ROS (c-d and j-k) and GSH (e-f) measurement, data plotted were mean ± SEM (n = 3). *P < 0·05, **P < 0·01, ***P < 0·001 in comparison to respective DMSO group.
Interestingly, in C4–2 cells, when we replaced ASC-J9® with AR-shRNA to suppress AR protein in the comet assay, we failed to see the obvious DNA damage effect (Fig. 5g), suggesting ASC-J9®-enhanced DNA damage may function via an AR-independent signal. Furthermore, treating the C4–2 (Fig. 5h) and CWR22Rv-1 (Fig. 5i) cells with CAD and Enz also failed to boost the DNA damage (increased tail/body ratio) or alter the ROS generation (Fig. 5j–k).
Together, results from Fig. 5a–k showing that ASC-J9®, and not AR-shRNA, CAD, or Enz, could modulate the ROS to alter the DNA damage, indicated that ASC-J9® may function via its unique polyphenols structure, and not an anti-AR mechanism, to modulate oxidative stress [36]. Further study of this ASC-J9® AR-independent mechanism to alter the DNA damage may help us to better understand these interesting findings.
3.6. Preclinical study using the in vivo mouse model to demonstrate that ASC-J9® can increase radiosensitivity to better suppress PCa progression
To demonstrate all above in vitro data showing ASC-J9® could increase radiosensitivity to better suppress PCa progression in the in vivo mouse model, we xenografted 5 × 105 C4-2 cells into nude mice, and mice were castrated 2 weeks after tumors formed. On the 14th day after castration, mice were treated with i.p. injections of ASC-J9® at 75 mg/kg/on days 28, 30, and 32 (after cells injection), with exposure to 8-Gy dose radiation on day 31 (Fig. 6a). The results revealed that both IR alone or ASC-J9® treatment alone decreased PCa tumor progression. Importantly, IR combined with ASC-J9® led to better suppression of PCa progression (Fig. 6b) with the quantitation shown in Fig. 6c. In addition, the tumors excised after sacrifice showed the stronger apoptosis marker, cleavage of PARP (Fig. 6d).
Fig. 6.
Tumor regrowth and apoptosis in xenograft nude mouse model. C4–2 cells were subcutaneously xenografted on the flanks of nude mice for 4 treatment groups, including non- IR, 8Gy IR, 75 mg/kg ASC-J9®, and 8Gy IR + J9. (a) Photograph for treatment of xenografted nude mouse model. (b) Representative images of each group at Day 0 and Day 24 (the middle circles contain group information and growth ratio of representative mice). (c) Tumor growth curve over time (n = 5), final tumor growth ratio was normalized with Day 0 value and compared by unpaired student t -test. (d) IHC staining of cleaved PARP-1 at 4 days after IR treatment in C4–2 cells, *P < 0·05, **P < 0·01, ***P < 0·001.
4. Discussion
One of the major limitations of RT in suppressing solid tumors, including PCa, is that the solid tumor cells are in a hypoxic environment [37] that reduces the efficacy for RT. Radiation's ability to kill cancer cells rapidly decreases in areas of oxygen depletion, because production of free radicals is reduced. Cancer cells with lower ROS status might be 2–3 times more resistant to RT than those in the normal condition [38], thereby reducing survival rates in patients. Our results clearly revealed that in PCa cells, adding ASC-J9® before IR dramatically increased ROS and decreased GSH generation compared to IR alone (Fig. 5c–f). Hence altered ROS status is one of the key mechanisms by which ASC-J9® enhances IR efficacy in our studies.
The use of neoadjuvant ADT together with RT has been used to increase overall survival and cure rates. In addition, ADT also reduced PCa tumor sizes to allow using lower doses of IR to suppress PCa [39]. Other reports indicated that ADT in combination with RT might also be able to delay the development of castration resistant PCa [[39], [40], [41]]. However, most, if not all, current ADT using antiandrogens may function via preventing or reducing androgens from binding to AR and have little capacity to suppress AR expression [42]. Importantly, accumulating evidence demonstrates that androgen effects are not equal to AR effects, because other factors, such as growth factors, kinases, cytokines or co-regulators, can also function in a similar manner as androgens to transactivate AR in the castration resistant condition [[43], [44], [45], [46], [47]]. In addition, AR variants (ARVs) have been identified to contribute to radioresistance [48]. Although the current ADT and antiandrogens have certain effects to enhance RT efficacy [49], these compounds failed to suppress ARVs mediated DDR. Therefore, targeting the remaining functional AR/ARVs after ADT may have better therapeutic efficacy in suppressing PCa progression during the castration resistant stage [50,51].
Our unexpected finding that RT could enhance AR expression raised very important clinical questions as to whether the RT-enhanced AR might interrupt/reduce the continuation of RT therapy efficacy and if the RT-enhanced AR might also be able to reduce the efficacy of subsequent ADT with various antiandrogens after the development of the RT resistance. Our results reveal that using RT plus anti-AR agents, such as ASC-J9® or AR-shRNA (Fig. 2a–d), yield better efficacies to suppress PCa. Since ASC-J9® is a much smaller molecular compound, as compared to AR-shRNAs or microRNAs, it can easily be used for in vivo delivery [[52], [53], [54], [55]], and early in vivo mice studies using i.p injection or oral gavage with ASC-J9® have demonstrated clearly the AR degradation with few adverse effects [42,[56], [57], [58], [59], [60], [61]]. Importantly, results reported here further identified ASC-J9® not only has an AR degradation effect, it also has an AR independent mechanism to increase IR efficacy with little adverse effects or indeed, a slightly protective effect on the neighboring normal bladder cells during IR.
As RT with a higher dose of γ-radiation may also result in damage to normal tissues, especially normal bladder tissue, several radiosensitizers including Bevacizumab [62], Isoflavones [63,64], Panobinostat [65] and Sunitinib [66], have been developed to enhance the RT efficacy without increasing the γ-radiation dosage. Mechanistic studies suggested that these radiosensitizers might be able to function through multiple signals to alter the RT efficacy. For example, soy isoflavones were found to be able to enhance the efficacy of RT via inhibiting cell survival pathways with extra anti-oxidant and anti-inflammatory activities [63,64]. Results from ASC-J9® studies suggest that this drug might function through multiple mechanisms including alteration of the pATR/CHK1 pathway to accelerate the RT-induced apoptosis, suppressing DDR system and inducing the ROS production.
Among the above three major mechanisms we studied, we found replacing ASC-J9® with AR-shRNA to knock down AR allowed us to obtain similar results in activating cell apoptosis effects (Fig. 3g) and suppressing the ATR/CHK1 pathway (Fig. 3h), but not the ratio of tail/body in the comet assay (Fig. 5g). One of the possible mechanisms that underlie these contrasting results could be that knocking-down of AR with AR-shRNA failed to alter the intracellular GSH level as compared to ASC-J9® treatment, which might then fail to boost the ROS levels during combining IR with AR-shRNA treatment. These contrasting results suggest that ASC-J9® may have additional non-AR-mediated functions, beyond degrading AR, to enhance IR efficacy.
Finally, to better explain why ASC-J9® can increase IR efficacy to suppress PCa cells, and have few adverse effects on the normal bladder cells that neighbor the PCa cells, we hypothesize that there may be higher basal levels of ROS existing in PCa cells than in the neighboring normal bladder cells [67]. Combining ASC-J9® plus IR may result in an overall increase in endogenous ROS in PCa cells, which may exceed their tolerance threshold and suppress PCa cell survival even in AR negative PC-3 cells (Supplementary Fig. S3). In contrast, the neighboring normal bladder cells with a much lower basal level of ROS [68] may have much higher tolerance for ASC-J9®-induced ROS. We also found that normal bladder cells generated much less ROS during the combined therapy of ASC-J9® treatment with IR (Supplementary Fig. S4).
In summary, this preclinical study demonstrated that in both in vitro cell lines and the in vivo mouse model, a regimen that combines IR with ASC-J9® treatment not only provided better efficacy over the current conventional RT + ADT with antiandrogen treatment to reduce PCa tumor size, it also resulted in no adverse effects on normal bladder cells. The limitation of this study is that even if ASC-J9®, as a radiosensitizer, truly improves RT outcomes, the effect is likely limited to decreasing local recurrences. For patients who eventually have distant rather than local recurrence, we did not set up an ideal model to evaluate the risk. In addition, with the in vivo model (subcutaneous implantation) in our study, we still cannot precisely evaluate the impact on the bladder function. The image guiding RT for small animal plus orthotopic PCa model, and bladder functional tests can help us to better address this question in future studies.
These results suggest that combining ASC-J9® treatment with IR has the potential to be a novel therapy to help clinicians to better suppress PCa progression in humans.
The following are the supplementary data related to this article.
Supplementary Fig. S1.
a–c. Clonogenic forming ability under serial doses of ASC-J9®. 14 days result in C4-2 (a), CWR22Rv-1 (b), and SV-HUC (c) cells after 6 h ASC-J9® treatment; Supplementary Fig. S2: Protein level of total ATR and total CHK1 change after IR. (a) total ATR (b) total CHK1; Supplementary Fig. S3: ASC-J9® treatment has partially effect in AR negative PCa cells. Clonogenic assay result of PC-3 cells; Supp Fig. S4: ROS levels in PCa and normal bladder cells. DCF-DA value in same numbers of C4–2 and SV-HUC cells before and after IR.
See Supplementary data.
Funding sources
This work was supported by NIH grant CA156700 and George Whipple Professorship Endowment, and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002).
Declarations of conflicts of interest
ASC-J9® was patented by the University of Rochester, University of North Carolina, and AndroScience, and then licensed to AndroScience. Both the University of Rochester and author C. Chang own royalties and equity in AndroScience. The remaining authors have nothing to disclose as individuals.
Contribution statement
FC (1st author) performed the experiments, designed studies, collected data, and wrote manuscript draft. YC (equal contribution author) designed the studies, and assisted with data interpretation. YS provided technical support. DC helped with paper revision. YN and GL worked on data analysis. PK helped with data interpretation. SY designed the studies and CC helped design studies and finished final manuscript writing.
Acknowledgments
We thank Mrs. Karen Wolf for help with editing and revision.
References
- 1.Center M.M., Jemal A., Lortet-Tieulent J., Ward E., Ferlay J., Brawley O. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012;61(6):1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Warde P., Mason M., Ding K., Kirkbride P., Brundage M., Cowan R. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378(9809):2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dal Pra A., Cury F., Souhami L. Combining radiation therapy and androgen deprivation for localized prostate cancer—a critical review. Curr. Oncol. 2010;17(5):28. doi: 10.3747/co.v17i5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki M., Amano M., Choi J., Park H.J., Williams B.W., Ono K. Synergistic effects of radiation and beta-lapachone in DU-145 human prostate cancer cells in vitro. Radiat. Res. 2006;165(5):525–531. doi: 10.1667/RR3554.1. [DOI] [PubMed] [Google Scholar]
- 5.Fang Y., DeMarco V.G., Nicholl M.B. Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis. Cancer Sci. 2012;103(6):1090–1098. doi: 10.1111/j.1349-7006.2012.02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazareth L.V., Weigel N.L. Activation of the human androgen receptor through a protein kinase A signaling pathway. J. Biol. Chem. 1996;271(33):19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 7.Mowszowicz I., editor. Mechanisms and paradoxical effects. Annales d'endocrinologie. 1988. Antiandrogens. [Google Scholar]
- 8.Magon N. Gonadotropin releasing hormone agonists: Expanding vistas. Indian J Endocrinol. Metabol. 2011;15(4):261. doi: 10.4103/2230-8210.85575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores E., Bratoeff E., Cabeza M., Ramirez E., Quiroz A., Heuze I. Steroid 5alpha-reductase inhibitors. Mini-Rev. Med. Chem. 2003;3(3):225–237. doi: 10.2174/1389557033488196. [DOI] [PubMed] [Google Scholar]
- 10.Launay-Vacher V., Ayllon J., Janus N., Spano J.P., Ray-Coquard I., Gligorov J. Drug management of prostate cancer: prevalence and consequences of renal insufficiency. Clin Genitourin Cancer. 2009;7(3):E83–E89. doi: 10.3816/CGC.2009.n.029. [DOI] [PubMed] [Google Scholar]
- 11.Brueggemeier R.W., Li P.K. Fundamentals of steroid chemistry and biochemistry. Burger's Med. Chem. Drug Discov. 2003 Jan 15:1–34. [Google Scholar]
- 12.Culig Z., Hobisch A., Cronauer M.V., Radmayr C., Trapman J., Hittmair A. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54(20):5474–5478. [PubMed] [Google Scholar]
- 13.Hobisch A., Eder I.E., Putz T., Horninger W., Bartsch G., Klocker H. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58(20):4640–4645. [PubMed] [Google Scholar]
- 14.Abreu-Martin M.T., Chari A., Palladino A.A., Craft N.A., Sawyers C.L. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol. Cell. Biol. 1999;19(7):5143–5154. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadar M.D. Androgen-independent induction of prostate-specific antigen gene expression via cross-talk between the androgen receptor and protein kinase A signal transduction pathways. J. Biol. Chem. 1999;274(12):7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z., Chang Y.-J., Yu I.-C., Yeh S., Wu C.-C., Miyamoto H. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat. Med. 2007;13(3):348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- 17.Ma W.L., Hsu C.L., Wu M.H., Wu C.T., Wu C.C., Lai J.J. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135(3) doi: 10.1053/j.gastro.2008.05.046. [947-55. e5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai K.-P., Huang C.-K., Chang Y.-J., Chung C.-Y., Yamashita S., Li L. New therapeutic approach to suppress castration-resistant prostate cancer using ASC-J9 via targeting androgen receptor in selective prostate cells. Am. J. Pathol. 2013;182(2):460–473. doi: 10.1016/j.ajpath.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamashita S., Lai K.-P., Chuang K.-L., Xu D., Miyamoto H., Tochigi T. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia. 2012;14(1):74–83. doi: 10.1593/neo.111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu J.-W., Hsu I., Xu D., Miyamoto H., Liang L., Wu X.-R. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am. J. Pathol. 2013;182(5):1811–1820. doi: 10.1016/j.ajpath.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spratt D.E., Evans M.J., Davis B.J., Doran M.G., Lee M.X., Shah N. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 2015 Nov 15;75(22):4688–4696. doi: 10.1158/0008-5472.CAN-15-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denham J.W., Steigler A., Lamb D.S., Joseph D., Turner S., Matthews J. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12(5):451–459. doi: 10.1016/S1470-2045(11)70063-8. [DOI] [PubMed] [Google Scholar]
- 23.Li Y., Chan S.C., Brand L.J., Hwang T.H., Silverstein K.A., Dehm S.M. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73(2):483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunne A.L., Price M.E., Mothersill C., McKeown S.R., Robson T., Hirst D.G. Relationship between clonogenic radiosensitivity, radiation-induced apoptosis and DNA damage/repair in human colon cancer cells. Br. J. Cancer. 2003;89(12):2277–2283. doi: 10.1038/sj.bjc.6601427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nava V.E., Cuvillier O., Edsall L.C., Kimura K., Milstien S., Gelmann E.P. Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Res. 2000;60(16):4468–4474. [PubMed] [Google Scholar]
- 26.Sancar A., Lindsey-Boltz L.A., Unsal-Kacmaz K., Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 27.Stracker T.H., Usui T., Petrini J.H. Taking the time to make important decisions: the checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair. 2009;8(9):1047–1054. doi: 10.1016/j.dnarep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinhardt H.C., Yaffe M.B. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr. Opin. Cell Biol. 2009;21(2):245–255. doi: 10.1016/j.ceb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heffernan T.P., Kawasumi M., Blasina A., Anderes K., Conney A.H., Nghiem P. ATR-Chk1 pathway inhibition promotes apoptosis after UV treatment in primary human keratinocytes: potential basis for the UV protective effects of caffeine. J. Invest. Dermatol. 2009;129(7):1805–1815. doi: 10.1038/jid.2008.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bedi A., Barber J.P., Bedi G.C., El-Deiry W.S., Sidransky D., Vala M.S. BCR-ABL-mediated inhibition of apoptosis with delay of G2/M transition after DNA damage: a mechanism of resistance to multiple anticancer agents. Blood. 1995;86(3):1148–1158. [PubMed] [Google Scholar]
- 31.Kuo L.J., Yang L.X. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In Vivo. 2008;22(3):305–309. [PubMed] [Google Scholar]
- 32.Polkinghorn W.R., Parker J.S., Lee M.X., Kass E.M., Spratt D.E., Iaquinta P.J. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013 Nov;3(11):1245–1253. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girdhani S., Bhosle S.M., Thulsidas S.A., Kumar A., Mishra K.P. Potential of radiosensitizing agents in cancer chemo-radiotherapy. J. Cancer Res. Ther. 2005;1(3):129–131. doi: 10.4103/0973-1482.19585. [DOI] [PubMed] [Google Scholar]
- 34.Collins A.R. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol. Biotechnol. 2004;26(3):249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 35.Mikkelsen R.B., Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22(37):5734–5754. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 36.Mileo A.M., Miccadei S. Polyphenols as modulator of oxidative stress in cancer disease: new therapeutic strategies. Oxidative Med. Cell. Longev. 2016;2016:17. doi: 10.1155/2016/6475624. Article ID 6475624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown J.M. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007;435:297–321. doi: 10.1016/S0076-6879(07)35015-5. [DOI] [PubMed] [Google Scholar]
- 38.Rockwell S., Dobrucki I.T., Kim E.Y., Marrison S.T., Vu V.T. Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr. Mol. Med. 2009;9(4):442–458. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horwitz E.M., Bae K., Hanks G.E., Porter A., Grignon D.J., Brereton H.D. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J. Clin. Oncol. 2008;26(15):2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 40.Kupelian P.A., Mohan D.S., Lyons J., Klein E.A., Reddy C.A. Higher than standard radiation doses (> or =72 Gy) with or without androgen deprivation in the treatment of localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2000;46(3):567–574. doi: 10.1016/s0360-3016(99)00455-1. [DOI] [PubMed] [Google Scholar]
- 41.Hara I., Miyake H., Yamada Y., Takechi Y., Hara S., Gotoh A. Neoadjuvant androgen withdrawal prior to external radiotherapy for locally advanced adenocarcinoma of the prostate. Int. J. Urol. 2002;9(6):322–328. doi: 10.1046/j.1442-2042.2002.00470_1.x. [discussion 8] [DOI] [PubMed] [Google Scholar]
- 42.Lai K.P., Huang C.K., Chang Y.J., Chung C.Y., Yamashita S., Li L. New therapeutic approach to suppress castration-resistant prostate cancer using ASC-J9 via targeting androgen receptor in selective prostate cells. Am. J. Pathol. 2013;182(2):460–473. doi: 10.1016/j.ajpath.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balk S.P. Androgen receptor as a target in androgen-independent prostate cancer. Urology. 2002;60(3 Suppl 1):132–138. doi: 10.1016/s0090-4295(02)01593-5. [discussion 8-9] [DOI] [PubMed] [Google Scholar]
- 44.Sharifi N., Farrar W.L. Androgen receptor as a therapeutic target for androgen independent prostate cancer. Am. J. Ther. 2006;13(2):166–170. doi: 10.1097/00045391-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Mostaghel E.A., Page S.T., Lin D.W., Fazli L., Coleman I.M., True L.D. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67(10):5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 46.Page S.T., Lin D.W., Mostaghel E.A., Hess D.L., True L.D., Amory J.K. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J. Clin. Endocrinol. Metab. 2006;91(10):3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 47.Yuan X., Balk S.P. Mechanisms mediating androgen receptor reactivation after castration. Urol. Oncol. 2009;27(1):36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin Y., Li R., Xu K., Ding S., Li J., Baek G. Androgen receptor variants mediate DNA repair after prostate cancer irradiation. Cancer Res. 2017 Sep 15;77(18):4745–4754. doi: 10.1158/0008-5472.CAN-17-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghashghaei M., Paliouras M., Heravi M., Bekerat H., Trifiro M., Niazi T.M. Enhanced radiosensitization of enzalutamide via schedule dependent administration to androgen-sensitive prostate cancer cells. Prostate. 2018;78(1):64–75. doi: 10.1002/pros.23445. [DOI] [PubMed] [Google Scholar]
- 50.Sadar M.D. Advances in small molecule inhibitors of androgen receptor for the treatment of advanced prostate cancer. World J. Urol. 2012;30(3):311–318. doi: 10.1007/s00345-011-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mostaghel E.A., Plymate S.R., Montgomery B. Molecular pathways: targeting resistance in the androgen receptor for therapeutic benefit. Clin. Cancer Res. 2013 Feb 15;20(4):791–798. doi: 10.1158/1078-0432.CCR-12-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nadiminty N., Tummala R., Lou W., Zhu Y., Zhang J., Chen X. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J. Biol. Chem. 2012;287(2):1527–1537. doi: 10.1074/jbc.M111.278705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korpal M., Korn J.M., Gao X., Rakiec D.P., Ruddy D.A., Doshi S. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer Discov. 2013;3(9):1030–1043. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 54.Kim S.S., Cho H.J., Kang J.Y., Kang H.K., Yoo T.K. Inhibition of androgen receptor expression with small interfering RNA enhances cancer cell apoptosis by suppressing survival factors in androgen insensitive, late stage LNCaP cells. ScientificWorldJournal. 2013;2013:519397. doi: 10.1155/2013/519397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao X., Tang S., Thrasher J.B., Griebling T.L., Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol. Cancer Ther. 2005;4(4):505–515. doi: 10.1158/1535-7163.MCT-04-0313. [DOI] [PubMed] [Google Scholar]
- 56.Lin T.H., Izumi K., Lee S.O., Lin W.J., Yeh S., Chang C. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis. 2013;4 doi: 10.1038/cddis.2013.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin T.H., Lee S.O., Niu Y., Xu D., Liang L., Li L. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/Enzalutamide versus anti-androgen receptor ASC-J9(R) Lead to promotion versus suppression of prostate cancer metastasis. J. Biol. Chem. 2013;288(27):19359–19369. doi: 10.1074/jbc.M113.477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyamoto H., Yang Z., Chen Y.T., Ishiguro H., Uemura H., Kubota Y. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl. Cancer Inst. 2007;99(7):558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 59.Soh S.F., Huang C.-K., Lee S.O., Xu D., Yeh S., Li J. Determination of androgen receptor degradation enhancer ASC-J9® in mouse sera and organs with liquid chromatography tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014;88:117–122. doi: 10.1016/j.jpba.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamashita S., Lai K.P., Chuang K.L., Xu D., Miyamoto H., Tochigi T. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia. 2012;14(1):74–83. doi: 10.1593/neo.111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Z., Chang Y.J., Yu I.C., Yeh S., Wu C.C., Miyamoto H. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat. Med. 2007;13(3):348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- 62.Vuky J., Pham H.T., Warren S., Douglass E., Badiozamani K., Madsen B. Phase II study of long-term androgen suppression with bevacizumab and intensity-modulated radiation therapy (IMRT) in high-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012;82(4):e609–e615. doi: 10.1016/j.ijrobp.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad I.U., Forman J.D., Sarkar F.H., Hillman G.G., Heath E., Vaishampayan U. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr. Cancer. 2010;62(7):996–1000. doi: 10.1080/01635581.2010.509839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pendleton J.M., Tan W.W., Anai S., Chang M., Hou W., Shiverick K.T. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8:132. doi: 10.1186/1471-2407-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rathkopf D.E., Picus J., Hussain A., Ellard S., Chi K.N., Nydam T. A phase 2 study of intravenous panobinostat in patients with castration-resistant prostate cancer. Cancer Chemother. Pharmacol. 2013;72(3):537–544. doi: 10.1007/s00280-013-2224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corn P.G., Song D.Y., Heath E., Maier J., Meyn R., Kuban D. Sunitinib plus androgen deprivation and radiation therapy for patients with localized high-risk prostate cancer: results from a multi-institutional phase 1 study. Int. J. Radiat. Oncol. Biol. Phys. 2013;86(3):540–545. doi: 10.1016/j.ijrobp.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 68.Gupta S.C., Hevia D., Patchva S., Park B., Koh W., Aggarwal B.B. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox Signal. 2012;16(11):1295–1322. doi: 10.1089/ars.2011.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See Supplementary data.